Abstract
The major barrier for using small interfering RNA (siRNA) as cancer therapeutics is the inadequate delivery and transfection in solid tumors. We have previously shown that paclitaxel tumor priming, by inducing apoptosis, expands the tumor interstitial space, improves the penetration and dispersion of nanoparticles and siRNA-lipoplexes in 3-dimensional tumor histocultures, and promotes the delivery and transfection efficiency of siRNA-lipoplexes under the locoregional setting in vivo (i.e., intraperitoneal treatment of intraperitoneal tumors). The current study evaluated whether tumor priming is functional for systemically delivered siRNA via intravenous injection, which would subject siRNA to several additional delivery barriers and elimination processes. We used the same pegylated cationic (PCat)-siRNA lipoplexes as in the intraperitoneal study to treat mice bearing subcutaneous human pancreatic Hs766T xenograft tumors. The target gene was survivin, an inducible chemoresistance gene. The results show single agent paclitaxel delayed tumor growth but also significantly induced the survivin protein level in residual tumors, whereas addition of PCat-siSurvivin completely reversed the paclitaxel-induced survivin and enhanced the paclitaxel activity (p<0.05). In comparison, PCat-siSurvivin alone did not yield survivin knockdown or antitumor activity, indicating the in vivo effectiveness of intravenous siRNA-mediated gene silencing requires paclitaxel cotreatment. Additional in vitro studies showed that paclitaxel promoted the cytoplasmic release of siGLO, a 22 nucleotide double-stranded RNA that has no mRNA targets, from its PCat lipoplex and/or endosomes/lysosomes. Taken together, our earlier and current data show paclitaxel tumor priming, by promoting the interstitial transport and cytoplasmic release, is critical to promote the delivery and transfection of siRNA in vivo. In addition, because paclitaxel has broad spectrum activity and is used to treat multiple types of solid tumors including the hard-to-treat pancreatic cancer, the synergistic paclitaxel+siSurvivin combination represents a potentially useful chemo-gene therapy.
Keywords: Intravenous siRNA delivery, Survivin, Paclitaxel, Pancreatic Cancer, Chemo-gene therapy
Graphical Abstract

1. Introduction
Small interfering RNA (siRNA) is an attractive option for post-transcriptional silencing of a target gene. The most critical barrier for using siRNA as cancer therapeutics is the inadequate delivery and transfection in vivo [1-3]. siRNA, typically comprising 20-27 nucleotides, has high negative charge that is unfavorable for cellular uptake. siRNA molecules are readily degraded by serum endonucleases and removed by glomerular filtration, resulting in short plasma half-lives of <10 min after intravenous administration [4]. Cationic liposomes form lipoplex with siRNA, which protects siRNA from degradation and facilitates cellular binding and internalization of siRNA [3,5-7].
The concept of tumor priming, i.e., using apoptosis-inducing drugs to transiently expand the interstitial space and thereby promote the intratumoral diffusion of drugs, was first described in our 1999 publication [8]. This proof-of-principle study used an ex vivo tumor histoculture model that is devoid of vasculature. Hence, the enhanced intratumoral particle transport is due to enhanced diffusion and not due to enhanced convection or enhanced extravasation. The utility of tumor priming to enhance the nanoparticle delivery in vitro and in vivo, achieved with several cytotoxic agents, was demonstrated in subsequent publications [9-14]. For example, we have shown that (a) tumor priming using paclitaxel to induce apoptosis and expand the tumor interstitial space improves the penetration and dispersion of nanoparticles (up to at least 200 nm diameters) in 3-dimensional tumor histocultures and in animal tumors, and (b) tumor priming is tumor-specific and the improvement in nanoparticle delivery is sufficient to enhance the therapeutic efficacy of doxorubicin liposomes (85 nm diameter) in tumor-bearing animals. We have since extended this approach to siRNA delivery, using lipoplex of siRNA with pegylated cationic liposomes (PCat) [11,13,15,16]. The results show paclitaxel priming promotes (a) the delivery of siRNA-lipoplexes in 3-dimensional tumor cultures and (b) the siRNA-mediated gene knockdown of the chemoresistance gene survivin in mice, resulting in superior antitumor activity for the paclitaxel+PCat-siSurvivin combination compared to single agents. In the latter in vivo study, both paclitaxel and PCat-siSurvivin were administered by intraperitoneal (IP) injections. Compared to IP treatments, intravenously administered siRNA are subjected to additional processes that are prominent in siRNA elimination, e.g., entrapment and elimination by the reticuloendothelial system. This consideration, together with the fact that intravenous injection is the major administration route for cancer therapeutics, prompted the present study to determine the effectiveness of tumor priming in intravenous siRNA therapy.
The present study investigated the siRNA transfection efficiency and therapeutic activity of intravenous paclitaxel+PCat-siSurvivin in mice bearing subcutaneous human pancreatic Hs766T xenograft tumors. The transfection efficiency was monitored by the changes in survivin protein levels in tumors. The therapeutic activity was evaluated by tumor growth inhibition and treatment-induced antiproliferation and apoptotic effects. We used the same PCat carrier and the same target gene survivin as in the previous IP study. Survivin was selected because (a) it is highly and selectively expressed in human pancreatic cancer [17,18], (b) high survivin expression is correlated with more extensive metastases and shorter overall survival of pancreatic cancer patients [17-20], (c) high level survivin expression correlates with chemo/radio-resistance in multiple tumor types and its inhibition enhances cell death induced by chemo/radio-therapy [15,21-26], and (d) paclitaxel induces survivin expression whereas siSurvivin significantly increases paclitaxel-induced cell death [27]. As shown below, paclitaxel cotreatment promoted the transfection and effectiveness of intravenous PCat-siSurvivin in vivo. We further investigated whether paclitaxel, in addition to promoting the interstitial transport, can affect the cellular processing of PCat-siRNA; the in vitro results obtained using a non-functional, fluorescent siRNA indicate paclitaxel promoted its cytoplasmic release.
2. Materials and Methods
2.1. Chemicals and reagents
Paclitaxel was purchased from BioxelPharma (Quebec, Canada). Lipids (1,2-dioleoyl-3-trimethylammoniumpropane or DOTAP, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine or DOPE, 1,2-dioleoyl-sn-glycero-3-phospho-ethanolamine-N-(lissamine rhodamine B sulfonyl) ammonium salt or rhodamine-DOPE, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] or DSPE-PEG, and cholesterol were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL, USA), cefotaxime sodium from Hoechst-Roussel (Somerville, NJ), all chemicals including 4′,6-diamidino-2-phenylindole or DAPI from Sigma Chemical (St. Louis, MO) or Life Technologies (Carlsbad, CA), organic solvents from Fisher Scientific (Fair Lawn, NJ), gentamicin from Solo Pak Laboratories (Franklin Park, IL), and all other cell culture supplies from Life Technologies (Grand Island, NY). siGLO and nontarget siRNA (siNT, Non-Targeting siRNA #1) were purchased from Dharmacon (Chicago, IL); survivin siRNA (siSurvivin, human specific, #6351), survivin monoclonal antibody (71G4B7E), and caspase-3 polyclonal antibody (#9661) from Cell Signaling Technology (Danvers, MA), and Ki67 (MM1 clone) antigen kit from Novocastra Lab (Newcastle, UK). All chemicals and reagents were used as received.
