Abstract
Cancer immunotherapies such as sipuleucel-T and ipilimumab are promising new treatments that harness the power of the immune system to fight cancer and achieve long-lasting remission. Interleukin (IL)-27, a member of the IL-12 heterodimeric cytokine family, has pleiotropic functions in the regulation of immune responses with both pro-inflammatory and anti-inflammatory properties. Evidence obtained using a variety of preclinical mouse models indicates that IL-27 possesses potent antitumor activity against various types of tumors through multiple mechanisms without apparent adverse effects. These mechanisms include those mediated not only by CD8+ T cells, natural killer cells and macrophages, but also by antibody-dependent cell-mediated cytotoxicity, antiangiogenesis, direct antiproliferative effects, inhibition of expression of cyclooxygenase-2 and prostaglandin E2, and suppression of epithelial–mesenchymal transition, depending on the characteristics of individual tumors. However, the endogenous role of IL-27 subunits and one of its receptor subunits, WSX-1, in the susceptibility to tumor development after transplantation of tumor cell lines or endogenously arising tumors seems to be more complicated. IL-27 functions as a double-edged sword: IL-27 increases IL-10 production and the expression of programmed death ligand 1 and T-cell immunoglobulin and mucin domain-3, and promotes the generation of regulatory T cells, and IL-27 receptor α singling enhances transformation; IL-27 may augment protumor effects as well. Here, we review both facets of IL-27, antitumor effects and protumor effects, and discuss the potential clinical application of IL-27 as an antitumor agent.
Keywords: IL-27, antitumor effects, protumor effects, IL-10, Treg
Cancer Immunotherapy
Cancer immunotherapy has recently come into the spotlight as a promising new treatment that harnesses the power of the immune system; sipuleucel-T and ipilimumab were approved by the US Food and Drug Administration for patients with cancer in 2010 and 2011, respectively.1,2 Sipuleucel-T is a therapeutic vaccine using dendritic cells (DC) stimulated by a tumor-specific antigen combined with granulocyte-macrophage colony-stimulating factor.1 Ipilimumab is an inhibitory monoclonal antibody specific to one of the immune checkpoints, cytotoxic T-lymphocyte antigen 4.2 Currently, various therapeutic strategies to overcome tumor immune evasion and enhance antitumor immunity are also undergoing investigation.
Interleukin-12 Cytokine Family
The interleukin (IL)-12 cytokine family is unique in that it is a heterodimeric cytokine composed of two different subunits, and one subunit is often shared by another cytokine in the same family.3,4 These member cytokines are mainly produced by antigen-presenting cells such as DC and play critical roles in the regulation of differentiation into respective helper T (Th) cells and their functions. IL-12 is composed of p35 and p40 subunits and induces the differentiation of naive CD4+ T cells into Th1 cells to enhance the cellular immunity by augmentation of interferon (IFN)-γ production from natural killer (NK) cells and T cells and generation of cytotoxic T lymphocytes (CTL).3 The p40 subunit is also covalently bound with an IL-12 p35-related protein, p19, to form IL-23, which plays an important role in developing tissue-specific autoimmune diseases and inflammatory diseases by enhancing IL-17 production and maintaining pathogenic Th17 cells.4 IL-27 consists of an IL-12 p35-related protein, p28, which is also called IL-30, and an IL-12 p40-related protein, Epstein–Barr virus-induced gene 3 (EBI3)4–7 (Fig.1). IL-27 receptor (R) is composed of IL-27Rα (WSX-1/TCCR) and glycoprotein (gp) 130, a common receptor subunit for the IL-6 family of cytokines. EBI3 was previously reported to associate with p35 to form heterodimeric molecule EBI3/p35, and EBI3/p35 was recently found to be produced by regulatory T (Treg) cells and to play a suppressive role in the Treg cells; therefore, it is named IL-35.
Figure 1.
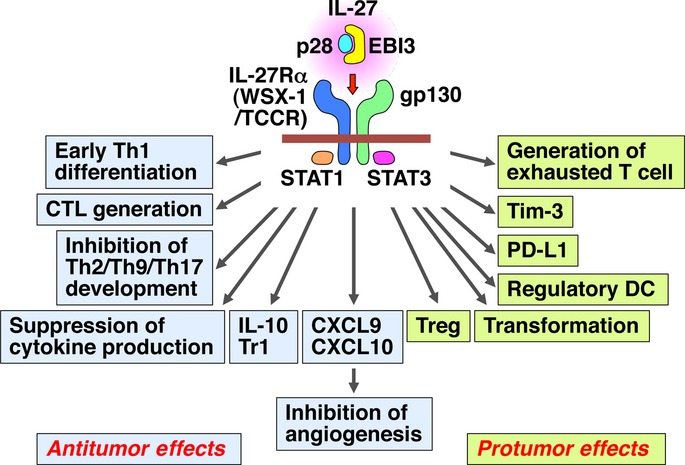
Antitumor and protumor effects of IL-27 depending on the type of cells that IL-27 stimulates and the tumor context. CTL, cytotoxic T lymphocyte; DC, dendritic cell; EBI3, Epstein–Barr virus-induced gene 3; gp, glycoprotein; IFN, interferon; IL, interleukin; NK, natural killer; PD-L1, programmed death ligand 1; R. receptor; STAT, signal transducer and activator of transcription; Th, helper T; Tim-3, T-cell immunoglobulin and mucin domain-3; Tr1, IL-10-producing regulatory T; Treg, regulatory T.
