Abstract
Differentially regulated microRNA (miRNA) are associated with hepatic fibrosis; however, their potential usefulness for blocking hepatic fibrosis has not been exploited fully. We examined the expression of miRNA in the liver of a transgenic mouse model in which platelet-derived growth factor C (PDGF-C) is overexpressed (Pdgf-c Tg), resulting in hepatic fibrosis and steatosis and the eventual development of hepatocellular carcinoma (HCC). Robust induction of miR-214 correlated with fibrogenesis in the liver of Pdgf-c Tg mice, atherogenic high-fat diet-induced NASH mice, and patients with chronic hepatitis B or C. Pdgf-c Tg mice were injected with locked nucleic acid (LNA)-antimiR-214 via the tail vein using Invivofectamine 2.0 and the degree of hepatic fibrosis and tumor incidence were evaluated. Pdgf-c Tg mice treated with LNA-antimiR-214 showed a marked reduction in fibrosis and tumor incidence compared with saline or LNA-miR-control-injected control mice. In vitro, LNA-antimiR-214 significantly ameliorated TGF-β1-induced pro-fibrotic gene expression in Lx-2 cells. MiR-214 targets a negative regulator of EGFR signaling, Mig-6. Mimic-miR-214 decreased the expression of Mig-6 and increased the levels of EGF-mediated p-EGFR (Y1173 and Y845) and p-Met (Tyr1234/1235) in Huh-7 cells. Conversely, LNA-antimiR-214 repressed the expression of these genes. In conclusion, miR-214 appears to participate in the development of hepatic fibrosis by modulating the EGFR and TGF-β signaling pathways. LNA-antimiR-214 is a potential therapy for the prevention of hepatic fibrosis.
Keywords: Hepatic fibrosis, hepatocellular carcinoma, locked nucleic acid, microRNA, platelet-derived growth factor C
Hepatic fibrosis develops in the setting of chronic inflammation such as in chronic hepatitis B or C, alcoholic liver disease, non-alcoholic steatohepatitis and autoimmune hepatitis. The progression of hepatic fibrosis is clinically associated with liver cell dysfunction and portal hypertension, and frequently with hepatocellular carcinoma (HCC).1
Hepatic fibrosis is initiated by the activation of stellate cells, which are stimulated by pro-fibrotic cytokines such as transforming growth factor (TGF)-β and platelet-derived growth factor C (PDGF-C) and various external stimuli such as oxidative stress. The overexpression of PDGF-C in the liver of a recently developed transgenic mouse model (Pdgf-c Tg) resulted in the progression of hepatic fibrosis and steatosis and the development of HCC.2 This mouse model showed the close association between hepatic fibrosis and the occurrence of HCC, and also suggested the possibility that inhibition of hepatic fibrosis could be an attractive therapy to reduce the incidence of HCC. Recently, we reported that the acyclic retinoid peretinoin prevented hepatic fibrosis and reduced the development of HCC by targeting PDGF signaling in Pdgf-c Tg mice.3
MicroRNA (miRNA) are small, endogenous, single-stranded, non-coding RNA that regulate gene expression and play an important role in various biological processes, including organ development and differentiation, cell death, and proliferation, as well as in various diseases including cancer.4
In the progression of hepatic fibrosis, several miRNA such as miR-34a, miR-155, miR-199a/b and miR-214 are upregulated, while miR-29, miR-150 and miR-194 are downregulated.5 However, the functional relevance of these miRNA in hepatic fibrosis has not been assessed fully in an in vivo model. In the present study, using Pdgf-c Tg mice, we evaluated the function and potential usefulness of miR-214 for improving hepatic fibrosis and preventing the development of HCC.
Materials and Methods
Detailed material and methods are described in the Data S1.
Clinical samples
Liver tissue samples were obtained from patients with chronic hepatitis C or B as previously described.6,7 Normal controls were histologically normal tissues and were obtained from patients who underwent partial hepatectomy for metastatic liver tumors as previously described.6,7 Informed consent was obtained from all patients and ethics approval for the study was obtained from the ethics committee for human genome/gene analysis research at Kanazawa University Graduate School of Medical Science.
Mouse studies
The generation and characterization of Pdgf-c Tg mice have been described previously.2,3 Wild-type (WT) and Pdgf-c Tg mice on a C57BL/6J background were maintained in a pathogen-free animal facility under a standard 12:12 h light:dark cycle. To analyze the development of liver fibrosis, 9-week-old male Pdgf-c Tg mice were injected with saline, locked nucleic acid (LNA)-antimiR-control (scramble) or LNA-antimiR-214 (Exiqon, Skelstedet, Denmark) containing Invivofectamine 2.0 (Invitrogen, Carlsbad, CA, USA) twice (with an interval of 1 week) via the tail vein. At 3 days after the second injection, the mice were killed for analysis (n = 5 for each group).
To analyze tumorigenesis in the liver, 32-week-old male Pdgf-c Tg mice were injected with LNA-antimiR-214, LNA-antimiR-control or saline via the tail vein for a total of six injections (50 μg each) with an interval of 3 weeks using Invivofectamine 2.0 (20 mg/200 μL). The mice were killed 1 week after the final injection. The incidence of hepatic tumors, maximum tumor size and liver weight were evaluated (n = 6 for each group). All animal experiments were carried out in accordance with the Guidelines for the Care and Use of Laboratory Animals at the Takara-Machi Campus of Kanazawa University, Japan.
Micro RNA expression in the liver of Pdgf-c Tg mice
Micro RNA expression levels in the liver of Pdgf-c Tg mice were evaluated using TaqMan Array Rodent MicroRNA A Cards v2.0 containing 384 miRNA assays (Applied Biosystems, Carlsbad, CA, USA). Total RNA containing miRNA was isolated from the liver tissues of WT and Pdgf-c Tg mice at 20 and 48 weeks of age (each n = 4) according to the protocol of the mirVana miRNA Isolation Kit (Invitrogen). cDNA was prepared by reverse transcription using 10 ng of each isolated total RNA and 3 μL of each reverse transcription primer with specific loop structures. Reverse transcription was performed using the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems) according to the manufacturer's protocol. Then, a mixture of 6.67 μL nuclease-free water, 10 μL TaqMan 2× Universal PCR Master Mix (No AmpErase UNG; Applied Biosystems) and 2 μL TaqMan MicroRNA Assay Mix, which was included in the kit, was prepared for each sample in a 384-well plate; 1.33 μL of the reverse transcription product was added to the mixture, and the amplification reaction was performed on an ABI PRISM 7900HT (Applied Biosystems). Relative expression levels were calculated after normalization to RNU44.
