Abstract
Malignant melanoma is an aggressive tumour of the skin with increasing incidence, frequent metastasis and poor prognosis. At the same time, it is an immunogenic type of cancer with spontaneous regressions. Most recently, the tumoricidal effect of plasmacytoid dendritic cells (pDC) and their capacity to overcome the immunosuppressive tumour microenvironment are being investigated. In this respect, we studied the effect of the infectious, but replication-deficient, herpes simplex virus 1 (HSV-1) d106S vaccine strain, which lacks essential immediate early genes, in pDC co-cultures with 11 melanoma cell lines. We observed a strong cytotoxic activity, inducing apoptotic and necrotic cell death in most melanoma cell lines. The cytotoxic activity of HSV-1 d106S plus pDC was comparable to the levels of cytotoxicity induced by natural killer cells, but required only a fraction of cells with effector : target ratios of 1 : 20 (P < 0·05). The suppressive activity of cell-free supernatants derived from virus-stimulated pDC was significantly neutralized using antibodies against the interferon-α receptor (P < 0·05). In addition to type I interferons, TRAIL and granzyme B contributed to the inhibitory effect of HSV-1 d106S plus pDC to a minor extent. UV-irradiated viral stocks were significantly less active than infectious particles, both in the absence and presence of pDC (P < 0·05), indicating that residual activity of HSV-1 d106S is a major component and sensitizes the tumour cells to interferon-producing pDC. Three leukaemic cell lines were also susceptible to this treatment, suggesting a general anti-tumour effect. In conclusion, the potential of HSV-1 d106S for therapeutic vaccination should be further evaluated in patients suffering from different malignancies.
Keywords: dendritic cells, viral, human, cytotoxicity, cancer
Introduction
Malignant melanoma is a highly aggressive skin tumour, which is characterized by increasing incidence, frequent metastasis and poor prognosis.1 Localized primary tumours can be treated by surgical removal, but metastatic disease is often treatment-refractory with < 20% of patients responding to chemotherapy and adjuvant administration of interferon-α (IFN-α) and/or interleukin 2 (IL-2).2,3 At the same time, malignant melanoma is a very immunogenic type of cancer, in which spontaneous regressions occur in 4–15% of primary lesions and 0·23% of melanoma metastases.4 For these reasons, metastatic melanoma came into the focus of immunotherapeutic approaches. Studies using monocyte-derived dendritic cells pulsed with melanoma-derived peptides or antigens have been performed in several hundred patients.5 Although the success of these immunotherapies has been limited so far with response rates not exceeding 5–10%,6 progression-free survival significantly correlated with the development of tumour-specific cytotoxic T cells.7 New approaches in immunotherapy have targeted immunosuppressive networks within the tumour microenvironment. In this respect, improved survival has been observed in patients suffering from malignant melanoma when antibodies against the co-inhibitory molecules CTLA-48 and programmed cell death 1 were administered9.
In the last few years, the tumoricidal potential of plasmacytoid dendritic cells (pDC) has been evaluated. These cells were originally identified as major producers of IFN-α in the blood upon stimulation with influenza virus or herpes simplex virus (HSV).10,11 Type I IFNs are required for tumour-specific T-cell priming and rejection of highly immunogenic tumours, as evident from studies in mice deficient in the IFN-α receptor (IFN-αR).12 Peptide-pulsed and CD40 ligand-matured pDC can prime expansion and IFN-γ production of melanoma-specific CD8+ T cells.13 Plasmacytoid DC can take up opsonized antigens via their Fc receptors and stimulate CD4+ and CD8+ T-cell proliferation.14 Other studies have described cross-presentation of exogenous antigens to CD8+ T cells by pDC after exposure to influenza and measles viruses,15,16 cell debris from apoptotic cells,17 and particulate antigen.18 Notably, tumour peptide-loaded pDC synergize with myeloid dendritic cells (mDC) in inducing antigen-specific CD8+ T-cell cytotoxic responses and in restricting tumour cell growth.19
Besides these indirect anti-tumour effects, activated pDC can mount direct cytotoxicity against malignant melanoma.20 In a mouse model, topical administration of imiquimod, a synthetic Toll-like receptor (TLR) 7 agonist, induced melanoma cell killing independent of adaptive immunity, through a mechanism dependent on type I IFNs, TRAIL, and granzyme B.21 TRAIL- and cell-contact-dependent cytotoxicity were also observed in human pDC after stimulation with TLR7/9 agonists and IFN-α.22 The pDC were also activated by some clinical-grade prophylactic vaccines, among them inactivated tick-borne encephalitis virus.23 The latter was used to stimulate tumour-peptide loaded pDC, which were then injected into the lymph nodes of 15 patients with metastatic melanoma.24 This was the first clinical trial to show significantly prolonged overall survival of melanoma patients after injection of activated pDC.
We observed strong activation and maturation of pDC after exposure to a clinical HSV-1 isolate.25 These data prompted us to test the efficacy of an infectious, but replication-deficient, HSV-1 isolate in 11 different melanoma cell lines. We show here, for the first time, that the HSV-1 d106S vaccine vector strain26 in combination with pDC develops strong cytotoxic activities against tumour cells that are at least equivalent or superior to effects induced by synthetic TLR7 and TLR9 agonists. Therefore, the potential of this virus for the therapeutic vaccination of patients suffering from malignant melanoma should be further evaluated.
Materials and methods
Tumour cell lines
Five established human melanoma cell lines from primary cutaneous tumour (IGR-39) and subcutaneous (SK-MEL-30) and lymph node (IGR-1, IGR-37, SK-MEL-3) metastases were obtained from the Leibniz Institute DSMZ – German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany. Six other melanoma cell lines (AXBI, LIWE-7, ARST-1, ICNI-5li, HV18MK, UMBY-1) were established from metastatic lesions of patients at the Department of Dermatology, University Hospital Erlangen, Germany. Generation of such cell lines was as follows: after surgical removal of melanoma metastases and informed consent of the patient (approved by the ethics committee of the Medical Faculty, Friedrich-Alexander-Universität Erlangen-Nürnberg, EK 4602), a small sample of the excised tumour was cut into small pieces and put into a cell culture dish in RPMI-1640 medium supplemented with 20% human pooled serum, amino acids and gentamycin. Half of the medium was replaced once a week. After one to several weeks, melanoma cells grew out and were frozen down at low passage numbers. After thawing, all melanoma cell lines except HV18MK were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco/Life Technologies, Darmstadt, Germany), supplemented with 10% heat-inactivated (56°, 60 min) fetal calf serum (Sigma-Aldrich, Taufkirchen, Germany), 0·3 mg/ml glutamine, 200 U/ml penicillin and 90 U/ml streptomycin. HV18MK cells and the leukaemic cell lines Daudi, U937 and KG-1 (American Type Culture Collection, Manassas, VA) were cultured in supplemented RPMI-1640 medium (Gibco/Life Technologies). Human foreskin fibroblasts (HFF), cultured in supplemented DMEM, served as a non-melanoma control cell line.
