Abstract
BACKGROUND
Autoimmune thyroiditis occurs in 10–25% of patients with type 1 diabetes (T1D). Most of these patients are also positive for thyroid peroxidase (TPO) antibodies. Thyroid dysfunction complicates T1D metabolic control and is a component of the autoimmune polyglandular syndrome (APS, type 2 or 3). Previous studies of isolated T1D and of T1D combined with other autoimmune disorders showed genetic susceptibility for alleles in HLA-DQB1 and -DRB1 and also CTLA4 and PTPN22.
RESEARCH DESIGN AND METHODS
We analyzed the Type 1 Diabetes Genetics Consortium Autoantibody Workshop data by differentiating those T1D probands with and without TPO antibodies or thyroid disease with respect to polymorphisms in HLA, CTLA4, INS, PTPN22, and VDR, taking into account the ethnic origin. Genotype and clinical/immunogenic phenotype data were analyzed by gene counting methods and logistic regression analysis.
RESULTS
The presence of TPO antibodies (25.2%) and thyroid disease (8.4%) was associated with older age, female sex, and presence of other autoantibodies (GAD65, ATPase, 21-OH) (all P < 0.001). The highest prevalence was in patients of Hispanic ancestry (31%) and the lowest in those of African ancestry (8%). In T1D non-Hispanic whites, HLA-DRB1*0101 is significantly (P < 0.0001) less frequent in TPO-positive than in TPO-negative individuals, whereas HLA-DRB1*0404, -DQB1*0301, and -DPB1*0201 are significantly (P < 0.0001) more frequent. Subjects with a high titer of TPO autoantibodies and with thyroid disease were associated with female sex and older age and negatively associated with DRB1*0401-DQB1*0302 (P < 0.0001). No significant differences were observed for an association of TPO positivity or thyroid disease with single nucleotide polymorphisms in the INS, CTLA4, or VDR loci, with nominal significance (P = 0.01) for PTPN22 R620W variant.
CONCLUSIONS
Thyroid autoimmunity is highly prevalent in T1D patients of non-Hispanic white, Asian, or Hispanic origin. The strongest disease risk is conferred by female sex and older age. This risk is modulated by HLA-DRB1 and HLA-DPB1 loci. The immunogenetic profile for T1D with thyroid autoimmunity may identify distinct pathways regulating polyglandular autoimmunity and disease.
Introduction
Type 1 diabetes (T1D) results from the β-cell–specific destruction mediated by T cells in genetically susceptible individuals after triggering events. This organ-specific destruction is not just confined to the pancreatic islets of Langerhans but may also affect the thyroid and other tissue targets leading to the autoimmune polyglandular syndrome type 2 (APS 2) (1). These immune-mediated disorders may occur combined in the same individual but also may be isolated diseases clustered in families (2). A shared genetic predisposition has been shown to be common in all autoimmune endocrinopathies, specifically for T1D and thyroid autoimmunity (3).
Autoimmune thyroid diseases (AITDs) are among the most common immune-mediated disorders and they enhance the risk to develop β-cell autoimmunity or overt T1D (4). Thyroid dysfunction worsens diabetes as thyroid hormones play a crucial role in energy and metabolic regulation, with recent data pointing to both central as well as peripheral mechanisms involving AMPK (5,6). Furthermore, the pathogenesis of thyroid and β-cell autoimmunity displays similar T-cell infiltration and presumed activation signals (7,8). Signatures of T-cell imbalance can also be observed in the peripheral blood as indirect markers of the immune response (9).
The Type 1 Diabetes Genetics Consortium (T1DGC) generated a large biobank of renewable (e.g., Epstein Barr virus–transformed B-cell lines) and nonrenewable (e.g., serum and DNA) resources to clarify the genetic basis for T1D. The T1DGC has identified more than 40 susceptibility loci by genome-wide association and linkage studies (www.t1dbase.org). It also analyzed humoral immunity in affected sibling pairs by measuring thyroid peroxidase (TPO) autoantibodies (TPO-Ab) as indicators of AITD. These data have permitted, for the first time, an estimation of the prevalence of AITD in a large sample of familial T1D. Furthermore, the unique collection of ethnic subpopulations permitted an ethnic-specific characterization of T1D with associated diseases as well as TPO antibody status.
Research Design and Methods
In total, 7,083 subjects with T1D were screened for TPO-Ab; 7,055 were genotyped for HLA class I (HLA-A, -B, and -C) and class II (HLA-DRB1, -DQA1, -DQB1, -DPA1, and -DPB1) alleles. Genotypes were assigned by high-resolution analysis using a PCR-based sequence-specific oligonucleotide probe system (10) for the eight distinct HLA genes. A total of 2,182 subjects were genotyped for T1D-associated single nucleotide polymorphisms (SNPs), including those in CTLA4, INS, PTPN22, and VDR. Targeted SNP typing used an Illumina custom array. The largest ethnic group comprised 5,219 non-Hispanic whites (NHWs) with the HLA genotype.
TPO-Ab were measured by radioimmunoassay (KRONUS, Star, ID), and thresholds for positivity were defined according to the manufacturer’s instructions. TPO antibody titers were documented from 6,836 T1D subjects, while positivity or negativity for TPO was recorded from only 247 participants. Presence or absence of thyroid disease was defined in a previously collected detailed clinical questionnaire for T1DGC study participants.