Paclitaxel has low aqueous solubility. For the in vitro experiments that required relatively low drug concentrations, we first dissolved paclitaxel in ethanol; the stock solution (10 μM) was diluted in cell culture medium to the final concentrations of 2-5 nM. For the in vivo experiment that required higher drug concentrations, we dissolved paclitaxel in 50:50 (v/v) Cremophor and ethanol (diluted 1:10 in physiological saline prior to injection).
2.2. Preparation of liposomes and siRNA lipoplexes
PCat, comprising cationic lipid (DOTAP), neutral lipids (cholesterol, DOPE) and pegylated lipid (DSPE-PEG2000) at a molar ratio of 50:30:19:1, was prepared using the dry film and extrusion method as previously described [15]. Briefly, lipids were combined and dissolved in 9:1 v/v mixture of chloroform and methanol. The organic phase was evaporated under nitrogen to yield a thin lipid film that was dried under vacuum. The film was hydrated with RNase-free buffer, and the resulting liposomal suspension was passed through a liposome extruder with a 100 nm pore size membrane to yield PCat [15,16]. PCat-siRNA lipoplex was formed by gently mixing PCat with an aqueous siRNA solution (10 μM) at room temperature, at 4:1 DOTAP:siRNA charge ratio. For the study of PCat-siRNA lipoplex stability in fetal bovine serum (FBS), lipoplex (300 μl of 10 μM siRNA mixed with 63 μl of 10 mg/ml PCat) was incubated with equal volume of FBS at 37°C for 10 min to 24 h. An aliquot of the mixture was diluted 10-fold in nuclease-free water and analyzed for particle size distribution using Zetasizer Nano ZS90 (Malvern, Westborough, MA).
2.3. Uptake of PCat-siRNA in Hs766T cells
Cellular uptake and intracellular localization of PCat-siRNA lipoplex were studied using confocal microscopy and flow cytometry. The PCat liposomes in these studies were labeled with rhodamine (red fluorescence) by substituting 1 mol% DOPE with 1 mol% rhodamine-DOPE. The siRNA in these studies was the green fluorescent siGLO, a 22 nucleotide double-stranded RNA that has no mRNA targets, does not interfere or compete with functional siRNA, and contains a nuclear translocation sequence that causes cytoplasm-to-nucleus translocation. Hence, the presence of red fluorescence indicates liposome internalization and the green fluorescence indicates siGLO internalization, whereas the co-localized red and green signals (yellow) indicates intact lipoplex and the presence of separate signals indicates release of siGLO from the lipoplex. Because only the free siGLO (i.e., dissociated from PCat), with its nuclear translocation sequence, can enter the nucleus, the presence of green fluorescence in the nucleus indicates the release of siGLO from lipoplex, endosomes and/or lysosomes.
Human pancreatic Hs766T cells (gift from Dr. B. Ryu, Johns Hopkins University, Baltimore, MD) were maintained in Dulbecco's Modified Eagle's Medium supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, and antibiotics (90 μg/ml gentamicin plus 90 μg/ml cefotaxime sodium or 100 units/ml penicillin plus 100 μg/ml streptomycin), at 37°C in a humidified atmosphere with 5% CO2. Cells were seeded on a 6-well plate, in some cases on a round coverslip, and allowed to attach overnight. The cells were then washed and placed in serum/antibiotic-free Dulbecco's Modified Eagle's Medium. PCat/siGLO lipoplex (100 nM) and paclitaxel (2-5 nM) were added. Six h later, the medium was replaced with serum/antibiotic-containing medium plus paclitaxel and incubated for an additional 10 h. For confocal microscopic examination, cells grown on coverslips were washed once with ice-cold phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde for 20 min at room temperature in the dark. Cell nuclei were stained with DAPI (0.6 μg/ml in PBS) at room temperature for 3 min. Afterwards, cells were washed three times with ice-cold PBS and the coverslip was mounted on a glass slide and examined under a confocal microscope (Olympus FluoView™ FV1000, Hamburg, Germany). The excitation/emission wavelengths were 405/430-470 nm for DAPI, 488/535-565 nm for siGLO, and 543/560-620 nm for rhodamine. For the quantitative analysis of siGLO in nucleus, Hs766T cells were harvested by trypsinization and centrifugation. The cell pellet was resuspended and fixed in 1 ml 4% paraformaldehyde as above. After centrifugation, the cell pellet was resuspended in 1 ml cold nuclei extraction buffer (320 mM sucrose, 5 mM MgCl2, 10 mM HEPES, 1% Triton X-100, at pH 7.4), vortexed gently and incubated on ice for 10 min, following by centrifugation at 2000× g; these procedures were repeated three times. The resulting nuclei were suspended in cold PBS containing 5 mM MgCl2 and analyzed by flow cytometry within 1 h; untreated control samples (without fluorescence), similarly processed, were used to establish the background fluorescence for flow cytometry gating. The fraction of total nuclei with signals above the background fluorescence was determined.
2.4. Concentration-effect relationship of PCat-siRNA
MCF7 cells (ATCC, Manassas, VA) were cultured in RPMI1640 containing 10% FBS, glutamine and antibiotics, allowed to incubate overnight at 37°C, 5% CO2 and 95% humidity. Cells (106) were seeded on a 10 cm cell culture dish (Corning, NY), in serum-containing medium and treated with PCat-siSurvivin (final concentration of 0, 3, 10, 30, 100, 300, and 1000 nM siRNA). After 22 h, cells were washed twice with cold PBS, scraped, and centrifuged at 5000×g for 30 sec. The cell pellet was stored at −80°C until analysis. Total RNA was extracted using the E.Z.N.A® HP Total RNA Kit (Omega Biotek, Norcross, GA), and reverse transcribed to cDNA using qScript™ cDNA SuperMix (Quanta Biosciences, Gaithersburg, MD). Real-time RT-PCR (triplicate samples, 5 μL cDNA per reaction) was performed with PerfeCTa® MultiPlex qPCR SuperMix (Quanta Biosciences) in a CFX96™ Real-Time PCR Detection Systems (Bio-Rad, Hercules, CA). The primer probe sets (Integrated DNA Technologies, Coralville, IA) used were: survivin, forward: 5′-CAACCGGACGAATGCTTTT-3′; reverse: 5′-AAGAACTGGCCCTTCTTGGA-3′; probe: 5′-/5HEX/CCAGATGAC/ZEN/GACCCCATAGAGGAA/3IABkFQ/-3′; GAPDH, forward: 5′ – AATCCCATCACCATCTTCCAG – 3′; reverse: 5′ – AAATGAGCCCCAGCCTTC – 3′; probe: 5′ – /5Cy5/CCAGCATCGCCCCACTTGATTTT/3IAbRQSp/ – 3′. The multiplex thermal reaction program was: 3 min at 95°C, 40 cycles of 15 sec at 95°C and 1 min at 61°C. Survivin mRNA expression relative to GAPDH expression was calculated using the ΔΔCt-method.
