Abstract
Zinc transporter 8 autoantibodies (ZnT8A) were analyzed in sera from 1,504 subjects as part of the Type 1 Diabetes Genetics Consortium (T1DGC) Autoantibody Workshop. For these participants with type 1 diabetes (T1D), samples were collected within 3 years of T1D diagnosis. ZnT8A were detected in 862 subjects (57.3%), with the highest frequencies and median titers being associated with the shortest duration of disease. ZnT8A were present at similar frequencies in non-Hispanic whites, non-Hispanic blacks, and Hispanics, but significantly less prevalent in those of Asian ancestry. Sera containing ZnT8A selectively recognizing at least one of the SLC30A8 single nucleotide polymorphisms (encoding ZnT8A) were detected in all populations; however, Trp-specific sera were much less frequent in non-Hispanic blacks, consistent with the anticipated lower frequency of the SLC30A8 rs13266634 T allele in African American populations. ZnT8A positivity was associated with HLA-DQ8, but this was primarily due to the DRB1*0404-DQ8 haplotype. This was in contrast to autoantibodies to IA-2 that were strongly associated with DRB1*0401-DQ8. These effects appeared essentially independent of racial or ethnic background. The DRB1*0401-DQ8 and DRB1*0404-DQ8 haplotypes were associated with T1D subjects positive for GAD65, IA-2, and ZnT8A. In contrast to DRB1*0401-DQ8, there was no significant association of DRB1*0404-DQ8 with single or dual autoantibody positivity. The DRB1*0404-DQ8 haplotype was also associated with T1D subjects whose sera recognized both polymorphic variants of zinc transporter 8, an effect not seen for DRB1*0401-DQ8.
Introduction
The insulin secretory granule zinc transporter SLC30A8 (ZnT8) is a major target of cell-mediated (1–3) and humoral (4–8) autoimmunity in human type 1 diabetes (T1D). ZnT8 autoantibodies (ZnT8A) are present in 60–80% of Caucasian patients with T1D at onset (5,8) and, together with autoantibodies to insulin (IAA), GAD65 (GADA), and IA-2 (IA-2A), can confirm diagnosis of autoimmune diabetes in over 94% of subjects. In genetically at-risk first-degree relatives of T1D cases followed prospectively, ZnT8A appear at a median age of 3–4 years (4,8) and generally persist until onset of clinical disease. In young individuals, ZnT8A typically develop later than IAA and GADA and, together with IA-2A and ZnT8A positivity, can identify subjects with prediabetes at greatest risk of rapid progression to clinical T1D (6,7). Postonset ZnT8A titers decline rapidly, likely reflecting the continuing loss of β-cell mass (9–11).
Epitope mapping experiments have revealed the presence of at least one autoantibody binding site in the ZnT8 N-terminal intracellular domain that precedes the six transmembrane–spanning regions and at least two distinct sites in the 95aa C-terminal cytosolic domain comprising residues 275-369 (8,12–14). SLC30A8 exon 13, which encodes amino acid residues 318-369, is the site of single nucleotide polymorphism (SNP) rs13266634 (C/T), a nonsynonymous substitution in the first base of the codon encoding residue 325 that results in either an Arg or Trp at this position. This residue determines the binding specificity of a subset of ZnT8A (14). Genome-wide association studies (GWAS) have shown that the major allele (encoding Arg) is associated with increased susceptibility to type 2 diabetes (15–18). No direct association between this SNP and T1D risk has been detected (19). Presence of the rs13266634 major allele may confer an increased susceptibility of developing autoimmune diabetes at a younger age (20) and homozygosity at this locus can stratify risk in ZnT8A+, but not ZnT8A−, individuals (4). The second base of the codon encoding residue 325 is the site of rs16889462 (G/A), a second nonsynonymous SNP that encodes a Gln at this position. This allele is virtually absent in individuals of European descent but is detectable at a low minor allele frequency in sub-Saharan African, African American, and Asian populations (http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?rs=16889462).
A key concern of current human T1D research is to develop improved metrics that can stratify risk in subjects with prediabetes, identify those individuals most likely to benefit from particular therapeutic interventions, or act as secondary biomarkers of therapeutic efficacy. Given that autoimmune diabetes is believed to result from an inappropriate response to an environmental trigger in the context of genetic susceptibility that leads, initially, to immune dysregulation and, ultimately, to insulin insufficiency (21–23), it is of paramount importance to fully understand the extent to which genetic variation influences the behavior of the most commonly used biochemical markers of disease.
The Type 1 Diabetes Genetics Consortium (T1DGC) is an international study whose primary aims are to discover genes that modify diabetes risk and to provide an expanded genetic and sample resource for research (24). Within the T1DGC (https://www.niddkrepository.org/studies/t1dgc/), there are sera from more than 1,500 T1D-affected individuals that were collected within 3 years of clinical onset—a time period over which ZnT8A typically persist (10,11). The significant sample size and genetically diverse population represented by the T1DGC can both provide increased statistical power over our previous studies and permit identification of regional environmental influences that might otherwise confound the results obtained.
We now report a detailed study of genetic associations between positivity for ZnT8A and other biochemical markers of islet autoimmunity close to clinical onset. Moreover, given the common association of T1D with other autoimmune endocrinopathies (25), we investigated associations between ZnT8A and markers of Addison disease (steroid 21-hydroxylase autoantibodies [21-OHA]), autoimmune thyroiditis (thyroid peroxidase autoantibodies [TPOA]), autoimmune gastritis (autoantibodies to the α-subunit of ATPase 4 [ATP4A-A]), and celiac disease (transglutaminase autoantibodies [TGA]).
Research Design and Methods
Subjects
The T1DGC sample and data repository is derived from affected sibling pairs, trio families, cases and control subjects from four regional networks (Asia-Pacific, European, North American, and U.K.) (24). Previous studies have shown that ZnT8A prevalence and their titers fall rapidly after T1D onset, with an average half-life approximating 90 days (9–11). Consequently, an inclusion criterion for our study was that the serum sample had been drawn within 3 years of clinical diagnosis of T1D. At the time that this study commenced the repository contained 6,749 sera from subjects with T1D, of which 1,504 met our entry criteria (420 year 0; 596 year 1; 488 year 2). Demographics of the included subjects are shown in Supplementary Table 1. For the purpose of racial/ethnic comparisons, non-Hispanic whites (NHWs) are defined as whites of European descent who failed to self-identify as Hispanic and include both subjects who specifically self-identified as non-Hispanic and those who did not specifically express an ethnicity. Similarly, non-Hispanic blacks (NHBs) are defined as blacks who failed to self-identify as Hispanic.