Isolation of pDC, natural killer cells, and myeloid dendritic cells
Peripheral blood mononuclear cells of healthy donors were recovered from leucoreduction system chambers obtained after a routine platelet apheresis procedure,27 approved by the ethics committee of the Medical Faculty, Friedrich-Alexander-Universität Erlangen-Nürnberg (Ref. no. 177_12B). Leucoreduction system chambers were purchased from the Transfusion Medicine and Hemostasis Department, University Hospital Erlangen, Germany. After standard Biocoll (Biochrom AG, Berlin, Germany) density gradient centrifugation, pDC and natural killer (NK) cells were purified from peripheral blood mononuclear cells by positive and negative selection using BDCA4 and NK Cell Isolation Kits from Miltenyi Biotec (Bergisch Gladbach, Germany), respectively, as previously described.28 For mDC purification, the CD1c (BDCA-1)+ dendritic cell isolation kit was applied according to the manufacturer’s recommendations (Miltenyi Biotec). Cell viability was controlled by trypan blue staining and was usually > 95% directly after isolation. The pDC were cultured with 20 ng/ml IL-3 (R&D Systems, Wiesbaden-Nordenstadt, Germany).
Generation of virus stocks
The HSV-1 strain d106S is infectious, but replication-deficient due to deletions of essential viral genes and promoter regions,26 and is derived from the HSV-1 d106 virus by backcrossing to obtain an acyclovir-sensitive phenotype.29 It expresses green fluorescent protein (GFP) under the control of a cytomegalovirus promoter. The complementing cell line E11, which provides the deleted proteins ICP4 and ICP27/47 in trans, was propagated in DMEM with supplements. E11 cells were infected with d106S virus for 2–3 days, then harvested and centrifuged at 600 g for 10 min. Cell pellets were subjected to two freeze–thaw cycles, resuspended in 5 ml Dulbecco’s Phosphate-Buffered Saline (DPBS), and disrupted by Dounce homogenization 20 times. After centrifugation at 600 g to remove cell debris, supernatants were loaded onto a continuous sucrose gradient (30–15% sucrose in virus standard buffer; 0·05 m Tris–HCl, 0·012 m KCl, 0·005 m EDTA, 0·1% BSA) and centrifuged at 50 000 g for 30 min. The visible viral layer was harvested and centrifuged at 78 000 g for 90 min. Virus pellets were resuspended in RPMI-1640, filtered through 0·22-μm pores, and stored at −80°. Some virus aliquots were inactivated by application of 1 Joule/cm2 using the Bio-Link 254 UV crosslinker (Vilber Lourmat, Eberhardzell, Germany). The 50% tissue culture infective dose was determined using the method of Reed and Munch.
Stimulation of melanoma cells
Melanoma cells were exposed to 0·1 μm taxol (Sigma-Aldrich), 4 ng/ml human recombinant IFN-α2b (Miltenyi Biotec), serum-free medium, HSV-1 d106S at a multiplicity of infection (MOI) of 0·5–1 or corresponding volumes of UV-inactivated virus, and supernatants from unstimulated or stimulated pDC. For these purposes, 500 000 freshly isolated pDC were exposed to wild-type HSV-130 at 37° for 3 hr, washed with DPBS, and then incubated with trypsin/EDTA at 37° for 15 min to remove all viral particles. Cells were washed with supplemented RPMI-1640 and incubated for 18 hr before supernatants were harvested and frozen at −20°. For stimulation, supernatants were used at a concentration of 4 ng/ml IFN-α2a/2b, as measured by the IFN-α ELISA module set (see below). In co-cultures, pDC were added to melanoma cells at ratios of 0·5–1 : 1, unless indicated otherwise. In some experiments, cells were stimulated with the endotoxin-free oligodeoxynucleotides (ODN) CpG-A 6016 (5′-T*C-G-A-C-G-T-C-G-T-G-G*G*G*G-3′, where * stands for phosphorothioate and – for phosphodiester bonds, 2·5 μm) and CpG-B 10103 (T*C*G*T*C*G*T*T*T*T*T*C*G*G*T*C*G*T*T*T*T, 0·25 μm), provided by Coley Pharmaceutical GmbH – A Pfizer Company (Düsseldorf, Germany), and the TLR7 agonist S-27609 at 5 μm, provided by 3m Pharmaceuticals (St Paul, MN).
Infection of melanoma cells by HSV-1 d106S
A total of 20 000 melanoma cells were cultured in 500 μl supplemented DMEM overnight. After infection with HSV-1 d106S (MOI = 1) for 2 hr, cells were washed with DPBS and trypsinized at 37° for 15 min to remove all viral particles from the cell surface. Cells were seeded in supplemented DMEM, and GFP expression by HSV-1 d106S was measured by flow cytometry at different time-points post infection.
Analysis of melanoma cell growth
Melanoma cells were seeded in 24-well plates at 10 000 cells/250 μl supplemented DMEM. After overnight culture, 10 000 pDC or other stimuli were added in 250 μl RPMI-1640 plus supplements and IL-3. After 4 days of culture, supernatants were replaced by water. Plates were frozen at −80° to disrupt cell membranes, thawed, and lysed using 0·2% Triton X-100 (Carl Roth GmbH, Karlsruhe, Germany) for 2 hr. DNA concentration was determined using the Qubit dsDNA high sensitivity assay kit with Qubit fluorometer (Invitrogen/Life Technologies, Karlsruhe, Germany) as described by the manufacturer. To adjust for the aneuploidy of tumour cells, each cell line was normalized to the DNA content of the uncultivated cells, and further transformed logarithmically (log2) to obtain the number of theoretical cell divisions.
Analysis of melanoma cell viability
Cell viability was analysed using the Trevigen 3-[4,5-dimethylthiazol-2yl]-2,5-diphenyl-tetrazolium bromide (MTT) based assay (R&D Systems). Melanoma cells were seeded in flat-bottomed 96-well plates at 10 000 cells per well and incubated overnight before different stimuli were added. After 48 hr of stimulation, 10 μl MTT reagent was added per well and incubated at 37° for 3 hr, before 100 μl of detergent solution was added to solubilize the formazan crystals. Plates were incubated at 37° overnight and then measured photometrically at 550/650 nm. All wells were analysed with reference to the optical density of untreated melanoma cells, which was set at 100%. The optical density in wells with pDC co-cultures was adjusted for the enhanced metabolic turnover by subtracting the optical density of pDC only.
Light microscopy
Light microscopic images were recorded using the Nikon Eclipse TS100 instrument with CMOS TCA3·0C camera (Ample Scientific, Norcross, GA) and Tsview7 (Fuzhou Tucsen Image Technology, Fuzhou, Fujian, China) software.
FACS analysis
For cell proliferation analysis, 1 × 106 cells were washed twice in pre-warmed DPBS and stained with the cell proliferation dye eFluor 450 (10 μm) (eBioscience, Frankfurt, Germany) at 37° for 10 min. Labelling was stopped by adding cold DMEM plus supplements and incubating cells on ice for 5 min. Cells were washed three times, seeded at 20 000 cells/well in 24-well plates, and incubated overnight. After addition of different stimuli, cells were harvested daily from 0 to 4 days, using Accutase solution (Sigma-Aldrich) at 37° for 3 min. For cell death analysis, cells were incubated in 1× binding buffer with allophycocyanin-conjugated Annexin V for 5 min and 7-AAD staining for another 5 min (all eBioscience). Cells were immediately processed and analysed by flow cytometry using an LSR-II flow cytometer with automated compensation and FACSDiva software (BD Biosciences, Heidelberg, Germany). Data were analysed using FCS Express 3 Software (De Novo Software, Los Angeles, CA).
Neutralization experiments
Neutralization experiments were performed using murine IgG1 antibodies to IL-1β (clone 8516), tumour necrosis factor-α (clone 28401), and TRAIL (clone 75411) with IgG1 isotype control (clone 11711) (all R & D Systems); and murine IgG2a antibody to human IFN-αR (clone MMHAR-2; PBL Assay Science, Piscataway, NJ) with mouse IgG2a isotype (clone PPV-04) (both purchased from Acris Antibodies, Herford, Germany), at a concentration of 15 μg/ml. The irreversible, cell-permeable inhibitor of granzyme B, Z-AAD-CMK (Enzo Life Sciences, Lörrach, Germany), was used at 5 μg/ml, dissolved in DMSO. The SuperKillerTRAIL protein (Enzo Life Sciences) was used at a concentration of 500 ng/ml.
