Abstract
Generally, cancer tissue is palpated as a hard mass. However, the elastic nature of cancer tissue is not well understood. The aim of the present study was to evaluate the clinical utility of measuring the elastic modulus (EM) in colorectal cancer tissue. Using a tactile sensor, we measured the EM of 106 surgically resected colorectal cancer tissues. Data on the EM were compared with clinicopathological findings, including stromal features represented by Azan staining and the α-SMA positive area ratio of the tumor area. Finally, a cDNA microarray profile of the tumors with high EM were compared with the findings of tumors with low EM. A higher EM in tumors was associated with pathological T, N, and M-stage tumors (P < 0.001, P = 0.001 and P = 0.011, respectively). Patients with high EM tumors had shorter disease-free survival than had patients with low EM. The EM showed strongly positive correlation with the Azan staining positive area ratio (r = 0.908) and the α-SMA positive area ratio (r = 0.921). Finally, the cDNA microarray data of the tumors with high EM revealed a distinct gene expression profile compared with data from those tumors with low EM. The assessment of the elasticity of colorectal cancer tissue may allow a more accurate clinical stage and prognosis estimation. The distinct phenotypical features of the high EM tumors and their strong association with stromal features suggest the existence of a biological mechanism involved in this phenomenon that may contribute to future therapy.
Keywords: Colorectal cancer, disease-free survival, elasticity, pathological, phenotypical
In general, although cancer tissue is palped as a hard mass, what is the nature of cancer elasticity is elusive. The elastic modulus (EM) is a physical parameter1 that characterizes the elasticity of a material. It has been applied for the measurement of the elasticity of human tissues such as skin, bone, breast and brain.2–8 Different organs have a wide range of elasticity, and the elasticity of a specific organ can be altered as a result of various disease processes. For instance, Samani et al.7 found that the mean EM of normal breast tissue was 1.9 kPa, that of fibroadenoma was 11.42 kPa, and that of invasive ductal carcinoma was 22.55 kPa. However, there have been few reports on the elasticity of gastrointestinal cancer tissue.9
Most clinical studies in cancer have been performed using ultrasound elastography, which provides a semiquantitative assessment of the EM of cancer tissue.10–15 The data suggest that cancer elasticity is correlated with histological or clinicopathological findings, including scirrhous subtype or prognosis and direct and quantitative evaluation of EM may provide a more detailed analysis between tumor elasticity and clinicopathological features.
We recently established a quantitative assessment method for EM in human tissue using a tactile sensor.16 In that report we could measure the EM of pancreatic tissue without any deformation of the resected specimen. Furthermore, that report has demonstrated that the assessment of the EM of the pancreatic cut edge is correlated with histological fibrosis or clinical result of postoperative pancreatic fistula formation. Therefore, in this study, we measured the elasticity of colorectal cancer tissue by tactile sensor to determine the correlation between these data and the clinicopathological or histological findings; we then used these data to estimate the clinical utility of quantitative EM assessment. Furthermore, cancer tissue elasticity seemed to correlate to cancer cell invasion or migration, but the gene expression profile of cancer tissue with high elasticity was not known. Therefore, we performed a global gene expression analysis using cancer specimens with high and low EM. We compared the gene expression profile between them to help understand the biological nature of elasticity in cancer tissue. The aims of this study were to investigate the clinical utility of assessing the EM of colorectal cancer and to evaluate the pathological or phenotypical relevance of such data.
Materials and Methods
Patients and clinical data collection
This study was approved by the institutional review board of the National Cancer Center (2012-067). The population in this study consisted of 106 patients who had undergone colorectal cancer surgery at the Department of Colorectal and Pelvic Surgery, National Cancer Center Hospital East, between February and December 2012 (67 men, 39 women; mean age, 66.0 years; range, 35–89 years; Table1). Patients who had received neoadjuvant therapies were excluded from the study. Clinicopathological data were reviewed from medical records. During this period, the operations and postoperative management were standardized. Patients were followed up by using a standardized protocol (including clinical examination with computed tomography of the chest, abdomen and pelvis; and measurement of tumor marker levels) every 4 months for the first 2 years, then every 6 months for 3 years, then annually thereafter. A total colonoscopy was performed at 2 and 5 years after surgery.
Table 1.
Clinicopathological characteristics
| Characteristics | Patients (n = 106) |
|---|---|
| n (%) | |
| Age (year) | 66.0 ± 10.5†(range, 35–89) |
| Sex | |
| Men | 67 (63.2) |
| Women | 39 (36.8) |
| Site of the tumor | |
| Colon | 67 (63.2) |
| Rectum | 39 (36.8) |
| Size of the tumor (mm) | 42.0 ± 23.9†(range, 10.0–138) |
| Stage | |
| I | 22 (20.8) |
| II | 35 (33.0) |
| III | 38 (35.8) |
| IV | 11 (10.4) |
| T-Stage | |
| 1 | 6 (5.7) |
| 2 | 23 (21.7) |
| 3 | 59 (55.6) |
| 4 | 18 (17.0) |
| Lymph node metastasis | |
| No | 61 (57.5) |
| Yes | 45 (42.5) |
| Distant metastasis | |
| No | 95 (89.6) |
| Yes | 11 (10.4) |
Mean ± SD.