Cell culture
Human embryonic kidney (HEK) 293AD, immortalized hepatocytes (THTL-5b and TTNT),8,9 human hepatocellular carcinoma (HCC) cell lines (Huh-7, Huh-1, HepG2, Hep3B, HLE and HLF) and human Lx-2 stellate cells (kindly provided by Dr Scott Friedman, Mount Sinai School of Medicine, New York, NY, USA) were maintained in DMEM (Gibco, Grand Island, NY, USA) supplemented with 10% FBS (Gibco), 1% l-glutamine (Gibco) and 1% penicillin/streptomycin (Gibco) in a humidified atmosphere of 5% CO2 at 37°C. Cultured cells in 12-well plates were grown to 70% confluence and transfected with LNA-antimiR (10 nM; Exiqon), mimic miRNA (50 nM; Invitrogen) and anti-mitogen-inducible gene 6 (Mig-6) siRNA (40 nM; Invitrogen) using Lipofectamine RNAiMAX according to the manufacturer's instructions. Transfected cells were serum-depleted for 9 h and then treated with 3 ng/mL recombinant human transforming growth factor (TGF)-β1 (Merck KGaA, Darmstadt, Germany) for 24 h.
Results
Upregulated micro RNA in the progression of hepatic fibrosis in Pdgf-c Tg mice
To determine whether specific miRNA were correlated with PDGF-C-induced liver fibrosis, we analyzed miRNA expression in whole liver from Pdgf-c Tg and non-transgenic WT mice at 20 and 48 weeks of age using TaqMan quantitative real-time detection-PCR (RTD-PCR). Out of the 381 miRNA tested, 17 were upregulated and 16 were downregulated (P < 0.05). Thirteen representative miRNA are shown in Table1 (P < 0.005). MiR-214 was the most significantly upregulated; therefore, we focused on miR-214 for further study. We confirmed that miR-214 was also upregulated with the progression of hepatic fibrosis in patients with chronic hepatitis C or B (Fig. S1a).10 We also confirmed that miR-214 was upregulated in an atherogenic high-fat diet (Ath + HF) mouse model that induces oxidative stress and steatohepatitis with cellular ballooning and fibrosis (Fig. S1b,c).11
Table 1.
Upregulated micro RNA in liver of PDGF-C Tg mice compared with wild-type (WT) mice at 20 and 48 weeks
| Number | miRNA | PDGF-C Tg/WT (20 weeks) | PDGF-C Tg/WT (48 weeks) | ||
|---|---|---|---|---|---|
| n = 4 P-value | n = 4 Fold | n = 4 P-value | n = 4 Fold | ||
| Upregulated | |||||
| 1 | mmu-miR-214 | 8.39E-05 | 4.17 | 8.55E-05 | 3.70 |
| 2 | mmu-miR-195 | 2.04E-04 | 2.94 | 2.62E-04 | 2.94 |
| 3 | mmu-miR-497 | 2.78E-04 | 3.03 | 4.84E-04 | 3.33 |
| 4 | mmu-miR-146b | 3.47E-04 | 3.45 | 7.06E-03 | 3.45 |
| 5 | mmu-miR-34a | 5.36E-04 | 2.86 | 5.65E-04 | 2.70 |
| 6 | mmu-miR-132 | 8.28E-04 | 2.63 | 1.65E-04 | 3.45 |
| 7 | mmu-miR-199a-3 | 1.10E-03 | 2.56 | 1.33E-03 | 1.92 |
| 8 | mmu-miR-155 | 2.65E-03 | 3.57 | 1.06E-04 | 2.86 |
| 9 | mmu-miR-223 | 3.48E-03 | 4.35 | 1.53E-04 | 3.03 |
| Downregulated | |||||
| 1 | mmu-miR-486 | 1.76E-03 | 0.45 | 1.53E-04 | 0.36 |
| 2 | mmu-miR-192 | 4.33E-03 | 0.52 | 4.19E-03 | 0.57 |
| 3 | mmu-miR-122 | 4.40E-03 | 0.61 | 2.83E-02 | 0.65 |
| 4 | mmu-miR-451 | 4.85E-03 | 0.39 | 1.46E-03 | 0.37 |
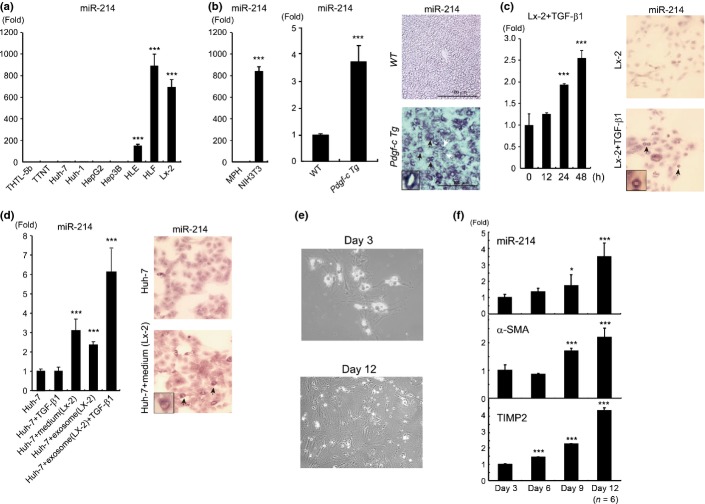
To explore which cell type expresses miR-214, we evaluated its expression in the following cell lines: two human immortalized hepatocyte cell lines (THTL-5b and TTNT), four human HCC cell lines (Huh-7, HepG2, HLE and HLF) and a human stellate cell-derived cell line (Lx-2; Fig.1a). The relative expression of miR-214 was high in two HCC cell lines (HLE and HLF) and stellate cells (Lx-2). In murine cells, the expression of miR-214 in a mouse embryo-derived fibroblast cell line (NIH3T3) was significantly higher than in mouse primary hepatocytes (Fig.1b, left). These results suggested that miR-214 was mainly expressed in mesenchymal cells, but only expressed at a low level in parenchymal hepatocytes. The expression of miR-214 in the liver of Pdgf-c Tg mice increased by threefold to fourfold compared with WT mice (Table1 and Fig.1b middle). Interestingly, however, in situ hybridization analysis demonstrated the increased expression of miR-214 in hepatocytes (black arrows) as well as mesenchymal cells (white arrows) from Pdgf-c Tg mice (Fig.1b, right). In vitro, TGF-β1 (3 ng/mL) significantly induced the expression of miR-214 in Lx-2 cells, which was confirmed by RTD-PCR and in situ hybridization (Fig.1c); however, the same concentration of TGF-β1 did not induce miR-214 expression in Huh-7 cells (Fig.1d). Therefore, we examined whether miR-214 was transferred through exosomes from Lx-2 cells to Huh-7 cells. The amount of miR-214 in exosomes was 10-fold higher in cultured medium from Lx-2 cells than from Huh-7 cells (Fig. S2a). The expression of miR-214 in Huh-7 cells significantly increased by approximately threefold by co-culture with Lx-2 cells and it was increased further by co-culture with TGF-β1-stimulated Lx-2 cells (Fig. S2a). When culture medium from Lx-2 cells or purified exosomes from the culture medium of Lx-2 cells were added to the culture medium of Huh-7 cells, intracellular miR-214 levels in Huh-7 cells were significantly upregulated, and they were upregulated further by the addition of TGF-β1 (Fig.1d). These results indicated that exosomes containing miR-214 were released from Lx-2 cells and taken up by Huh-7 cells. Interestingly, the expression of vimentin, cyclin D1 and alpha-fetoprotein (AFP) was slightly but significantly increased in Huh-7 cells by co-culture with Lx-2 cells, Moreover, Huh-7 cells formed a number of spheroids when co-cultured with Lx-2 cells (Fig. S2b,c). These data implied that Huh-7 cells acquired a more malignant tumor cell phenotype by co-culture with Lx-2 cells. Therefore, it could be speculated that the increased levels of miR-214 in the hepatocytes of Pdgf-c Tg mice might be derived from surrounding stellate cells or fibroblasts and increased miR-214 levels might play a role in the transformation of hepatocytes.