Statistical analysis was performed using BiAS for Windows, epsilon 2007, for calculating allele frequencies. For each of the 47 most common (minor allele frequency >3%) alleles, allelic association was tested for seven related phenotypes (TPO, TD [thyroid disease], TPO+TD+, TPO+TD−, TPO−TD+, TPO-Ab++, and TPO-Ab++TD++) by logistic regression, clustered by family, with age and sex as covariates. Significance levels were determined using robust variance estimators. To correct for multiple testing, a P < 0.001 threshold was used for statistical significance. Screening for HLA allele associations was restricted to the 5,266 NHW subjects with HLA genotypes. Allelic associations identified in NHWs were tested in other ethnic groups and combined using the DerSimonian and Laird random-effects method to assess heterogeneity. Analyses were carried out using Stata (version 11.2; StataCorp, College Station, TX). Phased DR-DQ haplotypes were obtained from family data, in most cases including both parents as well as unaffected siblings.
Results
Phenotypes of TPO Positivity and Thyroid Disease in T1D
Six clinical/immunogenic phenotype groups were distinguished by antibody profile and diagnosis of thyroid disease: 1) patients with or without TPO antibodies (TPO+ vs. TPO−), 2) those with or without thyroid disease (TD+ vs. TD−), 3) those with both TPO–Ab and thyroid disease (TPO+TD+), 4) those with TPO-Ab but without thyroid disease (TPO+TD−), 5) those with thyroid disease without TPO-Ab (TPO−TD+), and, as the reference group for the latter three phenotypes, 6) those T1D patients without thyroid disease or TPO autoantibody positivity (TPO−TD−). It should be noted that TPO positivity was found in both low titer (TPO-Ab+ <75 units/L) and high titer (TPO-Ab++ >75 units/L) subject samples. Out of 7,083 T1D cases, there were 1,785 (25.2%) who tested positive for TPO antibodies, and 596 T1D cases (8.4%) reported a concomitant thyroid disorder.
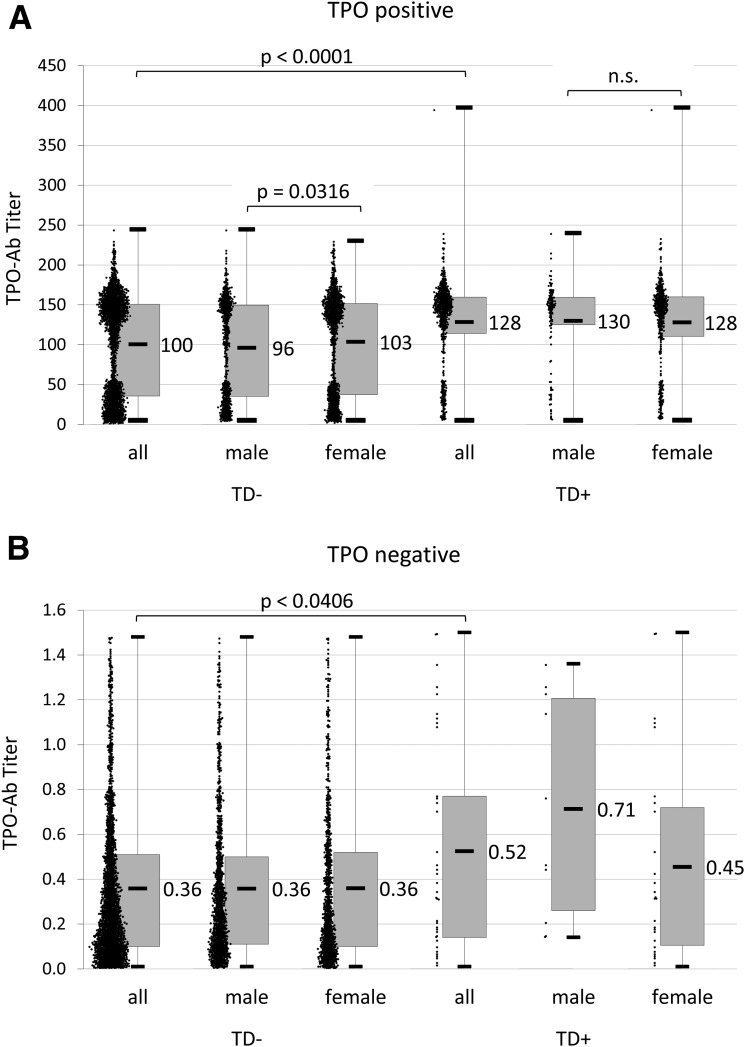
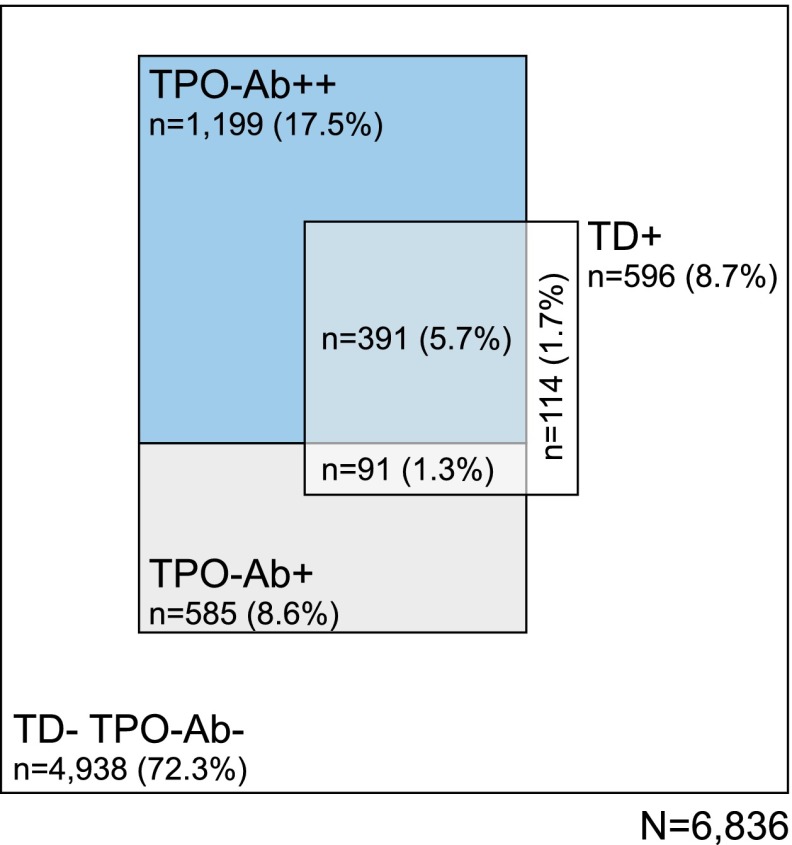
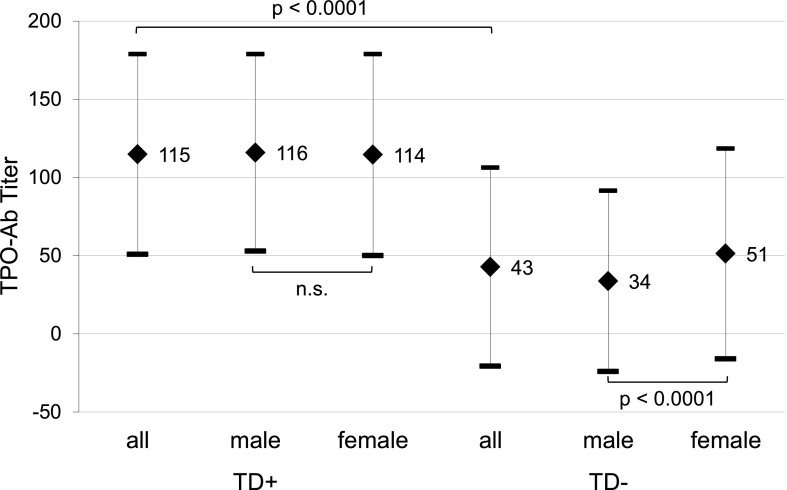