Quantification of IFN-α
Interferon-α2a/2b levels in cell culture supernatants were measured using an ELISA module set as recommended by the manufacturer (eBioscience). Samples were diluted appropriately and measured within the linear range of the assay.
Statistics
The number of independently performed experiments (n) is given in the figure legends. Data sets were compared using the paired Student’s t-test or analysis of variance (Figs 1d and 5f). Two-sided P-values < 0·05 were considered significant. Data are presented as mean and standard error of the mean; *P < 0·05, **P < 0·01, ***P < 0·001.
Results
HSV-1 d106S plus pDC inhibit melanoma cell growth
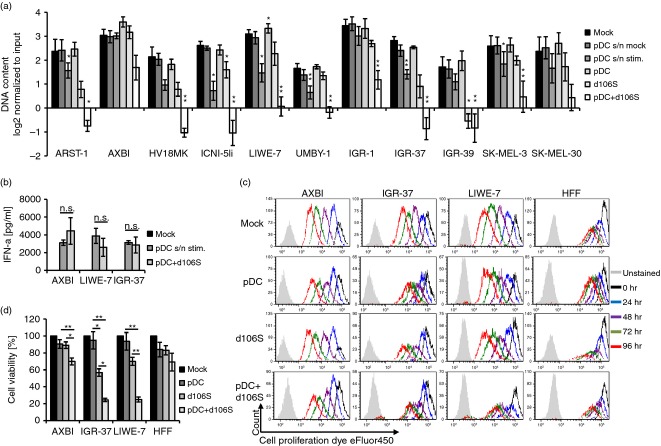
Recombinant IFN-α is used as adjuvant therapy in patients suffering from malignant melanoma.3 To evaluate the effect of this cytokine in vitro, a total of 11 melanoma cell lines were exposed to human recombinant IFN-α2b for 4 days, using the DNA content of the cultures as read-out for cell growth. Significant reduction of melanoma cell growth was observed in serum-free medium and with the anti-mitotic drug taxol, which arrests the cell cycle at the G2/M phase (P < 0·05) (see Supplementary material, Fig. S1). Human recombinant IFN-α2b significantly reduced the growth of SK-MEL-30, which was described as susceptible to IFN-α,31 and of ARST-1 at concentrations of 4 ng/ml (P < 0·05). In contrast, supernatants of HSV-1-exposed pDC containing similar amounts of IFN-α2a/2b reduced growth of 6 of the 11 melanoma cell lines (P < 0·05) (Fig.1a). To compare the inhibitory potential of pDC with the effect of cell-free supernatants, co-cultures of pDC and melanoma cells were stimulated with the infectious, but replication-deficient HSV-1 d106S (MOI = 1). The IFN-α 2a/2b concentrations in these co-cultures were comparable to the conditions described above (Fig.1b). Exposure to virus in the presence of pDC drastically reduced the DNA content in 9 of 11 melanoma cell lines (P < 0·05), while stimulation with HSV-1 d106S alone was effective in two cell lines only (Fig.1a). For further analyses, we selected three cell lines that either remained largely unaffected (AXBI) or responded to virus-stimulated pDC (IGR-37, LIWE-7). Staining of these cell lines in co-cultures with HSV-1 d106S plus pDC revealed continuous dilution of the CFSE-derivative eFluor450 dye in AXBI and control HFF cells over 4 days, but much less in IGR-37 and LIWE-7 cells (Fig.1c). The effect of HSV-1 d106S on melanoma cell proliferation was negligible, while pDC plus HSV-1 d106S had a significant inhibitory effect (P < 0·05) (see Supplementary material, Fig. S2). Similar data for pDC plus HSV-1 d106S were obtained using an MTT-based assay, while HSV-1 d106S significantly decreased viability of IGR-37 cells (P < 0·05) (Fig.1d). HFF viability was not affected under either condition. Altogether, soluble factors in pDC supernatants, but in particular pDC plus HSV-1 d106S inhibited proliferation and viability of susceptible melanoma cell lines.
Figure 1.
Herpes simplex virus 1 (HSV-1) d106S plus plasmacytoid dendritic cells (pDC) inhibit melanoma cell growth. (a) Proliferation of 11 melanoma cell lines in the presence of supernatants of unstimulated (pDC s/n mock) or virus-stimulated pDC (pDC s/n stim.), unstimulated pDC, HSV-1 d106S (MOI = 1), and pDC plus HSV-1 d106S, as measured by DNA concentration 4 days post stimulation (n = 4). Values were compared with the mock control using the paired Student’s t-test. (b) Interferon-α (IFN-α) 2a/2b concentrations after exposure of three melanoma cell lines to supernatants from virus-stimulated pDC (MOI = 1) for 18 hr or in co-cultures of melanoma cells and pDC stimulated likewise (n = 4). Paired Student’s t-test. (c) Dilution of the CFSE-derivative eFluor450 in three melanoma cell lines and human foreskin fibroblasts (HFF) at 0, 24, 48, 72 and 96 hr post co-culture with pDC, HSV-1 d106S, and pDC plus HSV-1 d106S (MOI = 1) compared with untreated cells. Gating on melanoma cells excluded cell debris and pDC. Data represent three independent experiments. (d) MTT-based viability of three melanoma cell lines and HFF in the presence of unstimulated pDC, HSV-1 d106S (MOI = 1), and pDC plus HSV-1 d106S, at 2 days post stimulation (n = 7). Statistics were performed using analysis of variance. *P < 0·05, **P < 0·01.
HSV-1 d106S plus pDC induce melanoma cell death
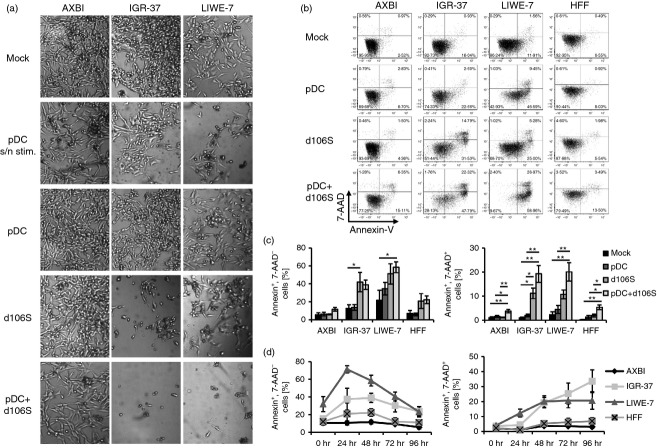
Microscopic images of melanoma cell cultures exposed to different stimuli suggested not only reduced proliferation, but also cell death (Fig.2a). Although AXBI remained largely unaffected, IGR-37 responded well to HSV-1 d106S and virus-stimulated pDC, whereas LIWE-7 cells were more susceptible to pDC supernatants and virus-stimulated pDC. To characterize the type of cell death, melanoma cells were stained with Annexin-V and 7-AAD as markers for apoptosis and necrosis, respectively (Fig.2b). In this analysis, IGR-37 developed apoptosis and necrosis with HSV-1 d106S alone and with virus-stimulated pDC, while LIWE-7 cells showed increased apoptosis after exposure to virus-stimulated pDC. Apoptosis was more pronounced in LIWE-7 than IGR-37 cells, but necrosis was observed in both cell lines (Fig.2c). Apoptosis occurred in LIWE-7 and IGR-37 cells within 24–48 hr, but not in AXBI and HFF cells (Fig.2d). In conclusion, HSV-1 d106S, but in particular HSV-1 d106S plus pDC induced apoptotic and necrotic melanoma cell death within a few days.
