Abstract
TLR-9 agonists are immunostimulating agents that have antitumor effects in animal models. A phase I trial was conducted to define the safety profile of subcutaneous injections, combined with intrathecally administration of CpG-28, a TRL 9 agonist, in patients with neoplastic meningitis (NM). Cohorts of 3–6 patients with NM were treated for 5 weeks with escalating doses of CpG-28. The primary endpoint was tolerance. Secondary endpoints were progression free survival (PFS) and overall survival (OS). Twenty-nine patients were treated with CpG-28. The primary cancers were malignant glioma, lung carcinoma, breast cancer, melanoma or melanocytoma, ependymoma, and colorectal cancer. The median age was 56 years and median Karnovsky Performance status (KPS) was 70%. The treatment was well tolerated. Adverse effects that were possibly or probably related to the studied drug were grade 2 lymphopenia, anemia and neutropenia, local erythema at injection sites, fever and seizure. There were five serious adverse events: two confusions, two infections of ventricular devices and one grade 4 thrombopenia and neutropenia. The median PFS was 7 weeks and median OS was 15 weeks. Interestingly, the median survival was slightly (but not significantly) higher in the eight patients who were concomitantly treated with bevacizumab (19 weeks vs 15 weeks; P = 0.11). CpG-28 was well tolerated at doses up to 0.3 mg/kg subcutaneously and 18 mg intrathecally. Additional trials are warranted.
Keywords: CpG ODN, immunotherapy, neoplastic meningitis, phase I, TLR-9
Neoplastic meningitis (NM) is a devastating disease, with no effective therapies available. Despite the combination of systemic treatment with intra-cerebrospinal fluid (CSF) chemotherapy and radiotherapy to the sites of bulky disease, the prognosis of NM remains poor, with a median survival of 2–3 months, and only 15% of patients surviving at 12 months.1
Oligodeoxynucleotides containing unmethylated cytosine-guanosine motifs (CpG-ODN) activate both the innate and the adaptive immune system through activation of Toll-like receptor 9 (TLR-9).2 In humans, TLR-9 is mainly expressed by plasmacytoid dendritic cells (pDCs) and B cells.3 Plasmacytoid DCs are present in human CSF and are elevated in various neuroinflammatory conditions.4 TLR-9 is also expressed in human microglial cells,5 and in human glioblastomas (GBM).5,6
Several animal models have shown that immune stimulation by a TLR-9 agonist such as CpG-28 can induce tumor rejection through activation of both innate and specific immunity, especially when injected directly into the tumor.7,8 Phase I and II clinical trials have been conducted in patients harboring recurrent GBM where CpG-28 has been locally administered into the tumor mass; both showed a good safety profile and some cases of minor responses.9,10
This phase I trial was conducted in patients with NM to define the tolerance of CpG-28 injected both subcutaneously and intrathecally.
Materials and Methods
Patient eligibility criteria
The eligibility criteria were defined as follows: age ≥18 years; histologically proven systemic cancer or primitive brain tumor; diagnosis of neoplastic meningitis (positive cerebrospinal fluid cytology and/or clinical symptoms of NM and MRI findings consistent with NM); absence of any visible CSF blockage on cerebro-spinal MRI; a modified Rankin index >3; life expectancy >2 months; and adequate contraception. The exclusion criteria were pregnancy; breast-feeding; history of autoimmune disease; breast cancer NM either responding to or not previously treated with intra-CSF methotrexate; lymphomatous meningitis; contraindication to MRI; platelets <80 000/mm3; neutrophils <500/mm3; lymphocytes <300/mm3; coagulation disorders. All patients signed an informed consent form, which was approved by the institutional review board (Comité de Protection des Personnes participant à une Recherche Biomédicale de Paris-Pitié- Salpetrière; Protocole No. P060103).
Treatment and drug administration
The TLR-9 agonist CpG-28 (sequence 5′-TAAACGTTATAACGTTATGACGTCAT-3′), synthesized with a wholly phosphorothioate backbone was supplied by Oligovax (Paris, France) and AGEPS (Agence Générale des Equipements et Produits de Santé). Cohorts of 3–6 patients were treated for 5 weeks with escalating doses of CpG-28 following a Fibonacci design (level 1: 0.1 mg/kg per week subcutaneously (SC); level 2: 0.3 mg/kg per week SC; level 3: 0.3 mg/kg SC associated with 3 mg intra-CSF every other week; level 4: 0.3 mg/kg SC associated with 7 mg intra-CSF every other week; level 5: 0.3 mg/kg SC associated with 12 mg intra-CSF every other week; and level 6: 0.3 mg/kg SC associated with 18 mg intra-CSF every other week). After level 4, because the dose-limiting toxicity (DLT) was not reached, the protocol was amended to add two supplementary levels (5 and 6) and to allow additional intrathecal injections (1 per month) for the patients showing clinical and/or radiological improvement.