Pro-Inflammatory and Anti-Inflammatory Properties of Interleukin-27
Interleukin-27 is one of the pleiotropic cytokines with both pro-inflammatory and anti-inflammatory properties acting on various types of cells, such as T cells, B cells, macrophages and DC, depending on the context (Fig.1).4–6 IL-27 efficiently activates both signal transducer and activator of transcription (STAT) 1 and STAT3, which bind to distinct IL-27R subunits WSX-1 and gp130, respectively. IL-27 promotes the early induction of Th1 differentiation and generation of CTL,8,9 but it inhibits the differentiation of naive CD4+ T cells into Th2 cells, Th9 cells and Th17 cells, and suppresses the production of pro-inflammatory cytokines, leading to amelioration of allergic diseases and autoimmune diseases. In addition, IL-27 augments IL-10 production from these Th cells and generation of IL-10-producing regulatory (Tr1) T cells,10–12 whereas the role of IL-27 in induction of Treg cells is still controversial. IL-27 was initially shown to suppress the differentiation of inducible Treg cells from naive CD4+ T cells,13–15 and transfer of TCCR-deficient CD4+CD45RBhi cells in a colitis model was demonstrated to reduce development of colitis with an increased percentage of Treg cells in the gut.16 Contrary to the antagonistic effects of IL-27 on Treg cells, recent reports have paradoxically revealed that IL-27 does not inhibit the generation of Treg cells, but rather promotes it and produces a distinct Treg cell population expressing T-cell-specific T-box transcription factor (T-bet) and CXCR3 to control Th1-mediated immunity at the local sites of inflammation.17 Moreover, IL-27 was recently demonstrated to exert immunosuppressive functions via induction of the programmed death ligand 1 (PD-L1) on CD4+ T cells and DC.18–20 IL-27-mediated induction of PD-L1 inhibits Th17 cell differentiation and reduces pathology in experimental autoimmune encephalomyelitis.20
Therapeutic Potential of Interleukin-27 as an Antitumor Agent
Antitumor effects of interleukin-27 against transplanted tumors genetically engineered to secrete interleukin-27
The antitumor efficacy of IL-27 was first demonstrated in 2004 using a transplanted mouse tumor genetically engineered to secrete IL-27 before transplantation as a preclinical tumor model.21 Since then, accumulating evidence has revealed that IL-27 possesses potent antitumor activity against a variety of tumor models through multiple mechanisms without apparent adverse effects.21,22 The mechanisms are those mediated by CD8+ T cells,21,23–30 NK cells22,24,25,31 and macrophages,32 as well as antibody-dependent cell-mediated cytotoxicity (ADCC),33 anti-angiogenesis,34–40 direct antiproliferative effects,36,41–43 inhibition of expression of cyclooxygenase-2 (COX-2) and prostaglandin E2 (PGE2),44 and suppression of epithelial–mesenchymal transition (EMT),32,39 depending on the characteristics of individual tumors (Fig.2 and Table1). Transfection of highly immunogenic tumors such as mouse colon carcinoma (colon 26) with IL-27 expression vector greatly reduced tumor growth, which was mainly mediated by CD8+ T cells.21 IL-27 plays a particularly important role in the generation of CTL by enhancing the survival of tumor-specific CD8+ T cells and the differentiation into effector and memory cells.8,9 In contrast, the antitumor effects of IL-27 against poorly immunogenic tumors such as mouse melanoma B16F10 were mediated by multiple mechanisms via angiogenesis,34 NK cells,22,25,31 and its direct antiproliferative effects on tumors.32,39,41,44 IL-27 stimulated human umbilical vein endothelial cells and induced production of anti-angiogenic chemokines such as CXCR9 and CXCR10 to elicit strong anti-angiogenic activity against melanomas, which contributed to its antitumor and antimetastatic activities.34 In addition, IL-27 has potent direct antiproliferative activity on melanomas through WSX-1/STAT1 and, in part, through interferon regulatory factor (IRF)-1 and tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL).41,43 It also directly restrained tumorigenicity of mouse Lewis lung carcinoma LLC by suppressing expression of COX-2 and PGE2.44 Moreover, IL-27 activated NK cells and augmented tumor-specific immunoglobulin production, which cooperatively exerted ADCC activity against mouse head and neck squamous cell carcinoma SCCVII,33 and inhibited EMT and angiogenic factor production in a STAT1-dominant pathway in human non-small cell lung cancer.32,39 Similar suppression of tumor growth was observed with human tumors such as esophageal carcinoma45 and pancreatic carcinoma.42
Figure 2.
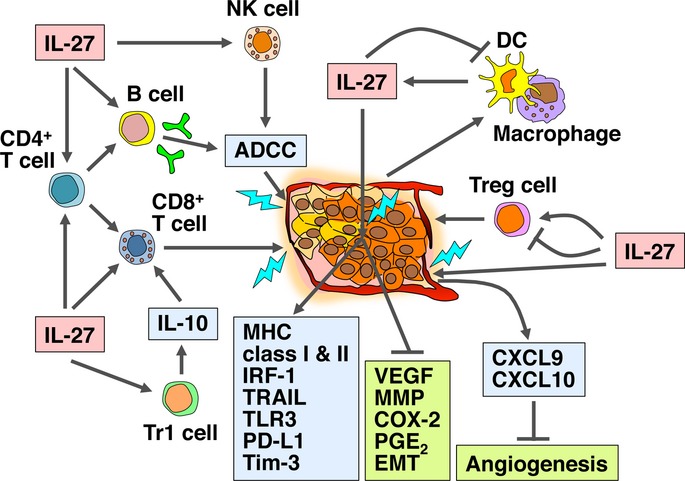
Potent antitumor activity of IL-27 through multiple mechanisms that are mediated by CD8+ T cells, NK cells, macrophages, macrophages, ADCC, anti-angiogenesis, direct anti-proliferative effect, inhibition of COX-2 and PGE2 expression, and suppression of EMT, depending on the characteristics of individual tumors. ADCC, antibody-dependent cell-mediated cytotoxicity; COX-2, cyclooxygenase-2; CTL, cytotoxic T lymphocyte; DC, dendritic cell; EBI3, Epstein-Barr virus–induced gene 3; EMT, epithelial–mesenchymal transition; IRF, interferon regulatory factor; IL, interleukin; MHC, major histocompatibility complex; MMP, matrix metalloproteinase; NK, natural killer; PGE2, prostaglandin E2; PD-L1, programmed death ligand 1; Tim-3, T-cell immunoglobulin and mucin domain-3; TLR, Toll-like receptor; Tr1, IL-10-producing regulatory T; TRAIL, tumor necrosis factor–related apoptosis-inducing ligand; Treg, regulatory T; VEGF, vascular endothelial growth factor.
Table 1.
