Abstract
Interleukin-17 (IL-17) is a cytokine with critical functions in multiple autoimmune diseases. However, its roles in type I diabetes and the underlying mechanisms remain to be fully elucidated. In the current study, we investigated the impact of IL-17 deficiency on streptozotocin (STZ) -induced diabetes. Il-17−/− mice exhibited attenuated hyperglycaemia and insulitis after STZ treatment compared with control mice. The Il-17−/− mice had fewer CD8+ cells infiltrating the pancreas than wild-type controls after STZ injection. Wild-type mice showed increased percentage and number of splenic CD8+ cells and decreased Gr1+ CD11b+ myeloid-derived suppressor cells (MDSC) after STZ treatment, but Il-17−/− mice maintained the percentages and numbers of splenic CD8+ cells and MDSC, suggesting that IL-17 is implicated in STZ-induced cellular immune responses in the spleen. We further purified the MDSC from spleens of STZ-treated mice. Il-17−/− MDSC showed increased ability to suppress CD8+ cell proliferation in vitro compared with wild-type MDSC. Transfer of MDSC to diabetic mice showed that MDSC from Il-17−/− mice could ameliorate hyperglycaemia. Moreover, recipients with MDSC from Il-17−/− mice had a decreased percentage of CD8+ cell in the spleen compared with recipients with MDSC from wild-type mice. These data suggest that IL-17 is required in splenic MDSC function after STZ delivery. In summary, our study has revealed a pathogenic role of IL-17 in an STZ-induced diabetes model with important implications for our understanding of IL-17 function in autoimmune diseases.
Keywords: diabetes, interleukin-17A, myeloid-derived suppressor cells, streptozotocin
Introduction
Interleukin-17 (IL-17) is an inflammatory cytokine secreted by T cells. Six IL-17 family members (IL-17A–F) have been described, and the prototype member of the family is IL-17A, often termed IL-17 in literature. Interleukin-17 mediates pro-inflammatory responses and induces many other cytokines, such as IL-6, tumour necrosis factor-α, transforming growth factor-β, in various cell types.1 It is important in proliferation, maturation and invasion of neutrophils2 and is one of the major effectors of T helper type 17 cells, which exhibited pivotal roles in autoimmune disease. Adoptively transferred T helper type 17 cells could stimulate CD8+ cytotoxic T lymphocytes and promote diabetes development.3 Moreover, increasing evidence showed that other IL-17-producing cells also play important roles in diabetes.4,5
However, the roles of IL-17 in different diabetes models remained controversial. In non-obese diabetic (NOD) mouse models, IL-17 neutralization treatment could prevent the development of diabetes.6 Interleukin-17-deficient (Il-17−/−) NOD mice showed delayed onset of diabetes.7 On the other hand, an IL-17 knockdown NOD line created by directly introducing a short hairpin RNA construct did not show altered diabetes susceptibility.8 Neutralization of IL-17 in vivo did not protect NOD-severe combined immunodeficiency mice against diabetes transferred by diabetic splenocytes.9 Moreover, IL-17 production was undetectable in CD4+ or CD8+ T cells in the CD8-driven lymphocytic choriomeningitis virus-induced model of type 1 diabetes.10 At the same time, in vitro data showed that IL-17 markedly promoted induced nitric oxide production and nitric oxide-dependent toxicity in mouse pancreatic islet cells.11 However, IL-17 may be more involved in affecting immune responses in secondary lymphoid tissues rather than directly mediating beta cell injury, as the IL-17-positive cells were relatively rare in the pancreas during diabetes development.6
Myeloid-derived suppressor cells (MDSC) represent a population of immature myeloid cells with the ability to suppress various T-cell functions.12 MDSC with hallmarks of surface Gr-1 and CD11b in mice accumulate during many pathological conditions including cancer and autoimmunity.13 MDSC have been shown to play a protective role in diabetes.14,15 In tumours, IL-17 was reported to promote MDSC recruitment16 and mediate MDSC-induced tumour immune responses.17 In diabetes, reports describing the potential link between IL-17 and MDSC in diabetes are currently lacking.
In this study, we examined the effects of IL-17 deficiency on streptozotocin (STZ) -induced diabetes. Our findings indicate that IL-17 is implicated in STZ-induced immune responses in both pancreas and spleen.
Materials and methods
Animal models
Six- to eight-week-old Il-17−/− mice from Dr Yoichiro Iwakura18 and wild-type C57BL/6 mice were used for diabetes models. Mice after 4 hr fast were injected intraperitoneally with STZ (50 mg/kg of body weight) in citrate buffer (Sigma, St Louis, MO, USA) or an equivalent volume of citrate buffer daily for five consecutive days. Blood glucose levels were measured before first injection and after final injection between 9 a.m. and 10 a.m. All mice were housed in pathogen-free facilities on a 12-hr light- dark cycle. All protocols and procedures in this study were approved by the Ethics Committee of Wuhan University (Permission No. SYXK2008-0013).