The bimodal distribution of TPO antibody titers (TPO-Ab++/TPO-Ab+) was observed in both sexes and was not different when stratified by age of T1D onset (Fig. 1). The majority of TPO-positive T1D patients were TPO-Ab++ (17.5%), only 8.6% were TPO-Ab+. As shown in Fig. 2, only small groups had TPO-Ab+ and thyroid disease (1.3%) or were TPO−TD+ patients (1.7%). T1D patients with thyroid disease had a significantly (P < 0.0001) higher mean titer of TPO-Ab (115 units/L) compared with those without thyroid disease (43 units/L) (Fig. 3).
Figure 1.
A: TPO-Ab titers in TPO-Ab+ T1D patients. B: TPO-Ab titers in TPO-Ab− T1D patients.
Figure 2.
The relative proportions of T1D patients either with thyroid autoimmunity and TPO-Ab+ or TPO-Ab++ and/or thyroid disease are represented by the areas within the quadrangle.
Figure 3.
T1D patients with thyroid disease have significantly higher TPO-Ab titers than those without a thyroid disorder.
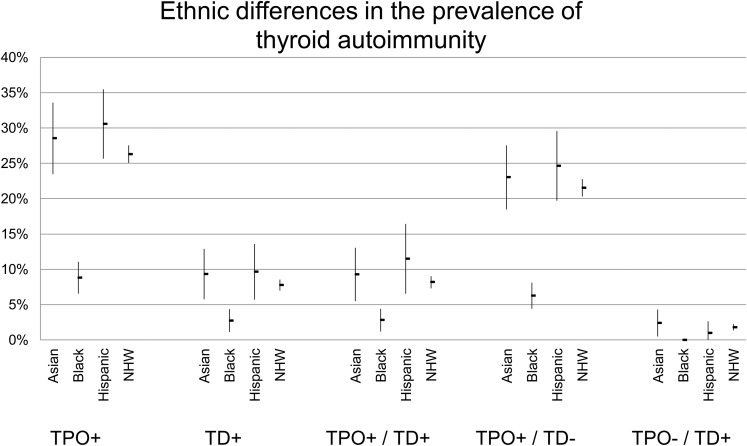
TPO positivity was associated with both age at sampling time and with female sex. Compared with the total study population, T1D patients who were TPO+ were significantly older (23.6 vs. 20.8 years) and more often female (63.9 vs. 50%), as were T1D TD+ patients (28.8 years and 74.7% female) (Table 1). For the combined phenotypes, TPO−TD− are the youngest (19.5 years), followed by TPO+TD− (22.0 years), TPO+TD+ (27.7 years), and TPO−TD+ (32.4 years) (P < 0.001). Female sex also predisposes to thyroid disease (OR 2.27, P < 0.001), regardless of TPO antibodies (TPO+TD+, 74.5% female; TPO−TD+, 75.3% female; P < 0.001). The sex bias is less apparent, although still significant, for TPO+TD− patients (59.9% female, P < 0.001). Moreover, there were differences within the ethnic groups (Table 2 and Fig. 4), with the highest prevalence of TPO autoantibody positivity in Hispanics (29.7%), which is consistent with the highest prevalence in TPO+TD+ patients (12%) despite their younger mean age (14 years in Hispanics vs. 22.5 years in NHWs).
Table 1.
Association of thyroid autoimmunity with age and sex
| Phenotype | n | Age (years) |
Sex |
|||
|---|---|---|---|---|---|---|
| Mean | SD | P | % Female | P | ||
| Total | 7,083 | 20.8 | 12.5 | 50.0 | ||
| TPO+ | 1,785 | 23.6 | 13.2 | <0.001 | 63.9 | <0.001 |
| TD+ | 596 | 28.8 | 14.4 | <0.001 | 74.7 | <0.001 |
| TPO+TD+ | 482 | 27.7 | 14.1 | <0.001 | 74.5 | <0.001 |
| TPO+TD− | 1,303 | 22.0 | 12.4 | <0.001 | 59.9 | <0.001 |
| TPO−TD+ | 93 | 32.4 | 13.9 | <0.001 | 75.3 | <0.001 |
| TPO−TD− | 4,943 | 19.5 | 11.9 | 44.5 | ||
Table 2.
