Abstract
Inflammation contributes to the development and perpetuation of several disorders and T lymphocytes orchestrate the inflammatory immune response. Although the role of T cells in inflammation is widely recognized, specific therapies that tackle inflammatory networks in disease are yet to be developed. CD4+CD28null T cells are a unique subset of helper T lymphocytes that recently shot back into the limelight as potential catalysts of inflammation in several inflammatory disorders such as autoimmunity, atherosclerosis and chronic viral infections. In contrast to conventional helper T cells, CD4+CD28null T cells have an inbuilt ability to release inflammatory cytokines and cytotoxic molecules that can damage tissues and amplify inflammatory pathways. It comes as no surprise that patients who have high numbers of these cells have more severe disease and poor prognosis. In this review, I provide an overview on the latest advances in the biology of CD4+CD28null T cells. Understanding the complex functions and dynamics of CD4+CD28null T cells may open new avenues for therapeutic intervention to prevent progression of inflammatory diseases.
Keywords: autoimmunity, apoptosis, atherosclerosis, co-stimulation, inflammation, CD4+CD28null T lymphocytes, helper T cells
Introduction
Several subsets of T and B lymphocytes with highly specialized functions have been characterized. Some lymphocytes promote inflammation, while others have anti-inflammatory roles, and an optimal balance between these two opposing sets of lymphocytes is critical for immune homeostasis. We have recently characterized a pro-inflammatory subset of CD4+ T helper 1 (Th1) lymphocytes known as CD4+CD28null T cells because they characteristically lack CD28, a co-stimulatory receptor critical for the activation and function of T cells.1,2 CD4+CD28null T cells expand in several diseases associated with chronic inflammation (e.g. autoimmunity, atherosclerosis, etc.), whereas in healthy individuals they are almost undetectable.3 High frequencies of CD4+CD28null T cells correlate with disease severity and poor prognosis, which led to suggestions that these lymphocytes may be involved in the pathogenesis of chronic inflammatory disorders.4 This review provides an overview on the ‘signature’ features that distinguish CD4+CD28null T cells from conventional CD4+CD28+ T lymphocytes, the diseases in which these cells have been identified, the mechanisms that underlie expansion of this cell subset and potential therapeutic strategies that could be employed to target CD4+CD28null T cells.
The ABC of CD4+CD28null T-cell biology
The main feature of CD4+CD28null T cells is the loss of CD28, a co-stimulatory receptor that is critical for the activation, proliferation and survival of CD4+ T cells.5 For most CD4+ T cells, and especially for naive CD4+ T lymphocytes, lack of CD28-transduced signals during activation induces an anergic state and renders lymphocytes unable to respond to antigen at subsequent encounters.6 However, this is not the case with CD4+CD28null T cells that instead of being anergic have enhanced effector functions and can be (re)-stimulated by antigens.7 Indeed, in several diseases in which this subset expands it has been shown that CD4+CD28null T cells have oligoclonal antigen receptors with restricted antigen diversity, suggesting that repeated antigen stimulation may facilitate expansion of these lymphocytes.8,9 Moreover, CD4+CD28null T lymphocytes display potent effector functions such as secretion of inflammatory cytokines interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α).1,10,11 Another feature that markedly differentiates CD4+CD28null from conventional CD4+CD28+ T lymphocytes is the expression of the cytotoxic molecules perforin and granzyme B,1,12 which are physiologically employed by natural killer cells and cytotoxic CD8+ T lymphocytes to kill target cells (Table1). Not only do CD4+CD28null T cells express cytotoxic molecules but they release them and kill endothelial cells, at least in vitro.13 Another contrasting feature to conventional helper CD4+CD28+ T lymphocytes is the expression of activating natural killer cell receptors such as NKG2D.14 Moreover, CD4+CD28null T cells lose their sensitivity to apoptosis induction2 and are resistant to the suppressive actions of regulatory T (Treg) cells,15 compared with CD28+ counterparts. Treg cells have pivotal roles in keeping pro-inflammatory lymphocytes in check and, by this, maintaining immunological tolerance and preventing excessive immune responses.16 By becoming less susceptible to suppression, CD4+CD28null T cells may escape from the control of Treg cells and so drive inflammation unimpeded.
Table 1.
The ABC of CD4+CD28null T-cell biology
| CD4+CD28null T cells | CD4+CD28+ helper T cells | Ref. | |
|---|---|---|---|
| CD28 co-stimulatory receptor | Absent | Present | 3 |
| OX40/4-1BB co-stimulatory receptors | Up-regulated | Absent1 | 1 |
| T-cell receptors (TCR) | Oligoclonal | Polyclonal | 8,9 |
| Activating natural killer cell receptors (e.g. NKG2D) | Present | Absent | 14 |
| CX3CR1 (fractalkine receptor) | Present | Absent | 36 |
| ‘Signature’ cytokines | interferon-γ, tumour necrosis factor-α | Variable2 | 1,11 |
| Cytolytic enzymes (perforin, granzymes) | Present | Absent | 1,12 |
| Cytotoxic function | Present | Absent | 1,13 |
| Resistance to apoptosis induction | Yes | No | 2,58 |
| Ability to provide help signals to B cells | No | Yes | 11 |
| Sensitivity to suppression by regulatory T cells | Decreased | Yes | 15 |
OX40 and 4-1BB expression are induced only after T-cell activation.
Depending on the T helper (Th) cell subset (e.g. interferon-γ for Th1; interleukin-4 for Th2; interleukin-17 for Th17, etc.).
It remains controversial whether CD4+CD28null T cells are antigen specific and which are the precise antigens that trigger and/or drive their expansion. It has been suggested that CD4+CD28null T lymphocytes are auto-reactive and that repeated stimulation by auto-antigens drives the expansion of this cell subset. However, CD4+CD28null T cells often respond to ubiquitous antigens such as heat-shock proteins and viral antigens, while failing to respond to well-known auto-antigens such as collagen in rheumatoid arthritis (RA) or oxidized low-density lipoprotein in atherosclerosis.15,17 Indeed, some studies suggested that infection with cytomegalovirus (CMV) might drive expansion of CD4+CD28null T cells, as this virus is well known to induce loss of CD28 in CD8+ T cells.18 However, other studies failed to find any relationship between CD4+CD28null T-cell proliferation and CMV-seropositivity.17,19 Another proposed antigen is human heat-shock protein 60, as CD4+CD28null T cells from patients with myocardial infarction were found to respond to this antigen in vitro.17 Myelin basic protein, which is the target of the autoimmune response in multiple sclerosis, has also been suggested to induce proliferation of CD4+CD28null T cells isolated from patients with multiple sclerosis and to enhance IFN-γ production from these cells.20 However, other studies failed to identify myelin basic protein reactivity in CD4+CD28null T cells.15 An alternative hypothesis for what drives CD4+CD28null T-cell expansion is that other cues (e.g. ligands for co-stimulatory and/or natural killer cell receptors, chemokines, adhesion molecules) rather than antigens may be sufficient to activate and induce effector functions in CD4+CD28null T lymphocytes in the disease setting.