Genotyping
Eight HLA loci (HLA-A, -B, -C, -DRB1, -DQA1, -DQB1, -DPA1, and -DPB1) were genotyped at four-digit resolution in all T1DGC affected sibling pair and trio families and cases and control subjects, as previously described (26). SNP markers in the INS (−23HphI, rs689) and CTLA4 (T17A, rs231775) genes were also genotyped for all of the 1,504 subjects in our substudy.
Autoantibody Assays
T1DGC laboratories performed assays for GADA, IA-2A, 21-OHA, TGA, and TPOA using established assays (27). ATP4A-A and ZnT8A were assayed using in-house radioimmunoprecipitation assays, as previously described (8–11,14,28). For ZnT8, three 35S-radiolabeled probes were generated, each containing two copies of the C-terminal domain (amino acids 275-369) joined by a flexible linker (Pro-Ser-Thr-Pro-Pro-Gly-Ser-Gly-Gly-Gly-Ala-Ser). Probes varied solely with respect to the amino acids at position 325 in each monomer. Thus, probe CR/CW is a heterodimer of ZnT8 C-terminal domains containing sequentially Arg325 and Trp325, while CR/CR and CW/CW are homodimers containing two copies of Arg325 or Trp325, respectively. Aliquots of each sera (2.5 μL) were incubated overnight at 4°C with labeled probe (nominally 20,000 cpm) and immune complexes were captured with protein A Sepharose (GE Healthcare, Piscataway, NJ), extensively washed, and bound radioactivity quantified by liquid scintillation counting. To allow for standardization, each assay plate also contained eight human negative control samples and two positive controls, comprising a rabbit polyclonal anti-ZnT8 and a human reference serum that was pan-reactive for the three ZnT8 probes. Each sample was assayed in duplicate and results are expressed as immunoprecipitation indices of the means (sample − negative control)/(rabbit standard − negative control). The threshold for positivity for each assay was determined from the 99th percentile of a series of >200 sera from subjects without T1D matched for age, sex, and race, run alongside >100 “new-onset” sera from T1D patients attending the Barbara Davis Center for Diabetes (Aurora, CO). The derived indices were 0.0129 (CR/CW), 0.0132 (CR/CR), and 0.0110 (CW/CW). Discordant replicates that spanned the cutoff value were routinely repeated. Unless otherwise specified, ZnT8A refers to results obtained with the CR/CW probe.
Classification of “unrestricted,” “Arg-only,” “Trp-only,” or “Hetero-only” sera was based on the concordance in signals between the CR/CW, CR/CR, and CW/CW probes. Thus, unrestricted sera are positive for all probes, Arg-only immunoreactivity is defined as CR/CR >0.0132 and CR/CW >0.0129 but CW/CW ≤0.0110, and Trp-only sera as CR/CR ≤0.0132 but CW/CW >0.0110 and CR/CW >0.0129. ZnT8 is naturally dimeric (29), raising the possibility that in rs13266634 heterozygous individuals epitopes that selectively recognize ZnT8 heterodimers may exist. Such Hetero-only sera are defined as CR/CW >0.0129 but CW/CW ≤0.0110 and CR/CR ≤0.0132.
Statistical Analysis
The relationship of individual SLC30A8 (ZnT8A) variants to age, age of diabetes onset, duration of diabetes, sex, and classical HLA alleles, INS and CTLA4 SNPs, was examined using logistic regression with robust variance estimators. Duration and ethnicity were included as covariates and familial correlations were included to accommodate the pedigree structure. A multinomial regression model was used in association analyses of the number of islet autoantibodies. Screening for genetic associations was based upon a single SNP in each of INS and CTLA4 and 38 common (minor allele frequency >5%) HLA alleles. HLA class II haplotypes were reconstructed using MENDEL (version 12.0) (30,31) to assign the parental origin of HLA-DRB1, -DQA1, and -DQB1 alleles. In most cases, parental origin of the alleles at all three loci was assigned unambiguously from family data including both parents and unaffected siblings. HLA haplotypes with unphased alleles were inferred based upon known linkage disequilibrium (LD) within populations. Stepwise logistic regression was used to distinguish HLA allele associations that are likely secondary to LD with other HLA alleles. Regression coefficients, odds ratios (ORs), standard errors, significance levels (Wald test), and prevalence estimates were calculated using STATA (version 11.2; StataCorp LP; College Station, TX). A stringent criterion for statistical significance of genetic association tests was taken as P < 0.00125 (0.05/40), using a Bonferroni correction that assumes all of the 38 common HLA alleles are independent. However, given the strong LD of all eight HLA loci, a significance level of 0.005 (0.05/10) was considered suggestive evidence of association.
Results
ZnT8A were detected in 862 (57.3%) of the 1,504 T1DGC subjects who had sera drawn within 3 years of clinical T1D onset. Prevalence of ZnT8A decreased from 64.5% in subjects with 0–1 year’s duration of diabetes to 51.0% in subjects with 2–3 years’ duration. This decrease in prevalence was mirrored by the duration-dependent decline in the median indices of ZnT8A+ subjects from 0.23 (range 0.013–0.919) in the “year 0” group to 0.09 (range 0.013–0.882) in the “year 2” group (P = 0.0001). ZnT8A showed an expected age-dependent bimodal distribution, being less prevalent in individuals aged 0–5 years or over 20 years of age at blood draw, as compared with those aged 6–20 years (8,32,33). Prevalence of ZnT8A was similar in males (57.4%) and females (57.3%, P = 1.00). ZnT8A were less common among T1D cases of Asian ancestry (26.0%, P < 1.0 × 10−8), with similar frequencies observed among NHW (59.7%), Hispanic (60.5%), and NHB (57.9%) groups (Table 1). Of the 862 ZnT8A+ subjects, 66.9% were unrestricted, 21.8% were Arg-only, 9.3% were Trp-only, and 2.0% were Hetero-only. There were no significant racial/ethnic differences in the pattern of autoreactivity among ZnT8A+ subjects (P = 0.29) (Table 2). The relative frequency of unrestricted autoreactivity decreased from 68.6% in subjects with less than 1 year’s duration of T1D to 59.4% with 2–3 years’ duration (P = 0.001). There were corresponding increases in the relative frequencies of Arg-only (from 23.3% to 24.9%), Trp-only (from 7.8% to 10.4%), and Hetero-only (from 0.4% to 5.2%) subjects (Supplementary Table 2).
