Abstract
The immune system plays an important role in the pathogenesis of pulmonary tuberculosis–type 2 diabetes mellitus (PTB-DM) co-morbidity. However, the phenotypic profile of leucocyte subsets at homeostasis in individuals with active or latent tuberculosis (LTB) with coincident diabetes is not known. To characterize the influence of diabetes on leucocyte phenotypes in PTB or LTB, we examined the frequency (Fo) of leucocyte subsets in individuals with TB with (PTB-DM) or without (PTB) diabetes; individuals with latent TB with (LTB-DM) or without (LTB) diabetes and non-TB-infected individuals with (NTB-DM) or without (NTB) diabetes. Coincident DM is characterized by significantly lower Fo of effector memory CD4+ T cells in LTB individuals. In contrast, DM is characterized by significantly lower Fo of effector memory CD8+ T cells and significantly higher Fo of central memory CD8+ T cells in PTB individuals. Coincident DM resulted in significantly higher Fo of classical memory B cells in PTB and significantly higher Fo of activated memory and atypical B cells in LTB individuals. Coincident DM resulted in significantly lower Fo of classical and intermediate monocytes in PTB, LTB and NTB individuals. Finally, DM resulted in significantly lower Fo of myeloid and plasmacytoid dendritic cells in PTB, LTB and NTB individuals. Our data reveal that coincident diabetes alters the cellular subset distribution of T cells, B cells, dendritic cells and monocytes in both individuals with active TB and those with latent TB, thus potentially impacting the pathogenesis of this co-morbid condition.
Keywords: B cells, dendritic cells, diabetes, monocytes, T cells, tuberculosis
Introduction
Tuberculosis (TB) is an enormous public health challenge with over 9 million new cases worldwide and 1·5 million deaths in 2013.1 Exposure to Mycobacterium tuberculosis can result in a variety of outcomes, including resistance to infection, latent infection without active disease, active pulmonary disease (PTB) or active extra-pulmonary disease.2 Although 2 billion people worldwide are infected with M. tuberculosis, only 5–10% of these individuals develop active disease, and the mechanisms by which most individuals with latent infection are protected against the development of active disease are still not well understood.2 Diabetes is a major risk factor for TB.3 The global prevalence of diabetes reached 382 million in 2013 and is predicted to escalate to 592 million by 2035.4 Interestingly, approximately 80% of people with diabetes currently live in low-income and middle-income countries, where TB is also highly endemic.4 The convergence of diabetes and TB and the heightened morbidity and mortality associated with co-morbid disease poses major healthcare problems in these countries. Diabetes is associated with an approximately threefold to fivefold increase in the risk of active TB3 and it is now estimated that 15% of the TB burden worldwide is attributable to diabetes.5
Previous studies have clearly shown that TB–type 2 diabetes mellitus (TB-DM) co-morbidity is typically characterized by elevated systemic and antigen-specific levels of pro-inflammatory cytokines especially type 1 and type 17 cytokines,6,7 cytokines that appear to play an important role in pathogenesis. Moreover, CD4+ and CD8+ T cells appear to be activated to a greater degree in TB-DM individuals with higher frequencies of T helper type 1 and type 17 cytokine production.8,9 In addition, the monocyte phenotype in TB-DM co-morbidity is also altered with monocytes exhibiting diminished phagocytic ability and increased expression of the chemokine receptor, CCR2.10,11 The role of dendritic cell (DC) subsets in TB-DM co-morbidity has not been examined. Very few studies have examined the innate or adaptive immune responses in the interaction of latent TB (LTB)-DM co-morbidity. We have previously shown that LTB-DM is characterized by diminished systemic and antigen-specific production of type 1 and type 17 cytokines in marked contrast to the findings in TB-DM co-morbidity.12 Hence, the effects of DM on the innate and adaptive cytokine responses appear to be divergent, with exaggerated cytokine responses in PTB and diminished cytokine responses in LTB.
We, therefore, examined the ex vivo phenotypic profile of T-cell, B-cell, DC and monocyte subsets in six different groups of individuals: PTB with diabetes or without diabetes; LTB with diabetes or without diabetes and non-TB-infected (NTB) with diabetes or without diabetes. Our data reveal that while the effect of coincident diabetes on phenotypic profile of T-cell and B-cell subsets is only moderate, it profoundly alters the frequencies of monocyte and DC subsets in active and latent TB.