2.5. In vivo antitumor activity
Immunodeficient athymic Nu/Nu female mice (National Cancer Institute, Frederick, MD), 6-8 weeks old, were cared for in accordance to Institutional Animal Care and Use Committee-approved protocols. Subconfluent Hs766T cells were harvested and implanted subcutaneously into the left and right flanks (1 million cells per side). Treatments were initiated when tumors reached at least 3 mm in width. Mice were randomized according to initial tumor size and body weight, to six groups to receive intravenous injections of vehicle (control), single agents (20 mg/kg paclitaxel per dose, 1 nmole PCat-siNT or PCat-siSurvivin per dose), or their combinations (paclitaxel plus PCat-siNT, paclitaxel plus PCat-siSurvivin). Paclitaxel was given on day 0 and 4 for a total of 2 doses and a single dose of PCat-siSurvivin was given on day 3 (i.e., within the 24-96 h time window required for effective paclitaxel tumor priming). Control animals received the same vehicle used in the treated groups, i.e., 50:50 Cremophor:ethanol diluted in physiological saline for when paclitaxel was given and PCat for when PCat-siRNA was given.
The 1 nmole PCat-siSurvivin dose was estimated to yield an initial concentration of 500-1000 nM in blood and, in view of the sustained mRNA and protein knockdown found in our earlier study [15], was expected to be sufficient to yield biological activity.
Antitumor activities were evaluated by tumor size changes (tumor volume was calculated as the product of 0.5 × (width)2 × (length). We adopted the clinical concept of Response Evaluation Criteria in Solid Tumors (RECIST) to analyze the tumor size data. Progression-free survival time (PFS) was the length of time required for disease progression. Overall survival time was the length of time required for the tumor to grow to 3-times the initial tumor volume. Some animals were euthanized during the experiment due to large tumor size (≥2 cm). For these animals, their final tumor size/volume values were used to calculate the mean values at subsequent time points. Increase in lifespan (ILS) was calculated as (difference between the median survival time (MST) of the treated and control groups) divided by (MST of controls) and multiplied by 100%.
2.6. In vivo cellular and molecular pharmacodynamic endpoints
Tumor-bearing animals were treated as described above. At 72 h after the second paclitaxel treatment or 96 h after the PCat-siSurvivin treatment, tumors were excised from anesthetized mice, fixed with 10% formalin, processed, embedded in paraffin, and cut into 6-8 μm histologic sections using a microtome. Treatment effects were monitored by measuring changes in survivin protein levels, apoptotic cells (caspase 3 staining), and proliferating cells (Ki67 staining), using previously described immunohistochemical methods [28]. Briefly, tumor sections were deparaffinized, rehydrated, boiled in 10 mM sodium citrate buffer (pH 6) for 15 min for antigen retrieval, and washed. After blocking with 10 mg/ml bovine serum albumin, tissue samples were incubated with primary antibody (1:500 dilution for Ki67, 1:200 dilution for survivin, and 1:100 dilution for caspase 3) at room temperature for 2 h, followed by incubation with biotinylated or fluorescent secondary antibody for 30 min. For Ki67 and caspase 3 staining, slides were also incubated with 3% hydrogen peroxide for 5 min after antigen retrieval to quench the endogenous peroxidase activity, followed by incubation with streptavidin-peroxidase complex for 30 min after secondary antibody treatment, and then detection using 3,3’-diaminobenzidine as the chromogen. Survivin staining was detected by monitoring the fluorescent secondary antibody. The histological sections were counterstained with hematoxylin, dehydrated, and mounted using Permount® (Fisher). Sections for survivin analysis were mounted with Prolong Diamond Antifade Mountant with DAPI. For negative controls, the primary antibody was replaced with blocking reagent.
Microscopic fields (400× magnification for Ki67 and caspase 3, and 630× for survivin) were randomly selected from the most intensively labeled areas of the tumor section. We evaluated 3-5 microscopic fields per sample, 2 samples per mouse, and 4-5 mice per treatment group. On average, >1100 tumor cells were counted per sample for control groups, and >800 cells for treated groups for the counting of Ki67- and caspase 3-positive cells. The quantitative image analysis of survivin expression levels in solid tumors was performed using Colocalizer Pro software (Boise, ID). The background pixel intensity was determined from cell-free regions, and signals that exceeded 3 times the background level was quantified as the total intensity of survivin; the results were normalized to the number of nuclei in the same field.
2.7. Data and statistical Analysis
Survival data of different treatment groups were analyzed with the log rank test and Kaplan-Meier plot using SAS (Cary, NC). Differences in antitumor activity between groups were determined using repeated measures analysis of variance (ANOVA). Differences in molecular pharmacodynamics endpoints (survivin, Ki67, caspase 3) among multiple treatment groups were analyzed with the Tukey test after one way ANOVA. p values of less than 0.05 were considered statistically significant.
3. Results
3.1. Properties of PCat-siRNA lipoplexes
The average diameter of PCat liposomes was 110±1 nm (range, 109-110 nm, n=3) and the average surface charge was 60±7 mV (range, 53-66 mV), which were respectively changed to 187±29 nm (range, 170-221 nm) and 38±8 mV (range, 33-47 mV) upon mixing with siRNA at a 4:1 DOTAP:siRNA charge ratio. The lipoplex size was further increased by the incubation with FBS (50%) to about 225 nm after 10 min without further changes at 12 and 24 h.