Ethnic differences in the prevalence of thyroid autoimmunity
| Age (years) |
Sex |
|||||
|---|---|---|---|---|---|---|
| Ethnicity | n | Frequency (%) | Mean | SD | Male | Female |
| Other | 20 | 0.2 | 10.7 | 4.7 | 16 (80) | 4 (20) |
| Asian | 380 | 5.4 | 17.4 | 7.6 | 183 (48.2) | 197 (51.8) |
| Black | 710 | 10.0 | 14.8 | 8.2 | 311 (43.8) | 399 (56.2) |
| Hispanic | 495 | 7.0 | 14.0 | 7.9 | 245 (49.5) | 250 (50.5) |
| NHW | 5,478 | 77.3 | 22.5 | 13.1 | 2,788 (50.9) | 2,690 (49.1) |
| Total | 7,083 | 20.8 | 12.5 | |||
Figure 4.
Ethnic differences in the prevalence of thyroid autoimmunity.
TPO positivity is associated with GAD65 positivity and ATPase positivity. In those TPO-positive T1D subjects, more than one-half in each ethnic group is also positive for GAD65 (52.8% NHWs, 57.7% Hispanics, 73.0% blacks, and 57.8% Asians, all P < 0.004) (Table 3). TPO positivity is less associated with ATPase positivity (39.5% NHWs, 42.2% Hispanics, 44.9% blacks, and 26.2% Asians, all P < 0.001) (Table 4). TPO positivity is also associated with 21-hydroxylase (21-OH) in NHWs (P < 0.001) and Hispanics (P = 0.008) but not in blacks (P = 0.287) (Table 5). None of the 359 Asians tested were positive for 21-OH. There is no evidence for association between TPO positivity and IA-2 (Table 6) or TG (Table 7) positivity in any ethnic group.
Table 3.
Association between TPO and GAD65 autoantibody positivity
| TPO | GAD65, n (%) |
P | ||
|---|---|---|---|---|
| Negative | Positive | |||
| NHW | Negative | 2,288 (60) | 1,525 (40) | <0.001 |
| Positive | 688 (47.2) | 769 (52.8) | ||
| Hispanic | Negative | 194 (57.7) | 142 (42.3) | 0.003 |
| Positive | 60 (42.3) | 82 (57.7) | ||
| Black | Negative | 290 (46.8) | 330 (53.2) | 0.001 |
| Positive | 20 (27) | 54 (73) | ||
| Asian | Negative | 148 (59.2) | 102 (40.8) | 0.004 |
| Positive | 46 (42.2) | 63 (57.8) | ||
Table 4.
Association between TPO and ATPase autoantibody positivity
| TPO | ATPase, n (%) |
P | ||
|---|---|---|---|---|
| Negative | Positive | |||
| NHW | Negative | 3,152 (86.3) | 501 (13.7) | <0.001 |
| Positive | 835 (60.5) | 545 (39.5) | ||
| Hispanic | Negative | 257 (79.8) | 65 (20.2) | <0.001 |
| Positive | 78 (57.8) | 57 (42.2) | ||
| Black | Negative | 487 (82.8) | 101 (17.2) | <0.001 |
| Positive | 38 (55.1) | 31 (44.9) | ||
| Asian | Negative | 218 (90.1) | 24 (9.9) | <0.001 |
| Positive | 76 (73.8) | 27 (26.2) | ||
Table 5.
Association between TPO and 21-OH autoantibody positivity
| TPO | 21-OH, n (%) |
P | ||
|---|---|---|---|---|
| Negative | Positive | |||
| NHW | Negative | 3,771 (98.9) | 42 (1.1) | <0.001 |
| Positive | 1,403 (96.3) | 54 (3.7) | ||
| Hispanic | Negative | 336 (100) | 0 (0) | 0.008 |
| Positive | 138 (97.2) | 4 (2.8) | ||
| Black | Negative | 618 (99.7) | 2 (0.3) | 0.287 |
| Positive | 73 (98.6) | 1 (1.4) | ||
| Asian | Negative | 250 (100) | 0 (0) | — |
| Positive | 109 (100) | 0 (0) | ||
Table 6.
Association between TPO and IA-2 autoantibody positivity
| TPO | IA-2, n (%) |
P | ||
|---|---|---|---|---|
| Negative | Positive | |||
| NHW | Negative | 2,036 (53.4) | 1,777 (46.6) | 0.734 |
| Positive | 786 (53.9) | 671 (46.1) | ||
| Hispanic | Negative | 127 (37.8) | 209 (62.2) | 0.681 |
| Positive | 57 (40.1) | 85 (59.9) | ||
| Black | Negative | 286 (46.1) | 334 (53.9) | 0.902 |
| Positive | 33 (44.6) | 41 (55.4) | ||
| Asian | Negative | 189 (75.6) | 61 (24.4) | 0.588 |
| Positive | 86 (78.9) | 23 (21.1) | ||
Table 7.
