Abstract
It has become evident that P-cadherin, one of the classical cadherins, contributes to the malignant behavior of several types of cancer. In this study, we analyzed the expression of P-cadherin and its clinicopathological and prognostic values in intrahepatic cholangiocarcinoma (ICC) and pancreatic cancer. Furthermore, we investigated the functional role of P-cadherin in these cancer cells by knockdown and overexpression in vitro and by analyzing the correlation between the P-cadherin expression and its promoter methylation status. Thirty of 59 ICC cases (51%) and 36 of 73 pancreatic cancer cases (49%) stained positive for P-cadherin with mainly membranous distribution in tumor cells by immunohistochemistry. P-cadherin expression was significantly correlated with several clinicopathological factors, which reflect tumor behavior, and was identified as an independent adverse prognostic factor for disease-free survival in patients with ICC (relative risk [RR] 2.93, P = 0.04) and pancreatic cancer (RR 2.68, P = 0.005) via multivariate analyses. P-cadherin downregulation by siRNA suppressed migration and invasion, and P-cadherin overexpression induced the opposite effects in both ICC and pancreatic cancer cells, without any effects on cell proliferation. P-cadherin expression was related to its promoter methylation status in both cell lines and cancer tissues. In summary, P-cadherin overexpression may serve as a useful biomarker of invasive phenotype and poor prognosis; P-cadherin expression was found to be regulated by its promoter methylation. These results suggest that P-cadherin represents a novel therapeutic target for the treatment of ICC and pancreatic cancer.
Keywords: Intrahepatic cholangiocarcinoma, invasiveness and migration, pancreatic cancer, P-cadherin, promoter hypomethylation
Intrahepatic cholangiocarcinoma (ICC) is the second most common type of primary hepatic malignancy after hepatocellular carcinoma, and accounts for 10–15% of primary liver cancers.1 In Japan, mortality rates of ICC was increased by approximately 20% between 1980 and 2000. Both the incidence and mortality rate of ICC have increased drastically in recent years.2 Although surgical resection is the only current curative treatment for long-term survival, surgery is not suitable for most patients. Pancreatic cancer is the fourth leading cause of cancer-related mortality, and has a poor prognosis, with an overall 5-year survival rate of 6%.3 Although surgical resection provides the only potentially curative treatment for patients with pancreatic cancer, only 10% of patients with pancreatic cancer are treated with curative resections; the remaining patients are treated conservatively.4 Even among patients who undergo surgical resection with curative intent, only 20–25% survive longer than 5-years.5 Information on the molecular pathogenesis of ICC and pancreatic cancer is limited, and new therapeutic targets need to be identified urgently.
Cadherins are key cell adhesion molecules that affect organ development and maintenance. They display tissue-specific distribution, and are identified as epithelial (E), neuronal (N) and placental (P) cadherin isoforms.6 P-cadherin was first identified in mouse placenta.7 P-cadherin mediates cell–cell adhesion via calcium-dependent hydrophilic interactions.8 P-cadherin is linked to the actin cytoskeleton through catenin, thereby establishing the cadherin–catenin complex.9 These molecules play important roles in both cellular adhesion and in signal transduction activities that influence several important biological processes, such as tissue development, cell migration, cell scattering and tumorgenesis.8 In humans, P-cadherin expression is restricted to the basal epithelial layers, which indicates a role in cell growth and differentiation.10 Although P-cadherin expression and its significance have been investigated for several malignancies, its prognostic value remains controversial. P-cadherin overexpression is associated with aggressiveness and/or poor prognosis in breast,11,12 endometrial,13 ovarian14 and colorectal cancers,15 but with better prognosis in oral squamous cell16,17 and gastric cancers,18 and melanomas.19 Promoter methylation of the P-cadherin gene (CDH3) is a proposed mechanism of its overexpression in cancer tissues.18 Our previous study demonstrated that P-cadherin is overexpressed in a wide range of malignancies, including ICC and pancreatic cancer, and that it may represent a promising target for the immunotherapy of these cancers.20
To our knowledge, no study has focused on the relevance of P-cadherin expression to clinicopathological features, long-term outcomes, or the relationship between its expression and promoter methylation status in ICC and pancreatic cancer patients. In this study, we elucidated the clinical and biological significance of P-cadherin and the regulation mechanism of its expression by CDH3 promoter methylation in these cancers.
Material and Methods
Patients and tissue specimen
We enrolled 59 patients with ICC and 73 patients with pancreatic cancer who underwent surgical resection with curative intent from 1993 to 2010 at the Kumamoto University Hospital, Kumamoto, Japan. Patients with combined hepatocellular and cholangiocarcinoma or R2 resections were excluded from this study. Of the 59 patients with ICC, 3 were classified as stage I, 17 as stage II, 16 as stage III, 10 as stage IVa, and 13 as stage IVb. The median follow-up period after surgery was 39.6 months (range: 3.5–241.0). Of the 73 patients with pancreatic cancer, 8 were classified as stage I, 2 as stage II, 27 as stage III, 27 as stage IVa and 9 as stage IVb, based on the basis of the International Union against Cancer tumor-node-metastasis classification.21 The median follow-up period after surgery was 27.0 months (range: 2.5–96.4). Signed informed consent was obtained from all patients. In addition, we collected 27 frozen pancreatic cancer tissues for methylation-specific PCR (MSP) analysis. The study protocol was approved by the Human Ethics Review Committee of the Graduate School of Life Sciences, Kumamoto University.
Immunohistochemical staining and scoring
Immunohistochemical staining was performed on 3-μm, formalin-fixed, paraffin-embedded sections, as described previously.20,22 Briefly, endogenous peroxidase activity was blocked using 3% H2O2 for 5 min. Sections were incubated with mouse anti-human P-cadherin antibody (clone 56; BD Biosciences, Tokyo, Japan) overnight at 4°C, and then incubated with a biotin-free HRP-labeled polymer of the EnVision Plus detection system (Dako, Tokyo, Japan). Positive reactions were visualized with diaminobenzidine solution, followed by counterstaining with Mayer's hematoxylin. P-cadherin staining was scored on the basis of the percentage of positively stained cells by counting >500 cancer cells. When the cut-off value was defined as the median value, ≤15% staining was classified as low expression (P-cadherinlow) and ≥15% as high expression (P-cadherinhigh) in ICC and pancreatic cancer patients. Assessment of immunohistochemical results was based on a semiquantitative evaluation, which did not include staining intensity.