Figure 1.
Regulation of miR-214 expression in hepatocytes and hepatic stellate cells. (a) The expression levels of miR-214 in different cell lines (n = 6). (b) The expression levels of miR-214 in mouse primary hepatocytes (MPH) and NIH3T3 cells (left panel). The expression levels of miR-214 in the liver of non-transgenic wild-type (WT) and Pdgf-c Tg mice (middle; n = 6). In situ hybridization of miR-214 in the liver of WT and Pdgf-c Tg mice (right panel; black arrows indicate hepatocytes and white arrows indicate mesenchymal cells). (c) Time course of miR-214 levels in Lx-2 cells following stimulation with transforming growth factor (TGF)-β1 (3 ng/mL; left; n = 6). In situ hybridization showing the intracellular accumulation of miR-214 in Lx-2 cells following TGF-β1 treatment (24 h; right). (d) Intracellular miR-214 expression in Huh-7 cells by various factors. Huh-7 cells were stimulated with TGF-β1 (3 ng/mL), medium (of Lx-2 cells) and exosomes (derived from the medium of Lx-2 cells). After 24 h stimulation, intracellular miR-214 expression was evaluated (n = 6; left). In situ hybridization of the intracellular accumulation of miR-214 in Huh-7 cells exposed to the medium of Lx-2 cells (right). (e) Morphology of freshly isolated quiescent hepatic stellate cells (day 3) and activated myofibroblast-like cells (day 12). (f) The expression of miR-214, α-SMA and TIMP2 was upregulated during spontaneous trans-differentiation of stellate cells to myofibroblast-like cells. The TaqMan assay was performed in duplicate and repeated three times. Values are means ± SD. *P < 0.05, **P < 0.01, ***P < 0.001. In situ hybridization was repeated three times.
We isolated hepatic stellate cells (HSC) from normal mouse liver and evaluated the expression of miR-214 in HSC (Fig.1e). The expression of miR-214 was significantly upregulated during the progression of trans-differentiation from HSC to myofibroblast-like cells (Fig.1f). Together with this trans-differentiation, the expression of α-smooth muscle actin (SMA) and tissue inhibitor of metalloproteinase (TIMP) 2 was significantly upregulated.
Functional relevance of miR-214 in fibrogenesis in vitro
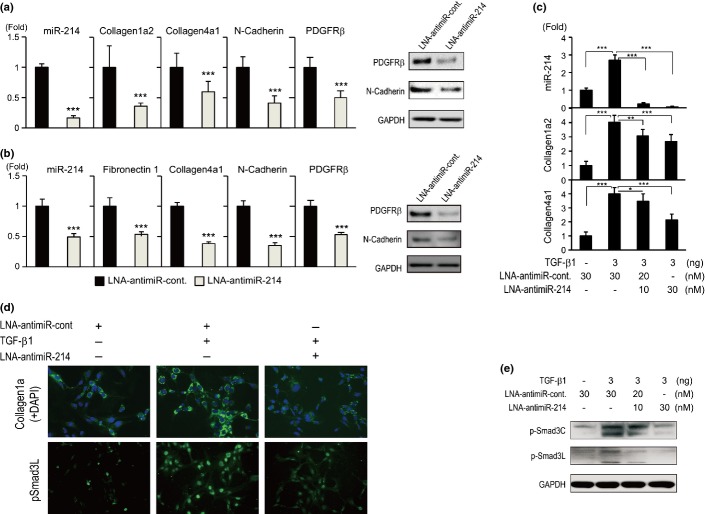
To examine the functional relevance of miR-214 in fibrogenesis, we knocked down the expression of miR-214 using LNA-antimiR-214 in Lx-2 and HLF cells (Fig.2a,b). The expression of collagen 1a2, collagen 4a1, fibronectin 1, PDGF receptor β (PDGFRβ) and N-cadherin was significantly repressed following the repression of miR-214, which was confirmed by TaqMan RTD-PCR and western blotting. Furthermore, LNA-antimiR-214 significantly ameliorated the TGF-β1-induced expression of collagen 1a2, collagen 4a1, phosphorylated (p)-mothers against DPP homolog (Smad) 3C and p-Smad3L in Lx-2 cells. These findings were analyzed by RTD-PCR (Fig.2c), immunofluorescence staining (Fig.2d) and western blotting (Fig.2e).
Figure 2.
Functional roles of miR-214 expression in hepatocytes and hepatic stellate cells. (a, b) Depletion of miR-214 in Lx-2 cells (a) and HLF cells (b) decreased the expression of pro-fibrotic and epithelial–mesenchymal transition-related genes. Gene and protein expression was examined by TaqMan real-time detection-PCR (RTD-PCR; left) or western blotting (right), respectively (n = 6). (c) Locked nucleic acid (LNA)-antimiR-214 ameliorated the TGF-β1-induced expression of collagen 1a2 and 4a1 in Lx-2 cells in a dose-dependent manner (TaqMan RTD-PCR; n = 6). (d) Immunofluorescence staining of collagen 1 and pSmad3L in Lx-2 cells. TGF-β1 (3 ng/mL) stimulation for 24 h increased the expression of collagen 1 and pSmad3L, while transfection of LNA-antimiR-214 canceled the increase in the expression of these genes. (e) Western blotting of p-Smad3 and p-Smad3L in Lx-2 cells in the same experiment. The TaqMan assay was performed in duplicate and repeated three times. Values are means ± SD. *P < 0.05, **P < 0.01, ***P < 0.001. Western blotting and immunofluorescence staining were repeated three times.
Gene expression profiling in Lx-2 cells in which miR-214 was knocked down with LNA-antimiR-214 was obtained using an Affymetrix GeneChip. A total of 1437 genes were downregulated and 880 genes were upregulated (P < 0.005). The changes in the levels of representative genes are shown in Table S1. The expression of collagen 1a2, collagen 4a1, EGFR, endothelin, α-SMA, TGF-β2, Smad3 and PDGFRβ was significantly downregulated by repression of miR-214. Pathway analysis of these genes by functional enrichment analysis using MetaCore (Thomson Reuters, New York, NY, USA) showed that neurogenesis, cytoskeleton, ErbB, cell adhesion and epithelial–mesenchymal transition (EMT) signaling were downregulated, while transcription and translation signaling were activated (Fig. S2). These results showed the functional relevance of miR-214 in fibrogenesis.