It is tempting to speculate that CD4+CD28null T cells cross the classic boundaries of innate and adaptive immune cells and, by doing so, share features with innate-like T lymphocytes. Several populations of innate-like T cells have been described, including invariant natural killer T cells, γδ T cells, and mucosa-associated invariant T cells.21–23 Responses mediated by innate-like T cells occur in the early stages of infectious and inflammatory disorders and shape the subsequent adaptive responses.24 The main characteristics of innate-like T cells that set them apart from traditional adaptive T lymphocytes are: relatively restricted antigen receptor repertoire; potent and rapid cytokine production (due to constitutive transcription of cytokine genes); and cytolytic activity. Indeed, in patients with inflammatory disorders it has been shown that CD4+CD28null T cells have oligoclonal antigen receptors,8,9 produce high levels of inflammatory cytokines and express cytotoxic molecules, features similar to those of innate-like T cells.
CD4+CD28null T cells – senescent versus divergent?
Highly proliferative cells such as fibroblasts and T lymphocytes are susceptible to entering a state of arrested cell division termed cellular senescence. Characteristically, senescent cells irreversibly lose their capacity to proliferate, while remaining viable and metabolically active. Senescent T lymphocytes have been suggested to accumulate with age. In addition to growth arrest, senescent cells are often resistant to apoptosis, have altered expression of genes that regulate cell cycle entry and progression, and express senescence markers (e.g. β-galactosidase, p16).25 Additionally, loss of CD28 has been proposed to identify senescent T lymphocytes. At birth, nearly all T lymphocytes express CD28 in humans, whereas with ageing, CD8+CD28null T cells and, to a lesser extent CD4+CD28null T cells, are detected in increasing numbers in the circulation.7 Interestingly, for reasons that are not known, the increase in circulating CD28null T lymphocytes occurs only in humans and primates but has not been detected in aged mice. Previous studies have shown that CD4+CD28null T cells lack CD28 mRNA, suggesting that CD28 loss is the result of a block in transcription, which is regulated by binding of nuclear protein complexes to α and β motifs in the minimal promoter of the CD28 gene.26 However, loss of CD28 is not a specific senescence marker as CD4+CD28null T cells are a heterogeneous population including not only senescent but also different types of non-senescent effector T lymphocytes.27 Importantly, in contrast to the marked expansion of CD8+CD28null T cells in aged individuals, CD4+CD28null T-cell expansion is rarely detected in most elderly subjects in the absence of inflammatory co-morbidities7, suggesting that CD8+ T cells are more susceptible to replicative senescence. Reduced binding of nuclear proteins to the β but not α motif of the CD28 promoter is characteristic of replicative senescence.26 In comparison to CD4+ T cells, CD8+ T cells contain a single β-bound protein complex, which explains why they are more susceptible to complete loss of nuclear proteins bound to the β motif of the CD28 promoter and subsequent CD28 down-regulation.26 CD27 is also progressively lost during T-cell differentiation and it has been proposed to identify senescent lymphocytes that have lost the ability to proliferate.7 CD4+CD28null T cells that lose expression of CD27 have been suggested to represent end-stage senescent lymphocytes that have marked telomere shortening and impaired proliferation. CD4+CD28nullCD27− T cells have been described in CMV-seropositive individuals but were absent in CMV-seronegative subjects.28 The inability of CD4+CD27− T cells to proliferate is mediated, at least in part, by activation of the p38 kinase.27 However, not all CD4+CD28null T cells lose CD27,29 and the CD27 expression profile on CD4+CD28null T cells in patients with autoimmunity or atherosclerosis has not been investigated. Previous studies suggested that although proliferation may be affected in senescent lymphocytes, certain effector functions (e.g. production of inflammatory cytokines, cytotoxicity) are preserved, which may enable these cells to damage tissues and amplify inflammation. Of note, we recently found that CD4+CD28null T cells maintain their ability to proliferate in vitro in response to anti-CD3 antibodies, albeit with a slower division rate compared with CD4+CD28+ T cells, which indicates that CD4+CD28null T cells do not have replicative senescence.2 Whether truly senescent or not, it is clear that CD4+CD28null T cells have properties that are different from those ascribed to immune-exhausted senescent lymphocytes induced by chronic re-stimulation by viruses. Another important aspect is that T-cell senescence may not always be irreversible. Indeed, senescence of a subset of effector memory CD4+CD27− T cells characterized by re-expression of CD45RA (also known as EMRA CD4+ T cells) was reversed in vitro by inhibition of p38 signalling.30 In view of these recent findings, it remains to be clarified whether CD4+CD28null T cells that expand in various inflammatory diseases are indeed senescent or simply diverge phenotypically and functionally from helper CD4+ T lymphocytes. Additionally, if CD4+CD28null T cells are senescent, it would be important to know whether their senescence is irreversible or whether it can be reversed by inflammatory cues.