Materials and methods
Study population
We studied a group of 300 individuals: 100 with PTB (50 PTB-DM and 50 PTB); 100 with LTB (50 LTB-DM and 50 LTB) and 100 with NTB (50 NTB-DM and 50 NTB). These individuals were recruited by screening 1500 individuals for TB infection/disease and DM status. The baseline characteristics including demographic and biochemical features of the study population are shown in Table1. PTB was diagnosed on the basis of sputum smear and culture positivity. LTB diagnosis was based on Quantiferon Gold-in Tube ELISA and tuberculin skin test positivity, absence of chest radiography abnormalities or pulmonary symptoms and negative sputum smears and cultures. NTB individuals were asymptomatic with normal chest radiographs and negative sputum smears, cultures, negative Quantiferon results and tuberculin skin test induration. DM was diagnosed on the basis of glycated haemoglobin (HbA1c) levels, according to the American Diabetes Association criteria (DM > 6·5%). All the individuals were HIV seronegative. All individuals were anti-tuberculous treatment naive. Anthropometric measurements, including height, weight and biochemical parameters, including random plasma glucose and HbA1c were obtained using standardized techniques as detailed elsewhere.13 No significant differences in age or gender were observed between the respective groups. All individuals were examined as part of a natural history study protocol approved by the Institutional Review Board of the National Institute of Research in Tuberculosis (NCT01154959), and informed written consent was obtained from all participants.
Table 1.
Demographics, biochemistry and immunology profile of study individuals
| Study demographics | PTB | LTB | NTB | |||
|---|---|---|---|---|---|---|
| DM | NDM | DM | NDM | DM | NDM | |
| No. of subjects recruited | 50 | 50 | 50 | 50 | 50 | 50 |
| Gender (M/F) | 31/19 | 27/23 | 27/23 | 31/19 | 28/22 | 21/29 |
| Median age (range) | 44 (33–70) | 45 (28–65) | 47 (33–72) | 33 (19–60) | 47 (33–72) | 33 (19–60) |
| Smear grade 1+/2+/3+ | 16/12/22 | 21/15/14 | NA | NA | NA | NA |
| Interferon-γ release assay | Positive | Positive | Negative | Negative | ||
| Random blood glucose, mg/dl | 287 (200–653) | 89 (68–137) | 208 (178–588) | 81 (71–125) | 257 (185–553) | 79 (67–127) |
| HbA1c, % | 11·3 (8·01–16·16) | 5·12 (4·05–5·25) | 9·3 (7·01–12·58) | 5·1 (4·57–5·36) | 10·3 (7·78–12·16) | 5·12 (4·25–5·31) |
The values represent geometric means and range (except for age where median and range are shown).
DM, type 2 diabetes mellitus; LTB, latent tuberculosis; NDM, non-diabetic; NTB, non-tuberculosis-infected; PTB, pulmonary tuberculosis.
Ex vivo analysis
All antibodies used in the study were from BD Biosciences (San Jose, CA), BD Pharmingen (San Diego, CA), eBioscience (San Diego, CA), or R&D Systems (Minneapolis, MN). Whole blood was used for ex vivo phenotyping and it was performed on all 300 individuals. Briefly, to 250-μl aliquots of whole blood was added a cocktail of monoclonal antibodies specific for various immune cell types. T-cell phenotyping was performed using antibodies directed against CD45-Peridinin chlorophyll protein (PerCP; clone 2D1, BD), CD3-AmCyan (clone SK7; BD), CD4-phycoerythrin (PE) Cy7 (clone SK3; BD), CD8-allophycocyanin (APC) H7 (clone SK1; BD), CD45RA-Pacific Blue (clone H1100; Biolegend, Cambridge, UK), and CCR7-FITC (clone 3D12; eBioscience). Naive cells were classified as CD45RA+ CCR7+, central memory cells as CD45RA− CCR7+, and effector memory cells as CD45RA− CCR7−. B-cell phenotyping was performed using antibodies directed against CD45-PerCP (clone 2D1, BD), CD19-Pacific Blue (clone H1B19; Biolegend) CD27-APC-Cy7 (clone M-T271; BD), CD21-FITC (clone B-ly4; BD) CD20-PE (clone 2H7; BD) and CD10-APC (clone H110a; BD). Naive