Abstract
MAS1 is a receptor for angiotensin 1-7 (A1-7), which is derived from angiotensin II (A-II) by the action of angiotensin converting enzyme (ACE) 2. MAS1 induces anti-A-II phenotypes, such as vessel dilation and depression of blood pressure. Using immunohistochemistry, we examined the role of MAS1 in 132 cases of invasive ductal carcinoma (IDC) of the breast. While benign mammary tissues expressed MAS1 at high levels, MAS1 expression was attenuated in all IDC, especially in scirrhous IDC. The decrease in MAS1 expression was associated with tumor growth, lymph node metastasis, and grade. MAS1 expression was inversely associated with the proliferation index and epidermal growth factor receptor and human epidermal growth factor receptor-2 expression. Of the 132 cases, 12 (9.1%) were triple-negative breast cancer (TNBC) cases. All TNBC cases (the 12 cases and the additional 36 cases using a tissue array) expressed MAS1. Using the TNBC cell lines 4T1 and MDA-MB-468, which expresses MAS1, we found that cell growth, anti-apoptotic survival and invasion were suppressed by MAS1 activation with A1-7 treatment and enhanced by MAS1 knockdown. In contrast, synergic effect was found between tamoxifen and A1-7 in a luminal A breast cancer cell line, MCF-7. Combination treatment with cisplatin, an ACE2 activator, and an A-II type 1 receptor blocker showed synergic effects on tumor growth inhibition of 4T1 tumors in a syngeneic mouse model. These findings suggest that MAS1 might act as an inhibitory regulator of breast cancer and may be a possible molecular target for this malignancy.
Keywords: ACE2, angiotensin, angiotensin1-7, breast cancer, MAS1
The renin-angiotensin system (RAS) is a blood pressure controlling mechanism consisting of multiple peptides, enzymes and receptors.1,2 Angiotensin-II (A-II), the major active peptide, activates A-II type 1 receptor (AT1R) to provide vasoconstriction and hypertension. In contrast, activation of the alternative A-II receptor, AT2R, elicits the opposite effects.3 In cancer, A-II has protumoral effects and has been demonstrated to enhance cell proliferation, survival, invasion, angiogenesis and subsequent metastasis through AT1R.2–5 Moreover, anti-RAS treatment has been shown to reduce colorectal cancer (CRC) liver metastasis in a mouse model.6 In contrast, AT2R shows antitumoral effects at high A-II concentrations.7
Through other pathways, angiotensin 1-7 (A1-7) is produced from A-I by CD10 (neprilysin) or from A-II by ACE2.8 MAS1 is a receptor for A1-7 and A-II, which elicits the opposite effects on vasculature compared to AT1R; namely, vasodilatation and hypotension.8,9 The MAS1–A1-7 axis has, moreover, been implicated in certain cancers10 such as CRC.11
Breast cancer is the fourth leading cause of cancer death in Japanese women.12 The treatment of breast cancer commonly includes a combination of surgery, radiation, chemotherapy, hormone therapy and targeted therapy against human epidermal growth factor receptor-2 (HER2).13 When adopting hormone therapy or HER2 targeted therapy, the expression of estrogen receptor (ER), progesterone receptor (PgR) or HER2 should be evaluated. In triple-negative breast cancer (TNBC), expressions of ER, PgR and HER2 are lost in all cancer cells.14 TNBC represent approximately 15% of all breast cancers,14 TNBC shows rapid growth and a high frequency of recurrence and metastasis. Accordingly, it is associated with high mortality and poor prognosis.14,15 Currently, adjuvant therapies, including targeting of epidermal growth factor receptor (EGFR) and poly (ADP-ribose) polymerase 1, are being developed to treat this breast cancer subtype.16,17 Identifying a novel molecular target is an important issue for the treatment of TNBC.
In the present study, we aim to elucidate the expression and tumor-inhibitory role of MAS1, and discuss its potential as a molecular target for treatment of breast cancer, including TNBC.
Materials and Methods
Surgical specimens
Formalin-fixed surgical specimens from 158 patients (21 benign mammary lesions, including mastopathy, usual duct hyperplasia or intraductal papilloma, 10 ductal carcinomas in situ [DCIS], and 132 invasive ductal carcinomas [IDC]) diagnosed at the Department of Molecular Pathology, Nara Medical School were randomly selected. Because written informed consent was not obtained, any identifying information was removed from the samples before analysis to ensure strict privacy protection (unlinkable anonymization). All procedures were performed in accordance with the Ethical Guidelines for Human Genome/Gene Research enacted by the Japanese Government, which was approved by the Ethics Committee of Nara Medical University (Authorization Number 937).
Tissue array
Tissue array slides of TNBC were obtained from US Biomax (BR487a, Rockville, MD, USA). Quadriplet slides were immunostained with ER, PgR, Her2 and MAS1. IDC cases with triple negative expression (36 cases) were used for comparison between clinicopathological parameters and MAS1 expression.
Cell culture and reagents
The 4T1 mouse breast cancer, LL2 mouse lung cancer, MCF-7 human breast cancer (luminal A phenotype) and MDA-MB-468 human TNBC (basal phenotype) cell lines were purchased from DS Pharma (Tokyo, Japan).18 The CT26 mouse colon cancer cell line was a kind gift from Professor I. J. Fidler (MD Anderson Cancer Center). The cell lines were maintained in DMEM (Sigma Chemical, St. Louis, MO, USA) containing 10% FBS (Sigma) in a 5% CO2 atmosphere at 37°C. The following reagents were purchased: charcoal stripped FBS (Sigma), tamoxifen (TMX; Sigma), angiotensin I and II (A-I and II; Abgent, San Diego, CA, USA), angiotensinogen (ATG; Calbiochem, Darmstadt, Germany), angiotensin 1-7 (A1-7; California Peptide Research, Napa, CA, USA), cisplatin (CDDP; Alexis) and xanthenone (XTN; Alfa Aesar, Ward Hill, MA, USA).