Cell lines and culture
The cholangiocarcinoma cell lines HuCCT-1 and HuH28 were purchased from the Japanese Collection of Research Bioresources (Osaka, Japan); and the RBE, SSP25 and YSCCC cell lines from the RIKEN Bioresource Center (Ibaraki, Japan). RBE, HuCCT-1, HuH28, SSP25 and YSCCC cell lines were cultured in RPMI-1640 (Invitrogen, Tokyo, Japan) containing 10% FBS, and the OZ cell line was cultured in Williams' E medium (Invitrogen) containing 10% FBS. The pancreatic cancer cell lines, such as Panc1, PK-8, PK-59, KLM-1 and MiaPaCa-2, were purchased from the RIKEN Bioresource Center (Ibaraki, Japan), and the Hs700T cell line from the American Type culture collection (Manassas, VA, USA). Panc1, PK-8, PK-59 and KLM-1 cell lines were cultured in RPMI-1640 containing 10% FBS, and the MiaPaCa-2 and Hs700T cells were cultured in D-MEM (Invitrogen) containing 10% FBS. All cultures were maintained in a 5% CO2 air-humidified atmosphere at 37°C.
Depletion of P-cadherin by synthetic small-interfering RNA
Two individual P-cadherin-specific siRNA were chemically synthesized with the following sequences: (#1) 5′-CACUGAUUGAUGUCAAUGAtt-3′, 5-UCAUUGACAUCAAUCAGUGtt-3′ and (#2) 5′-CCCUCUUGGUGUUCGACUAtt-3′, 5′-UAGUCGAACACCAAGAGGGtg-3′ (Ambion). Stealth RNAi negative control (Invitrogen) was used as a negative control. The dose of siRNA was set at 100 nM to cause ≤20% inhibition of P-cadherin compared with the negative control. Twenty-four hours after plating, cells were transfected with 3.0 nM P-cadherin-siRNA or control siRNA using Lipofectamine transfection reagent RNAiMAX (Invitrogen) in accordance with the manufacturer's instructions. At 48 h after transfection, cells were harvested and subjected to western blotting. Each transfection was performed in triplicate and repeated three times, as described previously.23
Lentiviral gene transfer
Lentiviral vector-mediated gene transfer was performed as described previously.24 Briefly, 17 μg of CSII-CMV-RfA and CSIIEFRfA self-inactivating vectors,20 carrying CDH3 cDNA and 10 μg of pCMV-VSV-G-RSV-Rev and pCAG-HIVgp were transfected into 293T cells grown in a 10-cm culture dish by using Lipofectamine 2000 reagent (Invitrogen). At 60 h after transfection, medium was recovered and viral particles were pelleted by ultracentrifugation (50 000 g, 2 h). The pellet was suspended in 50 μL of RPMI 1640, and 10 μL of viral suspension was added to 5 × 104 RBE or MiaPaCa-2 cells per well in a flat-bottomed 96-well plate. Expression of the transfected P-cadherin gene was confirmed by western blotting.
Western blotting
To isolate the proteins, cells collected from 6-well plates were washed once in PBS and lysed in RIPA buffer (50 mM Tris/HCl [pH 7.5], 150 mM NaCl, 1% [v/v] Nonidet P-40, 0.5% sodium deoxycholate and 0.1% sodium dodecyl sulfate [SDS]) and protease inhibitor. Each protein sample (12 μg) was resolved by SDS-polyacrylamide gel electrophoresis, transferred onto a polyvinylidene difluoride membrane, and incubated with P-cadherin mouse monoclonal antibody (1:500 dilution). Signals were detected by incubation with secondary antibodies labeled with the ECL Detection System (GE Healthcare, Little Chalfont, UK).
Matrigel invasion assay
Biocoat Matrigel-coated invasion chambers (BD Biosciences, CA, USA) were used to examine cell invasiveness. Transfected cells were grown to 90% confluence in 6-well plates and seeded. Briefly, 5 × 104 HuCCT-1 or MiaPaCa-2 cells and 1 × 105 PK-59 or RBE cells in 500 μL of serum-free medium were added to the upper chamber. Medium containing 10% FBS was added to the lower chamber. Serum-free medium was added to the lower chamber of control wells. Cells were allowed to invade the Matrigel for 24 h at 37°C in 5% CO2 atmosphere. After 22 h, non-invading cells were removed with a cotton swab, and the invading cells were stained with 1% toluidine blue and counted under a microscope at ×20 magnification.
Scratch assay
Transfected cells were grown to 90% confluence. Subsequently, these cells were moved to 6-well plates and were grown to 95% confluence. A wound was created by scratching cells with a sterile 1000 μL pipette tip. Cells were washed with serum-free medium to remove floating cells, and fresh serum-free culturing medium was added. Photographs of the wound were obtained under ×10 magnification at appropriate times. Wound distances were measured and averaged from three points per wound area as a baseline width, and the width of the mean wound distance was calculated. To evaluate wound closures, the points along each wound were marked, and the horizontal distance the migrating cells traveled into the wound was measured.
Proliferation assay
Transfected cells were grown to 90% confluence in 6-well plates and were seeded in 96-well plates at a density of 1500 cells per well. Medium in each well was changed daily. Viable cholangiocarcinoma cell numbers were measured for 0, 24, 48, 72, 96 or 120 h with a Cell Counting Kit-8 that contained 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium (Dojin Laboratories, Kumamoto, Japan), according to the manufacturer's instructions. Optical density was measured at 450 nm using an automatic microplate reader (Molecular Devices, Osaka, Japan). Each experiment was performed in triplicate.
Methylation-specific PCR analysis
Genomic DNA was extracted from the cholangiocarcinoma and pancreatic cancer cell lines and ICC tumor tissues using standard proteinase-K digestion followed by QI Amp DAN FFPE Tissue Kit (Qiagen, Tokyo, Japan). Extracted genomic DNA was modified with sodium bisulfite using an EpiTect Bisulfite kit (Qiagen). Bisulfite modified DNA was amplified with the primers used for the detection of unmethylated DNA (product size, 149 bp): 5′-TTGTGAGGGGGTGGGATTTTGTGGT-3′ (forward), 5′-ATAAAACAACTACCACAACAACAAACCAA-3′ (reverse) and methylated DNA (141 bp) 5′-CGAGGGGGCGGGATTTCGTG-3′ (forward), 5′-ACAACTACCGCGACGACGAACCGA-3′ (reverse). The following PCR conditions were used for unmethylated DNA: 10 min at 95°C, followed by 35 cycles of 95°C (30 s), 61°C (30 s), 72°C (90 s), and final extension at 72°C for 7 min. Conditions for methylated DNA were similar, but the annealing temperature was maintained at 64°C; MSP was performed twice.