cells were classified as CD45+ CD19+ CD21+ CD27−; classical memory B cells as CD45+ CD19+ CD21+ CD27+; activated memory B cells as CD45+ CD19+ CD21− CD27+; atypical memory B cells as CD45+ CD19+ CD21− CD27−; immature B cells as CD45+ CD19+ CD21+ CD10+; and plasma cells as CD45+ CD19+ CD21− CD20−.14,15 Phenotyping of DC was performed using antibodies directed against HLA-DR, lineage cocktail (CD3, CD14, CD16, CD19, CD20, CD56) FITC (clone SJ25C1, SK7, MΦp9, L27, NCAM16.2, 3G8 BD) CD123-PE (clone 9F5; BD) CD11c-APC (clone S-HCL-3; BD). Plasmacytoid DCs were classified as (Lin– HLA-DR+ CD123+); myeloid DCs as (Lin– HLA-DR+ CD11c+). Monocyte phenotyping was performed using antibodies directed against CD45-PerCP (clone 2D1; BD), CD14-Pacific Blue (clone M5E2; Biolegend) HLA-DR-PE-Cy7 (clone L243; BD) and CD16-APC-Cy7 (clone 3G8; BD). Classical monocytes were classified as CD45+ HLA-DR+ CD14hi CD16−; intermediate monocytes as CD45+ HLA-DR+ CD14hi CD16dim and non-classical monocytes were classified as CD45+ HLA-DR+CD14dimCD16hi. Following 30 min of incubation at room temperature erythrocytes were lysed using 2 ml of FACS lysing solution (BD Biosciences Pharmingen), and cells were washed twice with 2 ml of 1× PBS and suspended in 200 μl of PBS (Lonza, Walkersville, MD). Eight-colour flow cytometry was performed on a FACSCanto II flow cytometer with facsdiva software, version 6 (Becton Dickinson). The gating was set by forward and side scatter, and 100 000 gated events were acquired. Data were collected and analysed using flow jo software (TreeStar, Ashland, OR). A representative flow cytometry plot showing the gating strategies for T-cell, B-cell, DC and monocyte subsets is shown in the Supplementary material (Fig. S1).
Statistical analysis
Geometric means were used for measurements of central tendency. Comparisons were made using the Mann–Whitney U-test with Holm’s correction for multiple comparisons. Analyses were performed using graphpad prism Version 6 (GraphPad, San Diego, CA).
Results
NTB-DM status is associated with altered frequency of T-cell, B-cell, DC and monocyte subsets
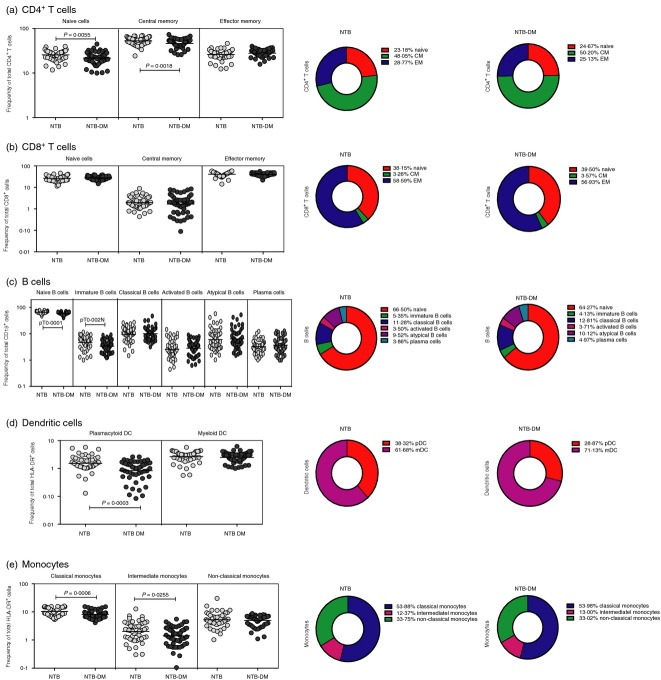
To determine the influence of DM on leucocyte phenotypic profiles of non-TB-infected individuals, we measured the frequency (Fo) of T-cell, B-cell, DC and monocyte subsets in NTB-DM and NTB individuals. As shown in Fig.1(a), NTB-DM individuals exhibited significantly lower Fo of naive and central memory but not effector memory CD4+ T cells compared with NTB individuals. In contrast, NTB-DM individuals did not exhibit any significant difference in the Fo of naive, central memory and effector memory CD8+ T-cell subsets compared with NTB individuals (Fig.1b). In addition, NTB-DM individuals exhibited significantly lower Fo of naive and immature B cells compared with NTB individuals (Fig.1c). NTB-DM individuals also exhibited significantly lower Fo of plasmacytoid DC compared with NTB individuals (Fig.1d). Finally, as shown in Fig.1(e), NTB-DM individuals exhibited significantly lower Fo of classical and intermediate monocytes in comparison with NTB individuals. Hence, diabetes per se is associated with profound alterations in the Fo of leucocyte subsets in the periphery, independent of TB.
Figure 1.
Non-tuberculosis-infected diabetic (NTB-DM) status is associated with altered frequency (Fo) of T-cell, B-cell, dendritic cell (DC) and monocyte subsets. (a) Fo of CD4+ T-cell (b); CD8+ T-cell; (c) B-cell; (d) DC and (e) monocyte subsets in NTB-DM (n = 50) and NTB (n = 50) individuals. The data are represented as scatter plots with each circle representing a single individual. P values were calculated using the Mann–Whitney U-test. The pie charts depict the geometric mean Fo of the individual subset of leucocytes in the two groups.