Cell treatment, cell growth, apoptosis and in vitro invasion assay
The cells were treated with TMX (2 μg/mL), ATG (20 nM), A-I (1 ng/mL), A-II (1 ng/mL), A1-7 (100 nM) or CDDP (10 μM) for 24 h before analyses. Cells were seeded at a density of 10 000 cells per well in 12-well tissue culture plates. Cell growth was assessed by cell counting using an autocytometer (Sysmex, Kobe, Japan). Apoptosis was assessed by staining with Hoechst 33258 fluorescent dye (Wako Pure Chemical, Osaka, Japan). The number of apoptotic cells was determined by examining 1000 cells.19 A modified Boyden chamber assay was performed to examine breast cancer cell invasion in vitro.20 All experiments were performed in triplicate.
Animal model
Female BALB/c mice (5 weeks old) purchased from Japan SLC (Shizuoka, Japan) were used for the in vivo experiments. The mice were maintained according to the institutional guidelines approved by the Committee for Animal Experimentation of Nara Medical University. Single-cell suspensions of 4T1 cells (1 × 106) in Hanks balanced saline solution were injected into the mammary pad of each mouse. MAS1 siRNA (siRNA) or control scramble siRNA (10 pmol, encapsulated with cationic liposome [EL-C-01; Nippon-Oil & Fats, Tokyo, Japan]),21,22 CDDP (3 mg/kg body weight, once a week) and XTN (0.5 mg/kg body weight, once a week)23 were injected into the peritoneal cavity. Losartan (ARB; Toronto Research Chemicals, Toronto, ON, Canada; 6 mg/kg body weight, once a week) was administered intragastrically. Four weeks post-inoculation, the mice were killed and the size and number of tumors in the primary site and lungs were determined.
Immunohistochemistry
Consecutive 4-μm sections were immunohistochemically stained using the immunoperoxidase technique, as described previously.24 MAS1 antibody (Novocastra, Newcastle upon Tyne, UK) was used at a concentration of 0.5 μg/mL. Color development was performed using 3-3′-diaminobenzidine (DAKO, Carpinteria, CA, USA), and the specimens were counterstained with Meyer's hematoxylin (Sigma) to visualize the nuclei. To confirm the specificity of antibody immunoreactivity, cardiac muscle and colonic mucosa were used as positive and negative controls, respectively. After immunostaining, all slides were assessed according to the immunoreactive score.25 This score was originally proposed for evaluation of ER expression, although it has also been used to evaluate membranous and/or cytosolic staining in many studies.19 The score was expressed as four grades: “none,” score 0; “trace,” score 1–2; “weak,” score 3–5; and “strong,” score 6–8.
Reverse transcription-PCR
Total RNA (1 μg) was synthesized using the ReverTra Ace quantitative PCR (qPCR) RT Kit (Toyobo, Osaka, Japan). The primer sets for amplification are listed in Table1. The PCR conditions were set according to the provider's instructions. PCR products were electrophoresed on a 2% agarose gel and visualized using ethidium bromide.
Table 1.
RT-PCR primer sequences
| Forward primer | Reverse primer | Reference | |
|---|---|---|---|
| Human | |||
| MAS1 | tgagctttcttctggccatt | gaccaatgccgactggtact | BC110454.2 |
| Mouse | |||
| MAS1 | catctaggactgggcagagc | agtcaggaggtggagagcaa | NM_008552.4 |
| Cathepsin D | tcaggaagcctctctgggta | cccaagatgccatcaaactt | NM_009983.2 |
| ACE2 | ctacaggcccttcagcaaag | tgcccagagcctagagttgt | AB053182.1 |
| CD10 | gaaattcagccaaagcaagc | tgctgagcactgaagaatgg | BC034092.1 |
| ER1 | aagggcagtcacaatgaacc | gccaggtcattctccacatt | NM_007956.4 |
| PgR | ggtggaggtcgtacaagcat | ctcatgggtcacctggagtt | NM_008829.2 |
| HER2 | gctgctggacattgatgaga | gggatcccatcgtaaggttt | BC053078.1 |
ACE2, angiotensin converting enzyme 2; CD10, neprilysin (cluster of differentiation 10); ER1, estrogen receptor 1; HER2, human epithelial growth factor receptor-related 2; PgR, progesterone receptor; RT-PCR, reverse transcriptase polymerase chain reaction.
Quantitative reverse transcription-polymerase chain reaction
Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and total RNA (1 μg) was synthesized with the ReverTra Ace qRT Kit (Toyobo). Quantitative reverse transcription-PCR was performed using StepOne Plus Real-Time PCR Systems (Applied Biosystems, Foster City, CA, USA) using TaqMan Fast Universal PCR Master Mix (Applied Biosystems). The values were compensated by the relative standard curve quantification method. PCR condition was according to the manufacturer's instructions and the ACTB mRNA level was amplified for internal control. TaqMan Gene Expression Assay of MAS1 was purchased from Applied Biosystems. PCR was done in triplicate.
siRNA transfection
FlexiTube siRNA for MAS1 was purchased from Qiagen Genomics (Bothell, WA, USA). AllStars Negative Control siRNA was used as a control (Qiagen). Cells were transfected with 50 nM siRNA for each gene using Lipofectamine 2000 (Invitrogen, Invitrogen Corp., Carlsbad, CA) according to the manufacturer's instructions.
ELISA and examination of ACE2 activity
Frozen mouse tissues were sonicated in lysis buffer, and the supernatants were collected and used for ELISA. The concentrations of A1-7 and ACE2 were determined using the A1-7 ELISA kit (Cusabio Biotech, Wuhan, China) and the SensoLyte 390 ACE2 Activity Assay Kit (AnaSpec, Fremont, CA, USA), respectively, according to the manufacturer's instructions.
Statistical analysis
Statistical analyses of experimental data were performed using the Mann–Whitney U-test, analysis of variance and the χ2-test. Nonparametric correlations were examined using the Spearman rank correlation test. Statistical significance was defined as a two-sided P-value of <0.05.