Susceptibility of mice transplanted with tumors expressing IL-27 and mice injected with IL-27 to development of tumors
| Treatment | Susceptibility | Tumor | Reference |
|---|---|---|---|
| IL-27 transfectant | Reduced tumor growth in vivo by augmented antitumor CTL | Mouse colon carcinoma (Colon 26) | Hisada et al. 200421 |
| IL-27 transfectant | Reduced tumor growth in vivo by augmented antitumor CTL | Mouse orthotopic primary and metastatic neuroblastoma (TBJ) | Salcedo et al. 200423 |
| IL-27 transfectant | Reduced tumor growth in vivo by enhanced T-cell-dependent and NK-cell-dependent antitumor immunity | Mouse colon carcinoma (Colon 26) | Chiyo et al. 200424 |
| IL-27 transfectant | Reduced tumor growth in vivo by upregulation of CXCL9 and CXCL10 and inhibition of angiogenesis | Mouse melanoma (B16F10) lung metastasis | Shimizu et al. 200634 |
| IL-27 transfectant | Reduced tumor growth in vivo by NK-cell-mediated antitumor immunity independent of IFN-γ | Mouse melanoma (B16F10) | Oniki et al. 200622 |
| IL-27 transfectant | Reduced tumor growth in nude mice by NK cells through Fas/FasL pathway | Human esophageal carcinoma (Eca109) | Liu et al. 200845 |
| In vitro treatment | Reduced tumor growth in vitro by direct antiproliferative activity through WSX-1/STAT1/IRF-1 signaling | Mouse melanoma (B16F10) Human melanoma (SK-MEL-13, 28, 37) | Yoshimoto et al. 200841 |
| IL-27 transfectant | Reduced tumor growth in nude mice by augmented IFN-γ production and NK activity | Human esophageal carcinoma (Eca109) | Liu et al. 200831 |
| IL-27 transfectant | Reduced tumor growth in vivo by NK cell–mediated ADCC | Mouse head and neck squamous cell carcinoma (SCCVII) | Matsui et al. 200933 |
| IL-27 transfectant | Reduced tumor growth in vivo by CD8+ and CD4+ T cells, IFN-γ and NK cells | Mouse hepatocellular carcinoma (MM45T.Li) | Hu et al. 200925 |
| IL-27 plasmid injection | Therapeutic synergy of hydrodynamic injection of IL-27 plasmid and IL-2 by augmented generation of tumor-specific CTLs but suppressed expansion of Treg cells | Mouse disseminated metastatic neuroblastoma (TBJ) in liver | Salcedo et al. 200926 |
| IL-27 transfectant | Restraint of tumorigenicity in vivo by suppressing the expression of COX-2 and PGE2 | Mouse Lewis lung carcinoma (LLC) | Ho et al. 200944 |
| IL-27 plasmid injection | Elimination of distal aggressive tumor by IL-12 and IL-27 sequential gene therapy via intramuscular electroporation through T and NK cells | Mouse colon carcinoma (Colon 26) Mouse adenocarcinoma (4T1) | Zhu et al. 201027 |
| IL-27 protein injection | Damped tumorigenicity of human multiple myeloma cell lines in NOD/SCID mice through inhibition of angiogenesis | Human multiple myeloma (NCI-H929 and U266) | Cocco et al. 201035 |
| IL-27 protein injection | Inhibition of leukemia cell spreading in NOD/SCID/IL-2Rγ−/− mice through suppression of proliferation, angiogenesis, miR-155 expression and induction of apoptosis | Human B-ALL | Canale et al. 201136 |
| IL-27 gene therapy | Inhibition of tumor growth by sonoporation delivery of IL-27 gene therapy through enhanced accumulation of effector cells | Mouse prostate cancer (TCR2) | Zolochevska et al. 201128 |
| IL-27 protein injection | Inhibition of tumor growth in NOD/SCID mice through suppressed angiogenesis | Human follicular lymphoma and diffuse large B-cell lymphoma (SU-DHL-4) | Cocco et al. 201237 |
| IL-27 transfectant by retroviral infection | Inhibition of tumor growth in nude mice through induction of apoptosis and cell-cycle arrest | Human pancreatic carcinoma (AsPC1) | Liu et al. 201242 |
| IL-27 protein injection | Inhibition of tumor growth in NOD/SCID/IL-2Rγ−/− mice through suppression of angiogenesis and spreading related genes | Human AML | Zorzoli et al. 201238 |
| IL-27 transfectant | Enhanced antitumor CTL responses in vivo via programming tumor antigen-specific CD8+ T cells into IL-10-producing memory precursor-like effector cells characterized by a greater survival advantage | Mouse plasmacytoma (J558) | Liu et al. 201329 |
| IL-27 transfectant vaccine | T-cell-mediated antitumor effects by tumor vaccine engineered to secrete IL-27 by means of cationic liposome | Mouse Lewis lung carcinoma (LLC) | Zhang et al. 201330 |
| IL-27 protein injection | Inhibition of tumor growth in NOD/SCID mice by upregulation of TRAIL and TLR3 in cooperation with a TLR3 agonist poly(I:C) in a partly TRAIL-dependent manner | Human melanoma (SK-ME-13, 28, 37) | Chiba et al. 201343 |
| In vitro treatment | Inhibition of EMT and angiogenic factor production in a STAT1-dominant pathway | Human non-small cell lung cancer | Kachroo et al. 201339 |
| IL-27 protein injection | Inhibition of tumor growth in athymic nude mice through reduced proliferation and vascularization by downregulation of pro-angiogenesis-related genes (FLT1, PTGS1/COX-1, FGFR3) and upregulation of anti-angiogenesis-related genes (CXCL10, TIMP3) | Human prostate cancer (PC3 and DU145) | Di Carlo et al. 201440 |
| IL-27 protein injection | Inhibition of tumor growth in athymic nude and NOD/SCID mice through granulocyte-driven and macrophage-driven colliquative necrosis, CXCL3 production, and reduced pluripotency-related and EMT-related gene expression | Human non-small cell lung cancer, particularly adenocarcinoma (Calu-6) and squamous cell carcinoma (SK-MES) | Airoldi et al. 201532 |
ADCC, antibody dependent cell-mediated cytotoxicity; AML, acute myeloid leukemia; B-ALL, B-acute lymphoblastic leukemia; COX-2, cyclooxygenase-2; CTL, cytotoxic T lymphocyte; EBI3, Epstein–Barr virus-induced gene 3; EMT, epithelial–mesenchymal transition; FGFR, fibroblast growth factor receptor; FLT, fms-related tyrosine kinase; IFN, interferon; IRF, interferon regulatory factor; IL, interleukin; NK, natural killer; LLC, Lewis lung carcinoma; NOD/SCID, nonobese diabetic/severe combined immunodeficient; PGE2, prostaglandin E2; poly(I:C), polyinosinic-polycytidylic acid; PTGS1, prostaglandin G/H synthase 1; STAT, signal transducer and activator of transcription; TLR, Toll-like receptor; TRAIL, tumor necrosis factor–related apoptosis-inducing ligand; Treg, regulatory T; TIMP3, tissue inhibitor of metalloproteinase 3.