In addition to their usual treatment, patients received clobazam (10 mg before every intrathecal injection and 10 mg during the 3 days after the intra-CSF injection) to prevent seizures and at least 20 mg of prednisolone to prevent arachnoiditis. Concomitant treatments with antiepileptics and any other approved chemotherapeutic agents were allowed if deemed appropriate by the physician in charge of the patient.
Evaluation of the patients
Patients were assessed every week during the initial 5 weeks, and then every 15 days for a month, and then monthly until progression. At each visit, neurological and general examinations, complete blood count, serum biochemistry, and liver function tests were performed. The CSF was examined at each intrathecal injection. Antinuclear antibody titers were measured at baseline, day 30 and 60. Toxicity was graded according to the NCI expanded Common Toxicity Criteria (NCI CTC 3.0).
Spinal and cerebral MRI were performed 4 and 8 weeks after treatment, and then every other month. A complete response was defined as the complete disappearance of all enhancing lesions on MRI for at least 4 weeks on two consecutive images, and a negative CSF cytology, confirmed on three different consecutive CSF studies on days 0, 3 and 30, if the initial cytology was positive. A partial response was defined as a ≥ 50% decrease of the sum of products of diameters of all measurable enhancing lesions on MRI for at least 4 weeks, without any new lesions. Progression was defined as a ≥ 25% increase in the sum of the products of diameters of enhancing lesions, the appearance of any new lesions on MRI. Stable disease applied to patients who did not qualify for complete or partial response, or progression.
Study design and quality insurance
This phase I trial was designed as an open-label, non-randomized study. The first three patients at a dose level were observed for 4 weeks after CpG-28 administration. If no DLT was observed, the dose was then escalated to the next level. If one instance of DLT was observed among the initial three patients, an additional three patients had to be treated at that dose level with no further DLT for dose escalation to proceed. If two instances of DLT were observed at a dose level, a total of six patients had to be treated at the previous level. The maximum tolerated dose (MTD) was the highest dose to cause DLT in no more than one of six patients. DLT was defined as grade 4 toxicity occurring within 1 month after administration of CpG-28. Evidence for severe autoimmune diseases was also considered to be DLT, irrespective of the delay after drug administration. An independent scientific committee that approved each dose escalation monitored all recorded data.
Statistical analysis and end points
Demographic and baseline characteristics were recorded as medians (with ranges) for continuous variables and proportions for categorical variables. The progression-free survival (PFS) was measured from the date of enrolment to the first date of disease progression or of death from any cause. The overall survival (OS) was measured from the date of enrolment to the date of death from any cause.
IP-10 assay
Venous blood samples, drawn into EDTA-containing Vacutainer tubes, were centrifuged and the supernatants were stored at −70°C. IP-10 concentration was assessed using a human IP-10 ELISA Kit (RayBiotech, Norcross, GA, USA).
Results
Patient characteristics
Thirty patients were included between March 2007 and May 2012, but one patient did not receive the experimental drug because of persisting abnormal liver functions after the screening. The characteristics of the 29 treated patients are summarized in Table1.
Table 1.
Patients’ characteristics
| Patients characteristics | No. patients | % |
|---|---|---|
| 29 | ||
| Sex | ||
| Male | 12 | 41 |
| Female | 17 | 59 |
| Age, years | ||
| Median (range) | 56 (21–71) | |
| KPS | ||
| Median (range) | 80 (50–100) | |
| ≥70% | 21 | 72 |
| <70% | 8 | 28 |
| Primary cancer | ||
| Glioma (WHO grade III and IV) | 15 | 51.5 |
| Breast cancer | 3 | 10.3 |
| Small cells lung cancer | 2 | 6.9 |
| Non small cells lung cancer | 6 | 20.7 |
| Colorectal adenocarcinoma | 1 | 3.4 |
| Melanoma | 1 | 34 |
| Melanocytoma | 1 | 3.4 |
| Ependymoma | 1 | 3.4 |
| Time from diagnosis of NM to inclusion, months | ||
| Median (range) | 0.8 (0.1–101.9) | |
| Prior therapy for NM | ||
| WBRT | 4 | 14 |
| Chemotherapy | 8 | 28 |
| None | 18 | 62 |
| Route of administration (n = 23) | ||
| Ventricular device | 5 | 22 |
| Lumbar puncture | 15 | 65 |
| Ventricular device or lumbar puncture | 3 | 13 |
KPS, Karnovsky Performance Status; NM, neoplastic meningitis; WBRT, whole brain radiation therapy; WHO, World Health Organization.