PTB-DM is associated with altered Fo of T-cell, B-cell, DC and monocyte subsets
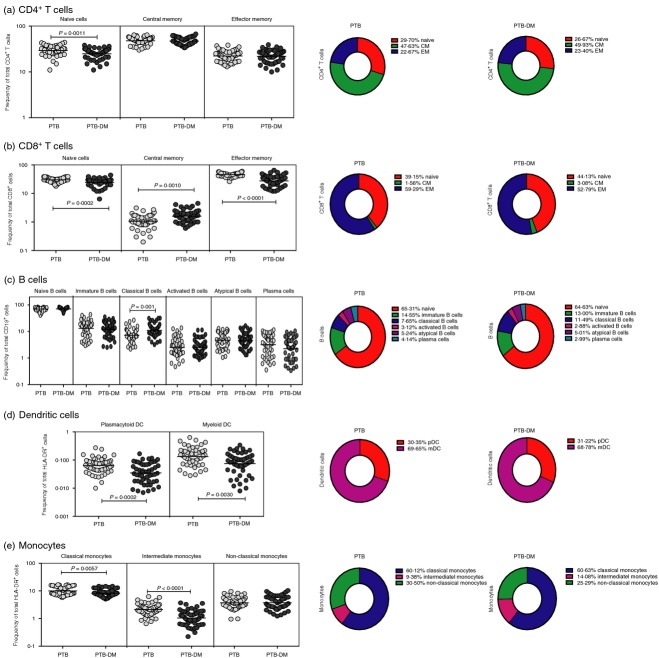
To determine the influence of PTB-DM co-morbidity on leucocyte phenotypic profiles, we measured the Fo of T-cell, B-cell, DC and monocyte subsets in PTB-DM and PTB individuals. As shown in Fig.2(a), PTB-DM individuals exhibited significantly lower Fo of naive but not central or effector memory CD4+ T cells compared with PTB individuals. Similarly, PTB-DM individuals exhibited significantly lower Fo of naive and effector memory but significantly higher Fo of central memory CD8+ T cells compared with PTB individuals (Fig.2b). In addition, PTB-DM individuals exhibited significantly higher Fo of classical memory B cells compared with PTB individuals (Fig.2c). PTB-DM individuals also exhibited significantly lower Fo of both DC subsets (myeloid DC and plasmacytoid DC) compared with PTB individuals (Fig.2d). Finally, as shown in Fig.2(e), PTB-DM individuals exhibited significantly lower Fo of classical and intermediate monocytes in comparison to PTB individuals. Hence, PTB with coincident diabetes is associated with profound alterations in the Fo of leucocyte subsets in the periphery.
Figure 2.
Pulmonary tuberculosis–diabetes (PTB-DM) co-morbidity is associated with altered frequency (Fo) of T-cell, B-cell, dendritic cell (DC) and monocyte subsets. (a) Fo of CD4+ T-cell (b); CD8+ T-cell; (c) B-cell; (d) DC and (e) monocyte subsets in PTB-DM (n = 50) and PTB (n = 50) individuals. The data are represented as scatter plots with each circle representing a single individual. P values were calculated using the Mann–Whitney U-test. The pie charts depict the geometric mean Fo of the individual subset of leucocytes in the two groups.