Antitumor effects of interleukin-27 protein injection against human tumors transplanted in immunodeficient mice
Moreover, IL-27 has demonstrated potent antitumor activity in a variety of human therapeutic models through injection of IL-27 protein into immunodeficient mice such as nude mice, nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice and NOD/SCID/IL-2Rγ−/− mice after transplantation of human tumors. The human tumors reported on so far are melanoma,43 multiple myeloma,35 B-acute lymphoblastic leukemia (B-ALL),36 follicular lymphoma,37 diffuse large B-cell lymphoma,37 acute myeloid leukemia (AML),38 prostate cancer40 and non-small cell lung cancer32,39 (Table1). IL-27 strongly inhibited in vitro tumor growth of human melanoma, multiple myeloma, follicular lymphoma and diffuse large B-cell lymphoma through suppression of angiogenesis and induction of apoptosis, and the tumorigenicity of these tumors transplanted in NOD/SCID mice was greatly hampered by IL-27.35,37,43 Similarly, IL-27 suppressed leukemic spreading of B-ALL cells and leukemia dissemination of AML cells transplanted in NOD/SCID/IL-2Rγ−/− because of significant reduction of angiogenic and spreading-related genes, including vascular endothelial growth factors, angiopoietins and matrix metalloproteinases (MMP), and also because of upregulation of angiostatic molecules, such as tissue inhibitor of MMP.36,38 Direct antiproliferative effects of IL-27 in collaboration with polyinosinic-polycytidylic acid [poly(I:C)], one of the Toll-like receptor 3 (TLR3) ligands whose expression was revealed to be upregulated by IL-27, was also observed in NOD/SCID mice transplanted with human melanoma.43 In addition, IL-27 was recently shown to inhibit tumor growth of human prostate cancers in athymic nude mice through reduced proliferation and vascularization by downregulation of pro-angiogenesis-related genes and upregulation of anti-angiogenesis-related genes.40 Inhibition of tumor growth of human non-small cell lung cancers was also demonstrated to be mediated by granulocyte-driven and macrophage-driven colliquative necrosis, CXCL3 production, and reduced pluripotency-related and EMT-related gene expression.32,39
Endogenous Role of IL-27 in the Susceptibility to Development of Tumors
Antitumor and protumor effects of endogenous WSX-1
To gain further insight into the antitumor effects of IL-27, it is important to clarify its endogenous role in the exertion of antitumor effects or protumor effects. Therefore, mice deficient in IL-27 subunits and receptor subunits were analyzed for susceptibility to tumor development (Table2). WSX-1-deficient mice overall showed more excessive tumor growth of melanoma B16 injected subcutaneously than did WT mice.46 However, this phenotype appears to be the sum of the effects of lacking WSX-1 in different immune responses, such as generation of CTL and antigen-presenting capacity of DC after maturation. Tumor-specific CTL generation was lower in WSX-1-deficient mice than in WT mice, and CTL induction in WSX-1-deficient mice was not restored by transfer of WT DC pulsed with tumor antigen, indicating that IL-27 is directly required for generation of tumor-specific CTL.46 In contrast, when transferred into tumor-bearing mice, WSX-1-deficient DC pulsed with tumor antigen were more potent than WT DC in the inhibition of tumor growth and generation of CTL, indicating the suppressive effects of IL-27 on DC function.46 It is also reported that WSX-1-deficient mice had reduced resistance to endogenously arising mouse tumor models, 3-methylcholanthrene (MCA)-induced fibrosarcoma and polyoma middle T antigen (PyMT)-induced mammary carcinoma.47 This reduced resistance was accompanied by decreased IFN-γ production from CD4+ and CD8+ T cells and an increased number of Treg cells. In marked contrast, however, it was recently demonstrated that WSX-1-deficient mice showed more attenuated tumor growth of B16F10 and LLC than did WT mice.48 This augmented antitumor effect was explained by the reduced number of T-cell immunoglobulin and mucin domain-3 (Tim-3)+ programmed death-1 (PD-1)+ CD8+ T cells, which are the most exhausted T-cell population among tumor-infiltrating lymphocytes, indicating IL-27 signaling as a key regulator of effector T-cell responses via induction of Tim-3.48 Taken together, these results suggest that IL-27/WSX-1 signaling plays critical roles in both generation and exhaustion of CTL, together with suppression of DC function.
Table 2.
Susceptibility of mice deficient in WSX-1, EBI3 and p28 to development of tumors
| Deficient mice | Susceptibility | Tumor | Reference |
|---|---|---|---|
| WSX-1 | Overall reduced resistance to tumor growth due to impaired antitumor CTL generation but accompanied with augmented antitumor immunity by DC | Melanoma (B16) | Shinozaki et al. 200846 |
| Reduced resistance to endogenously arising tumor growth due to decreased IFN-γ production by CD4+ and CD8+ T cells and increased numbers of Treg cells | MCA-induced fibrosarcoma PyMT-induced mammary carcinoma | Natividad et al. 201347 | |
| Augmented resistance to tumor growth due to reduced number of the most exhausted Tim-3+PD-1+CD8+ T cells among TILs | Melanoma (B16F10) Lewis lung carcinoma (LLC) | Zhu et al. 201548 | |
| EBI3 | Augmented resistance to tumor growth due to increased numbers of IFN-γ-producing killer DC with T-bet-mediated antitumor CD8+ T-cell responses in the lung | Melanoma (B16F10) lung metastasis | Sauer et al. 200849 |
| DC-specific conditional p28 | Reduced resistance to tumor growth due to impairment of IL-27-mediated CXCL10 expression in MDSCs accompanied by reduced recruitment and activation of NK and NKT cells | MCA-induced fibrosarcoma Melanoma (B16) | Wei et al. 201353 |
| Enhanced antitumor immune responses due to impairment of IL-27-mediated CCL22 expression in DC with reduced infiltration of Treg cells into tumors | Melanoma (B16F10) Lymphoma (EL-4) MCA-induced fibrosarcoma | Xia et al. 201454 |
CTL, cytotoxic T lymphocyte; DC, dendritic cells; EBI3, Epstein–Barr virus-induced gene 3; IFN, interferon; IL, interleukin; MCA, 3-methylcholanthrene; MDSC, myeloid-derived suppressor cell; NK, natural killer; PD-1, programmed death-1; PyMT, polyoma middle T antigen; T-bet, T-cell-specific T-box transcription factor; TIL, tumor-infiltrating lymphocyte; Tim-3, T-cell immunoglobulin and mucin domain-3; Treg, regulatory T.