Measurement of the resected colorectal cancer tissue elastic modulus
We measured the EM within 30 min after the colorectal cancer was resected. The EM, also known as Young's modulus (kilopascals), is a measure of the stiffness of an elastic material.1 The EM of colorectal cancer tissue was defined as the Young's modulus of the colorectal cancer tissue as calculated by a tactile sensor of the Venustron system (Axiom, Fukushima, Japan). Details of the theory and the calculation of the EM were as described previously.16 During measurement the resected intestinal tract was opened longitudinally (Fig.1a). The specimen was placed on a board with sufficient stability, and the mucosal side was placed upward so that the probe could be directed vertically toward this surface (Fig.1b,c). The Venustron system was used to calculate the EM of specimen for every 0.005 mm of indentation depth using a travel range of 4 mm and a resonant frequency of 50 Hz. We determined the EM using 1, 2, 3 and 4-mm travel ranges, and used the mean for the analysis. The EM of the colorectal cancer tissue was measured in three areas of the center of the ulcer floor (Fig.1a). Measurements of the EM were performed three times at each area. The EM of the colorectal cancer tissue sample was defined as the mean EM of those three results. Assessments of the EM of normal tissue more than 3 cm distant from the tumor were measured from three areas, taking the mean of the three measurements (Fig.1a). The EM of the colorectal cancer tissue was then analyzed in relation to the patient's clinical and histological characteristics. Of these characteristics, tumor size was divided into large and small groups using the mean size of 42 mm. Serum CEA and SC19-9 was also divided into two groups using the values of our hospital normal range (5.0 ng/mL and 37.0 U/mL, respectively).
Figure 1.
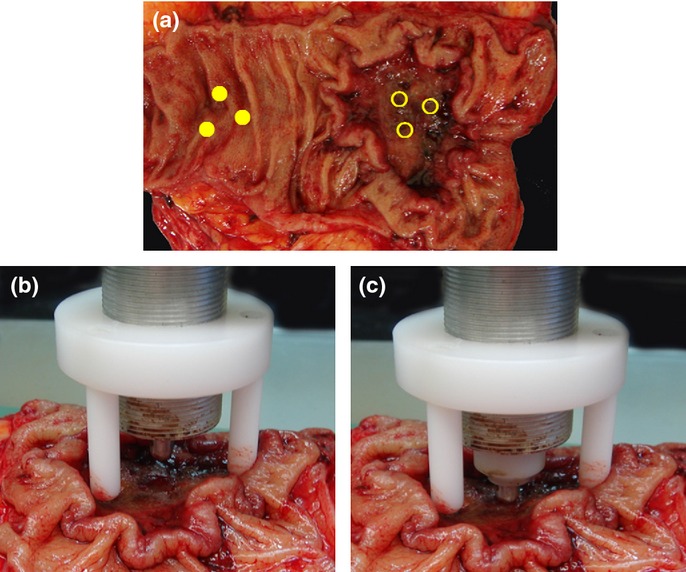
The measurement of the elastic modulus (EM) with Venustron system. (a) Each of three points are measured for the EM of the cancer tissue and normal tissue. The center of the ulcer floor is selected for the cancer tissue. A point more than 3 cm distant from the tumor is selected for normal tissue. (b, c) The EM of the specimen was calculated automatically by Venustron system. The probe is pressing vertically toward the mucosal side surface of the cancer with a depth of pressure of 4 mm and a resonant frequency of 50 Hz.
Histologic evaluation
The colorectal cancer was assessed using the Union for International Cancer Control (UICC) TNM Classification System (7th edition). Formalin-fixed, paraffin-embedded specimens obtained from a colorectal stump were cut into 3-μm-thick serial sections. The sections were stained by H&E and Azan-Mallory (Azan), and by immunohistochemical staining for α-smooth muscle actin (α-SMA). Immunohistochemical staining for α-SMA was performed automatically on a Ventana Benchmark ULTRA (Ventana Medical Systems, AZ, USA). Monoclonal anti-human α-SAM antibody (Dako, Glostrup, Denmark) was used at a dilution of 1:100, and the conditions for antigen retrieval and primary antibody incubation were set at 91°C for 8 min and 35°C for 60 min, respectively. The slides were photographed using a NanoZoomer Digital Pathology Virtual Slide Viewer (Hamamatsu Photonics, Hamamatsu, Japan) and subjected to morphometric analysis as previously reported.16,17 For the evaluation of Azan expression and immunocytochemical α-SAM expression, ×40 magnification images were taken and saved as JPEG files. The ratios of Azan-positive area and α-SAM positive area in an image were calculated using morphometric software (WinRoof, Mitani, Corporation, Fukui, Japan). We randomly selected each set of three images without muscle tissue from <1, 1–3 or >3 mm depth from the surface of the cancer tissue. The ratios of Azan-positive area and α-SAM positive area were calculated from the mean of these nine areas. Histologic analyses of a case are shown in Figure2(a,b). We show examples of images with high or low EM in Supplementary Figures S1–S4. One investigator (S.K.) carried out all of the histologic analyses under the supervision of an experienced pathologist (M.K.).
Figure 2.
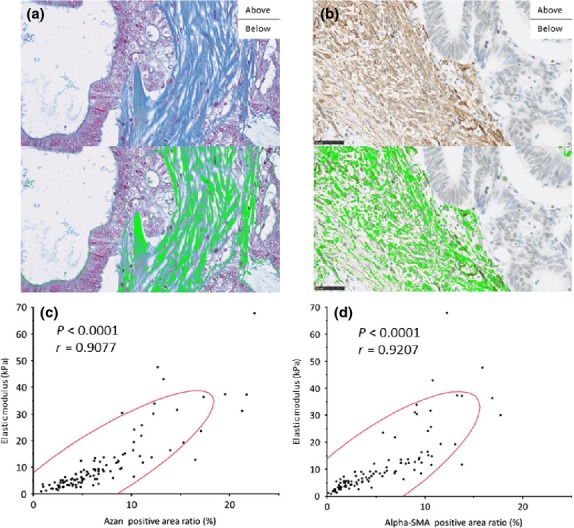
The correlation between the elastic modulus (EM) of cancer tissue and morphometric factors. The Azan positive area was determined as the visualized area stained with aniline blue (a-above) and identified as bright green in this image by using the color-detecting algorithm of the software (a-below). The α-SMA positive area (b-above) was identified as bright green using the color-detecting algorithm of the software (b-below). (c) There was a strong correlation with the EM of colorectal cancer tissue and the Azan positive area (P < 0.0001; r = 0.9077). (d) There was also a strong correlation with the EM of colorectal cancer tissue and the α-SMA positive area (P < 0.0001; r = 0.9207).