CD4+CD28null T cells in inflammatory diseases
CD4+CD28null T cells were originally identified in patients with RA.9,31 Since then, this lymphocyte subset has been found in the circulation and/or tissues in several chronic inflammatory disorders (e.g. autoimmunity, atherosclerosis, viral infections). A high proportion of patients with RA have increased frequencies of circulating CD4+CD28null T cells and in some patients this subset has also been identified in the synovial fluid.12 However, subsequent studies failed to confirm the presence of CD4+CD28null T cells in the synovial fluid or membrane.32 Moreover, the frequency of CD4+CD28null T cells correlated with RA severity and extra-articular involvement (e.g. vascular inflammation).33,34 In vitro experiments showed that CD4+CD28null T cells from RA patients support synoviocyte proliferation better than conventional CD4+CD28+ T lymphocytes.35,36 Excessive proliferation of synovial cells causes cartilage erosion and bone destruction in RA, indicating that CD4+CD28null T cells could potentially contribute to this process. Another autoimmune disorder in which CD4+CD28null T cells have been described is multiple sclerosis.20 In this disease, CD4+CX3CR1+ T lymphocytes had similar functional features to those ascribed to CD4+CD28null T cells in RA (i.e. IFN-γ production, expression of perforin and granzyme).37 CD4+CD28null T cells have also been identified in systemic lupus erythematosus, ankylosing spondylitis, Crohn’s disease, Wegener’s granulomatosis (currently known as granulomatosis with polyangiitis), Graves’ disease and autoimmune myopathy.38–44 In addition to autoimmunity, CD4+CD28null T cells have been characterized in chronic viral infections (e.g. CMV,18 HIV45 and hepatitis B virus29), and in end-stage and chronic kidney disease, including chronic rejection of kidney transplants.46
Advances in the biology and role of CD4+CD28null T cells in atherosclerosis
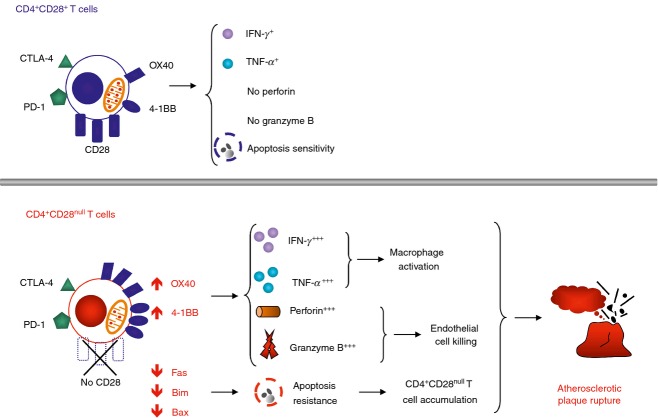
Following their identification in RA, CD4+CD28null T cells have been found to expand in patients with myocardial infarction or unstable angina (often grouped under the term of acute coronary syndrome, ACS).10 Patients with stable angina also have higher frequencies of CD4+CD28null T cells compared with healthy individuals, albeit lower than the frequencies identified in patients with myocardial infarction.10 Interestingly, CD4+CD28null T cells were identified preferentially in unstable compared with stable atherosclerotic lesions, suggesting that they may trigger plaque rupture and life-threatening acute coronary events.8 The role of CD4+CD28null T cells in plaque rupture was later reinforced by observations that these cells can kill endothelial cells in vitro.13 Moreover, activated CD4+ T lymphocytes isolated from the circulation or atherosclerotic plaques of patients with myocardial infarction are known to kill vascular smooth muscle cells,47,48 which may also be the case for CD4+CD28null T cells, although direct experimental proof is lacking. Another study implicating these lymphocytes in the pathogenesis of myocardial infarction found that patients with high percentages of CD4+CD28null T cells have increased risk of recurrent acute coronary events and poor outcome compared with patients with low CD4+CD28null T-cell frequencies.49 Patients with diabetes, a well-known risk factor for myocardial infarction, also have increased circulating CD4+CD28null T cells. Moreover, high frequencies of these lymphocytes associate with the occurrence of the first acute cardiovascular event and poor outcome following myocardial infarction in diabetic patients.50 We demonstrated that CD4+CD28null T cells from patients with ACS have features that markedly distinguish them from conventional CD4+CD28+ T cells (Fig.1). First, both resting and in vitro activated CD4+CD28null T cells produce more IFN-γ and TNF-α than their CD4+CD28+ counterparts.1 Second, in stark contrast to conventional CD4+CD28+ T cells, we showed that resting CD4+CD28null T cells express perforin and granzymes. Moreover, activation of CD4+CD28null T cells induced a decrease in perforin and granzyme, which was accompanied by expression of the degranulation marker CD107a on the cell surface. These findings indicate that in patients with myocardial infarction CD4+CD28null T cells are equipped with a potent pro-inflammatory and cytotoxic arsenal that may enable them to amplify the inflammatory response and kill cells in the vascular wall with potentially devastating consequences for the stability of atherosclerotic lesions.
Figure 1.
Characteristics of CD4+CD28null T cells in atherosclerosis. In patients that develop myocardial infarction due to coronary atherosclerosis CD4+CD28null T cells have features that distinguish them from conventional helper CD4+CD28+ T lymphocytes. Specifically, CD4+CD28null T cells express higher levels of alternative co-stimulatory receptors OX40 and 4-1BB, whereas co-inhibitory receptors (CTLA-4 and PD-1) are present in similar levels to those on CD28+ counterparts. OX40 and 4-1BB regulate the production of inflammatory cytokines [tumour necrosis factor-α (TNF-α) and interferon-γ (IFN-γ)] and the release of cytotoxic molecules (perforin, granzymes) by CD4+CD28null T cells. Moreover, in these patients the expression of the death receptor Fas and of pro-apoptotic mitochondrial proteins Bim and Bax is significantly reduced in CD4+CD28null T cells. This endows CD4+CD28null T lymphocytes with resistance to apoptosis, which may allow these cells to accumulate and amplify inflammation and cause vessel wall damage, which may contribute to atherosclerotic plaque rupture.
We demonstrated for the first time that alternative co-stimulatory receptors are crucial for the pro-inflammatory and tissue-damaging functions of CD4+CD28null T cells in atherosclerosis. We found that the expression of alternative co-stimulatory receptors OX40 and 4-1BB is significantly higher in CD4+CD28null T cells compared with conventional CD4+CD28+ T cells.1 The up-regulation of co-stimulatory receptors was present only in CD4+CD28null T cells from patients with myocardial infarction but was absent in stable angina, suggesting that this altered phenotype was specific for myocardial infarction. Furthermore, using blocking antibodies we clearly demonstrated that OX40 and 4-1BB control the production of inflammatory cytokines and perforin from CD4+CD28null T cells. Alternative co-stimulatory receptors may facilitate direct stimulation of CD4+CD28null T lymphocytes in peripheral tissues and render them independent of activation by professional dendritic cells in secondary lymphoid organs. Indeed, we have shown that atherosclerotic plaques express OX40L and 4-1BBL and that the phenotype of CD4+CD28null T lymphocytes isolated from plaques resembled that of CD4+CD28null T cells activated in vitro, with high levels of OX40 and 4-1BB.2 Local activation of CD4+CD28null T cells in peripheral tissues may amplify inflammation in targeted organs and lead to breakage of self-tolerance and autoimmune responses. Overall, our identification of alternative co-stimulatory receptors in CD4+CD28null T cells provided a mechanism that regulates the harmful actions of CD4+CD28null T cells in atherosclerosis and a proof of concept that therapeutic agents targeting OX40 and 4-1BB co-stimulation may be beneficial in this disease.51
How do CD4+CD28null T cells expand?
