Abstract
The P2X7 receptor, an ATP-gated plasma membrane ion channel, is involved in inflammation, apoptosis and cell proliferation, and thereby plays a crucial role during oncogenic transformation in various malignancies. This study aims to evaluate the impact of P2X7 receptor expression on postoperative cancer-specific survival of patients with clear-cell renal cell carcinoma (ccRCC). A total of 273 patients with ccRCC undergoing nephrectomy at a single institution were retrospectively enrolled in this study, among which 86 patients died of this disease and six patients died of other causes. Clinicopathologic features and cancer-specific survival (CSS) were recorded. P2X7 expression was assessed by immunohistochemistry in clinical specimens. Kaplan–Meier method with log rank test was performed to compare survival curves. Cox regression models were used to evaluate the prognostic values of variables on CSS. Concordance index was calculated to assess prognostic accuracy of prognostic models. Median follow-up period was 90 months (range, 11–120 months). Intratumoral P2X7 expression was significantly lower than peritumoral tissues (P < 0.001). Moreover, high intratumoral P2X7 expression, which was significantly associated with shorten CSS (P < 0.001), high TNM stage (P = 0.038), Fuhrman grade (P = 0.035), SSIGN (stage, size, grade, and necrosis) score (P = 0.021) and University of California Integrated Staging System (UISS) score (P = 0.007), was indicated to be an independent prognostic factor for CSS (hazard ratio [HR], 1.693; P = 0.034). The prognostic accuracy of TNM stage, UISS and SSIGN scoring models was improved when intratumoral P2X7 expression was added. Intratumoral P2X7 expression is a potential independent adverse prognostic indicator for postoperative CSS of patients with ccRCC.
Keywords: Clear-cell renal cell carcinoma, cancer-specific survival, extracellular ATP receptor, P2X7, prognostic biomarker
Kidney cancer caused nearly 13 680 deaths and had 65 150 new cases in the USA according to 2013 statistics.1 Most kidney cancers are categorized as clear-cell renal cell carcinoma (ccRCC), accounting for 2–3% of all adult malignancies, and its incidence and mortality has also been arising 2–3% per decade worldwide.2,3 ccRCC can be cured by surgery if detected at early stage. However, 30–40% of patients still experience recurrence or metastasis and approximate 102 000 deaths are caused annually.4 The natural history of ccRCC is complicated and clinical outcome can be varied even with similar pathological features. Thus, to screen out high-risk patients for extra appropriate postoperative therapy and surveillance planning, it is of high priority to establish an accurate outcome prediction model for patients who undergo curative intended nephrectomy.
Currently, several prognostic models for RCC patients have been established. Besides the TNM staging system being last modified in 2009, the other two major models include the University of California Integrated Staging System (UISS), which combines TNM stage, Fuhrman grade and performance status,5,6 and the stage, size, grade, and necrosis (SSIGN) score developed by Mayo Clinic.7 Although these models have good prognostic abilities, they still have potential to be more accurate. Studies show that in ccRCC, biomarkers, such as B7-H1, Survivin, Ki-67, could improve the prognostic accuracy of UISS and SSIGN.8 These results suggest that current prognostic models may be improved by incorporating novel biomarkers.
Besides genetic mechanism, recent studies implied that inflammatory pathway might also contribute to ccRCC growth and immune escape.9 P2X7 receptor is an ATP-gated ion channel and plays a key role in the activation of inflammatory pathway by binding with ATP.10 Extracellular ATP is known as a member of danger associated molecular patterns (DAMPs), and could be a response towards endogenous danger signals arising from tumors during malignant transformation.11 Once inflammasomes are activated via P2X7 receptors, the autocleavage of pro-caspase-1 will start, and then mature pro-inflammatory cytokines, such as interleukin-1β (IL-1β) and IL-18, will release, and will cause sterile inflammation that is directly linked to many cancers.12 Therefore, P2X7 receptor may act like a “hallmark” of cancer progression of many cancers such as chronic lymphocytic leukemia, melanoma, prostate, breast, skin and thyroid cancer.13 In ccRCC, pro-inflammatory cytokines IL-1β was reported to promote tumor development.14 Expression of P2X7 receptor was also discovered in kidney and renal tracts.15 Meanwhile, Adinolfi reported that expression of P2X7 receptors in human embryonic kidney cells exhibited a higher possibility of tumorigenic as well.16 In the same study, the expression of this receptor was found upregulated in ccRCC tissues by immunohistochemistry staining. These results lead us to suspect the expression of P2X7 receptor as a mediator of inflammasomes activation in ccRCC and therefore affect the patients’ outcome by assisting tumor progression. However, the potential prognostic value of P2X7 receptors in ccRCC remains to be elucidated.
In this study, we analyzed expression of P2X7 receptors by immunohistochemistry in both ccRCC intratumoral tissues and peritumoral tissues, and we also evaluated their associations with clinicopathologic characteristics. Furthermore, we assessed the prognostic value of intratumoral P2X7 expression in patients’ cancer-specific survival (CSS) by conducting subgroup analyses and Kaplan–Meier estimates. Finally, we verified the independent prognostic value of P2X7 expression by univariate and multivariate analyses and its ability to improve the prognostic accuracy of current well-established prognostic models, such as TNM staging system, UISS and SSIGN.