Results
Expression of MAS1 in breast cancer
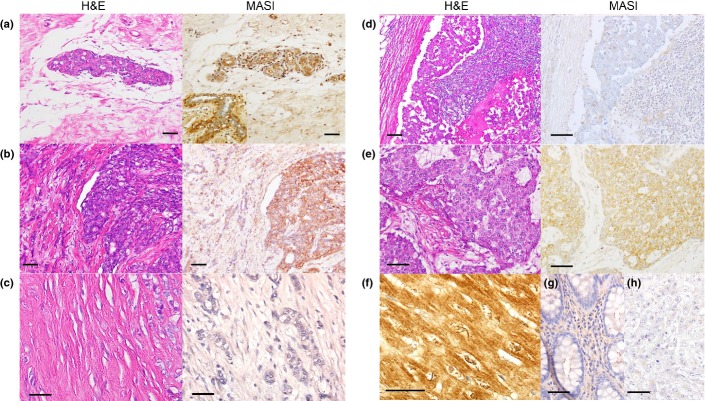
A total of 21 benign breast tissues, 10 DCIS and 132 IDC cases were examined by immunohistochemistry (Table2). MAS1 expression was strongly observed in the benign mammary duct (Fig.1a). In some cases, MAS1 expression was emphasized in the myoepithelial cells (inset). In Figure1(b), the cribriform type DCIS expressed MAS1 at strong grade, whereas surrounding invading nests expressed MAS1 at traceable-weak grade. Scirrhous IDC and the lymph node metastasis expressed MAS1 at “none” grade (Fig.1c,d), In Figure1(e), solid-tubular type TNBC was shown, which expressed MAS1 at weak grade. Thus, MAS1 expression was repressed in the order from benign duct, DCIS to IDC, but not TNBC. The positive control, cardiomyocytes, showed very high MAS1 expression in the cytoplasm and plasma membrane (Fig.1f), whereas colonic epithelium, and no primary antibody-staining as the negative controls, showed no MAS1 expression (Fig.1g,h).
Table 2.
Relationship between MAS1 expression and clinicopathological parameters of breast cancer samples
| N | MAS1 expression | Weak | Strong | P | ||
|---|---|---|---|---|---|---|
| None | Trace | |||||
| Benign tissue | 21 | 0 | 0 | 2 | 19 | |
| DCIS | 10 | 0 | 0 | 3 | 7 | NS |
| IDC | 132 | 24 | 50 | 58 | 0 | <0.0001 |
| Papillotubular | 35 | 3 | 9 | 23 | 0 | |
| Solid-tubular | 55 | 6 | 22 | 27 | 0 | |
| Scirrhous | 42 | 15 | 19 | 8 | 0 | 0.0002 |
| Grade 1 | 53 | 4 | 18 | 31 | 0 | |
| Grade 2 | 37 | 4 | 15 | 18 | 0 | |
| Grade 3 | 42 | 16 | 17 | 9 | 0 | 0.0003 |
| pT1 | 55 | 1 | 21 | 33 | 0 | |
| pT2 | 41 | 7 | 17 | 17 | 0 | |
| pT3 | 21 | 8 | 6 | 7 | 0 | |
| pT4 | 15 | 8 | 6 | 1 | 0 | <0.0001 |
| pN0 | 104 | 1 | 45 | 58 | 0 | |
| pN1 | 28 | 23 | 5 | 0 | 0 | <0.0001 |
P-value was calculated by χ2-test. DCIS, ductal carcinomas in situ; IDC, invasive ductal carcinoma; NS, not significant.
Figure 1.
Expression of MAS1 in breast cancer. MAS1 expression was examined by immunohistochemistry in (a) benign mammary ducts (inset; prominent MAS1 expression was observed in the myoepithelial cells), (b) ductal carcinoma in situ (right side) with invasive ductal carcinoma (IDC) component (left side), (c) scirrhous type IDC, (d) lymph node metastasis, (e) triple-negative breast cancer; solid-tubular type, (f) cardiac muscle as a positive control, (g) colonic mucosa as a negative control and (h) technical negative control by no MAS1 antibody treatment. The scale bars, 50 μm.
Relationship between MAS1 expression and clinicopathological parameters
The MAS1 expression levels in IDC were significantly lower than those in benign tissues (Table2). However, DCIS did not show a significant reduction in MAS1 expression compared to the non-cancerous tissues. DCIS also showed MAS1 expression at higher grades than that of IDC (P < 0.0001). The six cases of 10 DCIS surrounded IDC (Table3). Comparing the MAS1 expression between DCIS and IDC in the same tumor, the expression levels in IDC were lower than those in DCIS.
Table 3.
DCIS cases
| Age | Grade | MAS1 | ER | PgR | HER2 | MAS1 in IDC |
|---|---|---|---|---|---|---|
| 30 | Low | Strong | 2 | 1 | 2+ | Traceable |
| 38 | Low | Strong | 3 | 2 | 0 | Weak |
| 48 | Intermediate | Strong | 3 | 3 | 1+ | NA |
| 63 | Low | Strong | 3 | 3 | 0 | Traceable |
| 71 | Low | Strong | 3 | 3 | 1+ | NA |
| 47 | Intermediate | Strong | 3 | 1 | 2+ | Weak |
| 38 | Intermediate | Strong | 2 | 2 | 1+ | None |
| 45 | Low | Weak | 3 | 2 | 0 | Traceable |
| 67 | High | Weak | 2 | 3 | 2+ | NA |
| 58 | High | Weak | 3 | 3 | 2+ | NA |
Estrogen receptor (ER) and progesterone receptor (PgR) were scored by J-Score system. Human epithelial growth factor receptor-related (HER2) was scored according to the Guide for HER2 Testing by Japanese Breast Cancer Society. NA, not applicable, no IDC was accompanied.
All histological types of IDC showed reduced MAS1 expression compared to non-cancerous tissues, with scirrhous carcinomas showing the most prominent reduction in MAS1 expression (Table2). A decrease in MAS1 expression was associated with histological grade, tumor growth (T factor) and lymph node metastasis (N factor).
Relationship between MAS1 expression and molecular parameters
Next, the relationships between the expression levels of MAS1 and various molecular markers, including the MIB-1 proliferation index and the expression of ER, PgR, epidermal growth factor receptor (EGFR) and HER2, were examined (Table4). MAS1 expression was inversely associated with the MIB-1 proliferation index and EGFR and HER2 expression, whereas no association with the expression of the hormone receptors (ER and PgR) was noted.
Table 4.
