Paraneoplastic cerebellar degeneration (PCD) is one of the most frequent paraneoplastic syndromes affecting the CNS. It is associated with antibodies targeting intracellular neuronal antigens (Hu, Yo, Ri, CV2/CRMP5), which are not thought to be directly pathogenic, and surface antigens (DNER, mGluR1, VGCC), which are potentially pathogenic.1,2 However, in many patients the immunologic target remains unidentified, resulting in diagnostic and therapeutic challenges. We report a patient with PCD and a squamous cell lung carcinoma with antibodies to a novel neuronal surface antigen, plasticity-related gene 5 (PRG5).
Case description.
A 72-year-old man presented with a 2-week history of progressive dizziness, vomiting, oscillopsia, dysarthria, and ataxia. Five months earlier he had been diagnosed with a poorly differentiated squamous cell lung carcinoma, which was radically resected. On examination, he had a symmetrical horizontal gaze-evoked nystagmus and saccadic pursuits. He had severe cerebellar dysarthria and trunk and limb ataxia and was unable to walk.
CT of the thorax showed a recurrence of the tumor in the mediastinum. MRI of the brain was normal. CSF examination showed pleocytosis (25 white blood cells/μL), elevated protein (1.05 g/L), normal glucose, and normal cytologic findings. All tests for classical paraneoplastic and published surface antibodies were negative (supplementary data at Neurology.org/nn). The patient received irradiation to the mediastinum after which his neurologic syndrome stabilized. He died 4 months after onset of disease.
Results.
Immunohistochemistry (IHC) of rat brain slices showed neuropil staining of cerebellum and hippocampus with patient serum and CSF but not control serum and CSF (figure 1A). Both serum and CSF labeled the surface of rat hippocampal neurons and stained the tips of dendritic spines (figure 1B). Using immunoprecipitation of whole rat brain lysate with patient serum and CSF followed by mass spectrometry analysis (as described in reference 3), we identified PRG5 as the autoantigen. PRG5 is a transmembrane protein enriched in plastic areas of the adult brain and involved in neurite outgrowth and the formation of dendritic spines.4,5
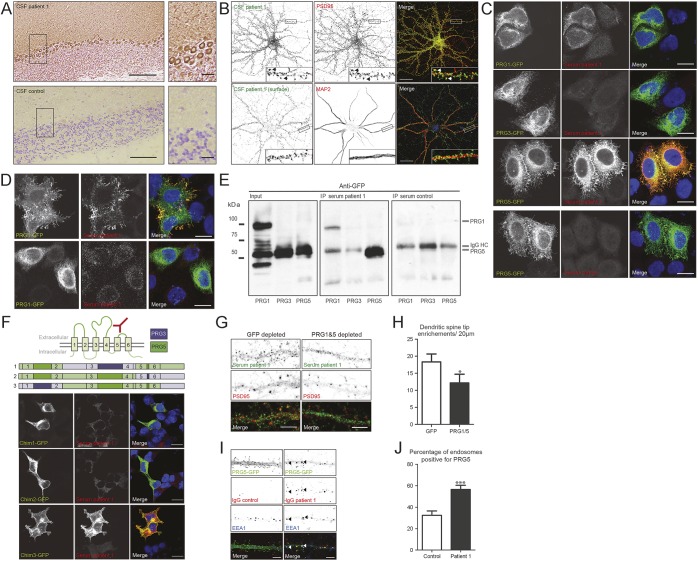
Figure 1. Identification and characterization of plasticity-related gene 5 (PRG5) as a neuronal surface autoantigen.