Repression of miR-214 with locked nucleic acid-antimiR-214 ameliorates hepatic fibrosis in Pdgf-c Tg mice
We evaluated the effect of repressing miR-214 in vivo using Pdgf-c Tg mice. Pdgf-c Tg mice at 9 weeks of age were injected with LNA-antimiR-214 via the tail vein twice (50 μg each) at an interval of 7 days by using Invivofectamine 2.0 (Fig.3a).12 Three days after the second injection, the mice were killed for evaluation.
Figure 3.
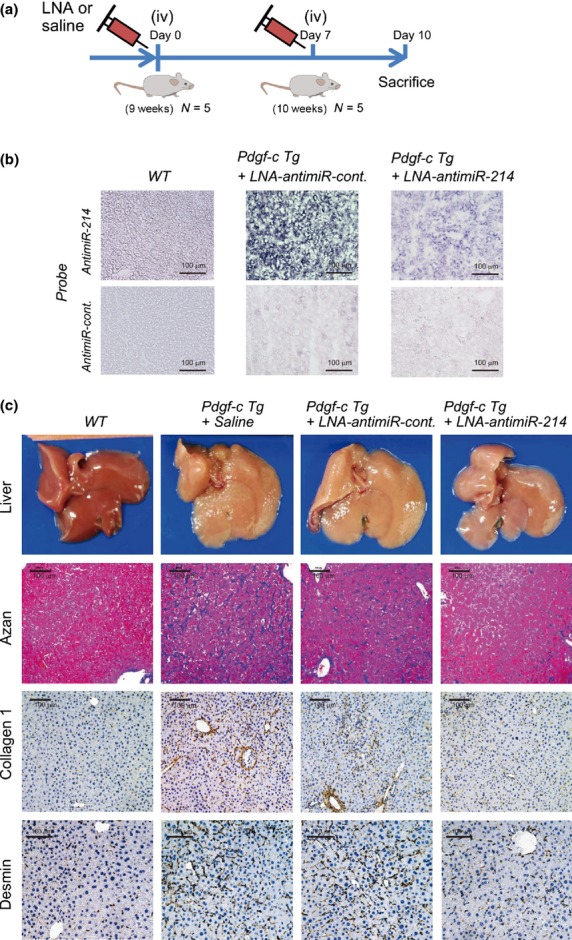
In vivo evaluation of hepatic fibrosis in Pdgf-c Tg mice injected with locked nucleic acid (LNA)-antimiR-214. (a) Experimental design: Pdgf-c Tg mice at 9 weeks of age were injected with saline, LNA-antimiR-control or LNA-antimiR-214 via the tail vein twice (50 μg each; n = 5). Three days after the second injection, the mice were killed for evaluation. (b) In situ hybridization of miR-214 in wild-type (WT) and Pdgf-c Tg liver injected with LNA-antimiR-control or LNA-antimiR-214. (c) Macroscopic findings of WT and Pdgf-c Tg liver injected with saline, LNA-antimiR-control or LNA-antimiR-214 (upper). Azan staining (2nd) and immunohistochemical staining of collagen 1 (3rd) and desmin (bottom) at sacrifice are shown.
In situ hybridization of miR-214 in the liver of Pdgf-c Tg mice showed a marked decrease in the expression of miR-214 by LNA-antimiR-214 (Fig.3b). Analysis of the gross appearance of the liver of Pdgf-c Tg mice injected with LNA-antimiR-214 showed a smooth surface of the liver compared with control liver injected with saline or LNA-antimiR-control (scramble; Fig.3c). Azan staining of fibrotic tissues and immunohistochemical (IHC) staining of collagen 1 and desmin showed a significant improvement of the area of fibrosis in the liver of Pdgf-c Tg mice injected with LNA-antimiR-214 compared with saline or LNA-antimiR-control (Fig.3c).
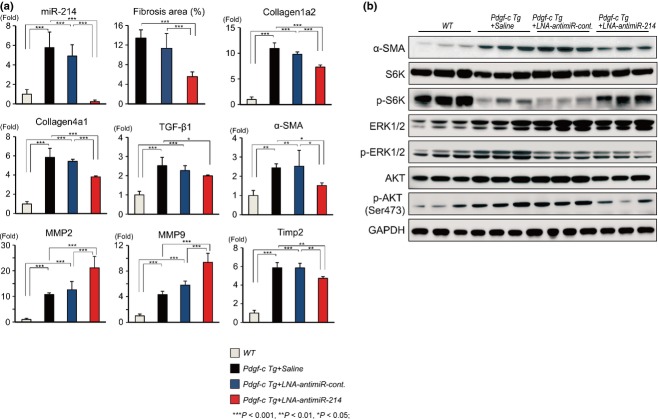
These findings were also confirmed by TaqMan RTD-PCR and western blotting (Fig.4a). The upregulation of miR-214 was efficiently repressed by LNA-antimiR-214, while the expression of collagen 1a2, collagen 4a1, TGF-β1, α-SMA and TIMP2 was significantly repressed in the liver of Pdgf-c Tg mice injected with LNA-antimiR-214 compared with mice injected with saline or LNA-antimiR-control. Conversely, the expression of matrix metallopeptidase 2 (MMP2) and MMP9 was significantly upregulated by LNA-antimiR-214. Correlated with these results, western blotting analysis showed that the increased levels of α-SMA and the phosphorylated forms of extracellular signal-regulated kinase (ERK) 1/2 and V-Akt murine thymoma viral oncogene homolog (AKT; p-ERK1/2 and p-AKT) were decreased in the liver of Pdgf-c Tg mice following injection with LNA-antimiR-214 (Fig.4b). Conversely, p-S6K levels were decreased in the liver of Pdgf-c Tg mice and were recovered by injection with LNA-antimiR-214.
Figure 4.
Quantitative evaluation of the expression of pro-fibrotic genes using TaqMan real-time detection-PCR (RTD-PCR) and western blotting. (a) Quantitative analysis of the expression of pro-fibrotic genes using TaqMan RTD-PCR. Fibrotic areas in mouse liver were measured semi-quantitatively by densitometry analysis (n = 6 each). (b) Western blotting analysis of the expression of pro-fibrotic genes (α-SMA, ERK 1/2, p-ERK 1/2, AKT and p-AKT) and protein synthesis (S6K and p-S6K).