Materials and Methods
Patients
A total of 273 patients (146 patients with pairs of peritumor/tumor specimens and 127 patients with single tumor specimens) with ccRCC undergoing radical nephrectomy at Zhongshan Hospital, Shanghai, China from 2001 to 2004 were retrospectively enrolled in this study, among which 86 patients died of this disease and six patients died of other causes. Zhongshan Hospital's ethics committee approved this study and informed consent was obtained from each patient. Patients were selected according to the following criteria: (i) confirmed post-operative histopathology diagnosis; (ii) no bilateral disease and familial RCC; (iii) complete available follow-up data; (iv) no preoperative neoadjuvant and/or postoperative adjuvant therapy; and (v) tumor stage classification was carried out according to 2010 AJCC TNM classification.17 For each patient, the following clinicopathologic information was collected: age, gender, tumor size, presence of histologic tumor necrosis, TNM stage, Fuhrman grade, and Eastern Cooperative Oncology Group Performance Status (ECOG-PS). UCLA Integrated Staging System (UISS) score and stage, size, grade and necrosis (SSIGN) score were also calculated according to previous reports.5,6 CSS was measured from the date of surgery to death caused by cancer. All the survival data were updated in October 2013.
Immunohistochemistry
Tissue microarray construction and immunohistochemistry protocol were described in the Supplemental Methods (Suppl. Doc. S1). From the 273 patients we enrolled, 273 intratumoral tissues and 146 peritumoral tissues were obtained. Primary antibodies against human P2X7 receptor (1:200; Abcam, Cambridge, MA, USA) were applied in the procedure. The immunostaining was evaluated by two pathologists (C. Fu and S. Zhen) without the knowledge of patients’ information and clinical outcome. A semiquantitative immunohistochemistry score on a scale of 0–300 was calculated for each sample by multiplying the staining intensity (0, no staining; 1, weak; 2, moderate; and 3, strong) and the percentage of cells (0–100%) at each intensity level. The expression of P2X7 was dichotomized into low and high according to the median value of the semiquantitative score.
Statistical analysis
Correlations between immunohistochemical variables and clinicopathologic characteristics were analyzed with χ2 tests for categorical variables, student's t-tests for continuous variables. Kaplan–Meier method with log-rank test was applied to compare survival curves. Subgroup analyses were conducted by univariate analysis expressing hazard ratios (HR). All statistical tests were two-sided and performed at a significance level of 0.05. Univariate and multivariate Cox regression models were used to analyze the impact of prognostic factors on CSS, and P < 0.05 was considered statistically significant. The prognostic accuracy of various Cox regression models was quantified by Harrell's concordance index (C-index), which ranges from 0.5 (no predictive meaning) to 1 (perfect prediction). Data were analyzed using SPSS 21 (IBM Corporation, Armonk, NY, USA), Stata 12.0 (StataCorp, College Station, TX, USA) and R software version 3.0.2 and the “rms” package (R Foundation for Statistical Computing, Vienna, Austria).
Results
Immunohistochemistry detection of P2X7 receptor and correlations with clinicopathological features
P2X7 receptor was stained in both intratumoral tissues and peritumoral tissues. According to the immunohistochemistry scoring method described before, both representative images of high and low density of P2X7 expression in intratumoral and peritumoral tissues are illustrated in Figure1. The average staining score of peritumoral P2X7 expression (115) was significantly higher than intratumoral P2X7 expression (88) (P < 0.001, Fig.1).
Figure 1.
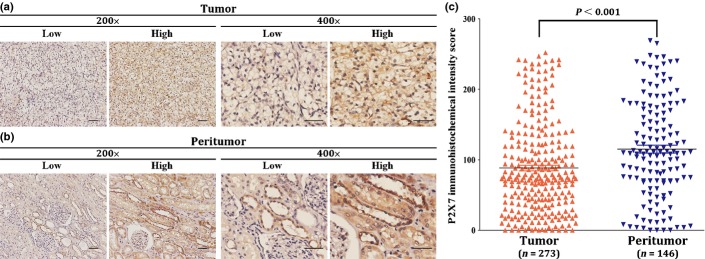
Immunohistochemistry detection and scoring of P2X7 expression in clear-cell renal cell carcinoma (ccRCC). Representative microphotographs of P2X7 expression in intratumoral tissues (a) and in peritumoral tissues (b). Original magnification: ×200, ×400 respectively. Scale bar: 50 μm. (c) Scatter plots for IHC staining score in unpaired intratumoral tissues (n = 273) and peritumoral tissues (n = 146). P-value is determined by non-parametric Mann–Whitney test.
The median intensity score of peritumoral P2X7 expression was 110 (range, 0–269), and 146 patients were dichotomized into high expression (n = 73) and low expression (n = 73) groups, respectively. Meanwhile, the median intensity score of intratumoral P2X7 expression was 75 (range, 0–252), and 273 patients were dichotomized into high expression (n = 138) and low expression (n = 135) groups, respectively.
Correlations between the intratumoral P2X7 expression level and clinicopathological features are listed in Table1. Intratutomral P2X7 expression was positively correlated with TNM stage (P = 0.038), Fuhrman grade (P = 0.035), SSIGN score (P = 0.021) and UISS score (P = 0.007). The other clinicopathological features did not present significant correlation with intratumoral P2X7 expression. In addition, peritumoral P2X7 expression was not correlated with any clincopathological features except tumor size (P = 0.044) (Supl. Table S1).
Table 1.