Toxicity
There were 159 adverse events (AEs) reported; 72 were considered as possibly being related to the procedure or studied drug, according to the investigator in charge of the patient, and five of which were considered as severe AE (SAE). AEs ≥grade 2 are summarized in Table2.
Table 2.
Adverse events (≥grade 2) probably or possibly related to the procedure of studied drug according to the investigator in charge of the patient
| Adverse event | Dose level | |||||
|---|---|---|---|---|---|---|
| 0.1 mg/kg per week SC | 0.3 mg/kg per week SC | 0.3 mg/kg per week SC 3 mg/15 days IT | 0.3 mg/kg per week SC 7 mg/15 days IT | 0.3 mg/kg per week SC 12 mg/15 days IT | 0.3 mg/kg per week SC 18 mg/15 days IT | |
| n = 3 | n = 3 | n = 3 | n = 12 | n = 3 | n = 5 | |
| Clinical | ||||||
| Fever > 38.0°C | 2 | 1 | 1 | 6 | – | 1 |
| Seizure | – | – | 1 | 5 | 4 | 2 |
| Local erythema (SC injection) | 3 | 1 | 1 | 6 | 1 | 4 |
| Ommaya reservoir infection | – | – | – | 1† | 1† | – |
| Arachnoiditis | – | – | – | – | 1† | 1† |
| Intra-cerebral hematoma | – | – | – | – | 1† | – |
| Biological | ||||||
| Lymphopenia | ||||||
| Grade 2 | 2 | 2 | – | 4 | 1 | 2 |
| Grade 3 | 2 | 1 | 1 | – | – | 2 |
| Grade 4 | – | – | 1 | – | – | 1 |
Severe adverse event (SAE).
Temperature over 38°C during 24–48 h after CpG-28 injection was noted in 10 patients, was well tolerated and disappeared within 5 days without antibiotics. A relationship with the studied drug is probable.
Local erythema after the subcutaneous injections was seen in 16 patients, was well tolerated and disappeared within 1 week without any treatment. In nine patients, this local erythema was observed after each subcutaneous injection.
Seven patients experienced grade 3 lymphopenia (<500/mm3); four of them already had a grade 2 lymphopenia at the time of inclusion. All of these patients had chemotherapy between 1 and 4 months before study enrolment and/or received concomitant chemotherapy during the protocol, leaving the relationship with the studied drug uncertain.
Within 7 days following administration of CpG-28 into the CSF, 11 patients experienced short partial seizures, which were possibly related to the treatment. All seizures resolved without recurrence following adjustments with anticonvulsant medication.
In addition, there were five SAEs considered as possibly or probably related to the protocol. Two SAEs (arachnoiditis, occurring within 24 h after intra-CSF injection) were considered to be related to the infusion of CpG. Patient 22 had confusion and fever 24 h after the second CpG-28 intrathecal injection. Patient 28 had confusion and fever (above 38.5°C) 8 h after the first CpG-28 intrathecal injection. Both patients fully recovered once the steroid dosage was increased. There were two infections of the Ommaya reservoir (patients 18 and 24). Patient 19 had a subarachnoid hemorrhage 7 days after the first injection, in association with a grade 4 thrombopenia and a grade 4 neutropenia, probably related to concomitant chemotherapy with fotemustine.
There were no treatment-related deaths, and we found no evidence for autoimmune disease after administration of CpG-28.
Dose escalation and dose-limiting toxicity
In level 4, three patients (patients 10, 12 and 15) died prematurely before the end of the protocol, due to tumor progression, and one patient (patient 19) stopped the treatment at day 7 because of an SAE that was not deemed to be related to the study drug. The independent scientific committee recommended the inclusion of six supplementary patients. Because no DLT was seen at this dose level, the protocol was then amended to add two supplementary levels (5 and 6).