Protumor effects of endogenous Epstein–Barr virus-induced gene 3
In contrast to both the antitumor and protumor effects of WSX-1, whose deficiency seems to highly agree with loss of representative IL-27 functions, EBI3-deficient mice showed augmented antitumor activity against lung metastasis of B16F10 (Table2).49 This effect was induced by expansion of a newly described cell subset called IFN-γ-producing killer DC in the lung of EBI3-deficient mice, and these DC then activated CD8+ T cells to produce IFN-γ and TNF-α, resulting in tumor apoptosis in a T-bet-dependent manner. This phenotype is consistent with increased EBI3 expression in serum and tumor tissue correlating with higher risk of cancer progression.50,51 Although EBI3-deficient mice showed abrogated IL-35-mediated Treg activity52 and also may be devoid of IL-27-mediated regulation of Treg cells, if any, the endogenous role of EBI3 likely contributes more strongly to the protumor activity mediated by EBI3 alone than the antitumor activity by IL-27.
Both antitumor and protumor effects of endogenous p28
So far, there have been two seemingly opposite articles regarding the endogenous role of p28 in the induction of antitumor immune responses53,54 (Table2). DC-specific conditional p28-deficient mice were initially demonstrated to have reduced resistance to progression of tumors such as MCA-induced fibrosarcoma and transplanted melanoma B16.53 These effects were due to impairment of IL-27-mediated CXCL10 expression in myeloid-derived suppressor cells (MDSC) and IL-12 production from DC, which led to recruitment and activation of NK and NKT cells, resulting in immunological control of tumors.53 In contrast, using the same p28-deficient mice, the same group also reported that IL-27 plays a critical role in tumor infiltration of Treg cells.54 In tumor-associated DC, IL-27 promoted the expression of CCL22, which is critical for recruitment of peripheral Treg cells into tumors, and the p28-deficient mice showed reduced tumor infiltration of Treg cells.54 Because IL-27, but not p28, restored the respective phenomena in the p28-deficient mice, the endogenous role of p28 seems to strongly agree with IL-27 functions. However, the role of IL-27 in the induction of Treg cells still appears to be controversial in the tumor microenvironment, as described later.
Potential Protumor Effects of Interleukin-27
Contribution of interleukin-27-mediated upregulation of interleukin-10 to its antitumor effects
Because IL-27 possesses both pro-inflammatory and anti-inflammatory activities, some such as IL-27-mediated augmentation of IL-10 production10–12 and upregulation of PD-L118–20 and Tim-348 may function against its antitumor effects. However, the role of IL-10 in the induction of antitumor immune responses is often controversial, and increasing evidence supports a positive role of IL-10 for induction of antitumor CTL responses, which were demonstrated in IL-10-deficient mice and IL-10 transgenic mice.55–57 IL-27 was recently demonstrated to significantly enhance the survival of activated tumor-specific CD8+ T cells in vitro and in vivo and to induce a unique memory precursor cell phenotype in tumor antigen-specific CD8+ T cells, characterized by, for instance, upregulation of IL-10.29 The IL-27-mediated IL-10 production from CTL contributed to the induction of memory precursor cell phenotype, CTL memory and, consequently, tumor rejection.29
Contribution of interleukin-27-mediated upregulation of programmed death ligand 1 and T-cell immunoglobulin and mucin domain-3 to its protumor effects
Exhausted T cells are characterized by their sustained expression of inhibitory receptors such as PD-1 and Tim-3.58–60 The PD1/PD-L1 pathway has emerged as a central player in immune regulation, and cancer cells that express PD-L1 promote tumor progression through inhibition of PD-1 expressing effector cells.61 Co-expression of PD-1 and other inhibitory receptors such as Tim-3 contributes to the induction of T-cell exhaustion and defines T cells with more deeply exhausted phenotype.60 IL-27 was recently shown to induce expression of PD-L1 in not only T cells and DC18–20 but also tumors.62 Histological analyses revealed that when IL-27 was detected in primary cutaneous melanomas, its expression was maintained in metastatic lesions.62 In addition, IL-27 induced PD-L1 expression in melanoma cell lines and the in situ expression of IL-27 in melanoma correlated with those of PD-L1 and IL-10.62 Thus, it is highly conceivable that IL-27-induced PD-L1 expression in T cells and tumors could contribute to the protumor effects of IL-27, whose possibility remains to be directly clarified. Moreover, IL-27 was recently demonstrated to induce the expression of the transcription factor nuclear factor, IL-3 regulated (NFIL3), which cooperates with T-bet, to induce the expression of Tim-3.48 IL-27 signaling was required for the induction of Tim-3+ exhausted T cells and promotion of tumor growth.48 Thus, IL-27-mediated upregulation of inhibitory molecules such as PD-L1 and Tim-3 likely contributes to tumor progression.
Positive and negative effects of interleukin-27 on Treg cells
The role of IL-27 in the induction of Treg cells in the context of tumorigenesis is also controversial. The induction of Treg cells54,63 and the suppression of them47 by IL-27 have both been reported. Initially, apoptotic tumor cells were demonstrated to induce IL-27 release from human DC to activate Treg cells that express CD69 and CD39 to generate adenosine, thereby suppressing cytotoxicity of CD73-expressing CD8+ T cells and limiting antitumor immunity.63 Moreover, the effect of endogenous IL-27 on Treg cells in the tumor microenvironment was investigated using IL-27p28 conditional knockout mice. IL-27 induced the expression of CCL22 on tumor-associated DC, which mediates tumor infiltration of Treg cells, resulting in tumor progression.54 In contrast, it was also demonstrated that tumor development and growth are accelerated in WSX-1-deficient mice.47 The enhanced tumor growth in both carcinogen-induced fibrosarcoma and oncogene-driven mammary carcinoma was associated with increased number of Treg cells and decreased IFN-γ-production by CD4+ and CD8+ T cells.47 Collectively, if IL-27 were to promote the generation of Treg cells, the antitumor effects of IL-27 could be weakened. In contrast, if IL-27 were to inhibit it, the antitumor effects of IL-27 could be augmented, but side effects such as development of autoimmune diseases might be expected. Further studies are necessary to elucidate the precise effect of IL-27 on Treg cells in the tumor microenvironment.
