Abstract
Type 1 diabetes (T1D) and celiac disease (CeD) cluster in families and can occur in the same individual. Genetic loci have been associated with susceptibility to both diseases. Our aim was to explore the genetic differences between individuals developing both these diseases (double autoimmunity) versus those with only one. We hypothesized that double autoimmunity individuals carry more of the genetic risk markers that are shared between the two diseases independently. SNPs were genotyped in loci associated with T1D (n = 42) and CeD (n = 28) in 543 subjects who developed double autoimmunity, 2,472 subjects with T1D only, and 2,223 CeD-only subjects. For identification of loci that were specifically associated with individuals developing double autoimmunity, two association analyses were conducted: double autoimmunity versus T1D and double autoimmunity versus CeD. HLA risk haplotypes were compared between the two groups. The CTLA4 and IL2RA loci were more strongly associated with double autoimmunity than with either T1D or CeD alone. HLA analyses indicated that the T1D high-risk genotype, DQ2.5/DQ8, provided the highest risk for developing double autoimmunity (odds ratio 5.22, P = 2.25 × 10−29). We identified a strong HLA risk genotype (DQ2.5/DQ8) predisposing to double autoimmunity, suggesting a dominant role for HLA. Non-HLA loci, CTLA4 and IL2RA, may also confer risk to double autoimmunity. Thus, CeD patients who carry the DQ2.5/DQ8 genotype may benefit from periodic screening of autoantibodies related to T1D.
Introduction
Type 1 diabetes (T1D) and celiac disease (CeD) are immunologic disorders, affecting between 0.5% and 1% of the general population (1,2). They are both multifactorial diseases arising from a combination of multiple genetic and environmental factors. In addition, these two diseases co-occur in families, and even in single patients, more often than expected by chance (3). Approximately 4–9% of patients with T1D also have CeD (4), while patients with CeD are at increased risk of developing T1D (5). Since the genetic contribution within each disease is high, there may be an overlap in their etiology due to shared genetic risk factors (6) or due to synergistic effects of the genes involved in each disease separately (7).
Both T1D and CeD are seen mainly in populations of European ancestry, although they occur at a lower prevalence in African, Asian, and Latin American populations (2,8,9). The underlying autoimmune processes share some features, but the autoreactive T cells and autoantibodies are directed against different autoantigens: insulin, GADA65, and IA-2 in T1D and tissue transglutaminase and endomysial antibody in CeD (10). In most patients, preislet and celiac autoimmunity develop in early childhood, although both diseases can also develop later in life (11,12).
The class II genes explain a major component of familial clustering in both T1D and CeD, in particular the HLA-DRB1, HLA-DQA1, and HLA-DQB1 genes (13). For T1D, alleles of HLA class II genes can confer both disease susceptibility and disease protection. Individuals carrying both the DR3-DQ2 (DRB1*03-DQB1*0201) and DR4-DQ8 haplotype (DRB1*04-DQB1*0302) are at the highest risk for developing T1D (14). Its presence marks a 55% risk of developing overt diabetes by age 12 years (15); however, only 20–50% of patients with T1D carry this genotype. For CeD, the most prominent association is with HLA-DQ2.5 (DQA1*0501-DQB1*0201) (16). Individuals homozygous for the DQB1*02 allele (i.e., carriers of DQ2.5/DQ2.5 and DQ2.5/DQ2.2) are at high risk of developing CeD (17).
Genome-wide association studies (GWAS) have revolutionized the identification of additional predisposing risk factors to these diseases outside the HLA region. To date, more than 40 non-HLA loci for T1D and 26 non-HLA loci for CeD have been identified by GWAS (summarized at www.t1dbase.org [18–20]). It is noteworthy that many of the non-HLA loci are shared between various autoimmune diseases (7,21). GWAS and cross-disease studies have identified the same regions, or even the same single nucleotide polymorphisms (SNPs), as associated with both T1D and CeD, including the HLA, TAGAP, IL18RAP, SH2B3, CTLA4, CCR5, IL2/21, BACH2, UBASH3A, and PTPN2 loci (7,22).
Individuals affected by more than one autoimmune disorder may have an immune response more disturbed than those with only one disease. Specific genetic factors already identified as contributors to risk of T1D and CeD individually could be critical for double autoimmunity. Thus, our aim was to examine the genetic differences between individuals developing both T1D and CeD with respect to the genetic risk associated with having only one of these diseases.
Research Design and Methods
Patients and Control Participants
Informed consent was obtained for all samples used, and the project was approved by the ethics committees of each of the institutions involved. T1D-only samples were collected from the Type 1 Diabetes Genetics Consortium (T1DGC), and CeD-only samples were collected from previous studies (19,23,24). Samples from individuals with both T1D and CeD (double autoimmunity) were collected from T1DGC, the Barbara Davis Center, and the VU University Medical Centre (Amsterdam, the Netherlands) (Table 1). The identification of T1D only was based on self-reports, evaluation of medical records, and, when indicated, C-peptide determination using a standard protocol of the T1DGC.