One adverse event was considered as a probable DLT at level 6 (patient 28). This patient developed a grade 4 arachnoiditis. As no other DLTs were observed at this last dose level, the MTD was not reached in this study, as could be expected with a non-cytotoxic drug.
Serum IP-10 assessment and CSF studies
In 10 patients, we evaluated the plasma IP-10 levels immediately before and 24 h after the first CpG-28 SC administration (Fig.1). A significant increase was seen 24 h after CpG-28 administration (median IP-10 14 before vs 95 pg/mL after CpG-28 administration, P = 0.02, Mann–Whitney test) and IP-10 levels returned to pre-injection or baseline concentrations at day 7 (data not shown). No clear correlation was found between the plasmatic IP-10 levels and the intensity of local injection site reactions or pyrexia.
Figure 1.
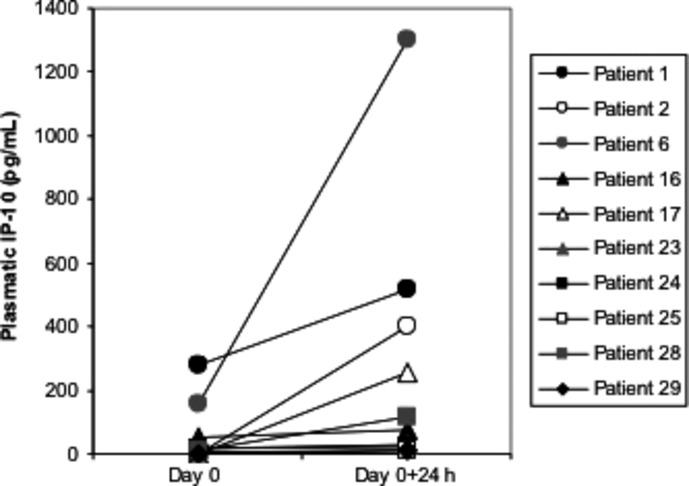
Assessment of interferon-inducible protein-10 (IP-10) in the plasma of 10 patients (two in level 1; one in level 3; two in level 4 and five in level 6), immediately before and 24 h after the first CpG-ODN administration. IP-10 levels were determined by ELISA.
No significant decrease in proteinorachia or pleiocytosis was seen during the protocol. None of the seven patients showing malignant cells at the time of inclusion achieved a complete clearing of malignant cells.
PFS and radiological response
The median PFS was 7 weeks (range, 1.0–81.0; Table3). Four patients improved clinically (patients 7, 8, 9 and 18) and three radiological responses were observed (patients 8, 17 and 18; Table3). These patients were concomitantly treated with chemotherapy or radiotherapy, making the role of CpG-28 unclear.
Table 3.
Efficacy of CpG-28 and concomitant chemotherapy received by the patients during the study
| Dose | Patient | Primary cancer | KPS improvement | Time until neurological progression (weeks) | Survival (weeks) | Concomitant oncological treatment | |
|---|---|---|---|---|---|---|---|
| Radiotherapy | Chemotherapy | ||||||
| 0.1 mg/kg per week SC | #01 | Melanocytoma | No | 9 | 15 | ||
| #02 | Ependymoma WHO grade III | No | 9 | 300 (alive) | |||
| #03 | Breast | No | 5 | 7 | Vinblastine + Cyclophosmamid | ||
| 0.3 mg/kg per week SC | #04 | Breast | No | 4 | 5 | ||
| #05 | Glioblastoma | No | 13 | 16 | |||
| #06 | Optic Astrocytoma WHO III | No | 29 | 29 | |||
| 0.3 mg/kg per week SC 3 mg/15 days IT | #07 | NSCLC | Yes | 4 | 8 | ||
| #08 | SCLC | Yes | 21 | 23 | Spinal | ||
| #09 | Anaplastic oligodendroglioma WHO grade III | Yes | 23 | 30 | Carmustine | ||
| 0.3 mg/kg per week SC 7 mg/15 days IT | #10 | NSCLC | No | 1 | 3 | Erlotinib | |
| #11 | Breast | No | 6 | 18 | Cytarabine + Cisplatine | ||
| #12 | Colorectal | No | 2 | 3 | |||