ELISA
Mouse serum samples were obtained and stored at −20°C until the ELISA. Insulin concentrations were determined using ELISA kits (Millipore, Billerica, MA, USA) as recommended by the assay manufacturer.
Histology
Pancreatic slides from three mice in each group on Day 35 after injection were analysed. A minimum of 30 islets stained with haematoxylin & eosin from each mouse were examined microscopically by two different observers for the presence of insulitis. The severity of insulitis was scored as follows: 0, no lymphocytic infiltration; 1, lymphocytic infiltration occupying < 25% of the total islet cell area; 2, lymphocytic infiltration occupying 25–49% of the total islet cell area; 3, lymphocytic infiltration occupying 50–75% of the total islet cell area; 4, lymphocytic infiltration occupying > 75% of the total islet cell area, or small retracted islets.
Flow cytometry
Peripheral blood samples were collected and red cells were removed using Red Blood Cell Lysing Buffer (Sigma). Splenocytes were isolated from freshly obtained spleen specimens after grinding and filtering. After depleting red cells, single splenic cell suspensions were collected for further staining. Single pancreatic cell suspensions were prepared as previous described.19 For surface staining, cells were stained for 30 min with fluorescently labelled antibodies against surface molecules: CD4 (clone: RM4-5), CD8 (clone: H35-17.2), Gr-1 (clone: RB6-8C5), Ly6G (clone: 1A8), Ly6C (clone: HK1.4), F4/80 (clone: BM8), CD11b (clone: M1/70), and NK1.1 (clone: PK136) (eBioscience, San Diego, CA, USA). For Foxp3 (clone: FJK-16s) staining, cells were fixed, permeabilized using buffers and then stained with labelled antibodies (eBioscience). The cell suspensions were analysed or sorted by FACS (BD Aria III; BD, Franklin Lakes, NJ, USA). Gates were set based on the staining profile of the isotype controls.
Adoptive transfer
The MDSC (Gr1+ and CD11b+) were purified by flow cytometric sorting from the spleens of Il-17−/− mice and wild-type mice treated with STZ on Day 24. The MDSC (2 × 105) from one mouse were intravenously injected into the tail vein of another wild-type diabetic mouse treated with STZ on Day 13. There were 12 recipients in total, with six in each group.
CD8+ cells and MDSC co-culture
The culture medium is RPMI-1640 plus 10% heat inactivated fetal calf serum (Gibco, Gaithersburg, MD, USA) and 10 mm l-glutamine. 96-well plates were pre-coated with 50 μl PBS per well containing 5 μg/ml LEAF™ purified anti-mouse CD3 and CD28 (Biolegend, San Diego, CA, USA) at 4°C overnight. FACS-sorted splenic CD8+ cells from diabetic mice were labelled with 5 μm CFSE (Sigma) for 5 min, washed and divided into 100 μl (2 × 105 cells) per well. FACS-sorted splenic Gr1+ CD11b+ cells (MDSC) from STZ-treated mice were collected and added into CD8+ cells (100 μl, 2 × 105 cells per well). The cells were cultured in a humidified atmosphere with 5% CO2 at 37°C for 96 hr. Then, 50 μl of supernatant was collected for interferon-γ ELISA (eBioscience). Cells were collected and analysed by FACS. Proliferation Indices were analysed using modfit lt software (Verity Software House, Topsham, ME, USA).
Quantitative RT-PCR
RNA was extracted using Rneasy mini Kit (Qiagen, Hilden, Germany) and reverse transcribed to cDNA using an RT Kit (Invitrogen, Carlsbad, CA, USA). Quantitative PCR analyses were performed using the SYBR Green PCR Master Mix (Takara, Shiga, Japan). The relative RNA levels of each gene were normalized against β-actin. Primers used were: Arg1: 5′-TAC AAG ACA GGG CTC CTT TCA G-3′ and 5′-CCG TTG AGT TCC GAA GCA AG-3′. iNOS: 5′-TCC TGG ACA TTA CGA CCC CT-3′ and 5′-CTC TGA GGG CTG ACA CAA GG-3′. Il-10: 5′-GGT TGC CAA GCC TTA TCG GA-3′ and 5′-GGG GAG AAA TCG ATG ACA GC-3′. β-actin: 5′-TGA AGA TCA AGA TCA TTG CTC CTC-3′ and 5′-CCT GCT TGC TGA TCC ACA TC-3′.
Statistical analysis
All values are expressed as mean ± SE of the mean. Differences were analysed using Student’s t-test and P < 0·05 was considered statically significant. The severity of the insulitis was analysed by Wilcoxon signed rank test.