LTB-DM is associated with altered Fo of T-cell, B-cell, DC and monocyte subsets
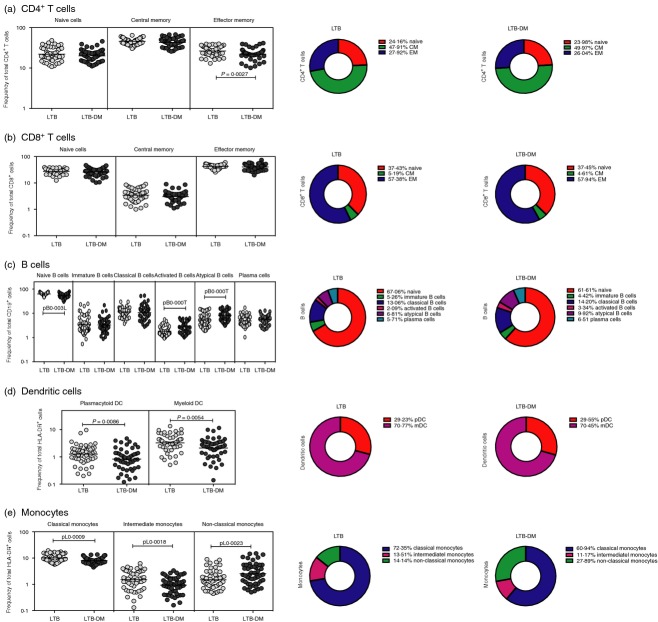
To determine the influence of LTB-DM co-morbidity on leucocyte phenotypic profiles, we measured the Fo of T-cell, B-cell, DC and monocyte subsets in LTB-DM and LTB individuals. As shown in Fig.3(a), LTB-DM individuals exhibited significantly lower Fo of effector memory but not naive or central memory CD4+ T cells compared with LTB individuals. In contrast, LTB-DM individuals did not exhibit any significant difference in the Fo of naive, central memory and effector memory CD8+ T-cell subsets compared with LTB individuals (Fig.3b). In addition, LTB-DM individuals exhibited significantly higher Fo of activated memory and atypical B cells but significantly lower Fo of naive B cells compared with LTB individuals (Fig.3c). LTB-DM individuals also exhibited significantly lower Fo of both DC subsets (myeloid DC and plasmacytoid DC) compared with LTB individuals (Fig.3d). Finally, as shown in Fig.3(e), LTB-DM individuals exhibited significantly lower Fo of classical and intermediate monocytes and significantly higher Fo of non-classical monocytes in comparison to LTB individuals. Hence, LTB with coincident diabetes is associated with profound alterations in the Fo of leucocyte subsets in the periphery.
Figure 3.
Latent tuberculosis–diabetes mellitus (LTB-DM) co-morbidity is associated with altered frequency (Fo) of T-cell, B-cell, dendritic cell (DC) and monocyte subsets. (a) Fo of CD4+ T-cell (b); CD8+ T-cell; (c) B-cell; (d) DC and (e) monocyte subsets in LTB-DM (n = 50) and LTB (n = 50) individuals. The data are represented as scatter plots with each circle representing a single individual. P values were calculated using the Mann–Whitney U-test. The pie charts depict the geometric mean Fo of the individual subset of leucocytes in the two groups.
Discussion
Susceptibility to active TB is influenced by many host factors, including DM.16 Tuberculosis infection and disease are known to induce alterations in innate and adaptive immune responses, modulations that determine the status of infection (latent versus active) as well as the degree of pathology.17 Establishment of a pro-inflammatory environment both locally and systemically is a hallmark of chronic TB disease.18 Similarly, DM is also characterized by a chronic state of low-grade inflammation due to activation of pro-inflammatory mediators and increased formation of advanced glycation end products.19,20 In addition DM can also profoundly modulate cells of the innate and adaptive immune system, most notably monocytes/macrophages and neutrophils.21 Finally, T-cell responses are also known to be altered in DM with hyperactivation of T cells being a major feature.22 However, the role of innate and adaptive immune cells in diabetes with coincident TB has not been explored in detail. Our study is the first study, to our knowledge, to explore the ex vivo phenoptype of leucocyte subsets in TB-DM co-morbidity.
The major subsets of CD4+ and CD8+ T cells can be defined by the expression of CD45RA and CCR7.23 Typically, both CD4+ and CD8+ T cells are classified as naive, central memory or effector memory based on the expression pattern of the above markers. Both CD4+ and CD8+ T cells are essential for immunity to TB;24 and LTB is thought to be associated with expansion of effector and central memory T cells, while active TB is associated with expansion of only central memory T cells.25 T cells in TB-DM are known to be hyper-activated, although the exact memory phenotype of these cells is not known. Our data show that PTB profoundly alters the memory repertoire of CD8+ T cells but not CD4+ T cells, whereas LTB has only a minimal effect on both compartments and DM alone primarily alters the memory repertoire of CD4+ T cells but not CD8+ T cells. Also, our data show that latent and active TB cannot be differentiated based on the CD4+ T-cell memory subsets alone because very few changes in these subsets were observed. Hence, TB-DM co-morbidity is characterized by major alterations in the homeostatic levels of CD4+ and CD8+ T-cell memory subsets.