Table 1.
Samples and data sets used in our analyses
| Double autoimmunity case subjects | Control subjects with T1D only | Control subjects with CeD only | Total | |
|---|---|---|---|---|
| Origin (by center) | ||||
| Barbara Davis Center | 313 | 313 | ||
| T1DGC | 147 | 2,472 | 2,619 | |
| VU University Medical Centre | 51 | 51 | ||
| UMCG | 32 | 2,223 | 2,255 | |
| Origin (by country) | ||||
| U.S. | 460 | 2,472 | 2,932 | |
| The Netherlands | 83 | 1,134 | 1,217 | |
| U.K. | 1,089 | 1,089 |
The identification of double autoimmunity individuals among patients first diagnosed with T1D was based initially upon self-reporting and confirmed by having high and persistent levels of IgA transglutaminase (IgA tissue transglutaminase) autoantibodies or confirmed by biopsy (25). T1D was identified in patients first diagnosed with CeD according to the guidelines of an American Diabetes Association position statement (26). The patients with CeD only were identified with autoantibody testing, confirmed by an intestinal biopsy (27). Control subjects of Caucasian ancestry were also included (23). In total, 543 individuals with double autoimmunity were identified, 3,098 patients with T1D only, 12,480 CeD-only patients, and 11,023 control subjects. All samples were genotyped using the ImmunoChip (23). The hybridization and processing of the CeD samples and part of the double autoimmunity samples (those not from T1DGC) were performed in the Department of Genetics, University Medical Centre Groningen (UMCG), while the genotyping of the T1D samples and the double autoimmunity samples from T1DGC was performed at the Genome Sciences Laboratory in the Center for Public Health Genomics at the University of Virginia. A total of 28 non-HLA SNPs associated with CeD and 42 SNPs with T1D were selected, all at genome-wide significance (P < 5 × 10−8) (19,20,23,28–33). After quality control, 66 SNPs remained for our analysis: 21 non-HLA SNPs associated with CeD-only, 33 SNPs associated with T1D-only, and 12 SNPs from eight loci shared between the two diseases (Supplementary Table 1). For prediction of whether an individual carries HLA-DQ2 (DQ2.5 or DQ2.2) and/or DQ8 alleles, five of the six tagging SNPs described by Monsuur et al. (34) were used. We failed to predict the HLA-DQ7 haplotype, as the sixth SNP (rs4639334) failed quality-control metrics.
Study Groups and Quality Control
Two data sets were assembled and two independent analyses performed to identify SNPs contributing to double autoimmunity. Individuals in the first analysis consisted of “case” subjects with double autoimmunity and “control” subjects with T1D only (T1D+CeD/T1D). Individuals in the second analysis consisted of “case” subjects with double autoimmunity and “control” subjects with CeD only (T1D+CeD/CeD).
The quality-control assessment protocols were conducted for each study group independently. Individuals were excluded with call rate <99.5% or sex inconsistency or if there was a first- or second-degree relationship with the index case. SNPs were excluded with a genotyping rate <99%, minor allele frequency <0.05%, and failure of Hardy-Weinberg equilibrium assumptions (P < 5 × 10−6). The latter analysis was performed using KING, version 1.4, software (35). Owing to the different ethnic backgrounds present in the sample (samples from North America, Europe, U.K., and Asia Pacific in the T1DGC data set and from Europe and India in the CeD data set), a principal components analysis was applied to each of the data sets with the aim of identifying and excluding possible ethnicity outliers and to reduce the possibility of population stratification. This analysis was performed sequentially using EIGENSTRAT, version 4.2, software (36) and removing outliers at each step. After quality control, the data set included 2,955 individuals for the T1D+CeD/T1D analysis (1,451 males and 1,504 females) and 2,655 individuals for the T1D+CeD/CeD analysis (865 males and 1,790 females)—all the samples with Caucasian origin.
Statistical Analysis
The association analysis was conducted separately for HLA and non-HLA risk loci. For the HLA locus, the analysis was performed on the predicted haplotypes and genotypes of DQ2.5 (DQA1*0501, DQB1*0201, and DRB1*03), DQ2.2 (DQA1*0201, DQB1*0202, and DRB1*07), and DQ8 (DQA1*03, DQB1*0302, and DRB1*04) and including the first five principal components as covariates. These haplotypes are well-known risk factors for both T1D and CeD. The absence of any of these haplotypes was classified as “other.” The HLA analyses were divided into an analysis of the number of haplotypes per individual (whether an individual was carrying 0, 1, or 2 copies of the tested haplotype) and of genotypes (whether an individual was carrying combinations of risk haplotypes).
