Abstract
Previous reports have indicated that reprogramming technologies may be useful for altering the malignant phenotype of cancer cells. Although somatic stem cells in normal tissues are more sensitive to reprogramming induction than differentiated cells, it remains to be elucidated whether any specific subpopulations are sensitive to reprogramming in heterogeneous tumor tissues. Here we examined the susceptibility of pancreatic cancer stem cells (CSC) and non-CSC to reprogramming. To characterize CSC populations, we focused on c-Met signaling, which has been identified as a marker of CSC in mouse experiments in vivo. Cells that expressed high levels of c-Met showed higher CSC properties, such as tumor-initiating capacity, and resistance to gemcitabine. Real-time reverse transcription-polymerase chain reaction in cells expressing high levels of c-Met revealed endogenous expression of reprogramming factors, such as OCT3/4, SOX2, KLF4 and cMYC. Introduction of these four factors resulted in higher alkaline phosphatase staining in cells with high c-Met expression than in controls. Therefore, the study results demonstrate that cellular reprogramming may be useful for extensive epigenetic modification of malignant features of pancreatic CSC.
Keywords: Cancer stem cell, cancer therapy, induced pluripotent stem cell, pancreatic cancer, reprogramming
Takahashi and Yamanaka1 first discovered that adult mice fibroblasts could be reprogrammed into a pluripotent state using only four key transgenes (4F: Oct3/4, Sox2, Klf4 and cMyc) and termed these cells “induced pluripotent stem (iPS) cells.” Thereafter, numerous studies have shown that various types of human differentiated cells from normal tissues can also be induced into iPS cells; thus, iPS cell technology has now become the most promising innovation for regenerative medicine.2,3 In malignant cells, Carette et al.4 first applied 4F reprogramming to chronic myeloid leukemia cells and showed that ectopic expression of 4F-enabled differentiation into three germ layer cell types. Our previous studies have demonstrated that serial gastrointestinal cancer cell lines can also be reprogrammed and that iPS-like cancer (iPC) cells and their malignant phenotypes, such as those that are chemoresistant and tumorigenic, were effectively decreased by reprogramming in vitro and in vivo.5,6 These results suggest that reprogramming of gastrointestinal cancer cells modifies their malignant phenotypes.
Many types of malignant tumors, including pancreatic cancer, have poor clinical outcomes despite advances in chemotherapeutic agents.7,8 Many studies have indicated that this is partly because of the existence of a specific subpopulation of cells known as cancer stem cells (CSC), which are characterized by their quiescence, self-renewal capacity, heterogeneity, and multipotency rather than pluripotency, similar to the properties of normal somatic stem cells. In addition, CSC are now well known to show relative resistance to most conventional chemotherapeutic agents.9 Hence, to create a durable clinical response, it is very important to develop effective treatments that target and eliminate CSC. Although our previous studies have demonstrated that reprogramming of serial gastrointestinal cancer cells can decrease their chemoresistance and tumorigenicity, no previous reports have shown whether CSC, which should be targeted, are sensitive to reprogramming.
To isolate CSC from a heterogeneous population prospectively, many cell surface markers are commonly used in various types of malignant cells. Li et al.10 report that in pancreatic cancer, c-Met, a member of the receptor tyrosine kinase family, is currently the most specific marker of pancreatic CSC in vitro and in vivo. Here we studied the susceptibility of pancreatic CSC and non-CSC to reprogramming using the CSC marker c-Met and demonstrated that pancreatic CSC are favorable targets for reprogramming.
Material and Methods
RNA isolation, RT-PCR and quantitative RT-PCR analysis
Total RNA was prepared using TRIzol reagent (Life Technologies, Gaithersburg, MD, USA), and RT was performed using the SuperScript III RT First-Strand Synthesis System according to the manufacturer's instructions (Life Technologies). To confirm PCR amplification, 35 cycles of PCR were performed using a PCR kit (Takara, Kyoto, Japan) on the GeneAmp PCR System 9600 (PE Applied Biosystems, Foster City, CA, USA) under the following conditions: 95°C for 10 s, 60°C for 10 s and 72°C for 60 s. An 8-μL aliquot of each reaction mixture was size-fractionated on a 2% agarose gel and visualized using ethidium bromide staining. To confirm RNA quality, PCR amplification was performed for GAPDH using specific primers. For quantitative assessment, gene expression was evaluated by quantitative RT-PCR (qRT-PCR) using the LightCycler CYBR Green Master kit (Roche Diagnostics, Mannheim, Germany) for cDNA amplification of specific target genes. The expression of mRNA copies was normalized against GAPDH mRNA expression. All primer sequences are listed in Table S1.