(A) Immunohistochemistry of adult rat cerebellum using patient CSF (top panel) and control CSF (bottom panel). The patient's CSF stains the neuropil of the cerebellar molecular layer and Purkinje cell cytoplasm. Scale bars: 100 μm in the overview, 20 μm in the magnification. (B) Immunocytochemistry of cultured rat hippocampal neurons (18 days in vitro [DIV]). The top panel shows a permeabilized staining with patient CSF (green) and anti-PSD95 (red) to mark the post synapse. The patient CSF labels the tips of both mature and immature dendritic spines. Arrows indicate colocalization between the patient CSF and PSD95 in the tips of mature dendritic spines. The bottom panel shows a neuron surface labeled with patient CSF (green) followed by permeabilized staining with anti-MAP2 (red) to mark the dendrites. The patient CSF recognizes an extracellular epitope located along the dendrites. Scale bars: 20 μm. (C) HeLa cells expressing PRG1, 3, or 5 tagged with green fluorescent protein (GFP) (green) were permeabilized and stained with patient or healthy control serum (red). The patient serum strongly recognizes PRG5 and to a lesser extent PRG1. Scale bars: 10 μm. (D) HeLa cells expressing PRG1 or 5 tagged with GFP (green) were surface stained with patient serum (red). The patient serum strongly recognizes an extracellular epitope on PRG5. Scale bars: 10 μm. (E) Immunoprecipitation (IP) of GFP-tagged PRG1, 3, and 5 using patient or healthy control (HC) serum. The sample was run on SDS-PAGE and subsequently stained with anti-GFP. The patient serum, but not HC serum, strongly pulls down PRG5 and to a lesser extent PRG1. Bands visible in the control blot at 50 kDa are background bands representing the IgG heavy chain. (F) Schematic representation of PRG5 (based on reference 4). GFP-tagged chimeric proteins (green) of PRG3 (schematic purple) and PRG5 (schematic green) expressed in human embryonic kidney cells and stained with patient serum (red). The patient serum only recognizes chimera 3, containing the second and third extracellular loop of PRG5. Scale bars: 10 μm. (G) Permeabilized immunofluorescent staining of rat hippocampal neurons (18 DIV) with anti-PSD95 (red) and serum (green) depleted of PRG1 and 5 antibodies or GFP as a control. The specific labeling of dendritic spine tips is diminished if the serum is depleted of PRG1 and 5 antibodies. Scale bars: 5 μm. (H) Quantification of depletion. Bars represent the number of enrichments in dendritic spine tips per 20-μm dendrite. N = 18 cells/condition. Error bars = SEM; p = 0.0301 (Mann-Whitney test). (I) Rat hippocampal neurons (20 DIV) transfected with PRG5-GFP treated for 24 hours with purified patient IgGs or healthy control IgGs (10 ng/μL). Cells were acid washed to remove all protein from the cell surface and stained to visualize human IgG (red) and anti-EEA1 (blue) to mark early endosomes. Upon incubation with patient IgGs, PRG5-GFP is internalized from the dendritic spine tips and moves to early endosomes. The arrows indicate triple colocalization of PRG5-GFP, IgGs, and EEA1. Scale bars: 5 μm. (J) Percentage of EEA1-positive early endosomes containing PRG5-GFP in the soma of hippocampal neurons. N = 17 cells/condition. Error bars = SEM; p = 0.007 (Mann-Whitney test).
To validate the antigen, serum and CSF were used in cell-based assays (CBAs) for PRG1, 3, and 5. Both samples showed strong reactivity to PRG5, weak reactivity to PRG1, and no reactivity to PRG3 (figure 1C). PRG5 was also recognized under nonpermeabilizing conditions (figure 1D). Anti-PRG5 titers were >1:200,000 (serum and CSF in CBA). We did not find additional patients with anti-PRG5 antibodies among 214 patient sera (98 with cerebellar ataxia, 95 with neuropil staining on IHC, and 21 with PCD and small cell lung carcinoma) and 137 control sera (supplementary data).
The patient serum recognized a conformational epitope on PRG5 as it immunoprecipitated PRG5 from lysate, but it no longer recognized the epitope when denatured on Western blot (figure 1E, figure e-1). Using chimeric proteins of PRG3 and PRG5, this epitope was mapped to the second and third extracellular loop (figure 1F). When staining with patient serum depleted of both PRG1 and PRG5 antibodies, specific labeling of dendritic spine tips was diminished (figure 1, G and H).
To study the effects of anti-PRG5 antibodies, mature cultured hippocampal neurons were treated for 4 days with purified healthy control IgGs or patient IgGs. No changes occurred in dendritic spine number, spine morphology, synapse number, or dendritic branching (data not shown). However, 24-hour treatment of hippocampal neurons with patient IgGs (not healthy control IgGs) induced internalization of green fluorescent protein (GFP)-tagged PRG5 (figure 1I). Internalized PRG5-GFP accumulated within EEA1-positive early endosomes in the soma of the neurons (figure 1J).
Discussion and conclusion.