Repression of miR-214 with locked nucleic acid-antimiR-214 reduces tumor incidence in Pdgf-c Tg mice
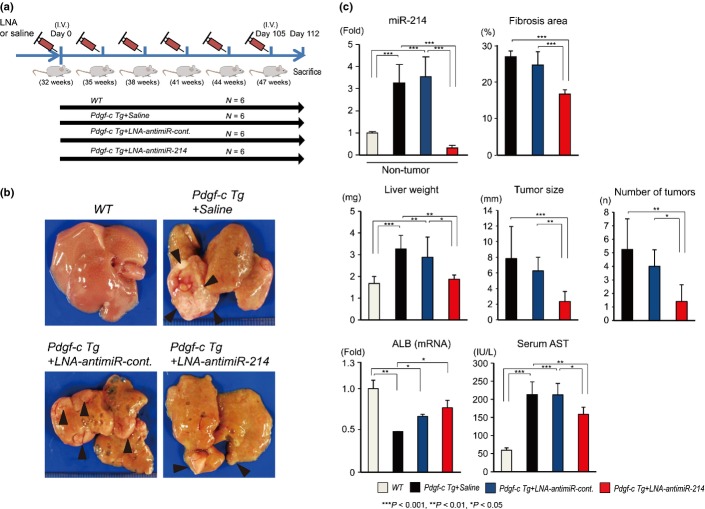
We evaluated whether repression of miR-214 with LNA-antimiR-214 could reduce tumor incidence in Pdgf-c Tg mice. Pdgf-c Tg mice develop dysplastic foci by 6–7 months of age, and preneoplastic foci were detected in all Tg mice by 12 months.2 Thirty-two-week-old Pdgf-c Tg and WT mice were injected with LNA-antimiR-214, LNA-antimiR-control or saline via the tail vein for a total of six injections (50 μg each) at an interval of 3 weeks by using Invivofectamine 2.0 (Fig.5a). At 1 week after the final injection, the mice (each group = 6) were killed. LNA-antimiR-214 substantially repressed the expression of miR-214 in non-tumor and tumor lesions. Liver weight, tumor size and tumor number were significantly reduced following injection with LNA-antimiR-214 compared with saline or LNA-antimiR-control (Fig.5b,c). The fibrotic area in the liver of control Pdgf-c Tg mice (saline group) increased from 13% at 10 weeks to 28% at 47 weeks (Figs4a, 5c and S3a). Repeated injection with LNA-antimiR-214 reduced the fibrotic area from 28% to 17%, indicating that LNA-antimiR-214 was effective on advanced stage liver fibrosis. Moreover, serum aspartate transaminase (AST) levels were significantly reduced, while, conversely, the expression of albumin was significantly increased following injection with LNA-antimiR-214 (Fig.5c). We did not observe obvious histological changes in the lung, heart, kidney or spleen following repeated injection with LNA-antimiR-214 for 15 weeks (data not shown).
Figure 5.
Repeated injection of locked nucleic acid (LNA)-antimiR-214 and tumor incidence in Pdgf-c Tg mice. (a) Pdgf-c Tg or WT mice at 32 weeks of age were injected repeatedly with LNA-antimiR-214, LNA-antimiR-control or saline via the tail vein for a total of six times. One week after the final injection, the mice (each group = 6) were killed. (b) Gross appearance of livers from Pdgf-c Tg or WT mice injected repeatedly with LNA-antimiR-214, LNA-antimiR-control or saline (n = 6). (c) Evaluation of fibrosis, tumors, albumin (mRNA) and serum AST in the liver of Pdgf-c Tg mice injected repeatedly with LNA-antimiR-214, LNA-antimiR-control, or saline (n = 6).
Mir-214 increases EGFR signaling through repression of Mig-6
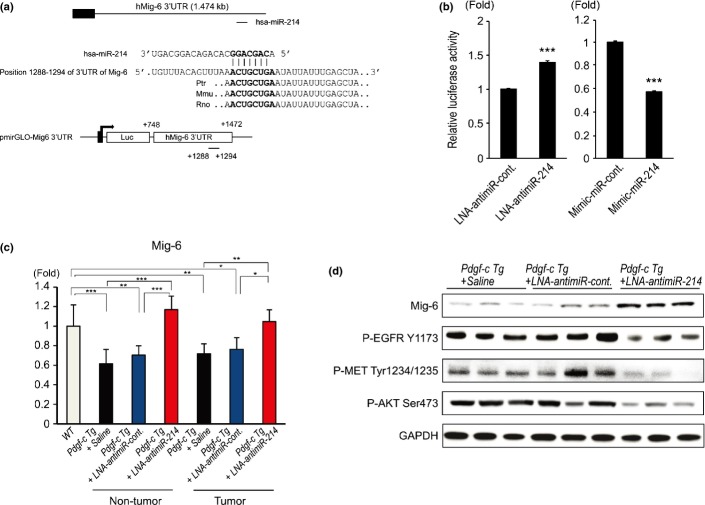
To explore the underlying mechanisms by which miR-214 reduced liver growth in Pdgf-c Tg mice, we searched for target genes of miR-214. We found that Mig-6, a negative regulator of EGFR signaling,13,14 contained a putative miR-214 binding site at nucleotides (nt) 1288–1294 of its 3′-untranslated region (UTR; Fig.6a). To examine whether the regulation of Mig-6 is mediated through its 3′-UTR, we constructed a reporter plasmid, pmirGLO-Mig-6, consisting of a luciferase reporter gene fused to a portion of the 3′-UTR of Mig-6 (nt 748–1472) that included the putative miR-214 binding site (Fig.6a). Transfection of pmirGLO-Mig-6 and LNA-antimiR-214 into 293AD cells significantly increased luciferase activity, while the transfection of pmirGLO-Mig-6 and mimic-miR-214 significantly decreased luciferase activity compared with the control (LNA-antimiR-control or mimic-miR-control; Fig.6b). In vivo, the expression of Mig-6 was significantly decreased in the liver of Pdgf-c Tg mice injected with saline or LNA-antimiR-control, while it recovered following injection with LNA-antimiR-214 (Fig.6c). Western blotting analysis showed that the expression of EGFR and the levels of p-EGFR (Y1173), p-MET (Tyr1234/1235) and p-AKT (Ser473) were decreased in the liver of Pdgf-c Tg mice following injection with LNA-antimiR-214 (Fig.6d). In clinical samples, there was a significant negative correlation between the expression of miR-214 and Mig-6 in the liver of patients with chronic hepatitis C (Figs S1a and S5a).
Figure 6.
MiR-214 targets the EGFR signaling inhibitor Mig-6. (a) Sequence alignment of putative binding sites of miR-214 in the 3′-UTR sequence of Mig-6 and the construction of the reporter plasmid (pmirGLO-Mig-6). (b) Locked nucleic acid (LNA)-antimiR-214 significantly increased the luciferase activity of pmirGLO-Mig-6, a reporter plasmid containing the putative binding site of miR-214 in the 3′-UTR of Mig-6, while mimic-214 decreased the luciferase activity of pmirGLO-Sema6A (n = 6). (c) Changes in the expression of Mig-6 in the liver of Pdgf-c Tg mice that were injected with saline, LNA-antimiR-control or LNA-antimiR-214 (n = 5). (d) Western blotting of EGFR-related genes in the liver of Pdgf-c Tg mice that were injected with saline, LNA-antimiR-control or LNA-antimiR-214. All experiments were performed in duplicate and repeated three times. *P < 0.05, **P < 0.01, ***P < 0.001, Values are means ± SD.
