Abstract
Multiple studies have shown that diabetes mellitus (DM) can affect the efficacy of revascularization therapies and subsequent clinical outcomes. Selection of the appropriate myocardial revascularization strategy is critically important in the setting of multivessel coronary disease. Optimal medical therapy is an appropriate first-line strategy in patients with DM and mild symptoms. When medical therapy does not adequately control symptoms, revascularization with either PCI or CABG may be used. In patients with treated DM, moderate to severe symptoms and complex multivessel coronary disease, coronary artery bypass graft surgery provides better survival, fewer recurrent infarctions and greater freedom from re-intervention. Decisions regarding revascularization in patients with DM must take into account multiple factors and as such require a multidisciplinary team approach (‘heart team’).
Introduction
Diabetes mellitus (DM) has reached epidemic proportions worldwide, and its prevalence is rising [1]. Estimates predict an increase in the prevalence of DM from 6.4% (affecting 285 million adults) in 2010 to 7.7% (439 million adults) in 2030 [2]. Type 1 DM accounts for 5–10% of the total cases of diabetes worldwide, and its prevalence is approximately 1 in 300 adults in the US [3,4].
Coronary artery disease (CAD) is the main cause of death in both type 1 and type 2 DM [5]. The adverse macrovascular consequences of DM are well recognized, as is the accompanying accelerated rate of atherosclerosis that predisposes patients to occlusive CAD and myocardial infarction (MI). Atherosclerosis in patients with DM is diffuse and rapidly progressive, and more likely to require complete revascularization [6]. In the United States, ~one third of all percutaneous coronary intervention (PCI) procedures are performed on patients with DM, and ~25% of patients undergoing coronary artery bypass graft (CABG) surgery have DM [5]. Over 90% of these patients have type 2 DM [7].
The unique pathophysiology of atherosclerosis in patients with DM modifies the response to arterial injury, with profound clinical consequences. The metabolic alterations in DM, including hyperglycemia, hyperinsulinemia and insulin resistance, affect not only the clinical outcome after bare metal stents (BMS) implantation [8,9], but also differentially modulate the vessel wall response (and relative clinical outcomes with respect for the risk of restenosis and stent thrombosis) to different types of drug eluting stents (DES) [10]. The impact of DM on the clinical efficacy of revascularization procedures becomes critically important in the setting of complex multivessel CAD.
Revascularization versus Medical Therapy
Patients with DM have worse outcomes after surgical and catheter-based revascularization [11]. Compared with patients without DM, early and long-term morbidity and mortality are higher in patients with DM after CABG, with increased risk of postoperative complications (Table 1). A recent meta-analysis of more than 100,000 patients showed that all-cause mortality is 1.6 to 1.8-fold greater in patients with DM at all times after CABG from 1 month to 10 years[12]. As such the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI-2D) trial tested the hypothesis that in DM patients and stable coronary disease, prompt revascularization, CABG or PCI, would reduce long-term rates of death and cardiovascular events as compared with medical therapy alone. BARI-2D randomized patients with demonstrated ischemia who were asymptomatic or who had mild to moderate symptoms, and documented CAD by angiography. The appropriate method of revascularization for each patient (PCI or CABG) was determined a priori by the responsible physician, resulting in a population of patients with a much greater atherosclerotic burden in the CABG stratum. The 5-year survival rate was 88.3% among patients in the revascularization group and not statistically different in the medical-therapy group (87.8%). Similarly, major cardiovascular event rate did not differ significantly between the revascularization and the medical-therapy. Prompt revascularization did however reduce MIs (only with CABG), rates of worsening angina (8% vs. 13%; P<0.001), new angina (37% vs. 51%; P<0.001), and subsequent coronary revascularizations (18% vs. 33%; P<0.001) and maintained freedom angina (66% vs. 58%; P<0.003) during 3-year of follow-up. The benefits of revascularization were documented mostly during the year after the intervention, and most importantly were noticeably greater in patients undergoing CABG than PCI [13,14]. Similarly, in COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation), the addition of early PCI to optimal medical therapy did not significantly reduce the risk of death or MI regardless of DM status [15].
Table 1.
| Complication |
|---|
| Sternal infection |
| Stroke |
| Postoperative acute renal failure |
| Prolonged ICU stay |
| 30-day mortality (or in-hospital) |
| Blood transfusion |
| Saphenous vein graft failure |
CABG versus PCI in Multivessel CAD
The Bypass Angioplasty Revascularization Investigation (BARI) study compared multivessel angioplasty to CABG in patients with medically treated DM and found a near doubling of mortality at 5-years with PCI (35% vs. 19%, P=0.003) [16]. The survival benefit of CABG in patients with diabetes persisted at 10 years (PTCA 45.5% vs. CABG 57.8%, P=0.025) [17]. In an analysis based on pooled individual patient data from 10 randomized trials comparing CABG with PCI (median follow-up of 5.9 years), mortality among patients with DM was 30% lower in the CABG group than in the PCI group (hazard ratio [HR] 0.70; 95% CI 0.56–0.87; P=0.014 for the interaction between DM and type of revascularization) [18]. These large differences in mortality underscore the importance of the appropriate decisions regarding the mode of revascularization in DM. However, many observers suggested that the advent of DES had made these earlier results obsolete.