Table 1.
Frequency of ZnT8A positivity in T1DGC subjects recruited within 3 years of clinical diagnosis
| Group | Characteristics | Diabetes duration | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Prevalence | Male | Female | P* | 0–1 year | 1–2 years | 2–3 years | P† | |
| Total | 1,504 | 57.31 | 57.36 | 57.26 | 1.000 | 64.52 (0.23) | 57.38 (0.145) | 51.02 (0.09) | 0.0002 |
| 0.0001 | |||||||||
| NHW | 991 | 59.74 | 59.78 | 59.69 | 1.000 | 67.16 (0.275) | 59.85 (0.14) | 53.42 (0.09) | 0.003 |
| Hispanic | 167 | 60.48 | 57.69 | 62.92 | 0.528 | 68.52 (0.25) | 58.57 (0.18) | 53.49 (0.17) | 0.281 |
| NHB | 240 | 57.92 | 57.26 | 58.54 | 0.896 | 62.16 (0.14) | 56.47 (0.12) | 55.56 (0.09) | 0.666 |
| Asian | 100 | 26.00‡ | 32.69 | 18.75 | 0.170 | 27.27 (0.035) | 31.58 (0.05) | 20.00 (0.095) | 0.515 |
| Other | 6 | 66.67 | 50.00 | 100 | 0.467 | 100 nd | 50 nd | 50 nd | nd |
Data are n, %, or % (median ZnT8A+ index). nd, not determined.
*, sex association;
†, association with disease duration;
‡, P = 7.47 × 10−9 relative to other racial/ethnic subgroups.
Table 2.
Relative frequency of ZnT8A stratified by homodimer reactivity
| Group | n | Unrestricted | Arg-only | Trp-only | Hetero-only |
|---|---|---|---|---|---|
| Total | 862 | 66.94 (583) | 21.81 (184) | 9.28 (78) | 1.97 (17) |
| NHW | 592 | 65.54 (388) | 21.62 (128) | 10.98 (65) | 1.86 (11) |
| Hispanic | 101 | 72.28 (73) | 20.79 (21) | 5.94 (6) | 0.99 (1) |
| NHB | 139 | 73.38 (102) | 20.14 (28) | 2.88 (4) | 3.60 (5) |
| Asian | 26 | 69.23 (18) | 19.23 (5) | 11.54 (3) | 0 |
| Other | 4 | 50.00 (2) | 50.00 (2) | 0 | 0 |
Data are n or % (number of positive subjects). Results are expressed as the percentage of ZnT8A+ subjects in each group.
Among the 1,504 T1DGC subjects, 61.5% were positive for GADA and 66.8% positive for IA-2A. The prevalence of GADA, IA-2A, and ZnT8A varied with race/ethnicity, although there were marked differences in the patterns of variation. GADA were more widespread among NHBs (70.0%, P = 3.9 × 10−4) and IA-2A were more frequent among Hispanics (83.2%, P = 2.6 × 10−4) and less common among Asians (34.0%, P = 1.4 × 10−9) (Fig. 1). Like ZnT8A, the frequency of IA-2A was similar in males and females; however, GADA showed a female bias (67.9% vs. 55.7%, P = 0.003). In contrast to the distinct decline in ZnT8A prevalence with duration of disease (Table 1), the prevalence of IA-2A was relatively stable over the time range studied, decreasing from 67.6% in subjects with 0–1 year’s duration to 65.8% in subjects with 2–3 years’ duration (P = 0.83) (Supplementary Table 3). A decline in the prevalence of GADA, from 67.6% in subjects with 0–1 year’s duration to 56.6% in subjects with 2–3 years’ duration, was also observed (P = 0.003) (Supplementary Table 4).
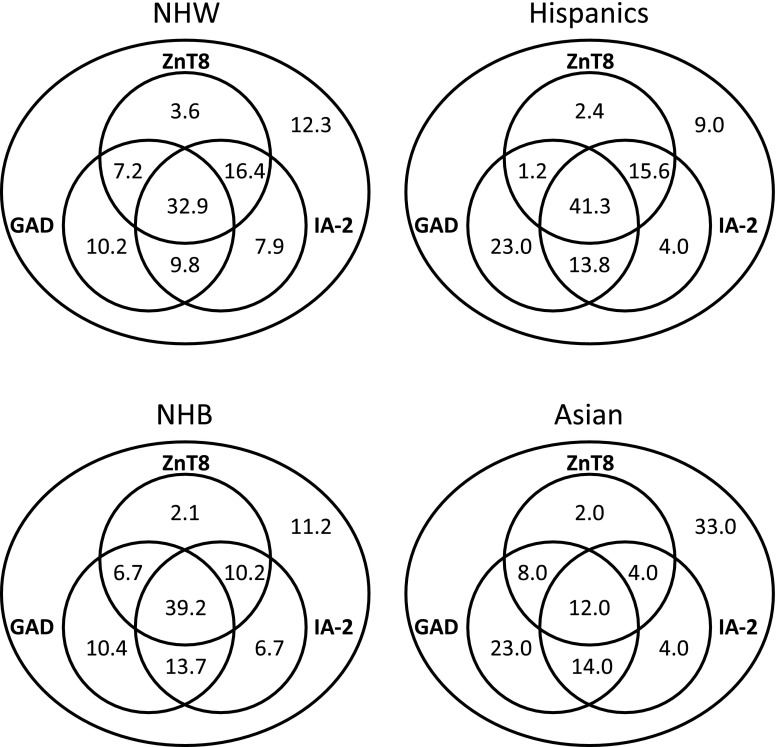
Figure 1.