Results
Il-17 deficiency attenuates STZ-induced diabetes
To understand the function of IL-17 in diabetes, we established multiple low-dose STZ models of Il-17−/− mice and the blood glucose levels were monitored (Fig.1a). Both Il-17−/− and wild-type mice showed comparably increased glucose levels after STZ injection for 15 days. Wild-type control mice maintained a hyperglycaemic state (> 15 mm) from Day 18 to Day 35 before being killed (19·0 ± 3 mm). In contrast, Il-17−/− mice treated with STZ exhibited dramatically lower glucose levels from Day 18 to Day 35 before being killed (12·7 ± 2 mm).
Figure 1.
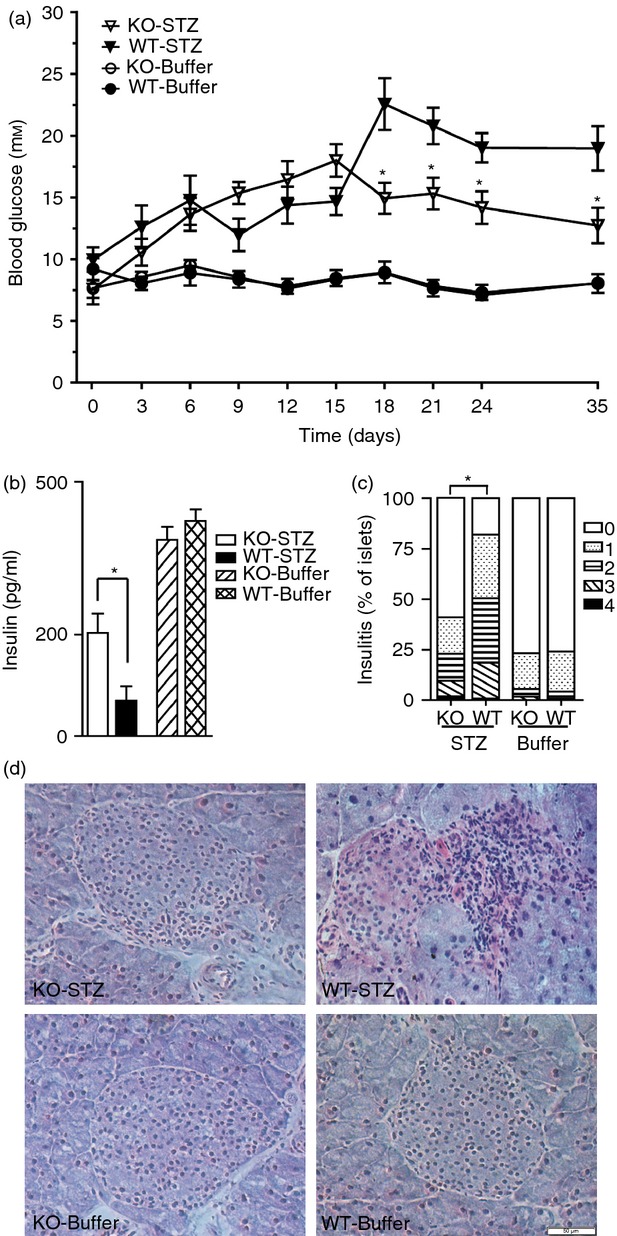
Il-17 deficiency attenuated streptozotocin (STZ) -induced diabetes. Multiple low dose of STZ were injected into Il-17−/− (KO) and wild-type (WT) mice; citrate buffer was injected as control. (a) Blood glucose levels were measured before the first injection (Day 0) and after the final injection from Days 1 to 35. (b) The serum insulin levels of four group mice were determined on Day 35. Each group n = 10. Student’s t-tests were performed between the STZ-treated groups. (c) The severity of insulitis was scored as described in the Materials and methods section. Levels of insulitis: 0 (white), 1 (dotted), 2 (horizontal stripes), 3 (diagonal stripes), and 4 (black). Wilcoxon signed rank test analysis was performed to analysis the insulitis between the STZ-treated groups. *P < 0·05. (d) Haematoxylin & eosin staining of pancreatic sections of four group mice. Images are representative of major islets in each group. Bar = 50 μm.
In agreement with the blood glucose changes, Il-17−/− mice had greatly increased levels of serum insulin compared with wild-type mice on Day 35 after STZ treatment (Il-17−/−: 203 ± 31 pg/ml versus wild-type: 70 ± 11 pg/ml) (Fig.1b). We then examined the impact of Il-17 knockout on the progression of insulitis. Wild-type mice showed dramatically increased leucocyte infiltration into the islets after STZ treatment. In contrast, Il-17−/− mice exhibited moderate insulitis after STZ treatment (Fig.1c,d). Together, Il-17−/− mice showed attenuated multiple low doses of STZ-induced diabetes.