Potential transforming activity of interleukin-27Rα
A point mutation in Janus kinase (JAK)2 (JAK2-V617F), which has an increased inherent kinase activity, was identified in a number of neoplastic myeloproliferative disorders.64 From a patient with acute myeloid leukemia, IL-27Rα was recently identified as a gene that can induce ligand-independent transformation of hematopoietic cells via JAK-dependent pathways.65 In addition, it was demonstrated that IL-27Rα induces the tyrosine phosphorylation of JAK2 and its downstream target STAT5, and transforms cytokine-dependent hematopoietic cells to cytokine independence when co-expressed with mutated JAK2-V617F but not wild-type JAK2.65,66 This activating effect on JAK2-V617F by IL-27Rα was dependent on a functional IL-27Rα Box 1 motif but was independent of the gp130 co-receptor, suggesting that IL-27Rα may be functioning as a homodimer.65 Intriguingly, we have previously shown that IL-27 stimulates and expands hematopoietic stem cells to differentiate into myeloid cells in IL-27 transgenic mice.67 Taken together, aberrant IL-27Rα signaling may enhance myeloid cell growth, potentially leading to myeloproliferative neoplasms, although higher incidence of development of spontaneous tumors was not observed in the IL-27 transgenic mice.67
Future Prospects and Concluding Remarks
Cytokine-based cancer immunotherapies currently used include systemic administration, local delivery, vaccination with tumor cells engineered to secrete cytokine, adjuvants for cancer vaccine and adoptive therapy of immune cells expanded by cytokines. Because IL-27 is a multifunctional cytokine acting with a double-edged sword, both antitumor and protumor effects of IL-27 are conceivably expected depending on the type of cells that IL-27 stimulates and the tumor context. Currently, antibody drugs have become very popular and are one of the most promising cancer immunotherapies. Therefore, to overcome the protumor effects of IL-27, it is highly possible that IL-27-mediated antitumor effects could be further augmented by antibodies against immune checkpoints, such as PD-L1 and Tim-3, and also by antibodies against Treg cells, such as cytotoxic T-lymphocyte-associated protein 4. These possibilities remain to be investigated in the near future. In addition, IL-27 was shown to have lower toxicity in mouse models, probably because of low induction of IFN-γ;21,22 therefore, the combination of IL-27 treatment with other cancer immunotherapies to overcome the protumor effects of IL-27 could be a promising therapeutic strategy against cancer.
Disclosure Statement
The authors have no conflict of interest to declare.
References
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–46. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–42. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- Villarino AV, Huang E, Hunter CA. Understanding the pro- and anti-inflammatory properties of IL-27. J Immunol. 2004;173:715–20. doi: 10.4049/jimmunol.173.2.715. [DOI] [PubMed] [Google Scholar]
- Mizoguchi I, Higuchi K, Mitobe K, Tsunoda R, Mizuguchi J, Yoshimoto T. Interleukin-27: Regulation of immune responses and disease development by a pleiotropic cytokine with pro- and anti-inflammatory properties. In: Yoshimoto T, Yoshimoto T, editors. Cytokine Frontiers: Regulation of Immune Responses in Health and Disease. Tokyo: Springer; 2013. pp. 353–75. [Google Scholar]
- Yoshida H, Hunter CA. The immunobiology of interleukin-27. Annu Rev Immunol. 2015;33:417–43. doi: 10.1146/annurev-immunol-032414-112134. [DOI] [PubMed] [Google Scholar]
- Morishima N, Owaki T, Asakawa M, Kamiya S, Mizuguchi J, Yoshimoto T. Augmentation of effector CD8+ T cell generation with enhanced granzyme B expression by IL-27. J Immunol. 2005;175:1686–93. doi: 10.4049/jimmunol.175.3.1686. [DOI] [PubMed] [Google Scholar]
- Schneider R, Yaneva T, Beauseigle D, El-Khoury L, Arbour N. IL-27 increases the proliferation and effector functions of human naive CD8+ T lymphocytes and promotes their development into Tc1 cells. Eur J Immunol. 2011;41:47–59. doi: 10.1002/eji.201040804. [DOI] [PubMed] [Google Scholar]
- Awasthi A, Carrier Y, Peron JP, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–9. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- Fitzgerald DC, Zhang GX, El-Behi M, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–9. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- Stumhofer JS, Silver JS, Laurence A, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–71. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- Neufert C, Becker C, Wirtz S, et al. IL-27 controls the development of inducible regulatory T cells and Th17 cells via differential effects on STAT1. Eur J Immunol. 2007;37:1809–16. doi: 10.1002/eji.200636896. [DOI] [PubMed] [Google Scholar]
- Huber M, Steinwald V, Guralnik A, et al. IL-27 inhibits the development of regulatory T cells via STAT3. Int Immunol. 2008;20:223–34. doi: 10.1093/intimm/dxm139. [DOI] [PubMed] [Google Scholar]
- Wojno ED, Hosken N, Stumhofer JS, et al. A role for IL-27 in limiting T regulatory cell populations. J Immunol. 2011;187:266–73. doi: 10.4049/jimmunol.1004182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JH, Kljavin NM, Ramamoorthi N, Diehl L, Batten M, Ghilardi N. IL-27 promotes T cell-dependent colitis through multiple mechanisms. J Exp Med. 2011;208:115–23. doi: 10.1084/jem.20100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AO, Beiting DP, Tato C, et al. The cytokines interleukin 27 and interferon-gamma promote distinct Treg cell populations required to limit infection-induced pathology. Immunity. 2012;37:511–23. doi: 10.1016/j.immuni.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakhanova S, Bedke T, Enk AH, Mahnke K. IL-27 renders DC immunosuppressive by induction of B7-H1. J Leukoc Biol. 2011;89:837–45. doi: 10.1189/jlb.1209788. [DOI] [PubMed] [Google Scholar]