As previously mentioned, expansion of the CD4+CD28null T-cell subset in patients affected by autoimmune disorders or ACS has been linked to the severity of disease and an unfavourable prognosis.34,49 Hence, deciphering the mechanisms responsible for the accumulation of these cells may lead to the identification of novel strategies to target CD4+CD28null T cells. Following T-cell activation and expansion, unwanted and potentially harmful T lymphocytes are purged through apoptosis, which ensures homeostasis and prevents a build-up of inflammatory T cells. Apoptosis induction is tightly regulated by the balance of pro- and anti-apoptotic signals induced in response to environmental queues.52 Apoptotic cell death regularly proceeds either via the extrinsic (death-receptor-dependent) or intrinsic (mitochondria-dependent) pathway, both of which culminate in activation of caspases that cleave DNA and other essential cellular components, followed by the demise of the cell.52 The extrinsic apoptosis pathway is initiated by ligation of death receptors such as Fas (CD95) that relay death signals through proteins that associate with their intracellular death domain.53 The central molecules in the mitochondrial pathway belong to the Bcl-2 (B cell lymphoma-2) family,54 which includes anti-apoptotic proteins (e.g. Bcl-2, Bcl-xL) and pro-apoptotic proteins (e.g. Bim, Bax).55,56 One model proposes that anti-apoptotic Bcl-2 molecules form complexes with the pro-apoptotic Bax in the mitochondrial membrane, which prevents apoptosis induction unless these complexes are disintegrated.55 This function is carried out by the pro-apoptotic molecule Bim, which in response to death triggers, displaces Bcl-2/Bcl-xL, and releases Bax to exercise its death-inducing effects.57
We have recently identified that CD4+CD28null T cells from patients with myocardial infarction have defects in apoptosis regulation. We demonstrated that, in contrast to conventional CD4+CD28+ T lymphocytes, CD4+CD28null T cells resist apoptosis induction through Fas ligation or ceramide treatment in vitro.2 The loss of apoptosis sensitivity in CD4+CD28null T cells was mediated by a marked reduction in Fas and the pro-apoptotic mitochondrial molecules Bim and Bax. Previous studies in RA suggested that CD4+CD28null T-cell expansion is primarily due to up-regulation of the anti-apoptotic molecule Bcl-2.58,59 Moreover, in this disease CD4+CD28null T cells displayed similar levels of Fas, Bcl-xL and Bax compared with CD4+CD28+ T cells. Notably, these results were generated using CD4+CD28null T-cell lines/clones instead of direct analysis of primary CD4+CD28null T cells. The levels of molecules regulating apoptosis pathways are perturbed in T cells expanded in culture, as cells are subjected to several rounds of re-activation to extend their survival and therefore may not model with sufficient fidelity the in vivo status of apoptotic molecules. Interestingly, in patients with myocardial infarction we did not identify any differences in the anti-apoptotic molecules Bcl-2 and Bcl-xL in freshly analysed primary CD4+CD28null T cells, implying that these molecules do not have a central role in apoptosis resistance of CD4+CD28null T lymphocytes in this disease. Whether the mechanisms that underlie the apoptosis resistance of CD4+CD28null T cells diverge in RA and myocardial infarction or are the result of using different types of cells remains to be established in future studies.
Tissue tropism of CD4+CD28null T cells
CD4+CD28null T cells have been identified not just in the circulation of patients with inflammatory diseases but also in target tissues. A better understanding of the mechanisms that guide tissue recruitment and re-circulation of this cell subset bears potential translational implications. Chemokine receptors and adhesion molecules tightly regulate the migration of T lymphocytes during physiological immune responses and have crucial roles in inflammatory diseases.60 Some studies suggest that expression of chemokine receptors and adhesion molecules by CD4+CD28null T cells drives their ability to infiltrate tissues, which may enable these cells to cause local inflammation and tissue damage. CD8+CD28null T cells have been found to express high levels of the adhesion molecule lymphocyte function-associated antigen-1 (LFA-1), which is possibly the result of changes in DNA methylation induced by ageing.61 LFA-1 is an integrin that regulates T-cell recruitment at inflammatory sites and their interaction with antigen-presenting cells through binding to intercellular adhesion molecule-1 and it lowers the activation threshold of T cells. Moreover, as intercellular adhesion molecule-1 is also expressed by cells in various tissues (e.g. synoviocytes), it has been proposed that LFA-1 may enable CD8+CD28null T cells to stimulate synoviocytes. Whether LFA-1 carries out similar functions in CD4+CD28null T cells remains to be investigated. A chemokine receptor that has been identified preferentially on CD4+CD28null T cells is CX3CR1 (the fractalkine receptor).36 This receptor enables CD4+CD28null T cells to interact with synoviocytes which express fractalkine, which in turn increases proliferation, IFN-γ production and release of cytotoxic molecules from CD4+CD28null T lymphocytes in vitro. Moreover, this interaction enhanced the proliferation of synoviocyte cell lines generated from the synovium of RA patients.35 CX3CR1 has also been suggested to facilitate CD4+CD28null T-cell infiltration in peripheral target tissues. However, direct identification of these cells in situ is less accurate compared with the peripheral circulation as their main feature is the lack of the CD28 receptor and other unique markers that could distinguish CD4+CD28null T cells from other CD4+ T lymphocytes are not available. It has been suggested that CD4+CD28null T cells are present in the brain of some patients with multiple sclerosis, based on detection of CD4+CX3CR1+ T cells, which should include predominantly CD4+CD28null T cells as other subsets of CD4+ T cells do not usually express this chemokine receptor.62 Noteworthy, fractalkine the ligand for CX3CR1, is upregulated in the serum and cerebrospinal fluid of patients with multiple sclerosis as well as in multiple sclerosis brain lesions.62 Fractalkine levels are also elevated in urine in kidney transplant recipients during graft rejection,63 suggesting that this chemokine receptor/ligand pair has an important role in guiding the migration of CD4+CD28null T cells into target tissues. CD4+CD28null T cells have also been identified in the lung in RA patients with pneumonitis64, in the muscle in patients with autoimmune myopathies39,42 as well as in lamina propria of patients with Crohn’s disease,65 primarily based on identification of CD4+NKG2D+ T cells in these tissues, as NKG2D is a marker preferentially expressed by CD4+CD28null and not by CD4+CD28+ T cells), although the mechanisms that guide recruitment of this cell subset in these tissues remain unknown.
Potential strategies to target CD4+CD28null T cells
Blockade of inflammatory cytokines
Considering that CD4+CD28null T-cell expansion is a consistent feature of chronic inflammatory conditions, several attempts have been made to identify strategies for targeting this cell subset. One of the initial studies targeted the pro-inflammatory cytokine TNF-α. Bryl et al.66 found that in in vitro treatment with TNF-α down-regulated the expression of CD28 on CD4+CD28+ T-cell clones by affecting the transcription of the CD28 gene. In a later study, the same group described how TNF-α blockade with Infliximab induced a recovery of CD28 expression on peripheral blood mononuclear cells from patients with RA.67 Similar effects of Infliximab on CD28 expression were generated by treatment of whole blood from patients with unstable angina and elevated frequencies of CD4+CD28null T cells, which resulted in an increase in CD28 levels on T cells.68 Based on these findings TNF-α blockade has been proposed to reduce the frequency of CD4+CD28null T cells in patients with RA. However, a recent study found that the percentage of CD4+CD28null T cells remained unchanged in patients with RA who were treated with Infliximab or Etanercept over a period of 1 year.69 Interestingly, we have recently found that TNF-α treatment failed to down-regulate CD28 in primary CD4+CD28+ T cells from both healthy individuals and patients with myocardial infarction (Bullenkamp J. and Dumitriu I.E., manuscript in preparation). Moreover, due to severe adverse effects, use of TNF-α inhibitors is currently not justified in patients with myocardial infarction and the potential atheroprotective effects of TNF-α blockade remain to be precisely established in this disease. Another cytokine that has been proposed to enable CD28 re-expression is interleukin-12 (IL-12). CD4+CD28null T-cell clones re-stimulated in the presence of IL-12 were also shown to up-regulate CD28.70 In contrast to TNF-α, IL-12 on its own failed to up-regulate CD28. Whether IL-12 could induce CD28 re-expression in primary CD4+CD28null T cells is not known. Importantly, whether inflammatory cytokine blockade alters the function of CD4+CD28null T cells in addition to re-expression of CD28 has not been investigated.