| #13 | Mixte glioma WHO grade III | Yes | 9 | 26 | Carboplatin + Etoposide | ||
| #14 | NSCLC | No | 5 | 10 | Topotecane | ||
| #15 | NSCLC | No | 2 | 4 | Carboplatin + Pemetrexed | ||
| #16 | NSCLC | No | 21 | 23 | Erlotinib, Pemetrexed | ||
| #17 | Anaplastic oligoastrocytoma WHO Grade III | Stable | 22 | 55 | Carmustine, Bevacizumab | ||
| #18 | Glioblastoma | Yes | 36 | 40 | Lomustine, Bevacizumab | ||
| #19 | Melanoma | Stable | 10 | 84 | Fotemustine | ||
| #21 | Glioblastoma | No | 4 | 4 | Bevacizumab | ||
| #22 | Glioblastoma | No | 3 | 9 | Bevacizumab, Lomustine | ||
| 0.3 mg/kg per week SC 12 mg/15 days IT | #23 | Glioma WHO grade III | Stable | 81 | 81 | Bevacizumab | |
| #24 | Glioblastoma | No | 9 | 14 | |||
| #25 | Glioblastoma | No | 5 | 6 | Spinal thoracic | Bevacizumab | |
| 0.3 mg/kg per week SC 7 mg/15 days IT | #26 | NSCLC | Stable | 19 | 28 | Docetaxel | |
| #27 | Glioblastoma | No | 7 | 15 | Lomustine | ||
| #28 | Glioblastoma | No | 4 | 7 | |||
| #29 | Glioblastoma | No | 4 | 8 | Bevacizumab | ||
| #30 | Glioblastoma | Stable | 11 | 29 | Bevacizumab | ||
KPS, Karnovsky Performance Status; NSCLC, non-small cell lung cancer; SC, subcutaneously; SCLC, small cell lung cancer; WHO, World Health Organization.
At level 4, two patients (patients 17 and 18) had clinical or radiological responses. Because of this improvement, in agreement with the independent scientific committee, these patients were allowed to receive CpG-ODN intrathecally every month until clinical progression.
Three patients showed a remarkable evolution. Patient 2, who had a grade III ependymoma (with a chronic evolution already before inclusion) was stable for 9 weeks during the protocol, and is still alive 6 years after inclusion. Patient 17 showed a clinical and a radiological improvement after the association of CpG-28 and bevacizumab (Fig.2). He remained stable for 5 months, and died at 12.5 months. Patient 18 had a clinical improvement after the concomitant treatment with CpG-28 and bevacizumab, remained stable for 8 months and died at 8.8 months.
Figure 2.
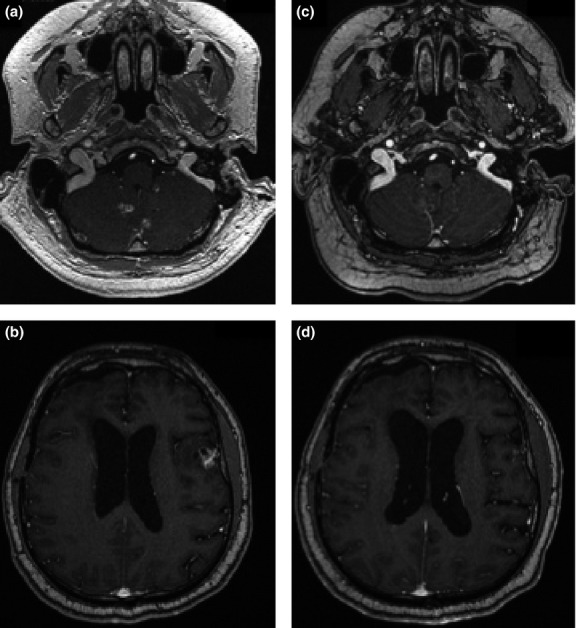
Radiological evolution of MRI (axial post-contrast T1 weighted image) in patient 17. (a) and (b): at screening; c and d: after 7 intrathecal CpG-28 injections combined with 11 cycles of bevacizumab (7.8 months later).
Survival and patient follow-up
The median overall survival in the 29 patients was 15 weeks (range, 3–300; Fig.3a). Interestingly, the median survival tended to be higher in the patients (n = 8) who were concomitantly treated with bevacizumab and intrathecal CpG28, although this trend was not significant (19 weeks vs 15 weeks for patients treated with CpG-28 and bevacizumab vs CpG-28 without bevacizumab; P = 0.11; Fig.3b).
Figure 3.