Statistical analysis
Statistical analyses were performed using the JMP program (SAS Institute, Cary, NC, USA). Quantitative data were expressed as mean ± standard deviation, unless stated otherwise. The χ2-test was used to analyze relationships between categorical variables. Kaplan–Meier survival plots were generated and comparisons were made using log-rank tests. To estimate risk factors for recurrence and survival by Cox proportional hazards regression analysis, continuous variables were converted into binary. P < 0.05 was considered significant.
Results
P-cadherin expression is associated with clinicopathological features and poor prognosis in patients with intrahepatic cholangiocarcinoma or pancreatic cancer
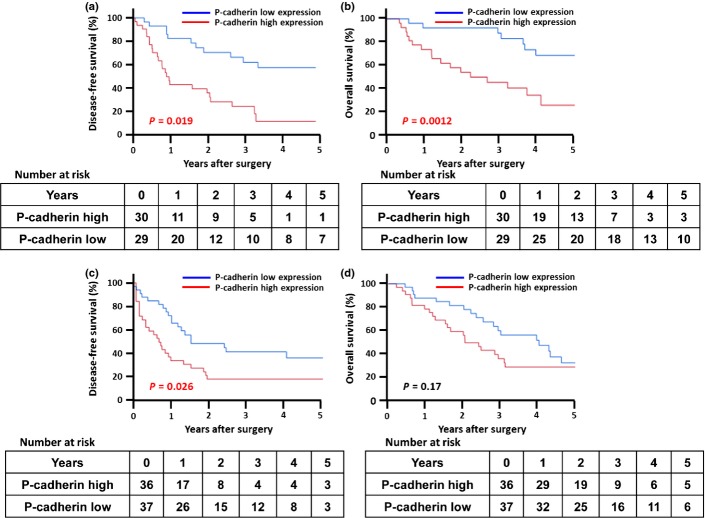
We examined the relationship between P-cadherin expression and clinicopathological features in 59 patients with ICC and 73 with pancreatic cancer by immunohistochemistry. Thirty of 59 ICC cases (51%) and 36 of 73 pancreatic cancer cases (49%) stained positive for P-cadherin, with mainly membranous distribution in tumor cells (Fig.1). No staining was detected in their normal ductal or acinar cells. P-cadherin expression was significantly correlated with tumor diameter (P = 0.0006) and lymph node (LN) metastasis (P = 0.044) in ICC (Suppl. Table S1), and with gender (P = 0.026), posterior tissue invasion (P = 0.046) and other organ invasion (P = 0.015) in pancreatic cancer (Suppl. Table S2). Survival analyses revealed that the cumulative disease-free survival (DFS) and overall survival (OS) were significantly shorter in patients with P-cadherin positive tumors than in those with P-cadherin negative tumors in ICC (P = 0.019, Fig.2a; P = 0.0012, Fig.2b, respectively). In patients with pancreatic cancer, although DFS was significantly worse in patients with P-cadherin positive tumors (P = 0.026, Fig.2c), OS did not differ significantly between the groups (P = 0.17, Fig.2d). Multivariate analyses identified high P-cadherin expression to be an independent predictor for DFS in both ICC (hazard ratio [HR] 2.93, P = 0.044, Table1) and pancreatic cancer (HR 2.68, P = 0.005, Table2). Although univariate analysis revealed that high expression levels of P-cadherin affects OS in ICC, its significance was not retained in multivariate analysis (HR 2.70, P = 0.07, Table1).
Figure 1.
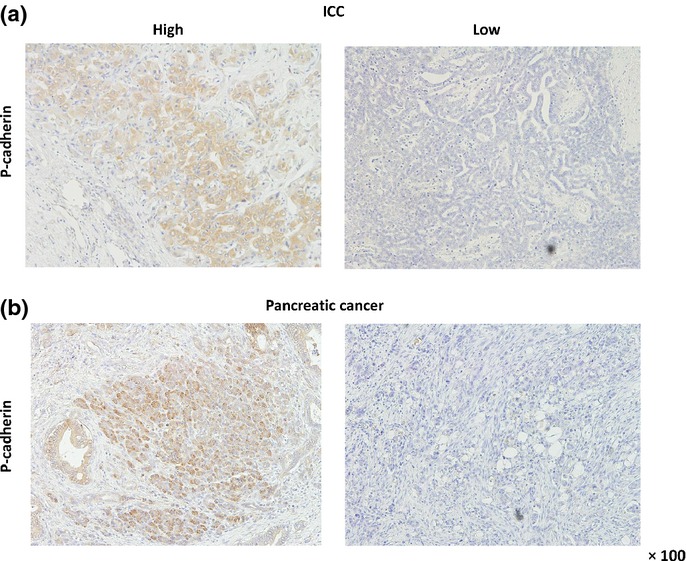
P-cadherin expression shown by immunohistochemistry in intrahepatic cholangiocarcinoma (ICC) and pancreatic cancer tissues. Representative images of positive and negative P-cadherin staining in patients with ICC (a) and pancreatic cancer (b). Staining shows mainly membranous distribution of P-cadherin in tumor cells.
Figure 2.
Cumulative DFS and overall survival OS according to P-cadherin expression. Kaplan–Meier curves show that P-cadherin expression is significantly associated with DFS (a, P = 0.019) and OS (b, P = 0.0012) among patients with ICC; however, among patients with pancreatic cancer, Kaplan–Meier curves show that P-cadherin expression is significantly associated with DFS (c, P = 0.026), but not with OS (d, P = 0.17). DFS, disease-free survival; ICC, intrahepatic cholangiocarcinoma; OS, overall survival.
Table 1.