Gene expression analysis using microarray
Frozen samples were obtained from 82.4% of the cases. Tumor elasticity, clinical stage and RNA integrity number (RIN) for all cases are shown in Supplementary Table S1. We selected four samples from the highest and the lowest EM with a RIN >6.0, measured with a 2100 Bioanalyzer (Aligent Tochnologies, Santa Clara, CA, USA), and at stage II (pTNM pathologic classification). We excluded 1 sample in each group because of high GAPDH levels. The groups were named EM-high and EM-low. We used GeneChip Human Genome U133 Plus 2.0 arrays (Affymetrix, Santa Clara, CA, USA). Target cDNA was generated from 100 to 200 ng of total RNA extracted from each sample using a 39 IVT Express Kit (Affymetrix, Santa Clara, CA, USA). The procedures for target hybridization, washing and staining for signal amplification were performed according to the supplier's protocols. The arrays were scanned with a Gene Chip Scanner 3000 (Affymetrix), and the intensity of each feature of the array was calculated using GeneChip Operating Software, version 1.1.1 (Affymetrix). The average intensity was standardized to the target intensity, which was set equal to 1000, to reliably compare different arrays. The values were log transformed and median centered. The programs GeneSpring (Agilent Technologies, Santa Clara, CA, USA) and Excel (Microsoft Corporation, Redmond, WA, USA) were used to perform the numerical analyses for gene selection.
Cancer dataset
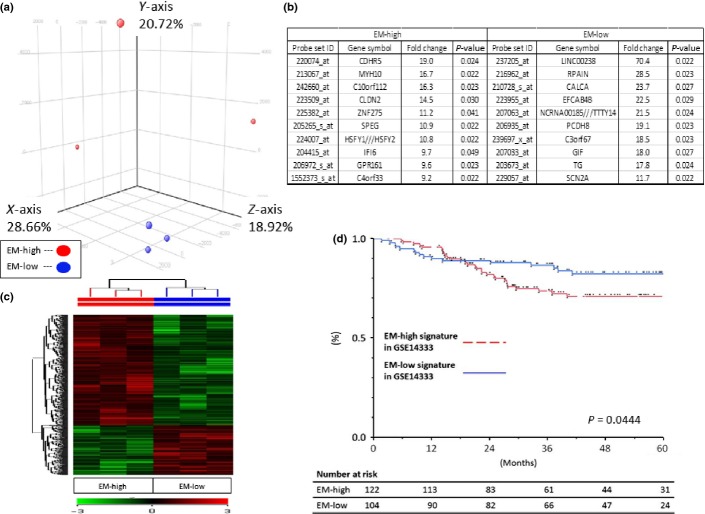
To evaluate the phenotypical features of the EM-high or EM-low groups, we used a cancer dataset of the Gene Expression Omnibus (GEO) repository (https://array.nci.nih.gov/caarray/). The colorectal cancer dataset we used was GSE14333.18 The 559 genes related to the EM level in this study were compared with a published set of genes periodically expressed during the Hela cell cycle.18 The 45 genes that overlapped with the cell cycle gene list were removed because of the previous study, and 514 signature genes related to EM level were determined (Suppl. Fig. S5a).19 Using these 514 genes, we were able to categorize the 226 patients with colorectal cancer by GSE14333 into EM-high signature in GSE14333 and EM-low signature in GSE14333 (Suppl. Table S2, Suppl. Fig. S5b).20
Statistical analysis
Comparison between the EM of the cancer tissue and the patient's clinical and histologic characteristics was performed using a Mann–Whitney U-test. Correlation between the EM and morphometric analysis was evaluated using Spearman's correlation coefficient “r.” Disease-free-survival (DFS) rates were calculated using Kaplan–Meier methods. Differences between curves were evaluated with the log-rank test. All analyses were performed using JMP version 7.0 (SAS Institute, Cary, NC, USA). All calculated P-values were two-sided and P < 0.05 was considered statistically significant. In the microarray analysis, gene expression data were analyzed using GeneSpring GX12.5 (Agilent Technologies). Row data were summarized by using MAS5 and normalized by log transformation and median centering for numerical analyses for gene selection. For principal component analysis (PCA), we used probe sets that were reliably measured and varied threefold above the global median in at least 2 samples (approximately 10%); analyses were performed using GeneSpring GX12.5. The differentially expressed probe sets used in supervised hierarchical clustering were selected based on P < 0.05 and fold change (FC) >2.0. P-values were calculated using one way ANOVA with Benjamini and Hochberg FDR multiple testing correction.
Results
Correlation between elastic modulus and clinicopathological findings
The median EM of the colorectal cancer tissue for the 106 cases was 7.51 kPa. It was significantly higher than that of normal colorectal tissue (P < 0.0001; Fig.3a). The EM of the colorectal cancer tissue was correlated with each T, N and M-stage of the UICC TNM classification (P < 0.0001, P = 0.0010 and P = 0.0111, respectively; Fig.3b–d). In T-stage, the EM of T3 cancer tissue was higher than that of T2, and that of T4 was higher than that of T3 (P < 0.0001 and P = 0.0055, respectively). The EM of colorectal cancer tissue was correlated with pathological findings (e.g. tumor size, venous invasion, perineural invasion, poorly differentiated clusters and elastic laminal invasion) and clinical findings (e.g. serum carcinoembryonic antigen levels and obstructive tumor; Table2). Furthermore, the EM was positively correlated with the ratio of Azan positivity (r = 0.9077; P < 0.0001) and the ratio of α-SMA positivity (r = 0.9207; P < 0.0001; Fig.2c,d). The Azan positive area showed collagen fibers21 and the α-SMA positive area showed myofibroblasts.22,23 These correlations suggest that the EM was influenced by the character of the cancer stroma.