Relationship between MAS1 expression and molecular markers in breast cancer
| Slope | R | P | |
|---|---|---|---|
| MIB-1 | −0.706 | 0.322 | 0.0033 |
| ER | −0.136 | 0.1363 | NS |
| PgR | −0.002 | 0.0217 | NS |
| HER2 | −0.392 | 0.2599 | 0.0149 |
| EGFR | −0.223 | 0.2335 | 0.0259 |
P: Nonparametric correlations were examined using the Spearman rank correlation test. EGFR, epidermal growth factor receptor; ER, estrogen receptor; HER2, human epithelial growth factor receptor-related; NS, not significant; PgR, progesterone receptor.
Role of MAS1 in MCF-7 and MDA-MB-468 human breast cancer cells
The expression of MAS1 was examined in MCF-7 human breast cancer cells (luminal A phenotype) and MDA-MB-468 TNBC cells (basal phenotype) (Fig.2a,b). MCF-7 cells expressed MAS1, which was increased by estradiol deprivation for 48 h. In contrast, estradiol deprivation did not affect MAS1 expression in MDA-MB-468 cells. MAS1 expression was decreased to a traceable level by knockdown in both cell lines.
Figure 2.
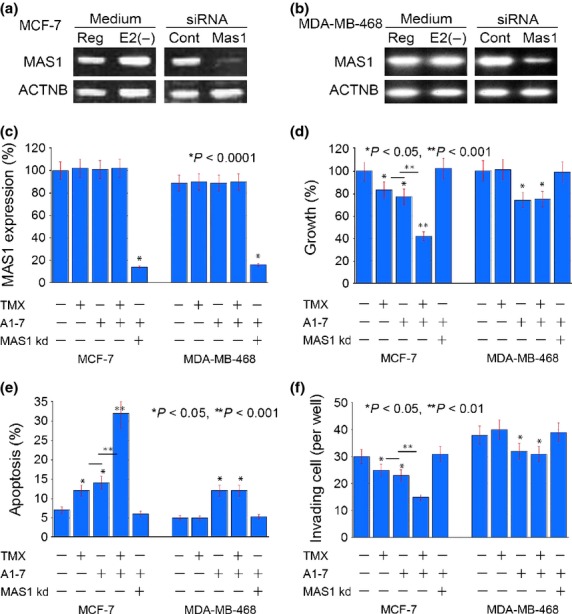
Effect of MAS1 activation by A1-7 with or without TMX or MAS1 knockdown in human breast cancer cell lines. (a, b) Expression of MAS1 gene in MCF-7 and MDA-MB-468 cells. Reg, regular medium; E2(−), cells cultured with estradiol-free medium supplemented with charcoal stripped FBS for 48 h; ACTNB, beta-actin; cont, control. (c–f) Effects of MAS1 knockdown and treatment with TMX and/or A1-7 on the MAS1 expression (c), growth (d), apoptosis (e) and invasion (f) of MCF-7 and MDA-MB-468 cells. Bar: standard deviation from three independent experiments. P was calculated by Mann–Whitney U-test.
Referring to the observation, we examined the effect of MAS1 activation by A1-7 with or without TMX or MAS1 knockdown (Fig.2c–f). MAS1 expression was increased by TMX treatment in MCF-7 cells but not in MDA-MB-468 cells. TMX alone and A1-7 alone showed inhibition of growth, survival and invasion in MCF-7 cells. Moreover, concurrent treatment of TMX and A1-7 showed synergic effects in MCF-7 cells. In contrast with MCF-7 cells, MDA-MB-468 cells showed that A1-7 treatment inhibited growth, survival and invasion, but TMX did not. The effects by A1-7 were abrogated by MAS1 knockdown. Thus, A1-7 treatment is effective in both non-TNBC and TNBC cells; however, a synergic effect with anti-estrogen treatment might be expected in non-TNBC cells.
Role of MAS1 in 4T1 mouse triple-negative breast cancer cells
The expression of MAS1 and other RAS factors was examined in 4T1 mouse breast cancer, LL2 mouse lung cancer and CT26 mouse colon cancer cells (Fig.3a). The 4T1 cells were found to express MAS1 but not the A1-7 producing enzymes ACE2 and CD10. In contrast, LL2 and CT26 cells expressed ACE2 and CD10, whereas MAS1 expression was very low. Upon MAS1 knockdown, MAS1 expression disappeared in 4T1 cells (Fig.3b).
Figure 3.
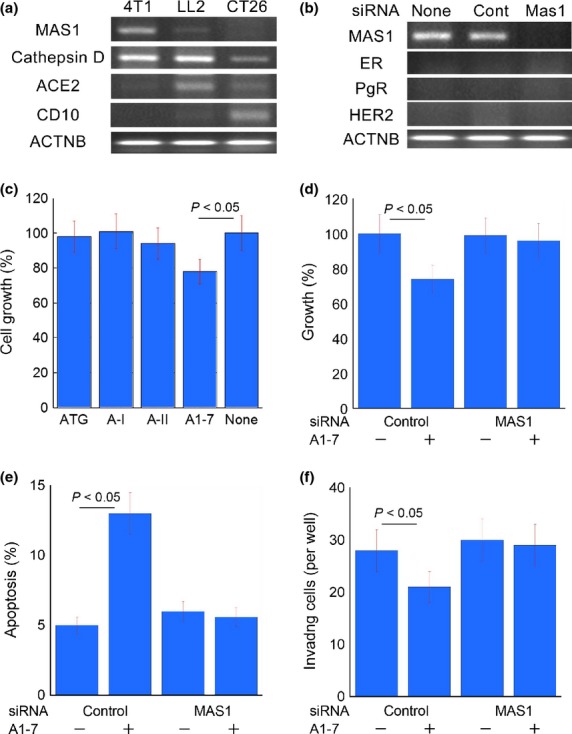
Effects of MAS1 knockdown on the tumor properties of 4T1 mouse breast cancer cells. (a) Expression of renin-angiotensin system-associated genes in 4T1, LL2 and CT26 cells. (b) Knockdown of MAS1 in 4T1 cells. (C) Effects of angiotensin family factors on the growth of 4T1 cells. (d–f) Effects of MAS1 knockdown on the growth (d), apoptosis (e), and invasion (f) of 4T1 cells. Bar: standard deviation from three independent experiments. P was calculated by Mann–Whitney U-test. A, angiotensin; ACE2, angiotensin converting enzyme 2; ACTNB, beta-actin; ATG, angiotensinogen; CD10, neprilysin (cluster of differentiation 10); cont, control; ER, estrogen receptor; HER2, human epithelial growth factor receptor-related 2; PgR, progesterone receptor.