Figure 2.
Herpes simplex virus 1 (HSV-1) d106S plus plasmacytoid dendritic cells (pDC) induce melanoma cell death. (a) Light microscopy of three melanoma cell lines in the presence of supernatants from virus-stimulated pDC (pDC s/n stim.), unstimulated pDC, HSV-1 d106S (MOI = 1), and pDC plus HSV-1 d106S. One representative of 12 independent experiments is shown. (b and c) Apoptosis and necrosis of three melanoma cell lines and human foreskin fibroblasts (HFF) at 48 hr post stimulation with unstimulated pDC, HSV-1 d106S (MOI = 1), and pDC plus HSV-1 d106S, analysed by Annexin-V and 7-AAD staining. We show (b) one representative flow cytometry experiment and (c) mean and standard error of five independent runs. Analysis of variance was used to compare the effects of all stimuli to each other with respect to each cell line. (d) Kinetics of apoptotic and necrotic cell death at 0, 24, 48, 72 and 96 hr post stimulation with HSV-1 d106S plus pDC (n = 5). *P < 0·05, **P < 0·01.
HSV-1 d106S plus pDC develop cytotoxic activity comparable to NK cells
The activity of virus-stimulated pDC described above resembled the effects of NK cells. To exclude that the observed effects were in fact due to contaminating NK cells in the pDC preparations, we titrated pDC and NK cells in melanoma cell co-cultures. In contrast to NK cells, unstimulated pDC did not affect melanoma cell viability. After exposure to HSV-1 d106S plus pDC, the viability of IGR-37 and LIWE-7 cells was significantly reduced at pDC : melanoma cell ratios of 1 : 1 down to even 1 : 20 (P < 0·05) (Fig.3a) compared with virus only treatment. In contrast, virus-stimulated NK cells were only active at ratios of 1 : 1 and 1 : 2 (Fig.3b), pointing to a much stronger activity of virus-stimulated pDC. The degree of cytotoxicity obtained with HSV-1 d106S plus pDC was comparable to the effect of NK cells, but lower pDC numbers were required to achieve this cytotoxicity. Preparations of pDC were contaminated on average by 0·17% (0·13–0·78%) NK cells, as reported previously.25 This percentage would be equal to 13–78 contaminating NK cells in 10 000 pDC, which excluded the possibility that the observed effects of pDC in infected melanoma cell co-cultures were the result of contaminating NK cells.
Figure 3.
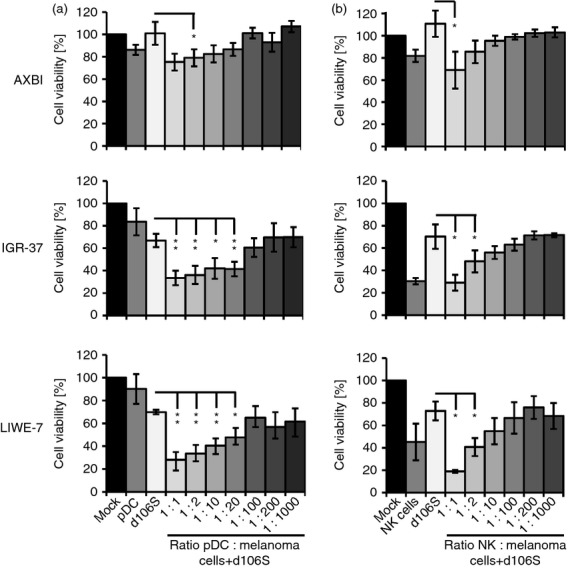
Virus-stimulated plasmacytoid dendritic cells (pDC) develop natural killer (NK) cell-like cytotoxic activity. MTT viability analysis of three herpes simplex virus 1 (HSV-1) d106S-exposed melanoma cell lines in the presence of decreasing numbers of (a) pDC and (b) NK cells (n = 3). Controls included unstimulated melanoma cells (mock), co-cultures with unstimulated pDC and unstimulated NK cells, and melanoma cells exposed to HSV-1 d106S only. Statistics were performed using paired t-tests in comparison to HSV-1 d106S-exposed melanoma cells. *P < 0·05, **P < 0·01.
Type I IFNs contribute to soluble and cell-associated effects of pDC
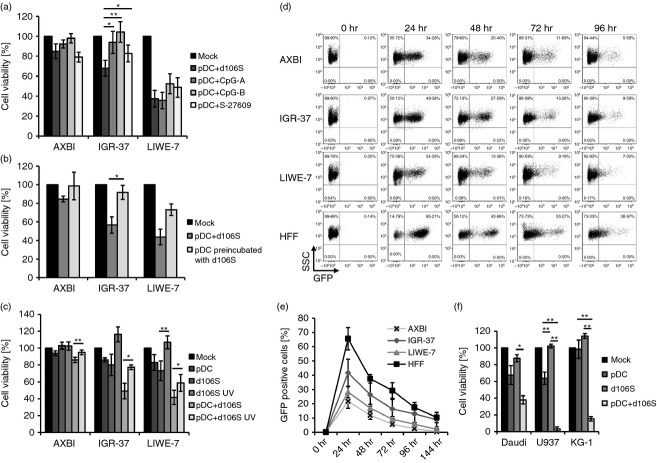
Our results so far indicated that virus-stimulated pDC acted on melanoma cells through soluble and cell-associated factors. To identify the mechanism(s) that are responsible for the suppressive effect, co-cultures of pDC and melanoma cells were stimulated in the presence of neutralizing antibodies or inhibitors against molecules involved in cytotoxicity. Neutralizing IFN-αR antibodies abrogated the inhibitory effects of cell-free pDC supernatants on IGR-37 and LIWE-7 cells almost completely (P < 0·01) (Fig.4a). IFN-aR antibodies blocked the effect of all type I IFNs, indicating that the observed melanoma cell inhibition strongly depends on this family of cytokines. The effect of IFN-αR antibodies on virus-stimulated pDC in melanoma cell co-cultures was also significant (P < 0·05) (Fig.4a), demonstrating that type I IFNs also played a crucial role in this context. Two other cytokines, namely tumour necrosis factor-α and IL-1β, which are secreted by HSV-1-stimulated peripheral blood mononuclear cells,28 did not appear to be involved in pDC cytotoxicity, because neutralization of both molecules did not affect suppressive activity of pDC supernatants or virus-stimulated pDC (results not shown). Next, we investigated TRAIL and granzyme B, which both mediate tumour cell killing by imiquimod-stimulated pDC.21 Neutralization of TRAIL reduced the suppressive effects of HSV-1 d106S plus pDC on LIWE-7 cells to a minor extent (P < 0·05) (Fig.4b). Correspondingly, LIWE-7 cells were susceptible to stimulation with SuperKillerTRAIL (see Supplementary material, Fig. S3). A small effect was also observed in IGR-37 cells after inhibition of granzyme B activity by Z-AAD-CMK (P < 0·05) (Fig.4c). Altogether, type I IFNs, and to a minor extent TRAIL and granzyme B, contribute to the cytotoxic effect of HSV-1 d106S plus pDC.
Figure 4.