Venn diagrams showing the frequency of GADA, IA-2A, and ZnT8A among T1DGC subjects stratified by racial/ethnic group.
Despite these differences, the vast majority (94.5%) of the 862 subjects who were ZnT8A+ were also positive for either GADA or IA-2A, with over half (58.3%) positive for both specificities. This concordance appeared independent of the pattern of immunoreactivity to the different ZnT8 polymorphic variants (87.5% among Trp-only subjects; 92.6% among Arg-only subjects). The association between ZnT8A and IA-2A (detected in 83.2% of ZnT8A+ subjects vs. 44.7% ZnT8A− subjects [P = 1.2 × 10−8]) appeared stronger than that for GADA (detected in 69.7% of ZnT8A+ subjects vs. 50.5% of ZnT8A− subjects [P = 1.3 × 10−8]). Concordance rates for islet antibody positivity among 171 sibling pairs from 167 families were 69.6% for ZnT8A, 70.8% for GADA, and 77.2% for IA-2A, which is significantly higher than expected for random pairing of the 342 subjects (P = 2.2 × 10−6, P = 2.3 × 10−7, P = 1.3 × 10−9, respectively). However, there was no evidence for familial clustering of immunoreactivity to specific SLC30A8 SNPs, with sibling concordance of 56.0% among 75 ZnT8A+ sibling pairs—a value very similar to the expected concordance for random pairs of 150 subjects (53.8%).
Although T1D is often associated with other organ-specific autoimmune endocrinopathies, there was minimal evidence of association of either ZnT8A or IA-2A with markers of Addison disease (21-OHA), autoimmune thyroiditis (TPOA), pernicious anemia (ATP4A-A), or celiac disease (TGA), notwithstanding modest increases in TGA frequencies from 4.7% to 7.3% between subjects with and without ZnT8A (P = 0.04) and from 4.4% to 7.1% between subjects with and without IA-2A (data not shown). In contrast, GADA were strongly associated with a subset of other organ-specific autoantibodies including TPOA (12.9% among GADA− subjects vs. 22.6% in GADA+ subjects, P = 2.7 × 10−6) and ATP4A-A (10.8% among GADA− subjects vs. 16.9% in GADA+ subjects, P = 0.002), despite the near-equal frequencies of TGA between subjects with or without GADA (Supplementary Table 5).
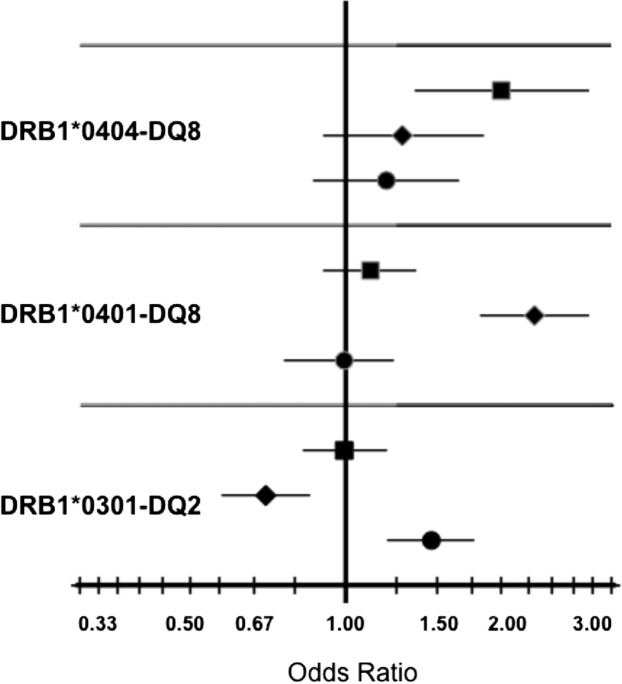
Association analyses were performed to assess the effects of HLA alleles and haplotypes on the presence of islet autoantibodies. Common alleles and haplotypes of HLA-A, -B, -Cw, -DRB1, -DQA1, -DQB1, -DPA1, and -DPB1 were screened for association with each of ZnT8A, GADA, and IA-2A. All three markers were strongly associated with DR and DQ alleles known to confer the highest risk for T1D; however, the pattern of association differed markedly for the different autoantibodies (Fig. 2). Whereas GADA showed a strong positive association with DRB1*0301-DQ2 (OR 1.46, P = 1.2 × 10−4), IA-2A showed an equally strong negative association with this haplotype (OR 0.70, P = 3.9 × 10−4). Similarly, the DQ8 association with ZnT8A was with DRB1*0404-DQ8 (OR 1.97, P = 0.001), while the DQ8 association with IA-2A was with DRB1*0401-DQ8 (OR 2.31, P = 9.1 × 10−12). In both cases, the DR4 alleles significantly increased the risk for ZnT8A beyond that conferred by DQ8. There was no evidence of association between DQ8 and GADA, although this specificity was strongly associated with DPA1*0103 (OR 1.42, P = 1.1 × 10−4) and DPB1*0301 (OR 1.55, P = 0.001). These associations were not seen for either ZnT8A or IA-2A. There was also no evidence for association of the highest T1D risk DQ2/DQ8 genotype with either ZnT8A (OR 1.10, P = 0.459), GAD65A (OR 1.21, P = 0.116), or IA-2A (OR 1.25, P = 0.103). Similarly, there was no evidence of association with SNPs in the T1D-associated candidate genes INS (ZnT8: OR 0.88, P = 0.188; GAD65A: OR 0.90, P = 0.300; IA-2A: OR 1.14, P = 0.211) or CTLA4 (ZnT8: OR 1.09, P = 0.282; GAD65A: OR 1.01, P = 0.927; IA-2A: OR 1.16, P = 0.083).
Figure 2.
OR plots illustrating positive associations between specific HLA-DR-DQ haplotypes and individual T1D autoantibodies. ORs and confidence limits for the 1,504 included subjects are shown. GAD65A (circles), IA-2A (diamonds), and ZnT8A (squares).