Two contemporary trials comparing PCI with DES to CABG in patients with DM attempted to address this renewed controversy (Figure 1). The pre-specified DM-subgroup analysis (n=452) of SYNTAX (SYNergy Between PCI With TAXus and Cardiac Surgery) showed that the 1-year composite MACCE rate was significantly higher in patients with DM after PCI compared with CABG mainly from an increased risk of repeat revascularization [19]. Importantly, compared with CABG, mortality was higher after PCI for DM patients with highly complex lesions (SYNTAX score ≥33; 4.1% vs. 13.5%, P= 0.04). At 3-years follow-up, presence of diabetes was associated with increased rates of repeat revascularization (12.9% vs. 28%, P=0.001) and, consequently, MACCE (22.9% vs. 37%, P=0.002) in the PCI arm, with a trend toward increased cardiac death (4.8 vs. 8.8%, P=0.10) [20].
Figure 1.
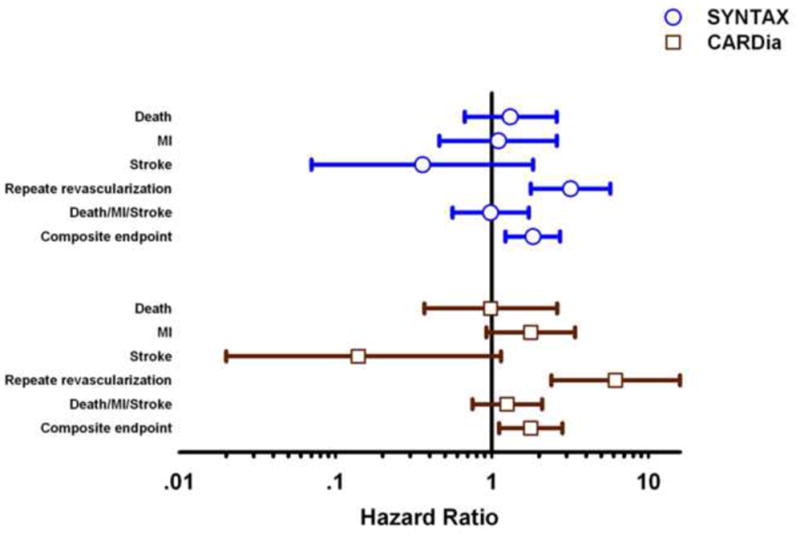
1-year clinical outcomes of contemporary trials comparing CABG and PCI in patients with DM.
The CARDia (Coronary Artery Revascularization in Diabetes) trial enrolled patients with DM (n=510) with either multivessel CAD or complex single-vessel CAD (ostial or proximal left anterior descending artery disease) in whom coronary revascularization was recommended on clinical grounds [7]. The primary end point was a composite of death, MI, and stroke with a major secondary end point of the composite of the primary outcome and repeat revascularization using a noninferiority design. SES were used in 69% of patients in the PCI arm. The study could not demonstrate noninferiority of PCI for the primary endpoint (10.5% with CABG compared with 13.0% with PCI) (Figure 1). The data are not complete however. A “catch-up” phenomenon has been described with DES in the DM population - repeat revascularizations and myocardial infarctions continue to accumulate beyond the first year after DES implantation, and may approach BMS-treated patients at 5 years [21]. Furthermore, large-scale clinical investigations showed that DM (especially insulin-treated) is an independent predictor of DES thrombosis [9,11].
In summary, data from SYNTAX and CARDia and from nonrandomized comparisons [21,22] demonstrate equivalent short-term mortality of PCI with DES and CABG in patients with diabetes, except for patients with the most complex disease (SYNTAX score ≥33) [19]. Importantly, follow-up at 1-year may not yet reflect the true long-term differences between CABG and DES treatment of patients with DM because previous reports demonstrated reduced long-term mortality in CABG compared with PCI [17,18]. Finally, few data are available with regard to the long-term clinical implications and risks associated with of the ‘softer’ endpoint of repeat revascularization. The belief that CABG can always be safely deferred in favor of an initial strategy of PCI in multivessel disease is probably erroneous, as prior PCI seems to increase the risk of subsequent CABG [23,24].
Explaining the mortality benefit of CABG
Several mechanisms may account for the protective effect of CABG in diabetes, including the increased restenosis following PCI in diabetes and incomplete revascularization associated with multivessel angioplasty [9,25], the overlapping effects of multiple bypass grafts to different coronary arteries and the relative resistance of internal mammary arterial grafts to accelerated atherosclerosis [26,27]. Cardiac mortality post CABG was 2.9% when an IMA graft was used and rose 6-fold to 18.2% when only vein grafts were employed [16].