Relationship between intratumoral P2X7 receptor expression and clinicopathological characteristics
| Variable | Patients | P2X7 expression† | |||
|---|---|---|---|---|---|
| No. | % | High (n = 138) | Low (n = 135) | P | |
| Age (years)‡ | 0.578 | ||||
| Mean ± SD | 56.78 ± 12.47 | 57.30 ± 13.42 | 56.46 ± 11.60 | ||
| Gender | |||||
| Male | 191 | 70.0 | 93 | 98 | 0.349 |
| Female | 82 | 30.0 | 45 | 37 | |
| Tumor size‡ | 0.839 | ||||
| Mean ± SD | 4.73 ± 2.62 | 4.73 ± 2.62 | 4.79 ± 2.66 | ||
| TNM stage | |||||
| I | 164 | 60.1 | 76 | 88 | 0.038 |
| II | 33 | 12.1 | 14 | 19 | |
| III | 59 | 21.6 | 35 | 24 | |
| IV | 17 | 6.2 | 13 | 4 | |
| Fuhrman grade | |||||
| 1 | 50 | 18.3 | 22 | 28 | 0.035 |
| 2 | 122 | 44.7 | 54 | 68 | |
| 3 | 66 | 24.2 | 38 | 28 | |
| 4 | 35 | 12.8 | 24 | 11 | |
| Necrosis | |||||
| Absent | 208 | 76.2 | 102 | 106 | 0.372 |
| Present | 65 | 23.8 | 36 | 29 | |
| ECOG-PS | |||||
| 0 | 220 | 80.6 | 107 | 113 | 0.198 |
| ≥1 | 53 | 19.4 | 31 | 22 | |
| SSIGN score | |||||
| 0–3 | 182 | 66.7 | 84 | 98 | 0.021 |
| 4–7 | 61 | 22.3 | 32 | 29 | |
| ≥8 | 30 | 11.0 | 22 | 8 | |
| UISS score | |||||
| 1 | 98 | 35.9 | 38 | 60 | 0.007 |
| 2 | 145 | 53.1 | 80 | 65 | |
| ≥3 | 30 | 11.0 | 20 | 10 | |
†Split by median. ‡Student's t-test; χ2 test for all the other analyses. ECOG-PS, Eastern Cooperative Oncology Group performance status; SSIGN, stage, size, grade and necrosis; UISS, UCLA Integrated Staging System.
The bold values indicate that these P values are considered statistically significant.
Correlation of P2X7 expression with clinical outcome of patients with ccRCC
Further analyses were performed to investigate the prognostic value of P2X7 expression on CSS in ccRCC patients. Median follow-up period was 90 months (range, 11–120 months). According to Kaplan–Meier survival analysis, ccRCC patients with high intratumoral P2X7 expression had worse cancer-specific survival than those with low intratumoral P2X7 expression (P < 0.001, Fig.2). On the other hand, peritumoral P2X7 expression did not present a prognostic value on CSS (Suppl. Fig. S1).
Figure 2.
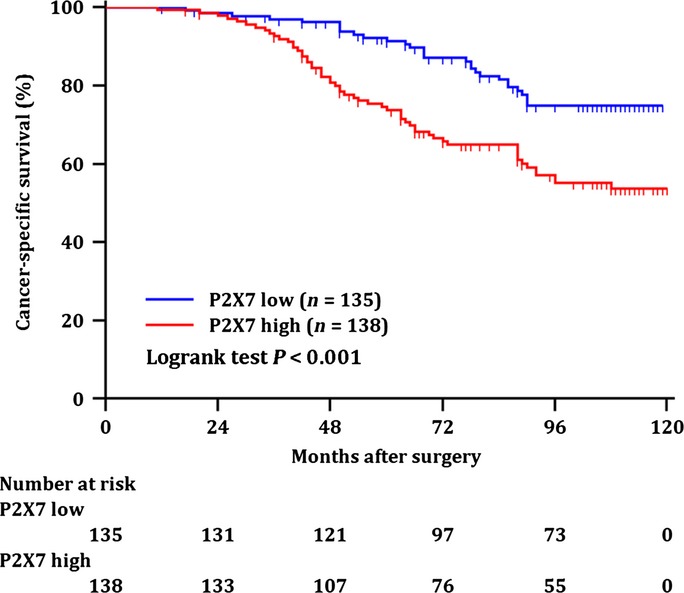
Kaplan–Meier analysis for cancer-specific survival (CSS) of all patients with clear-cell renal cell carcinoma (ccRCC) according to the intratumoral P2X7 expression. Kaplan–Meier analysis of CSS of all patients with ccRCC by intratumoral P2X7 expression. P-value was calculated by log-rank test.
High intratumoral P2X7 expression is an independent prognostic factor for CSS in ccRCC patients
Univariate and multivariate analyses were conducted to evaluate the prognostic significance of intratumoral P2X7 expression on CSS of ccRCC patients. As summarized in Table2, intratumoral P2X7 expression was identified as a risk factor (P = 0.002) with other factors, such as tumor size (P < 0.001), TNM stage (P < 0.001), Fuhrman Grade (P < 0.001), necrosis (P = 0.031), and ECOG-PS (P = 0.001). Moreover, multivariate analysis further identified TNM stage (P < 0.001), Fuhrman grade (P < 0.001), ECOG-PS (P = 0.018), and P2X7 expression (P = 0.034) as independent prognostic factors for CSS.
Table 2.