Univariate and multivariate analyses of predictive factors for disease-free and overall survival in ICC
| Factors | n | Disease free survival | Overall survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | ||||||
| P value | HR | 95% CI | P value | P value | HR | 95% CI | P value | ||
| Age† (years) | |||||||||
| <66 | 28 | 0.0048* | 1.21 | 0.48–2.94 | 0.68 | 0.012* | 1.19 | 0.48–2.9 | 0.70 |
| ≥66 | 31 | ||||||||
| Gender | |||||||||
| Female | 26 | 0.91 | 0.36 | ||||||
| Male | 33 | ||||||||
| CA19-9† (U/ml) | |||||||||
| ≥ 38 | 30 | 0.66 | 0.53 | ||||||
| < 38 | 29 | ||||||||
| Stage | |||||||||
| Stage III/IV | 39 | 0.030* | 0.040* | ||||||
| Stage I/II | 20 | ||||||||
| Tumor diameter† (mm) | |||||||||
| ≥38 | 30 | 0.027* | 3.81 | 1.41–11.11 | 0.0080* | 0.054 | 3.52 | 1.22–10.69 | 0.019* |
| <38 | 29 | ||||||||
| Tumor number | |||||||||
| Multiple | 18 | 0.022* | 1.02 | 0.37–2.91 | 0.98 | 0.055 | 1.01 | 0.34–2.63 | 0.99 |
| Solitary | 41 | ||||||||
| Macroscopic type | |||||||||
| Other types‡ | 19 | 0.97 | 0.92 | ||||||
| Mass forming type | 40 | ||||||||
| Lymph node metastasis§ | |||||||||
| Positive | 15 | 0.0053* | 1.22 | 0.19–2.56 | 0.76 | 0.0011* | 1.21 | 0.19–2.53 | 0.76 |
| Negative | 44 | ||||||||
| Tumor differentiation§ | |||||||||
| Poor | 14 | 0.52 | 0.62 | ||||||
| Well/Mod | 45 | ||||||||
| Vascular invasion§ | |||||||||
| Positive | 31 | 0.0082* | 1.03 | 0.45–2.30 | 0.95 | 0.014* | 1.34 | 0.48–4.80 | 0.60 |
| Negative | 28 | ||||||||
| P-cadherin expression | |||||||||
| High | 30 | 0.019* | 2.93 | 1.03–8.49 | 0.044* | 0.0012* | 2.70 | 0.92–8.03 | 0.070 |
| Low | 29 | ||||||||
*P < 0.05. †Cutoff value defined as the median value. ‡Periductal infiltrating type and intraductal growth type. §Pathological diagnosis. 95% CI, confidence interval; CA19-9, carbohydrate antigen 19-9; mod, moderately-differentiated; poor, poorly differentiated; HR, hazard ratio; well, well-differentiated.
Table 2.
Univariate and multivariate analyses of predictive factors for disease-free and overall survival in pancreatic cancer
| Factors | n | Disease free survival | Overall survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | ||||||
| P-value | HR | 95% CI | P-value | P-value | HR | 95% CI | P-value | ||
| Age† (years) | |||||||||
| <72 | 37 | 0.031* | 1.72 | 0.79–3.84 | 0.17 | 0.23 | |||
| ≥72 | 36 | ||||||||
| Gender | |||||||||
| Male | 35 | 0.5 | 0.83 | ||||||
| Female | 38 | ||||||||
| Operative method | |||||||||
| Pancreatoduodenectomy | 46 | 0.082 | 0.022* | 1.29 | 0.47–3.51 | 0.61 | |||
| Distal pancreatectomy | 27 | ||||||||
| Combined resection of surrounding organs | |||||||||
| Yes | 26 | 0.90 | 0.28 | ||||||
| No | 47 | ||||||||
| Tumor differentiation‡ | |||||||||
| Poor | 11 | 0.019* | 1.73 | 0.58–4.76 | 0.31 | 0.17 | |||
| Well/Mod | 62 | ||||||||
| Interstitial connective tissue‡ | |||||||||
| Scirrhous type | 26 | 0.0011* | 1.23 | 0.59–2.56 | 0.57 | 0.0077* | 1.23 | 0.57–2.64 | 0.59 |
| Medullary/intermediate type | 47 | ||||||||
| Growth patterns of tumors infiltrating surrounding tissue | |||||||||
| INF γ | 32 | 0.0001* | 4.14 | 1.72–10.49 | 0.001* | 0.0003* | 2.3 | 1.03–5.37 | 0.042* |
| INF α/β | 41 | ||||||||
| Lymph duct invasion | |||||||||
| ly 2/3 | 35 | 0.0002* | 1.05 | 0.44–2.39 | 0.91 | 0.0067* | 1.66 | 0.64–4.01 | 0.29 |
| ly 0/1 | 38 | ||||||||
| Venous invasion | |||||||||
| v 2/3 | 41 | 0.0001* | 3.89 | 1.31–12.26 | 0.014* | 0.0003* | 1.73 | 0.65–4.99 | 0.28 |
| v 0/1 | 32 | ||||||||
| Intrapancreatic nerve invasion | |||||||||
| ne 2/3 | 46 | 0.0012* | 1.4 | 0.53–3.72 | 0.49 | 0.0011* | 1.45 | 0.52–4.19 | 0.48 |
| ne 0/1 | 27 | ||||||||
| Tumor size‡ (mm) | |||||||||
| ≥40 | 16 | 0.14 | 0.16 | ||||||
| <40 | 57 | ||||||||
| Pancreatic bile duct invasion | |||||||||
| Positive | 23 | 0.0051* | 2.77 | 1.22–6.27 | 0.015* | 0.066 | |||
| Negative | 50 | ||||||||
| Duodenum invasion‡ | |||||||||
| Positive | 31 | 0.085 | 0.0079* | 1.89 | 0.82–4.64 | 0.14 | |||
| Negative | 42 | ||||||||
| Superior tissue invasion | |||||||||
| Positive | 33 | 0.032* | 1.51 | 0.72–3.18 | 0.28 | 0.069 | |||
| Negative | 40 | ||||||||
| Posterior tissue invasion | |||||||||
| Positive | 36 | 0.008* | 3.42 | 1.37–8.67 | 0.0085* | 0.0059* | 1.53 | 0.68–3.48 | 0.30 |
| Negative | 37 | ||||||||
| Portal vein invasion‡ | |||||||||
| Positive | 24 | 0.15 | 0.086 | ||||||
| Negative | 49 | ||||||||
| Artery invasion | |||||||||
| Positive | 6 | 0.0001* | 4.99 | 1.42–15.97 | 0.014* | 0.0001* | 7.1 | 1.96–23.54 | 0.004* |
| Negative | 67 | ||||||||
| Plexus infiltration‡ (PL) | |||||||||
| Positive | 22 | 0.0004* | 1.73 | 0.74–4.03 | 0.20 | 0.0046* | 1.31 | 0.58–2.99 | 0.52 |
| Negative | 51 | ||||||||
| Other organs invasion‡ | |||||||||
| Positive | 4 | 0.48 | 0.29 | ||||||
| Negative | 69 | ||||||||
| Local development degree‡ | |||||||||
| T 3/4 | 60 | 0.008* | 0.0007* | ||||||
| T 1/2 | 13 | ||||||||
| Lymph node metastasis‡ | |||||||||
| Positive | 44 | 0.001* | 1.03 | 0.37–2.81 | 0.95 | 0.0068* | 1.10 | 0.42–2.85 | 0.85 |
| Negative | 29 | ||||||||
| P-cadherin expression | |||||||||
| High | 36 | 0.026* | 2.68 | 1.35–5.41 | 0.005* | 0.17 | 1.50 | 0.78–2.92 | 0.22 |
| Low | 37 | ||||||||
*P < 0.05. †Cutoff value defined as the median value. ‡Pathological diagnosis. CI, confidence interval; HR, hazard ratio; mod, moderately-differentiated; poor, poorly differentiated; well, well-differentiated.