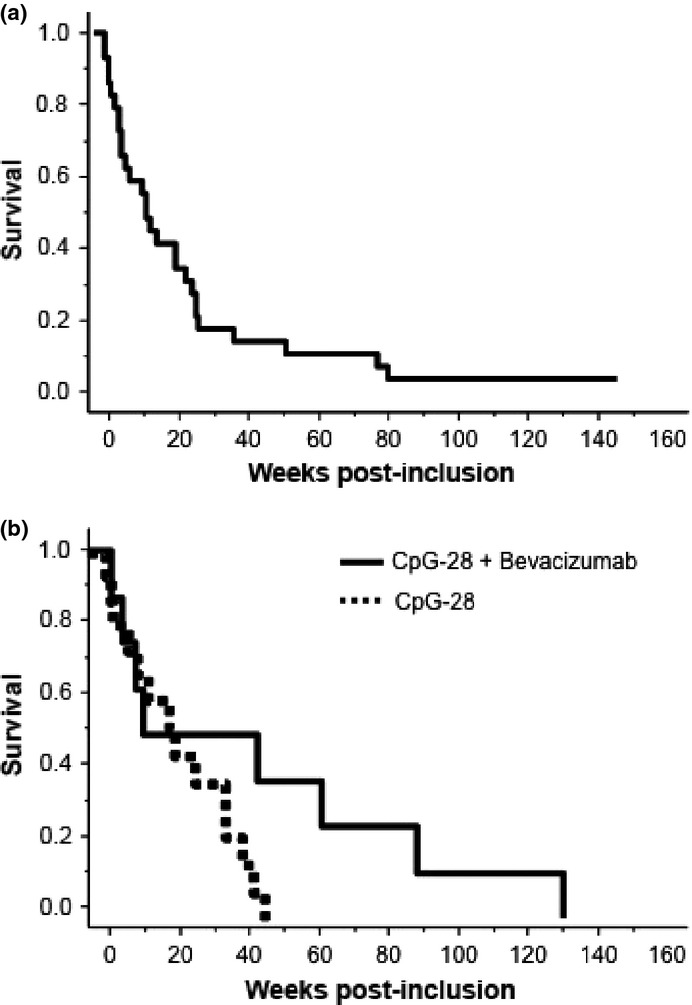
(a) Kaplan–Meier survival curve of patients treated within the protocol. (b) Kaplan–Meier survival curve of patients with glioma treated with combined subcutaneous and intrathecal CpG-28 (levels 3–6), without (n = 15) or concomitantly with (n = 8) bevacizumab (P = 0.11).
Discussion
Neoplastic meningitis (NM) remains challenging for clinical oncologists, because of its resistance to treatments and poor outcome.1,11 As most therapeutic agents are not able to cross the blood–brain barrier, direct intrathecal administration has been proposed as a local approach to circumvent the blood-CSF barrier. However, the number of anticancer drugs that can be safely administered by the intrathecal route is limited. CpG ODNs are powerful new immunostimulating agents that are currently in clinical trials in cancer patients.9,10 In this study, we demonstrated the feasibility of administering CpG-ODN intrathecally for patients with NM.
Safety was the primary endpoint of this trial. Subcutaneous and intrathecal administration of CpG-28 was generally well tolerated and the toxicity profile was very similar to those reported in other clinical trials using CpG-ODN.9,10,12 Symptoms relating to the inflammatory properties of CpG-28 were the most significant toxicity observed. These inflammatory reactions were local in the case of subcutaneous injections (55% of patients), and could induce mild fever (38% of patients). When CpG-28 was injected into the CSF, arachnoiditis was observed in two patients, which was self-limited in one patient and reversible with increased steroid levels for both patients who experienced this toxicity. This arachnoiditis was severe enough in one patient to be considered as a DLT. Short partial seizures, possibly related to arachnoiditis, also occurred in 11 patients (41%) within 7 days of CpG-28 administration, but were easily manageable with anticonvulsant treatments. Asymptomatic lymphopenia was the most frequent adverse event (66%). It remains unclear whether the lymphopenia was related to the studied drug or to previous treatments with chemotherapy. This is a common biological event seen in CpG trials that is counterintuitive with a drug that stimulates lymphoproliferation.9,10,12–14 The mechanisms underlying these lymphopenia remain unclear and might be related to a margination process.15 In summary, a DLT was observed in only one patient, and the MTD was therefore not reached.