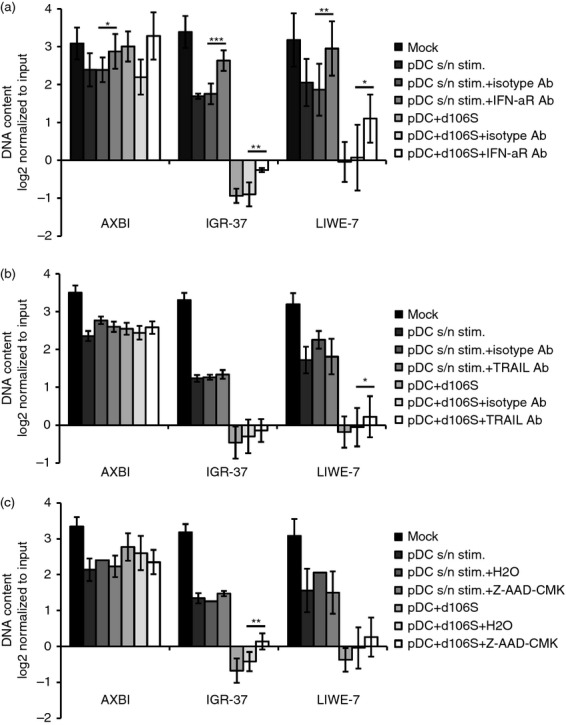
Soluble and cell-associated factors contribute to the suppressive activity of herpes simplex virus 1 (HSV-1) d106S plus plasmacytoid dendritic cells (pDC). Effects of neutralizing antibodies against (a) the interferon-α receptor (IFN-aR Ab) (n = 4) and (b) TRAIL (TRAIL Ab) (n = 5), as well as (c) a cell-permeable inhibitor of granzyme B (Z-AAD-CMK) (n = 4), on the proliferation of three melanoma cell lines, which were stimulated with supernatants of virus-stimulated pDC (pDC s/n stim.) or pDC plus HSV-1 d106S. Paired t-tests comparing the respective inhibitor/antibody with solvent/isotype control. *P < 0·05, **P < 0·01, ***P < 0·001. Results for IFN-α receptor and TRAIL were reproduced using the MTT assay (data not shown).
HSV-1 d106S exerts a virus-induced cytolytic effect on melanoma cells
Cytotoxic effects of pDC against melanoma cells were also reported after exposure of pDC to synthetic TLR7 and TLR9 agonists.21,22 To find out how the effects of virus infection compare with other stimuli, co-cultures of melanoma cells and pDC were exposed in parallel to HSV-1 d106S, CpG-A, CpG-B and the synthetic TLR7 agonist S-27609. All stimuli were comparably active on LIWE-7 cells, whereas HSV-1 d106S proved to be superior in inducing pDC cytotoxicity on IGR-37 cells (P < 0·05) (Fig.5a). This pointed to an important effect of the virus in the co-cultures. To find out whether HSV-1 d106S needed to be present in the co-cultures to induce this effect, pDC were pre-incubated with virus and then washed and trypsinized to remove all viral particles. With this procedure, the suppressive effects on IGR-37 and LIWE-7 cells were reduced (Fig.5b), suggesting that maximal cytotoxicity required infection of melanoma cells. Stocks of UV-inactivated HSV-1 d106S with cross-linked viral DNA showed a decreased effect on melanoma cell viability, both with and without pDC (Fig.5c). Hence, HSV-1 d106S was required in co-cultures to obtain fully suppressive pDC activity, suggesting that infection of melanoma cells by HSV-1 d106S contributed to the effect. Therefore, we studied infection of melanoma cells. As shown by GFP expression, all melanoma cell lines and HFF cells were infected by HSV-1 d106S (HFF > IGR-37 > LIWE-7 > AXBI) (Fig.5d). GFP signals peaked at 24 hr post infection, representing initial infection, and decreased thereafter with no evidence of productive viral replication (Fig.5e). Notably, the degree of infection in the different melanoma cell lines reflected the induction of cell death after exposure to HSV-1 d106S plus pDC, which was not the case for HFF cells (Fig.2b). Altogether, HSV-1 d106S, although not inducing a lytic replication cycle, exerted virolytic activity on melanoma cells that rendered them more susceptible to the pDC attack. To find out whether the effect of HSV-1 d106S plus pDC was limited to melanoma cells, we performed similar experiments using the Daudi B-lymphoblast cell line, the U937 histiocytic lymphoma cell line, and the KG-1 myeloid leukaemia cell line. Most notably, the effects on melanoma cells were reproduced in these cell lines (Fig.5f), suggesting that the effect of HSV-1 d106S plus pDC extends to other malignancies.
Figure 5.
Herpes simplex virus 1 (HSV-1) d106S exerts virolytic effects on melanoma cells. (a) Viability of melanoma cell lines in co-cultures with plasmacytoid dendritic cells (pDC), stimulated with HSV-1 d106S, CpG-A ODN 6016, CpG-B ODN 10103, and the synthetic Toll-like receptor 7 (TLR7) agonist S-27609 (n = 6). Paired t-test for the comparison of pDC plus HSV-1 d106S to synthetic TLR ligands. (b) Effect of pDC on the viability of melanoma cell lines, analysed with HSV-1 d106S in co-cultures, versus pDC which were pre-incubated with the virus for 3 hr, washed and trypsinized before melanoma cell co-culture (n = 6). Paired t-test. (c) Viability of melanoma cell lines exposed to unstimulated pDC, infectious and UV-inactivated HSV-1 d106S, and pDC plus infectious or UV-inactivated virus (n = 6). Paired t-tests comparing the effect of UV-inactivated versus infectious virus. (d and e). Infection of melanoma cell lines and human foreskin fibroblasts (HFF) with HSV-1 d106S as measured by GFP expression at the indicated time-points post infection, shown as (d) one representative flow experiment and (e) mean and standard error of four independent experiments. (f) Viability of three leukaemic cell lines after exposure to unstimulated pDC, HSV-1 d106S, and pDC with HSV-1 d106S (n = 4). The effects of all stimuli were compared using anova. *P < 0·05, **P < 0·01.
Discussion
The success of immunotherapies using monocyte-derived dendritic cells for advanced stages of melanoma has been limited. We aimed to improve these tumour-vaccination approaches using virus-activated pDC, hypothesizing that, upon viral stimulation with HSV-1, pDC mount cytotoxic immune responses against melanoma cells. Our study indeed showed that HSV-1 d106S plus pDC induced a strong inhibitory activity on the majority of melanoma cell lines as well as against three leukaemic cell lines (Figs1a and 5f). The inhibitory effect of HSV-1 d106S plus pDC was superior to HSV-1 d106S alone and also to supernatants from stimulated pDC (Fig.1a, d; Fig.3, Fig.5f; see Supplementary material, Fig. S2).
The effects of supernatants and virus-stimulated pDC could at least partially be neutralized by IFN-αR antibodies (Fig.4a), pointing to the anti-proliferative and pro-apoptotic role of type I IFNs.32 Interferon-α has become a standard adjuvant immunotherapy in melanoma patients, although response rates do not exceed 10–20%, and adverse events often result in discontinuation of therapy.3 Remarkably, the three melanoma cell lines that responded to neutralization of the IFN-α receptor (Fig.4), showed no sensitivity to recombinant IFN-α2b (see Supplementary material, Fig. S1), although IFN-α2a/2b amounts were similar under all conditions (Fig.1b). An explanation may be that HSV-1-stimulated pDC secrete other forms of type I IFNs, which all bind to the IFN-α receptor.