Association between TPO and TG autoantibody positivity
| TPO | TG, n (%) |
P | ||
|---|---|---|---|---|
| Negative | Positive | |||
| NHW | Negative | 3,537 (92.8) | 276 (7.2) | 0.292 |
| Positive | 1,339 (91.9) | 118 (8.1) | ||
| Hispanic | Negative | 318 (94.6) | 18 (5.4) | 0.304 |
| Positive | 131 (92.3) | 11 (7.7) | ||
| Black | Negative | 600 (96.8) | 20 (3.2) | 0.153 |
| Positive | 74 (100) | 0 (0) | ||
| Asian | Negative | 234 (93.6) | 16 (6.4) | 0.276 |
| Positive | 98 (89.9) | 11 (10.1) | ||
Allele Frequencies of the MHC
In the series of HLA class I and class II genes in the MHC, 10 alleles showed significant allele frequency differences in one or more phenotypic subgroups of thyroid autoimmunity (Table 8) as identified by logistic regression analysis: class I HLA-A*2402 and class II HLA-DQA1*0101 and -DQA1*0301; HLA-DQB1*0301, -DQB1*0302, and -DQB1*0501; HLA-DRB1*0101, -DRB1*0401, and -DRB1*0404; and HLA-DPB1*0201. However, DRB1*0404 is in complete linkage disequilibrium (LD) with DQA1*0301-DQB1*0302, and also DRB1*0101 is in complete LD with DQA1*0101-DQB1*0501. In both cases, the HLA-DQ associations were secondary to LD with the HLA-DRB1 allele. Of the remaining alleles, the effects of A*2402, DQB1*0301, DRB1*0404, DPB1*0201 are independent, whereas the effects of DQA1*0301 and DRB1*0401 are secondary to LD with DQB1*0301.
Table 8.
Association of thyroid autoimmunity and thyroid disease with HLA alleles
| TPO−TD− (7,450)* | TPO+TD+ (822)* | TPO+TD− (2,088)* | TPO-TD+ (172)* | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HLA allele | % (n) | % (n) | P | OR (95% CI) | % (n) | P | OR (95% CI) | % (n) | P | OR (95% CI) |
|
A*2402 |
12.0 (893) |
7.3 (60) |
<0.0001 |
0.59 (0.44–0.80) |
10.6 (222) |
0.139 |
0.88 (0.74–1.04) |
11.6 (20) |
>0.5 |
1.02 (0.64–1.62) |
|
DRB1*0101† |
7.3 (545) |
3.9 (32) |
<0.001 |
0.51 (0.34–0.77) |
4.4 (92) |
1E-05 |
0.58 (0.45–0.74) |
11.0 (19) |
>0.1 |
1.49 (0.91–2.44) |
|
DRB1*0404‡ |
4.9 (362) |
7.8 (64) |
<0.0001 |
1.78 (1.31–2.43) |
8.6 (179) |
5E-09 |
1.87 (1.51–2.30) |
5.8 (10) |
>0.3 |
1.48 (0.70–2.66) |
|
DQB1*0301 |
4.1 (302) |
5.7 (47) |
<0.05 |
1.43 (1.03–2.00) |
6.5 (136) |
7E-06 |
1.87 (1.30–2.00) |
6.4 (11) |
>0.2 |
1.43 (0.81–2.53) |
| DPB1*0201 | 14.0 (1,045) | 19.6 (161) | <0.0001 | 1.50 (1.22–1.84) | 17.6 (367) | 3E-04 | 1.30 (1.23–1.49) | 22.7 (39) | <0.0001 | 1.95 (1.35–2.81) |
*Data in parentheses are number of alleles in each group.
†Haplotype DRB1*0101-DQA1*0101-DQB1*0501.
‡Haplotype DRB1*0404-DQA1*0301-DQB1*0302.
For most of the alleles associated with thyroid autoimmunity, the pattern of association is similar for different phenotypic subgroups (Table 9). There is consistent association of DPB1*0201 both with patients with TPO positivity and manifest thyroid disease (22.7% TPO−TD+) and with cases with thyroid disease but without TPO antibodies (10.6% TPO+TD−) versus those without TPO antibodies and thyroid disease (14% TPO−TD−) (odds ratio [OR] 1.95, P = 4 × 10−4 and OR 1.30, P = 3 × 10−4, respectively). The protective effect of A*2402 has the strongest effect in individuals with manifest thyroid disease who are also TPO positive (7.3% TPO+TD+ vs. 12.0% TPO−TD−; OR 0.59, P < 0.0001). There was no evidence supporting an effect of A*2402 in manifest thyroid disease without TPO antibodies (11.6% TPO−TD+ vs. 12.0% TPO−TD−; OR 1.02, P > 0.5). The DRB1*0401 and DQB1*0302 alleles were significantly less frequent in subjects with TPO-Ab++ than with TPO-Ab+. In addition, they were also significantly less frequent in TPO+ subjects with high titers and thyroid disease (TPO-Ab++TD+) than in TPO+ subjects with low titers and no thyroid disease (TPO-Ab+TD−). This was seen for the DRB1*0401-DQB1*0302 haplotype but was not observed when DRB1*0401 was present on the same haplotype with DQB1*0301 or when DQB1*0302 was present on the same haplotype as DRB1*0404 (data not shown).
Table 9.
Association of thyroid autoimmunity with HLA alleles in NHWs
| TPO− (7,622)* |
TPO+ (2,910)* |
TD− (9,918)* |
TD+ (1,030)* |
|||||
|---|---|---|---|---|---|---|---|---|
| HLA allele | % (n) | % (n) | P | OR (95% CI) | % (n) | % (n) | P | OR (95% CI) |
| A*2402 | 12.0 (913) | 6.3 (183) | 0.006 | 0.80 (0.68–0.94) | 11.7 (1,159) | 8.4 (87) | 0.006 | 0.72 (0.56–0.92) |
| DRB1*0101† | 7.4 (564) | 4.3 (124) | <0.001 | 0.55 (0.44–0.68) | 6.7 (661) | 5.2 (54) | >0.1 | 0.78 (0.57–1.06) |
| DRB1*0404‡ | 4.9 (372) | 8.3 (243) | <0.0001 | 1.84 (1.52–2.23) | 5.7 (565) | 7.8 (80) | <0.005 | 1.45 (1.12–1.88) |
| DQB1*0301 | 4.1 (313) | 6.3 (183) | <0.0001 | 1.52 (1.26–1.84) | 4.6 (459) | 5.9 (61) | >0.1 | 1.23 (0.94–1.61) |
| DPB1*0201 | 14.2 (1,084) | 18.1 (528) | <0.0001 | 1.31 (1.16–1.49) | 14.9 (1,480) | 19.8 (204) | <0.0001 | 1.37 (1.14–1.63) |
*Data in parentheses are number of alleles in each group.