Immunocytochemistry
The immunocytochemical examination was performed using antibodies to detect pluripotent markers (anti-Oct3/4, anti-Sox2, anti-Nanog or anti-Tra-1-60) in accordance with the manufacturer's instructions (Cell Signaling Technology, Beverly, MA, USA).
Other methods
Additional methods are described in Data S1.
Results
PANC-1 cells induced with reprogramming factors presented induced pluripotent stem-like phenotype
We previously reported that reprogramming using retroviral or lentiviral vectors, which are DNA viruses, altered their malignant phenotypes.5 However, potential risks of exogenous genomic insertion remain. Because Sendai viral vectors, which are RNA viruses, have the advantage of avoiding undesirable genomic alteration,11 we applied Sendai viral vectors for reprogramming and estimated the optimum conditions needed.
PANC-1 cells were transfected with Sendai virus carrying blue fluorescent protein (BFP) within a certain range of multiplicity of infection (MOI; 0, 3, 30, 100) on day 0. Fluorescence microscopy confirmed that >80% of PANC-1 cells efficiently expressed BFP at an MOI of 30 by day 3 (Fig. S1). In contrast, <30% of PANC-1 cells expressed BFP at an MOI of 3. Thus, we used an MOI dose of 30 in accordance with our protocol (Fig.1a). We infected PANC-1 cells using a series of Sendai viruses carrying four defined transgenes, OCT3/4, SOX2, KLF4 and cMYC, on day 0. RT-PCR detected expression of these four transgenes on day 3 (Fig.1b). According to our reprogramming protocol, PANC-1 cells were cultured on a mouse embryo fibroblast feeder with human ES medium supplemented with 0.5 μM PD0325901, 3 μM CHIR99021 and 0.5 μM A83-01 from day 7. By day 21, a number of iPC cell colonies with a clear, flat, well-defined appearance and high density of granulated cells had gradually formed (Fig.1c).
Figure 1.
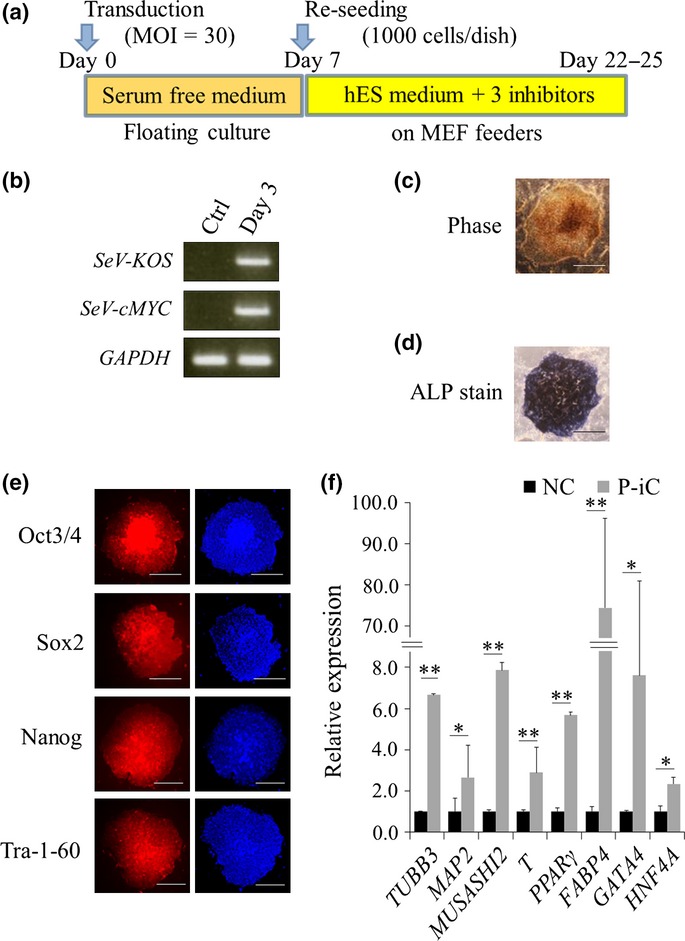
Induction of pluripotent cancer cells from PANC-1 cells. (a) Schematic representation of the reprogramming protocol. (b) RT-PCR analysis of transgenes for reprogramming. (c) Representative image of induced pluripotent stem (iPS)-like colonies. Phase, phase contrast. Bar = 500 μm. (d) Representative image of alkaline phosphatase (ALP)-positive colonies. Bar = 500 μm. (e) Immunofluorescence staining of ES cell markers in reprogrammed colonies. Right panels, Hoechst33342. (f) qRT-PCR of differentiation markers. n = 3. Data are represented as the mean ± SD and were analyzed by two-tailed unpaired t-tests. (*P<0.05, **P<0.01, P-iC vs. NC), P-iC, Post-iPC; NC, Negative control.Bar = 500 μm. ALP, alkaline phosphatase; Ctrl, control; Day 3, post-transduced PANC-1 cells on day 3; iPS, induced pluripotent stem; SeV-cMYC, Sendai virus carrying cMYC; SeV-KOS, Sendai virus carrying polycistronic transgenes KLF4-OCT3/4-SOX2.