Il-17 deficiency inhibits CD8+ T-cell infiltration into pancreas after STZ treatment
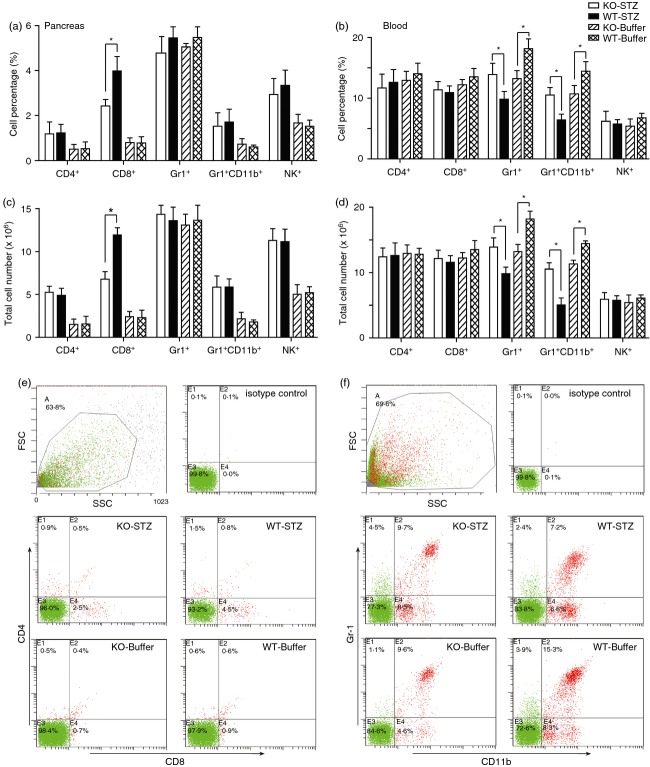
To characterize the inflammatory cellular response induced by STZ treatment in Il-17−/− mice, we quantified several immune cell subsets in the pancreas. Both Il-17−/− and wild-type mice showed increased percentages and numbers of CD4+, CD8+, Gr-1+ CD11b+ and NK+ cells on Day 24 after STZ injection (Fig.2a and c). However, Il-17−/− mice had significantly lower percentages and numbers of CD8+ cells than wild-type mice after STZ treatment (Fig.2a, c and e). We further quantified the immune cell subsets in peripheral blood. The Il-17−/− and wild-type mice had similar percentages and numbers of CD4+, CD8+ and NK+ cells in peripheral blood (Fig.2b and d). Il-17−/− mice showed lower percentages and lower numbers of Gr1+ cells and Gr1+ CD11b+ (MDSC) in peripheral blood than wild-type mice. After STZ injection, while Il-17−/− mice maintained the levels of Gr1+ cells and MDSC, wild-type mice had dramatically decreased Gr1+ cells and MDSC. In outcome, Il-17−/− mice exhibited even higher percentages and numbers of Gr1+ cells and MDSC than wild-type controls after STZ treatment (Fig.2b, d and f). Together with other reports,6 we speculated that IL-17 may be more involved in affecting immune responses in the spleen than in the lesion tissue.
Figure 2.
Il-17 deficiency inhibited CD8+ cell infiltration into pancreas after streptozotocin (STZ) treatment. FACS analysis results of pancreatic cells (a and c) and peripheral blood cells (b and d) after CD4, CD8, Gr-1, CD11b, or NK1.1 staining. Single pancreatic cells and peripheral blood cells were prepared from Il-17−/− and wild-type mice on Day 24 after STZ or buffer injections. Each group consisted of at least three mice. *P < 0·05. Representative plots from FACS analysis of pancreatic cells (e) and peripheral blood cells (f) are shown.