A-II and A-I, which were converted by cathepsin D expressed in 4T1 cells, were found to enhance the growth of these cells (Fig.3c). In contrast, the growth of 4T1 cells was suppressed by A1-7 treatment. In the knockdown study, control siRNA-exposed 4T1 cells showed suppression of cell growth and invasion and induction of apoptosis upon A1-7 treatment (Fig.3d–f); however, these effects were abrogated by MAS1 siRNA-treatment.
Expression of MAS1 in triple-negative breast cancer
Of the 132 cases of IDC, 12 (9.1%) showed no expression of ER, PgR and HER2, and were, hence, designated as TNBC (Table5a). The 12 TNBC cases expressed MAS1 at “weak” grade, whereas 48 cases of the 120 non-TNBC cases expressed MAS1 at “weak” grade (P < 0.0001). Importantly, all TNBC expressed MAS1, although the levels of MAS1 expression in TNBC and non-TNBC samples did not differ significantly (Table5b). Spearman's correlation between MAS1 score and clinicopathological parameters was calculated for evaluation of the role of MAS1 in progression of TNBC cases. As shown in Table5(a), MAS1 expression was correlated with pT and pN.
Table 5.
Expression of MAS1 in triple-negative breast cancer (a) Relationship between MAS1 expression and clinicopathological parameter in triple-negative breast cancer cases; (b) Triple negative cancer versus other cancer and (c) Relationship between MAS1 expression in TNBC tissue array cases
| (a) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Histology | Grade | pT | pN | ER (%) | PR (%) | HER2 | p53 (%) | EGFR | MIB1 (%) | MAS1 | MAS1 score | |
| 48 | IDC | ST | 2 | 1 | 0 | 0 | 0 | 0 | 30 | Weak | 25 | Trace | 1 |
| 42 | IDC | ST | 1 | 2 | 0 | 0 | 0 | 0 | 60 | Moderate | 25 | Trace | 1 |
| 44 | IDC | SC | 3 | 3 | 1 | 0 | 0 | 0 | 55 | Weak | 25 | Trace | 2 |
| 36 | IDC | SC | 1 | 1 | 0 | 0 | 0 | 0 | 45 | None | 35 | Weak | 3 |
| 54 | IDC | SC | 2 | 1 | 0 | 0 | 0 | 0 | 20 | None | 5 | Weak | 3 |
| 44 | IDC | ST | 2 | 1 | 0 | 0 | 0 | 0 | 50 | Weak | 5 | Weak | 3 |
| 79 | IDC | PT | 2 | 2 | 0 | 0 | 0 | 0 | 20 | Moderate | 10 | Weak | 4 |
| 56 | IDC | SC | 3 | 2 | 0 | 0 | 0 | 0 | 20 | Weak | 15 | Weak | 3 |
| 49 | IDC | SC | 3 | 2 | 1 | 0 | 0 | 0 | 0 | None | 10 | Weak | 4 |
| 36 | IDC | PT | 2 | 3 | 1 | 0 | 0 | 0 | 15 | None | 15 | Weak | 5 |
| 56 | IDC | ST | 2 | 4 | 1 | 0 | 0 | 0 | 70 | None | 40 | Weak | 5 |
| 37 | IDC | ST | 3 | 4 | 1 | 0 | 0 | 0 | 95 | Weak | 60 | Weak | 5 |
| P | NS | <0.05 | <0.05 | NS | NS | NS | |||||||
| (b) | |
|---|---|
| Parameter | P-value** |
| Age | NS |
| Grade | 0.0322 |
| Histology | NS |
| pT | NS |
| pN | NS |
| MAS1 | <0.0001 |
| (c) | |||
|---|---|---|---|
| MAS1 score | P | ||
| Parameter | 1–3 | 4–5 | |
| N | 27 | 9 | |
| Age | 50.4 ± 2.7 | 47.9 ± 1.8 | NS |
| Histology | |||
| PT | 9 | 2 | |
| ST | 10 | 3 | |
| SC | 8 | 4 | NS |
| Grade | |||
| 1 | 6 | 0 | |
| 2 | 15 | 4 | |
| 3 | 6 | 5 | NS |
| T | |||
| 1–2 | 23 | 3 | |
| 3–4 | 4 | 6 | <0.01 |
| N | |||
| 0 | 12 | 2 | |
| 1–2 | 15 | 7 | <0.005 |
| Stage | |||
| I | 1 | 0 | |
| II | 22 | 3 | |
| III–IV | 4 | 6 | <0.05 |
(a) P: Nonparametric correlations were examined using the Spearman rank correlation test.
(b) P-values were calculated by Mann–Whitney U-test** or χ2-test.
(c) P-values were calculated by Mann–Whitney U-test or χ2-test. EGFR, epidermal growth factor receptor; ER, estrogen receptor; HER2, human epithelial growth factor receptor-related; IDC, invasive ductal carcinoma; NS, not significant; PR, progesterone receptor; PT, papillotubular; SC, scirrhous; ST, solid tubular.
To confirm the MAS1 expression in TNBC, we analyzed the tissue array specimens. The 36 cases of triple-negative IDC were subjected to comparison of MAS1 expression with the clinicopathological parameters (Table5c). All cases expressed MAS1 at score 1–5 levels (i.e. traceable to weak), which were correlated with pT, pN and stage.