Unlike PCI bypass grafts to mid-coronary vessels treats culprit lesions and protects against proximal disease progression or plaque rupture proximal to patent graft. All of these effects reduce the area of jeopardized or ischemic myocardium with CABG [28].
Graft Selection and Patency
DM does not appear to adversely affect patency of internal thoracic artery (ITA) grafts [26,27]. Nonrandomized analyses indicate that bilateral internal thoracic artery (BITA) grafting appears to be particularly important in the diabetic population [29]. However, the use of BITA results in greater sternal wound complications in patients with DM (especially insulin-treated) [30,31]. Harvesting skeletonized ITA may reduce the risk of sternal wound complications associated with BITA by minimizing the risk of devascularization of the sternum as compared with removal with an attached muscle pedicle [29,32]. Therefore, some surgeons believe that DM is not a contraindication for skeletonized BITA [33]. Notwithstanding, presently, it is unclear whether selective referral of patients with DM for skeletonized BITA grafting despite higher risks of sternal infection is justified [11].
The radial artery (RA) conduits obtained from patients with DM has greater tendency to spasm compared with RAs from patients without DM, and exhibit impaired endothelial function [34]. In a randomized trial comparing angiographic RA graft patency vs. SVG patency at 1-year after CABG, there was a significant interaction between graft type and DM; RA grafts had lower patency rate than SVGs in patients with DM and the reverse was true in patients without DM [35].
Tight perioperative glycemic control
Many patients without the pre-op diagnosis of DM acquire perioperative insulin resistance and profound hyperglycemia after CABG [36] (Figure 2). Poorly controlled hyperglycemia is associated with increased morbidity, mortality, and worsening health outcomes following cardiac surgery [37]. However, tight perioperative glucose control remains controversial. Vigilant control arose from the Portland Diabetic Project and other trials which suggest benefit [37], but severe hypoglycemia (<2.2 mmol/L [40 mg/dL]) has been reported in 5%–17% of more recent trails with continuous insulin infusion [38]. Moreover, in a meta-analysis of 10 trials, intensive insulin therapy (IIT) did not improve important clinical outcomes increased the risk of hypoglycemia 6-fold [39]. In the NICE-SUGAR randomized controlled trial, glycemic control, below 108 mg/dL (6.0 mmol/L) increased all-cause mortality in ICU patients, surgical and nonsurgical [40], and other trials demonstrated that at least one glucose level <40 mg/dl independently predicts mortality in patient after cardiac surgery [41].
Figure 2.
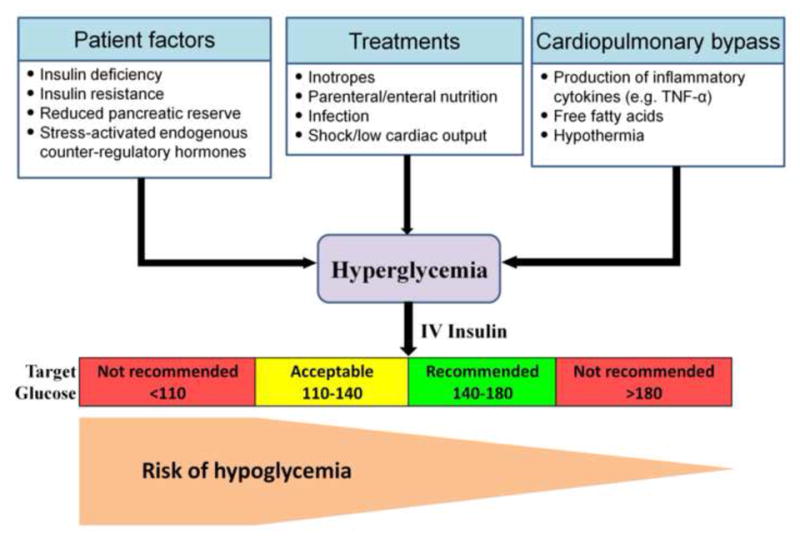
Perioperative hyperglycemia.
Although the relationship between hypoglycemia and cardiovascular outcomes is complex, in patients with established CAD, especially in the setting of acute ischemia, hypoglycemia is associated with adverse cardiovascular outcomes [42]. In this context, hypoglycemia has been shown to lower myocardial blood flow reserve [43]. In patients undergoing cardiac surgery, moderate postoperative glycemic control (120–170 mg/dL) may provide the best clinical outcome [44]. Based on recent studies, the American Diabetes Association recommends initiating insulin therapy for treatment of persistent hyperglycemia, starting at a threshold of no greater than 180 mg/dl (10.0 mmol/l), and aiming for glucose range of 140–180 mg/dl (7.8–10.0 mmol/l) for the majority of critically ill patients [45]. The American College of Physicians recommends avoiding IIT to normalize blood glucose in SICU/MICU patients with or without diabetes mellitus [46]. While the precise range for optimal blood glucose levels is unknown, a target blood glucose level of 7.8–11.1 mmol/L (140–to 200 mg/dL) is recommended if insulin therapy is used in SICU/MICU patients. At this range, IIT is associated with similar mortality outcomes as IIT targeted at blood glucose levels of 4.4–to 6.1 mmol/L (80–110 mg/dL) and is associated with a lower risk for hypoglycemia.