The granuloma, which is characteristic of organized immunity against TB, has been shown to contain B cells – a finding that suggests their involvement in orchestrating the containment of infection.26 The role of B cells in the granuloma appears to be immunomodulation through the secretion of cytokines such as interleukin-10, and the production of immunoglobulin to engage Fcγ antibody receptors (FcγRs) expressed on mononuclear phagocytes.27 B cells are also known to regulate inflammation in DM by expressing an inflammatory cytokine profile.28 Circulating B cells are classified into different subsets based on their characteristic expression of surface markers including various B-cell memory compartments, such as classical memory B cells, activated memory B cells and atypical memory B cells.14 Hence, both HIV and malaria are characterized by the expansion of an atypical memory B-cell compartment.15,29 Our previous study on the expression pattern of these subsets showed no alterations of any of these subsets in active PTB compared with LTB.30 Our data, here reveal that although DM per se does not influence the memory B-cell subsets, LTB-DM co-morbid individuals exhibit an expansion of activated memory B cells and atypical memory B cells and PTB-DM individuals exhibit an expansion of classical memory B cells. Interestingly, no differences were observed in the plasma cell compartments between the different groups of individuals. Therefore, our data indicating a perturbation in the memory B-cell compartment, imply a potential impact on the ability of these cells to rapidly respond to secondary stimulation as well as on the immunoregulatory role of these subsets. This needs to be explored in more detail in the future.
Circulating blood DCs have been identified as HLA-DR+ cells (but negative for lineage-specific markers) and are divided into cells of the myeloid lineage (CD11c+) or the plasmacytoid lineage (CD123+).23 Investigation of the function of these subsets in active TB shows that co-operation between M. tuberculosis-infected myeloid DCs and plasmacytoid DCs favours the stimulation of CD4+ T cells.31 In addition, the cross-talk between these subsets is also known to promote antibacterial activity and CD8+ T-cell stimulation.32 Therefore, it is clear that both subsets of DCs play an important role in the immune response to TB. Our data on the DC subset phenotypes in TB-DM co-morbidity show that DM alone induces alterations in the plasmacytoid DC compartments with plasmacytoid DC frequencies being decreased in DM compared with non-DM individuals. Our data also reveal that both PTB and LTB induce a profound diminution in the frequencies of myeloid DCs and plasmacytoid DCs in the presence of coincident DM. Hence, the potential to activate the adaptive immune response by initiating antigen presentation in the periphery could potentially be affected in TB-DM co-morbidity. This fits with the current picture of TB-DM in animal models, wherein it has been clearly shown that DC activation, migration and antigen presentation to CD4+ T cells are significantly delayed.17
Human monocyte subsets can be categorized based on the dichotomous expression of the markers – CD14 and CD16 – into three major subsets, classical, intermediate and non-classical.33 Previous studies have shown that these subsets have divergent biological roles in TB infection and disease,34 with non-classical monocytes being expanded in active TB and correlating with disease severity,35 and classical monocytes contributing to the anti-mycobacterial response and being expanded in individuals with latent TB.34 Our study illustrates that DM per se alters the equilibrium of these three subsets with diminished frequencies of classical and intermediate subsets, with this pattern remaining the same in the presence of coincident PTB or LTB. In addition, only LTB appears to have a significant effect on the proportion of non-classical monocytes, although subtle alterations in this compartment in the presence of PTB cannot be ruled out. Hence, a more in-depth study of the functional effects of the alterations of monocyte subsets in LTB and PTB is warranted. Therefore, TB-DM co-morbidity is also characterized by an altered proportion of monocyte subsets, with the potential to influence innate and adaptive immunity to TB. This is reminiscent of previous data showing alterations in phagocytic and chemotactic functions of monocytes in TB-DM individuals.36
Our study suffers from the limitation of being an observational study depicting only associations and no functional consequences or cause–effect relationships. In addition, another limitation of using frequencies within a defined subset is that it is not possible to determine which subset actually causes the alteration. Nevertheless, our study clearly delineates a profound impact of diabetes on not only on individuals with active and latent TB but also on non-TB-infected individuals. Although this effect is mainly focused on antigen-presenting cells rather than on T or B cells, the potential impact of such alteration could have major detrimental effects on the host immune responses in TB-DM. This detailed knowledge on qualitative and quantitative alterations in the presence or absence of TB infection/disease and the perturbations of these parameters in the presence of DM co-morbidity have important implications for the both host immunity and metabolic responses. In conjunction with our earlier data on exaggerated T-cell and cytokine responses in PTB-DM co-morbidity and diminished T-cell and cytokine responses in LTB-DM co-morbidity, our current findings on alterations in homeostatic frequencies of innate and adaptive immune cells suggests an important effect of metabolic perturbation on the constitutive composition of the immune system and portends important effects on responses to exogenous challenge.6,12 Future research on how the alterations in leucocyte phenotypes in DM functionally impact TB outcomes could potentially be very useful in elucidating the pathogenesis of this complex disease interaction.
Acknowledgments
We thank the staff of the Department of Clinical Research and the Department of Social Work, NIRT especially Ms Kalaiselvi and Government Stanley Hospital, Chennai, for valuable assistance in recruiting the patients for this study, R. Anuradha, V. Gopinath and Jovvian George of the NIH–ICER for technical assistance. This study was funded by the Division of Intramural Research, NIAID, NIH.