The 4T1 cell line did not express ER, PgR and HER2, which meant that the cells were classified as TNBC (Fig.3b). In the syngeneic BALB/c mouse model, MAS1-knockdown of 4T1 cells resulted in increased tumor growth in the mammary pads and lung metastasis by tail vein inoculation (Fig.4a,b). CDDP has been reported to be effective against TNBC in clinical studies,26 and, accordingly, we used CDDP as a chemotherapeutic agent in this study. In the in vitro study, CDDP or A1-7 treatment resulted in mild growth inhibition, whereas CDDP plus A1-7 co-treatment showed synergic inhibitory effects in 4T1 cells exposed to control siRNA (Fig.4c). In contrast, only CDDP showed an effect in 4T1 cells transfected with MAS1 siRNA. In the mouse model, XTN, an ACE2 activator, was used for activation of MAS1 (Fig.4d). Since CDDP and XTN treatment both inhibited tumor growth individually, co-treatment with CDDP and XTN showed additive effects on growth inhibition in cells treated with control siRNA. In contrast, the tumors in mice treated with MAS1 siRNA were larger than those in mice treated with control siRNA, and CDDP showed less pronounced inhibitory effects in these mice.
Figure 4.
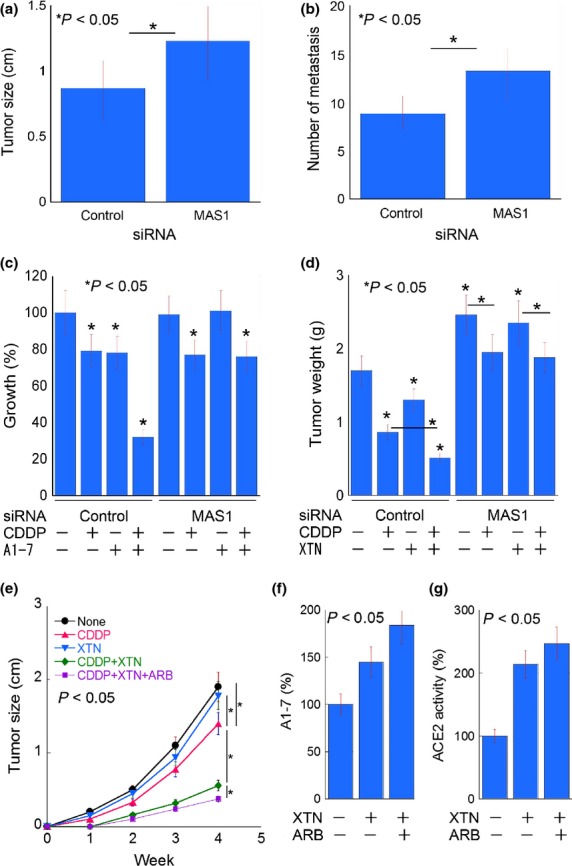
Effect of MAS1 targeting on the antitumoral activity of cisplatin (CDDP) in a mouse tumor model. (a, b) Tumors were treated with MAS1 siRNA. (a) Tumor size after 4T1 tumor cell inoculation in the mammary pad. (b) Number of metastatic foci in the lungs from 4T1 cells inoculated in the tail vein. (c) In vitro treatment of 4T1 cells with CDDP and/or angiotensin 1-7 (A1-7) and control or MAS1 siRNA. (d) In vivo treatment of 4T1 cells in the mouse mammary pad with CDDP and/or xanthenone and control siRNA or MAS1 siRNA. (e) Tumor growth by intramammary inoculation of 4T1 cells. (f) Angiotensin converting enzyme 2 activity and A1-7 concentration in inoculated 4T1 cells and the surrounding stromal cells of the mammary pads. ARB, losartan. P was calculated from three independent experiments or five mice by Mann–Whitney U-test.
In the last set of experiments, we examined concurrent treatment with XTN and angiotensin-II type 1 receptor blocker (ARB) (Fig.4e). ARB plus XTN treatment showed more pronounced inhibitory effects than XTN treatment alone. Moreover, CDDP plus ARB plus XTN showed synergic inhibitory effects, which were even more pronounced than the treatments with CDDP alone or CDDP plus XTN.
To elucidate the effects of XTN, tumor cells and the surrounding mammary stromal cells were separately examined for ACE2 activity (Fig.4f). In tumor cells, ACE2 activity was not significantly increased by XTN, whereas stromal cells showed marked increases in ACE2 activity by XTN.
Discussion
In the present study, MAS1 expression was found to be inversely associated with histological type, grade, tumor growth (T factor) and nodal metastasis (N factor). These findings suggest that MAS1 may possess tumor-inhibitory properties. The in vitro findings supported these results, with MAS1 knockdown resulting in increased 4T1 cell growth, survival and invasion. Thus, reduced MAS1 expression might be a relevant marker for the malignant potential of breast cancer.
MAS1 is expressed in non-cancerous breast tissues; however, here, we found that the expression was significantly decreased in most IDC cases. The cause of MAS1 repression has not been elucidated. In contrast, MAS1 is upregulated in CRC tissue compared to non-cancerous mucosa. Moreover, in breast cancer, MAS1 shows antitumoral effects, whereas in CRC, MAS1 has been demonstrated to be refractory to A1-7 and A-II.11 Gene mutations or intracellular signal alterations might explain the differences in MAS1 expression and activity between these malignancies. Under normal conditions, MAS1 produces a functional complex with the other A-II receptors, AT1R and AT2R, to maintain vascular homeostasis.27 Certain cancers might result in deviations in vascular homeostasis by causing an imbalance in the expression of the angiotensin-related receptors.
Importantly, in the present study, MAS1 expression was retained in TNBC compared to non-TNBC. The 4T1 mouse breast cancer cell line showed no ER, PgR or HER2 expression, which indicates that it is a TNBC cell line. MDA-MB-468 human breast cancer cell line is known as TNBC.18 In 4T1 MDA-MB-468 and cells, activation of MAS1 by A1-7 inhibited cell growth. Moreover, a synergic inhibition by co-treatment with A1-7 and CDDP was observed in 4T1 cells in a syngeneic animal model.