Glycemic control after CABG surgery
The results of the pharmacological arm of the BARI-2D trial are particularly relevant to the management of patients with DM and multivessel CAD after CABG. In general both insulin-sensitization and insulin-provisional approaches are both appropriate [47]. Mortality and cardiovascular event rates were identical whether patients received insulin-sensitizing agents or insulin itself - one year survival was 88% in both groups and freedom from cardiovascular disease events was 78% and 75% respectively [14]. However, as has been seen in other settings insulin-sensitization therapy was associated with lower body mass index, higher high-density lipoprotein cholesterol level, and lower rates of severe hypoglycemia.
All insulin sensitizing agents are not identical. Metformin is recommended as the initial pharmacological therapy, in the absence of specific contraindications, for its effect on glycemia and absence of weight gain or hypoglycemia. Concerns raised that sulfonylureas as a drug class may increase CAD mortality in type 2 DM were not substantiated by the UKPDS or ADVANCE studies. Sulfonylureas can be added if lifestyle intervention and metformin fail to achieve or sustain the glycemic goals [48]. A recent meta-analysis of clinical trials of rosiglitazone (Avandia), a thiazolidinedione, pointed to an increased risk of myocardial infarction [49], which fueled debate regarding the safety of this agent. The BARI-2D trial assessed therapeutic strategies rather than any specific drug. No safety concerns were seen for the insulin-sensitizing group, in which >60% received thiazolidinediones, predominantly rosiglitazone (55%) [14]. Notwithstanding, given that other options are now available, the use of rosiglitazone is not recommended [48]. Newer antidiabetic agents, such as, agonists of the glucagon-like peptide-1 (GLP1) receptor or dipeptidyl peptidase 4 (DPP4) inhibitors, which prevent the breakdown of endogenous GLP1, have shown beneficial effects in patients undergoing angioplasty and CABG in small studies [50,51]. These agents are currently being tested in large randomized trials of patients with CAD [52].
Approach to coronary revascularization in patients with DM
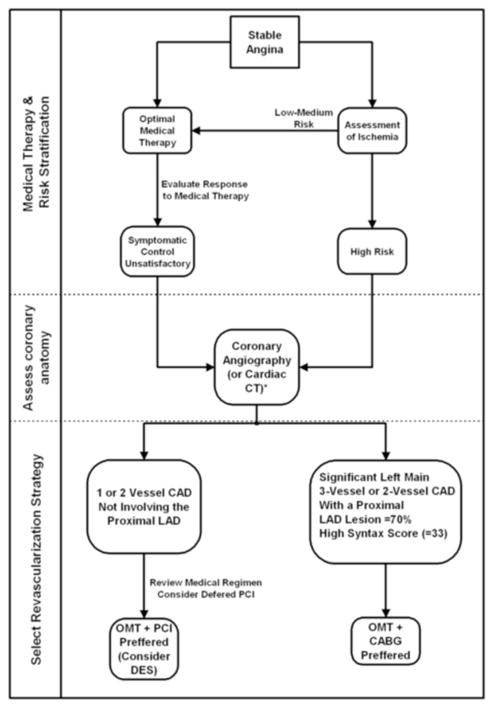
Revascularization aims to reduce subsequent acute coronary events, improve survival and reduce anginal symptoms. Based on the BARI-2D trial, optimal medical therapy rather than coronary revascularization is an excellent first-line strategy in patients with DM who are asymptomatic [53] or with mild symptoms (CCS Class I or II) and less severe CAD (single-vessel or two-vessel CAD not involving the proximal left anterior descending artery) [14,54]. For these patients, it is unlikely that an initial revascularization strategy is better than medical therapy even in a rare patient [55]. Revascularization can reduce anginal symptoms [13] and may be applied later if medical therapy does not adequately control symptoms (Figure 3). In patients who require intervention after optimization of medical therapy there is a significantly higher risk of repeat revascularizations with PCI. DES, albeit, is superior to BMS in ischemia-driven repeat revascularization procedures (target lesion revascularization) [9,11], DES studies have consistently shown higher repeat revascularization rates after PCI compared with surgical revascularization in patients with DM [7,19,21]. Further study must however confirm that the superiority of DES is maintained at long points in time. Notwithstanding, PCI with DES or BMS is a reasonable approach in these patients (Figure 3).
Figure 3.
Revascularization strategy in patients with DM and stable angina.
* may be useful to exclude significant CAD
The surgical approach has better survival, fewer recurrent infarctions and greater freedom from re-intervention for patients with treated DM, moderate to severe symptoms and multivessel CAD [19,56], or significant involvement of the proximal left anterior descending or left main coronary artery [14,54,56] (Figure 3).