3.2. Paclitaxel promoted cytoplasmic release of siGLO from lipoplex
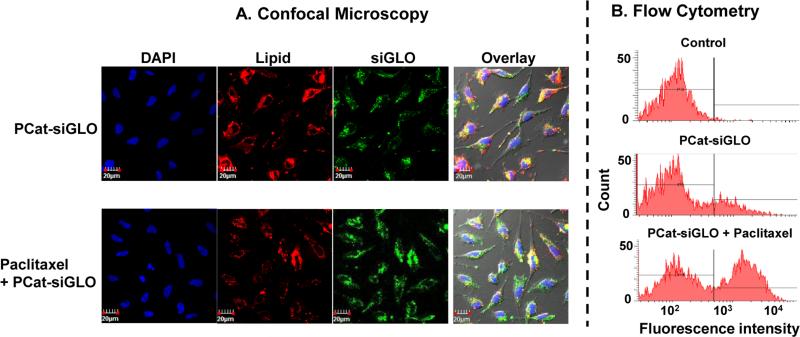
Figure 1A shows the presence of PCat-siGLO in Hs766T cells. The vehicle control samples showed intact lipoplex in the cytoplasm and free siGLO in the nucleus. Cotreatment with paclitaxel (2 nM) altered the intracellular localization such that there were visibly less intact lipoplex in the cytoplasm and greater amount of free siGLO in the nucleus.
Figure 1. Paclitaxel enhanced the cytoplasmic release of siGLO from PCat-siRNA lipoplex and/or endosome/lysosome.
Cells were incubated with PCat-siGLO, with or without paclitaxel (2 nM) cotreatment. Cell nuclei were stained with DAPI (blue). PCat, which contained rhodamine-labeled DOPE, showed red fluorescence. siGLO showed green fluorescence. siGLO contains a nucleus translocation sequence and, upon release from lipoplex siRNA and/or endosomes, enters the nucleus. (A) Confocal microscopy results. Colocalized red and green signals indicate intact PCat-siGLO. Separate green signals indicate siGLO dissociated from the lipoplex. (B) Flow cytometry analysis of siGLO-containing nuclei. The background fluorescence for flow cytometry gating was established using untreated controls (indicated by vertical line). The fraction of nuclei with fluorescence signals above the background fluorescence (indicated by horizontal line) was determined.
Figure 1B shows the flow cytometry results. The control samples were used to establish the background fluorescence of nuclei without siGLO treatment; the cut-off is indicated by the vertical line in the plot of frequency (counts) vs. fluorescence intensity (i.e., only 1% of control nuclei were located to the right of the vertical line). In comparison, the samples treated with PCat-siGLO showed ~19-times higher fraction of nuclei with fluorescence intensity above the cut-off, confirming the flow cytometry gating was appropriate for identifying the siGLO-containing nuclei. Cotreatment with paclitaxel at 2, 3, 4 and 5 nM further increased the fraction of siGLO-containing nuclei to ~50% (range, 38-68%). Because only free siGLO can enter the nucleus, these data indicate paclitaxel enhanced the release of siGLO from the lipoplex and/or endosomes/lysosomes.
3.3. Concentration-effect relationship of PCat-siSurvivin
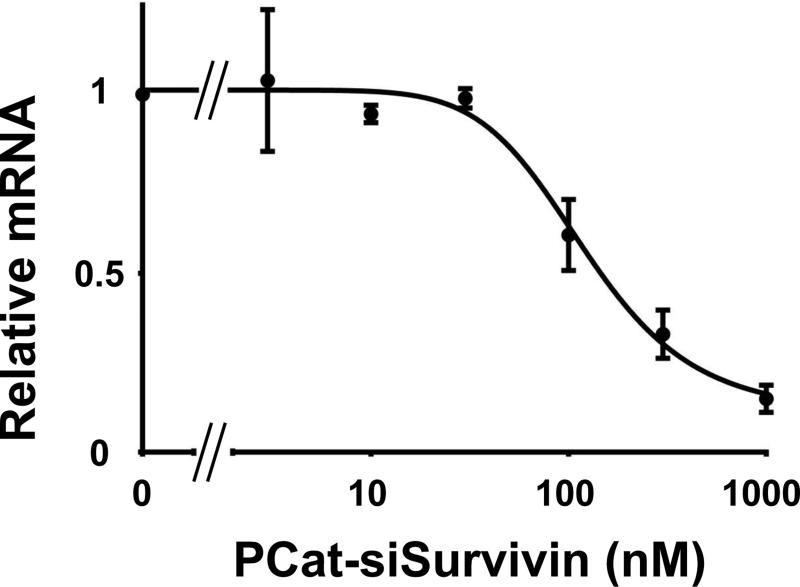
Our previous study established that 100 nM PCat-siSurvivin was sufficient to produce significant gene knockdown in Hs766T cells sustained for at least 48 h, in serum-free medium [15]. Because Hs766T cells produces mucin, the current study evaluated the concentration-effect relationship in human breast cancer MCF7 cells in serum-containing medium. Figure 2 shows that PCat-siSurvivin reduced the survivin mRNA level in a dose-dependent manner; the PCatsiSurvivin concentration that produced 50% inhibition (IC50) at 22 h was about 145 nM.
Figure 2. Concentration-effect relationship of PCat-siSurvivin.
Cells were treated with PCat-siSurvivin in serum-containing media, for 22 h. Cells were harvested and the total RNA was extracted, reverse transcribed to cDNA and analyzed using real-time RT-PCR. Survivin mRNA expression relative to GAPDH expression was calculated using the ΔΔCt-method.
3.4. In vivo antitumor activity
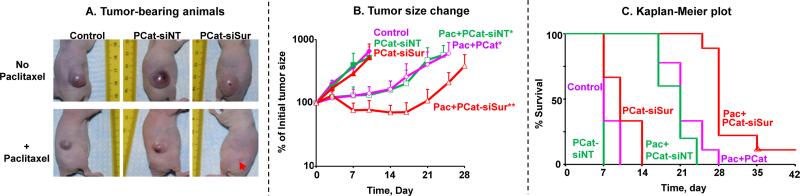
Figures 3A and 3B show the tumor size changes over time. The vehicle control group showed continuous tumor growth, reaching 653% of the initial size on day 10 (day 0 is day of first treatment). Tumor growth was not affected by PCat-siNT or PCat-siSurvivin (p>0.05 compared to untreated control), indicating neither PCat nor single agent siSurvivin had antitumor activity. In comparison, single agent paclitaxel (2 doses) caused significant tumor growth delay to about 140% of the initial size on day 10 and about 600% on day 25 (p<0.05 compared to the vehicle control and two single agent siRNA groups). The addition of PCat-siNT to paclitaxel had no effect, whereas the addition of PCat-siSurvivin (1 nmole single dose) significantly improved the efficacy with a 29% tumor regression on day 14 and delayed tumor regrowth (211% of initial size on day 25) (p<0.05 compared to paclitaxel with and without PCat-siNT). The difference in treatment outcomes between paclitaxel+PCat-siNT and paclitaxel+PCat-siSurvivin indicates survivin silencing enhanced the paclitaxel efficacy.
Figure 3. Synergy between paclitaxel and PCat-siSurvivin in vivo.