Because many types of pluripotent stem cells, including iPS cells, are well known to exhibit alkaline phosphatase (ALP) activity, an ALP staining assay is often used as a screening for undifferentiated status.12,13 We subjected the iPC colonies to the ALP staining assay that showed strongly positive ALP activity (Fig.1d). The ALP-positive colonies were also tested for expression of other undifferentiated cell markers, Oct3/4, Sox2, Nanog and Tra-1-60, by immunofluorescence staining (Fig.1e). Efficient expression of not only exogenous Oct3/4 and Sox2 but also endogenous Nanog and Tra-1-60 in the iPC cells was observed.
To assess the differentiation potential of the iPC colonies, the colonies were picked up and exposed to 5 days of floating culture conditions followed by 7 days of adherent culture conditions. We termed the cells arising from the subsequent culture “post-iPC cells” and compared them with their parental cells using qRT-PCR. The relative expression levels of three serial germ layer markers (TUBB3 [ectoderm], T [mesoderm] and GATA4 [endoderm]) were significantly higher in the post-iPC cells than in their parental cells (Fig.1f). Thus, induced PANC-1 cells were positive for ALP activity and other undifferentiated markers, and simultaneously exhibited potential for differentiation into the other two germ layer derivatives, which indicated that they had become pluripotent stem cells.
c-Met is a good marker of cancer stem cells in pancreatic cancer
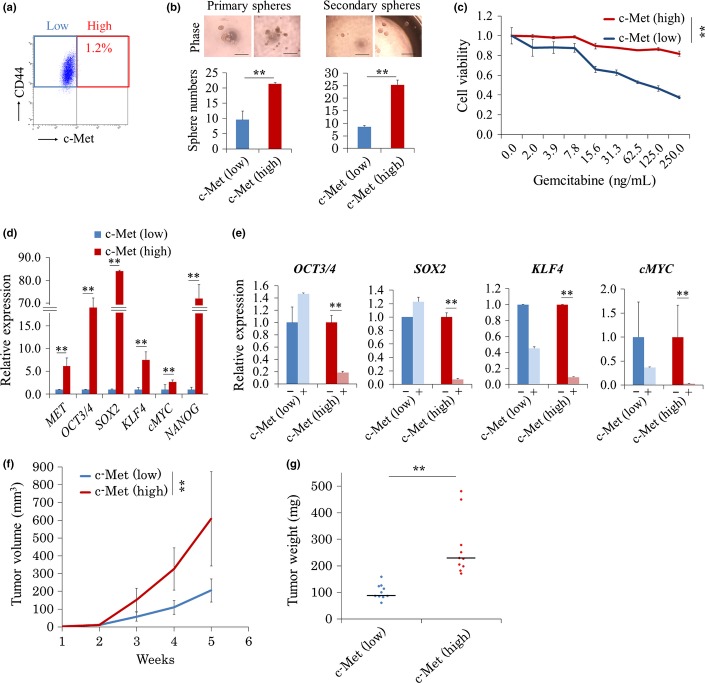
Recent studies have reported that c-Met, or the combination of c-Met and CD44, is one of the most specific markers of CSC in pancreatic cancer.10 Therefore, we aimed to separate pancreatic CSC using these two cell surface markers by flow cytometry (Fig.2a). The population expressing c-Met accounted for 1.2% of all cells. In contrast, almost all PANC-1 cells expressed CD44; thus, this marker was not suitable for isolating CSC. Consequently, for further analysis, we focused on c-Met as a pancreatic CSC marker.