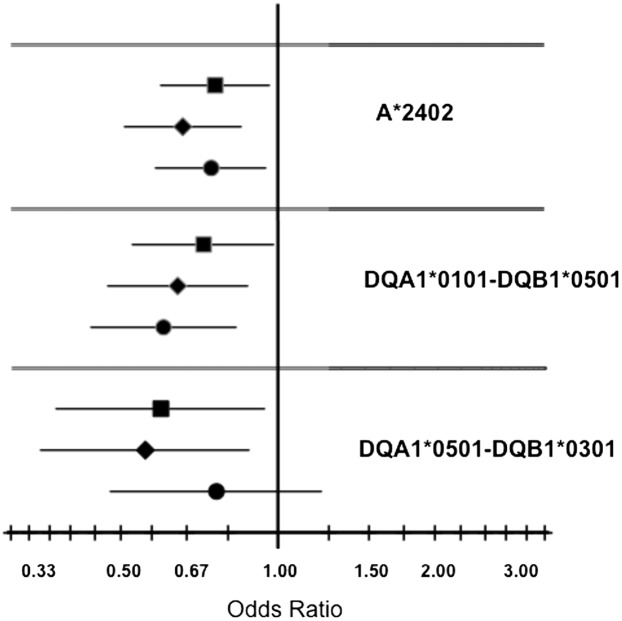
In addition to the strong positive (risk) associations with HLA class II haplotypes, suggestive negative (protective) associations were observed for ZnT8A with DQB1*0301 (OR 0.63, P = 0.004), for GAD65A with DQ5 (DQA1*0101-DQB1*0501: OR 0.60, P = 0.002), and for IA-2A with A*2402 (OR = 0.66, P = 0.002), DQA1*0102 (OR 0.61, P = 0.001), DQ5 (OR 0.64, P = 0.005), and DRB1*0101 (OR 0.70, P = 0.001). The DRB1*0101 association is based upon LD with DQ5 (P = 0.117 when added to a model that included DQ5) (Fig. 3). The ZnT8A association with DQB1*0301 was driven by the DQ7.5 haplotype (DQA1*0501-DQB1*0301: OR 0.59, P = 0.027), with no evidence for association of ZnT8A with DQ7.3 (DQA1*0301-DQB1*0301: OR 0.88, P = 0.615).
Figure 3.
OR plots illustrating negative associations between specific HLA-DR-DQ haplotypes and individual T1D autoantibodies. ORs and confidence limits for the 1,504 included subjects are shown. GAD65A (circles), IA-2A (diamonds), and ZnT8A (squares).
Of the three major haplotypes containing DQA1*0102 (DQA1*0102-DQB1*0602, DQA1*0102-DQB1*0502, and DQA1*0102-DQB1*0604), the DQA1*0102-DQB1*0602 haplotype had the strongest association with IA-2A (OR 0.37, P = 0.014), although all three haplotypes were relatively uncommon in this data set (MAF <3%). Although they did not reach significance, there were also trends toward negative associations of ZnT8A and GADA with A*2402, of ZnT8A with HLA-DQ5, and of IA-2A with HLA-DQ7.5, consistent with a recent report (34).
Association analyses were also conducted based on the number of islet autoantibodies present, a metric known to predict T1D risk more accurately than the presence of any single autoantibody (35). The groups defined by the number of autoantibodies for which they were seropositive showed consistent trends for age of onset and duration, modest differences by sex, and racial/ethnic differences limited to a lower frequency of antibody positivity among Asian ancestry (maximum P = 0.012 across all categories of antibody positivity) (Table 3). Stratifying by age, single autoantibody positive subjects formed the youngest group with the earliest onset of T1D (P < 0.01 compared with GADA−, IA-2A−, and ZnT8A− subjects), followed by those with two autoantibodies (P < 0.01), those who were GADA+, IA-2A+, and ZnT8A+, and finally the oldest individuals in the triple-autoantibody positive group (also with the most recent onset of diabetes) (P = 0.006 comparing duration for three vs. no autoantibodies).
Table 3.
Association analyses between HLA and number of islet autoantibodies (GADA, IA-2A, and ZnT8A)
| Number of islet autoantibodies | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
|||||||
| OR | CI | P | OR | CI | P | OR | CI | P | |
| DR4(01)-DQ8 | 1.73 | 1.18–2.55 | 0.005 | 1.99 | 1.38–2.87 | 0.005 | 2.09 | 1.45–3.00 | 8.5 × 10−5 |
| DR4(04)-DQ8 | 1.06 | 0.56–1.99 | 0.857 | 1.25 | 0.70–2.23 | 0.444 | 1.88 | 1.08–3.20 | 0.26 |
| DR3(01)-DQ2 | 1.60 | 1.17–2.18 | 0.003 | 1.40 | 1.04–1.90 | 0.028 | 1.23 | 0.90–1.68 | 0.194 |
| DQ2/DQ8 | 2.05 | 1.31–3.20 | 0.002 | 1.92 | 1.24–2.96 | 0.004 | 1.84 | 1.19–2.86 | 0.006 |
| A*2402 | 0.98 | 1.68–1.41 | 0.917 | 0.63 | 0.44–0.92 | 0.015 | 0.57 | 0.39–0.83 | 0.003 |
Significance levels are calculated from multinomial regression using 0 autoantibodies as baseline, and including age or age at onset (but not both), duration, and race/ethnicity as covariates.