Univariate and multivariate Cox regression analyses of potential prognostic factors for cancer-specific survival (CSS)
| Variable | Univariate analyses | Multivariate analyses | ||
|---|---|---|---|---|
| HR (95%CI)† | P | HR (95%CI)† | P | |
| Tumor size (cm) | 1.175 (1.099–1.255) | <0.001 | 1.079 (0.976–1.193) | 0.110 |
| TNM stage | <0.001 | <0.001 | ||
| II vs. I‡ | 2.060 (1.057–4.012) | 0.032 | 1.381 (0.620–3.079) | 0.392 |
| III vs. I‡ | 3.337 (2.025–5.497) | <0.001 | 2.146 (1.219–3.779) | 0.018 |
| IV vs. I‡ | 14.328 (7.192–28.544) | <0.001 | 9.779 (4.370–21.883) | 0.001 |
| Fuhrman grade | <0.001 | <0.001 | ||
| 2 vs. 1‡ | 1.706 (0.749–3.886) | 0.201 | 1.596 (0.683–3.734) | 0.335 |
| 3 vs. 1† | 3.232 (1.407–7.423) | 0.008 | 3.834 (1.609–9.132) | 0.008 |
| 4 vs. 1‡ | 7.432 (3.163–17.463) | <0.001 | 4.130 (1.621–10.519) | 0.004 |
| Necrosis (present vs. absence‡) | 1.674 (1.056–2.654) | 0.031 | 0.996 (0.600–1.651) | 0.984 |
| ECOG-PS (≥1 vs. 0‡) | 3.465 (2.241–5.357) | 0.001 | 1.903 (1.170–3.097) | 0.018 |
| P2X7 expression (high vs. low‡) | 2.248 (1.437–3.517) | 0.002 | 1.693 (1.051–2.728) | 0.034 |
†All HR and 95%CI were calculated from 1000 bootstrap samples protected from overfitting. ‡Reference group. CI, confidence interval; ECOG-PS, Eastern Cooperative Oncology Group performance status; HR, Hazard Ratio.
The bold values indicate that these P values are considered statistically significant.
Subgroup analysis for prognostic value of P2X7 expression in ccRCC patients
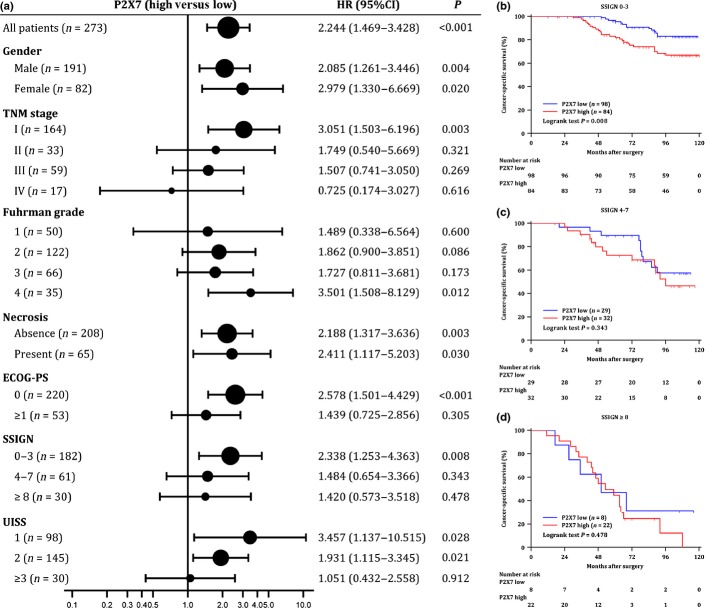
To further determine the prognostic value of intratumoral P2X7 expression in patients with different clinical status, subgroup analyses were conducted using univariate analysis. As showed in Figure3(a), high expression of P2X7 showed a significantly higher risk for cancer-specific death in early TNM stage (stage I), low score subgroups of ECOG-PS, SSIGN and UISS. Additionally, high expression of intratumoral P2X7 also presented a significant higher risk in Fuhrman grade 4 subgroup.
Figure 3.
Subgroup analyses using intratumoral P2X7 expression. (a) Comparison of hazard ratios (HR) for cancer-specific survival (CSS) of clear-cell renal cell carcinoma (ccRCC) patients in different subgroups divided by clinicopathological features. Sizes of the circles are proportional to the number of patients (n), with horizontal lines indicating 95% confidential intervals (95%CI). Kaplan–Meier analysis for CSS of patients with ccRCC in the SSIGN (stage, size, grade, and necrosis) 0–3 category (n = 182) (b), SSIGN 4–7 category (n = 61) (c) and SSIGN≥8 category (n = 30) (d), respectively. P-value was calculated by log-rank test.
As the SSIGN scoring system is originally developed to predict the cancer specific survival of patients with ccRCC, we further performed Kaplan–Meier survival method to evaluate the association between P2X7 expression and clinical outcome of ccRCC patients in different SSIGN subgroups. As presented in Figure3(b–d), ccRCC patients could be stratified by P2X7 expression in low risk subgroup (P = 0.008, Fig.3b), but could not be stratified in intermediate and high risk subgroup (P = 0.343, Fig.3c; P = 0.478, Fig.3d, respectively). Similar results were also observed in TNM staging and UISS scoring system with high P2X7 expression significantly predicting shortened CSS in early TNM stage (stage I) and low UISS score (score 1 and 2) subgroups (Suppl. Fig. S2). Moreover, subgroup analysis also was conducted in each Fuhrman grade. Patients could not be stratified by P2X7 expression in Fuhrman grade 1 (P = 0.600), Fuhrman grade 2 (P = 0.086) and Fuhrman grade 3 (P = 0.173), but could be significantly stratified by P2X7 expression in Fuhrman grade 4 (P = 0.012) (Suppl. Fig. S3).