Figure 3.
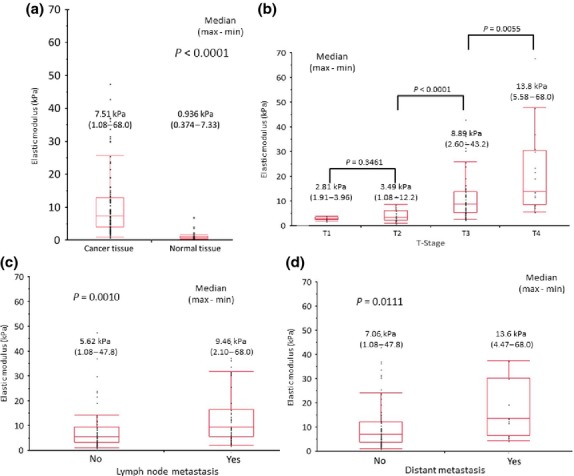
The correlation between the elastic modulus (EM) of cancer tissue and normal tissue, and TNM classification. (a) The EM of the cancer tissue is much higher than that seen for the normal tissue (P < 0.0001). (b) The EM of colorectal cancer was strongly correlated with T stage. The EM of T3 tumors was much higher than that seen in T2 (P < 0.0001). The EM of T4 was higher than that seen in T3 (P = 0.0055). (c) The EM of the tumors with lymph node metastasis was higher than that seen in tumors without lymph node metastasis (P = 0.0010). (d) The EM of the tumors with distant metastasis was higher than that seen in tumors without distant metastasis (P = 0.0111).
Table 2.
Comparing elastic modulus with clinicopthological factors
| Characteristics | n (%) | Stiffness (kPa) | P-value | ||
|---|---|---|---|---|---|
| Median | Min | Max | |||
| Size of the tumor (mm) | |||||
| <42 mm | 62 (58.5) | 5.17 | 1.08 | 31.8 | <0.0001 |
| ≧42 mm | 44 (41.5) | 11.4 | 3.27 | 68.0 | |
| Lymphatic invasion | |||||
| Negative | 46 (43.4) | 5.77 | 1.41 | 47.8 | 0.1206 |
| Positive | 60 (56.6) | 8.48 | 1.08 | 68.0 | |
| Venous invasion | |||||
| Negative | 29 (27.4) | 5.61 | 1.08 | 37.3 | 0.0116 |
| Positive | 77 (72.6) | 8.89 | 1.91 | 68.0 | |
| Perineural invasion | |||||
| Negative | 76 (71.7) | 5.97 | 1.08 | 43.2 | 0.0088 |
| Positive | 30 (28.3) | 12.1 | 1.41 | 68.0 | |
| Tumor budding | |||||
| Negative | 49 (46.2) | 7.11 | 1.08 | 43.2 | 0.2871 |
| Positive | 57 (53.8) | 7.69 | 1.91 | 68.0 | |
| Poorly differentiated clusters | |||||
| Negative | 52 (49.1) | 5.67 | 1.08 | 43.2 | 0.0050 |
| Positive | 54 (50.9) | 9.19 | 1.91 | 68.0 | |
| Elastic laminal invasion | |||||
| No | 42 (62.7) | 5.71 | 1.08 | 37.5 | <0.0001 |
| Yes | 25 (37.3) | 13.6 | 5.58 | 47.8 | |
| Serum carcinoembryonic antigen (ng/mL) | |||||
| <5.0 | 57 (53.8) | 5.81 | 1.47 | 36.5 | 0.0090 |
| ≧5.0 | 49 (46.2) | 9.23 | 1.08 | 68.0 | |
| Serum carcinoma 19-9 (U/mL) | |||||
| <37.0 | 76 (71.7) | 7.08 | 1.08 | 36.5 | 0.0688 |
| ≧37.0 | 30 (28.3) | 10.8 | 3.06 | 68.0 | |
| Obstructive Tumor | |||||
| No | 83 (78.3) | 5.81 | 1.08 | 43.2 | <0.0001 |
| Yes | 23 (21.7) | 13.8 | 4.61 | 68.0 | |
Association with disease-free survival
In this study, 7 patients were excluded because of residual tumors after the operation. The EM cut-off value was determined using the receiver operating characteristic (ROC) curve for its relationship with the tactile sensation. The area under the curve for the correlation between EM and recurrence was 0.631. When 16.6 kPa was set as the cut-off value for the EM, sensitivity, specificity, positive predictive value and negative predictive value were 40.0, 88.1, 37.5 and 89.2%, respectively. This cut-off value made two groups defined as the hard tumor group (HTG) and the soft tumor group (STG). The median follow-up time was 23 months. The DFS of the HTG was shorter than that seen in the STG (P = 0.0071; Fig.4). The 2-year recurrence rates of the HTG and STG were 38.1 and 11.5%, respectively.
Figure 4.
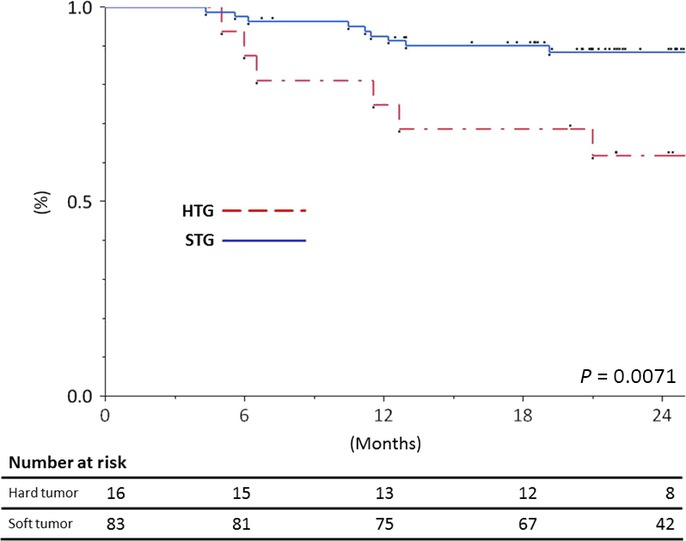
Kaplan–Meier curves for for disease-free survival (DFS) according to the hard tumor group (n = 16) and soft tumor group (n = 83). The DFS of the hard tumor group was shorter than that seen in the soft tumor group (P = 0.0071).