Antitumor activity was evaluated in immunodeficient mice bearing subcutaneous Hs766T human pancreatic xenograft tumors. All treatments were administered by intravenous injections. Day 0 represents the day of treatment initiation. The paclitaxel dose was 20 mg/kg, given on day 0 and 4 for a total of 2 doses. A single PCat-siSurvivin dose (1 nmole) was given on day 3. Animals were maintained for up to 38 days after treatment initiation, with tumor size measurements taken every 3 or 4 days. Control animals received the same vehicle used in the treated groups, i.e., 50:50 Cremophor:ethanol diluted in physiological saline for when paclitaxel was given and PCat for when PCat-siRNA was given. Control (filled diamonds, n=3), PCat-siNT (filled squares, n=3), PCat-siSurvivin (PCat-siSur; filled triangles, n=3), paclitaxel+blank PCat (Pac; open diamonds, n=9), paclitaxel+PCat-siNT (Pac+PCat-siNT; open squares, n=5), paclitaxel+PCat-siSurvivin (Pac+PCat-siSur; open triangles, n=9). *p<0.05 vs. control, single agent PCat-siNT or single agent PCat-siSurvivin groups. **p<0.05 vs. all other groups. (A) Tumor-bearing animals after treatments. Note the small tumors in the paclitaxel+siSurvivin animals (indicated by red arrow). (B) Tumor growth. Mean+SD. (C) Kaplan-Meier plot of overall survival.
For PFS, the vehicle control and two single agent siRNA groups showed identical values of 4 days, indicating no appreciable activity for single agent PCat-siSurvivin. In comparison, paclitaxel alone and paclitaxel+PCat-siNT prolonged the PFS to 10 and 7 days, respectively (p<0.05 for both, compared to vehicle control). Adding PCat-siSurvivin to paclitaxel further increased PFS to 25 days (p<0.001 compared to all other groups). Figure 3C shows the Kaplan-Meier plot for overall survival. The control and two single agent siRNA groups showed MST of 7 and 10 days, respectively. In comparison, paclitaxel alone and paclitaxel+PCat-siNT prolonged the MST to 21 days (i.e., 110-200% ILS for both groups). Adding PCat-siSurvivin to paclitaxel further increased MST to 28 days or 180-300% ILS (p<0.001 compared to all other groups). The paclitaxel+PCat-siSurvivin combination was the only group that had a surviving animal at the time of experiment termination (38 days).
With respect to treatment toxicity, PCat-siSurvivin did not cause additional body weight loss compared to the vehicle control group. Paclitaxel treatments resulted in slight body weight loss of 3% on day 6 followed by rapid recovery after 1 day, whereas the addition of PCat-siSurvivin did not increase the paclitaxel toxicity (2% weight loss on day 6).
3.5. In vivo cellular and molecular pharmacodynamic effects
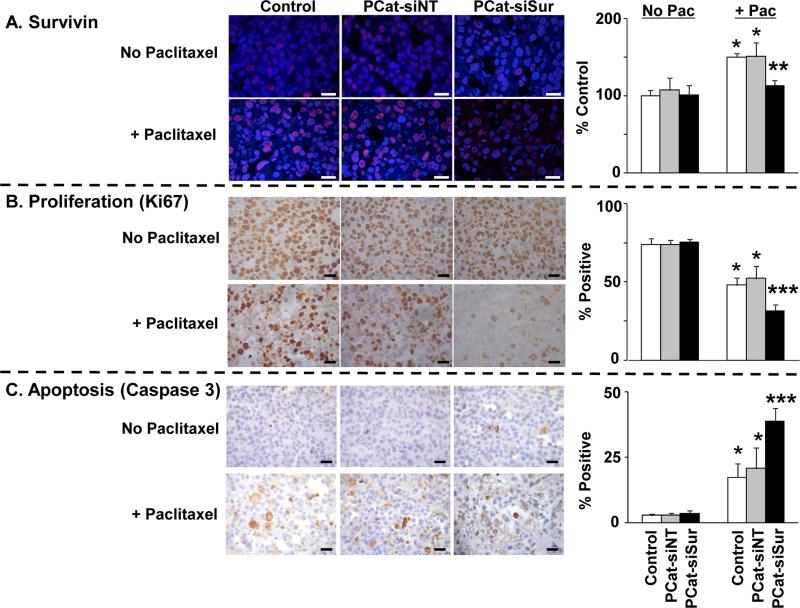
Figure 4A shows the micrographs of tumors stained for survivin and the corresponding image analysis results expressed as survivin staining intensity per cell. The two single agent siRNA groups, i.e., PCat-siNT and PCat-siSurvivin, showed similar values as the vehicle control, indicating neither siNT nor siSurvivin had an effect. In comparison, single agent paclitaxel and the paclitaxel+PCat-siNT combination caused significant increases in survivin levels (about 150% of control, p<0.05). This is consistent with the literature reports that chemotherapeutic agents including paclitaxel induce survivin expression [29-31]. In contrast, the paclitaxel+PCat-siSurvivin combination showed identical survivin level as in the vehicle control. Collectively, these data indicate PCat-siSurvivin completely reversed the survivin induction by paclitaxel.
Figure 4. Paclitaxel enhanced the delivery and transfection of PCat-siSurvivin in tumors:Cellular and molecular pharmacodynamic endpoints.
Immunodeficient mice were implanted with tumors and treated as described in Figure 3. Tumors were excised 72 h after the second dose of paclitaxel treatment (96 h after siRNA treatment), and processed for immunohistochemical staining and quantitative image analysis of survivin levels and of Ki67- and caspase 3-positive cells as described in Methods. Mean±SD (3-5 microscopic fields per tumor, 2 tumors per mouse, 4-5 mice per treatment group). Pac: paclitaxel. PCat-siSur: PCat-siSurvivin. *, p<0.05 compared to vehicle control, single agent PCat-siNT or single agent PCat-siSurvivin groups. **, p<0.05 compared to all other paclitaxel-treated groups. ***p<0.05 compared to all other groups. White or black bars: 25 μm.
Figures 4B and 4C respectively show the micrographs of tumors stained for the proliferation marker Ki67 and the apoptosis marker caspase 3, and the corresponding image analysis results. As expected, paclitaxel significantly inhibited proliferation and induced apoptosis (p<0.05 in both cases). Addition of PCat-siNT did not improve the paclitaxel activity, whereas the addition of PCat-siSurvivin significantly enhanced the paclitaxel-induced antiproliferation and apoptosis (p<0.05).