Extension of established prognostic models with intratumoral P2X7 expression
Since intratumoral P2X7 expression had been identified as an independent prognostic factor described above, we combined P2X7 expression with three major ccRCC prognostic models, TNM stage, UISS and SSIGN systems, to investigate whether P2X7 expression could improve their prognostic accuracy. Harrell's concordance index (c-index) was calculated to evaluate the prognostic accuracy. As shown in Table3, for CSS, the c-index for original TNM stage, UISS and SSIGN systems were 0.648, 0.679 and 0.710, respectively, which were significantly improved to 0.698 (P < 0.001), 0.719 (P = 0.001) and 0.728 (P = 0.039) when P2X7 expression (c-index = 0.607) was integrated into these conventional models.
Table 3.
Comparison of prognostic accuracy of P2X7 expression, TNM stage, UISS and SSIGN scoring system
| Prognostic model | C-index | P-value |
|---|---|---|
| P2X7 expression | 0.607 | |
| TNM stage | 0.648 | |
| TNM stage + P2X7 expression | 0.698 | <0.001 |
| UISS score | 0.679 | |
| UISS score + P2X7 expression | 0.719 | 0.001 |
| SSIGN score | 0.710 | |
| SSIGN score + P2X7 expression | 0.728 | 0.039 |
C-index, Harrell's concordance index; SSIGN, stage, size, grade and necrosis; UISS, UCLA Integrated Staging System.
The bold values indicate that these P values are considered statistically significant.
Discussion
To our knowledge, this is the first study to identify high intratumoral expression of P2X7 receptor as a negative prognostic factor for CSS of patients with ccRCC after nephrectomy. High expression of P2X7 receptors in ccRCC tissues indicates shortened survival time, especially for patients with low SSIGN score, TNM stage I and low UISS score. Moreover, this study also identified the ability of intratumoral P2X7 expression to improve predicative accuracy of TNM staging system, UISS and SSIGN scoring models. These integrated prognostic models with intratumoral P2X7 expression are useful in filtering patients for additional treatment and planning follow-up. For instance, patients with high intratumoral P2X7 expression may need adjuvant treatment and a closer follow-up strategy even they are classified as early stage, such as SSIGN score below 3.
Due to the complicated and various outcomes of ccRCC patients, establishing an accurate prognostic model is critical important in clinical practice. Compared with traditional prognostic models based on histopathological features, genetic and molecular biomarkers may have advantage in accurate prognosis. Recently, several biomarkers are reported to be involved in ccRCC prognosis predicting. These newly reported biomarkers include gene expression signature ClearCode34,18 serum cell-free DNA,19 and loss of BAP1 protein,20 which represent different strategies for seeking for biomarkers: gene-based, blood-based and molecule-based ways. Furthermore, molecular-based, or in another word, protein-based biomarkers, not only have advantages in predicting prognosis, but also have potential ability for application of targeted therapies.
As a receptor for extracellular ATP, one of the DAMPs, P2X7 receptors have a complicated and even controversial role in the interplay between cancer and immunity. Although P2X7 receptor is long well known as its cytotoxicity that has anti-tumor effects, recent studies show this receptor may also promote tumor growth and activate inflammasomes that directly linking to many cancers.13,21,22 Adinolfi et al. found that human embryonic kidney cells over-expressing P2X7 presented a more tumorigenic and anaplastic phenotype than control cells.16 Another study also demonstrated that the stimulation of P2X7 by ATP could activate a proliferative pathway in ovarian carcinoma cells.23 In addition, the work of Jelassi et al.24 determined that P2X7 could enhance cancer cell invasiveness, which mainly occurred in advanced stage tumors. Thus P2X7 might accelerate cell survival and cell proliferation in early stage and promote migration and invasiveness in advanced stage. At present, this study revealed that the ccRCC patients in early stage, rather than in advanced stage, could be significantly stratified by P2X7 expression, which indicates the pivotal role of P2X7 in cell proliferation and cell growth during tumorigenesis. To date, studies had determined that P2X7 exerts pro-cancerous functions probably through PI3K/Akt and HIF1α/VEGF signaling.25,26 Yet the exact molecular mechanism adopted by P2X7 in RCC remains to be elucidated, which merits our further investigation. Since P2X7 receptor played a pivotal role in inflammatory pathway, many studies had focused on the function of P2X7 receptors in cancer. Histological evidences have been discovered that the expression of P2X7 receptor was increased in prostate,27 breast and skin cancer,28,29 neuroblastoma,30 leukemia31 and thyroid papillary carcinoma.32 The prognostic value of a polymorphism of P2X7 receptor gene was evaluated in chronic lymphocytic leukemia (CLL) according to Thunberg.33 Nevertheless, in ccRCC, P2X7 receptor was only determined to be highly expressed,16 the prognostic value of P2X7 receptor in ccRCC remains to be revealed. This study evaluated its predicting ability in ccRCC and may enrich prognostic markers of ccRCC. Since the well-known marker of inflammation, C-reactive protein (CRP), also had been identified as a strong prognostic indicator in RCC,34–36 P2X7 might be closely related with CRP under the inflammatory microenvironment during tumor development of ccRCC. Combination of P2X7 expression with CRP level might produce a more robust prognostic indicator for ccRCC, which also merits further exploration.
Our data presented that the expression level of P2X7 was significantly higher in peritumoral than intratumoral tissues, which drew our attention. As we inferred above, P2X7 high expression in intratumoral tissues itself could promote tumor cell proliferation and growth and then initiate tumor development. Meanwhile, P2X7 expressing tumor cells might release cytokines, induce hypoxia and inflammatory microenvironment in surrounding peritumoral tissues. It has been reported that interleukin-1β released by tumor cells and hypoxia are inducers of P2X7 expression.37,38 P2X7 has been documented to mediate T cell responses, control the functions of immune cells such as monocytes, macrophages, dendritic cells and lymphocytes and induce the production of numerous pro-tumorigenic factors such as extracellular matrix-degrading enzymes.25,39 Hence, the tumor-educated high P2X7 expressing peritumoral tissues might construct a pro-cancerous environment for the tumor cells’ survival and outward expansion.