Correlation of the phenotypical differences with elastic modulus-high and elastic modulus-low groups
The phenotypical differences between EM-high and EM-low groups were compared. The PCA revealed that EM-high and EM-low cases formed two distinct clusters (Fig.5a). Supervised cluster analysis with the 514 genes related to the EM also revealed 2 distinct clusters (Fig.5c). We show the 10 most related genes in Figure5(b). Contraction associated gene of myosin, heavy chain 10 (MYH10) or cell-adhesion associated gene of cadherin-related family member 5 (CDHR5) or protocadherin-8 (PCDH8) were found. This result revealed EM-dependent phenotypical diversity in the cancer tissue. Therefore, the association between EM-dependent gene expression profiles and prognosis was evaluated. The DFS of the patients with EM-high signatures in GSE14333 was worse than that of those patients with EM-low signatures in GSE14333 (P = 0.0444; Fig.5d), which indicated that EM-associated gene expression profiles included clinically important factors.
Figure 5.
Phenotypical differences between elastic modulus (EM)-high and EM-low. (a, c) The principal cluster analysis and heat map of 514 gene expression profiles showed there were phenotypical differences between EM-high and EM-low tissues. (b) Each of the 10 most related genes are shown here. (d) Kaplan–Meier curves for disease-free survival (DFS) according to EM-high signatures in GSE14333 (n = 122) and EM-low signatures in GSE14333 (n = 104) suggest that DFS of patients with tumors with high EM was shorter than that seen in patients with low EM (P = 0.0444).
Discussion
Recently, we reported the utility of a tactile sensor of the Venustron system for measuring the elasticity of normal human tissue.16 This method was found to be easy, objective and quantitative. We safely measured more than 100 cancer tissues using the Venustron system, and no tissue deformation or destruction as a result of the contact of the sensor was observed. In addition, the measuring process took under 5 min. Furthermore, concordant with the previous report, the EM was strongly correlated with clinicopathological and morphometric factors. These results suggest the utility and validity of our method.
Concordant with the previous report on breast and prostate cancer tissues, we also found a high elasticity of cancer tissue compared to that of normal tissue.7,24 In addition, our quantitative data were useful in elucidating the correlation between the elasticity of human cancer tissue and clinicopathological factors. Similar to previous reports on prostate,25 breast,10,11 and head and neck,14 our data also revealed that EM is useful for estimating T, N and M-staging, which indicates future clinical implications. EM evaluation during endoscopy may enable physicians to predict metastasis before an operation in gastrointestinal cancers. As for the strong association with T-stage, the histoanatomic dependent diversity of fibroblasts and their different reaction to cancer stimuli have been reported previously.17 We speculate that heterogenous fibroblasts that reside in different histoanatomical sites (e.g. submucosa or subserosa) can be an origin to establish T-stage dependent elasticity. Therefore, we performed a detailed histological analysis that revealed a strong association with EM. The Azan positive area, stained with anilin blue, reflected the amount of collagen fibers.21 The α-SMA positive area reflected the fibroblast proliferation.22,23 This suggested that the EM was affected by the nature of the origin of the cancer stroma. Importantly, subperitoneal fibroblasts were known to show α-SMA and collagen upregulation after cancer medium stimulation.17 These biological findings seem to be concordant with the higher elasticity in pT4 colorectal cancer which have contact with subperitoneal fibroblasts.
Cancer cell growth or migration is mainly governed by EM of the surrounding microenvironment.26,27 In breast cancer, the amounts of collagen identified histologically corresponded with the stiffness of the tumor.28 A greater amount of collagen content has been reported to increase the risk of regional lymph node metastasis.29,30 Several in vitro reports show that the pathogenesis of tumor invasion and metastasis is linked to increased stiffness of tumor cells and the matrix.26,31 In our study of colorectal cancer tissue, the EM was related not only to the features of stromal cells but also to those of cancer cells, which included venous invasion or poorly differentiated clusters. These data seem to suggest the biological importance of the interaction between cancer cells and stromal cells.
As shown in Table2 and Figure3, the elasticity of the tumors is related with the tumor size. In general, the center of the tumor contains the oldest lesions, such as fibrosis, and the surface ulcers with much exudate. Therefore, the elasticity of the tumor may be related to the aging of the tumor.
Strong association between EM and pathological features seem to reflect the interaction between cancer cells and stromal cells. Of these, surprisingly, tumor budding did not, but poorly differentiated clusters showed strong correlation with EM values. In this study, tumor budding and poorly differentiated clusters were assessed using a method suggested by Ueno et al.32 Tumor budding and poorly differentiated clusters shared similar morphology without glandular formation with the difference in cell number of cluster. Both of these factors were associated with poor clinical outcome. Only tumor budding was reported to be associated with biological epithelial–mesenchymal transition or cancer stem cells, biological differences between tumor budding and poorly differentiated clusters were not demonstrated. Our results that high EM values are associated with not tumor budding but with poorly differentiated clusters could be a clue to elucidate the biological differences between them. Finally, inter-observer variation in the evaluation of tumor budding and poorly differentiated clusters has been reported.33 Further investigation will be required to assess the pathological relationships with EM values.
The DFS of patients with high EM tumors was also shorter than those of patients with low EM tumors. This is the first report that demonstrates EM as a prognostic marker in human cancer. Data accumulation and long-term follow up will be available in the future to establish the clinical utility of this procedure as a prognostic marker.