IP-10 is an immune chemokine induced by TLR-9 activation.2,15 In our patients, treatment with CpG-28 modulated plasma IP-10 levels. This is consistent with the TH1-like pattern of activation of the innate immune system previously observed after subcutaneous injections of CpG-ODN in healthy humans2,16 or in cancer patients.17,18 Interestingly, the response to CpG-28 was heterogeneous among patients, some responding by a marked elevation of plasma IP-10 (and CSF levels, data not shown), whereas the others responded poorly. This is in contrast with the studies performed on healthy volunteers in which every subject showed an increase in serum IP-10 levels after SC CpG-ODN administration.2 This discrepancy may be related to the health of the patient, given that impaired immunity is commonly observed in patients with cancer, especially when treated with chemotherapy. No consistent association was observed between IP-10 plasmatic levels, local injection site reactions, hyperthermia and tumor response. This result is in line with the other clinical studies evaluating CpG-ODN for the treatment of different tumors.17,18 However, the small sample size in this study may have confounded these results, and further investigations in larger patient cohorts are required for confirmation of our results.
No conclusion on the efficacy of CpG in NM can be drawn from this phase 1 study with escalating doses. In addition, most patients were concomitantly treated with chemotherapy, which was allowed, for ethical reasons, when deemed necessary. Eight patients (levels 4, 5 and 6) with meningeal gliomatosis were treated concomitantly with bevacizumab. Interestingly, the median survival was higher in these patients when compared to the other patients, although not significantly so. In addition, two of these patients showed radiological and a prolonged PFS (22 and 36 weeks). It is difficult to conclude whether the improvement was related to the combination of CpG-28 and bevacizumab or to bevacizumab alone. A pilot study on 15 patients with NM treated with bevacizumab reported some radiological responses, but the PFS was 6 weeks and the overall survival was 14 weeks.19 A synergy between CpG-ODN and bevacizumab may be explained by immunomodulating effects as bevacizumab was recently reported to inhibit Treg accumulation in peripheral blood of patients with mCRC.20 A synergistic effect between local treatment with CpG-28 and anti-Vascular Endothelial Growth Factor treatments was also observed in a murine model of glioblastoma.21
In conclusion, this study demonstrated the safety of administering CpG-28 intrathecally to patients with NM. Additional trials are warranted.
Acknowledgments
Study supported by: the Association Oligocyte, the Association pour le développement des neurosciences à Avicenne (ADNA), the Assistance Publique – Hôpitaux de Paris (sponsor of the trial) and Oligovax Inc. (drug supply).
Disclosure Statement
The Assistance Publique–Hôpitaux de Paris and A.F. Carpentier hold a patent position in CpG ODN. A.F. Carpentier holds shares in Oligovax and is a consultant for Roche.
References
- Le Rhun E, Taillibert S, Chamberlain MC. Carcinomatous meningitis: leptomeningeal metastasis in solid tumors. Surg Neurol Int. 2013;4(Suppl 4):S265–88. doi: 10.4103/2152-7806.111304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg AM, Efler SM, Wittpoth M, Al Adhami MJ, Davis HL. Induction of systemic TH1-like innate immunity in normal volunteers following subcutaneous but not intravenous administration of CPG 7909, a synthetic B-class CpG oligodeoxynucleotide TLR9 agonist. J Immunother. 2004;27:460–71. doi: 10.1097/00002371-200411000-00006. [DOI] [PubMed] [Google Scholar]
- Takeshita S, Takeshita F, Haddad DE, Janabi N, Klinman DM. Activation of microglia and astrocytes by CpG oligodeoxynucleotides. NeuroReport. 2001;12:3029–32. doi: 10.1097/00001756-200110080-00010. [DOI] [PubMed] [Google Scholar]
- Pashenkov M, Huang YM, Kostulas V, Haglund M, Söderström M, Link H. Two subsets of dendritic cells are present in human cerebrospinal fluid. Brain. 2001;124(Pt 3):480–92. doi: 10.1093/brain/124.3.480. [DOI] [PubMed] [Google Scholar]