The frequency of DQ2/DQ8 was significantly increased among subjects with one or more of GADA, IA-2A, or ZnT8A when compared with GADA−, IA-2A−, and ZnT8A− subjects (OR 1.84 to OR 2.05, 0.002 ≤ P ≤ 0.006). This feature of DQ2/DQ8 was the same as for the DRB1*0401-DQ8 haplotype (OR 1.73 to OR 2.09, 8.2 × 10−5 ≤ P ≤ 0.005). The frequency of DQA1*0102-DQB1*0602 was significantly decreased among subjects with one or more islet autoantibodies (OR 0.14 to OR 0.50, 1.6 × 10−7 < P < 0.012). In contrast, the effect of the DRB1*0301-DQ2 haplotype was restricted to the group with only one autoantibody, while the effect of the DRB1*0404-DQ8 haplotype was restricted to those with three islet autoantibodies (OR 1.88, P = 0.026, with no autoantibody as baseline; OR 1.82, P = 0.007, with one autoantibody as baseline) (Table 3). A negative association was observed between the number of antibody specificities and HLA-A*2402 (OR 0.98, P = 0.917 for one antibody; OR 0.63, P = 0.015 for two antibodies; OR 0.57, P = 0.003 for three antibodies). The association between DRB1*0404-DQ8 and the persistent expression of multiple islet autoantibodies was reflected in the increased frequency of this haplotype in ZnT8A+ subjects reacting to both the CR/CR and CW/CW probes as compared with Arg-only or Trp-only subjects (exact test of independence P = 0.019), an effect not seen for the DRB1*0401-DQ8 or DRB1*0301-DQ2 haplotypes (or DQ2/DQ8 genotype) (Supplementary Table 6).
Conclusions
Autoantibodies to islet antigens are currently the most reliable biomarkers of T1D (36) and, as such, are generally central to defining entry criteria for clinical trials designed to prevent or delay onset of overt disease (37). However, it is abundantly clear that there are significant differences between individuals in their humoral autoresponses to specific autoantigens. Such differences may be the result of dissimilar temporal or environmental factors but may also reflect genetic variation. If the contribution of genetics could be better understood, the utility of these key biomarkers could be improved. The goal of the current study was to identify genetic markers associated with the generation of ZnT8A in the subjects enrolled by the T1DGC as part of the T1DGC Autoantibody Workshop and compare them to markers linked to GADA and IA-2A.
Previous studies have demonstrated that ZnT8A titers decline significantly after onset of T1D, often in parallel with the loss of C-peptide (9–11). This decline suggests that ZnT8A might be a surrogate indicator of residual β-cell mass, yet it also limits its utility as a biomarker in individuals with long-standing disease. Accordingly, we restricted our analyses to subjects with disease duration of less than 3 years at blood draw. Consistent with expectations, both the overall frequency and median titer of ZnT8A in positive subjects were significantly reduced in the samples provided 2–3 years after onset relative to those taken during the first year of T1D. The decline was most evident in the NHW and Hispanic groups, who also had the highest median titers in year 0. The significantly lower prevalence of ZnT8A among T1D participants of Asian ancestry (26.0%) in the T1DGC, a subgroup derived predominantly from the southern Asian peninsula and Indian subcontinent, is remarkably consistent with previous studies reporting ZnT8A positivity in 27.8% of Japanese (38) and in 24.1% of Chinese patients with T1D (39). However, a more recent study in a second Japanese cohort reported marked variability across clinical subtypes of T1D, ranging from 0% among patients with fulminant T1D to 77% among young-aged (<10 years) patients with acute onset T1D (33).
The C-terminal domain of SLC30A8 (ZnT8) contains multiple autoantibody epitopes (13,14) that can broadly be separated into two classes depending on the significance of residue 325. Class “A” epitopes have an absolute dependence on residue 325 and are directly related to the subject's rs13266634 genotype and antibodies that recognize them can bind one of the homodimeric probes, but not both. Sera defined as Arg-only or Trp-only exclusively contain antibodies to class A epitopes. In contrast, class “B” epitopes are independent of residue 325 and likely involve ZnT8 surfaces that do not include this residue (13) and antibodies that recognize them can bind both types of homodimers as well as the heterodimer. Any one ZnT8A+ sera may contain antibodies recognizing either only class A, only class B, or a mixture of both. Thus, unrestricted sera may contain antibodies exclusively to class B epitopes, a mixture of class A and class B, or, in the case of individuals heterozygous at rs13266634, a mixture of class A epitopes of each variant. The latter individuals may additionally contain Hetero-only antibodies. Within an individual subject, each antibody specificity will behave in a related but independent manner and after T1D onset may decline at distinct rates. Evidence to support such independence was obtained in a Swedish study that examined ZnT8A decline after onset (11), and two lines of evidence from the current study give additional support to this conclusion. First, there was no evidence for familial clustering of immunoreactivity to specific SLC30A8 variants beyond chance, despite the shared environmental exposures and increased genetic sharing from affected sibling pairs. Second, together with the decline in titer, there was a distinct disease duration-related decrease in the proportion of unrestricted sera in the ZnT8A+ subjects. As our previous postonset study gave no evidence for the acquisition of new specificities through epitope spreading during this phase of the disease (10), the most likely explanation is that the postonset decline in ZnT8A is associated with a simplification of the epitope specificities that remain detectable. There was, however, an unexpectedly large duration-dependent increase in the frequency of Hetero-only sera, suggesting that antibodies to these epitopes are preferentially preserved.
A key feature of this study was the inclusion of subjects drawn from multiple locations and ethnic backgrounds. Although a strength, this heterogeneity could be considered a weakness, as unidentified ancestral subgroups can increase the risk of detecting spurious associations. The T1DGC Autoantibody Workshop resource contrasts with the less diverse local populations investigated in the majority of previous studies of ZnT8A (4,8,9,11,38,39,40,41,42); in particular, we were able to document for the first time the behavior of ZnT8A in an African ancestry population (232/240 NHB subjects attended North American clinics). Overall, there was a similar prevalence to the NHW subjects, although the median titer of ZnT8A in positive subjects was lower and there appeared to be a slower rate of decline. Consistent with the much lower frequency of rs13266634 T allele in African American populations, the frequency of Trp-only sera was reduced relative to other populations. The relative low number of autoantibody-positive NHB subjects precluded a definitive analysis of HLA associations; however, the overall trends in these subgroups were similar to those in the entire cohort, with positive associations of ZnT8A with the DRB1*0404-DQ8 haplotype, of IA-2A with the DRB1*0401-DQ8 haplotype, and of GADA with the DRB1*0301-DQ2 haplotype. Overall, the consistency of our results with previously reported HLA associations in IA-2A, GAD65A, and ZnT8A, together with similar prevalence estimates in ZnT8A across subgroups (excepting Asian ancestry), adds strength to our novel findings showing differential effects of DR4-DQ8 haplotypes and possible negative associations with DQ5, DQ7.5, and DQ6.2.