Besides prognostic power, biomarkers have latent ability to be developed into therapeutic targets. Current therapeutic targets of ccRCC applied in clinical practice mainly involved in VEGF and mTOR pathways.40 However, cytotoxicity of these pathways’ inhibitors is an interference of curative effect, so a new strategy of exploring target pathway might be needed. The results of this study identified intratumoral P2X7 as a potential unfavorable prognostic factor for CSS in ccRCC patients. This finding suggests intratumoral P2X7-mediated activation of inflammasomes might accelerate early ccRCC progression by providing an inflammatory microenvironment. Therefore, intervention of P2X7 signal activation might be a novel molecular targeted strategy for patients with higher intratumoral P2X7 expression, especially for early stage patients. Application of P2X7 blockers has also been found to inhibit tumor progression.22 Another study reported a P2X7 antagonist, brilliant blue G (BBG), was able to suppress tumor growth in a C6 glioma brain tumor model.41 Together with these finding, the present study raised a strong potential of P2X7 blockers or antagonists might be a new therapeutic option for ccRCC, which needs further exploration and validation.
There are some limitations in our study. Firstly, although the sample size is not small, validation set and external validations of prognostic value of intratumoral P2X7 expression based on a larger population are required. Secondly, as metastatic ccRCC patients in our study are relatively rare, the prognostic value of P2X7 receptor in ccRCC with advanced-stage is required to be validated. Thirdly, our patient group exhibited limited racial/ethnic diversity (>99% Chinese), further validation of our results on various races may needed.
In summary, this study is the first to demonstrate that intratumoral P2X7 receptor expression is an independent prognostic factor in ccRCC, especially in patients with early stage disease. High expression of intratumoral P2X7 predicts shortened CSS. In addition, high intratumoral P2X7 expression may also improve prognostic accuracy of current TNM stage, UISS and SSIGN models. We provide a potential prognostic factor and a novel idea of molecular target for ccRCC to individualized clinical approach and follow-up program more properly.
Acknowledgments
We thank Ms Haiying Zeng (Department of Pathology, Zhongshan Hospital, Shanghai Medical College of Fudan University) for technical assistance. This study was funded by grants from National Basic Research Program of China (2012CB822104), National Natural Science Foundation of China (31100629, 31270863, 81471621, 81472227, J1210041), Program for New Century Excellent Talents in University (NCET-13-0146) and Shanghai Rising-Star Program (13QA1400300). All these study sponsors have no roles in the study design, in the collection, analysis, and interpretation of data.
Glossary
Abbreviations:
- BBG
brilliant blue G
- ccRCC
clear-cell renal cell carcinoma
- CI
confidence interval
- C-index
Harrell's concordance index
- CLL
chronic lymphocytic leukemia
- CSS
cancer-specific survival
- DAMPs
danger associated molecular patterns
- ECOG PS
Eastern Cooperative Oncology Group performance status
- HR
hazard ratio
- IL-1β
interleukin-1β
- IL-18
interleukin-18
- SSIGN
stage, size, grade, and necrosis score
- UISS
University of California Integrated Staging System
Disclosure Statement
The authors have no conflict of interest.
Supporting Information
Additional supporting information may be found in the online version of this article:
Fig. S1. Kaplan–Meier analysis for cancer-specific survival (CSS) of patients with clear-cell renal cell carcinoma according to peritumoral P2X7 expression. Kaplan–Meier analysis for CSS of patients with ccRCC by peritumoral P2X7 expression. P-value was calculated by log-rank test.
Fig. S2. Kaplan–Meier analysis for cancer-specific survival (CSS) of patients with clear-cell renal cell carcinoma according to the intratumoral P2X7 expression in each TNM stage and University of California Integrated Staging System (UISS) risk level. Kaplan–Meier analysis for CSS of patients in TNM stage I (n = 164) (A), TNM stage II (n = 33) (B), TNM stage III+IV (n = 76) (C), UISS = 1 (n = 98) (D), UISS = 2 (n = 145) (E), UISS ≥ 3 (n = 30) (F). P-value was calculated by log-rank test.
Fig. S3. Kaplan–Meier analysis for cancer-specific survival (CSS) of patients with clear-cell renal cell carcinoma according to the intratumoral P2X7 expression in each Fuhrman grade. Kaplan–Meier analysis for CSS of patients in Fuhrman grade 1 (n = 50) (A), Fuhrman grade 2 (n = 122) (B), Fuhrman grade 3 (n = 66) (C), Fuhrman grade 4 (n = 35) (D). P-value was calculated by log-rank test.
Table S1. Relationship between peritumoral P2X7 receptor expression and clinicopathological characteristics.