Notably, HSV-1 d106S plus pDC not only reduced melanoma cell proliferation, but also developed cytotoxic activity. Reduced DNA content after exposure of melanoma cells to virus-stimulated pDC already suggested cell death, which was confirmed by MTT assays, light microscopy and up-regulation of markers for apoptosis and necrosis in flow cytometry (Fig.2). Cytotoxic effects of HSV-1 d106S on the pDC were excluded (see Supplementary material, Fig. S4a–c). Several groups have similarly reported pDC cytotoxicity upon stimulation with CpG ODN and the synthetic TLR7 agonist imiquimod.21,22,33 Others have used monocyte-derived dendritic cells, killer monocytes and pDC at high effector : target ratios up to 100 : 1,34 whereas we used ratios of 1 : 1 in our experiments, and even ratios of 1 : 20 still reduced melanoma cell viability (Fig.3a). The degree of cytotoxicity exerted by HSV-1 d106S plus pDC was comparable to that by NK cells, but required fewer cells (Fig.3b), pointing to a stronger pDC effect on a cell-to-cell basis. Plasmacytoid DC also proved to exert superior cytotoxic activity compared with mDC in co-cultures with HSV-1 d106S and melanoma cells (see Supplementary material, Fig. S4d). It has to be considered, though, that the cytotoxic activity of cells in other studies is usually measured within hours, whereas we measured within 2 days (MTT) or 4 days (Qubit). For these reasons, we stimulated pDC in parallel with CpG ODN, a synthetic TLR7 agonist, and HSV-1 d106S for 48 hr (Fig.5a). This side-by-side comparison revealed that the cytotoxic activity of pDC plus HSV-1 d106S was at least comparable to other stimuli. For one melanoma cell line, the effect was significantly better (P < 0·05), suggesting that pDC stimulation by virus infection may be superior to synthetic TLR7 and TLR9 ligation.
Besides IFN-α, we found involvement of TRAIL and Granzyme B in the cytotoxic effects of HSV-1 d106S plus pDC on LIWE-7 and IGR-37 cells, respectively (Fig.4). In contrast to other studies,21,22 both molecules played only a minor role for the cytotoxicity of virus-stimulated pDC. Rather, type I IFNs in combination with viral infection of tumour cells (Fig.5d) appeared to be crucial for the cytotoxic effect, suggesting that either pDC expand their cytotoxic potential upon HSV-1 stimulation or infection with HSV-1 d106S sensitizes tumour cells to apoptotic and necrotic cell death. The latter mechanism may explain why AXBI cells, which were infected to a lesser extent compared with the other melanoma cell lines (Fig.5e), were largely resistant to the cytotoxic activity of HSV-1 d106S plus pDC. A virus-specific effect has also been postulated for oncolytic virotherapy, in which defective antiviral defences of tumour cells are exploited.35 The infectious, but replication-incompetent HSV-1 d106S still expresses the immediate early proteins ICP0 and ICP6.26 Expression of ICP0 by infected cells was sufficient for recognition of NK cells by natural cytotoxicity receptors.36,37 After UV irradiation, viral DNA is cross-linked and the virus no longer expresses immediate early proteins. In our experiments, irradiated virus stocks were significantly less active than infectious particles, both with and without pDC (Fig.5c). Hence, expression of ICP0 by HSV-1 d106S-infected melanoma cells appears to be important to induce cytotoxicity in co-culture with pDC. HSV-1 d106S does not induce a lytic replication cycle, but it is sufficiently active to endow pDC with a ‘license to kill’, and thus fulfils the criteria of a suitable vaccine construct.
Which would be the route of administration of HSV-1 d106S? Apart from stimulating patient-derived pDC ex vivo, which has been reported for the inactivated FSME vaccine followed by intranodal injection in patients with metastatic melanoma,24 intradermal, subcutaneous and intralymphatic routes of application are conceivable. Plasmacytoid DC are not present in healthy skin, but accumulate in peritumoral areas, and can occasionally be detected in close association to infiltrating CD8+ T cells within melanoma lesions13 or in sentinel lymph nodes.38 Unstimulated pDC appear to induce a T helper type 2 tolerogenic immune response and even propagate melanoma cell growth.39,40 Activated pDC, however, overcome the immunosuppressive tumour microenvironment and limit melanoma cell growth.33,41–43 In this respect, peri- and intralesional injections of HSV-1 d106S appear to be attractive, because immature pDC, which reside in or around melanoma lesions, may thereby be optimally stimulated. HSV-1 has already been used as an oncolytic viral vector in melanoma patients in phase II and III trials.35,44 In this vector as well as in HSV-1 d106S, the blocker of antigen presentation, ICP47, is deleted. HSV-1 d106S has an improved safety profile as additional deletions of ICP4 and ICP27 abrogate viral replication (Fig.5d), and should therefore be suitable for in vivo applications.
The HSV-1 d106S is engineered to incorporate foreign genes in the place of the cytomegalovirus-driven GFP.26,29 Via a transfer plasmid, genes coding for specific tumour antigens can be inserted into the virus by homologous recombination. In rhesus monkeys, which were vaccinated with recombinant HSV-1 d106S expressing SIV envelope and Nef antigens, cellular and humoral immunity against the transgenes were induced and helped to control SIV infection.45,46 It would be interesting to see how the in vitro effects of our study may translate into tumour models in vivo. Inserting genes for melanoma-specific antigens may help to attack the tumour from two sides: inducing innate pDC cytotoxicity and activating adaptive immune responses such as cytotoxic T lymphocytes. Plasmacytoid DC are known to link innate and adaptive immunity. They can take up opsonized antigen and thereby activate CD4+ and CD8+ T cells14 and efficiently cross-present viral antigen to CD8+ T cells.15,17,24,47 The pDC synergize with mDC in inducing a tumour-antigen specific CD8+ cytotoxic T-lymphocyte response.19 Type I IFNs are indispensable for tumour-specific T-cell priming and rejection of immunogenic tumours in the mouse model.12 All of these different qualities support the use of pDC in anti-tumour therapies. A superior effect on patient survival may be accomplished by combining HSV-1 d106S with CTLA-4 and PD-1 blockade.
Herpes simplex virus-1 d106S plus pDC exerted a distinct cytotoxic activity also on three leukaemic cell lines (Fig.5f), supporting the notion that the effect of virus-stimulated pDC may go beyond highly immunogenic tumours such as malignant melanoma.48 By providing a strong danger signal,49 HSV-1 d106S in combination with pDC has the potential to induce a general anti-tumour mechanism which should be evaluated in further studies.
Authors’ contributions
S.T. performed the experiments, J.B.B. and K.V. provided technical support, D.M.K., N.D., S.G. and B.S.T provided reagents and contributed to the discussion, S.T., P.S., and B.S. designed the experiments and wrote the manuscript.
Acknowledgments
We thank Bernhard Fleckenstein, Erlangen, and André Gessner, Regensburg, for continuous support and providing laboratory space. Ingrid Müller-Fleckenstein, Institute of Clinical and Molecular Virology, Erlangen, is acknowledged for providing protocols and helpful advice in preparing viral stocks. CpG-A ODN 6016 and CpG-B ODN 10103 were kindly contributed by Jörg Vollmer, Pfizer Oligonucleotide Therapeutics Unit – Coley Pharmaceutical GmbH, Düsseldorf, Germany, and the synthetic TLR7 agonist S-27609 by 3m Pharmaceuticals, St. Paul, MN. Michael Nevels and Christina Paulus contributed the ATCC cell lines Daudi, U937, and KG-1, and Martin Ehrenschwender provided the SuperKillerTRAIL protein. Part of this work was supported by the doctoral training programmes GRK1660 (‘Key signals of adaptive immune response’; to S.T.) and 1071 (‘Viruses of the immune system’; to J.B.B. and K.V.), the ‘Akademie der Wissenschaften und Literatur zu Mainz’ (to B.S.), and by NIH grant AI 057552 (to D.M.K).