Association analyses were performed for each study group using a genetic-based matching score. Pairwise comparisons of identity by descent were calculated for all samples, and then individuals were matched and clustered in homogeneous groups of case and control subjects to reduce false-positive associations owing to population stratification. With the results from the calculated clusters, a Cochran-Mantel-Haenszel analysis was performed, correcting the association for the genomic control inflation factor (λ). Nominal statistical significance of P < 0.05 was used as the threshold for association, as the analyzed SNPs had been associated in previous GWAS and replicated at genome-wide significance. The analyses were performed using PLINK, version 1.07, and the statistical suite R, version 3.1.0 (37,38).
Results
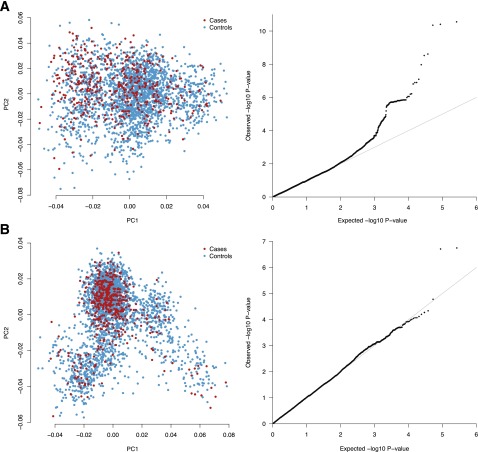
After completion of quality-control procedures and removal of outliers, a total of 2,955 samples (483 case and 2,472 control subjects) were included for the first T1D+CeD/T1D analysis; a total of 2,655 samples were included for T1D+CeD/CeD analysis (432 case and 2,223 control subjects). There was no evidence of significant inflation in the results of association (λT1D+CeD/T1D = 0.99; λT1D+CeD/CeD = 1.04) for either set of analyses (Fig. 1).
Figure 1.
Principal components and Q-Q plot of each group of analyses. The principal components analysis was used to cluster the most homogeneous samples for association analysis. The shape of the clusters differs because of the different origins of the merged samples; however, it is still possible to observe a good match between case and control subjects. A: Double autoimmunity vs. CeD-only patients. B: Double autoimmunity vs. patients with T1D only. PC1, principal component 1; PC2, principal component 2.
Comparing Known Risk Alleles Across Diseases
We first aimed to investigate the status of established CeD and T1D loci across the published GWAS data sets (19,20,28–33) considering only those loci with at least one reported risk allele–associated genome-wide significance (P < 5 × 10−8) and confirmation in independent samples. Across 28 non-HLA SNPs from CeD and 42 non-HLA SNPs from T1D, representing 60 distinct risk loci, eight loci (represented by 12 SNPs) are shared between both diseases (Supplementary Table 1). Four of the reported SNPs in CeD and/or T1D (rs13010713-ITGA4, rs11755527-BACH2, rs1265564-CUX2, and rs917997-IL18RAP) were removed based on quality-control metrics, with one SNP proxy inserted (rs917997 was replaced by rs7559479 for IL18RAP). In total, 66 SNPs were included in the association analysis.
Genetic Association in Double Autoimmunity Patients
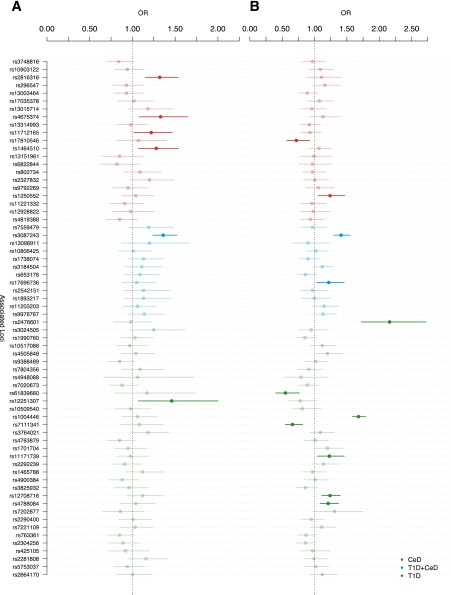
Results of the association analysis for each of the 66 SNPs that passed our quality control in the two diseases are shown in Fig. 2 (odds ratio [OR] and 95% CI). Of the 21 CeD-only SNPs, 6 (28.6%) were associated (P < 0.05) with risk of double autoimmunity (Table 2). Similarly, of 33 T1D-only SNPs, 8 (24.2%) from six loci were associated (P < 0.05) with double autoimmunity (Table 3).
Figure 2.
ORs and CIs for all the variants evaluated. ORs and 95% CIs for all the SNPs associated with CeD, T1D, or both that passed our quality controls. A: Double autoimmunity vs. T1D. B: Double autoimmunity vs. CeD. Highlighted markers correspond with those with a significant P value: <0.05. It was not possible to detect an enrichment of CeD or T1D variants associated with double autoimmunity based on the analysis of both data sets.