Expression of P-cadherin in cholangiocarcinoma and pancreatic cancer cell lines
We investigated P-cadherin expression in 6 cholangiocarcinoma and 6 pancreatic cancer cell lines by western blotting (Fig.3a). P-cadherin was highly expressed in HuCCT-1, YSCCC and OZ cholangiocarcinoma cells, and in PK-8, PK-59 and KLM-1 pancreatic cancer cells. For subsequent RNA interference (RNAi) analyses, we generated two Silencer Select siRNA that target different regions of the P-cadherin transcript. Both siRNA efficiently blocked P-cadherin expression in HuCCT-1 and PK59 (Fig.3b). We used P-cadherin-siRNA#2 in later experiments. Furthermore, we transfected a human P-cadherin expression vector into RBE and MiaPaCa-2 cells, both of which do not express P-cadherin. P-cadherin overexpression was confirmed by western blotting in these two cell lines (Fig.3c).
Figure 3.
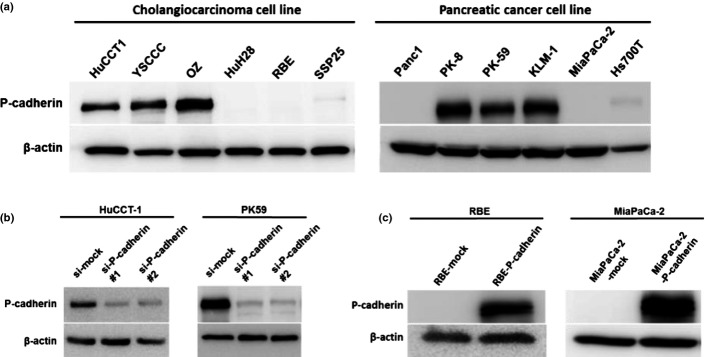
Knockdown and overexpression of P-cadherin in cholangiocarcinoma and pancreatic cancer cell lines. (a) P-cadherin expression in cholangiocarcinoma and pancreatic cancer cell lines is shown by western blot. (b) Knockdown of P-cadherin expression by siRNA in HuCCT-1 and PK59 cancer cells is shown by western blot. Expression was compared 48 h after transfection with P-cadherin-targeting siRNA against mock-transfected control cells. (c) RBE and MiaPaca-2 cells were transfected with cDNA that encoded full-length P-cadherin, and P-cadherin expression was confirmed by western blot against mock-transfected control cells.
Effects of P-cadherin expression on invasiveness migration, and proliferation in cancer cells
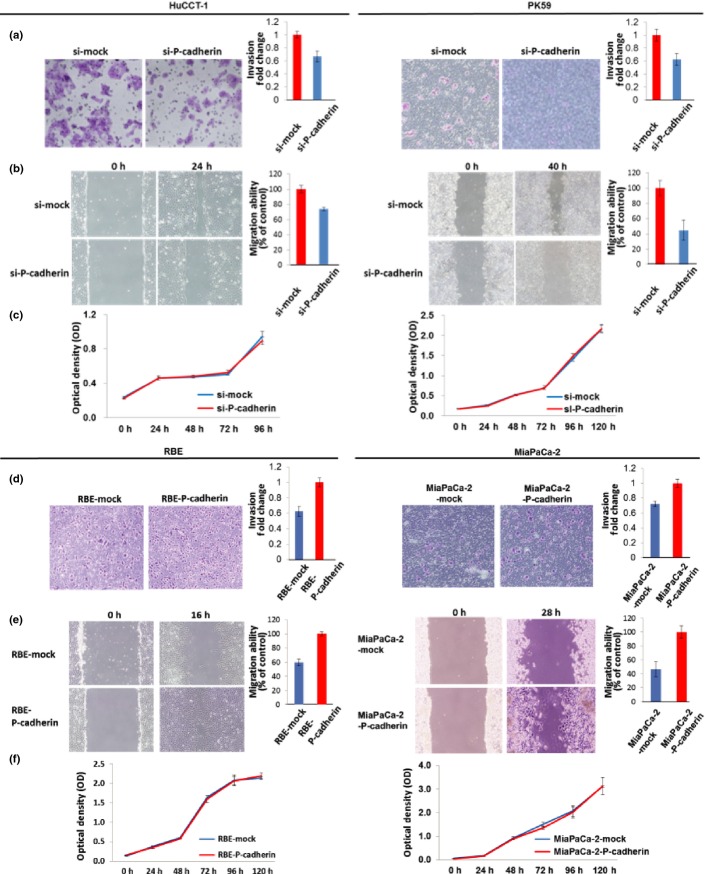
We examined the effect of RNAi-mediated P-cadherin repression on the invasiveness, migration and growth of cholangiocarcinoma (HuCCT1) and pancreatic cancer cells (PK59). Inhibition of P-cadherin led to significantly fewer invasive cells in the Matrigel cell invasion assay (P = 0.014 in HuCCT1 and P = 0.026 in PK59; Fig.4a). Inhibition of P-cadherin resulted in significantly decreased cell migration (P = 0.002 in HuCCT1 and P = 0.009 in PK59; Fig.4b). Inhibition of P-cadherin had no effect on cell proliferation (P = 0.33 in HuCCT1, P = 0.38 in PK59; Fig.4c).
Figure 4.
Effects of P-cadherin expression on invasion, migration and proliferation in cholangiocarcinoma and pancreatic cancer cells. (a) Inhibition of P-cadherin led to significant decrease in invasive cells as seen in a Matrigel cell invasion assay (P = 0.014 in HuCCT1 and P = 0.026 in PK59 cells). (b) Cell migration assay revealed that inhibition of P-cadherin resulted in decrease of cell migration (P = 0.002 in HuCCT1 and P = 0.009 in PK59). (c) Cell proliferation assay revealed that P-cadherin inhibition did not affect cancer cell proliferation in either HuCCT1 or PK59 cells. (d) P-cadherin overexpression led to increase of invasive cells using Matrigel cell invasion assay (P = 0.0061 in RBE and P = 0.019 in MiaPaCa-2). (e) Cell migration assay showed that P-cadherin overexpression resulted in increase of cell migration (P = 0.0005 in RBE and P = 0.006 in MiaPaCa-2). (f) Cell proliferation assay showed that P-cadherin overexpression did not affect cancer cell proliferation in either RBE or MiapaCa-2 cells.
Subsequently, we examined the effect of P-cadherin overexpression on invasiveness, migration and proliferation in cancer cells. P-cadherin overexpression led to increased invasiveness (P = 0.0061 in RBE, P = 0.019 in MiaPaCa-2; Fig.4d) and migration (P = 0.0005 in RBE, P = 0.006 in MiaPaCa-2; Fig.4e), but had no effect on cell proliferation (P = 0.26 in RBE, P = 0.74 in MiaPaCa-2; Fig.4f).