The functional role of miR-214 in EGFR signaling was analyzed in two human HCC cell lines (Huh-7 and HLF) in which the basal expression level of miR-214 was low and high, respectively (Fig.1a). The MTS assay showed a significant increase in the number of Huh-7 cells following stimulation with EGF or miR-214 overexpression (Fig.7a). EGF further augmented the proliferation of Huh-7 cells in which miR-214 was overexpressed. Mimic-miR-214 decreased the expression of Mig-6 and increased the levels of EGF-mediated p-EGFR (Y1173 and Y845) and p-Met (Tyr1234/1235; Fig.7b). When the expression of Mig-6 was repressed using two different siRNA (siMig-6-1 and siMig-6-2), the growth of Huh-7 cells was significantly increased (Fig.7c) and the levels of EGF-mediated p-EGFR (Y1173 and Y845) and p-Met (Tyr1234/1235) were substantially increased (Fig.7d). Conversely, when the expression of miR-214 was repressed in HLF cells by LNA-antimiR-214, their proliferation was significantly repressed (Fig.7e) and the levels of EGF-mediated p-EGFR (Y1173 and Y845) and p-Met (Tyr1234/1235) were substantially decreased (Fig.7f). The combination of LNA-antimiR-214 and siMig-6 (siMig-6-1 and siMig-6-2) partially, but not significantly, rescued the growth of HLF cells (Fig. S5b).
Figure 7.
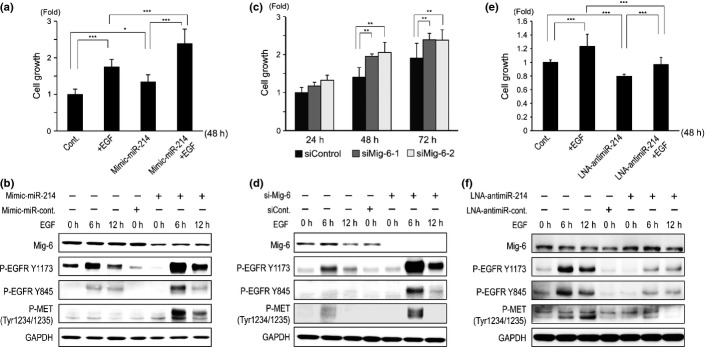
Mir-214 regulates tumor cell growth through EGFR signaling in Huh-7 and HLF cells. (a) Huh-7 cells were grown in 96-well plates under serum-free condition with/without recombinant EGF. Huh-7 cells were transfected with mimic-miR-214 at 24 h prior to the proliferation assay. After 48 h of culture, cell number was assessed by the MTS assay (n = 6). (b) Western blotting of Mig-6, p-EGFR, and p-MET in Huh-7 cells in which miR-214 was over-expressed with/without recombinant EGF. (c) Cell proliferation assay of Huh-7 cells in which Mig-6 was repressed using two different siRNA to Mig-6 (n = 6). (d) Western blotting of Mig-6, p-EGFR, and p-MET in Huh-7 cells in which Mig-6 was repressed with/without recombinant EGF. (e) HLF cells were grown in 96-well plates under serum-free conditions with/without recombinant EGF. HLF cells were transfected with LNA-antimiR-214 at 24 h prior to the proliferation assay. After 48 h of culture, cell number was assessed by the MTS assay (n = 6). (f) Western blotting of Mig-6, p-EGFR and p-MET in Huh-7 cells in which miR-214 was repressed with/without recombinant EGF. *P < 0.05, **P < 0.01, ***P < 0.001, All experiments were performed in duplicate and repeated three times. Values are means ± SD.
Discussion
Micro RNA are small regulatory RNA that play important roles in developmental processes and various diseases, including infection and cancer, and could, therefore, be potential targets for therapeutic intervention. The therapeutic use of the repression of specific miRNA using LNA-modified DNA phosphorothioate antisense oligonucleotides has been applied successfully to liver-expressed miR-122 in humans. This approach could be applicable to hepatic fibrosis induced by various etiologies.15
In this study, we focused on miR-214, which was the most significantly upregulated miRNA in the liver of Pdgf-c Tg mice. Moreover, we showed that the expression of miR-214 was upregulated with the progression of hepatic fibrosis in a diet-induced (Ath + HF) NASH mouse model11 and in patients with chronic hepatitis B or C (Fig. S1a,b). The upregulation of miR-214 was reported in a methionine choline-deficient diet mouse model16 and a dimethylnitrosamine-induced hepatic fibrosis rat model.17 However, others reported the downregulation of miR-214 in toxin-induced liver injury that was induced by ethanol feeding16 and exposure to CCl4 and thioacetamide.18 The precise mechanism of the differential regulation of miR-214 has not been characterized; however, miR-214 was reportedly upregulated in old mice and was related to decreased liver function, such as alcohol metabolism and glutathione metabolism.19 Further studies should be performed to reveal the differential regulation of miR-214 in different liver fibrosis models.
Interestingly, we found that many hepatocytes and HCC-derived cells (THTL-5b, TTNT, Huh-7, Huh-1, HepG2 and Hep3B) expressed low levels of miR-214, while some HCC cells (HLE and HLF) expressed abundant levels. Recently, we reported that there are two cancer stem cell marker-positive cell types (EpCAM+ or CD90+) in HCC cell lines and the gene expression profiles and characteristics of these cells are different. EpCAM+ cells (Huh-7, Huh-1, HepG2 and Hep3B) have a polygonal and epithelial cell shape, while CD90+ cells (HLE and HLF) have a spindle cell shape. EpCAM+ cells express genes with a known function in hepatocytes, while CD90+ cells express endothelial and mesenchymal markers and possess high metastatic capacity.20 Therefore, these findings supported the present result that miR-214 was mainly expressed in mesenchymal cells.
In the present study, we showed the possibility of a unique induction system of miR-214 in hepatocytes. We hypothesize that miR-214 is transferred to hepatocytes by exosomes released from surrounding mesenchymal cells with the progression of hepatic fibrosis. We found that co-culture of Huh-7 cells with Lx-2 cells significantly increased the intracellular levels of miR-214 in Huh-7 cells. Furthermore, by the addition of medium or exosomes derived from Lx-2 cells to the culture medium of Huh-7 cells, we showed the significant increase in intracellular miR-214 levels in Huh-7 cells (Fig.1d). Interestingly, gene expression analysis and a spheroid formation assay showed that Huh-7 cells acquired a more malignant tumor cell phenotype when co-cultured with Lx-2 cells. These results demonstrate that miR-214 is transferred to hepatocytes by exosomes, which are released from activated stellate cells and increased miR-214 levels might play a role in the transformation of hepatocytes. A recent report showed that endothelial cells release miR-214-containing exosomes to stimulate angiogenesis through the silencing of ataxia telangiectasia mutated in neighboring target cells.21 It could be speculated that this unique miRNA distribution system would be beneficial for the long-lasting efficacy of miRNA as they are protected from degradation processes. However, as exosomes contain unknown mRNA and proteins, it should be noted that such unknown factors could upregulate the levels of intrinsic miR-214.