Figure 2.
c-Met (high) population among PANC-1 cells represents high CSC-like phenotypes. (a) Typical FACS plot showing c-Met (high) and CD44 (+) frequencies in PANC-1 cells. (b) Representative images of spheres. Bar = 50 μm. Total number of spheres. N = 6 per group. Data are represented as the mean ± SD and were analyzed by two-tailed unpaired t-tests (**P < 0.01, c-Met [high] vs c-Met [low]). (c) Cell sensitivities to gemcitabine. n = 3. Data are represented as the mean ± SD and were analyzed by two-way measures analysis of variance (**P < 0.01, c-Met [high] vs c-Met [low]). (d) qRT-PCR of endogenous reprogramming transcription factors. n = 3. Data are represented as the mean ± SD and were analyzed by two-tailed unpaired t-tests (**P < 0.01, c-Met [high] vs c-Met [low]). (e) Quantitative RT-PCR of endogenous reprogramming transcription factors. n = 3. Data are represented as the mean ± SD and were analyzed by two-tailed unpaired t-tests (**P < 0.01, c-Met [high] vs c-Met [low]). −, negative control; +, 500 nM SU11274 treatment. (f) 1 × 104 of c-Met (high) and c-Met (low) cells were transplanted in NOD/SCID mice, and the xenograft tumor volume was monitored up to week 5. n = 10. Data are represented as the mean ± SD and were analyzed by two-way repeated measures analysis of variance (**P < 0.01, c-Met [high] vs c-Met [low]). (g) The weights of tumors were measured on week 5 after resection. n = 10. Data are represented as the mean ± SD and were analyzed using the Mann–Whitney U-test (**P < 0.01, c-Met [high] vs c-Met [low]). CSC, cancer stem cell.
To determine the CSC characteristics of cells expressing high levels of c-Met (c-Met [high] population) and of those exhibiting low c-Met expression (c-Met [low] population), we performed sphere formation assays that reflected the cells' self-renewal capacity in vitro. The sphere formation assays demonstrated that the c-Met (high) population formed twice as many spheres as primary spheres (P < 0.01) and three times as many spheres as secondary spheres as did the c-Met (low) population (P < 0.01; Fig.2b). We also tested cell viability on exposure to a chemotherapeutic agent, gemcitabine, using the MTT assays. The MTT assays showed that although gemcitabine inhibited proliferation of both cell populations in a dose-dependent manner, the c-Met (high) cells exhibited greater resistance to gemcitabine than did the c-Met (low) cells (P < 0.05; Fig.2c).
Cancer stem cells are also known to endogenously express stemness genes, such as OCT3/4, SOX2 and NANOG, in many cancers.14,15 We quantified the expression levels of endogenous OCT3/4, SOX2, KLF4, cMYC and NANOG in the two populations. The results demonstrated that the expression levels of these key genes were significantly higher in c-Met (high) cells than in c-Met (low) cells (Fig.2d).
Depending on its ligand, hepatocyte growth factor, c-Met not only is a CSC marker but also functions as a tyrosine kinase. Li et al.14 previously reported that c-Met signaling induced expression of reprogramming transcription factors associated with stemness, including OCT3/4, SOX2 and NANOG. We tested the efficacy of c-Met signaling inhibition on expression of reprogramming genes by treating cells with a specific c-Met inhibitor, SU11274. The maximum tolerated dose of SU11274 for c-Met signaling inhibition was determined to be 500 nM using the MTT assays (Fig. S2a). Expression of endogenous reprogramming factors was shown by qRT-PCR to be inhibited in the c-Met (high) population after treatment with 500 nM SU11274 for 12 h, whereas no effect on gene expression was observed in the c-Met (low) population (Fig.2e). Furthermore, to evaluate stem cell-like phenotypes in the c-Met (high) population, we compared the gene profiles of PANC-1 cells between these two populations. Gene set enrichment analysis revealed that genes upregulated during early stages of differentiation of embryoid bodies from embryonic stem cells tended to be upregulated in c-Met (high) populations relative to those in c-Met (low) populations (Fig. S2b), and genes downregulated during late stages of differentiation of embryoid bodies from embryonic stem cells also tended to be upregulated in c-Met (high) populations relative to those in c-Met (low) populations (Fig. S2b), which suggested that higher CSC characteristics were present in the c-Met (high) population than in the c-Met (low) population.
The gold standard tests for demonstrating the tumor-initiating capacity of CSC are transplantation and the limiting dilution assay. We transplanted four different numbers of c-Met (high) and c-Met (low) cells (100, 500, 1 × 103 and 1 × 104) into 5-week-old NOD/SCID mice to compare the tumor-initiating capacity between these two populations. The limiting dilution assay resulted in significantly higher tumorigenicity of the c-Met (high) population than of the c-Met (low) population (500 cell injection; P < 0.01; Fig. S2c). Similarly, at week 5, the tumor volume of the c-Met (high) population was three times larger (608 vs 205 mm3, P < 0.01), and the weight of the c-Met (high) population was 2.4 times larger (228 vs 94 mg, P < 0.01) than that of their counter populations, respectively (Figs2f,g; Fig. S2d,e). Thus, in agreement with those in previous reports, our results indicated that c-Met is a good marker for pancreatic CSC and functions to maintain stem cell-like phenotypes.