P-cadherin overexpression is associated with promoter hypomethylation in cholangiocarcinoma and pancreatic cancer cells
To evaluate the association between P-cadherin expression and its promoter methylation in cholangiocarcinoma and pancreatic cancer cells, we investigated the promoter methylation status by MSP analysis. HuCCT-1, YSCCC and OZ cholangiocarcinoma cells, and PK-8, PK-59 and KLM-1 pancreatic cancer cells, which express high levels of P-cadherin, had completely hypomethylated CDH3 promoter regions in comparison with cells that express low levels of P-cadherin (Fig.5a). We treated RBE and MiaPaCa-2 cells, which present no expression of P-cadherin and methylated CDH3 promoter regions, with the demethylating agent 5-aza-2V-deoxycytidine (5 μmol/L, 5 days). Complete hypomethylation of the CDH3 promoter region was closely correlated with increased P-cadherin protein expression (Fig.5b).
Figure 5.
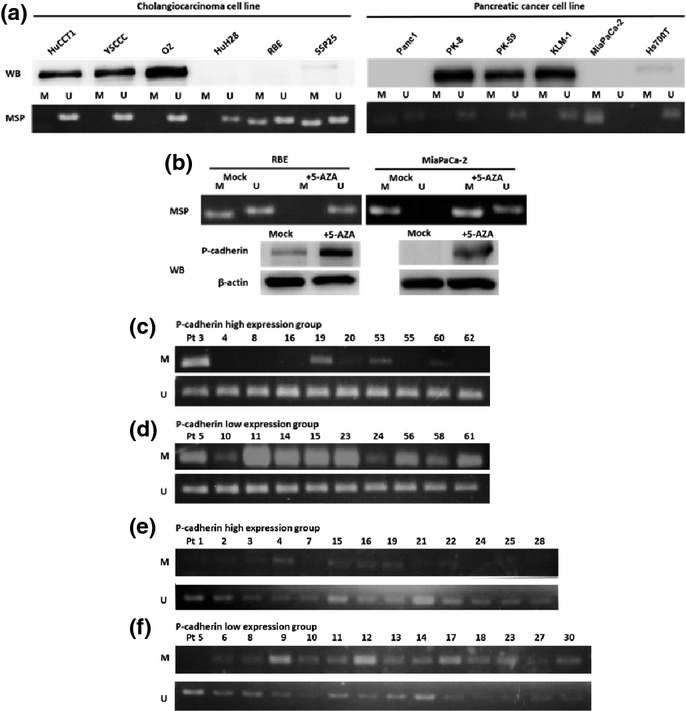
Relation of P-cadherin expression and promoter methylation. (a) Correlation between P-cadherin protein expression and promoter methylation status in HuCCT-1, YSCCC and OZ cholangiocarcinoma cells, and PK-8, PK-59 and KLM-1 pancreatic cancer cells. (b) Methylation status of RBE and MiaPaCa-2 cells, in the presence or absence of the demethylating agent 5-Aza-2V-deoxycytidine. (c,d) The analyses of CDH3 promoter methylation status by methylation-specific PCR in intrahepatic cholangiocarcinoma (ICC) tissues. Representative specimens from 10 P-cadherin high and 10 P-cadherin low tumors by immunohistochemistry were shown. In the 10 P-cadherin high cases, only 6 showed hypomethylation of CDH3 promoter region (c). In contrast, in the 10 P-cadherin low cases, all 10 cases had methylated CDH3 promoter regions (d). (e,f) The analyses of CDH3 promoter methylation status by methylation-specific PCR in pancreatic cancer tissues. Representative specimens from 13 P-cadherin high and 14 P-cadherin low tumors by immunohistochemistry are shown. In the 14 P-cadherin high cases, only 4 cases showed methylated CDH3 promoter region (e). In contrast, in the 14 P-cadherin low cases, 12 cases had methylated CDH3 promoter regions (f).
We then selected 20 resected ICC specimens, including 10 representative P-cadherin-positive and P-cadherin-negative cases each (as confirmed by immunohistochemistry), and examined their CDH3 promoter methylation status by MSP. Of the 10 P-cadherin-positive cases, only 4 contained methylated CDH3 promoter regions (Fig.5c), whereas all 10 P-cadherin-negative cases had methylated CDH3 promoter regions (Fig.5d). In addition, we selected 27 resected pancreatic cancer specimens from 2012, including 13 representative P-cadherin-positive and 14 representative P-cadherin-negative cases (as confirmed by immunohistochemistry), and examined their CDH3 promoter methylation status by MSP. Of the 13 P-cadherin positive cases, only 4 cases showed methylated CDH3 promoter regions (Fig.5e), whereas 12 of the 14 P-cadherin negative cases had methylated CDH3 promoter regions (Fig.5f).
Discussion
In the current study, P-cadherin expression was significantly correlated with several clinicopathological factors, and was identified as an independent adverse prognostic factor for DFS in both ICC and pancreatic cancer. Downregulation of P-cadherin by siRNA suppressed the migration and invasion, and overexpression of P-cadherin induced the opposite effects in both ICC and pancreatic cancer cells. P-cadherin expression was found to be related to its promoter hypomethylation.
To our knowledge, this is the first study to demonstrate the relationship between P-cadherin expression and clinicopathological features and prognosis in ICC and pancreatic cancer. P-cadherin expression was significantly correlated with tumor diameter (P = 0.0006) and LN metastasis (P = 0.044) in ICC (Suppl. Table S1), and with gender (P = 0.026), posterior tissue invasion (P = 0.046) and other organ invasion (P = 0.015) in pancreatic cancer (Suppl. Table S2). DFS was significantly shorter in patients with P-cadherin-positive tumors than in those with P-cadherin negative tumors in both ICC (P = 0.019) and pancreatic cancer (P = 0.026); these results were confirmed by multivariate analyses. Although the prognostic role of P-cadherin expression on clinicopathological features is controversial depending on the cancer type, our results demonstrate that P-cadherin expression reflects tumor aggressiveness and disease recurrence in ICC and pancreatic cancer.