Statins
In addition to their well-documented ability to down-regulate low-density lipoprotein-cholesterol, statins have anti-inflammatory and immune-modulatory effects.71 Statin treatment in patients with unstable angina was suggested to reduce the percentage of CD4+CD28null T cells, although the effect was rather limited (from an average of 3% to 2·3%, P = 0·022).72 Another group suggested that Rosuvastatin triggers apoptosis of CD4+CD28null T cells in patients with acute myocardial infarction and induces a contraction of this cell subset37. We recently investigated the effect of statins on CD4+CD28null T cells from patients with myocardial infarction. Treatment of sorted CD4+CD28null T cells with increasing doses of Atorvastatin or Rosuvastatin failed to induce apoptosis.2 This is not surprising as many patients with acute myocardial infarction or stable angina who are already on statin therapy still exhibit high numbers of CD4+CD28null T cells. Additionally, CD4+CD28null T-cell frequency remained unchanged for up to 2 years after an acute coronary event although statins are routinely prescribed following myocardial infarction13, indicating that statins do not affect the frequency of CD4+CD28null T cells, in line with our in vitro findings.
Modulation of co-stimulatory pathways
Another class of drugs that have the potential to modulate the deleterious functions of CD4+CD28null T cells target co-stimulation. Blockade of T-cell co-stimulation is being used in patients with RA. Abatacept, a CTLA-4Ig fusion protein that works by binding to B7 ligands CD80/CD86 and blocking their interaction with CD28 on T cells has shown some results in RA. Treatment with Abatacept for 1 year reduced the frequency of circulating CD8+CD28null T cells but had only a marginal and not statistically significant effect on CD4+CD28null T cells in RA patients, although a small correlation between CD4+CD28null T cells and the disease activity score DAS28 was found.73 Moreover, a study on a small group of RA patients showed that Abatacept did no alter the frequency of CD4+CD28null T cells in RA patients on long-term therapy with this drug (> 5 years),74 suggesting that any effects on this cell subset induced by Abatacept may be transient or the mere result of fluctuations in disease activity. In view of our findings that OX40 and 4-1BB co-stimulatory receptors are up-regulated on CD4+CD28null T cells in myocardial infarction, targeting these molecules may prove a more successful approach, especially as OX40 and 4-1BB belong to a different family of co-stimulatory receptors than CD28 and CTLA-4.75 Clinical targeting of OX40 and 4-1BB is being investigated in RA, multiple sclerosis, inflammatory bowel disease, asthma, transplantation and graft-versus-host disease.76 What makes OX40 and 4-1BB such attractive targets for modulation of co-stimulation pathways is their preferential expression on activated/effector T cells, whereas they are absent in naive/resting lymphocytes. This should facilitate specific targeting of effector T cells that mediate tissue damage without compromising the ability of naive T lymphocytes to mount appropriate immune responses to exogenous antigens.
Proteasomal inhibitors
Our findings that CD4+CD28null T cells have markedly reduced levels of the pro-apoptotic mitochondrial protein Bim, which has central roles in controlling apoptosis induction, made us investigate mechanisms responsible for Bim reduction in CD4+CD28null T cells to identify tools to revert their resistance to apoptosis. We showed that extracellular signal-regulated kinase 1/2 (ERK1/2), a protein kinase that has been implicated in regulating Bim levels by phosphorylating it, was constitutively activated in resting CD4+CD28null T cells from ACS patients.2 Moreover, ERK1/2 activation was further enhanced by CD4+CD28null T-cell activation. In line with this, CD4+CD28null T lymphocytes expressed significantly higher levels of phosphorylated Bim than conventional CD4+CD28+ counterparts. Moreover, ERK1/2 inhibition reduced phosphorylated Bim levels in activated CD4+CD28null T cells. Phosphorylation of Bim has been shown to tag this protein for degradation by the proteasome, which may explain the marked reduction in Bim that characterizes CD4+CD28null T cells in ACS. Proteasome inhibition increased phosphorylated Bim levels in both resting and activated CD4+CD28null T cells, confirming its central role in regulation of Bim levels.2 We were the first to show that treatment with the proteasome inhibitor MG-132 restored apoptosis sensitivity of CD4+CD28null T cells in ACS patients. The dose of proteasome inhibitor that restored apoptosis sensitivity in CD4+CD28null T cells was approximately a thousand times lower than the doses needed for induction of apoptosis in cancer cells, suggesting that re-sensitization of CD4+CD28null T cells to apoptosis could potentially be achieved in vivo in ACS at much lower doses than in cancer, with a lower adverse effect profile. Encouragingly, proteasome inhibition did not cause apoptosis in conventional T cells, indicating that the proteasome is an attractive target for selective elimination of CD4+CD28null T cells, while sparing their conventional counterparts and reducing bystander immunosuppression.
Other approaches to target CD4+CD28null T cells
Polyclonal anti-lymphocyte globulins have been recently suggested to reduce CD4+CD28null T-cell frequency in transplant recipients, possibly by triggering apoptosis, as demonstrated in vitro.77 Additionally, a recent study described that expression of Kv1.3 channels may confer the potential to target CD4+CD28null T cells, as specific blockade of these channels reduced the ability of CD4+CD28null T lymphocytes to produce IFN-γ and perforin in vitro.78 Interestingly, another recent in vitro study suggested that CD4+CD28null T cells might be resistant to the effects of the immunosuppressive drugs Tacrolimus and Everolimus, which may explain the expansion of this lymphocyte subset in chronic kidney allograft rejection.19 Better characterization of CD4+CD28null T cells should unveil other strategies to modulate the expansion and/or the function of this subset.