†Haplotype DRB1*0101-DQA1*0101-DQB1*0501.
‡Haplotype DRB1*0404-DQA1*0301-DQB1*0302.
Association analyses in T1D patients of Asian, African, and Hispanic ancestry exhibited similar trends in the pattern of allele frequencies for A*2402, DRB1*0101, DRB1*0404, DPB1*0201, DRB1*0401, and DQB1*0302 alleles. The pooled estimate of effect sizes remained statistically significant for each of these alleles, even when combined with results for NHWs. However, the association of DQB1*0301 with TPO phenotypes showed significant heterogeneity across ethnicities (P < 0.015), and the pooled estimate of the effect of DQB1*0301 was no longer significant (P > 0.4 for TPO+; P > 0.9 for TPO+TD−). There were weaker indications of population differences in the association of the DRB1*0401-DQB1*0302 haplotype with TPO antibody titers (P > 0.1). Although the association of both DRB1*0401 and DQB1*0302 alleles was consistent across populations, it remained significant (P < 0.001) in the pooled analyses.
Other Gene T1D Risk Loci
No significant association could be detected for SNPs in the CTLA4, INS, or VDR genes with association to TPO positivity in T1D subjects. Although there were only slight significant differences in minor allele frequency for some SNPs, this could not be confirmed by regression analysis at a significance criterion of P < 0.001 to correct for multiple testing. The PTPN22 SNP coding for the R620W variant (rs2476601) reached nominal significance for association with TPO positivity (TPO+; P < 0.01) in comparison with TPO-negative participants.
Conclusions
We found a high prevalence of thyroid autoimmunity as well as thyroid disorders in the population of T1D subjects participating in the T1DGC Autoantibody Workshop. Participants of African ancestry had a lower prevalence of thyroid autoimmunity compared with those of Asian ancestry who had the highest prevalence; NHWs were classified as intermediate for thyroid autoimmunity. Ethnic differences in polyendocrine autoimmunity of T1D have not yet been described and warrant further investigation. Due to the smaller numbers in the Hispanic and African ancestry groups, the specific genetic contributions are underpowered in our study to address genetic contribution. These findings, however, provide new insights into the polyendocrine autoimmunity of T1D.
The bimodal distribution of TPO positivity in low-titer and high-titer groups is certainly of interest. Latent thyroid autoimmunity can be distinguished from advanced forms that are more frequently associated with manifest thyroid disease. Follow-up testing of both TPO-Ab and thyroid function should, therefore, be advised for all T1D subjects who are found to be antibody positive but unaffected by thyroid disease. Whether thyroid autoimmunity will progress to overt disease cannot be predicted at present. Yet as the autoimmune pathophysiology runs a slow course with advancing age, more T1D patients will develop thyroid disease in the long run.
Recently, four gene loci were associated with autoantibody positivity at genome-wide significance in a large cross-sectional cohort of 6,160 T1D affected siblings (11). Brorsson et al. (11) tested for association with seven disease-specific autoantibodies and provided evidence that genetic control of antibody formation is distinct from T1D risk.
Conversely, testing for multiple islet autoantibodies was shown to be a useful predictive marker for risk of progression to T1D onset in patients with thyroid disease (12). Hereby, the immunogenetic profile for T1D with thyroid autoimmunity may identify distinct pathways regulating polyglandular autoimmunity and disease.
In the ethnic group of NHWs, the strongest genetic association for thyroid autoimmunity with T1D is observed with DPB1*0201, found in nearly a fifth of all T1D patients with a thyroid disorder and in 18.1% of those with TPO positivity. Only the risk for having the DRB1*0404 allele was higher in patients with TPO positivity (OR 1.84) and thus greater than in T1D cases with thyroid disorders (OR 1.45). In contrast, significant protection was found with DRB1*0101 (OR 0.55 for TPO) and A*2402 present (OR 0.80 for TPO, OR = 0.72 for TD) (Table 9).
These findings are in agreement with earlier reports of genetic susceptibility in thyroid autoimmune disease that is shared across the spectrum of several autoimmune disorders (2,3,13,14). Those results demonstrate that HLA genes in the MHC are linked with Graves disease, not only in Caucasians but also in Han Chinese from Taiwan (15,16). The largest component of genetic susceptibility for several autoimmune diseases resides in the MHC (13,17). The specific role of HLA-DP contributing to the risk in T1D has only recently been shown (10). Here, alleles of HLA-DP confer both significant susceptibility as well as protection. DPB1*0201 confers the highest risk in those T1D cases with thyroid disease but without antibodies and only slightly less risk in those with antibodies. In both groups, DPB1*0201 is the most prevalent risk allele (22.7% of TD patients without autoantibodies and 19.6% in TD cases with autoantibodies) that is otherwise observed only in 14% of T1D patients without thyroid autoimmunity.
The relatively high frequency of DPB1*0201 in Caucasian populations varies between 10–20% (www.allelefrequencies.net) (18). Some populations have higher frequencies, such as Italians and Sardinians (18–20%) in comparison with central Europeans (Germans and Austrians, 13–15%), Scandinavians (Norwegians and Swedes, 10–14%) or Bretons and British (10–15%). Whether these differences contribute to the regional variations in prevalence of thyroid autoimmunity should be investigated more thoroughly. HLA-DPB1 now comprises 155 alleles and its variability is increasingly recognized for its importance in bone marrow transplantation (19).