However, how P-cadherin affects tumor behavior is still unclear; there is a discrepancy in its role between different cancer types. Although previous studies have demonstrated an anti-invasive function for P-cadherin in melanoma25 and colon cancer,26 other studies have revealed pro-invasive functions in urinary bladder,26 breast,27 ovarian28 and pancreatic cancers.29 Taniuchi et al.26 demonstrate that P-cadherin overexpression promotes the motility of pancreatic cancer cells by interacting with p120ctn and, consequently, activating Rho-family GTPases, Rac1 and Cdc42. In the current study, P-cadherin downregulation suppressed migration and invasion, and P-cadherin overexpression induced the opposite effects in both ICC and pancreatic cancer cells, without affecting cell proliferation activity. These results suggest that P-cadherin overexpression in cancer cells is associated with invasive phenotype and malignant behavior of ICC and pancreatic cancer.
Cadherin-switching plays a crucial role in tumor progression in several types of cancer, including ovarian,28 bladder30,31 and prostate cancer.32 Cadherin-switching involves changes in the expression of different types of cadherin; switching from E-cadherin to N-cadherin or to P-cadherin is observed during tumorigenesis.15,33–35 E-cadherin downregulation is a hallmark of epithelial-mesenchymal transition (EMT), which affects cancer cell migration and invasion.36 Therefore, EMT may play a role in regulating cancer cell migration and invasion by P-cadherin expression. However, in previous studies on colon cancer and ICC, knockdown of P-cadherin in cancer cells did not induce any change in the expression of EMT markers, including vimentin, slug, snail and twist.15,37 Similarly, in the present study, E-cadherin and vimentin expression was not altered upon overexpression or downregulation of P-cadherin in ICC and pancreatic cancer cells (data not shown). These findings suggest that P-cadherin promotes migration and invasion of cancer cells in an EMT-independent manner.
Van Marck et al.26 reported that the role of P-cadherin in cancer cell adhesion and invasion depends on the cancer type. They demonstrate that, in colon cancer cells, P-cadherin functions as a pro-adhesive and anti-invasive/anti-migratory molecule, similar to E-cadherin. In contrast, in bladder cancer, P-cadherin functions as an anti-adhesive and pro-invasive/pro-migratory molecule. The latter phenomenon is more often observed in several types of cancer, including ICC and pancreatic cancer. These findings suggest that the role of P-cadherin and the significance of cadherin-switching during tumorigenesis should be determined individually in each cancer type.
DNA methylation of CpG islands on promoters is a major gene-regulation mechanism. P-cadherin expression is significantly correlated with promoter hypomethylation in breast,11 gastric,18 and colorectal cancers.38 In the present study, P-cadherin overexpression was correlated with hypomethylation of the CDH3 promoter, whereas treatment of cells that lacked P-cadherin expression with 5-aza-2′-deoxycytidine induced its expression, accompanied with hypomethylation of the CDH3 promoter region in cholangiocarcinoma and pancreatic cancer cells. These phenomena were also observed in specimens isolated from ICC and pancreatic cancer patients. Of the 10 high P-cadherin-expressing ICC tissues, only 4 contained methylated CDH3 promoter regions (Fig.5c), whereas all 10 low P-cadherin-expressing ICC tissues had methylated CDH3 promoter regions (Fig.5d). Similarly, of the 13 P-cadherin high P-cadherin-expressing pancreatic cancer tissues, only 4 contained methylated CDH3 promoter regions (Fig.5e), whereas 12 of the 14 P-cadherin negative cases had methylated CDH3 promoter regions (Fig.5f). Although the discrepancies observed in some cases imply that other mechanisms also mediate P-cadherin expression, our findings indicate that CDH3 promoter methylation is a major regulator of P-cadherin expression in ICC and pancreatic cancer.
In conclusion, our data demonstrate that P-cadherin overexpression is a potential biological marker for invasive phenotype and poor prognosis in ICC and pancreatic cancer, and that P-cadherin expression is possibly regulated by hypomethylation of the CDH3 promoter region. The present study suggests that P-cadherin and its hypomethylation process represent novel therapeutic targets for ICC and pancreatic cancer.
Disclosure Statement
The authors have no conflict of interest to declare.
Supporting Information
Additional supporting information may be found in the online version of this article:
Table S1. Association between P-cadherin expression and clinicopathological features in patients with intrahepatic cholangiocarcinoma.
Table S2. Association between P-cadherin expression and clinicopathological features in patients with pancreatic cancer.
References
- Olnes MJ, Erlich R. A review and update on cholangiocarcinoma. Oncology. 2004;66:167–79. doi: 10.1159/000077991. [DOI] [PubMed] [Google Scholar]
- Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg. 2014 doi: 10.1001/jamasurg.2013.5137. ; doi: 10.1001. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Iovanna J, Mallmann MC, Goncalves A, Turrini O, Dagorn JC. Current knowledge on pancreatic cancer. Front Oncol. 2012;2:6. doi: 10.3389/fonc.2012.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7:163–72. doi: 10.1038/nrclinonc.2009.236. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–34. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- Nose A, Takeichi M. A novel cadherin cell adhesion molecule: its expression patterns associated with implantation and organogenesis of mouse embryos. J Cell Biol. 1986;103:2649–58. doi: 10.1083/jcb.103.6.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock MJ, Johnson KR. Cadherins as modulators of cellular phenotype. Annu Rev Cell Dev Biol. 2003;19:207–35. doi: 10.1146/annurev.cellbio.19.011102.111135. [DOI] [PubMed] [Google Scholar]
- Behrens J. Cadherins and catenins: role in signal transduction and tumor progression. Cancer Metastasis Rev. 1999;18:15–30. doi: 10.1023/a:1006200102166. [DOI] [PubMed] [Google Scholar]
- Shimoyama Y, Hirohashi S, Hirano S, et al. Cadherin cell-adhesion molecules in human epithelial tissues and carcinomas. Cancer Res. 1989;49:2128–33. [PubMed] [Google Scholar]
- Paredes J, Albergaria A, Oliveira JT, Jeronimo C, Milanezi F, Schmitt FC. P-cadherin overexpression is an indicator of clinical outcome in invasive breast carcinomas and is associated with CDH3 promoter hypomethylation. Clin Cancer Res. 2005;11:5869–77. doi: 10.1158/1078-0432.CCR-05-0059. [DOI] [PubMed] [Google Scholar]