Glossary
- APC
allophycocyanin
- DC
dendritic cells
- DM
diabetes mellitus
- Fo
frequencies
- HbA1c
haemoglobin A1c
- LTB
latent tuberculosis
- NTB
non-tuberculosis-infected
- PE
phycoerythrin
- PerCP
peridinin chlorophyll protein
- PTB
pulmonary tuberculosis
- TB
tuberculosis
Disclosure
None reported.
Supporting Information
Figure S1. Gating strategy for estimating frequencies of CD4+ and CD8+ T-cell, B-cell, dendritic cell and monocyte subsets.
References
- WHO. 2014. Global Tuberculosis Report 2013 http://www.who.int/ [accessed on 31 May 2014]
- Walzl G, Ronacher K, Hanekom W, Scriba TJ, Zumla A. Immunological biomarkers of tuberculosis. Nat Rev Immunol. 2011;11:343–54. doi: 10.1038/nri2960. [DOI] [PubMed] [Google Scholar]
- Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5:e152. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IDF. IDF Diabetes Atlas. 5th edn. 2012. 2012 update. http://www.eatlasidforg/diabetesatlas/5e/ update 2012 [accessed on 1 December 2012] [Google Scholar]
- Lonnroth K, Roglic G, Harries AD. Improving tuberculosis prevention and care through addressing the global diabetes epidemic: from evidence to policy and practice. Lancet Diabetes Endocrinol. 2014;2:730–9. doi: 10.1016/S2213-8587(14)70109-3. [DOI] [PubMed] [Google Scholar]
- Kumar NP, Sridhar R, Banurekha VV, Jawahar MS, Fay MP, Nutman TB, Babu S. Type 2 diabetes mellitus coincident with pulmonary tuberculosis is associated with heightened systemic type 1, type 17, and other proinflammatory cytokines. Ann Am Thorac Soc. 2013;10:441–9. doi: 10.1513/AnnalsATS.201305-112OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo BI, Fisher-Hoch SP, Pino PA, Salinas A, Rahbar MH, Mora F, Cortes-Penfield N, McCormick JB. Tuberculosis in poorly controlled type 2 diabetes: altered cytokine expression in peripheral white blood cells. Clin Infect Dis. 2008;47:634–41. doi: 10.1086/590565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar NP, Sridhar R, Banurekha VV, Jawahar MS, Nutman TB, Babu S. Expansion of pathogen-specific T-helper 1 and T-helper 17 cells in pulmonary tuberculosis with coincident type 2 diabetes mellitus. J Infect Dis. 2013;208:739–48. doi: 10.1093/infdis/jit241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar NP, Sridhar R, Nair D, Banurekha VV, Nutman TB, Babu S. Type 2 diabetes mellitus is associated with altered CD8+ T and natural killer cell function in pulmonary tuberculosis. Immunology. 2015;144:677–86. doi: 10.1111/imm.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo BI, Twahirwa M, Rahbar MH, Schlesinger LS. Phagocytosis via complement or Fcγ receptors is compromised in monocytes from type 2 diabetes patients with chronic hyperglycemia. PLoS One. 2014;9:e92977. doi: 10.1371/journal.pone.0092977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stew SS, Martinez PJ, Schlesinger LS, Restrepo BI. Differential expression of monocyte surface markers among TB patients with diabetes co-morbidity. Tuberculosis. 2013;93(Suppl):S78–82. doi: 10.1016/S1472-9792(13)70015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar NP, George PJ, Kumaran P, Dolla CK, Nutman TB, Babu S. Diminished systemic and antigen-specific type 1, type 17, and other proinflammatory cytokines in diabetic and prediabetic individuals with latent Mycobacterium tuberculosis infection. J Infect Dis. 2014;210:1670–8. doi: 10.1093/infdis/jiu329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepa M, Pradeepa R, Rema M, Mohan A, Deepa R, Shanthirani S, Mohan V. The Chennai Urban Rural Epidemiology Study (CURES)–study design and methodology (urban component) (CURES-I) J Assoc Physicians India. 2003;51:863–70. [PubMed] [Google Scholar]
- Moir S, Ho J, Malaspina A, et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205:1797–805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss GE, Crompton PD, Li S, et al. Atypical memory B cells are greatly expanded in individuals living in a malaria-endemic area. J Immunol. 2009;183:2176–82. doi: 10.4049/jimmunol.0901297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronacher K, Joosten SA, van Crevel R, Dockrell HM, Walzl G, Ottenhoff TH. Acquired immunodeficiencies and tuberculosis: focus on HIV/AIDS and diabetes mellitus. Immunol Rev. 2015;264:121–37. doi: 10.1111/imr.12257. [DOI] [PubMed] [Google Scholar]