Il-17 deficiency affects CD8+ cell and MDSC percentages in spleens after STZ treatment
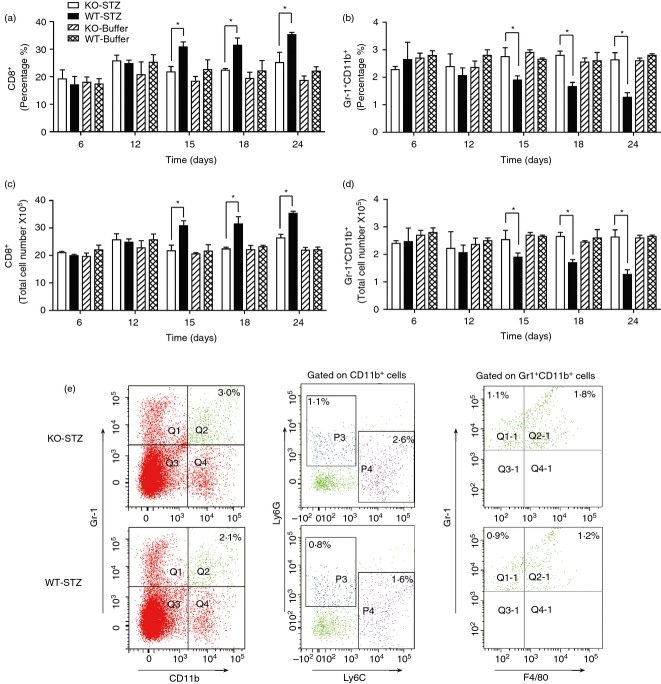
The four groups of mice (Il-17−/− mice treated with/without STZ, wild-type mice with/without STZ) had no significant differences in the percentages of CD4+, Gr-1+ and NK+ cells in the spleen (see Supplementary material, Fig. S1). Mice treated with STZ showed increased percentages of CD4+ Foxp3+ (regulatory T) cells after STZ treatment from Day 12, but no significant difference between Il-17−/− and wild-type groups (see Supplementary material, Fig. S1). Notably, wild-type mice exhibited gradually increased percentages and numbers of CD8+ cells from Day 15 after STZ injection, which was consistent with published literature on the important roles of CD8+ T cells in the STZ model.4 At the same time, Il-17−/− mice treated with STZ maintained the percentages and numbers of CD8+ cells at the basal levels found in control mice treated with buffer (Fig.3a and c). On the other hand, wild-type mice showed gradually decreased percentages and numbers of Gr1+ CD11b+ (MDSC) from Day 15 after STZ injection, and Il-17−/− mice treated with STZ maintained the percentages and numbers of MDSC at the basal levels of the control mice treated with buffer (Fig.3b and d). Hence, Il-17−/− mice exhibited lower percentages and numbers of CD8+ cells and higher MDSC than wild-type mice after STZ treatment. These data suggest that IL-17 is implicated in the regulation of splenic CD8+ cells and MDSC during STZ-induced diabetes development.
Figure 3.
Il-17 deficiency affected numbers of CD8+ cells and myeloid-derived suppressor cells (MDSC) in spleen after streptozotocin (STZ) treatment. FACS analysis results of splenocytes after CD8 (a and c), Gr-1 and CD11b (b and d) staining. Single splenic cells were prepared from Il-17−/− and wild-type mice at various time-points after STZ or buffer injection. (e) Representative plots from FACS analysis of the splenic Gr-1+ CD11b+ cells with Ly6C, Ly6G and F4/80. Splenic cells were prepared from Il-17−/− and wild-type mice on Day 15 after STZ injections. Each group consisted of at least three individual mice. *P < 0·05.
To further characterize the splenic MDSC from mice treated with STZ, we examined these cells with Ly6G, Ly6C and F4/80 staining. Splenic MDSC from both Il-17−/− and wild-type mice had Ly6G+ and Ly6C+ subsets with Ly6C+ cells as a major cell type (Fig.3e). Hence, CD11b+ Ly6C+ cells contributed mainly to the increased MDSC in Il-17−/− mice on Day 15 after STZ treatment. At the same time, there was no significant difference in the percentages of F4/80+ cells in the splenic MDSC between Il-17−/− and wild-type mice (61·5 ± 3·2% versus 58·4 ± 2·5%) (Fig.3e).
Il-17-deficient MDSC ameliorated STZ-induced hyperglycaemia
To characterize the roles of these MDSC, we purified Gr1+ CD11b+ cells from the spleens of Il-17−/− and wild-type mice on Day 24 after STZ treatment. As shown in Fig.4(a), MDSC from STZ-treated Il-17−/− mice expressed dramatically higher levels of Il-10, and similar levels of arg1 and iNOS compared with those in wild-type mice. We further co-cultured splenic CD8+ cells with the purified MDSC in vitro. The proliferation indices of CD8+ cells stimulated by CD3 and CD28 antibodies reached 8·43 ± 0·21. Whereas the proliferation indices of CD8+ cells decreased to 4·88 ± 0·39 after wild-type MDSC were added, they decreased even lower to 4·46 ± 0·31 after Il-17−/− MDSC were added (Fig.4b, and see Supplementary material, Fig. S2). At the same time, the interferon-γ production by CD8+ cells showed a slight decrease after MDSC addition, but no significant difference was found among the groups (Fig.4c). Next, we transferred the isolated MDSC into STZ-treated wild-type mice on Day 13 (Fig.4d). Both groups (recipients of Il-17−/− MDSC, recipients of wild-type MDSC) showed increased blood glucose levels. However, the recipients of Il-17−/− MDSC had significantly lower blood glucose levels than recipients of wild-type MDSC (Fig.4d). We further analysed the spleens of recipients on Day 24 after STZ treatment. The recipients of Il-17−/− MDSC exhibited increased expression levels of iNOS and Il-10 (Fig.4e) and a significantly lower percentage of CD8+ cells than recipients of wild-type MDSC (Fig4f). These data indicated that Il-17−/− MDSC could ameliorate STZ-induced hyperglycaemia with increased Il-10 expression and suppressed CD8+ cells involved.