High c-Met–expressing cells, which were enriched in cancer stem cells, showed higher reprogramming efficiency
Previous studies have shown that reprogramming efficiencies of somatic stem cells were higher than those of differentiated cells in normal tissues,16,17 and in malignant tumors, the CSC-enriched population mimicked the characteristics of somatic stem cells, such as self-renewal capacity and multipotency.14,18,19 This study also demonstrated that the c-Met (high) population showed higher self-renewal capacity and expressed endogenous reprogramming factors relatively higher than did the c-Met (low) population in PANC-1 cells. Accordingly, we hypothesized that CSC were more sensitive to reprogramming than were the other differentiated cancer cells in pancreatic cancer. To address this question, we separated the two cell populations according to c-Met expression using the autoMACS Pro Separator (Miltenyi Biotec, Bergisch Gladbach, Germany) and evaluated their reprogramming efficiency. In the present study, because we demonstrated that the c-Met inhibitor, SU11274, inhibited expression of endogenous reprogramming factors, we simultaneously evaluated the efficacy of c-Met signaling inhibition on the reprogramming efficiency in both cell populations (Fig.3a). ALP staining analysis demonstrated that the reprogramming efficiency was threefold higher in the c-Met (high) population than in the c-Met (low) population (P < 0.01), that the same result was observed with immunocytochemical staining assay of Nanog (Fig. S3a) and that SU11274 remarkably reduced the reprogramming efficiency in both populations (P < 0.01; Fig.3b).
Figure 3.
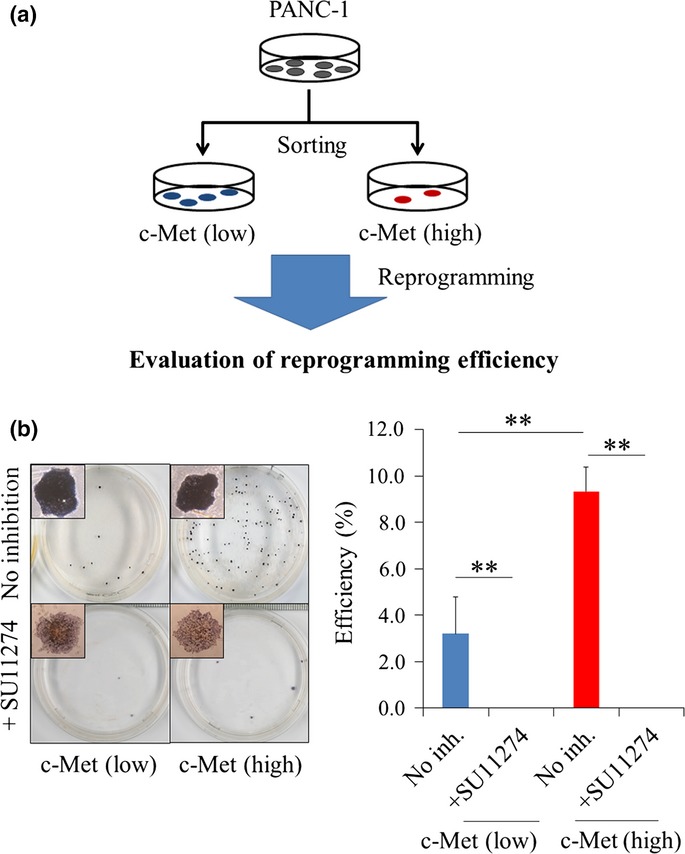
Reprogramming efficiency in high c-Met–expressing cells. (a) Schematic representation of the experimental protocol. (b) Representative images of ALP staining. N = 3. Data are represented as the mean ± SD and were analyzed by two-tailed unpaired t-tests (**P < 0.01, c-Met [high] vs c-Met [low]). ALP, alkaline phosphatase; NC, negative control.
Transfer efficiency and cell viability have been assumed to be critical for successful reprogramming. To test directly whether the immature status or the transfer efficiency of a given cell type affects reprogramming, PANC-1 cells were sorted into c-Met (high) and c-Met (low) populations followed by being transfected with SeV-BFP at MOI 30, respectively. Fluorescence imaging and flow cytometry analysis on day 3 revealed that >95% of PANC-1 cells were positive for BFP in two populations, and no significant difference in transfer efficiency was observed (Fig. S3b,c). Furthermore, to evaluate the cell viability of these two populations under floating culture conditions, the PANC-1 cells of these two populations cultured under 6-day floating conditions were reseeded at a density of 1000 cells/well onto six-well attachment plates supplemented with DMEM containing 10% FBS. After culturing for 3 days, the colonies that appeared were stained with crystal violet, and the number of stained colonies was counted. Crystal violet staining showed that there was no significant difference in the numbers of stained colonies between these two populations (Fig. S3d).