4. Discussion
We previously showed that PCat-siSurvivin was effective to knockdown survivin in cultured Hs766T cells, but not effective in in vivo tumors when given alone; this finding indicates inadequate delivery and/or transfection under in vivo conditions. We next found the delivery/transfection problem was overcome by using paclitaxel tumor priming, such that the paclitaxel+PCat-siSurvivin combination yielded synergy and the addition of PCat-siSurvivin reversed the survivin induction by paclitaxel, in the locoregional setting where both paclitaxel and siRNA were given by IP injections to treat peritoneal tumors [11,13,15]. The major goal of the present study was to investigate the potential utility of paclitaxel tumor priming under the systemic intravenous setting. As discussed below, the results demonstrate its effectiveness and provide several additional interesting observations.
The present study yielded qualitatively identical results in the systemic setting where both paclitaxel and PCat-siSurvivin were administered by intravenous injections to treat subcutaneous tumors as in the locoregional setting, in that paclitaxel tumor priming promoted the siRNA transfection in vivo and the addition of PCat-siSurvivin enhanced the antitumor activity of paclitaxel (i.e., greater tumor regression or delayed tumor regrowth, and greater apoptosis and antiproliferation). Because single agent PCat-siSurvivin was pharmacologically inert in vivo (i.e., it did not modulate the survivin expression), the greater antitumor activity for the combination indicates synergy. In addition, the molecular and cellular pharmacodynamic data indicate (a) two doses of intravenous paclitaxel were sufficient to induce survivin expression, and (b) because PCat-siSurvivin was administered between the first and second paclitaxel doses (72 h after the first and 24 h before the second), PCat-siSurvivin provided durable survivin knockdown and/or inhibited survivin induction for at least 96 h. The duration of in vivo knockdown is similar or longer compared to the duration of in vitro mRNA and protein knockdown in Hs766T cells we previously reported (maximum reduction of 45-75% between 6-16 h, followed by gradual recovery to pre-treatment baseline level at 54-78 h; [15]). The kinetics of gene silencing depends on the residence of siRNA on RNA-induced silencing complex and on the kinetics of mRNA and protein turnover. For example, we presented preliminary data showing that another PCat-siRNA (asymmetric siRNA against metadherin) remained associated with RNA-induced silencing complex for at least 78 h (about 30% of the peak level attained at 10 hr; [32]).
The major difference between the results of the previous IP study [15] and the current intravenous study is quantitative in that the IP study showed higher survivin induction by paclitaxel compared to the current study (433% of the control value vs. 150%, both at 72 h after paclitaxel treatment). The IP study also showed less complete reversal of survivin induction by siRNA (216% of the control value at 72 h vs. 115% at 96 h after PCat-siRNA treatment). These quantitative differences may be due to several differences in the experimental conditions and methodologies. The first is the different tumor exposures to paclitaxel, since paclitaxel exposure induces survivin [27] and paclitaxel resistance increases with drug concentration and treatment duration [33,34]. The IP study used the paclitaxel-loaded tumor-penetrating microparticles where paclitaxel was released locoregionally in close proximity of the tumors over several weeks, which yielded high micromolar and sustained paclitaxel concentrations in tumors for at least 7 days [35] and would therefore yield higher and more prolonged survivin upregulation. In contrast, the intravenous study used the paclitaxel Cremophor micelle solution that would result in a lower drug concentration and shorter drug exposure in the tumor, as only a small fraction of the intravenous dose reaches the tumor due to the short plasma half-life of about 2 h in mice [36]. Second, the IP study used the C19 polyclonal antibody, which stains both cytoplasmic and nuclear survivin (i.e., wild-type and other isoforms). The current study used the 71G4B7E monoclonal antibody specific for nuclear survivin, which yielded less variable readings compared to C19 antibody (e.g., standard deviations of 20% or less vs. 40-80%) but also generally lower readings. Third, as shown in the IP study, the survivin induction by paclitaxel is time-dependent. The different sampling times in the earlier and current studies might have contributed to the quantitative differences in the survivin levels.
The finding that paclitaxel tumor priming promotes the delivery and transfection of PCat-siSurvivin, in both locoregional and systemic settings, establishes its potential utility in the development of RNAi therapeutics. We propose this approach is translatable to the clinic because the three requirements for effective paclitaxel tumor priming are readily achievable in humans. The success of tumor priming depends on the paclitaxel dose (sufficient to induce 10% apoptosis in tumors), the time window between the administration of paclitaxel and siRNA, and the size of the siRNA carriers [11]. We have identified the effective tumor priming dose of paclitaxel as approximately 100 mg/m2 in humans, which is clinically achievable (i.e., lower than the standard-of-care doses of 125-175 mg/m2 of the two clinically used paclitaxel formulations, Cremophor micelles and paclitaxel-albumin nanoparticles). Based on the similar kinetics of paclitaxel-induced apoptosis in animal and human tumors, the 24-96 h window for priming is readily applicable in humans [11,37]. For particle size, we established 200 to 500 nm as the upper limit of carrier diameter in mouse tumors [11]. In view of the effective transfection for the 225 nm PCat-siRNA lipoplex in mouse tumors and the clinical efficacy of several nanoparticle drug products in the size range of 85-190 nm (e.g., 85 and 190 nm doxorubicin liposomes, 130 nm paclitaxel-albumin nanoparticles), lipoplexes in similar size range would benefit from tumor priming in humans. Because paclitaxel, a drug with broad spectrum activity, has been used to treat multiple types of solid tumors including ovarian, lung, breast and pancreatic cancer, paclitaxel tumor priming may have substantial clinical utility for chemo-gene therapy. Furthermore, we have shown that other apoptosis-inducing agents such as doxorubicin and mitomycin C are capable of expanding the interstitial space and promoting the intratumoral diffusion of drugs and PCat-siRNA [10,16]. Hence, we propose chemotherapy tumor priming may be a useful tool to deliver siRNA to silence other chemoresistance genes. Our group is investigating the use of tumor priming on metadherin, a gene responsible for chemoresistance and metastasis [32,38].
Results of the present study further hinted at complex biointerfacial interactions between PCat-siRNA lipoplex, serum and Cremophor. The present study used PCat, a 4-component, pegylated cationic liposomes, as siRNA carrier. The surface charge and % pegylation were optimized for endocytosis as shown in our previous study, e.g., the % pegylation is several-times lower compared to the commonly used cationic liposomes. The mixing of siRNA with PCat, due to the interaction between the positively charged nitrogen in the cationic lipid DOTAP and the negatively charged phosphate in the siRNA, resulted in lipoplexes with a larger size (187 vs. 110 nm). In the presence of FBS, the size further increased to about 225 nm, likely due to the binding of plasma proteins as reported for cationic lipid-DNA lipoplexes [39]. Cremophor also affected the particle sizes of PCat and lipoplex, but the Cremophor effects were dependent on FBS (unpublished results). A separate study on the effects of FBS and Cremophor on the stability and transfection efficiency of PCat-siRNA lipoplex, using both symmetrical and asymmetrical siRNA, is ongoing.