This study has demonstrated for first time the gene expression profiles that are associated with tumor elasticity. Furthermore, we elucidated its biological importance by associating it with clinical outcome. These global gene expression data will be available to search for prognostic biomarkers or future stromal target therapies. Based on previous reports and our pathological data, we speculated that EM was association with cell contractions or cell adhesion associated genes. Concordant with this speculation, we found that contraction-associated gene overexpression of MYH10 is overexpressed in high EM tumors. Furthermore, cell adhesion associated genes of CDHR5 or PCDH8 were differently expressed between high and low EM tumors. In this study, we performed global gene expression analysis using bulk tissue, which contain cancer cells, fibroblast, and other stromal cells. Therefore, the origin of overexpressed genes is not known. Further study will be required to confirm the origin of overexpressed genes.
The present study had certain limitations. First, the number of patients in the study is relatively small. Second, tumor EM values are not uniform and it is not clear whether the method of measuring EM presented in this study is optimal. A depth of 4 mm was the limit and we cannot measure the EM everywhere on the tumor. Third, reproducibility is an important consideration. Although the measurement of the EM was performed with an automatic indentation apparatus based on well-established physical rules, the surface of the cancer tissue is not completely flat, and the aqueous component of interstitial tissue at the indentation point may disperse into the surrounding area. In addition, physical characteristics that are not reflected in histologic features might create marked variability of data between cases. Moreover, the presence of plastic deformation and viscosity in the specimen cannot be ruled out, and, in fact, this is a characteristic of human organs. Further accumulation of cases with the global gene expression data and elasticity may help to clarify how characteristics other than pure elasticity may account for the properties of cancer tissue.
In conclusion, the present study has demonstrated the possibility of clinical utility of measuring the EM of colorectal cancer tissue to estimate accurate tumor stage and to predict clinical behavior. Moreover, we elucidated distinct gene expression profiles between the tumors with high and low EM, which were associated with clinical outcome. Our data of global gene expression profiles may allow us to search for new prognostic markers or stromal target therapies.
Acknowledgments
This work was funded by a Grant-in-Aid for Cancer Research A26-19 from the Ministry of Health, Labor and Welfare.
Disclosure Statement
The authors have no conflict of interest to declare.
Supporting Information
Additional supporting information may be found in the online version of this article:
Fig. S1. Representative colorectal cancer case with low EM. In a case, the elastic modulus (EM) of colorectal cancer tissue was 5.11 kPa as a case of tumor with low EM. (a) H&E staining (Loupe image). (b) Azan-Mallory staining (Loupe image). The two areas surrounded by yellow lines were the center of tumor and the margin of tumor. (c) The center of tumor with Azan-Mallory staining. (×40). (d) The margin of tumor with Azan-Mallory staining (×40). (e) The center of tumor with Azan-Mallory staining was evaluated using the color-detecting algorithm of the software. The Azan positive area was 7.32%. (f) The margin of tumor with Azan-Mallory staining was evaluated using the color-detecting algorithm of the software. The Azan positive area was 6.17%.
Fig. S2. Representative colorectal cancer case with low EM. In a case, the elastic modulus (EM) of colorectal cancer tissue was 5.11 kPa as a case of tumor with low EM. (a) H&E staining (Loupe image). (b) Immunohistochemical staining for α-smooth muscle actin (α-SMA; Loupe image). The two areas surrounded by yellow lines were the center of tumor and the margin of tumor. (c) The center of tumor with α-SMA. (×40). (d) The margin of tumor with α-SMA (×40). (e) The center of tumor with α-SMA was evaluated using the color-detecting algorithm of the software. The α-SMA positive area was 4.84%. (f) The margin of tumor with α-SMA staining was evaluated using the color-detecting algorithm of the software. The α-SMA positive area was 1.98%.
Fig. S3. Representative colorectal cancer case with high EM. In a case, the elastic modulus (EM) of colorectal cancer tissue was 30.6 kPa as a case of tumor with high EM. (a) H&E staining (Loupe image). (b) Azan-Mallory staining (Loupe image). The two areas surrounded by yellow lines were the center of tumor and the margin of tumor. (c) The center of tumor with Azan-Mallory staining. (×40). (d) The margin of tumor with Azan-Mallory staining (×40). (e) The center of tumor with Azan-Mallory staining was evaluated using the color-detecting algorithm of the software. The Azan positive area was 22.8%. (f) The margin of tumor with Azan-Mallory staining was evaluated using the color-detecting algorithm of the software. The Azan positive area was 10.9%.
Fig. S4. In a case, the elastic modulus (EM) of colorectal cancer tissue was 30.6 kPa as a case of tumor with high EM. (a) H&E staining (Loupe image). (b) Immunohistochemical staining for α-smooth muscle actin (α-SMA; Loupe image). The two areas surrounded by yellow lines were the center of tumor and the margin of tumor. (c) The center of tumor with α-SMA. (×40). (d) The margin of tumor with α-SMA (×40). (e) The center of tumor with α-SMA was evaluated using the color-detecting algorithm of the software. The α-SMA positive area was 22.1%. (f) The margin of tumor with α-SMA staining was evaluated using the color-detecting algorithm of the software. The α-SMA positive area was 18.3%.
Fig. S5. The elastic modulus (EM)-high and EM-low gene expression profiles. (a) A Venn diagram showing the 45 common gene expression profiles with that related to the Hela cell cycle and that related to elastic modulus EM-high or EM-low. (b) Heat map of 514 gene expression profiles of a public dataset showed there were phenotypical differences between EM-high and EM-low gene expression profiles.