Differentiation and reprogramming of cells are accompanied by drastic epigenetic modifications. Trimethylation of histone 3 lysine 4 (H3K4 me3) is frequently observed in the promoter regions of pluripotent stem cells and is known to be a hallmark of transcriptional activation in general. We measured H3K4 me3 occupancy in the promoter region of the stemness gene, SOX2, among c-Met (low) cells, c-Met (high) cells and iPC cells using ChIP analysis. The ChIP analysis showed that H3K4 me3 was almost absent in the promoter regions of SOX2 of c-Met (low) cells and was enriched up to 0.2% in c-Met (high) cells (P < 0.01; Fig. S3e). In addition, not surprisingly, H3K4 me3 occupancy in the promoter regions of SOX2 of iPC cells was dramatically increased relative to that in the other two populations (P < 0.01; Fig. S3e). This result was compatible with the results of the expression levels of the SOX2 gene in each cell type (P < 0.01; Fig. S3f).
Together, these data indicate that the c-Met signaling pathway affects CSC properties and has an important role in the susceptibility to reprogramming in PANC-1 cells.
Discussion
In this study, we demonstrated that PANC-1 cells, which represent a pancreatic cancer cell line, could be induced to form iPS-like colonies expressing embryonic stem cell markers homogeneously and to possess the potential to differentiate into three germ layer cells by 4F transduction. In addition, we showed that the c-Met (high) population, which was enriched in pancreatic CSC, was more susceptible to reprogramming than was the c-Met (low) counterparts. Although several studies, including ours, have demonstrated that several types of malignant tumor cells are capable of being induced into a pluripotent state by 4F reprogramming,4–6 no study has shown whether any specific subpopulations in heterogeneous tumor tissues may be susceptible to reprogramming.
Some studies have reported reprogramming efficiencies between multipotent somatic stem cells and differentiated cells in normal tissues. Eminli et al.16 show that hematopoietic stem/progenitor cells give rise to iPS cells up to 300 times more efficiently than do terminally differentiated B and T cells and yield reprogramming efficiencies of up to 28%. Wakao et al.17 also demonstrated that multilineage-differentiating stress-enduring (Muse) cells derived from human skin dermal fibroblasts, which are multipotent under some specific conditions, showed more than 30 times higher reprogramming efficiencies than did non-Muse cells. These results provide greater support for the elite model instead of the stochastic model in normal tissues and support our suggestion of an elite model in cancer by which multipotent CSC are poised to become pluripotent stem cells in heterogeneous populations.
The mechanism underlying the reprogramming efficiency in c-Met (high) pancreatic cancer cells remains unknown but may be related to the effects of c-Met signaling itself. The PI3K/Akt axis is a well-characterized downstream effector of c-Met signaling. Lin et al.20 studied in detail the roles of the PI3K/Akt axis associated with stemness using embryonal carcinoma cells. They demonstrated that phosphorylated Akt promotes phosphorylation of Oct3/4, and the resultant phosphorylated Oct3/4 had increased stability, which facilitated its nuclear localization and interaction with Sox2 to promote the transcription of the core stemness genes POU5F1 and NANOG. These results are consistent with our data (Figs2e,3b). The important role of c-Met signaling associated with CSC properties has also been reported in glioblastoma and prostate cancer,14,21 but there has been no reported study of the role of c-Met in reprogramming induction in these cancer types. However, it is assumed that c-Met signaling affects reprogramming efficiency, not directly but through stemness genes, such as OCT3/4 and SOX2. Interestingly, 3.5% of c-Met (low) cells were also positive for ALP staining. Of course, c-Met is not the master marker that can be used to distinguish pancreatic CSC from non-CSC completely, which may imply that reprogramming could detect unknown CSC in the c-Met (low) population.
We also studied reprogramming efficiency in other pancreatic cancer cell lines, MIAPaCa-2, PSN-1 and AsPC-1. However, we found that these cell lines were resistant to reprogramming. These results may be partly explained by innate genetic mutations related to the epithelial-to-mesenchymal transition pathway, such as in the transforming growth factor-β receptor and/or Smad family. Recent studies have shown that initiation of reprogramming requires mesenchymal-to-epithelial transition for cells with a mesenchymal status.22 Hence, we must consider further intervention in addition to simple 4F transduction to study reprogramming efficiency in these cell lines.