Glossary
- GFP
green fluorescent protein
- HFF
human foreskin fibroblasts
- HSV
herpes simplex virus
- IFN
interferon
- IFN-αR
interferon-α receptor
- IL
interleukin
- MOI
multiplicity of infection
- NK cell
natural killer cell
- ODN
oligodeoxynucleotide
- pDC
plasmacytoid dendritic cells
- TLR
Toll-like receptor
- UV
ultraviolet
Disclosures
D.M.K. is a co-inventor on a US patent ‘Replication-defective HSV vaccines’ that describes the use of HSV replication-defective viruses for immunization and immunotherapy.
Supporting Information
Figure S1. Effect of taxol, serum deprivation, and recombinant interferon-α 2b on melanoma cell proliferation.
Figure S2. Comparison of melanoma cell proliferation in the presence of (a) herpes simplex virus 1 (HSV-1) d106S and (b) HSV-1 d106S plus plasmacytoid dendritic cells (pDC).
Figure S3. Effect of soluble TRAIL on melanoma cell proliferation.
Figure S4. Comparison of the effect of herpes simplex virus 1 (HSV-1) d106S on plasmacytoid dendritic cells (pDC) and myeloid dendritic cells (mDC).
References
- Simard EP, Ward EM, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin. 2012;62:118–28. doi: 10.3322/caac.20141. [DOI] [PubMed] [Google Scholar]
- Agarwala SS, Kirkwood JM. Interferons in melanoma. Curr Opin Oncol. 1996;8:167–74. doi: 10.1097/00001622-199603000-00015. [DOI] [PubMed] [Google Scholar]
- Mouawad R, Sebert M, Michels J, Bloch J, Spano JP, Khayat D. Treatment for metastatic malignant melanoma: old drugs and new strategies. Crit Rev Oncol Hematol. 2010;74:27–39. doi: 10.1016/j.critrevonc.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Kalialis LV, Drzewiecki KT, Klyver H. Spontaneous regression of metastases from melanoma: review of the literature. Melanoma Res. 2009;19:275–82. doi: 10.1097/CMR.0b013e32832eabd5. [DOI] [PubMed] [Google Scholar]
- Engell-Noerregaard L, Hansen TH, Andersen MH, Thor Straten P, Svane IM. Review of clinical studies on dendritic cell-based vaccination of patients with malignant melanoma: assessment of correlation between clinical response and vaccine parameters. Cancer Immunol Immunother. 2009;58:1–14. doi: 10.1007/s00262-008-0568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesterhuis WJ, Aarntzen EH, De Vries IJ, Schuurhuis DH, Figdor CG, Adema GJ, et al. Dendritic cell vaccines in melanoma: from promise to proof? Crit Rev Oncol Hematol. 2008;66:118–34. doi: 10.1016/j.critrevonc.2007.12.007. [DOI] [PubMed] [Google Scholar]
- de Vries IJ, Bernsen MR, Lesterhuis WJ, Scharenborg NM, Strijk SP, Gerritsen MJ, et al. Immunomonitoring tumor-specific T cells in delayed-type hypersensitivity skin biopsies after dendritic cell vaccination correlates with clinical outcome. J Clin Oncol. 2005;23:5779–87. doi: 10.1200/JCO.2005.06.478. [DOI] [PubMed] [Google Scholar]
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14:3044–51. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–23. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–7. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salio M, Cella M, Vermi W, Facchetti F, Palmowski MJ, Smith CL, et al. Plasmacytoid dendritic cells prime IFN-γ-secreting melanoma-specific CD8 lymphocytes and are found in primary melanoma lesions. Eur J Immunol. 2003;33:1052–62. doi: 10.1002/eji.200323676. [DOI] [PubMed] [Google Scholar]
- Bjorck P, Beilhack A, Herman EI, Negrin RS, Engleman EG. Plasmacytoid dendritic cells take up opsonized antigen leading to CD4+ and CD8+ T cell activation in vivo. J Immunol. 2008;181:3811–7. doi: 10.4049/jimmunol.181.6.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pucchio T, Chatterjee B, Smed-Sorensen A, Clayton S, Palazzo A, Montes M, et al. Direct proteasome-independent cross-presentation of viral antigen by plasmacytoid dendritic cells on major histocompatibility complex class I. Nat Immunol. 2008;9:551–7. doi: 10.1038/ni.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillerme JB, Boisgerault N, Roulois D, Menager J, Combredet C, Tangy F, et al. Measles virus vaccine-infected tumor cells induce tumor antigen cross-presentation by human plasmacytoid dendritic cells. Clin Cancer Res. 2013;19:1147–58. doi: 10.1158/1078-0432.CCR-12-2733. [DOI] [PubMed] [Google Scholar]
- Hoeffel G, Ripoche AC, Matheoud D, Nascimbeni M, Escriou N, Lebon P, et al. Antigen crosspresentation by human plasmacytoid dendritic cells. Immunity. 2007;27:481–92. doi: 10.1016/j.immuni.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Tel J, Schreibelt G, Sittig SP, Mathan TS, Buschow SI, Cruz LJ, et al. Human plasmacytoid dendritic cells efficiently cross-present exogenous Ags to CD8+ T cells despite lower Ag uptake than myeloid dendritic cell subsets. Blood. 2013;121:459–67. doi: 10.1182/blood-2012-06-435644. [DOI] [PubMed] [Google Scholar]
- Lou Y, Liu C, Kim GJ, Liu YJ, Hwu P, Wang G. Plasmacytoid dendritic cells synergize with myeloid dendritic cells in the induction of antigen-specific antitumor immune responses. J Immunol. 2007;178:1534–41. doi: 10.4049/jimmunol.178.3.1534. [DOI] [PubMed] [Google Scholar]
- Chaperot L, Blum A, Manches O, Lui G, Angel J, Molens JP, et al. Virus or TLR agonists induce TRAIL-mediated cytotoxic activity of plasmacytoid dendritic cells. J Immunol. 2006;176:248–55. doi: 10.4049/jimmunol.176.1.248. [DOI] [PubMed] [Google Scholar]
- Drobits B, Holcmann M, Amberg N, Swiecki M, Grundtner R, Hammer M, et al. Imiquimod clears tumors in mice independent of adaptive immunity by converting pDCs into tumor-killing effector cells. J Clin Invest. 2012;122:575–85. doi: 10.1172/JCI61034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb ML, Glaser A, Stary G, Koszik F, Stingl G. TRAIL+ human plasmacytoid dendritic cells kill tumor cells in vitro: mechanisms of imiquimod- and IFN-α-mediated antitumor reactivity. J Immunol. 2012;188:1583–91. doi: 10.4049/jimmunol.1102437. [DOI] [PubMed] [Google Scholar]
- de Vries IJ, Tel J, Benitez-Ribas D, Torensma R, Figdor CG. Prophylactic vaccines mimic synthetic CpG oligonucleotides in their ability to modulate immune responses. Mol Immunol. 2011;48:810–7. doi: 10.1016/j.molimm.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Tel J, Aarntzen EH, Baba T, Schreibelt G, Schulte BM, Benitez-Ribas D, et al. Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res. 2013;73:1063–75. doi: 10.1158/0008-5472.CAN-12-2583. [DOI] [PubMed] [Google Scholar]