Ultimately no one paradigm can treat every patient with the confluence of chronic obstructive atherosclerosis and the profound divergent metabolic derangements of DM. Decisions regarding potential revascularization must take into account multiple factors and as such require a multidisciplinary team approach (‘heart team’). The heart team approach guarantees that all therapeutic options (i.e. optimal medical therapy, PCI, and CABG) are transparently discussed, and the individual patient preferences are considered in the decision-making process [56].
Highlights.
Selection of the optimal myocardial revascularization strategy is critically important in patients with diabetes
Optimal medical therapy is an appropriate initial strategy in patients with diabetes, mild symptoms and moderate coronary artery disease
Bypass surgery is superior to percutaneous intervention in treated diabetes and multivessel coronary disease
Bilateral internal thoracic artery grafting improves outcome but increases sternal wound complications
Strict perioperative glycemic control is associated with increased risk off hypoglycemia and is not associated with improved outcomes
Acknowledgments
This work was funded in part by R01 GM-49039 (ERE)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2. 7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 2.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32 (Suppl 1):S62–67. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39:481–497. doi: 10.1016/j.ecl.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry C, Tardif JC, Bourassa MG. Coronary heart disease in patients with diabetes: part II: recent advances in coronary revascularization. J Am Coll Cardiol. 2007;49:643–656. doi: 10.1016/j.jacc.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 6.Berry C, Tardif JC, Bourassa MG. Coronary heart disease in patients with diabetes: part I: recent advances in prevention and noninvasive management. J Am Coll Cardiol. 2007;49:631–642. doi: 10.1016/j.jacc.2006.09.046. [DOI] [PubMed] [Google Scholar]
- •7.Kapur A, Hall R, Malik I, Qureshi A, Butts J, de Belder M, Baumbach A, Angelini G, de Belder A, Oldroyd K, et al. Randomized Comparison of Percutaneous Coronary Intervention With Coronary Artery Bypass Grafting in Diabetic Patients: 1-Year Results of the CARDia (Coronary Artery Revascularization in Diabetes) Trial. J Am Coll Cardiol. 2010;55:432–440. doi: 10.1016/j.jacc.2009.10.014. A randomized trial comparing CABG to multivessel PCI with drug-eluting stents. [DOI] [PubMed] [Google Scholar]
- 8.Aronson D, Bloomgarden Z, Rayfield EJ. Potential mechanisms promoting restenosis in diabetic patients. J Am Coll Cardiol. 1996;27:528–535. doi: 10.1016/0735-1097(95)00496-3. [DOI] [PubMed] [Google Scholar]
- 9.Aronson D, Edelman ER. Revascularization for coronary artery disease in diabetes mellitus: angioplasty, stents and coronary artery bypass grafting. Rev Endocr Metab Disord. 2010;11:75–86. doi: 10.1007/s11154-010-9135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •10.Stone GW, Kedhi E, Kereiakes DJ, Parise H, Fahy M, Serruys PW, Smits PC. Differential clinical responses to everolimus-eluting and Paclitaxel-eluting coronary stents in patients with and without diabetes mellitus. Circulation. 2011;124:893–900. doi: 10.1161/CIRCULATIONAHA.111.031070. This study demonstrates that DES type may be important in patients with DM, especially insulin-treated. Everolimus-eluting stents were associated with substantial 2-year reductions in death, myocardial infarction, stent thrombosis, and target lesion revascularization as compared with paclitaxel-eluting stents resulted. [DOI] [PubMed] [Google Scholar]
- 11.Roffi M, Angiolillo DJ, Kappetein AP. Current concepts on coronary revascularization in diabetic patients. Eur Heart J. 2011 doi: 10.1093/eurheartj/ehr305. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Wu Z, Peng X, Wu A, Yue Y, Martin J, Cheng D. Prognosis of diabetic patients undergoing coronary artery bypass surgery compared with nondiabetics: a systematic review and meta-analysis. Journal of cardiothoracic and vascular anesthesia. 2011;25:288–298. doi: 10.1053/j.jvca.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 13.Dagenais GR, Lu J, Faxon DP, Kent K, Lago RM, Lezama C, Hueb W, Weiss M, Slater J, Frye RL. Effects of optimal medical treatment with or without coronary revascularization on angina and subsequent revascularizations in patients with type 2 diabetes mellitus and stable ischemic heart disease. Circulation. 2011;123:1492–1500. doi: 10.1161/CIRCULATIONAHA.110.978247. [DOI] [PubMed] [Google Scholar]