- Matta BM, Raimondi G, Rosborough BR, Sumpter TL, Thomson AW. IL-27 production and STAT3-dependent upregulation of B7-H1 mediate immune regulatory functions of liver plasmacytoid dendritic cells. J Immunol. 2012;188:5227–37. doi: 10.4049/jimmunol.1103382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirahara K, Ghoreschi K, Yang XP, et al. Interleukin-27 priming of T cells controls IL-17 production in trans via induction of the ligand PD-L1. Immunity. 2012;36:1017–30. doi: 10.1016/j.immuni.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisada M, Kamiya S, Fujita K, et al. Potent antitumor activity of interleukin-27. Cancer Res. 2004;64:1152–6. doi: 10.1158/0008-5472.can-03-2084. [DOI] [PubMed] [Google Scholar]
- Oniki S, Nagai H, Horikawa T, et al. Interleukin-23 and interleukin-27 exert quite different antitumor and vaccine effects on poorly immunogenic melanoma. Cancer Res. 2006;66:6395–404. doi: 10.1158/0008-5472.CAN-05-4087. [DOI] [PubMed] [Google Scholar]
- Salcedo R, Stauffer JK, Lincoln E, et al. IL-27 mediates complete regression of orthotopic primary and metastatic murine neuroblastoma tumors: role for CD8+ T cells. J Immunol. 2004;173:7170–82. doi: 10.4049/jimmunol.173.12.7170. [DOI] [PubMed] [Google Scholar]
- Chiyo M, Shimozato O, Yu L, et al. Expression of IL-27 in murine carcinoma cells produces antitumor effects and induces protective immunity in inoculated host animals. Int J Cancer. 2005;115:437–42. doi: 10.1002/ijc.20848. [DOI] [PubMed] [Google Scholar]
- Hu P, Hu HD, Chen M, et al. Expression of interleukins-23 and 27 leads to successful gene therapy of hepatocellular carcinoma. Mol Immunol. 2009;46:1654–62. doi: 10.1016/j.molimm.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Salcedo R, Hixon JA, Stauffer JK, et al. Immunologic and therapeutic synergy of IL-27 and IL-2: enhancement of T cell sensitization, tumor-specific CTL reactivity and complete regression of disseminated neuroblastoma metastases in the liver and bone marrow. J Immunol. 2009;182:4328–38. doi: 10.4049/jimmunol.0800471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Lee DA, Li S. IL-12 and IL-27 sequential gene therapy via intramuscular electroporation delivery for eliminating distal aggressive tumors. J Immunol. 2010;184:2348–54. doi: 10.4049/jimmunol.0902371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolochevska O, Xia X, Williams BJ, Ramsay A, Li S, Figueiredo ML. Sonoporation delivery of interleukin-27 gene therapy efficiently reduces prostate tumor cell growth in vivo. Hum Gene Ther. 2011;22:1537–50. doi: 10.1089/hum.2011.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Liu JQ, Talebian F, Wu LC, Li S, Bai XF. IL-27 enhances the survival of tumor antigen-specific CD8+ T cells and programs them into IL-10-producing, memory precursor-like effector cells. Eur J Immunol. 2013;43:468–79. doi: 10.1002/eji.201242930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Tian H, Li C, et al. Antitumor effects obtained by autologous Lewis lung cancer cell vaccine engineered to secrete mouse interleukin 27 by means of cationic liposome. Mol Immunol. 2013;55:264–74. doi: 10.1016/j.molimm.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Liu L, Wang S, Shan B, et al. IL-27-mediated activation of natural killer cells and inflammation produced antitumour effects for human oesophageal carcinoma cells. Scand J Immunol. 2008;68:22–9. doi: 10.1111/j.1365-3083.2008.02111.x. [DOI] [PubMed] [Google Scholar]
- Airoldi I, Tupone MG, Esposito S, et al. Interleukin-27 re-educates intratumoral myeloid cells and down-regulates stemness genes in non-small cell lung cancer. Oncotarget. 2015;6:3694–708. doi: 10.18632/oncotarget.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui M, Kishida T, Nakano H, et al. Interleukin-27 activates natural killer cells and suppresses NK-resistant head and neck squamous cell carcinoma through inducing antibody-dependent cellular cytotoxicity. Cancer Res. 2009;69:2523–30. doi: 10.1158/0008-5472.CAN-08-2793. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Shimamura M, Owaki T, et al. Antiangiogenic and antitumor activities of IL-27. J Immunol. 2006;176:7317–24. doi: 10.4049/jimmunol.176.12.7317. [DOI] [PubMed] [Google Scholar]
- Cocco C, Giuliani N, Di Carlo E, et al. Interleukin-27 acts as multifunctional antitumor agent in multiple myeloma. Clin Cancer Res. 2010;16:4188–97. doi: 10.1158/1078-0432.CCR-10-0173. [DOI] [PubMed] [Google Scholar]
- Canale S, Cocco C, Frasson C, et al. Interleukin-27 inhibits pediatric B-acute lymphoblastic leukemia cell spreading in a preclinical model. Leukemia. 2011;25:1815–24. doi: 10.1038/leu.2011.158. [DOI] [PubMed] [Google Scholar]
- Cocco C, Di Carlo E, Zupo S, et al. Complementary IL-23 and IL-27 anti-tumor activities cause strong inhibition of human follicular and diffuse large B-cell lymphoma growth in vivo. Leukemia. 2012;26:1365–74. doi: 10.1038/leu.2011.363. [DOI] [PubMed] [Google Scholar]
- Zorzoli A, Di Carlo E, Cocco C, et al. Interleukin-27 inhibits the growth of pediatric acute myeloid leukemia in NOD/SCID/Il2rg-/- mice. Clin Cancer Res. 2012;18:1630–40. doi: 10.1158/1078-0432.CCR-11-2432. [DOI] [PubMed] [Google Scholar]
- Kachroo P, Lee MH, Zhang L, et al. IL-27 inhibits epithelial-mesenchymal transition and angiogenic factor production in a STAT1-dominant pathway in human non-small cell lung cancer. J Exp Clin Cancer Res. 2013;32:97. doi: 10.1186/1756-9966-32-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Carlo E, Sorrentino C, Zorzoli A, et al. The antitumor potential of Interleukin-27 in prostate cancer. Oncotarget. 2014;5:10332–41. doi: 10.18632/oncotarget.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto T, Morishima N, Mizoguchi I, et al. Antiproliferative activity of IL-27 on melanoma. J Immunol. 2008;180:6527–35. doi: 10.4049/jimmunol.180.10.6527. [DOI] [PubMed] [Google Scholar]