Table 2.
Association of previously described variants for CeD and T1D in the data set of double autoimmunity versus CeD-only patients
| Chr | SNP | Position | A1 | Allele freq. | OR (95% CI) | P | Reported disease | Risk allele | OR reported | P reported | Gene reported | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | rs17810546 | 161147744 | G | 0.16 | 0.72 (0.57, 0.92) | 0.022 | CeD | G | 1.36 | 4 × 10−28 | IL12A | 19 |
| 10 | rs1250552 | 80728033 | G | 0.44 | 1.24 (1.05, 1.46) | 0.020 | CeD | NR | 1.12 | 9 × 10−10 | ZMIZ1 | 19 |
| 2 | rs3087243 | 204447164 | G | 0.58 | 1.41 (1.30, 1.55) | 0.001 | T1D+CeD | A | NR | 8 × 10−11 | CTLA4 | 20,22 |
| 12 | rs17696736 | 110971201 | G | 0.47 | 1.22 (1.03, 1.45) | 0.036 | T1D+CeD | G | 1.34 | 2 × 10−14 | SH2B3/LNK/TRAFD1/PTPN11 | 20 |
| 1 | rs2476601 | 114179091 | A | 0.12 | 2.16 (1.72, 2.72) | 2.4 × 10−9 | T1D | T | 1.98 | 2 × 10−80 | PTPN22 | 20 |
| 10 | rs61839660 | 6134703 | T | 0.09 | 0.55 (0.39, 0.76) | 0.001 | T1D | NR | 1.6 | 5 × 10−9 | IL2RA | 20 |
| 11 | rs1004446 | 2126719 | C | 0.61 | 1.68 (1.58, 1.79) | 1.2 × 10−7 | T1D | C | 1.61 | 4 × 10−9 | INS | 22 |
| 11 | rs7111341 | 2169742 | T | 0.27 | 0.66 (0.54, 0.81) | 4.6 × 10−4 | T1D | NR | NR | 4 × 10−48 | INS | 20 |
| 12 | rs11171739 | 54756892 | C | 0.43 | 1.23 (1.04, 1.45) | 0.026 | T1D | C | 1.34 | 1 × 10−11 | ERBB3 | 33 |
| 16 | rs12708716 | 11087374 | A | 0.64 | 1.24 (1.11, 1.39) | 0.034 | T1D | G/A | NR | 7 × 10−13 | CLEC16A/KIAA0350 | 22 |
| 16 | rs4788084 | 28447349 | G | 0.57 | 1.21 (1.08, 1.37) | 0.049 | T1D | G | 1.09 | 3 × 10−13 | IL27 | 20 |
A1, allele associated; Allele freq., allele frequency for which OR is reported; Chr, chromosome; Gene reported, the most plausible gene reported by the literature; NR, not reported; Position, position in base pair; Ref, reference.
Table 3.
Association of previously described variants for CeD and T1D in the data set of double autoimmunity versus patients with T1D only
| Chr | SNP | Position | A1 | Allele freq. | OR (95% CI) | P | Reported disease | Risk allele | OR reported | P reported | Gene reported | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | rs2816316 | 190803436 | A | 0.83 | 1.32 (1.15, 1.53) | 0.03086 | CeD | A | 1.25 | 2 × 10−17 | RGS1 | 19 |
| 2 | rs4675374 | 204510823 | A | 0.23 | 1.33 (1.08, 1.64) | 0.00728 | CeD | A | 1.14 | 6 × 10−9 | CTLA4/ICOS/CD28 | 19 |
| 3 | rs11712165 | 120601486 | C | 0.38 | 1.22 (1.01, 1.46) | 0.03101 | CeD | C | 1.13 | 8 × 10−9 | CD80/KTELC1 | 19 |
| 3 | rs1464510 | 189595248 | A | 0.46 | 1.28 (1.07, 1.54) | 0.006945 | CeD | A | 1.29 | 3 × 10−40 | LPP | 19 |
| 2 | rs3087243 | 204447164 | G | 0.61 | 1.36 (1.23, 1.51) | 0.00126 | T1D+CeD | G | 1.15 | 8 × 10−11 | CTLA4 | 20,22 |
| 10 | rs12251307 | 6163501 | T | 0.09 | 1.46 (1.06, 2.0) | 0.01756 | T1D | T | NR | 1 × 10−13 | IL2RA | 20 |
A1, allele associated; Allele freq., allele frequency for which OR is reported; Chr, chromosome; Gene reported, the most plausible gene reported by the literature; Position, position in base pair; Ref, reference.