Gaps in knowledge and future perspectives
Although CD4+CD28null T cells have been identified in several inflammatory disorders wherein they have enhanced effector function characterized by production of inflammatory factors and cytotoxicity, their precise contribution to the pathogenesis of these diseases is yet to be completely understood. Important questions that remain unanswered are which mechanisms drive the expansion of CD4+CD28null T cells, how are these cells recruited in tissues, and how can their deleterious effector function be down-modulated. Recent identification of molecules that regulate the production of inflammatory cytokines, cytotoxic proteins and the loss of apoptosis sensitivity in CD4+CD28null T cells may provide novel strategies for targeting CD4+CD28null T cells to tame this subset of lymphocytes and tackle inflammation.
Funding sources
Work in the author’s laboratory is funded by the British Heart Foundation (grant no. PG/10/50/28434, PG/13/24/30115 and PG/14/18/30724) and St George’s Hospital Charity, London, UK.
Disclosures
None.
References
- Dumitriu IE, Baruah P, Finlayson CJ, et al. High levels of costimulatory receptors OX40 and 4-1BB characterize CD4+CD28null T cells in patients with acute coronary syndrome. Circ Res. 2012;110:857–69. doi: 10.1161/CIRCRESAHA.111.261933. [DOI] [PubMed] [Google Scholar]
- Kovalcsik E, Antunes RF, Baruah P, Kaski JC, Dumitriu IE. Proteasome-mediated reduction in proapoptotic molecule Bim renders CD4+CD28null T cells resistant to apoptosis in acute coronary syndrome. Circulation. 2015;131:709–20. doi: 10.1161/CIRCULATIONAHA.114.013710. [DOI] [PubMed] [Google Scholar]
- Dumitriu IE, Araguas ET, Baboonian C, Kaski JC. CD4+CD28null T cells in coronary artery disease: when helpers become killers. Cardiovasc Res. 2009;81:11–9. doi: 10.1093/cvr/cvn248. [DOI] [PubMed] [Google Scholar]
- Dumitriu IE, Kaski JC. The role of T and B cells in atherosclerosis: potential clinical implications. Curr Pharm Des. 2011;17:4159–71. doi: 10.2174/138161211798764834. [DOI] [PubMed] [Google Scholar]
- Acuto O, Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat Rev Immunol. 2003;3:939–51. doi: 10.1038/nri1248. [DOI] [PubMed] [Google Scholar]
- Appleman LJ, Boussiotis VA. T cell anergy and costimulation. Immunol Rev. 2003;192:161–80. doi: 10.1034/j.1600-065x.2003.00009.x. [DOI] [PubMed] [Google Scholar]
- Weng NP, Akbar AN, Goronzy J. CD28– T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30:306–12. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzo G, Goronzy JJ, Yang H, et al. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation. 2000;101:2883–8. doi: 10.1161/01.cir.101.25.2883. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Martens PB, Weyand CM, Goronzy JJ. The repertoire of CD4+CD28– T cells in rheumatoid arthritis. Mol Med. 1996;2:608–18. [PMC free article] [PubMed] [Google Scholar]
- Liuzzo G, Kopecky SL, Frye RL, et al. Perturbation of the T-cell repertoire in patients with unstable angina. Circulation. 1999;100:2135–9. doi: 10.1161/01.cir.100.21.2135. [DOI] [PubMed] [Google Scholar]
- Weyand CM, Brandes JC, Schmidt D, Fulbright JW, Goronzy JJ. Functional properties of CD4+CD28– T cells in the aging immune system. Mech Ageing Dev. 1998;102:131–47. doi: 10.1016/s0047-6374(97)00161-9. [DOI] [PubMed] [Google Scholar]
- Namekawa T, Wagner UG, Goronzy JJ, Weyand CM. Functional subsets of CD4 T cells in rheumatoid synovitis. Arthritis Rheum. 1998;41:2108–16. doi: 10.1002/1529-0131(199812)41:12<2108::AID-ART5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Schulte S, Warrington KJ, et al. T-cell-mediated lysis of endothelial cells in acute coronary syndromes. Circulation. 2002;105:570–5. doi: 10.1161/hc0502.103348. [DOI] [PubMed] [Google Scholar]
- Warrington KJ, Takemura S, Goronzy JJ, Weyand CM. CD4+, CD28– T cells in rheumatoid arthritis patients combine features of the innate and adaptive immune systems. Arthritis Rheum. 2001;44:13–20. doi: 10.1002/1529-0131(200101)44:1<13::AID-ANR3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Thewissen M, Somers V, Hellings N, Fraussen J, Damoiseaux J, Stinissen P. CD4+CD28null T cells in autoimmune disease: pathogenic features and decreased susceptibility to immunoregulation. J Immunol. 2007;179:6514–23. doi: 10.4049/jimmunol.179.10.6514. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Zal B, Kaski JC, Arno G, et al. Heat-shock protein 60-reactive CD4+CD28null T cells in patients with acute coronary syndromes. Circulation. 2004;109:1230–5. doi: 10.1161/01.CIR.0000118476.29352.2A. [DOI] [PubMed] [Google Scholar]
- Hooper M, Kallas EG, Coffin D, Campbell D, Evans TG, Looney RJ. Cytomegalovirus seropositivity is associated with the expansion of CD4+CD28– and CD8+ CD28– T cells in rheumatoid arthritis. J Rheumatol. 1999;26:1452–7. [PubMed] [Google Scholar]
- Demmers MW, Baan CC, Janssen M, et al. Substantial proliferation of human renal tubular epithelial cell-reactive CD4+CD28null memory T cells, which is resistant to tacrolimus and everolimus. Transplantation. 2014;97:47–55. doi: 10.1097/01.TP.0000435697.31148.b2. [DOI] [PubMed] [Google Scholar]
- Markovic-Plese S, Cortese I, Wandinger KP, McFarland HF, Martin R. CD4+CD28– costimulation-independent T cells in multiple sclerosis. J Clin Invest. 2001;108:1185–94. doi: 10.1172/JCI12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol. 2013;13:101–17. doi: 10.1038/nri3369. [DOI] [PubMed] [Google Scholar]
- Gold MC, Lewinsohn DM. Co-dependents: MR1-restricted MAIT cells and their antimicrobial function. Nat Rev Microbiol. 2013;11:14–9. doi: 10.1038/nrmicro2918. [DOI] [PubMed] [Google Scholar]
- Vantourout P, Hayday A. Six-of-the-best: unique contributions of γδ T cells to immunology. Nat Rev Immunol. 2013;13:88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellabona P, Abrignani S, Casorati G. iNKT-cell help to B cells: a cooperative job between innate and adaptive immune responses. Eur J Immunol. 2014;44:2230–7. doi: 10.1002/eji.201344399. [DOI] [PubMed] [Google Scholar]
- Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–40. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- Vallejo AN, Brandes JC, Weyand CM, Goronzy JJ. Modulation of CD28 expression: distinct regulatory pathways during activation and replicative senescence. J Immunol. 1999;162:6572–9. [PubMed] [Google Scholar]
- Lanna A, Henson SM, Escors D, Akbar AN. The kinase p38 activated by the metabolic regulator AMPK and scaffold TAB 1 drives the senescence of human T cells. Nat Immunol. 2014;15:965–72. doi: 10.1038/ni.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JM, Vukmanovic-Stejic M, Dunne PJ, et al. Cytomegalovirus-specific CD4+ T cells in healthy carriers are continuously driven to replicative exhaustion. J Immunol. 2005;175:8218–25. doi: 10.4049/jimmunol.175.12.8218. [DOI] [PubMed] [Google Scholar]
- Wang Y, Bai J, Li F, et al. Characteristics of expanded CD4+CD28null T cells in patients with chronic hepatitis B. Immunol Invest. 2009;38:434–46. doi: 10.1080/08820130902943105. [DOI] [PubMed] [Google Scholar]
- Di Mitri D, Azevedo RI, Henson SM, et al. Reversible senescence in human CD4+ CD45RA+ CD27– memory T cells. J Immunol. 2011;187:2093–100. doi: 10.4049/jimmunol.1100978. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Goronzy JJ, Weyand CM. CD4+ CD7– CD28– T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J Clin Invest. 1996;97:2027–37. doi: 10.1172/JCI118638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasth AE, Snir O, Johansson AA, et al. Skewed distribution of proinflammatory CD4+CD28null T cells in rheumatoid arthritis. Arthritis Res Ther. 2007;9:R87. doi: 10.1186/ar2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens PB, Goronzy JJ, Schaid D, Weyand CM. Expansion of unusual CD4+ T cells in severe rheumatoid arthritis. Arthritis Rheum. 1997;40:1106–14. doi: 10.1002/art.1780400615. [DOI] [PubMed] [Google Scholar]
- Pawlik A, Ostanek L, Brzosko I, et al. The expansion of CD4+CD28– T cells in patients with rheumatoid arthritis. Arthritis Res Ther. 2003;5:R210–3. doi: 10.1186/ar766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai H, Park YW, He X, Goronzy JJ, Weyand CM. Fractalkine mediates T cell-dependent proliferation of synovial fibroblasts in rheumatoid arthritis. Arthritis Rheum. 2007;56:3215–25. doi: 10.1002/art.22919. [DOI] [PubMed] [Google Scholar]
- Sawai H, Park YW, Roberson J, Imai T, Goronzy JJ, Weyand CM. T cell costimulation by fractalkine-expressing synoviocytes in rheumatoid arthritis. Arthritis Rheum. 2005;52:1392–401. doi: 10.1002/art.21140. [DOI] [PubMed] [Google Scholar]
- Thewissen M, Somers V, Venken K, et al. Analyses of immunosenescent markers in patients with autoimmune disease. Clin Immunol. 2007;123:209–18. doi: 10.1016/j.clim.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Duftner C, Dejaco C, Kullich W, et al. Preferential type 1 chemokine receptors and cytokine production of CD28– T cells in ankylosing spondylitis. Ann Rheum Dis. 2006;65:647–53. doi: 10.1136/ard.2005.042085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasth AE, Dastmalchi M, Rahbar A, et al. T cell infiltrates in the muscles of patients with dermatomyositis and polymyositis are dominated by CD28null T cells. J Immunol. 2009;183:4792–9. doi: 10.4049/jimmunol.0803688. [DOI] [PubMed] [Google Scholar]
- de Garcia Tena J, Manzano L, Leal JC, San Antonio E, Sualdea V, Alvarez-Mon M. Active Crohn’s disease patients show a distinctive expansion of circulating memory CD4+ CD45RO+ CD28null T cells. J Clin Immunol. 2004;24:185–96. doi: 10.1023/B:JOCI.0000019784.20191.7f. [DOI] [PubMed] [Google Scholar]
- Moosig F, Csernok E, Wang G, Gross WL. Costimulatory molecules in Wegener’s granulomatosis (WG): lack of expression of CD28 and preferential up-regulation of its ligands B7-1 (CD80) and B7-2 (CD86) on T cells. Clin Exp Immunol. 1998;114:113–8. doi: 10.1046/j.1365-2249.1998.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya JM, Fasth AE, Zong M, et al. Expanded T cell receptor Vβ-restricted T cells from patients with sporadic inclusion body myositis are proinflammatory and cytotoxic CD28null T cells. Arthritis Rheum. 2010;62:3457–66. doi: 10.1002/art.27665. [DOI] [PubMed] [Google Scholar]
- Sun Z, Zhong W, Lu X, et al. Association of Graves’ disease and prevalence of circulating IFN-γ-producing CD28– T cells. J Clin Immunol. 2008;28:464–72. doi: 10.1007/s10875-008-9213-4. [DOI] [PubMed] [Google Scholar]
- Yang D, Wang H, Ni B, et al. Mutual activation of CD4+ T cells and monocytes mediated by NKG2D-MIC interaction requires IFN-γ production in systemic lupus erythematosus. Mol Immunol. 2009;46:1432–42. doi: 10.1016/j.molimm.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Fernandez S, French MA, Price P. Immunosenescent CD57+ CD4+ T-cells accumulate and contribute to interferon-γ responses in HIV patients responding stably to ART. Dis Markers. 2011;31:337–42. doi: 10.3233/DMA-2011-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlik A, Florczak M, Masiuk M, et al. The expansion of CD4+CD28– T cells in patients with chronic kidney graft rejection. Transplant Proc. 2003;35:2902–4. doi: 10.1016/j.transproceed.2003.10.061. [DOI] [PubMed] [Google Scholar]
- Pryshchep S, Sato K, Goronzy JJ, Weyand CM. T cell recognition and killing of vascular smooth muscle cells in acute coronary syndrome. Circ Res. 2006;98:1168–76. doi: 10.1161/01.RES.0000220649.10013.5c. [DOI] [PubMed] [Google Scholar]
- Sato K, Niessner A, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM. TRAIL-expressing T cells induce apoptosis of vascular smooth muscle cells in the atherosclerotic plaque. J Exp Med. 2006;203:239–50. doi: 10.1084/jem.20051062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzo G, Biasucci LM, Trotta G, et al. Unusual CD4+CD28null T lymphocytes and recurrence of acute coronary events. J Am Coll Cardiol. 2007;50:1450–8. doi: 10.1016/j.jacc.2007.06.040. [DOI] [PubMed] [Google Scholar]