- O’Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP. The immune response in tuberculosis. Annu Rev Immunol. 2013;31:475–527. doi: 10.1146/annurev-immunol-032712-095939. [DOI] [PubMed] [Google Scholar]
- Dorhoi A, Kaufmann SH. Perspectives on host adaptation in response to Mycobacterium tuberculosis: modulation of inflammation. Semin Immunol. 2014;26:533–42. doi: 10.1016/j.smim.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet. 2011;378:169–81. doi: 10.1016/S0140-6736(11)60614-4. [DOI] [PubMed] [Google Scholar]
- Nikolajczyk BS, Jagannathan-Bogdan M, Shin H, Gyurko R. State of the union between metabolism and the immune system in type 2 diabetes. Genes Immun. 2011;12:239–50. doi: 10.1038/gene.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolajczyk BS, Jagannathan-Bogdan M, Denis GV. The outliers become a stampede as immunometabolism reaches a tipping point. Immunol Rev. 2012;249:253–75. doi: 10.1111/j.1600-065X.2012.01142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the Human Immunology Project. Nat Rev Immunol. 2012;12:191–200. doi: 10.1038/nri3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasenosky LD, Scriba TJ, Hanekom WA, Goldfeld AE. T cells and adaptive immunity to Mycobacterium tuberculosis in humans. Immunol Rev. 2015;264:74–87. doi: 10.1111/imr.12274. [DOI] [PubMed] [Google Scholar]
- Achkar JM, Chan J, Casadevall A. B cells and antibodies in the defense against Mycobacterium tuberculosis infection. Immunol Rev. 2015;264:167–81. doi: 10.1111/imr.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J, Mehta S, Bharrhan S, Chen Y, Achkar JM, Casadevall A, Flynn J. The role of B cells and humoral immunity in Mycobacterium tuberculosis infection. Semin Immunol. 2014;26:588–600. doi: 10.1016/j.smim.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFuria J, Belkina AC, Jagannathan-Bogdan M, et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc Natl Acad Sci U S A. 2013;110:5133–8. doi: 10.1073/pnas.1215840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardava L, Moir S, Shah N, et al. Abnormal B cell memory subsets dominate HIV-specific responses in infected individuals. J Clin Invest. 2014;124:3252–62. doi: 10.1172/JCI74351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar NP, Sridhar R, Hanna LE, Banurekha VV, Nutman TB, Babu S. Decreased frequencies of circulating CD4+ T follicular helper cells associated with diminished plasma IL-21 in active pulmonary tuberculosis. PLoS One. 2014;9:e111098. doi: 10.1371/journal.pone.0111098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozza L, Farinacci M, Bechtle M, Staber M, Zedler U, Baiocchini A, Del Nonno F, Kaufmann SH. Communication between Human Dendritic Cell Subsets in Tuberculosis: requirements for Naive CD4+ T Cell Stimulation. Front Immunol. 2014;5:324. doi: 10.3389/fimmu.2014.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozza L, Farinacci M, Fae K, et al. Crosstalk between human DC subsets promotes antibacterial activity and CD8+ T-cell stimulation in response to bacille Calmette-Guérin. Eur J Immunol. 2014;44:80–92. doi: 10.1002/eji.201343797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KL, Yeap WH, Tai JJ, Ong SM, Dang TM, Wong SC. The three human monocyte subsets: implications for health and disease. Immunol Res. 2012;53:41–57. doi: 10.1007/s12026-012-8297-3. [DOI] [PubMed] [Google Scholar]
- Balboa L, Barrios-Payan J, Gonzalez-Dominguez E, et al. Diverging biological roles among human monocyte subsets in the context of tuberculosis infection. Clin Sci (Lond) 2015;129:319–30. doi: 10.1042/CS20150021. [DOI] [PubMed] [Google Scholar]
- Balboa L, Romero MM, Basile JI, et al. Paradoxical role of CD16+ CCR2+ CCR5+ monocytes in tuberculosis: efficient APC in pleural effusion but also mark disease severity in blood. J Leukoc Biol. 2011;90:69–75. doi: 10.1189/jlb.1010577. [DOI] [PubMed] [Google Scholar]
- Restrepo BI, Schlesinger LS. Host–pathogen interactions in tuberculosis patients with type 2 diabetes mellitus. Tuberculosis. 2013;93(Suppl):S10–4. doi: 10.1016/S1472-9792(13)70004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Gating strategy for estimating frequencies of CD4+ and CD8+ T-cell, B-cell, dendritic cell and monocyte subsets.