- Schuster P, Donhauser N, Pritschet K, Ries M, Haupt S, Kittan NA, et al. Co-ordinated regulation of plasmacytoid dendritic cell surface receptors upon stimulation with herpes simplex virus type 1. Immunology. 2010;129:234–47. doi: 10.1111/j.1365-2567.2009.03176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Broberg E, Watanabe D, Dudek T, Deluca N, Knipe DM. Genetic engineering of a modified herpes simplex virus 1 vaccine vector. Vaccine. 2009;27:2760–7. doi: 10.1016/j.vaccine.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser EF, Weidinger T, Zimmermann R, Ringwald J, Eckstein R. Recovery of white blood cells and platelets from leukoreduction system chambers of Trima Accel and COBE Spectra plateletpheresis devices. Transfusion. 2007;47:1943–4. doi: 10.1111/j.1537-2995.2007.01461.x. author reply 4–5. [DOI] [PubMed] [Google Scholar]
- Vogel K, Thomann S, Vogel B, Schuster P, Schmidt B. Both plasmacytoid dendritic cells and monocytes stimulate natural killer cells early during human HSV-1 infections. Immunology. 2014;132:588–600. doi: 10.1111/imm.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaniego LA, Neiderhiser L, DeLuca NA. Persistence and expression of the herpes simplex virus genome in the absence of immediate-early proteins. J Virol. 1998;72:3307–20. doi: 10.1128/jvi.72.4.3307-3320.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittan NA, Bergua A, Haupt S, Donhauser N, Schuster P, Korn K, et al. Impaired plasmacytoid dendritic cell innate immune responses in patients with herpes virus-associated acute retinal necrosis. J Immunol. 2007;179:4219–30. doi: 10.4049/jimmunol.179.6.4219. [DOI] [PubMed] [Google Scholar]
- Schaber B, Mayer P, Schreiner T, Rassner G, Fierlbeck G. Anti-proliferative activity of natural interferon-α, isotretinoin and their combination varies in different human melanoma cell lines. Melanoma Res. 1994;4:319–26. [PubMed] [Google Scholar]
- Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- Aspord C, Tramcourt L, Leloup C, Molens JP, Leccia MT, Charles J, et al. Imiquimod inhibits melanoma development by promoting pDC cytotoxic functions and impeding tumor vascularization. J Invest Dermatol. 2014;134:2551–61. doi: 10.1038/jid.2014.194. [DOI] [PubMed] [Google Scholar]
- Tel J, Anguille S, Waterborg CE, Smits EL, Figdor CG, de Vries IJ. Tumoricidal activity of human dendritic cells. Trends Immunol. 2014;35:38–46. doi: 10.1016/j.it.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30:658–70. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm SE, Howard K, Gomez MV, Reyburn HT. Expression of ICP0 is sufficient to trigger natural killer cell recognition of herpes simplex virus-infected cells by natural cytotoxicity receptors. J Infect Dis. 2007;195:1160–8. doi: 10.1086/512862. [DOI] [PubMed] [Google Scholar]
- Fitzgerald-Bocarsly P, Howell DM, Pettera L, Tehrani S, Lopez C. Immediate-early gene expression is sufficient for induction of natural killer cell-mediated lysis of herpes simplex virus type 1-infected fibroblasts. J Virol. 1991;65:3151–60. doi: 10.1128/jvi.65.6.3151-3160.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlini G, Urso C, Mariotti G, Di Gennaro P, Palli D, Brandani P, et al. Plasmacytoid dendritic cells represent a major dendritic cell subset in sentinel lymph nodes of melanoma patients and accumulate in metastatic nodes. Clin Immunol. 2007;125:184–93. doi: 10.1016/j.clim.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Aspord C, Leccia MT, Charles J, Plumas J. Plasmacytoid dendritic cells support melanoma progression by promoting Th2 and regulatory immunity through OX40L and ICOSL. Cancer Immunol Res. 2013;1:402–15. doi: 10.1158/2326-6066.CIR-13-0114-T. [DOI] [PubMed] [Google Scholar]
- Vermi W, Bonecchi R, Facchetti F, Bianchi D, Sozzani S, Festa S, et al. Recruitment of immature plasmacytoid dendritic cells (plasmacytoid monocytes) and myeloid dendritic cells in primary cutaneous melanomas. J Pathol. 2003;200:255–68. doi: 10.1002/path.1344. [DOI] [PubMed] [Google Scholar]
- Liu C, Lou Y, Lizee G, Qin H, Liu S, Rabinovich B, et al. Plasmacytoid dendritic cells induce NK cell-dependent, tumor antigen-specific T cell cross-priming and tumor regression in mice. J Clin Invest. 2008;118:1165–75. doi: 10.1172/JCI33583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamara F, Meindl S, Holcmann M, Luhrs P, Stingl G, Sibilia M. Identification and characterization of pDC-like cells in normal mouse skin and melanomas treated with imiquimod. J Immunol. 2004;173:3051–61. doi: 10.4049/jimmunol.173.5.3051. [DOI] [PubMed] [Google Scholar]
- Rothenfusser S, Hornung V, Ayyoub M, Britsch S, Towarowski A, Krug A, et al. CpG-A and CpG-B oligonucleotides differentially enhance human peptide-specific primary and memory CD8+ T-cell responses in vitro. Blood. 2004;103:2162–9. doi: 10.1182/blood-2003-04-1091. [DOI] [PubMed] [Google Scholar]
- Senzer NN, Kaufman HL, Amatruda T, Nemunaitis M, Reid T, Daniels G, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol. 2009;27:5763–71. doi: 10.1200/JCO.2009.24.3675. [DOI] [PubMed] [Google Scholar]
- Kaur A, Sanford HB, Garry D, Lang S, Klumpp SA, Watanabe D, et al. Ability of herpes simplex virus vectors to boost immune responses to DNA vectors and to protect against challenge by simian immunodeficiency virus. Virology. 2007;357:199–214. doi: 10.1016/j.virol.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CG, Lucas WT, Means RE, Czajak S, Hale CL, Lifson JD, et al. Vaccine protection against simian immunodeficiency virus by recombinant strains of herpes simplex virus. J Virol. 2000;74:7745–54. doi: 10.1128/jvi.74.17.7745-7754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui G, Manches O, Angel J, Molens JP, Chaperot L, Plumas J. Plasmacytoid dendritic cells capture and cross-present viral antigens from influenza-virus exposed cells. PLoS ONE. 2009;4:e7111. doi: 10.1371/journal.pone.0007111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speeckaert R, van Geel N, Vermaelen KV, Lambert J, Van Gele M, Speeckaert MM, et al. Immune reactions in benign and malignant melanocytic lesions: lessons for immunotherapy. Pigment Cell Melanoma Res. 2011;24:334–44. doi: 10.1111/j.1755-148X.2010.00799.x. [DOI] [PubMed] [Google Scholar]
- Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–5. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Effect of taxol, serum deprivation, and recombinant interferon-α 2b on melanoma cell proliferation.
Figure S2. Comparison of melanoma cell proliferation in the presence of (a) herpes simplex virus 1 (HSV-1) d106S and (b) HSV-1 d106S plus plasmacytoid dendritic cells (pDC).
Figure S3. Effect of soluble TRAIL on melanoma cell proliferation.
Figure S4. Comparison of the effect of herpes simplex virus 1 (HSV-1) d106S on plasmacytoid dendritic cells (pDC) and myeloid dendritic cells (mDC).