Article Information
Acknowledgments. This article is dedicated to the memory of George S. Eisenbarth, MD, PhD (1947–2012), and John C. Hutton, PhD (1948–2012), two giants in diabetes research who will be sorely missed.
Funding. This study used resources provided by the T1DGC, a collaborative clinical study sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the National Human Genome Research Institute, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and JDRF and supported by U01 DK062418. The study was also supported in part by grants from JDRF (4-2007-1056) and the National Institutes of Health (UCHSC Diabetes and Endocrinology Research Center) (P30 DK57516, DK32083, and AI50864).
Duality of Interest. J.M.W., J.C.H., and H.W.D. receive royalties from a patent relating to the diagnostic use of ZnT8A. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. J.M.W. researched data and wrote the manuscript. L.M.F. researched data. J.C.H. conceived the study, researched data, and contributed to discussion. P.R.F. wrote the manuscript and was responsible for the statistical analyses. H.W.D. wrote, reviewed, and edited the manuscript. H.W.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dcs15-2004/-/DC1.
This publication is based on the presentations from the Type 1 Diabetes Genetics Consortium (T1DGC) Autoantibody Workshop, which was held on 7 June 2011 in Bethesda, MD. The publication of this supplement was made possible by resources provided by the T1DGC, a collaborative clinical study sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the National Human Genome Research Institute, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and JDRF and supported by grant U01 DK062418.
References
- 1.Dang M, Rockell J, Wagner R, et al. Human type 1 diabetes is associated with T cell autoimmunity to zinc transporter 8. J Immunol 2011;186:6056–6063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Énée É, Kratzer R, Arnoux JB, et al. ZnT8 is a major CD8+ T cell-recognized autoantigen in pediatric type 1 diabetes. Diabetes 2012;61:1779–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scotto M, Afonso G, Larger E, et al. Zinc transporter (ZnT)8(186-194) is an immunodominant CD8+ T cell epitope in HLA-A2+ type 1 diabetic patients. Diabetologia 2012;55:2026–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Achenbach P, Lampasona V, Landherr U, et al. Autoantibodies to zinc transporter 8 and SLC30A8 genotype stratify type 1 diabetes risk. Diabetologia 2009;52:1881–1888 [DOI] [PubMed] [Google Scholar]
- 5.Andersson C, Larsson K, Vaziri-Sani F, et al. The three ZNT8 autoantibody variants together improve the diagnostic sensitivity of childhood and adolescent type 1 diabetes. Autoimmunity 2011;44:394–405 [DOI] [PubMed] [Google Scholar]
- 6.Gorus FK, Balti EV, Vermeulen I, et al.; Belgian Diabetes Registry . Screening for insulinoma antigen 2 and zinc transporter 8 autoantibodies: a cost-effective and age-independent strategy to identify rapid progressors to clinical onset among relatives of type 1 diabetic patients. Clin Exp Immunol 2013;171:82–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vermeulen I, Weets I, Asanghanwa M, et al.; Belgian Diabetes Registry . Contribution of antibodies against IA-2β and zinc transporter 8 to classification of diabetes diagnosed under 40 years of age. Diabetes Care 2011;34:1760–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wenzlau JM, Juhl K, Yu L, et al. The cation efflux transporter ZnT8 (SLC30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci U S A 2007;104:17040–17045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nielsen LB, Vaziri-Sani F, Pörksen S, et al.; Hvidoere Study Group on Childhood Diabetes . Relationship between ZnT8Ab, the SLC30A8 gene and disease progression in children with newly diagnosed type 1 diabetes. Autoimmunity 2011;44:616–623 [DOI] [PubMed] [Google Scholar]
- 10.Wenzlau JM, Walter M, Gardner TJ, et al. Kinetics of the post-onset decline in zinc transporter 8 autoantibodies in type 1 diabetic human subjects. J Clin Endocrinol Metab 2010;95:4712–4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaziri-Sani F, Oak S, Radtke J, et al. ZnT8 autoantibody titers in type 1 diabetes patients decline rapidly after clinical onset. Autoimmunity 2010;43:598–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lampasona V, Petrone A, Tiberti C, et al.; Non Insulin Requiring Autoimmune Diabetes (NIRAD) Study Group . Zinc transporter 8 antibodies complement GAD and IA-2 antibodies in the identification and characterization of adult-onset autoimmune diabetes: Non Insulin Requiring Autoimmune Diabetes (NIRAD) 4. Diabetes Care 2010;33:104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wenzlau JM, Frisch LM, Hutton JC, Davidson HW. Mapping of conformational autoantibody epitopes in ZNT8. Diabetes Metab Res Rev 2011;27:883–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wenzlau JM, Liu Y, Yu L, et al. A common nonsynonymous single nucleotide polymorphism in the SLC30A8 gene determines ZnT8 autoantibody specificity in type 1 diabetes. Diabetes 2008;57:2693–2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pascoe L, Tura A, Patel SK, et al.; RISC Consortium; U.K. Type 2 Diabetes Genetics Consortium . Common variants of the novel type 2 diabetes genes CDKAL1 and HHEX/IDE are associated with decreased pancreatic beta-cell function. Diabetes 2007;56:3101–3104 [DOI] [PubMed] [Google Scholar]
- 16.Scott LJ, Mohlke KL, Bonnycastle LL, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 2007;316:1341–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saxena R, Voight BF, Lyssenko V, et al.; Diabetes Genetics Initiative of Broad Institute of Harvard and MIT, Lund University, and Novartis Institutes of BioMedical Research . Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 2007;316:1331–1336 [DOI] [PubMed] [Google Scholar]