Doc.S1. Supplemental Methods: Tissue microarray (TMA) and immunohistochemistry.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- Escudier B, Eisen T, Porta C, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl. 7):vii65–71. doi: 10.1093/annonc/mds227. [DOI] [PubMed] [Google Scholar]
- Chiong E, Tay MH, Tan MH, et al. Management of kidney cancer in Asia: resource-stratified guidelines from the Asian Oncology Summit 2012. Lancet Oncol. 2012;13:e482–91. doi: 10.1016/S1470-2045(12)70433-3. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Zisman A, Pantuck AJ, Dorey F, et al. Improved prognostication of renal cell carcinoma using an integrated staging system. J Clin Oncol. 2001;19:1649–57. doi: 10.1200/JCO.2001.19.6.1649. [DOI] [PubMed] [Google Scholar]
- Zisman A, Pantuck AJ, Wieder J, et al. Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. J Clin Oncol. 2002;20:4559–66. doi: 10.1200/JCO.2002.05.111. [DOI] [PubMed] [Google Scholar]
- Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. An outcome prediction model for patients with clear-cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002;168:2395–400. doi: 10.1016/S0022-5347(05)64153-5. [DOI] [PubMed] [Google Scholar]
- Parker AS, Leibovich BC, Lohse CM, et al. Development and evaluation of BioScore: a biomarker panel to enhance prognostic algorithms for clear-cell renal cell carcinoma. Cancer. 2009;115:2092–103. doi: 10.1002/cncr.24263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vivar Chevez AR, Finke J, Bukowski R. The role of inflammation in kidney cancer. Adv Exp Med Biol. 2014;816:197–234. doi: 10.1007/978-3-0348-0837-8_9. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–67. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–89. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F, Ferrari D, Adinolfi E. P2X(7): a growth-promoting receptor-implications for cancer. Purinergic Signal. 2009;5:251–6. doi: 10.1007/s11302-009-9145-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrella BL, Vincenti MP. Interleukin-1 beta mediates metalloproteinase-dependent renal cell carcinoma tumor cell invasion through the activation enhancer binding protein beta. Cancer Med. 2012;1:17–27. doi: 10.1002/cam4.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman KA, Burnstock G, Unwin RJ. The P2X7 ATP receptor in the kidney: a matter of life or death? Nephron Exp Nephrol. 2005;101:e24–30. doi: 10.1159/000086036. [DOI] [PubMed] [Google Scholar]
- Adinolfi E, Raffaghello L, Giuliani AL, et al. Expression of P2X7 receptor increases in vivo tumor growth. Cancer Res. 2012;72:2957–69. doi: 10.1158/0008-5472.CAN-11-1947. [DOI] [PubMed] [Google Scholar]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg oOcol. 2010;17:1471–4. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- Brooks SA, Brannon AR, Parker JS, et al. ClearCode34: a prognostic risk predictor for localized clear-cell renal cell carcinoma. Eur Urol. 2014;66:77–84. doi: 10.1016/j.eururo.2014.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Martino M, Klatte T, Haitel A, Marberger M. Serum cell-free DNA in renal cell carcinoma: a diagnostic and prognostic marker. Cancer. 2012;118:82–90. doi: 10.1002/cncr.26254. [DOI] [PubMed] [Google Scholar]
- Joseph RW, Kapur P, Serie DJ, et al. Loss of BAP1 protein expression is an independent marker of poor prognosis in patients with low-risk clear-cell renal cell carcinoma. Cancer. 2014;120:1059–67. doi: 10.1002/cncr.28521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F. Purines, purinergic receptors, and cancer. Cancer Res. 2012;72:5441–7. doi: 10.1158/0008-5472.CAN-12-1600. [DOI] [PubMed] [Google Scholar]
- Roger S, Pelegrin P. P2X7 receptor antagonism in the treatment of cancers. Expert Opin Investig Drugs. 2011;20:875–80. doi: 10.1517/13543784.2011.583918. [DOI] [PubMed] [Google Scholar]
- Vazquez-Cuevas FG, Martinez-Ramirez AS, Robles-Martinez L, et al. Paracrine stimulation of P2X7 receptor by ATP activates a proliferative pathway in ovarian carcinoma cells. J Cell Biochem. 2014;115:1955–66. doi: 10.1002/jcb.24867. [DOI] [PubMed] [Google Scholar]
- Jelassi B, Chantome A, Alcaraz-Perez F, et al. P2X(7) receptor activation enhances SK3 channels- and cystein cathepsin-dependent cancer cells invasiveness. Oncogene. 2011;30:2108–22. doi: 10.1038/onc.2010.593. [DOI] [PubMed] [Google Scholar]
- Roger S, Jelassi B, Couillin I, Pelegrin P, Besson P, Jiang L. Understanding the roles of the P2X7 receptor in solid tumour progression and therapeutic perspectives. Biochim Biophys Acta. 2014 doi: 10.1016/j.bbamem.2014.10.029. ; doi: 10.1016/j.bbamem.2014.10.029. [DOI] [PubMed] [Google Scholar]
- Amoroso F, Capece M, Rotondo A, et al. The P2X7 receptor is a key modulator of the PI3K/GSK3beta/VEGF signaling network: evidence in experimental neuroblastoma. Oncogene. 2015 doi: 10.1038/onc.2014.444. ; doi: 10.1038/onc.2014.444. [DOI] [PubMed] [Google Scholar]