- ••14.Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, Goldberg SH, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. doi: 10.1056/NEJMoa0805796. The BARI-2D trial of type 2 DM patients with stable CAD showed no significant difference in the rates of death and major cardiovascular events between patients undergoing prompt revascularization and those receiving medical therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maron DJ, Boden WE, Spertus JA, Hartigan PM, Mancini GB, Sedlis SP, Kostuk WJ, Chaitman BR, Shaw LJ, Berman DS, et al. Impact of Metabolic Syndrome and Diabetes on Prognosis and Outcomes With Early Percutaneous Coronary Intervention in the COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) Trial. J Am Coll Cardiol. 2011;58:131–137. doi: 10.1016/j.jacc.2011.02.046. [DOI] [PubMed] [Google Scholar]
- 16.The Bypass Angioplasty Revascularization Investigation (BARI) Influence of diabetes on 5-year mortality and morbidity in a randomized trial comparing CABG and PTCA in patients with multivessel disease [see comments] Circulation. 1997;96:1761–1769. doi: 10.1161/01.cir.96.6.1761. [DOI] [PubMed] [Google Scholar]
- 17.The final 10-year follow-up results from the BARI randomized trial. J Am Coll Cardiol. 2007;49:1600–1606. doi: 10.1016/j.jacc.2006.11.048. [DOI] [PubMed] [Google Scholar]
- ••18.Hlatky MA, Boothroyd DB, Bravata DM, Boersma E, Booth J, Brooks MM, Carrie D, Clayton TC, Danchin N, Flather M, et al. Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet. 2009;373:1190–1197. doi: 10.1016/S0140-6736(09)60552-3. Analysis of pooled individual patient data from 10 randomized trials comparing CABG with PCI in the BMS era showed that mortality among patients with DM was 30% lower in the CABG group than in the PCI group. [DOI] [PubMed] [Google Scholar]
- 19.Banning AP, Westaby S, Morice MC, Kappetein AP, Mohr FW, Berti S, Glauber M, Kellett MA, Kramer RS, Leadley K, et al. Diabetic and nondiabetic patients with left main and/or 3-vessel coronary artery disease: comparison of outcomes with cardiac surgery and paclitaxel-eluting stents. J Am Coll Cardiol. 2010;55:1067–1075. doi: 10.1016/j.jacc.2009.09.057. [DOI] [PubMed] [Google Scholar]
- 20.Kappetein AP, Feldman TE, Mack MJ, Morice MC, Holmes DR, Stahle E, Dawkins KD, Mohr FW, Serruys PW, Colombo A. Comparison of coronary bypass surgery with drug-eluting stenting for the treatment of left main and/or three-vessel disease: 3-year follow-up of the SYNTAX trial. Eur Heart J. 2011;32:2125–2134. doi: 10.1093/eurheartj/ehr213. [DOI] [PubMed] [Google Scholar]
- 21.Onuma Y, Wykrzykowska JJ, Garg S, Vranckx P, Serruys PW. 5-Year follow-up of coronary revascularization in diabetic patients with multivessel coronary artery disease: insights from ARTS (arterial revascularization therapy study)-II and ARTS-I trials. JACC Cardiovasc Interv. 2011;4:317–323. doi: 10.1016/j.jcin.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Park DW, Kim YH, Song HG, Ahn JM, Oh J, Kim WJ, Lee JY, Kang SJ, Lee SW, Lee CW, et al. Long-term comparison of drug-eluting stents and coronary artery bypass grafting for multivessel coronary revascularization: 5-year outcomes from the Asan Medical Center-Multivessel Revascularization Registry. J Am Coll Cardiol. 2011;57:128–137. doi: 10.1016/j.jacc.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 23.Mack M. Does percutaneous coronary intervention compromise the outcome of subsequent coronary artery bypass grafting? JACC Cardiovasc Interv. 2009;2:765–766. doi: 10.1016/j.jcin.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Chocron S, Baillot R, Rouleau JL, Warnica WJ, Block P, Johnstone D, Myers MG, Calciu CD, Nozza A, Martineau P, et al. Impact of previous percutaneous transluminal coronary angioplasty and/or stenting revascularization on outcomes after surgical revascularization: insights from the imagine study. Eur Heart J. 2008;29:673–679. doi: 10.1093/eurheartj/ehn026. [DOI] [PubMed] [Google Scholar]
- 25.Detre KM, Lombardero MS, Brooks MM, Hardison RM, Holubkov R, Sopko G, Frye RL, Chaitman BR. The effect of previous coronary-artery bypass surgery on the prognosis of patients with diabetes who have acute myocardial infarction. Bypass Angioplasty Revascularization Investigation Investigators. N Engl J Med. 2000;342:989–997. doi: 10.1056/NEJM200004063421401. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz L, Kip KE, Frye RL, Alderman EL, Schaff HV, Detre KM. Coronary bypass graft patency in patients with diabetes in the Bypass Angioplasty Revascularization Investigation (BARI) Circulation. 2002;106:2652–2658. doi: 10.1161/01.cir.0000038885.94771.43. [DOI] [PubMed] [Google Scholar]