Another interesting and unexpected finding is that paclitaxel promoted the release of siGLO from the lipoplex and/or endosomes/lysosomes intracellularly. To our knowledge, this paclitaxel effect has not been previously reported. The release of intact siRNA to the cytoplasm is required to attain its gene silencing action (through RNA-induced Silencing Complex). Hence, this paclitaxel action might have contributed to the enhanced siSurvivin transfection in tumors. In view of the important role of intracellular trafficking in various aspects of molecular medicine, the mechanisms by which paclitaxel promoted the cytoplasmic siGLO release warrant additional studies.
In summary, our earlier and current studies collectively indicate that paclitaxel, by enhancing the interstitial transport of the lipoplex and the intracellular siRNA release from its carrier and/or endosome/lysosomes, promotes the in vivo transfection of PCat-siSurvivin in human pancreatic xenograft tumors. The paclitaxel-siRNA combination represents an attractive treatment modality for this highly chemoresistant cancer where the standard-of-care therapy with the intravenous paclitaxel-albumin nanoparticles+gemcitabine combination yields a relatively short survival time of 8.5 months [40].
Acknowledgements
Supported in part by research grants R43CA134047 (J. Wang), R01CA123159 (D. Cole), and R01CA158300 (G. Wientjes and J. Au) from the National Cancer Institute, NIH, DHHS.
Abbreviations used
- DAPI
4′,6-diamidino-2-phenylindole
- DC
liposomes comprising DOTAP:cholesterol in 50:50 molar ratio
- DOPE
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine
- DOTAP
1,2-dioleoyl-3-trimethylammoniumpropane
- DSPE-PEG
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000]
- FBS
fetal bovine serum
- ILS
increase in life span
- IP
intraperitoneal
- MST
median survival time
- PBS
phosphate-buffered saline
- PCat
liposomes comprising DOTAP:cholesterol:DOPE:DSPEPEG in 50:30:19:1 molar ratio
- PFS
progression-free survival time
- qPCR
quantitative real-time polymerase chain reaction
- siNT
nontarget siRNA
- siSurvivin
survivin siRNA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: J.L.-S. Au, M.G. Wientjes, Z. Lu and J. Wang have ownership interests in the siRNA delivery technology.
References
- 1.Au JL, Jang SH, Wientjes MG. Clinical aspects of drug delivery to tumors. J. Control Release. 2002;78:81–95. doi: 10.1016/s0168-3659(01)00488-6. [DOI] [PubMed] [Google Scholar]
- 2.Jang SH, Wientjes MG, Lu D, Au JL. Drug delivery and transport to solid tumors. Pharm. Res. 2003;20:1337–1350. doi: 10.1023/a:1025785505977. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Lu Z, Wientjes MG, Au JL. Delivery of siRNA Therapeutics: Barriers and Carriers. AAPS J. 2010;12:492–503. doi: 10.1208/s12248-010-9210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van de Water FM, Boerman OC, Wouterse AC, Peters JG, Russel FG, Masereeuw R. Intravenously administered short interfering RNA accumulates in the kidney and selectively suppresses gene function in renal proximal tubules. Drug Metab Dispos. 2006;34:1393–1397. doi: 10.1124/dmd.106.009555. [DOI] [PubMed] [Google Scholar]
- 5.Akhtar S, Benter IF. Nonviral delivery of synthetic siRNAs in vivo. J. Clin Invest. 2007;117:3623–3632. doi: 10.1172/JCI33494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh YK, Park TG. siRNA delivery systems for cancer treatment. Adv. Drug Deliv. Rev. 2009;61:850–862. doi: 10.1016/j.addr.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 7.Tseng YC, Mozumdar S, Huang L. Lipid-based systemic delivery of siRNA. Adv. Drug Deliv. Rev. 2009;61:721–731. doi: 10.1016/j.addr.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuh HJ, Jang SH, Wientjes MG, Weaver JR, Au JL. Determinants of paclitaxel penetration and accumulation in human solid tumor. J Pharmacol Exp Ther. 1999;290:871–880. [PubMed] [Google Scholar]
- 9.Jang SH, Wientjes MG, Au JL. Enhancement of paclitaxel delivery to solid tumors by apoptosis-inducing pretreatment: effect of treatment schedule. J Pharmacol Exp Ther. 2001;296:1035–1042. [PubMed] [Google Scholar]
- 10.Zheng JH, Chen CT, Au JL, Wientjes MG. Time- and concentration-dependent penetration of doxorubicin in prostate tumors. AAPS PharmSci. 2001;3:E15. doi: 10.1208/ps030215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu D, Wientjes MG, Lu Z, Au JL. Tumor priming enhances delivery and efficacy of nanomedicines. J Pharmacol Exp Ther. 2007;322:80–88. doi: 10.1124/jpet.107.121632. [DOI] [PubMed] [Google Scholar]
- 12.Lu Z, Tsai M, Lu D, Wang J, Wientjes MG, Au JL. Tumor penetrating microparticles for intraperitoneal therapy of ovarian cancer. J Pharmacol Exp Ther. 2008;327:673–682. doi: 10.1124/jpet.108.140095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong HL, Shen Z, Lu Z, Wientjes MG, Au JL. Paclitaxel tumor-priming enhances siRNA delivery and transfection in 3-dimensional tumor cultures. Mol. Pharm. 2011;8:833–840. doi: 10.1021/mp1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Lu Z, Yeung BZ, Wientjes MG, Cole DJ, Au JL. Tumor priming enhances siRNA delivery and transfection in intraperitoneal tumors. J Control Release. 2014;178:79–85. doi: 10.1016/j.jconrel.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Lu Z, Yeung BZ, Wientjes MG, Cole DJ, Au JL. Tumor priming enhances siRNA delivery and transfection in intraperitoneal tumors. J Control Release. 2014;178:79–85. doi: 10.1016/j.jconrel.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui M, Au JL, Wientjes MG, O'Donnell MA, Loughlin KR, Lu Z. Intravenous siRNA Silencing of Survivin Enhances Activity of Mitomycin C in Human Bladder RT4 Xenografts. J. Urol. 2015;194:230–237. doi: 10.1016/j.juro.2015.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kami K, Doi R, Koizumi M, Toyoda E, Mori T, Ito D, Fujimoto K, Wada M, Miyatake S, Imamura M. Survivin expression is a prognostic marker in pancreatic cancer patients. Surgery. 