- Giubilato S, Liuzzo G, Brugaletta S, et al. Expansion of CD4+CD28null T-lymphocytes in diabetic patients: exploring new pathogenetic mechanisms of increased cardiovascular risk in diabetes mellitus. Eur Heart J. 2011;32:1214–26. doi: 10.1093/eurheartj/ehq499. [DOI] [PubMed] [Google Scholar]
- Olofsson PS. Targeting T cell costimulation to prevent atherothrombosis. Circ Res. 2012;110:800–1. doi: 10.1161/CIRCRESAHA.112.265108. [DOI] [PubMed] [Google Scholar]
- Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nat Rev Immunol. 2007;7:532–42. doi: 10.1038/nri2115. [DOI] [PubMed] [Google Scholar]
- Krammer PH. CD95’s deadly mission in the immune system. Nature. 2000;407:789–95. doi: 10.1038/35037728. [DOI] [PubMed] [Google Scholar]
- Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–56. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- Antonsson B, Conti F, Ciavatta A, et al. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–2. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- O’Connor L, Strasser A, O’Reilly LA, et al. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 1998;17:384–95. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SN, Adams JM. Life in the balance: how BH3-only proteins induce apoptosis. Curr Opin Cell Biol. 2005;17:617–25. doi: 10.1016/j.ceb.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer M, Vallejo AN, Weyand CM, Goronzy JJ. Resistance to apoptosis and elevated expression of Bcl-2 in clonally expanded CD4+CD28– T cells from rheumatoid arthritis patients. J Immunol. 1998;161:1018–25. [PubMed] [Google Scholar]
- Vallejo AN, Schirmer M, Weyand CM, Goronzy JJ. Clonality and longevity of CD4+CD28null T cells are associated with defects in apoptotic pathways. J Immunol. 2000;165:6301–7. doi: 10.4049/jimmunol.165.11.6301. [DOI] [PubMed] [Google Scholar]
- Marelli-Berg FM, Cannella L, Dazzi F, Mirenda V. The highway code of T cell trafficking. J Pathol. 2008;214:179–89. doi: 10.1002/path.2269. [DOI] [PubMed] [Google Scholar]
- Azuma M, Phillips JH, Lanier LL. CD28– T lymphocytes. Antigenic and functional properties. J Immunol. 1993;150:1147–59. [PubMed] [Google Scholar]
- Broux B, Pannemans K, Zhang X, et al. CX3CR1 drives cytotoxic CD4+CD28– T cells into the brain of multiple sclerosis patients. J Autoimmun. 2012;38:10–9. doi: 10.1016/j.jaut.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Peng W, Chen J, Jiang Y, et al. Urinary fractalkine is a marker of acute rejection. Kidney Int. 2008;74:1454–60. doi: 10.1038/ki.2008.459. [DOI] [PubMed] [Google Scholar]
- Michel JJ, Turesson C, Lemster B, et al. CD56-expressing T cells that have features of senescence are expanded in rheumatoid arthritis. Arthritis Rheum. 2007;56:43–57. doi: 10.1002/art.22310. [DOI] [PubMed] [Google Scholar]
- Allez M, Tieng V, Nakazawa A, et al. CD4+ NKG2D+ T cells in Crohn’s disease mediate inflammatory and cytotoxic responses through MICA interactions. Gastroenterology. 2007;132:2346–58. doi: 10.1053/j.gastro.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Bryl E, Vallejo AN, Weyand CM, Goronzy JJ. Down-regulation of CD28 expression by TNF-α. J Immunol. 2001;167:3231–8. doi: 10.4049/jimmunol.167.6.3231. [DOI] [PubMed] [Google Scholar]
- Bryl E, Vallejo AN, Matteson EL, Witkowski JM, Weyand CM, Goronzy JJ. Modulation of CD28 expression with anti-tumor necrosis factor α therapy in rheumatoid arthritis. Arthritis Rheum. 2005;52:2996–3003. doi: 10.1002/art.21353. [DOI] [PubMed] [Google Scholar]
- Rizzello V, Liuzzo G, Brugaletta S, Rebuzzi A, Biasucci LM, Crea F. Modulation of CD4+CD28null T lymphocytes by tumor necrosis factor-α blockade in patients with unstable angina. Circulation. 2006;113:2272–7. doi: 10.1161/CIRCULATIONAHA.105.588533. [DOI] [PubMed] [Google Scholar]
- Pierer M, Rossol M, Kaltenhauser S, et al. Clonal expansions in selected TCR BV families of rheumatoid arthritis patients are reduced by treatment with the TNFα inhibitors etanercept and infliximab. Rheumatol Int. 2011;31:1023–9. doi: 10.1007/s00296-010-1402-9. [DOI] [PubMed] [Google Scholar]
- Warrington KJ, Vallejo AN, Weyand CM, Goronzy JJ. CD28 loss in senescent CD4+ T cells: reversal by interleukin-12 stimulation. Blood. 2003;101:3543–9. doi: 10.1182/blood-2002-08-2574. [DOI] [PubMed] [Google Scholar]
- Greenwood J, Mason JC. Statins and the vascular endothelial inflammatory response. Trends Immunol. 2007;28:88–98. doi: 10.1016/j.it.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugaletta S, Biasucci LM, Pinnelli M, et al. Novel anti-inflammatory effect of statins: reduction of CD4+CD28null T lymphocyte frequency in patients with unstable angina. Heart. 2006;92:249–50. doi: 10.1136/hrt.2004.052282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarsi M, Ziglioli T, Airo P. Decreased circulating CD28-negative T cells in patients with rheumatoid arthritis treated with abatacept are correlated with clinical response. J Rheumatol. 2010;37:911–6. doi: 10.3899/jrheum.091176. [DOI] [PubMed] [Google Scholar]
- Gomez-Garcia L, Ramirez-Assad C, Vargas A, et al. Reduced numbers of circulating CD28-negative CD4+ cells in patients with rheumatoid arthritis chronically treated with abatacept. Int J Rheum Dis. 2013;16:469–71. doi: 10.1111/1756-185X.12056. [DOI] [PubMed] [Google Scholar]
- Antunes RF, Kaski JC, Dumitriu IE. The role of costimulatory receptors of the tumour necrosis factor receptor family in atherosclerosis. J Biomed Biotechnol. 2012;2012:464532. doi: 10.1155/2012/464532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft M, Benedict CA, Ware CF. Clinical targeting of the TNF and TNFR superfamilies. Nat Rev Drug Discov. 2013;12:147–68. doi: 10.1038/nrd3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duftner C, Dejaco C, Hengster P, et al. Apoptotic effects of antilymphocyte globulins on human pro-inflammatory CD4+CD28– T-cells. PLoS ONE. 2012;7:e33939. doi: 10.1371/journal.pone.0033939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Cao M, Wu X, et al. Kv1.3 channels as a potential target for immunomodulation of CD4+CD28null T cells in patients with acute coronary syndrome. Clin Immunol. 2012;142:209–17. doi: 10.1016/j.clim.2011.10.009. [DOI] [PubMed] [Google Scholar]