- Liu L, Meng J, Zhang C, et al. Effects on apoptosis and cell cycle arrest contribute to the antitumor responses of interleukin-27 mediated by retrovirus in human pancreatic carcinoma cells. Oncol Rep. 2012;27:1497–503. doi: 10.3892/or.2012.1663. [DOI] [PubMed] [Google Scholar]
- Chiba Y, Mizoguchi I, Mitobe K, et al. IL-27 enhances the expression of TRAIL and TLR3 in human melanomas and inhibits their tumor growth in cooperation with a TLR3 agonist poly(I:C) partly in a TRAIL-dependent manner. PLoS ONE. 2013;8:e76159. doi: 10.1371/journal.pone.0076159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho MY, Leu SJ, Sun GH, Tao MH, Tang SJ, Sun KH. IL-27 directly restrains lung tumorigenicity by suppressing cyclooxygenase-2-mediated activities. J Immunol. 2009;183:6217–26. doi: 10.4049/jimmunol.0901272. [DOI] [PubMed] [Google Scholar]
- Liu LH, Shan BE, Shao LL, Wang SJ. Inhibitory effect of IL-27 gene on tumor formation activity of Eca109 cells in nude mice and its mechanisms. Ai Zheng. 2008;27:12–7. [PubMed] [Google Scholar]
- Shinozaki Y, Wang S, Miyazaki Y, et al. Tumor-specific cytotoxic T cell generation and dendritic cell function are differentially regulated by interleukin 27 during development of anti-tumor immunity. Int J Cancer. 2009;124:1372–8. doi: 10.1002/ijc.24107. [DOI] [PubMed] [Google Scholar]
- Natividad KD, Junankar SR, Mohd Redzwan N, et al. Interleukin-27 signaling promotes immunity against endogenously arising murine tumors. PLoS ONE. 2013;8:e57469. doi: 10.1371/journal.pone.0057469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Sakuishi K, Xiao S, et al. An IL-27/NFIL3 signalling axis drives Tim-3 and IL-10 expression and T-cell dysfunction. Nat Commun. 2015;6:6072. doi: 10.1038/ncomms7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer KA, Maxeiner JH, Karwot R, et al. Immunosurveillance of lung melanoma metastasis in EBI-3-deficient mice mediated by CD8+ T cells. J Immunol. 2008;181:6148–57. doi: 10.4049/jimmunol.181.9.6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino R, Takano A, Oshita H, et al. Identification of Epstein–Barr virus-induced gene 3 as a novel serum and tissue biomarker and a therapeutic target for lung cancer. Clin Cancer Res. 2011;17:6272–86. doi: 10.1158/1078-0432.CCR-11-0060. [DOI] [PubMed] [Google Scholar]
- Gonin J, Larousserie F, Bastard C, et al. Epstein-Barr virus-induced gene 3 (EBI3): a novel diagnosis marker in Burkitt lymphoma and diffuse large B-cell lymphoma. PLoS ONE. 2011;6:e24617. doi: 10.1371/journal.pone.0024617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collison LW, Workman CJ, Kuo TT, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–9. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- Wei J, Xia S, Sun H, et al. Critical role of dendritic cell-derived IL-27 in antitumor immunity through regulating the recruitment and activation of NK and NKT cells. J Immunol. 2013;191:500–8. doi: 10.4049/jimmunol.1300328. [DOI] [PubMed] [Google Scholar]
- Xia S, Wei J, Wang J, et al. A requirement of dendritic cell-derived interleukin-27 for the tumor infiltration of regulatory T cells. J Leukoc Biol. 2014;95:733–42. doi: 10.1189/jlb.0713371. [DOI] [PubMed] [Google Scholar]
- Mumm JB, Emmerich J, Zhang X, et al. IL-10 elicits IFNgamma-dependent tumor immune surveillance. Cancer Cell. 2011;20:781–96. doi: 10.1016/j.ccr.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Tanikawa T, Wilke CM, Kryczek I, et al. Interleukin-10 ablation promotes tumor development, growth, and metastasis. Cancer Res. 2012;72:420–9. doi: 10.1158/0008-5472.CAN-10-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groux H, Cottrez F, Rouleau M, et al. A transgenic model to analyze the immunoregulatory role of IL-10 secreted by antigen-presenting cells. J Immunol. 1999;162:1723–9. [PubMed] [Google Scholar]
- Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- Jones RB, Ndhlovu LC, Barbour JD, et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763–79. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin HT, Anderson AC, Tan WG, et al. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci USA. 2010;107:14733–8. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin HT, Ahmed R, Okazaki T. Role of PD-1 in regulating T-cell immunity. Curr Top Microbiol Immunol. 2011;350:17–37. doi: 10.1007/82_2010_116. [DOI] [PubMed] [Google Scholar]
- Gonin J, Carlotti A, Dietrich C, et al. Expression of IL-27 by tumor cells in invasive cutaneous and metastatic melanomas [corrected] PLoS ONE. 2013;8:e75694. doi: 10.1371/journal.pone.0075694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar D, Hahn C, Brune B, Roberts E, Weigert A. Apoptotic tumor cells induce IL-27 release from human DCs to activate Treg cells that express CD69 and attenuate cytotoxicity. Eur J Immunol. 2012;42:1585–98. doi: 10.1002/eji.201142093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuther GW. JAK2 activation in myeloproliferative neoplasms: a potential role for heterodimeric receptors. Cell Cycle. 2008;7:714–9. doi: 10.4161/cc.7.6.5567. [DOI] [PubMed] [Google Scholar]
- Pradhan A, Lambert QT, Reuther GW. Transformation of hematopoietic cells and activation of JAK2-V617F by IL-27R, a component of a heterodimeric type I cytokine receptor. Proc Natl Acad Sci USA. 2007;104:18502–7. doi: 10.1073/pnas.0702388104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan A, Lambert QT, Griner LN, Reuther GW. Activation of JAK2-V617F by components of heterodimeric cytokine receptors. J Biol Chem. 2010;285:16651–63. doi: 10.1074/jbc.M109.071191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seita J, Asakawa M, Ooehara J, et al. Interleukin-27 directly induces differentiation in hematopoietic stem cells. Blood. 2008;111:1903–12. doi: 10.1182/blood-2007-06-093328. [DOI] [PubMed] [Google Scholar]