We have identified a patient with PCD and autoantibodies to a novel extracellular conformational epitope on PRG5, a transmembrane protein expressed in the hippocampus and cerebellum and involved in dendritic spine formation.4,5 Its expression in the cerebellum is in line with the phenotype of our patient. Similar to what has been shown for anti-NMDA receptor antibodies,6 PRG5 is internalized and accumulates in early endosomes after treatment with patient IgGs. Because the localization of PRG5 in the plasma membrane is critical for its role in spine formation,4 its internalization could lead to neuronal dysfunction and neurologic symptoms.
Incubation of cultured hippocampal neurons with patient IgGs did not result in morphologic changes, possibly due to the biological redundancy of other PRG proteins. Cerebellar neurons may lack this redundancy; therefore, cerebellar slice cultures might be a better model system to study the pathologic effects of patient IgGs. Unfortunately, lack of serum sample precludes these experiments.
Although only 1 patient with PCD and PRG5 antibodies has been identified, we encourage physicians to consider this antigen, as extracellular antibodies are potentially pathogenic and the symptoms can improve or stabilize with aggressive treatment.
Supplementary Material
Acknowledgments
Acknowledgment: The authors thank N. Savaskan (Department of Neurosurgery, Medical Faculty of the Friedrich Alexander University, Erlangen-Nürnberg, Germany) for providing us with the PRG1, 3, and 5 DNA constructs; C. Wierenga (Department of Biology, div Cell Biology, Utrecht University, Utrecht, the Netherlands) for the use of her puncta analyzer macro; and L. McPhee (Linda McPhee Consulting) for editing the manuscript for nonintellectual content.
Footnotes
Supplemental data at Neurology.org/nn
Author contributions: M.H.v.C.-H.: data collection, analysis and interpretation, writing of the manuscript. E.d.G.: study design, data interpretation, and revision of the manuscript. M.J.T.: data interpretation and revision of the manuscript. E.H.: data collection and analysis. L.S.: data collection and analysis. C.C.H. and P.A.S.S.: study design, data interpretation, and revision of the manuscript.
Study funding: NUTS-OHRA (1104-034), Hersenstichting (2012(1)-141).
Disclosure: M. van Coevorden-Hameete reports no disclosures. E. de Graaff holds a patent for the detection of anti-DNER antibodies. M. Titulaer is on the scientific advisory board for MedImmune LLC, received travel funding from Sun Pharma, is on the editorial board for Neurology: Neuroimmunology & Neuroinflammation, and received research support from Netherlands Organisation for Scientific Research, ErasmusMC, and Dutch Epilepsy Foundation. E. Hulsenboom, L. Sabater, and C. Hoogenraad report no disclosures. P. Smitt holds a patent for the detection of anti-DNER and received research support from Euroimmun. Go to Neurology.org/nn for full disclosure forms. The Article Processing Charge was paid by Erasmus MC.
References
- 1.Dalmau J, Rosenfeld MR. Paraneoplastic syndromes of the CNS. Lancet Neurol 2008;7:327–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Coevorden-Hameete MH, de Graaff E, Titulaer MJ, Hoogenraad CC, Sillevis Smitt PA. Molecular and cellular mechanisms underlying anti-neuronal antibody mediated disorders of the central nervous system. Autoimmun Rev 2014;13:299–312. [DOI] [PubMed] [Google Scholar]
- 3.de Graaff E, Maat P, Hulsenboom E, et al. Identification of delta/notch-like epidermal growth factor-related receptor as the Tr antigen in paraneoplastic cerebellar degeneration. Ann Neurol 2012;71:815–824. [DOI] [PubMed] [Google Scholar]
- 4.Broggini T, Nitsch R, Savaskan NE. Plasticity-related gene 5 (PRG5) induces filopodia and neurite growth and impedes lysophosphatidic acid- and nogo-A-mediated axonal retraction. Mol Biol Cell 2010;21:521–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coiro P, Stoenica L, Strauss U, Brauer AU. Plasticity-related gene 5 promotes spine formation in murine hippocampal neurons. J Biol Chem 2014;289:24956–24970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moscato EH, Peng X, Jain A, Parsons TD, Dalmau J, Balice-Gordon RJ. Acute mechanisms underlying antibody effects in anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol 2014;76:108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.