Table S1. Clinical stage and RNA integrity number (RIN) of all our cases in stage II
Table S2. The 226 patients with colorectal cancer from public dataset (GSE14333)
References
- Phillips R. In retrospect: the feynman lectures on physics. Nature. 2013;504:30–1. doi: 10.1038/504030a. [DOI] [PubMed] [Google Scholar]
- Diridollou S, Vabre V, Berson M, et al. Skin ageing: changes of physical properties of human skin in vivo. Int J Cosmet Sci. 2001;23:353–62. doi: 10.1046/j.0412-5463.2001.00105.x. [DOI] [PubMed] [Google Scholar]
- Hara Y, Masuda Y, Hirao T, Yoshikawa N. The relationship between the Young's modulus of the stratum corneum and age: a pilot study. Skin Res Technol. 2013;19:339–45. doi: 10.1111/srt.12054. [DOI] [PubMed] [Google Scholar]
- Kaster T, Sack I, Samani A. Measurement of the hyperelastic properties of ex vivo brain tissue slices. J Biomech. 2011;44:1158–63. doi: 10.1016/j.jbiomech.2011.01.019. [DOI] [PubMed] [Google Scholar]
- Leng H, Reyes MJ, Dong XN, Wang X. Effect of age on mechanical properties of the collagen phase in different orientations of human cortical bone. Bone. 2013;55:288–91. doi: 10.1016/j.bone.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pailler-Mattei C, Bec S, Zahouani H. In vivo measurements of the elastic mechanical properties of human skin by indentation tests. Med Eng Phys. 2008;30:599–606. doi: 10.1016/j.medengphy.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Samani A, Zubovits J, Plewes D. Elastic moduli of normal and pathological human breast tissues: an inversion-technique-based investigation of 169 samples. Phys Med Biol. 2007;52:1565–76. doi: 10.1088/0031-9155/52/6/002. [DOI] [PubMed] [Google Scholar]
- Tilleman TR, Tilleman MM, Neumann MH. The elastic properties of cancerous skin: Poisson's ratio and Young's modulus. Isr Med Assoc J. 2004;6:753–5. [PubMed] [Google Scholar]
- Katsutoshi Miura SY. Histological image of gastric tumors by scanning acoustic microscope. BJAST. 2014;4:1–17. [Google Scholar]
- Evans A, Whelehan P, Thomson K, et al. Invasive breast cancer: relationship between shear-wave elastographic findings and histologic prognostic factors. Radiology. 2012;263:673–7. doi: 10.1148/radiol.12111317. [DOI] [PubMed] [Google Scholar]
- Yi A, Moon WK, Cho N, et al. Association of tumour stiffness on sonoelastography with axillary nodal status in T1 breast carcinoma patients. Eur Radiol. 2013;23:2979–87. doi: 10.1007/s00330-013-2930-y. [DOI] [PubMed] [Google Scholar]
- Itoh A, Ueno E, Tohno E, et al. Breast disease: clinical application of US elastography for diagnosis. Radiology. 2006;239:341–50. doi: 10.1148/radiol.2391041676. [DOI] [PubMed] [Google Scholar]
- Chang JM, Park IA, Lee SH, et al. Stiffness of tumours measured by shear-wave elastography correlated with subtypes of breast cancer. Eur Radiol. 2013;23:2450–8. doi: 10.1007/s00330-013-2866-2. [DOI] [PubMed] [Google Scholar]
- Furukawa MK, Furukawa M. Diagnosis of lymph node metastases of head and neck cancer and evaluation of effects of chemoradiotherapy using ultrasonography. Int J Clin Oncol. 2010;15:23–32. doi: 10.1007/s10147-009-0017-1. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Yamamoto Y, Ibusuki M, et al. Evaluation of tumor stiffness by elastography is predictive for pathologic complete response to neoadjuvant chemotherapy in patients with breast cancer. Ann Surg Oncol. 2012;19:3042–9. doi: 10.1245/s10434-012-2343-1. [DOI] [PubMed] [Google Scholar]
- Sugimoto M, Takahashi S, Kojima M, et al. What is the nature of pancreatic consistency? Assessment of the elastic modulus of the pancreas and comparison with tactile sensation, histology, and occurrence of postoperative pancreatic fistula after pancreaticoduodenectomy. Surgery. 2014;156:1204–11. doi: 10.1016/j.surg.2014.05.015. [DOI] [PubMed] [Google Scholar]
- Kojima M, Higuchi Y, Yokota M, et al. Human subperitoneal fibroblast and cancer cell interaction creates microenvironment that enhances tumor progression and metastasis. PLoS One. 2014;9:e88018. doi: 10.1371/journal.pone.0088018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield ML, Sherlock G, Saldanha AJ, et al. Identification of genes periodically expressed in the human cell cycle and their expression in tumors. Mol Biol Cell. 2002;13:1977–2000. doi: 10.1091/mbc.02-02-0030.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Sneddon JB, Alizadeh AA, et al. Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol. 2004;2:E7. doi: 10.1371/journal.pbio.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorissen RN, Gibbs P, Christie M, et al. Metastasis-associated gene expression changes predict poor outcomes in patients with Dukes stage b and c colorectal cancer. Clin Cancer Res. 2009;15:7642–51. doi: 10.1158/1078-0432.CCR-09-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallory FB. A contribution to staining methods: I. A differential stain for connective-tissue fibrillae and reticulum. II. Chloride of iron haematoxylin for nuclei and fibrin. III phosphotungstic acid haematoxylin for neuroglia fibres. J Exp Med. 1900;5:15–20. doi: 10.1084/jem.5.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherng S, Young J, Ma H. Alpha-Smooth Muscle Actin (α-SMA) J Am Sci. 2008;4:7–9. [Google Scholar]
- Storch KN, Taatjes DJ, Bouffard NA, Locknar S, Bishop NM, Langevin HM. Alpha smooth muscle actin distribution in cytoplasm and nuclear invaginations of connective tissue fibroblasts. Histochem Cell Biol. 2007;127:523–30. doi: 10.1007/s00418-007-0275-9. [DOI] [PubMed] [Google Scholar]
- Good DW, Stewart GD, Hammer S, et al. Elasticity as a biomarker for prostate cancer: a systematic review. BJU Int. 2014;113:523–34. doi: 10.1111/bju.12236. [DOI] [PubMed] [Google Scholar]