Of the 12 SNPs in eight loci that were shared across T1D and CeD, 10 SNPs (representing seven loci) exhibited the same trend of effect compared with the effect on individual disease risk in previous T1D or CeD GWAS. The IL12A locus SNP rs17810546 had an opposite effect in the double autoimmunity group (ORT1D+CeD/CeD 0.72; minor allele frequency 0.16, P = 0.022) than in the CeD GWAS (OR 1.36). There was only one locus shared between T1D and CeD (CTLA4 [rs3087243]) that was associated in both T1D+CeD/T1D (P = 0.001) and T1D+CeD/CeD (P = 0.0006). The association of double autoimmunity with IL2RA differed in the SNP for the two groups, with rs61839660 in T1D+CeD/CeD (P = 0.001) but rs12251307 in T1D+CeD/T1D (P = 0.0175). These two SNPs are in linkage disequilibrium (r2 = 0.543, D′ = 0.84); however, rs61839660 is located intronic in IL2RA, while rs12251307 is 5′ of the same gene.
Association of HLA Loci
None of the HLA haplotypes (HLA-DQ2.5, HLA-DQ2.2, or HLA-DQ8) were statistically significant for association of double autoimmunity with respect to CeD only (T1D+CeD/CeD). The HLA-DQ8 haplotype had the highest risk for double autoimmunity, though not significant, when the double autoimmunity individuals were compared with those with CeD only (OR 5.09, P = 0.16). In contrast, the HLA-DQ2.5 haplotype was significantly associated (P = 0.0003) with double autoimmunity relative to T1D only (OR 1.44). There was absence of association of double autoimmunity with “other” HLA risk haplotypes (Table 4).
Table 4.
Haplotype and genotype HLA association and frequency comparison between healthy control subjects and patients with double autoimmunity, T1D only, or CeD only
| Freq. control subjects | T1D+CeD/CeD |
T1D+CeD/T1D |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Freq. T1D+CeD | Freq. CeD only | OR (95% CI) | P | Freq. T1D+CeD | Freq. T1D only | OR (95% CI) | P | ||
| Haplotype | |||||||||
| DQ2.5 | 0.14 | 0.520 | 0.446 | 1.035 (0.860, 1.249) | 0.972 | 0.446 | 0.318 | 1.442 (1.189, 1.748) | 0.0003 |
| DQ2.2 | 0.094 | 0.047 | 0.155 | 0.255 (0.173, 0.374) | 0.422 | 0.046 | 0.040 | 1.201 (0.793, 1.821) | 0.381 |
| DQ8 | 0.1 | 0.350 | 0.064 | 5.086 (3.883, 6.662) | 0.163 | 0.346 | 0.392 | 0.939 (0.779, 1.131) | 0.520 |
| Other | 0.663 | 0.157 | 0.260 | 0.467 (0.366, 0.595) | 0.500 | 0.163 | 0.249 | 0.660 (0.530, 0.821) | 0.0001 |
| Genotype | |||||||||
| DQ2.5/DQ2.5 | 0.020 | 0.176 | 0.192 | 0.99 (0.96, 1.02) | 0.914 | 0.168 | 0.066 | 1.20 (1.14, 1.26) | 0.005 |
| DQ2.5/DQ2.2 | 0.032 | 0.039 | 0.232 | 0.84 (0.82, 0.87) | 7.29E-4 | 0.039 | 0.017 | 1.16 (1.06, 1.27) | 0.242 |
| DQ2.5/DQ8 | 0.027 | 0.350 | 0.067 | 1.47 (1.41, 1.53) | 3.31E-10 | 0.350 | 0.377 | 0.98 (0.95, 1.01) | 0.681 |
| DQ2.5/other | 0.184 | 0.150 | 0.357 | 0.87 (0.85, 0.90) | 1.9E-3 | 0.168 | 0.112 | 1.07 (1.03, 1.11) | 0.688 |
| DQ2.2/DQ2.2 | 0.012 | 0.005 | 0.004 | 1.03 (0.83, 1.28) | 0.905 | 0.004 | 0.002 | 1.18 (0.88, 1.58) | 0.169 |
| DQ2.2/DQ8 | 0.022 | 0.035 | 0.012 | 1.22 (1.10, 1.36) | 0.189 | 0.033 | 0.036 | 0.98 (0.92, 1.06) | 0.908 |
| DQ2.2/other | 0.111 | 0.012 | 0.059 | 0.87 (0.82, 0.92) | 0.129 | 0.010 | 0.025 | 0.91 (0.83, 0.99) | 0.326 |
| DQ8/DQ8 | 0.009 | 0.083 | 0.010 | 1.6 (1.46, 1.74) | 4.20E-4 | 0.083 | 0.078 | 1.00 (0.96, 1.05) | 0.886 |
| DQ8/other | 0.135 | 0.148 | 0.031 | 1.40 (1.32, 1.49) | 1.46E-4 | 0.143 | 0.216 | 0.94 (0.91, 0.97) | 0.175 |
| Other/other | 0.449 | 0.002 | 0.036 | 0.85 (0.79, 0.92) | 0.166 | 0.002 | 0.072 | 0.84 (0.80, 0.89) | 0.028 |
Freq., frequency.