Figure 4.
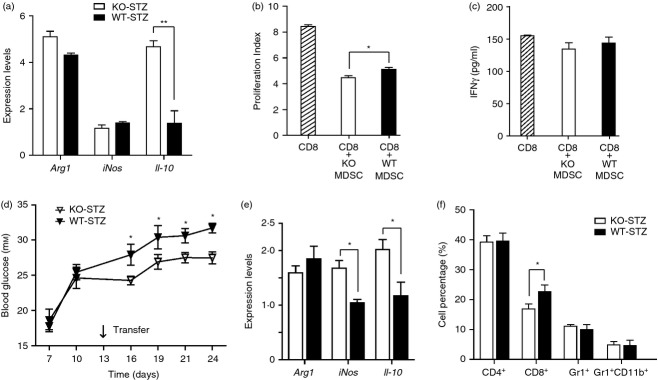
Il-17-deficient myeloid-derived suppressor cells (MDSC) ameliorated streptozotocin (STZ) -induced hyperglycaemia. (a) Quantitative RT-PCR analysis of the MDSC purified from spleens of Il-17−/− and wild-type mice on Day 24 after STZ treatment. Each group n = 3. (b) The proliferation indices of CD8 cells co-cultured with MDSC purified from spleens of Il-17−/− or wild-type mice on Day 24 after STZ treatment. (c) The concentrations of interferon-γ (IFN-γ) in the co-culture medium. Each group n = 3. (d) Blood glucose levels of the recipient mice were measured after final STZ injection from Days 7 to 24. On Day 13, the wild-type mice received MDSC (2 × 105/mouse) from Il-17−/− or wild-type mice treated with STZ. Each group n = 6. (e) Quantitative RT-PCR analysis of spleens from the recipients on Day 24. Each group n = 3. (f) FACS analysis results of splenocytes from the recipients on Day 24. Each group n = 3. *P < 0·05.
Discussion
Our current study indicates that IL-17 plays a pathogenic role in STZ-induced diabetes. Il-17−/− mice exhibited attenuated hyperglycaemia after Day 18 but not before Day 15 after STZ treatment, suggesting that IL-17 could be more critical in the late phase of STZ induced diabetes development rather than the onset. In supporting this statement, IL-17R knockout mice also had decreased levels of blood glucose and islet inflammation in the STZ model.4 However, IL-17 was reported to have different roles in NOD mouse models.6–9 The explanation could be that the NOD model is more complicated, developing spontaneous autoimmune diabetes with the various IL-17-producing T subset cells involved: Th17/Tc17,4 invariant natural killer T,5,20 γδT.9
Our findings indicate that IL-17 deficiency inhibits the increase of pancreatic and splenic CD8+ cells after STZ injection. The pancreatic infiltration of T cells is a critical process during the development of diabetes induced by multiple low doses of STZ.21 Moreover, the splenic immune response including CD8+ cells is also important in diabetes,22 and the administration of anti-CD8 antibodies resulted in a protective effect.23 In our study, the time-point (from Day 15 after STZ injection) of changed splenic CD8+ cell percentages was the same as that of the shift in blood glucose in Il-17−/− mice treated with STZ, suggesting that the decreased splenic CD8+ cells of Il-17−/− mice may be implicated in diabetes resistance. These data suggested that IL-17 plays important roles in both pancreatic and splenic immune responses during diabetes.
Our study provides evidence that IL-17 plays a role in STZ-induced changes of splenic MDSC. Under normal conditions, IL-17 deficiency alone is not sufficient to change the splenic MDSC (Fig.3). When STZ is administrated, auto-antigens are released from islets and the splenic MDSC percentage decreases. IL-17 deficiency could completely block this decrease, suggesting that IL-17 has an important role during this process. At the same time, IL-17 deficiency could affect the splenic MDSC function after STZ treatment. Il-17−/− MDSC showed increased ability to suppress CD8+ cell proliferation in vitro and a protective role after adoptive transfer in diabetic mice (Fig.4). These results were inconsistent with the fact that IL-17 is important for MDSC function in the tumour sites24,25 and infection.26,27 Further studies are needed, and our study represents a first step towards a better understanding of the connections of IL-17 and MDSC in diabetes.
In summary, our study revealed a pathogenic role of IL-17 in STZ-induced diabetes and the IL-17-dependent splenic immune response involved. Future study of the molecular signalling will shed new light on the systemic understanding of IL-17 function in diabetes.