- Peralta Soler A, Knudsen KA, Salazar H, Han AC, Keshgegian AA. P-cadherin expression in breast carcinoma indicates poor survival. Cancer. 1999;86:1263–72. doi: 10.1002/(sici)1097-0142(19991001)86:7<1263::aid-cncr23>3.3.co;2-u. [DOI] [PubMed] [Google Scholar]
- Stefansson IM, Salvesen HB, Akslen LA. Prognostic impact of alterations in P-cadherin expression and related cell adhesion markers in endometrial cancer. J Clin Oncol. 2004;22:1242–52. doi: 10.1200/JCO.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Tothill RW, Tinker AV, George J, et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res. 2008;14:5198–208. doi: 10.1158/1078-0432.CCR-08-0196. [DOI] [PubMed] [Google Scholar]
- Sun L, Hu H, Peng L, et al. P-cadherin promotes liver metastasis and is associated with poor prognosis in colon cancer. Am J Pathol. 2011;179:380–90. doi: 10.1016/j.ajpath.2011.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Guerra MF, Marazuela EG, Fernandez-Contreras ME, Gamallo C. P-cadherin expression reduced in squamous cell carcinoma of the oral cavity: an indicatior of poor prognosis. Cancer. 2005;103:960–9. doi: 10.1002/cncr.20858. [DOI] [PubMed] [Google Scholar]
- Lo Muzio L, Campisi G, Farina A, et al. P-cadherin expression and survival rate in oral squamous cell carcinoma: an immunohistochemical study. BMC Cancer. 2005;5:63. doi: 10.1186/1471-2407-5-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MA, Jung EJ, Lee HS, et al. P-cadherin expression in gastric carcinoma: its regulation mechanism and prognostic significance. Hum Pathol. 2010;41:877–85. doi: 10.1016/j.humpath.2009.04.031. [DOI] [PubMed] [Google Scholar]
- Bachmann IM, Straume O, Puntervoll HE, Kalvenes MB, Akslen LA. Importance of P-cadherin, beta-catenin, and Wnt5a/frizzled for progression of melanocytic tumors and prognosis in cutaneous melanoma. Clin Cancer Res. 2005;11:8606–14. doi: 10.1158/1078-0432.CCR-05-0011. [DOI] [PubMed] [Google Scholar]
- Imai K, Hirata S, Irie A, et al. Identification of a novel tumor-associated antigen, cadherin 3/P-cadherin, as a possible target for immunotherapy of pancreatic, gastric, and colorectal cancers. Clin Cancer Res. 2008;14:6487–95. doi: 10.1158/1078-0432.CCR-08-1086. [DOI] [PubMed] [Google Scholar]
- Uenishi T, Ariizumi S, Aoki T, et al. Proposal of a new staging system for mass-forming intrahepatic cholangiocarcinoma: a multicenter analysis by the Study Group for Hepatic Surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2014;21:499–508. doi: 10.1002/jhbp.92. [DOI] [PubMed] [Google Scholar]
- Okabe H, Beppu T, Ueda M, et al. Identification of CXCL5/ENA-78 as a factor involved in the interaction between cholangiocarcinoma cells and cancer-associated fibroblasts. Int J Cancer. 2012;131:2234–41. doi: 10.1002/ijc.27496. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Sakamoto Y, Okabe H, et al. Epigenetic therapy with the histone methyltransferase EZH2 inhibitor 3-deazaneplanocin A inhibits the growth of cholangiocarcinoma cells. Oncol Rep. 2014;31:983–8. doi: 10.3892/or.2013.2922. [DOI] [PubMed] [Google Scholar]
- Tahara-Hanaoka S, Sudo K, Ema H, Miyoshi H, Nakauchi H. Lentiviral vector-mediated transduction of murine CD34(−) hematopoietic stem cells. Exp Hematol. 2002;30:11–7. doi: 10.1016/s0301-472x(01)00761-5. [DOI] [PubMed] [Google Scholar]
- Van Marck V, Stove C, Van Den Bossche K, et al. P-cadherin promotes cell-cell adhesion and counteracts invasion in human melanoma. Cancer Res. 2005;65:8774–83. doi: 10.1158/0008-5472.CAN-04-4414. [DOI] [PubMed] [Google Scholar]
- Van Marck V, Stove C, Jacobs K, Van den Eynden G, Bracke M. P-cadherin in adhesion and invasion: opposite roles in colon and bladder carcinoma. Int J Cancer. 2011;128:1031–44. doi: 10.1002/ijc.25427. [DOI] [PubMed] [Google Scholar]
- Paredes J, Stove C, Stove V, et al. P-cadherin is up-regulated by the antiestrogen ICI 182,780 and promotes invasion of human breast cancer cells. Cancer Res. 2004;64:8309–17. doi: 10.1158/0008-5472.CAN-04-0795. [DOI] [PubMed] [Google Scholar]
- Cheung LW, Leung PC, Wong AS. Cadherin switching and activation of p120 catenin signaling are mediators of gonadotropin-releasing hormone to promote tumor cell migration and invasion in ovarian cancer. Oncogene. 2010;29:2427–40. doi: 10.1038/onc.2009.523. [DOI] [PubMed] [Google Scholar]
- Taniuchi K, Nakagawa H, Hosokawa M, et al. Overexpressed P-cadherin/CDH3 promotes motility of pancreatic cancer cells by interacting with p120ctn and activating rho-family GTPases. Cancer Res. 2005;65:3092–9. doi: 10.1158/0008.5472.CAN-04-3646. [DOI] [PubMed] [Google Scholar]
- Bryan RT, Tselepis C. Cadherin switching and bladder cancer. J Urol. 2010;184:423–31. doi: 10.1016/j.juro.2010.04.016. [DOI] [PubMed] [Google Scholar]
- Bryan RT, Atherfold PA, Yeo Y, et al. Cadherin switching dictates the biology of transitional cell carcinoma of the bladder: ex vivo and in vitro studies. J Pathol. 2008;215:184–94. doi: 10.1002/path.2346. [DOI] [PubMed] [Google Scholar]
- Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin Cancer Res. 2007;13:7003–11. doi: 10.1158/1078-0432.CCR-07-1263. [DOI] [PubMed] [Google Scholar]
- Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4:118–32. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J Cell Biol. 1999;147:631–44. doi: 10.1083/jcb.147.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel IS, Madan P, Getsios S, Bertrand MA, MacCalman CD. Cadherin switching in ovarian cancer progression. Int J Cancer. 2003;106:172–7. doi: 10.1002/ijc.11086. [DOI] [PubMed] [Google Scholar]
- Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–73. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- Baek S, Lee YW, Yoon S, Baek SY, Kim BS, Oh SO. CDH3/P-Cadherin regulates migration of HuCCT1 cholangiocarcinoma cells. Anat Cell Biol. 2010;43:110–7. doi: 10.5115/acb.2010.43.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milicic A, Harrison LA, Goodlad RA, et al. Ectopic expression of P-cadherin correlates with promoter hypomethylation early in colorectal carcinogenesis and enhanced intestinal crypt fission in vivo. Cancer Res. 2008;68:7760–8. doi: 10.1158/0008-5472.CAN-08-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Association between P-cadherin expression and clinicopathological features in patients with intrahepatic cholangiocarcinoma.
Table S2. Association between P-cadherin expression and clinicopathological features in patients with pancreatic cancer.