- Slater M, Danieletto S, Gidley-Baird A, Teh LC, Barden JA. Early prostate cancer detected using expression of non-functional cytolytic P2X7 receptors. Histopathology. 2004;44:206–15. doi: 10.1111/j.0309-0167.2004.01798.x. [DOI] [PubMed] [Google Scholar]
- Slater M, Danieletto S, Pooley M, Cheng Teh L, Gidley-Baird A, Barden JA. Differentiation between cancerous and normal hyperplastic lobules in breast lesions. Breast Cancer Res Treat. 2004;83:1–10. doi: 10.1023/B:BREA.0000010670.85915.0f. [DOI] [PubMed] [Google Scholar]
- Greig AV, Linge C, Healy V, et al. Expression of purinergic receptors in non-melanoma skin cancers and their functional roles in A431 cells. J Invest Dermatol. 2003;121:315–27. doi: 10.1046/j.1523-1747.2003.12379.x. [DOI] [PubMed] [Google Scholar]
- Raffaghello L, Chiozzi P, Falzoni S, Di Virgilio F, Pistoia V. The P2X7 receptor sustains the growth of human neuroblastoma cells through a substance P-dependent mechanism. Cancer Res. 2006;66:907–14. doi: 10.1158/0008-5472.CAN-05-3185. [DOI] [PubMed] [Google Scholar]
- Adinolfi E, Melchiorri L, Falzoni S, et al. P2X7 receptor expression in evolutive and indolent forms of chronic B lymphocytic leukemia. Blood. 2002;99:706–8. doi: 10.1182/blood.v99.2.706. [DOI] [PubMed] [Google Scholar]
- Solini A, Cuccato S, Ferrari D, et al. Increased P2X7 receptor expression and function in thyroid papillary cancer: a new potential marker of the disease? Endocrinology. 2008;149:389–96. doi: 10.1210/en.2007-1223. [DOI] [PubMed] [Google Scholar]
- Thunberg U, Tobin G, Johnson A, et al. Polymorphism in the P2X7 receptor gene and survival in chronic lymphocytic leukaemia. Lancet. 2002;360:1935–9. doi: 10.1016/S0140-6736(02)11917-9. [DOI] [PubMed] [Google Scholar]
- Karakiewicz PI, Hutterer GC, Trinh QD, et al. C-reactive protein is an informative predictor of renal cell carcinoma-specific mortality: a European study of 313 patients. Cancer. 2007;110:1241–7. doi: 10.1002/cncr.22896. [DOI] [PubMed] [Google Scholar]
- Komai Y, Saito K, Sakai K, Morimoto S. Increased preoperative serum C-reactive protein level predicts a poor prognosis in patients with localized renal cell carcinoma. BJU Int. 2007;99:77–80. doi: 10.1111/j.1464-410X.2006.06497.x. [DOI] [PubMed] [Google Scholar]
- Casamassima A, Picciariello M, Quaranta M, et al. C-reactive protein: a biomarker of survival in patients with metastatic renal cell carcinoma treated with subcutaneous interleukin-2 based immunotherapy. J Urol. 2005;173:52–5. doi: 10.1097/01.ju.0000146713.50673.e5. [DOI] [PubMed] [Google Scholar]
- Narcisse L, Scemes E, Zhao Y, Lee SC, Brosnan CF. The cytokine IL-1beta transiently enhances P2X7 receptor expression and function in human astrocytes. Glia. 2005;49:245–58. doi: 10.1002/glia.20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafani M, Schito L, Pellegrini L, et al. Hypoxia-increased RAGE and P2X7R expression regulates tumor cell invasion through phosphorylation of Erk1/2 and Akt and nuclear translocation of NF-{kappa}B. Carcinogenesis. 2011;32:1167–75. doi: 10.1093/carcin/bgr101. [DOI] [PubMed] [Google Scholar]
- Souza CO, Santoro GF, Figliuolo VR, et al. Extracellular ATP induces cell death in human intestinal epithelial cells. Biochim Biophys Acta. 2012;1820:1867–78. doi: 10.1016/j.bbagen.2012.08.013. [DOI] [PubMed] [Google Scholar]
- Michaelson MD. Combination of targeted agents in metastatic renal cell carcinoma: a path forward or a dead-end street? Cancer. 2012;118:1744–6. doi: 10.1002/cncr.26427. [DOI] [PubMed] [Google Scholar]
- Ryu JK, Jantaratnotai N, Serrano-Perez MC, McGeer PL, McLarnon JG. Block of purinergic P2X7R inhibits tumor growth in a C6 glioma brain tumor animal model. J Neuropathol Exp Neurol. 2011;70:13–22. doi: 10.1097/NEN.0b013e318201d4d4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Kaplan–Meier analysis for cancer-specific survival (CSS) of patients with clear-cell renal cell carcinoma according to peritumoral P2X7 expression. Kaplan–Meier analysis for CSS of patients with ccRCC by peritumoral P2X7 expression. P-value was calculated by log-rank test.
Fig. S2. Kaplan–Meier analysis for cancer-specific survival (CSS) of patients with clear-cell renal cell carcinoma according to the intratumoral P2X7 expression in each TNM stage and University of California Integrated Staging System (UISS) risk level. Kaplan–Meier analysis for CSS of patients in TNM stage I (n = 164) (A), TNM stage II (n = 33) (B), TNM stage III+IV (n = 76) (C), UISS = 1 (n = 98) (D), UISS = 2 (n = 145) (E), UISS ≥ 3 (n = 30) (F). P-value was calculated by log-rank test.
Fig. S3. Kaplan–Meier analysis for cancer-specific survival (CSS) of patients with clear-cell renal cell carcinoma according to the intratumoral P2X7 expression in each Fuhrman grade. Kaplan–Meier analysis for CSS of patients in Fuhrman grade 1 (n = 50) (A), Fuhrman grade 2 (n = 122) (B), Fuhrman grade 3 (n = 66) (C), Fuhrman grade 4 (n = 35) (D). P-value was calculated by log-rank test.
Table S1. Relationship between peritumoral P2X7 receptor expression and clinicopathological characteristics.
Doc.S1. Supplemental Methods: Tissue microarray (TMA) and immunohistochemistry.