- 27.Hwang HY, Choi JS, Kim KB. Diabetes does not affect long-term results after total arterial off-pump coronary revascularization. Ann Thorac Surg. 2010;90:1180–1186. doi: 10.1016/j.athoracsur.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 28.Kip KE, Alderman EL, Bourassa MG, Brooks MM, Schwartz L, Holmes DR, Jr, Califf RM, Whitlow PL, Chaitman BR, Detre KM. Differential influence of diabetes mellitus on increased jeopardized myocardium after initial angioplasty or bypass surgery: bypass angioplasty revascularization investigation. Circulation. 2002;105:1914–1920. doi: 10.1161/01.cir.0000014967.78190.bb. [DOI] [PubMed] [Google Scholar]
- 29.Kinoshita T, Asai T, Nishimura O, Suzuki T, Kambara A, Matsubayashi K. Off-pump bilateral versus single skeletonized internal thoracic artery grafting in patients with diabetes. Ann Thorac Surg. 2010;90:1173–1179. doi: 10.1016/j.athoracsur.2010.05.048. [DOI] [PubMed] [Google Scholar]
- 30.Pevni D, Uretzky G, Mohr A, Braunstein R, Kramer A, Paz Y, Shapira I, Mohr R. Routine use of bilateral skeletonized internal thoracic artery grafting: long-term results. Circulation. 2008;118:705–712. doi: 10.1161/CIRCULATIONAHA.107.756676. [DOI] [PubMed] [Google Scholar]
- 31.Nakano J, Okabayashi H, Hanyu M, Soga Y, Nomoto T, Arai Y, Matsuo T, Kai M, Kawatou M. Risk factors for wound infection after off-pump coronary artery bypass grafting: should bilateral internal thoracic arteries be harvested in patients with diabetes? J Thorac Cardiovasc Surg. 2008;135:540–545. doi: 10.1016/j.jtcvs.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Saso S, James D, Vecht JA, Kidher E, Kokotsakis J, Malinovski V, Rao C, Darzi A, Anderson JR, Athanasiou T. Effect of skeletonization of the internal thoracic artery for coronary revascularization on the incidence of sternal wound infection. Ann Thorac Surg. 2010;89:661–670. doi: 10.1016/j.athoracsur.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 33.Kinoshita T, Asai T, Suzuki T, Kambara A, Matsubayashi K. Off-pump bilateral versus single skeletonized internal thoracic artery grafting in high-risk patients. Circulation. 2011;124:S130–134. doi: 10.1161/CIRCULATIONAHA.110.010892. [DOI] [PubMed] [Google Scholar]
- 34.Choudhary BP, Antoniades C, Brading AF, Galione A, Channon K, Taggart DP. Diabetes mellitus as a predictor for radial artery vasoreactivity in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2007;50:1047–1053. doi: 10.1016/j.jacc.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Goldman S, Sethi GK, Holman W, Thai H, McFalls E, Ward HB, Kelly RF, Rhenman B, Tobler GH, Bakaeen FG, et al. Radial artery grafts vs saphenous vein grafts in coronary artery bypass surgery: a randomized trial. JAMA. 2011;305:167–174. doi: 10.1001/jama.2010.1976. [DOI] [PubMed] [Google Scholar]
- 36.Knapik P, Nadziakiewicz P, Urbanska E, Saucha W, Herdynska M, Zembala M. Cardiopulmonary bypass increases postoperative glycemia and insulin consumption after coronary surgery. Ann Thorac Surg. 2009;87:1859–1865. doi: 10.1016/j.athoracsur.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 37.Lazar HL, McDonnell M, Chipkin SR, Furnary AP, Engelman RM, Sadhu AR, Bridges CR, Haan CK, Svedjeholm R, Taegtmeyer H, et al. The Society of Thoracic Surgeons practice guideline series: Blood glucose management during adult cardiac surgery. Ann Thorac Surg. 2009;87:663–669. doi: 10.1016/j.athoracsur.2008.11.011. [DOI] [PubMed] [Google Scholar]
- ••38.Gandhi GY, Nuttall GA, Abel MD, Mullany CJ, Schaff HV, O’Brien PC, Johnson MG, Williams AR, Cutshall SM, Mundy LM, et al. Intensive intraoperative insulin therapy versus conventional glucose management during cardiac surgery: a randomized trial. Ann Intern Med. 2007;146:233–243. doi: 10.7326/0003-4819-146-4-200702200-00002. This randomized controlled trial of strict intraoperative glycemic control during cardiac surgery showd that lowering glucose concentrations to near normal levels intraoperatively by intravenous insulin infusion did not reduce shortterm death, morbidity, or length of stay in the ICU or hospital. The increased incidence of death and stroke in the intensive treatment group raised concern about routine implementation of this intervention. [DOI] [PubMed] [Google Scholar]
- 39.Griesdale DE, de Souza RJ, van Dam RM, Heyland DK, Cook DJ, Malhotra A, Dhaliwal R, Henderson WR, Chittock DR, Finfer S, et al. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ. 2009;180:821–827. doi: 10.1503/cmaj.090206. discussion 799–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 41.D’Ancona G, Bertuzzi F, Sacchi L, Pirone F, Stringi V, Arcadipane A, Bellazzi R, Pilato M. Iatrogenic hypoglycemia secondary to tight glucose control is an independent determinant for mortality and cardiac morbidity. Eur J Cardiothorac Surg. 2011;40:360–366. doi: 10.1016/j.ejcts.2010.11.065. [DOI] [PubMed] [Google Scholar]