In our animal model, we used XTN to activate ACE2, which converts A-II to A1-7. ACE2 activity was observed in the normal mammary pad tissue along with A1-7. Two weeks after inoculation in the animal model, no synergism between XTN and CDDP was observed. However, 4 weeks post-inoculation, synergism was, indeed, detected. We also treated the mice with ARB in addition to XTN and CDDP to increase the levels of A-II, which is unable to bind to AT1R. The addition of ARB to the XTN plus CDDP treatment regimen resulted in a 10% increase in growth inhibition. ARB is reported to increase A1-7 levels by conversion from A-II by ACE2.28
We examined an MCF-7 human breast cancer cell line possessing a luminal A phenotype. Interestingly, estrogen starvation from medium upregulated MAS1 expression in MCF-7 cells. TMX treatment also upregulated MAS1 expression, which inhibited cell growth, survival and invasion in synergism with MAS1 activation by A1-7. In rat pups, estradiol upregulated MAS1 in the brain;29 however, no reports were found on the effect of sex hormone on MAS1 expression in mature tissues or tumors. In our IDC cases, no correlation was detected in the expression levels between ER and MAS1 (data not shown). The precise mechanism requires further examination; however, anti-estrogen therapy might provide a synergic effect with MAS1 targeting in non-TNBC cases.
In the present study, MAS1 activation by A1-7 resulted in inhibition of cell growth, invasion and anti-apoptotic survival. MAS1 is a G-protein coupled receptor, which intracellular signal pathways are suppressing extracellular signal-regulated kinases1/2 (ERK1/2) and nuclear factor κB.30 MAS1 is known to activate Src homology 2-containing inositol phosphatase 2 (SHIP2), which inhibits activation of EGFR ERK1/2.31 Because TNBC have been reported to highly express EGFR as a basal phenotype,32 EGFR is one of the molecular targets in TNBC. Anti-EGFR activity of MAS1 might suggest that a combination molecular targeting of MAS1 and EGFR is relevant for TNBC treatment.
ATR2 shows also anti-angiotensin-II activity.2 The anti-tumoral effect of MAS1 was not affected by A-II, whereas ATR2 showed a biphasic effect; pro-tumoral in low A-II concentration and anti-tumoral in high A-II concentration.7
Interestingly, MAS1 expression was emphasized in the myoepithelial cells, or basal cells. Silencing of basal phenotype-associated FoxM1 decreases MAS1 expression.33 That possibly suggest that MAS1 expression might associated with myoepithelial, or basal phenotype. In the myocardial cells express MAS1 by hypomethylation of MAS1 gene promoter.34 Silencing of DNA methyltransferase 3a or tripartite motif protein 28 also causes MAS1 overexpression by epigenetic dysregulation35 Association of MAS1 expression with basal phenotype might explain the cause of MAS1 expression in TNBC, many of which bring basal phenotype. The cause of MAS1 repression in breast cancer has not been elucidated; however, epigenetic alteration and basal differentiation might be key subject in future study.
In the present study, we showed that activation of MAS1 could be achieved by ACE2 activation, which is enhanced by co-treatment with ARB, and that MAS1 activation resulted in increased CDDP antitumoral effects. MAS1 is a possible molecular target in breast cancer, including TNBC.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science, Japan and a Health and Labour Sciences Research Grant from the Ministry of Health, Labour and Welfare of Japan.
Disclosure Statement
The authors have no conflict of interest to declare.
References
- Escobar E, Rodriguez-Reyna TS, Arrieta O, Sotelo J. Angiotensin II, cell proliferation and angiogenesis regulator: Biologic and therapeutic implications in cancer. Curr Vasc Pharmacol. 2004;2:385–99. doi: 10.2174/1570161043385556. [DOI] [PubMed] [Google Scholar]
- Kuniyasu H. Multiple roles of angiotensin in colorectal cancer. World J Clin Oncol. 2012;3:150–4. doi: 10.5306/wjco.v3.i12.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyhrquist F, Saijonmaa O. Renin-angiotensin system revisited. J Intern Med. 2008;264:224–36. doi: 10.1111/j.1365-2796.2008.01981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshayes F, Nahmias C. Angiotensin receptors: a new role in cancer? Trends Endocrinol Metab. 2005;16:203–99. doi: 10.1016/j.tem.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Shimomoto T, Ohmori H, Luo Y, et al. Diabetes-associated angiotensin activation enhances liver metastasis of colon cancer. Clin Exp Metastasis. 2012;29:915–25. doi: 10.1007/s10585-012-9480-6. [DOI] [PubMed] [Google Scholar]
- Luo Y, Ohmori H, Shimomoto T, et al. Anti-angiotensin and hypoglycemic treatments suppress liver metastasis of colon cancer cells. Pathobiology. 2011;78:285–90. doi: 10.1159/000330169. [DOI] [PubMed] [Google Scholar]
- Zhou L, Luo Y, Sato S, et al. Role of two types of angiotensin II receptors in colorectal carcinoma progression. Pathobiology. 2014;81:169–75. doi: 10.1159/000362092. [DOI] [PubMed] [Google Scholar]
- Campbell DJ. Vasopeptidase inhibition: A double-edged sword? Hypertension. 2003;41:383–9. doi: 10.1161/01.HYP.0000054215.71691.16. [DOI] [PubMed] [Google Scholar]
- Regulska K, Stanisz B, Regulski M. The renin-angiotensin system as a target of novel anticancer therapy. Curr Pharm Des. 2013;19:7103–25. doi: 10.2174/13816128113199990508. [DOI] [PubMed] [Google Scholar]
- Johansson A, Helou K, Levan G. Cytogenetic localization of cancer-related genes in the rat and comparative mapping studies in human and mouse. Cytogenet Cell Genet. 1998;81:217–21. doi: 10.1159/000015034. [DOI] [PubMed] [Google Scholar]
- Bernardi S, Zennaro C, Palmisano S, et al. Characterization and significance of ACE2 and Mas receptor in human colon adenocarcinoma. J Renin Angiotensin Aldosterone Syst. 2012;13:202–9. doi: 10.1177/1470320311426023. [DOI] [PubMed] [Google Scholar]