Acknowledgments
This work was supported by a grant from National Natural Science Foundation of China to Zan Tong (No. 81301792). We thank Dr Zhinan Yin for kindly providing the Il-17−/− mice with Dr Yoichiro Iwakura’s permission. We also thank Dr Yu Wan for technical assistance during the experiments. Zan Tong designed the study, Zan Tong and Chen Dong wrote the paper, Weihuang Liu performed the FACS analysis. Huichao Yan established the mouse model.
Glossary
- FACS
fluorescence activated cell sorting
- MDSC
myeloid derived suppressor cells
- NOD
nonobese diabetic
- STZ
streptozotocin
Disclosures
The authors declare no commercial or financial conflict of interest.
Supporting Information
Figure S1. Il-17 deficiency did not affect CD4+, Foxp3+ and natural killer (NK) cell percentages in spleen after streptozotocin (STZ) treatment. FACS analysis results of splenocytes after CD4 (a), Foxp3 (b), Gr-1 (c) and NK (d) staining. Single splenic cells were prepared from Il-17−/− and wild-type mice at various time-points after STZ or buffer injection. Each group consisted of at least three individual mice. *P < 0·05.
Figure S2. Il-17 deficiency promoted the ability of myeloid-derived suppressor cells (MDSC) to suppress CD8+ cell proliferation in vitro. Representative FACS analysis data of CFSE-labelled CD8+ cells co-cultured with MDSC purified from spleens of Il-17−/− or wild-type mice on Day 24 after streptozotocin (STZ) treatment.
References
- Hemdan NY, Birkenmeier G, Wichmann G, Abu El-Saad AM, Krieger T, Conrad K, Sack U. Interleukin-17-producing T helper cells in autoimmunity. Autoimmun Rev. 2010;9:785–92. doi: 10.1016/j.autrev.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Reynolds JM, Angkasekwinai P, Dong C. IL-17 family member cytokines: regulation and function in innate immunity. Cytokine Growth Factor Rev. 2010;21:413–23. doi: 10.1016/j.cytogfr.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankathatti Munegowda M, Deng Y, Chibbar R, et al. A distinct role of CD4+ Th17- and Th17-stimulated CD8+ CTL in the pathogenesis of type 1 diabetes and experimental autoimmune encephalomyelitis. J Clin Immunol. 2011;31:811–26. doi: 10.1007/s10875-011-9549-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaochite JN, Caliari-Oliveira C, Davanso MR, et al. Dynamic changes of the Th17/Tc17 and regulatory T cell populations interfere in the experimental autoimmune diabetes pathogenesis. Immunobiology. 2013;218:338–52. doi: 10.1016/j.imbio.2012.05.010. [DOI] [PubMed] [Google Scholar]
- Li S, Joseph C, Becourt C, et al. Potential Role of IL-17-Producing iNKT Cells in Type 1 Diabetes. PLoS One. 2014;9:e96151. doi: 10.1371/journal.pone.0096151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emamaullee JA, Davis J, Merani S, Toso C, Elliott JF, Thiesen A, Shapiro AM. Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice. Diabetes. 2009;58:1302–11. doi: 10.2337/db08-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriya G, Uchida T, Akazawa S, et al. Double deficiency in IL-17 and IFN-γ signalling significantly suppresses the development of diabetes in the NOD mouse. Diabetologia. 2013;56:1773–80. doi: 10.1007/s00125-013-2935-8. [DOI] [PubMed] [Google Scholar]
- Joseph J, Bittner S, Kaiser FM, Wiendl H, Kissler S. IL-17 silencing does not protect nonobese diabetic mice from autoimmune diabetes. J Immunol. 2012;188:216–21. doi: 10.4049/jimmunol.1101215. [DOI] [PubMed] [Google Scholar]
- Han G, Wang R, Chen G, et al. Interleukin-17-producing γδ+ T cells protect NOD mice from type 1 diabetes through a mechanism involving transforming growth factor-β. Immunology. 2010;129:197–206. doi: 10.1111/j.1365-2567.2009.03166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Belle TL, Esplugues E, Liao J, Juntti T, Flavell RA, von Herrath MG. Development of autoimmune diabetes in the absence of detectable IL-17A in a CD8-driven virally induced model. J Immunol. 2011;187:2915–22. doi: 10.4049/jimmunol.1000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miljkovic D, Cvetkovic I, Momcilovic M, Maksimovic-Ivanic D, Stosic-Grujicic S, Trajkovic V. Interleukin-17 stimulates inducible nitric oxide synthase-dependent toxicity in mouse beta cells. Cell Mol Life Sci. 2005;62:2658–68. doi: 10.1007/s00018-005-5259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trikha P, Carson WE., 3rd Signaling pathways involved in MDSC regulation. Biochim Biophys Acta. 2014;1846:55–65. doi: 10.1016/j.bbcan.