T1D+CeD/CeD analysis identified a significant association with the heterozygote genotype DQ2.5/DQ8 (OR 1.47, P = 3.31 × 10−10) (Table 4). In the double autoimmunity group, we identified the haplotype DQ2.5/DQ2.5 (OR 1.2, P = 0.005) as significantly associated with risk compared with T1D only (Table 4).
Conclusions
It is possible that a subgroup of patients with T1D or CeD have certain characteristics that predispose them to develop both diseases. However, the larger percentage of individuals developing double autoimmunity than expected based on the prevalence of the individual diseases suggests that common genetic loci and common biological pathways are involved in the pathogenesis of double autoimmunity. By comparing the T1D and CeD GWAS results, we analyzed 12 shared genetic loci both within and outside the MHC-HLA region.
Targeted screening for CeD is recommended in high-risk groups such as children with T1D (27). Screening for CeD in children is recommended as soon as they develop T1D, and, in the case of a negative outcome, this test should be repeated at well-defined intervals for at least 10 years (10,39). Untreated CeD carries the risks of iron deficiency anemia, growth retardation, osteoporosis, neuropsychiatric disorders, fertility problems, and gastrointestinal malignancies such as intestinal lymphoma. Genetic risk profiling can contribute to identifying patients with T1D who are predisposed to develop CeD and who might benefit from closer monitoring, as in the majority of cases (>90%), the diagnosis of T1D precedes that of CeD.
Our aim in the T1DGC Autoantibody Workshop was to enhance the understanding of why a single patient develops two autoimmune diseases by investigating the associated genetic risk factors. In the future, this information might also aid in building genetic risk models to identify individuals with either T1D or CeD who are at high risk of developing double autoimmunity. In our analysis, the HLA locus still presents the most important association with double autoimmunity. However, our association study shows that the HLA haplotypes or genotypes that are related with the risk of double autoimmunity are not the same as those related to the risk of either T1D or CeD in isolation. Individuals with both diseases more closely resemble the patients with T1D only with respect to the frequency of the DQ2.5/DQ8 genotype, which is a well-known risk combination for T1D. The group of DQ2.5/DQ8 carriers is infrequent in the general population (∼2.5%), yet these individuals have a more than fivefold increased risk of developing either T1D or double autoimmunity. Thus, the periodic screening of T1D-related autoantibodies in predominantly CeD patients carrying DQ2.5/DQ8 could be helpful for identifying T1D at an early stage of the disease. The same approach applies to patients with T1D carrying DQ2.5/DQ2.5, who should be screened for CeD antibodies.
We did not observe a significant enrichment of the shared risk alleles in the group of double autoimmunity patients. In our analysis, we did observe a similar number of CeD-only or T1D-only loci for both study groups. We are aware of the lack of follow-up of the patients but, based on epidemiology, would not have expected a significant increase in the number of unnoticed double autoimmunity patients that could modify the results (40). We did not find any proof for our hypothesis that known shared genetic risk factors contribute to the coexistence of multiple diseases in the same individual. Nevertheless, CTLA4 has been associated with multiple autoimmune diseases and has a well-known role in the activation, differentiation, and proliferation of T cells (41). In our analysis, the CTLA4 SNP rs3087243 showed a significant association with double autoimmunity in both data sets. While this SNP has not been associated with CeD in GWAS reports, it is in linkage disequilibrium (r2 = 0.144; D′ = 0.86) with rs4675374, which is associated with CeD risk (19). These data suggest that CTLA4 can contribute to the development of double autoimmunity. We also observed the significant association of SNPs located in the IL2RA locus. The functional role of IL2RA is highly related to CTLA4, with a possibly synergistic role, for example, in regulating the activation and differentiation of CD4-positive T cells (42).
In conclusion, we have shown that there are different genetic associations between patients with double autoimmunity, T1D only, or CeD only. The impact of genetic risk is based, primarily, on specific alleles and genotypes in the HLA class II region, with some support for two genes (CTLA4 and IL2RA) that may be linked through a common immune pathway. The HLA and non-HLA loci found in this study can be used as stratification factors in the construction of risk models to predict double autoimmunity and for pathway enrichment analysis to enhance our understanding of the pathophysiology involved in the development of double autoimmunity. It should be noted that our analysis only included individuals of Caucasian origin. Hence, populations with other genetic backgrounds should be carefully checked, as the results may differ owing to differences in genetic background. The question of how these genetic factors influence the development of double autoimmunity requires further study.
Article Information
Acknowledgments. The authors thank all the participants in this study, all the doctors who collected blood, and Mathieu Platteel and Astrid Maatman for preparing and genotyping samples. The authors thank Jackie Senior, Department of Genetics, UMCG, for critically reading the manuscript.