Some studies on the deep relationships between core reprogramming factors and CSC phenotype or carcinogenesis have been reported. Ohshima et al.23 demonstrated that induction of OCT3/4, SOX2 and KLF4 to colon cancer cells and cultivation with serum-containing medium, not human ES medium, induced CSC-like phenotype cells. Ohnishi et al.24 also report that insufficient reprogramming to normal cells by 4F is linked to carcinogenesis with epigenetic alterations. However, if reprogramming is achieved, the malignant phenotypes of the reprogrammed cells may decrease. Our previous study demonstrated that reprogrammed PANC-1 cells decreased their proliferation in vitro and tumorigenicity in vivo,5 indicating the rationale for further studies to achieve the high therapeutic efficiency.
Pancreatic cancer cells induced by 4F have shown decreased malignancy in vitro and in vivo.5 Together with our previous data, the results of the present study indicate that reprogramming technology may be useful for extensive epigenetic modification of the malignant features of pancreatic cancer cells with the aim of targeting CSC.
Acknowledgments
We thank all members of our lab for fruitful discussions and technical assistance. This work was supported in part by: a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (http://www.mext.go.jp/english/; #23390199, #25112708, #25134711, #30253420, #26670604, P-Direct; MM, NN, MK, JK, KK and HI); a Grant-in-Aid from the Ministry of Health, Labor and Welfare (http://www.mhlw.go.jp/english/; #H23-003; MM and HI); a grant from the National Institute of Biomedical Innovation (http://www.nibio.go.jp/english/index.html; #12-4; MM and HI); and a grant from the Osaka University Drug Discovery Funds (http://www.osaka-u.ac.jp/en/index.html; MM and HI). Partial support was received from Takeda Science and the Medical Research Foundation (http://www.takeda-sci.or.jp/index.html; MM and HI), Princess Takamatsu Cancer Research Fund (http://www.ptcrf.or.jp/english; MM and HI), Suzuken Memorial Foundation (http://www.suzukenzaidan.or.jp; MK), Yasuda Medical Foundation (http://www.yasuda-mf.or.jp; NN), Pancreas Research Foundation (http://www.jprf.or.jp/shoreisho.html; KK), Nakatani Foundation (http://www.nakatani-foundation.jp; HI) and Nakatomi Foundation of Japan (https://www.nakatomi.or.jp/en/index.html; MK).
Disclosure Statement
Institutional endowments were received from Taiho Pharmaceutical. (http://www.taiho.co.jp/english/), the Evidence-Based Medical (EBM) Research Center (http://ebmrce.co.jp/index.html), Chugai (http://www.chugai-pharm.co.jp/english/index.html), Yakult Honsha (http://www.yakult.co.jp/english/index.html) and Merck (http://www.merck.co.jp/en/index.html); these funders had no role in providing the main experimental equipment, supply expenses, study design, data collection and analysis, decision to publish, or preparation of the manuscript in this work.
Supporting Information
Additional supporting information may be found in the online version of this article:
Fig. S1. Representative fluorescence images of PANC-1 cells expressing blue fluorescent protein.
Fig. S2. CSC properties in the c-Met (high) population. CSC, cancer stem cell.
Fig. S3. Reprogramming efficiency of the c-Met (high) and c-Met (low) populations.
Table S1. A list of primer sequences.
Data S1. Supporting Methods.