- Wang C, Cao S, Yan Y, et al. TLR9 expression in glioma tissues correlated to glioma progression and the prognosis of GBM patients. BMC Cancer. 2010;10:415. doi: 10.1186/1471-2407-10-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Kujas M, Marie Y, et al. Expression of TLR9 within human glioblastoma. J Neurooncol. 2008;88:19–25. doi: 10.1007/s11060-008-9536-2. [DOI] [PubMed] [Google Scholar]
- Carpentier AF, Chen L, Maltonti F, Delattre JY. Oligodeoxynucleotides containing CpG motifs can induce rejection of a neuroblastoma in mice. Cancer Res. 1999;59:5429–32. [PubMed] [Google Scholar]
- Carpentier AF, Xie J, Mokhtari K, Delattre JY. Successful treatment of intracranial gliomas in rat by oligodeoxynucleotides containing CpG motifs. Clin Cancer Res. 2000;6:2469–73. [PubMed] [Google Scholar]
- Carpentier A, Laigle-Donadey F, Zohar S, et al. Phase 1 trial of CpG ODN for patients with recurrent glioblastoma. Neuro Oncol. 2006;8:60–6. doi: 10.1215/S1522851705000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier A, Metellus P, Ursu R, et al. Intracerebral administration of CpG oligonucleotide for patients with recurrent glioblastoma: a phase II study. Neuro Oncol. 2010;12:401–8. doi: 10.1093/neuonc/nop047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz MJ, LaFollette S, Jaeckle KA, et al. Randomized trial of a slow-release versus a standard formulation of cytarabine for the intrathecal treatment of lymphomatous meningitis. J Clin Oncol. 1999;17:3110–6. doi: 10.1200/JCO.1999.17.10.3110. [DOI] [PubMed] [Google Scholar]
- Pashenkov M, Goëss G, Wagner C, et al. Phase II trial of a toll-like receptor 9 oligonucleotide in patients with metastatic melanoma. J Clin Oncol. 2006;24:5716–24. doi: 10.1200/JCO.2006.07.9129. [DOI] [PubMed] [Google Scholar]
- Hofmann MA, Kors C, Audring H, Walden P, Sterry W, Trefzer U. Phase 1 evaluation of intralesionally injected TLR9-agonist PF-3512676 in patients with basal cell carcinoma or metastatic melanoma. J Immunother. 2008;31:520–7. doi: 10.1097/CJI.0b013e318174a4df. [DOI] [PubMed] [Google Scholar]
- Link BK, Ballas ZK, Weisdorf D, et al. Oligodeoxynucleotide CpG 7909 delivered as intravenous infusion demonstrates immunologic modulation in patients with previously treated non-Hodgkin's lymphoma. J Immunother. 2006;29:558–68. doi: 10.1097/01.cji.0000211304.60126.8f. [DOI] [PubMed] [Google Scholar]
- Ursu R, Carpentier AF. Immunotherapeutic approach with oligodeoxynucleotides containing CpG motifs (CpG-ODN) in malignant glioma. Adv Exp Med Biol. 2012;746:95–108. doi: 10.1007/978-1-4614-3146-6_8. [DOI] [PubMed] [Google Scholar]
- Vicari AP, Schmalbach T, Lekstrom-Himes J, et al. Safety, pharmacokinetics and immune effects in normal volunteers of CPG 10101 (ACTILON), an investigational synthetic toll-like receptor 9 agonist. Antivir Ther. 2007;12:741–51. [PubMed] [Google Scholar]
- Weber JS, Zarour H, Redman B, et al. Randomized phase 2/3 trial of CpG oligodeoxynucleotide PF-3512676 alone or with dacarbazine for patients with unresectable stage III and IV melanoma. Cancer. 2009;115:3944–54. doi: 10.1002/cncr.24473. [DOI] [PubMed] [Google Scholar]
- Yamada K, Nakao M, Fukuyama C, et al. Phase I study of TLR9 agonist PF-3512676 in combination with carboplatin and paclitaxel in patients with advanced non-small-cell lung cancer. Cancer Sci. 2010;101:188–95. doi: 10.1111/j.1349-7006.2009.01361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves MD, DeGroot J, Tremont I, et al. A pilot study of systemically administered bevacizumab in patients with neoplastic meningitis/imaging, clinical, CSF, and biomarker outcomes. Neuro Oncol. 2011;13:iii85–91. (Abstract). [Google Scholar]
- Terme M, Pernot S, Marcheteau E, et al. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. J Cancer Res. 2013;73:539–49. doi: 10.1158/0008-5472.CAN-12-2325. [DOI] [PubMed] [Google Scholar]
- Banissi C, Maubant S, Rancic M, Carpentier AF. Combination of peritumoral administration of CpG-ODN with immunization against VEGF for treatement of malignant glioma. Neuro Oncol. 2012;14(Suppl 3):iii1–94. (Abstract). [Google Scholar]