- 42.Yakubovich N, Gerstein HC. Serious Cardiovascular Outcomes in Diabetes. Circulation. 2011;123:342–348. doi: 10.1161/CIRCULATIONAHA.110.948489. [DOI] [PubMed] [Google Scholar]
- 43.Rana O, Byrne CD, Kerr D, Coppini DV, Zouwail S, Senior R, Begley J, Walker JJ, Greaves K. Acute Hypoglycemia Decreases Myocardial Blood Flow Reserve in Patients With Type 1 Diabetes Mellitus and in Healthy Humans. Circulation. 2011 doi: 10.1161/CIRCULATIONAHA.110.992297. [DOI] [PubMed] [Google Scholar]
- 44.Bhamidipati CM, LaPar DJ, Stukenborg GJ, Morrison CC, Kern JA, Kron IL, Ailawadi G. Superiority of moderate control of hyperglycemia to tight control in patients undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2011;141:543–551. doi: 10.1016/j.jtcvs.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, Inzucchi SE, Ismail-Beigi F, Kirkman MS, Umpierrez GE. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32:1119–1131. doi: 10.2337/dc09-9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •46.Qaseem A, Humphrey LL, Chou R, Snow V, Shekelle P. Use of intensive insulin therapy for the management of glycemic control in hospitalized patients: a clinical practice guideline from the American College of Physicians. Annals of internal medicine. 2011;154:260–267. doi: 10.7326/0003-4819-154-4-201102150-00007. Recent guidelines on the management of glycemic control in hospitalized patients. [DOI] [PubMed] [Google Scholar]
- 47.Goldfine AB, Fonseca V. Management of diabetes mellitus in patients with cardiovascular disease in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Circulation. 2010;121:2447–2449. doi: 10.1161/CIRCULATIONAHA.109.925883. [DOI] [PubMed] [Google Scholar]
- 48.Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B American Diabetes A, European Association for Study of D. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 50.Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, Shannon RP. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–965. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- 51.Sokos GG, Bolukoglu H, German J, Hentosz T, Magovern GJ, Jr, Maher TD, Dean DA, Bailey SH, Marrone G, Benckart DH, et al. Effect of glucagon-like peptide-1 (GLP-1) on glycemic control and left ventricular function in patients undergoing coronary artery bypass grafting. Am J Cardiol. 2007;100:824–829. doi: 10.1016/j.amjcard.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 52.De Caterina R, Madonna R, Sourij H, Wascher T. Glycaemic control in acute coronary syndromes: prognostic value and therapeutic options. Eur Heart J. 2010;31:1557–1564. doi: 10.1093/eurheartj/ehq162. [DOI] [PubMed] [Google Scholar]
- 53.Young LH, Wackers FJ, Chyun DA, Davey JA, Barrett EJ, Taillefer R, Heller GV, Iskandrian AE, Wittlin SD, Filipchuk N, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA. 2009;301:1547–1555. doi: 10.1001/jama.2009.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chaitman BR, Hardison RM, Adler D, Gebhart S, Grogan M, Ocampo S, Sopko G, Ramires JA, Schneider D, Frye RL. The bypass angioplasty revascularization investigation 2 diabetes randomized trial of different treatment strategies in type 2 diabetes mellitus with stable ischemic heart disease: impact of treatment strategy on cardiac mortality and myocardial infarction. Circulation. 2009;120:2529–2540. doi: 10.1161/CIRCULATIONAHA.109.913111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC 2009 Appropriateness Criteria for Coronary Revascularization: a report by the American College of Cardiology Foundation Appropriateness Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, and the American Society of Nuclear Cardiology Endorsed by the American Society of Echocardiography, the Heart Failure Society of America, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2009;53:530–553. doi: 10.1016/j.jacc.2008.10.005. [DOI] [PubMed] [Google Scholar]
- •56.Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J, et al. Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2010;31:2501–2555. doi: 10.1093/eurheartj/ehq277. Recent european guidelines for coronary revascularization emphesizing the heart team approach. [DOI] [PubMed] [Google Scholar]
- 57.Marcheix B, Vanden Eynden F, Demers P, Bouchard D, Cartier R. Influence of diabetes mellitus on long-term survival in systematic off-pump coronary artery bypass surgery. The Annals of thoracic surgery. 2008;86:1181–1188. doi: 10.1016/j.athoracsur.2008.06.063. [DOI] [PubMed] [Google Scholar]
- 58.van Straten AH, Hamad MA, van Zundert AA, Martens EJ, Schonberger JP, de Wolf AM. Risk factors for deterioration of renal function after coronary artery bypass grafting. Eur J Cardiothorac Surg. 2010;37:106–111. doi: 10.1016/j.ejcts.2009.06.048. [DOI] [PubMed] [Google Scholar]