References
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Aasen T, Raya A, Barrero MJ, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26:1276–84. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- Sun N, Panetta NJ, Gupta DM, et al. Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. Proc Nat Acad Sci USA. 2009;106:15720–5. doi: 10.1073/pnas.0908450106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carette JE, Pruszak J, Varadarajan M, et al. Generation of iPSCs from cultured human malignant cells. Blood. 2010;115:4039–42. doi: 10.1182/blood-2009-07-231845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino H, Nagano H, Haraguchi N, et al. Hypoxia and TP53 deficiency for induced pluripotent stem cell-like properties in gastrointestinal cancer. Int J Oncol. 2012;40:1423–30. doi: 10.3892/ijo.2012.1346. [DOI] [PubMed] [Google Scholar]
- Miyoshi N, Ishii H, Nagai K, et al. Defined factors induce reprogramming of gastrointestinal cancer cells. Proc Nat Acad Sci USA. 2010;107:40–5. doi: 10.1073/pnas.0912407107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi R, Imamura M, Hosotani R, et al. Surgery versus radiochemotherapy for resectable locally invasive pancreatic cancer: final results of a randomized multi-institutional trial. Surg Today. 2008;38:1021–8. doi: 10.1007/s00595-007-3745-8. [DOI] [PubMed] [Google Scholar]
- Yokoyama Y, Nimura Y, Nagino M. Advances in the treatment of pancreatic cancer: limitations of surgery and evaluation of new therapeutic strategies. Surg Today. 2009;39:466–75. doi: 10.1007/s00595-008-3904-6. [DOI] [PubMed] [Google Scholar]
- Dawood S, Austin L, Cristofanilli M. Cancer stem cells: implications for cancer therapy. Oncology (Williston Park) 2014;28:1101–7. [PubMed] [Google Scholar]
- Li C, Wu JJ, Hynes M, et al. c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology. 2011;141:2218–27. doi: 10.1053/j.gastro.2011.08.009. e5. [DOI] [PubMed] [Google Scholar]
- Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85:348–62. doi: 10.2183/pjab.85.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor MD, Kardel MD, Iosfina I, et al. Alkaline phosphatase-positive colony formation is a sensitive, specific, and quantitative indicator of undifferentiated human embryonic stem cells. Stem Cells. 2008;26:1109–16. doi: 10.1634/stemcells.2007-0801. [DOI] [PubMed] [Google Scholar]
- Singh U, Quintanilla RH, Grecian S, Gee KR, Rao MS, Lakshmipathy U. Novel live alkaline phosphatase substrate for identification of pluripotent stem cells. Stem Cell Rev. 2012;8:1021–9. doi: 10.1007/s12015-012-9359-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li A, Glas M, et al. c-Met signaling induces a reprogramming network and supports the glioblastoma stem-like phenotype. Proc Nat Acad Sci USA. 2011;108:9951–6. doi: 10.1073/pnas.1016912108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonardo E, Hermann PC, Mueller MT, et al. Nodal/Activin signaling drives self-renewal and tumorigenicity of pancreatic cancer stem cells and provides a target for combined drug therapy. Cell Stem Cell. 2011;9:433–46. doi: 10.1016/j.stem.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Eminli S, Foudi A, Stadtfeld M, et al. Differentiation stage determines potential of hematopoietic cells for reprogramming into induced pluripotent stem cells. Nat Genet. 2009;41:968–76. doi: 10.1038/ng.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakao S, Kitada M, Kuroda Y, et al. Multilineage-differentiating stress-enduring (Muse) cells are a primary source of induced pluripotent stem cells in human fibroblasts. Proc Nat Acad Sci USA. 2011;108:9875–80. doi: 10.1073/pnas.1100816108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnekamp JF, Wang X, Medema JP, Vermeulen L. Colorectal cancer heterogeneity and targeted therapy: a case for molecular disease subtypes. Cancer Res. 2015;75:245–9. doi: 10.1158/0008-5472.CAN-14-2240. [DOI] [PubMed] [Google Scholar]
- Vanner RJ, Remke M, Gallo M, et al. Quiescent sox2(+) cells drive hierarchical growth and relapse in sonic hedgehog subgroup medulloblastoma. Cancer Cell. 2014;26:33–47. doi: 10.1016/j.ccr.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Yang Y, Li W, et al. Reciprocal regulation of Akt and Oct4 promotes the self-renewal and survival of embryonal carcinoma cells. Mol Cell. 2012;48:627–40. doi: 10.1016/j.molcel.2012.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida S, Hirohashi Y, Torigoe T, et al. Prostate cancer stem-like cells/cancer-initiating cells have an autocrine system of hepatocyte growth factor. Cancer Sci. 2013;104:431–6. doi: 10.1111/cas.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Liang J, Ni S, et al. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell. 2010;7:51–63. doi: 10.1016/j.stem.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Oshima N, Yamada Y, Nagayama S, et al. Induction of cancer stem cell properties in colon cancer cells by defined factors. PLoS ONE. 2014;9:e101735. doi: 10.1371/journal.pone.0101735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi K, Semi K, Yamamoto T, et al. Premature termination of reprogrammingin vivo leads to cancer development through altered epigenetic regulation. Cell. 2014;156:663–77. doi: 10.1016/j.cell.2014.01.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Representative fluorescence images of PANC-1 cells expressing blue fluorescent protein.
Fig. S2. CSC properties in the c-Met (high) population. CSC, cancer stem cell.
Fig. S3. Reprogramming efficiency of the c-Met (high) and c-Met (low) populations.
Table S1. A list of primer sequences.
Data S1. Supporting Methods.