Several reports showed that the expression of miR-214 was upregulated in activated HSC;22,23 however, one report described the downregulation of miR-214.18 Our study showed the upregulation of miR-214 in activated HSC (Fig.1a,b). TGF-β1 stimulated the expression of miR-214 in Lx-2 cells (Fig.1c). Moreover, repression of miR-214 in Lx-2 cells significantly decreased the expression of pro-fibrotic genes (collagen 1a2 and 4a1), EMT-related genes, and TGF-β1 signal-related genes at the mRNA and protein levels (Figs2 and S2).
To evaluate the therapeutic potential of the repression of miR-214 for anti-fibrosis in vivo, LNA-antimiR-214 was delivered efficiently into the liver using Invivofectamine 2.0 (Fig.3). We observed the colocalization of FAM-labeled LNA-antimiR-214 and α-SMA-, desmin-, F4/80- and AFP-positive cells stained using an APC-labeled antibody. These results suggested that LNA-antimiR-214 could be introduced into stellate cells, macrophages and hepatocytes (data not shown). LNA-antimiR-214 efficiently improved hepatic fibrosis in young (9 weeks old) and old (48 weeks old) mice (Figs2c and S4a). We observed a significant reduction in the mRNA levels of pro-fibrotic genes, such as collagen 1a2, collagen 4a1, TGF-β1 and α-SMA, which was associated with decreased levels of p-ERK1/2 and p-AKT (Fig.4). No obvious histological changes were observed in the lung, heart, kidney and spleen following six injections of LNA-antimiR-214. To our knowledge, this is the first report to demonstrate that LNA-antimiR treatment could prevent the progression of hepatic fibrosis in an in vivo mouse model. A recent report showed that genetic deletion of miR-214 significantly attenuated kidney interstitial fibrosis induced by unilateral ureteral obstruction.24
Interestingly, the repeated injection of LNA-antimiR-214 into Pdgf-c Tg mice significantly reduced tumor number and volume (Fig.5). The anti-tumor effect of LNA-antimiR-214 might be due to a secondary effect by reducing the fibrotic state or inflammation of the liver. A recent report showed that pharmacological inhibition of EGFR signaling by erlotinib effectively prevented the progression of hepatic fibrosis and blocked the development of HCC.25
We also found that Mig-6, an inhibitor of EGFR, is a putative target of miR-214 (Fig.6). Mig-6 inhibits EGFR catalytic activity by interfering with its dimerization.14 The expression of Mig-6 was repressed in Pdgf-c Tg mice and recovered by LNA-antimiR-214 (Fig.6c,d), which was consistent with the increased EGFR signaling by mimic-miR-214 and decreased EGFR signaling by LNA-antimiR-214 in cells (Fig.7b,f). Mig-6 could be a target of miR-214 in Lx-2 cells as well as in hepatocytes. Repression of miR-214 by LNA-antimiR-214 increased Mig-6 levels significantly and overexpression of Mig-6 significantly repressed the expression of collagen 1a2, collagen 4a1, PDGFRβ and N-cadherin in Lx-2 cells (Fig. S5c). Moreover, overexpression of Mig-6 repressed the levels of p-AKT (473), p-AKT (308) and p-ERK in Lx-2 cells (Fig. S5d). Thus, our results demonstrate that miR-214 is a pro-fibrotic miRNA by regulating TGF-β and EGFR signaling, and LNA-antimiR-214 resolves hepatic fibrosis in vitro and in vivo.
In addition to pro-fibrotic signaling, LNA-antimiR-214 substantially increased the levels of many ribosomal proteins (Fig. S2), including ribosomal protein S5 (RPS5; data not shown). A recent report showed that overexpression of RPS5 alleviated the progression of dimethylnitrosamine-induced and bile duct ligation-induced hepatic fibrosis.26 Moreover, the upregulation of ribosomal proteins by LNA-antimiR-214 might explain the improved protein synthesis and liver function, as demonstrated by the increased serum levels of albumin (Fig.5).
MiR-214 is involved in hedgehog signaling and targets the phosphatase and tensin homolog27 and p53,28 and its expression is upregulated in ovarian, stomach, pancreatic, cervical, lung and oral mucosal cancers, and malignant melanomas.27,29 Conversely, the decreased expression of miR-214 in HCC has been reported, and it might be related to reduced disease-free survival by upregulating the expression of hepatoma-derived growth factor.30 Although our model showed that reduced miR-214 levels improved hepatic fibrosis and reduced the incidence of hepatic tumors, the functional relevance of miR-214 in tumor cells should be explored further.
In summary, we demonstrated that LNA-antimiR-214 treatment could prevent the progression of hepatic fibrosis in an in vivo mouse model for the first time. Further study should be performed to assess the clinical application of LNA-antimiR-214 for hepatic fibrosis.
Acknowledgments
The authors are grateful to Dr Scott Friedman, Mount Sinai School of Medicine, for providing the Lx-2 cell line. The authors thank Mina Nishiyama for excellent technical assistance.
Disclosure Statement
The authors have no conflict of interest to declare.
Supporting Information
Additional supporting information may be found in the online version of this article:
The expression of miR-214 is upregulated in chronic liver disease.
Fig. S2. The changes of gene expression and phenotype of Huh-7 cells co-cultured with Lx-2 cells.
Fig. S3. Pathway analysis of differentially expressed genes in Lx-2 cells transfected with locked nucleic acid (LNA)-antimiR-214 or LNA-antimiR-control using MetaCore.
Fig. S4. Histopathology of the liver of Pdgf-c Tg and WT mice.
Fig. S5. The expression of miR-214 and Mig-6 in liver tissues and cell lines.
Table S1. Gene category and name of differentially expressed genes regulated by miR-214 in Lx-2 cells.
Data S1. Materials and methods of cell culture and gene expression analysis.