HLA-DPB1 alleles have been shown to be associated with Graves disease in a study of multiplex families from the U.K. (20,21) differentiating the risk of HLA-DR17 haplotypes. Graves disease also had a significant association with DPB1*0501, appearing as the major susceptibility gene with a population-attributable risk of 48.4% in a combined case-control and family study of Han Chinese (22). As Graves disease shows a slightly higher prevalence in Asians, the different frequencies of HLA risk and protection alleles may contribute to this geoepidemiological variation of autoimmune disease as well as to antibody formation (23–25). The varying frequencies of HLA-DR, -DQ, and -DP alleles across populations are paralleled by autoimmune disease incidence data with other risk factors such as higher birth weight (26) or viral infections (27). The high prevalence of TPO in the Asian T1D patients from the T1DGC cohort mirrors this enhanced ethnic susceptibility. Our analysis indicated the HLA class I Cw*1203 allele conferring an enhanced risk (data not shown) to develop thyroid autoimmunity in T1D patients. However, due to the comparatively low number of Asian individuals in this study, there was insufficient power to prove this.
T1D varies across populations with a somewhat slower course of onset in Japanese accompanied by more APS 3 variants (APS3v) and a female predominance. These APS3v patients show an association with DRB1*0405-DQB1*0401 (28). In the T1DGC Autoantibody Workshop data, we could not identify whether a later onset of T1D is associated with a higher frequency of thyroid autoimmunity. The major antigen in thyroid autoimmunity is TPO (29). Whether TPO epitopes engage in a specific interaction with the HLA-DR and/or HLA-DP peptide binding pocket in a similar manner as it has been presumed for GAD65, the major T1D antigen, is unknown (8,30,31). As the T1DGC recruited affected sibling pairs and only few simplex cases (as trios), we cannot assess whether the prevalence of AITD in multiplex T1D sibling relationships is higher than in simplex (i.e., sporadic) cases. However, the prevalence of TPO positivity is similar to data reported in earlier studies (14).
These findings have implications for the care of T1D patients, particularly those with Asian ancestry, as these individuals should be HLA genotyped and monitored for thyroid autoimmunity more carefully than the other groups investigated here. However, these results stress that all patients with T1D, particularly those with advancing age and women, need to have regular thyroid monitoring during follow-up investigations. Currently, genetic markers do not permit stratification of those with a higher risk with sufficient specificity. Nonetheless, the identification of risk or protective alleles represents the first step in the process of personalized medicine. Ultimately, unraveling the functional impact of these risk alleles will identify the mechanisms underlying multiple endocrine organ destruction.
Article Information
Funding. This study used resources provided by the T1DGC, a collaborative clinical study sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the National Human Genome Research Institute, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and JDRF and supported by U01 DK062418. P.R.F., P.B., and G.E. receive support from National Institutes of Healthgrants DK32083 and AI50864. P.B. is a Fellow of the Pediatric Scientist Development Program of the National Institutes of Health. K.B. is supported by the European Union’s 7th Framework Programme Natural Immunomodulators as Novel Immunotherapies for Type 1 Diabetes (NAIMIT), grant agreement 241447. The project described was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, award K12-HD000850.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. H.K. and P.R.F. researched data. P.B. reviewed the manuscript. G.E. contributed to the discussion and reviewed the manuscript. K.B. wrote the manuscript and researched data. K.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This publication is based on the presentations from the Type 1 Diabetes Genetics Consortium (T1DGC) Autoantibody Workshop, which was held on 7 June 2011 in Bethesda, MD. The publication of this supplement was made possible by resources provided by the T1DGC, a collaborative clinical study sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the National Human Genome Research Institute, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and JDRF and supported by grant U01 DK062418.
References
- 1.Eisenbarth GS, Gottlieb PA. Autoimmune polyendocrine syndromes. N Engl J Med 2004;350:2068–2079 [DOI] [PubMed] [Google Scholar]
- 2.Barker JM, Yu J, Yu L, et al. Autoantibody “subspecificity” in type 1 diabetes: risk for organ-specific autoimmunity clusters in distinct groups. Diabetes Care 2005;28:850–855 [DOI] [PubMed] [Google Scholar]
- 3.Badenhoop K, Walfish PG, Rau H, et al. Susceptibility and resistance alleles of human leukocyte antigen (HLA) DQA1 and HLA DQB1 are shared in endocrine autoimmune disease. J Clin Endocrinol Metab 1995;80:2112–2117 [DOI] [PubMed] [Google Scholar]
- 4.Pilia S, Casini MR, Cambuli VM, et al. Prevalence of type 1 diabetes autoantibodies (GAD and IA2) in Sardinian children and adolescents with autoimmune thyroiditis. Diabet Med 2011;28:896–899 [DOI] [PubMed] [Google Scholar]
- 5.López M, Varela L, Vázquez MJ, et al. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat Med 2010;16:1001–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duntas LH, Orgiazzi J, Brabant G. The interface between thyroid and diabetes mellitus. Clin Endocrinol (Oxf) 2011;75:1–9 [DOI] [PubMed] [Google Scholar]