- 18.Sladek R, Rocheleau G, Rung J, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 2007;445:881–885 [DOI] [PubMed] [Google Scholar]
- 19.Brorsson C, Bergholdt R, Sjögren M, et al. A non-synonymous variant in SLC30A8 is not associated with type 1 diabetes in the Danish population. Mol Genet Metab 2008;94:386–388 [DOI] [PubMed] [Google Scholar]
- 20.Gohlke H, Ferrari U, Koczwara K, Bonifacio E, Illig T, Ziegler AG. SLC30A8 (ZnT8) Polymorphism is Associated with Young Age at Type 1 Diabetes Onset. Rev Diabet Stud 2008;5:25–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boitard C. Pancreatic islet autoimmunity. Presse Med 2012;41(12 P 2):e636–e650 [DOI] [PubMed] [Google Scholar]
- 22.Gan MJ, Albanese-O’Neill A, Haller MJ. Type 1 diabetes: current concepts in epidemiology, pathophysiology, clinical care, and research. Curr Probl Pediatr Adolesc Health Care 2012;42:269–291 [DOI] [PubMed] [Google Scholar]
- 23.Lempainen J, Ilonen J. Influence of type 1 diabetes genes on disease progression: similarities and differences between countries. Curr Diab Rep 2012;12:447–455 [DOI] [PubMed] [Google Scholar]
- 24.Rich SS, Concannon P, Erlich H, et al. The Type 1 Diabetes Genetics Consortium. Ann N Y Acad Sci 2006;1079:1–8 [DOI] [PubMed] [Google Scholar]
- 25.Tsirogianni A, Pipi E, Soufleros K. Specificity of islet cell autoantibodies and coexistence with other organ specific autoantibodies in type 1 diabetes mellitus. Autoimmun Rev 2009;8:687–691 [DOI] [PubMed] [Google Scholar]
- 26.Mychaleckyj JC, Noble JA, Moonsamy PV, et al.; T1DGC . HLA genotyping in the international Type 1 Diabetes Genetics Consortium. Clin Trials 2010;7(Suppl.):S75–S87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bingley PJ, Williams AJ, Colman PG, et al.; T1DGC . Measurement of islet cell antibodies in the Type 1 Diabetes Genetics Consortium: efforts to harmonize procedures among the laboratories. Clin Trials 2010;7(Suppl.):S56–S64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wenzlau JM, Gardner TJ, Frisch LM, Davidson HW, Hutton JC. Development of a novel autoantibody assay for autoimmune gastritis in type 1 diabetic individuals. Diabetes Metab Res Rev 2011;27:887–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murgia C, Devirgiliis C, Mancini E, Donadel G, Zalewski P, Perozzi G. Diabetes-linked zinc transporter ZnT8 is a homodimeric protein expressed by distinct rodent endocrine cell types in the pancreas and other glands. Nutr Metab Cardiovasc Dis 2009;19:431–439 [DOI] [PubMed] [Google Scholar]
- 30.Lange K, Sinsheimer JS, Sobel E. Association testing with MENDEL. Genet Epidemiol 2005;29:36–50 [DOI] [PubMed] [Google Scholar]
- 31.Lange K, Weeks D, Boehnke M. Programs for Pedigree Analysis: MENDEL, FISHER, and dGENE. Genet Epidemiol 1988;5:471–472 [DOI] [PubMed] [Google Scholar]
- 32.Andersson C, Vaziri-Sani F, Delli A, et al.; BDD Study Group . Triple specificity of ZnT8 autoantibodies in relation to HLA and other islet autoantibodies in childhood and adolescent type 1 diabetes. Pediatr Diabetes 2013;14:97–105 [DOI] [PubMed] [Google Scholar]
- 33.Kawasaki E, Nakamura K, Kuriya G, et al. Differences in the humoral autoreactivity to zinc transporter 8 between childhood- and adult-onset type 1 diabetes in Japanese patients. Clin Immunol 2011;138:146–153 [DOI] [PubMed] [Google Scholar]
- 34.Long AE, Gillespie KM, Aitken RJ, Goode JC, Bingley PJ, Williams AJ. Humoral responses to islet antigen-2 and zinc transporter 8 are attenuated in patients carrying HLA-A*24 alleles at the onset of type 1 diabetes. Diabetes 2013;62:2067–2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verge CF, Gianani R, Kawasaki E, et al. Number of autoantibodies (against insulin, GAD or ICA512/IA2) rather than particular autoantibody specificities determines risk of type I diabetes. J Autoimmun 1996;9:379–383 [DOI] [PubMed] [Google Scholar]
- 36.Winter WE, Schatz DA. Autoimmune markers in diabetes. Clin Chem 2011;57:168–175 [DOI] [PubMed] [Google Scholar]
- 37.Thrower SL, Bingley PJ. Prevention of type 1 diabetes. Br Med Bull 2011;99:73–88 [DOI] [PubMed] [Google Scholar]
- 38.Kawasaki E, Uga M, Nakamura K, et al. Association between anti-ZnT8 autoantibody specificities and SLC30A8 Arg325Trp variant in Japanese patients with type 1 diabetes. Diabetologia 2008;51:2299–2302 [DOI] [PubMed] [Google Scholar]
- 39.Yang L, Luo S, Huang G, et al. The diagnostic value of zinc transporter 8 autoantibody (ZnT8A) for type 1 diabetes in Chinese. Diabetes Metab Res Rev 2010;26:579–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brorsson C, Vaziri-Sani F, Bergholdt R, et al.; Danish Study Group of Childhood Diabetes . Correlations between islet autoantibody specificity and the SLC30A8 genotype with HLA-DQB1 and metabolic control in new onset type 1 diabetes. Autoimmunity 2011;44:107–114 [DOI] [PubMed] [Google Scholar]
- 41.Andersen ML, Vaziri-Sani F, Delli A, et al. Association between autoantibodies to the arginine variant of the zinc transporter 8 (ZnT8) and stimulated C-peptide levels in Danish children and adolescents with newly diagnosed type 1 diabetes. Pediatr Diabetes 2012;13:454–462 [DOI] [PubMed] [Google Scholar]
- 42.Delli AJ, Vaziri-Sani F, Lindblad B, et al.; Better Diabetes Diagnosis Study Group . Zinc transporter 8 autoantibodies and their association with SLC30A8 and HLA-DQ genes differ between immigrant and Swedish patients with newly diagnosed type 1 diabetes in the Better Diabetes Diagnosis study. Diabetes 2012;61:2556–2564 [DOI] [PMC free article] [PubMed] [Google Scholar]