Funding. This study was supported by the Celiac Disease Consortium (an innovative cluster approved by the Netherlands Genomics Initiative and partly funded by the Dutch Government [grant BSIK03009 to C.W.]), the Netherlands Organisation for Scientific Research (NWO-VICI grant 918.66.620 to C.W.), the Dutch Digestive Diseases Foundation (MLDS WO11-30 to C.W.), the European Union Project PreventCD (FP6-2005-FOOD-4B-36383-PreventCD), the parent CEDAR grant (R01 DK050979), and the National Institutes of Health (5R1-DK-084568-02).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.G.-A., J.R., and C.W. designed the study. J.G.-A. and J.R. performed analysis. S.F.B., V.K., E.C.d.H., G.T., I.R.-P., A.S., the T1DGC, W.-M.C., S.O.-G., S.S., Diabeter, M.R., C.J.M., E.L., S.S.R., and C.W. collected samples and data. J.G.-A., J.R., and C.W. were the primary contributors to the writing of the manuscript. All other authors contributed to the writing of the manuscript. C.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dcs15-2007/-/DC1.
J.G.-A. and G.T. are currently affiliated with the Wellcome Trust Sanger Institute, Hinxton, Cambridge, U.K.
This publication is based on the presentations from the Type 1 Diabetes Genetics Consortium (T1DGC) Autoantibody Workshop, which was held on 7 June 2011 in Bethesda, MD. The publication of this supplement was made possible by resources provided by the T1DGC, a collaborative clinical study sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the National Human Genome Research Institute, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and JDRF and supported by grant U01 DK062418.
References
- 1.Biagi F, Klersy C, Balduzzi D, Corazza GR. Are we not over-estimating the prevalence of coeliac disease in the general population? Ann Med 2010;42:557–561 [DOI] [PubMed] [Google Scholar]
- 2.Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am 2010;39:481–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker JM. Clinical review: Type 1 diabetes-associated autoimmunity: natural history, genetic associations, and screening. J Clin Endocrinol Metab 2006;91:1210–1217 [DOI] [PubMed] [Google Scholar]
- 4.Lazzarotto F, Basso D, Plebani M, Moscon A, Zanchetta R, Betterle C. Celiac disease and type 1 diabetes. Diabetes Care 2003;26:248–249 [DOI] [PubMed] [Google Scholar]
- 5.Ludvigsson JF, Ludvigsson J, Ekbom A, Montgomery SM. Celiac disease and risk of subsequent type 1 diabetes: a general population cohort study of children and adolescents. Diabetes Care 2006;29:2483–2488 [DOI] [PubMed] [Google Scholar]
- 6.Zhernakova A, van Diemen CC, Wijmenga C. Detecting shared pathogenesis from the shared genetics of immune-related diseases. Nat Rev Genet 2009;10:43–55 [DOI] [PubMed] [Google Scholar]
- 7.Cotsapas C, Voight BF, Rossin E, et al.; FOCiS Network of Consortia . Pervasive sharing of genetic effects in autoimmune disease. PLoS Genet 2011;7:e1002254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cifuentes RA, Rojas-Villarraga A, Anaya JM. Human leukocyte antigen class II and type 1 diabetes in Latin America: a combined meta-analysis of association and family-based studies. Hum Immunol 2011;72:581–586 [DOI] [PubMed] [Google Scholar]
- 9.Park Y. Why is type 1 diabetes uncommon in Asia? Ann N Y Acad Sci 2006;1079:31–40 [DOI] [PubMed] [Google Scholar]
- 10.Sud S, Marcon M, Assor E, Palmert MR, Daneman D, Mahmud FH. Celiac disease and pediatric type 1 diabetes: diagnostic and treatment dilemmas. Int J Pediatr Endocrinol 2010;2010:161285 [DOI] [PMC free article] [PubMed]
- 11.Camarca ME, Mozzillo E, Nugnes R, et al. Celiac disease in type 1 diabetes mellitus. Ital J Pediatr 2012;38:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naik RG, Brooks-Worrell BM, Palmer JP. Latent autoimmune diabetes in adults. J Clin Endocrinol Metab 2009;94:4635–4644 [DOI] [PubMed] [Google Scholar]
- 13.Noble JA, Erlich HA. Genetics of type 1 diabetes. Cold Spring Harb Perspect Med 2012;2:a007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noble JA, Valdes AM. Genetics of the HLA region in the prediction of type 1 diabetes. Curr Diab Rep 2011;11:533–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aly TA, Ide A, Jahromi MM, et al. Extreme genetic risk for type 1A diabetes. Proc Natl Acad Sci U S A 2006;103:14074–14079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiao SW, Iversen R, Ráki M, Sollid LM. The adaptive immune response in celiac disease. Semin Immunopathol 2012;34:523–540 [DOI] [PubMed] [Google Scholar]
- 17.Romanos J, van Diemen CC, Nolte IM, et al. Analysis of HLA and non-HLA alleles can identify individuals at high risk for celiac disease. Gastroenterology 2009;137:834–840 [DOI] [PubMed]