- 7.McLachlan SM, Nagayama Y, Pichurin PN, et al. The link between Graves’ disease and Hashimoto’s thyroiditis: a role for regulatory T cells. Endocrinology 2007;148:5724–5733 [DOI] [PubMed] [Google Scholar]
- 8.Ellis RJ, Varela-Calvino R, Tree TI, Peakman M. HLA Class II molecules on haplotypes associated with type 1 diabetes exhibit similar patterns of binding affinities for coxsackievirus P2C peptides. Immunology 2005;116:337–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fountoulakis S, Vartholomatos G, Kolaitis N, Frillingos S, Philippou G, Tsatsoulis A. HLA-DR expressing peripheral T regulatory cells in newly diagnosed patients with different forms of autoimmune thyroid disease. Thyroid 2008;18:1195–1200 [DOI] [PubMed] [Google Scholar]
- 10.Erlich H, Valdes AM, Noble J, et al.; Type 1 Diabetes Genetics Consortium . HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes 2008;57:1084–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brorsson CA, Onengut S, Chen W-M, et al. ; Type 1 Diabetes Genetics Consortium. Novel association between immune-mediated susceptibility loci and persistent autoantibody positivity in type 1 diabetes. Diabetes 2015;64:3017–3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pietropaolo M, Peakman M, Pietropaolo SL, et al. Combined analysis of GAD65 and ICA512(IA-2) autoantibodies in organ and non-organ-specific autoimmune diseases confers high specificity for insulin-dependent diabetes mellitus. J Autoimmun 1998;11:1–10 [DOI] [PubMed] [Google Scholar]
- 13.Criswell LA, Pfeiffer KA, Lum RF, et al. Analysis of families in the Multiple Autoimmune Disease Genetics Consortium (MADGC) collection: the PTPN22 620W allele associates with multiple autoimmune phenotypes. Am J Hum Genet 2005;76:561–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Triolo TM, Armstrong TK, McFann K, et al. Additional autoimmune disease found in 33% of patients at type 1 diabetes onset. Diabetes Care 2011;34:1211–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heward JM, Allahabadia A, Daykin J, et al. Linkage disequilibrium between the human leukocyte antigen class II region of the major histocompatibility complex and Graves’ disease: replication using a population case control and family-based study. J Clin Endocrinol Metab 1998;83:3394–3397 [DOI] [PubMed] [Google Scholar]
- 16.Chen PL, Fann CS, Chang CC, et al. Linkage of Graves’ disease to the human leucocyte antigen region in the Chinese-Han population in Taiwan. Clin Endocrinol (Oxf) 2007;66:646–651 [DOI] [PubMed] [Google Scholar]
- 17.de Bakker PI, McVean G, Sabeti PC, et al. A high-resolution HLA and SNP haplotype map for disease association studies in the extended human MHC. Nat Genet 2006;38:1166–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez-Galarza FF, Christmas S, Middleton D, Jones AR. Allele frequency net: a database and online repository for immune gene frequencies in worldwide populations. Nucleic Acids Res 2011;39:D913–D919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson J, Mistry K, McWilliam H, Lopez R, Parham P, Marsh SG. The IMGT/HLA database. Nucleic Acids Res 2011;39:D1171–D1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratanachaiyavong S, Fleming D, Janer M, et al. HLA-DPB1 polymorphisms in patients with hyperthyroid Graves’ disease and early onset myasthenia gravis. Autoimmunity 1994;17:99–104 [DOI] [PubMed] [Google Scholar]
- 21.Ratanachaiyavong S, McGregor AM. HLA-DPB1 polymorphisms on the MHC-extended haplotypes of families of patients with Graves’ disease: two distinct HLA-DR17 haplotypes. Eur J Clin Invest 1994;24:309–315 [DOI] [PubMed] [Google Scholar]
- 22.Chen PL, Fann CS, Chu CC, et al. Comprehensive genotyping in two homogeneous Graves’ disease samples reveals major and novel HLA association alleles. PLoS One 2011;6:e16635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shapira Y, Poratkatz BS, Gilburd B, et al. Geographical differences in autoantibodies and anti-infectious agents antibodies among healthy adults. Clin Rev Allergy Immunol 2012;42:154–163 [DOI] [PubMed] [Google Scholar]
- 24.Shapira Y, Agmon-Levin N, Shoenfeld Y. Defining and analyzing geoepidemiology and human autoimmunity. J Autoimmun 2010;34:J168–J177 [DOI] [PubMed] [Google Scholar]
- 25.Shapira Y, Agmon-Levin N, Shoenfeld Y. Geoepidemiology of autoimmune rheumatic diseases. Nat Rev Rheumatol 2010;6:468–476 [DOI] [PubMed] [Google Scholar]
- 26.Järvinen TM, Harjutsalo V, Kinnunen L, Miettinen ME, Tuomilehto-Wolf E, Tuomilehto J. A population-specific diabetogenic haplotype HLA-A2,Cw1,B56,DR4,DQ8 is associated with high birthweight in Finnish diabetic families. Genes Immun 2008;9:207–213 [DOI] [PubMed] [Google Scholar]
- 27.Kubinak JL, Ruff JS, Hyzer CW, Slev PR, Potts WK. Experimental viral evolution to specific host MHC genotypes reveals fitness and virulence trade-offs in alternative MHC types. Proc Natl Acad Sci U S A 2012;109:3422–3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horie I, Kawasaki E, Ando T, et al. Clinical and genetic characteristics of autoimmune polyglandular syndrome type 3 variant in the Japanese population. J Clin Endocrinol Metab 2012;97:E1043–E1050 [DOI] [PubMed] [Google Scholar]
- 29.McLachlan SM, Rapoport B. Thyroid peroxidase as an autoantigen. Thyroid 2007;17:939–948 [DOI] [PubMed] [Google Scholar]
- 30.Menconi F, Osman R, Monti MC, Greenberg DA, Concepcion ES, Tomer Y. Shared molecular amino acid signature in the HLA-DR peptide binding pocket predisposes to both autoimmune diabetes and thyroiditis. Proc Natl Acad Sci U S A 2010;107:16899–16903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ge X, James EA, Reijonen H, Kwok WW. Differences in self-peptide binding between T1D-related susceptible and protective DR4 subtypes. J Autoimmun 2011;36:155–160 [DOI] [PMC free article] [PubMed] [Google Scholar]