2004;136:443–448. doi: 10.1016/j.surg.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 18.Lee MA, Park GS, Lee HJ, Jung JH, Kang JH, Hong YS, Lee KS, Kim DG, Kim SN. Survivin expression and its clinical significance in pancreatic cancer. BMC. Cancer. 2005;5:127. doi: 10.1186/1471-2407-5-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu BB, Wang WH. Survivin and pancreatic cancer. World J Clin Oncol. 2011;2:164–168. doi: 10.5306/wjco.v2.i3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tonini G, Vincenzi B, Santini D, Scarpa S, Vasaturo T, Malacrino C, Coppola R, Magistrelli P, Borzomati D, Baldi A, Antinori A, Caricato M, Nuzzo G, Picciocchi A. Nuclear and cytoplasmic expression of survivin in 67 surgically resected pancreatic cancer patients. Br. J Cancer. 2005;92:2225–2232. doi: 10.1038/sj.bjc.6602632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asanuma K, Moriai R, Yajima T, Yagihashi A, Yamada M, Kobayashi D, Watanabe N. Survivin as a radioresistance factor in pancreatic cancer. Jpn. J Cancer Res. 2000;91:1204–1209. doi: 10.1111/j.1349-7006.2000.tb00906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guan HT, Xue XH, Dai ZJ, Wang XJ, Li A, Qin ZY. Down-regulation of survivin expression by small interfering RNA induces pancreatic cancer cell apoptosis and enhances its radiosensitivity. World J Gastroenterol. 2006;12:2901–2907. doi: 10.3748/wjg.v12.i18.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kami K, Doi R, Koizumi M, Toyoda E, Mori T, Ito D, Kawaguchi Y, Fujimoto K, Wada M, Miyatake S, Imamura M. Downregulation of survivin by siRNA diminishes radioresistance of pancreatic cancer cells. Surgery. 2005;138:299–305. doi: 10.1016/j.surg.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Liu WS, Yan HJ, Qin RY, Tian R, Wang M, Jiang JX, Shen M, Shi CJ. siRNA directed against survivin enhances pancreatic cancer cell gemcitabine chemosensitivity. Dig. Dis. Sci. 2009;54:89–96. doi: 10.1007/s10620-008-0329-4. [DOI] [PubMed] [Google Scholar]
- 25.Shen YM, Yang XC, Song ML, Qin CH, Yang C, Sun YH. Growth inhibition induced by short hairpin RNA to silence survivin gene in human pancreatic cancer cells. Hepatobiliary. Pancreat. Dis. Int. 2010;9:69–77. [PubMed] [Google Scholar]
- 26.Yang J, Ouyang J, Ouyang L, Ouyang L, Chen Y. Inhibition of cell proliferation and increase of chemosensitivity by simultaneous knockdown of XIAP and survivin in pancreatic carcinoma cells. Oncol Res. 2013;21:43–50. doi: 10.3727/096504013X13793555706722. [DOI] [PubMed] [Google Scholar]
- 27.Ling X, Bernacki RJ, Brattain MG, Li F. Induction of survivin expression by taxol (paclitaxel) is an early event, which is independent of taxol-mediated G2/M arrest. J. Biol. Chem. 2004;279:15196–15203. doi: 10.1074/jbc.M310947200. [DOI] [PubMed] [Google Scholar]
- 28.Yu CC, Woods AL, Levison DA. The assessment of cellular proliferation by immunohistochemistry: a review of currently available methods and their applications. Histochem. J. 1992;24:121–131. doi: 10.1007/BF01047461. [DOI] [PubMed] [Google Scholar]
- 29.Lei Y, Geng Z, Guo-Jun W, He W, Jian-Lin Y. Prognostic significance of survivin expression in renal cell cancer and its correlation with radioresistance. Mol. Cell Biochem. 2010;344:23–31. doi: 10.1007/s11010-010-0525-3. [DOI] [PubMed] [Google Scholar]
- 30.Rodel F, Sprenger T, Kaina B, Liersch T, Rodel C, Fulda S, Hehlgans S. Survivin as a prognostic/predictive marker and molecular target in cancer therapy. Curr. Med. Chem. 2012;19:3679–3688. doi: 10.2174/092986712801661040. [DOI] [PubMed] [Google Scholar]
- 31.Zaffaroni N, Pennati M, Colella G, Perego P, Supino R, Gatti L, Pilotti S, Zunino F, Daidone MG. Expression of the anti-apoptotic gene survivin correlates with taxol resistance in human ovarian cancer. Cell Mol. Life Sci. 2002;59:1406–1412. doi: 10.1007/s00018-002-8518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeung BZ, Lu Z, Wientjes MG, Au JLS. A pharmacodynamic model of siRNA-mediated gene silencing. Proc. Am. Assoc. Pharm. Sci. 2014;2014:W5322. [Google Scholar]
- 33.Bhalla K, Huang Y, Tang C, Self S, Ray S, Mahoney ME, Ponnathpur V, Tourkina E, Ibrado AM, Bullock G. Characterization of a human myeloid leukemia cell line highly resistant to taxol. Leukemia. 1994;8:465–475. [PubMed] [Google Scholar]
- 34.Kavallaris M, Kuo DY, Burkhart CA, Regl DL, Norris MD, Haber M, Horwitz SB. Taxol-resistant epithelial ovarian tumors are associated with altered expression of specific beta-tubulin isotypes. J. Clin. Invest. 1997;100:1282–1293. doi: 10.1172/JCI119642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu Z, Tsai M, Wang J, Cole DJ, Wientjes MG, Au JL. Activity of drug-loaded tumor-penetrating microparticles in peritoneal pancreatic tumors. Curr. Cancer Drug Targets. 2014;14:70–78. doi: 10.2174/15680096113136660110. [DOI] [PubMed] [Google Scholar]
- 36.Yeh TK, Lu Z, Wientjes MG, Au JL. Formulating paclitaxel in nanoparticles alters its disposition. Pharm. Res. 2005;22:867–874. doi: 10.1007/s11095-005-4581-4. [DOI] [PubMed] [Google Scholar]
- 37.Au JLS, Li D, Gan Y, Gao X, Johnson AL, Johnston J, Millenbaugh NJ, Jang SH, Kuh HJ, Chen CT, Wientjes MG. Pharmacodynamics of immediate and delayed effects of paclitaxel: role of slow apoptosis and intracellular drug retention. Cancer Res. 1998;58:2141–2148. [PubMed] [Google Scholar]
- 38.Hu G, Chong RA, Yang Q, Wei Y, Blanco MA, Li F, Reiss M, Au JL, Haffty BG, Kang Y. MTDH activation by 8q22 genomic gain promotes chemoresistance and metastasis of poor-prognosis breast cancer. Cancer Cell. 2009;15:9–20. doi: 10.1016/j.ccr.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pozzi D, Caracciolo G, Capriotti AL, Cavaliere C, La BG, Anchordoquy TJ, Lagana A. Surface chemistry and serum type both determine the nanoparticle-protein corona. J. Proteomics. 2015;119:209–217. doi: 10.1016/j.jprot.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van CE, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]