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Montero CM, Finke J, Montero AJ. Myeloid-derived suppressor cells in cancer: therapeutic, predictive, and prognostic implications. Semin Oncol. 2014;41:174–84. doi: 10.1053/j.seminoncol.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin B, Ma G, Yen CY, et al. Myeloid-derived suppressor cells prevent type 1 diabetes in murine models. J Immunol. 2010;185:5828–34. doi: 10.4049/jimmunol.0903636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Liu H, He B, Fu Z. Resistance to streptozotocin-induced autoimmune diabetes in absence of complement C3: myeloid-derived suppressor cells play a role. PLoS One. 2013;8:e66334. doi: 10.1371/journal.pone.0066334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He D, Li H, Yusuf N, Elmets CA, Li J, Mountz JD, Xu H. IL-17 promotes tumor development through the induction of tumor promoting microenvironments at tumor sites and myeloid-derived suppressor cells. J Immunol. 2010;184:2281–8. doi: 10.4049/jimmunol.0902574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Zhang B, Li D, Lv M, Huang C, Shen GX, Huang B. Mast cells mobilize myeloid-derived suppressor cells and Treg cells in tumor microenvironment via IL-17 pathway in murine hepatocarcinoma model. PLoS One. 2010;5:e8922. doi: 10.1371/journal.pone.0008922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae S, Komiyama Y, Nambu A, et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–87. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- Tong Z, Liu W. IgG-positive cells surround pancreatic ducts and form multiple layers after streptozotocin treatment. Autoimmunity. 2013;46:369–74. doi: 10.3109/08916934.2013.773977. [DOI] [PubMed] [Google Scholar]
- Simoni Y, Gautron AS, Beaudoin L, et al. NOD mice contain an elevated frequency of iNKT17 cells that exacerbate diabetes. Eur J Immunol. 2011;41:3574–85. doi: 10.1002/eji.201141751. [DOI] [PubMed] [Google Scholar]
- O’Brien BA, Harmon BV, Cameron DP, Allan DJ. Beta-cell apoptosis is responsible for the development of IDDM in the multiple low-dose streptozotocin model. J Pathol. 1996;178:176–81. doi: 10.1002/(SICI)1096-9896(199602)178:2<176::AID-PATH433>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Satoh J, Takahashi K, et al. Complete suppression of insulitis and diabetes in NOD mice lacking interferon regulatory factor-1. J Autoimmun. 2001;17:119–25. doi: 10.1006/jaut.2001.0531. [DOI] [PubMed] [Google Scholar]
- Harlan DM, Barnett MA, Abe R, Pechhold K, Patterson NB, Gray GS, June CH. Very-low-dose streptozotocin induces diabetes in insulin promoter-mB7-1 transgenic mice. Diabetes. 1995;44:816–23. doi: 10.2337/diab.44.7.816. [DOI] [PubMed] [Google Scholar]
- Wu P, Wu D, Ni C, et al. γδT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity. 2014;40:785–800. doi: 10.1016/j.immuni.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa T, Shibata M, Gonda K, et al. Increased IL-17 production correlates with immunosuppression involving myeloid-derived suppressor cells and nutritional impairment in patients with various gastrointestinal cancers. Mol Clin Oncol. 2013;1:675–9. doi: 10.3892/mco.2013.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obregon-Henao A, Henao-Tamayo M, Orme IM, Ordway DJ. Gr1intCD11b+ myeloid-derived suppressor cells in Mycobacterium tuberculosis infection. PLoS One. 2013;8:e80669. doi: 10.1371/journal.pone.0080669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X, Sun R, Chen Y, Wei H, Tian Z. γδT cells drive myeloid-derived suppressor cell-mediated CD8+ T cell exhaustion in hepatitis B virus-induced immunotolerance. J Immunol. 2014;193:1645–53. doi: 10.4049/jimmunol.1303432. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Il-17 deficiency did not affect CD4+, Foxp3+ and natural killer (NK) cell percentages in spleen after streptozotocin (STZ) treatment. FACS analysis results of splenocytes after CD4 (a), Foxp3 (b), Gr-1 (c) and NK (d) staining. Single splenic cells were prepared from Il-17−/− and wild-type mice at various time-points after STZ or buffer injection. Each group consisted of at least three individual mice. *P < 0·05.
Figure S2. Il-17 deficiency promoted the ability of myeloid-derived suppressor cells (MDSC) to suppress CD8+ cell proliferation in vitro. Representative FACS analysis data of CFSE-labelled CD8+ cells co-cultured with MDSC purified from spleens of Il-17−/− or wild-type mice on Day 24 after streptozotocin (STZ) treatment.