- Wakao F, Nishimoto H, Katanoda K, Tsukuma H, Mikami H, editors. Cancer Statistics in Japan, 2013. 2013 edn. Tokyo: National Cancer Center; 2013. [Google Scholar]
- Yagata H, Kajiura Y, Yamauchi H. Current strategy for triple-negative breast cancer: appropriate combination of surgery, radiation, and chemotherapy. Breast Cancer. 2011;18:165–73. doi: 10.1007/s12282-011-0254-9. [DOI] [PubMed] [Google Scholar]
- Oakman C, Viale G, Di Leo A. Management of triple negative breast cancer. Breast. 2010;19:312–21. doi: 10.1016/j.breast.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Bagaria SP, Ray PS, Sim MS, et al. Personalizing breast cancer staging by the inclusion of ER, PR, and HER2. JAMA Surg. 2014;149:125–9. doi: 10.1001/jamasurg.2013.3181. [DOI] [PubMed] [Google Scholar]
- Brady-West DC, McGrowder DA. Triple negative breast cancer: therapeutic and prognostic implications. Asian Pac J Cancer Prev. 2011;12:2139–43. [PubMed] [Google Scholar]
- de Ruijter TC, Veeck J, de Hoon JP, van Engeland M, Tjan-Heijnen VC. Characteristics of triple-negative breast cancer. J Cancer Res Clin Oncol. 2011;137:183–92. doi: 10.1007/s00432-010-0957-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chekhun S, Bezdenezhnykh N, Shvets J, Lukianova N. Expression of biomarkers related to cell adhesion, metastasis and invasion of breast cancer cell lines of different molecular subtype. Exp Oncol. 2013;35:174–9. [PubMed] [Google Scholar]
- Kuniyasu H, Luo Y, Fujii K, et al. CD10 enhances metastasis of colorectal cancer by abrogating the anti-tumoural effect of methionine-enkephalin in the liver. Gut. 2010;59:348–56. doi: 10.1136/gut.2009.178376. [DOI] [PubMed] [Google Scholar]
- Kuniyasu H, Oue N, Wakikawa A, et al. Expression of receptors for advanced glycation end-products (RAGE) is closely associated with the invasive and metastatic activity of gastric cancer. J Pathol. 2002;196:163–70. doi: 10.1002/path.1031. [DOI] [PubMed] [Google Scholar]
- Chihara Y, Fujimoto K, Kondo H, et al. Anti-tumor effects of liposome-encapsulated titanium dioxide in nude mice. Pathobiology. 2007;74:353–8. doi: 10.1159/000110029. [DOI] [PubMed] [Google Scholar]
- Kuniyasu H, Oue N, Sasahira T, et al. Reg IV enhances peritoneal metastasis in gastric carcinomas. Cell Prolif. 2009;42:110–21. doi: 10.1111/j.1365-2184.2008.00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez Prada JA, Ferreira AJ, Katovich MJ, et al. Structure-based identification of small-molecule angiotensin-converting enzyme 2 activators as novel antihypertensive agents. Hypertension. 2008;51:1312–7. doi: 10.1161/HYPERTENSIONAHA.107.108944. [DOI] [PubMed] [Google Scholar]
- Kuniyasu H. The molecular pathology of gastric cancer. In: Kuriyama S, Yoshiji H, editors. New Perspectives in Cancer Research and Therapy 2005. Kerala: Research Signpost; 2005. pp. 85–98. [Google Scholar]
- Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987;8:138–40. [PubMed] [Google Scholar]
- Petrelli F, Coinu A, Borgonovo K, et al. The value of platinum agents as neoadjuvant chemotherapy in triple-negative breast cancers: a systematic review and meta-analysis. Breast Cancer Res Treat. 2014;144:223–32. doi: 10.1007/s10549-014-2876-z. [DOI] [PubMed] [Google Scholar]
- Castro CH, Santos RA, Ferreira AJ, Bader M, Alenina N, Almeida AP. Evidence for a functional interaction of the angiotensin-(1-7) receptor Mas with AT1 and AT2 receptors in the mouse heart. Hypertension. 2005;46:937–42. doi: 10.1161/01.HYP.0000175813.04375.8a. [DOI] [PubMed] [Google Scholar]
- Arumugam S, Thandavarayan RA, Palaniyandi SS, et al. Candesartan cilexetil protects from cardiac myosin induced cardiotoxicity via reduction of endoplasmic reticulum stress and apoptosis in rats: involvement of ACE2-Ang (1-7)-mas axis. Toxicology. 2012;291:139–45. doi: 10.1016/j.tox.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Karimi B, Hafidzi MN, Panandam JM, Fuzina NH. Comparison of effect of sex hormone manipulation during neonatal period, on mRNA expression of Slc9a4, Nr3c2, Htr5b and Mas1 in hippocampus and frontal cortex of male and female rats. J Biol Regul Homeost Agents. 2013;27:869–74. [PubMed] [Google Scholar]
- El-Hashim AZ, Renno WM, Raghupathy R, Abduo HT, Akhtar S, Benter IF. Angiotensin-(1-7) inhibits allergic inflammation, via the MAS1 receptor, through suppression of ERK1/2- and NF-kappaB-dependent pathways. Br J Pharmacol. 2012;166:1964–76. doi: 10.1111/j.1476-5381.2012.01905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio WO, Henrique de Castro C, Santos RA, Schiffrin EL, Touyz RM. Angiotensin-(1-7) counterregulates angiotensin II signaling in human endothelial cells. Hypertension. 2007;50:1093–8. doi: 10.1161/HYPERTENSIONAHA.106.084848. [DOI] [PubMed] [Google Scholar]
- Rao C, Shetty J, Prasad KH. Immunohistochemical profile and morphology in triple-negative breast cancers. J Clin Diagn Res. 2013;7:1361–5. doi: 10.7860/JCDR/2013/5823.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonsey DR, Follettie MT. Loss of the forkhead transcription factor FoxM1 causes centrosome amplification and mitotic catastrophe. Cancer Res. 2005;65:5181–9. doi: 10.1158/0008-5472.CAN-04-4059. [DOI] [PubMed] [Google Scholar]
- Kagara N, Huynh KT, Kuo C, et al. Epigenetic regulation of cancer stem cell genes in triple-negative breast cancer. Am J Pathol. 2012;181:257–67. doi: 10.1016/j.ajpath.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Whitelaw NC, Chong S, Morgan DK, et al. Reduced levels of two modifiers of epigenetic gene silencing, Dnmt3a and Trim28, cause increased phenotypic noise. Genome Biol. 2010;11:R111. doi: 10.1186/gb-2010-11-11-r111. [DOI] [PMC free article] [PubMed] [Google Scholar]