- Carson WC, Gerling GJ, Krupski TL, Kowalik CG, Harper JC, Moskaluk CA. Material characterization of ex vivo prostate tissue via spherical indentation in the clinic. Med Eng Phys. 2011;33:302–9. doi: 10.1016/j.medengphy.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Zaman MH, Trapani LM, Sieminski AL, et al. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc Natl Acad Sci U S A. 2006;103:10889–94. doi: 10.1073/pnas.0604460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilghman RW, Cowan CR, Mih JD, et al. Matrix rigidity regulates cancer cell growth and cellular phenotype. PLoS One. 2010;5:e12905. doi: 10.1371/journal.pone.0012905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner J, Stacer AC, Winterroth F, Johnson TD, Luker KE, Luker GD. Macroscopic stiffness of breast tumors predicts metastasis. Sci Rep. 2014;4:5512. doi: 10.1038/srep05512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakkad SM, Solaiyappan M, Argani P, et al. Collagen I fiber density increases in lymph node positive breast cancers: pilot study. J Biomed Opt. 2012;17:116017. doi: 10.1117/1.JBO.17.11.116017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson L, Czene K, Rosenberg L, Humphreys K, Hall P. Possible influence of mammographic density on local and locoregional recurrence of breast cancer. Breast Cancer Res. 2013;15:R56. doi: 10.1186/bcr3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraning-Rush CM, Califano JP, Reinhart-King CA. Cellular traction stresses increase with increasing metastatic potential. PLoS One. 2012;7:e32572. doi: 10.1371/journal.pone.0032572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno H, Konishi T, Ishikawa Y, et al. Prognostic value of poorly differentiated clusters in the primary tumor in patients undergoing hepatectomy for colorectal liver metastasis. Surgery. 2015;157:899–908. doi: 10.1016/j.surg.2014.12.025. [DOI] [PubMed] [Google Scholar]
- Ueno H, Hase K, Hashiguchi Y, et al. Novel risk factors for lymph node metastasis in early invasive colorectal cancer: a multi-institution pathology review. J Gastroenterol. 2014;49:1314–23. doi: 10.1007/s00535-013-0881-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Representative colorectal cancer case with low EM. In a case, the elastic modulus (EM) of colorectal cancer tissue was 5.11 kPa as a case of tumor with low EM. (a) H&E staining (Loupe image). (b) Azan-Mallory staining (Loupe image). The two areas surrounded by yellow lines were the center of tumor and the margin of tumor. (c) The center of tumor with Azan-Mallory staining. (×40). (d) The margin of tumor with Azan-Mallory staining (×40). (e) The center of tumor with Azan-Mallory staining was evaluated using the color-detecting algorithm of the software. The Azan positive area was 7.32%. (f) The margin of tumor with Azan-Mallory staining was evaluated using the color-detecting algorithm of the software. The Azan positive area was 6.17%.
Fig. S2. Representative colorectal cancer case with low EM. In a case, the elastic modulus (EM) of colorectal cancer tissue was 5.11 kPa as a case of tumor with low EM. (a) H&E staining (Loupe image). (b) Immunohistochemical staining for α-smooth muscle actin (α-SMA; Loupe image). The two areas surrounded by yellow lines were the center of tumor and the margin of tumor. (c) The center of tumor with α-SMA. (×40). (d) The margin of tumor with α-SMA (×40). (e) The center of tumor with α-SMA was evaluated using the color-detecting algorithm of the software. The α-SMA positive area was 4.84%. (f) The margin of tumor with α-SMA staining was evaluated using the color-detecting algorithm of the software. The α-SMA positive area was 1.98%.
Fig. S3. Representative colorectal cancer case with high EM. In a case, the elastic modulus (EM) of colorectal cancer tissue was 30.6 kPa as a case of tumor with high EM. (a) H&E staining (Loupe image). (b) Azan-Mallory staining (Loupe image). The two areas surrounded by yellow lines were the center of tumor and the margin of tumor. (c) The center of tumor with Azan-Mallory staining. (×40). (d) The margin of tumor with Azan-Mallory staining (×40). (e) The center of tumor with Azan-Mallory staining was evaluated using the color-detecting algorithm of the software. The Azan positive area was 22.8%. (f) The margin of tumor with Azan-Mallory staining was evaluated using the color-detecting algorithm of the software. The Azan positive area was 10.9%.
Fig. S4. In a case, the elastic modulus (EM) of colorectal cancer tissue was 30.6 kPa as a case of tumor with high EM. (a) H&E staining (Loupe image). (b) Immunohistochemical staining for α-smooth muscle actin (α-SMA; Loupe image). The two areas surrounded by yellow lines were the center of tumor and the margin of tumor. (c) The center of tumor with α-SMA. (×40). (d) The margin of tumor with α-SMA (×40). (e) The center of tumor with α-SMA was evaluated using the color-detecting algorithm of the software. The α-SMA positive area was 22.1%. (f) The margin of tumor with α-SMA staining was evaluated using the color-detecting algorithm of the software. The α-SMA positive area was 18.3%.
Fig. S5. The elastic modulus (EM)-high and EM-low gene expression profiles. (a) A Venn diagram showing the 45 common gene expression profiles with that related to the Hela cell cycle and that related to elastic modulus EM-high or EM-low. (b) Heat map of 514 gene expression profiles of a public dataset showed there were phenotypical differences between EM-high and EM-low gene expression profiles.
Table S1. Clinical stage and RNA integrity number (RIN) of all our cases in stage II
Table S2. The 226 patients with colorectal cancer from public dataset (GSE14333)