- 18.Burren OS, Adlem EC, Achuthan P, Christensen M, Coulson RM, Todd JA. T1DBase: update 2011, organization and presentation of large-scale data sets for type 1 diabetes research. Nucleic Acids Res 2011;39:D997–D1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubois PC, Trynka G, Franke L, et al. Multiple common variants for celiac disease influencing immune gene expression. Nat Genet 2010;42:295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrett JC, Clayton DG, Concannon P, et al.; Type 1 Diabetes Genetics Consortium . Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet 2009;41:703–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutierrez-Achury J, Coutinho de Almeida R, Wijmenga C. Shared genetics in coeliac disease and other immune-mediated diseases. J Intern Med 2011;269:591–603 [DOI] [PubMed] [Google Scholar]
- 22.Smyth DJ, Plagnol V, Walker NM, et al. Shared and distinct genetic variants in type 1 diabetes and celiac disease. N Engl J Med 2008;359:2767–2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trynka G, Hunt KA, Bockett NA, et al.; Spanish Consortium on the Genetics of Coeliac Disease (CEGEC); PreventCD Study Group; Wellcome Trust Case Control Consortium (WTCCC) . Dense genotyping identifies and localizes multiple common and rare variant association signals in celiac disease. Nat Genet 2011;43:1193–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rich SS, Akolkar B, Concannon P, et al. Overview of the Type I Diabetes Genetics Consortium. Genes Immun 2009;10(Suppl. 1):S1–S4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romanos J, Rosén A, Kumar V, et al.; PreventCD Group . Improving coeliac disease risk prediction by testing non-HLA variants additional to HLA variants. Gut 2014;63:415–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Diabetes Association. Standards of medical care in diabetes—2011. Diabetes Care 2010;34(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed]
- 27.Husby S, Koletzko S, Korponay-Szabó IR, et al.; ESPGHAN Working Group on Coeliac Disease Diagnosis; ESPGHAN Gastroenterology Committee; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition . European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr 2012;54:136–160 [DOI] [PubMed] [Google Scholar]
- 28.Hunt KA, Zhernakova A, Turner G, et al. Newly identified genetic risk variants for celiac disease related to the immune response. Nat Genet 2008;40:395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Heel DA, Franke L, Hunt KA, et al. A genome-wide association study for celiac disease identifies risk variants in the region harboring IL2 and IL21. Nat Genet 2007;39:827–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plagnol V, Howson JM, Smyth DJ, et al.; Type 1 Diabetes Genetics Consortium . Genome-wide association analysis of autoantibody positivity in type 1 diabetes cases. PLoS Genet 2011;7:e1002216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang J, Ellinghaus D, Franke A, Howie B, Li Y. 1000 Genomes-based imputation identifies novel and refined associations for the Wellcome Trust Case Control Consortium phase 1 data. Eur J Hum Genet 2012;20:801–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grant SF, Qu HQ, Bradfield JP, et al.; DCCT/EDIC Research Group . Follow-up analysis of genome-wide association data identifies novel loci for type 1 diabetes. Diabetes 2009;58:290–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wellcome Trust Case Control Consortium . Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 2007;447:661–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monsuur AJ, de Bakker PI, Zhernakova A, et al. Effective detection of human leukocyte antigen risk alleles in celiac disease using tag single nucleotide polymorphisms. PLoS One 2008;3:e2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manichaikul A, Mychaleckyj JC, Rich SS, Daly K, Sale M, Chen WM. Robust relationship inference in genome-wide association studies. Bioinformatics 2010;26:2867–2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 2006;38:904–909 [DOI] [PubMed] [Google Scholar]
- 37.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007;81:559–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ihaka R, Gentleman R. R: a language for data analysis and graphics. J Comput Graph Stat 1996;5:299–314 [Google Scholar]
- 39.Pham-Short A, Donaghue KC, Ambler G, Chan AK, Craig ME. Coeliac disease in type 1 diabetes from 1990 to 2009: higher incidence in young children after longer diabetes duration. Diabet Med 2012;29:e286–e289 [DOI] [PubMed]
- 40.Elfström P, Sundström J, Ludvigsson JF. Systematic review with meta-analysis: associations between coeliac disease and type 1 diabetes. Aliment Pharmacol Ther 2014;40:1123–1132 [DOI] [PubMed] [Google Scholar]
- 41.Stahl EA, Raychaudhuri S, Remmers EF, et al.; BIRAC Consortium; YEAR Consortium . Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat Genet 2010;42:508–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rudolph M, Hebel K, Miyamura Y, Maverakis E, Brunner-Weinzierl MC. Blockade of CTLA-4 decreases the generation of multifunctional memory CD4+ T cells in vivo. J Immunol 2011;186:5580–5589 [DOI] [PubMed] [Google Scholar]