References
- Friedman SL. Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol. 2010;7:425–36. doi: 10.1038/nrgastro.2010.97. [DOI] [PubMed] [Google Scholar]
- Campbell JS, Hughes SD, Gilbertson DG, et al. Platelet-derived growth factor C induces liver fibrosis, steatosis, and hepatocellular carcinoma. Proc Natl Acad Sci U S A. 2005;102:3389–94. doi: 10.1073/pnas.0409722102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H, Honda M, Campbell JS, et al. Acyclic retinoid targets platelet-derived growth factor signaling in the prevention of hepatic fibrosis and hepatocellular carcinoma development. Cancer Res. 2012;72:4459–71. doi: 10.1158/0008-5472.CAN-12-0028. [DOI] [PubMed] [Google Scholar]
- Esquela-Kerscher A, Slack FJ. Oncomirs – MicroRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–69. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- He Y, Huang C, Zhang SP, Sun X, Long XR, Li J. The potential of microRNAs in liver fibrosis. Cell Signal. 2012;24:2268–72. doi: 10.1016/j.cellsig.2012.07.023. [DOI] [PubMed] [Google Scholar]
- Honda M, Yamashita T, Ueda T, Takatori H, Nishino R, Kaneko S. Different signaling pathways in the livers of patients with chronic hepatitis B or chronic hepatitis C. Hepatology. 2006;44:1122–38. doi: 10.1002/hep.21383. [DOI] [PubMed] [Google Scholar]
- Ura S, Honda M, Yamashita T, et al. Differential microRNA expression between hepatitis B and hepatitis C leading disease progression to hepatocellular carcinoma. Hepatology. 2009;49:1098–112. doi: 10.1002/hep.22749. [DOI] [PubMed] [Google Scholar]
- Shinoda M, Tilles AW, Kobayashi N, et al. A bioartificial liver device secreting interleukin-1 receptor antagonist for the treatment of hepatic failure in rats. J Surg Res. 2007;137:130–40. doi: 10.1016/j.jss.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer AM, Cole KE, Smoot DT, et al. Simian virus 40 large tumor antigen-immortalized normal human liver epithelial cells express hepatocyte characteristics and metabolize chemical carcinogens. Proc Natl Acad Sci U S A. 1993;90:5123–7. doi: 10.1073/pnas.90.11.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–20. [PubMed] [Google Scholar]
- Matsuzawa N, Takamura T, Kurita S, et al. Lipid-induced oxidative stress causes steatohepatitis in mice fed an atherogenic diet. Hepatology. 2007;46:1392–403. doi: 10.1002/hep.21874. [DOI] [PubMed] [Google Scholar]
- Safdar H, Cheung KL, Vos HL, et al. Modulation of mouse coagulation gene transcription following acute in vivo delivery of synthetic small interfering RNAs targeting HNF4alpha and C/EBPalpha. PLoS ONE. 2012;7:e38104. doi: 10.1371/journal.pone.0038104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reschke M, Ferby I, Stepniak E, et al. Mitogen-inducible gene-6 is a negative regulator of epidermal growth factor receptor signaling in hepatocytes and human hepatocellular carcinoma. Hepatology. 2010;51:1383–90. doi: 10.1002/hep.23428. [DOI] [PubMed] [Google Scholar]
- Zhang X, Pickin KA, Bose R, Jura N, Cole PA, Kuriyan J. Inhibition of the EGF receptor by binding of MIG6 to an activating kinase domain interface. Nature. 2007;450:741–4. doi: 10.1038/nature05998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen HL, Reesink HW, Lawitz EJ, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368:1685–94. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- Dolganiuc A, Petrasek J, Kodys K, et al. MicroRNA expression profile in Lieber-DeCarli diet-induced alcoholic and methionine choline deficient diet-induced nonalcoholic steatohepatitis models in mice. Alcohol Clin Exp Res. 2009;33:1704–10. doi: 10.1111/j.1530-0277.2009.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WQ, Chen C, Xu MD, et al. The rno-miR-34 family is upregulated and targets ACSL1 in dimethylnitrosamine-induced hepatic fibrosis in rats. FEBS J. 2011;278:1522–32. doi: 10.1111/j.1742-4658.2011.08075.x. [DOI] [PubMed] [Google Scholar]
- Chen L, Charrier A, Zhou Y, et al. Epigenetic regulation of connective tissue growth factor by microRNA-214 delivery in exosomes from mouse or human hepatic stellate cells. Hepatology. 2013;59:1118–29. doi: 10.1002/hep.26768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes OC, An J, Sarojini H, Wang E. Murine microRNAs implicated in liver functions and aging process. Mech Ageing Dev. 2008;129:534–41. doi: 10.1016/j.mad.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Honda M, Nakamoto Y, et al. Discrete nature of EpCAM+ and CD90 + cancer stem cells in human hepatocellular carcinoma. Hepatology. 2013;57:1484–97. doi: 10.1002/hep.26168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Balkom BW, de Jong OG, Smits M, et al. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood. 2013;121:3997–4006. doi: 10.1182/blood-2013-02-478925. , S1–15. [DOI] [PubMed] [Google Scholar]
- Maubach G, Lim MC, Chen J, Yang H, Zhuo L. miRNA studies in in vitro and in vivo activated hepatic stellate cells. World J Gastroenterol. 2011;17:2748–73. doi: 10.3748/wjg.v17.i22.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Wu CQ, Zhang ZQ, Yao DK, Zhu L. Loss of expression of miR-335 is implicated in hepatic stellate cell migration and activation. Exp Cell Res. 2011;317:1714–25. doi: 10.1016/j.yexcr.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Denby L, Ramdas V, Lu R, et al. MicroRNA-214 antagonism protects against renal fibrosis. J Am Soc Nephrol. 2014;25:65–80. doi: 10.1681/ASN.2013010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs BC, Hoshida Y, Fujii T, et al. Epidermal growth factor receptor inhibition attenuates liver fibrosis and development of hepatocellular carcinoma. Hepatology. 2014;59:1577–90. doi: 10.1002/hep.26898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu WH, Hu HG, Tian Y, et al. Bioactive compound reveals a novel function for ribosomal protein S5 in hepatic stellate cell activation and hepatic fibrosis. Hepatology. 2014;60:648–60. doi: 10.1002/hep.27138. [DOI] [PubMed] [Google Scholar]
- Yang H, Kong W, He L, et al. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 2008;68:425–33. doi: 10.1158/0008-5472.CAN-07-2488. [DOI] [PubMed] [Google Scholar]
- Xu CX, Xu M, Tan L, et al. MicroRNA miR-214 regulates ovarian cancer cell stemness by targeting p53/Nanog. J Biol Chem. 2012;287:34970–8. doi: 10.1074/jbc.M112.374611. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ueda T, Volinia S, Okumura H, et al. Relation between microRNA expression and progression and prognosis of gastric cancer: a microRNA expression analysis. Lancet Oncol. 2010;11:136–46. doi: 10.1016/S1470-2045(09)70343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih TC, Tien YJ, Wen CJ, et al. MicroRNA-214 downregulation contributes to tumor angiogenesis by inducing secretion of the hepatoma-derived growth factor in human hepatoma. J Hepatol. 2012;57:584–91. doi: 10.1016/j.jhep.2012.04.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The expression of miR-214 is upregulated in chronic liver disease.
Fig. S2. The changes of gene expression and phenotype of Huh-7 cells co-cultured with Lx-2 cells.
Fig. S3. Pathway analysis of differentially expressed genes in Lx-2 cells transfected with locked nucleic acid (LNA)-antimiR-214 or LNA-antimiR-control using MetaCore.
Fig. S4. Histopathology of the liver of Pdgf-c Tg and WT mice.
Fig. S5. The expression of miR-214 and Mig-6 in liver tissues and cell lines.
Table S1. Gene category and name of differentially expressed genes regulated by miR-214 in Lx-2 cells.
Data S1. Materials and methods of cell culture and gene expression analysis.