Background: Sickle cell disease is an inherited blood disorder resulting in severe anemia.
Results: Limiting iron toxicity through disruption of intestinal HIF-2α improves the anemia.
Conclusion: Intestinal HIF-2α deficiency improves anemia in sickle cell disease.
Significance: Intestinal HIF-2α is a novel therapeutic target in sickle cell disease.
Keywords: hypoxia, hypoxia-inducible factor (HIF), intestinal epithelium, iron, iron metabolism, iron homeostasis, anemia, sickle cell disease, HIF-2α
Abstract
Sickle cell disease (SCD) is caused by genetic defects in the β-globin chain. SCD is a frequently inherited blood disorder, and sickle cell anemia is a common type of hemoglobinopathy. During anemia, the hypoxic response via the transcription factor hypoxia-inducible factor (HIF)-2α is highly activated in the intestine and is essential in iron absorption. Intestinal disruption of HIF-2α protects against tissue iron accumulation in iron overload anemias. However, the role of intestinal HIF-2α in regulating anemia in SCD is currently not known. Here we show that in mouse models of SCD, disruption of intestinal HIF-2α significantly decreased tissue iron accumulation. This was attributed to a decrease in intestinal iron absorptive genes, which were highly induced in a mouse model of SCD. Interestingly, disruption of intestinal HIF-2α led to a robust improvement in anemia with an increase in RBC, hemoglobin, and hematocrit. This was attributed to improvement in RBC survival, hemolysis, and insufficient erythropoiesis, which is evident from a significant decrease in serum bilirubin, reticulocyte counts, and serum erythropoietin following intestinal HIF-2α disruption. These data suggest that targeting intestinal HIF-2α has a significant therapeutic potential in SCD pathophysiology.
Introduction
Sickle cell disease (SCD)3 is an autosomal recessive hemolytic disorder. SCD is a major public health concern in the United States and in many other countries (1). In the United States, about 100,000 individuals are affected by SCD, the majority being African-American, and worldwide, more than 3,000,000 patients are born every year with this disorder (2). SCD is caused by a single amino acid substitution of valine for glutamic acid in the β-globin chain. The resultant hemoglobin is known as hemoglobin S (HbS). Upon deoxygenation, HbS results in decreased life span and sickling deformity of the RBC membrane (3). SCD presents with hemolytic anemia, tissue iron overload, and episodes of vaso-occlusive events (4–6). At present, hematopoietic stem cell transplantation is the definitive curative approach, but it is associated with significant morbidity and mortality as well as high cost (7). Neonatal screenings, penicillin prophylaxis for infection, and disease-modifying intervention such as hydroxyurea and blood transfusions have been shown to improve disease outcome (8). Blood transfusion is an important component of SCD management that increases oxygen-carrying capacity and replaces sickled RBCs. However, repeated blood transfusions contribute further to the existing iron overload (9) and necessitate iron chelation therapy (10). Cellular iron overload in SCD patients is associated with increased expression of inflammatory markers and mortality (11). Previous work has demonstrated that decreasing intestinal iron absorption either through treatment with hepcidin mimetics or through disruption of intestinal HIF-2α, the major local regulator of iron absorption, led to a decrease in iron overload in β-thalassemia mouse models. Paradoxically, the decrease in iron absorption also improved anemia (12, 13). This has been demonstrated to be through a decrease in iron-induced reactive oxygen species, leading to decreased RBC apoptosis. However, it is not clear whether limiting intestinal iron absorption may have beneficial effects in SCD. The present work demonstrates that disruption of intestinal HIF-2α limits intestinal iron absorption and improves anemia while decreasing systemic iron levels.
Materials and Methods
Humanized SCD Mouse Model
The mice utilized had the α- and β-globin genes replaced by fragments of the human α- and sickle βS-globin loci (14). Heterozygote mating of these mice produces homozygous SS SCD mice. Humanized SCD mice were bred and maintained as described previously (14). HPLC hemoglobin electrophoresis was performed on all mice to confirm sickle cell genotype (15). All animal experiments were approved by the University of Michigan Committee on Use and Care of Animals.
Bone Marrow Transplantation (BMT)
Total body irradiation and BMT were performed as described previously (12), and engraftment of the donor marrow was near 100%. All the mice were sacrificed 6 months following BMT.
Western Blot Analysis
Isolation of whole cell extracts from duodenal scrapings, followed by detection of HIF-2α (Novus Biologicals) and GAPDH (Santa Cruz Biotechnology), was performed as mentioned previously (16).
Real-time Quantitative PCR (qPCR)
RNA isolation, reverse transcription, qPCR, and primers used for qPCR were described previously (17).
Iron Staining
Tissue iron detection was performed in formalin-fixed paraffin-embedded sections stained with Perls' Prussian blue.
Tissue Iron Quantitation
Tissue nonheme iron was quantified as described previously (12).
Iron and Hematological Analysis
Serum iron was analyzed using the QuantiChrom iron assay kit (Bioassay Systems, Hayward, CA). The Unit for Laboratory Animal Medicine Pathology Core at The University of Michigan performed complete blood count analysis.
Histology
Histologic analysis was done on H&E-stained paraffin sections.
Statistics
Results are expressed as means ± S.D. p values were calculated by independent t test, one-way analysis of variance, and two-way analysis of variance.
Results and Discussion
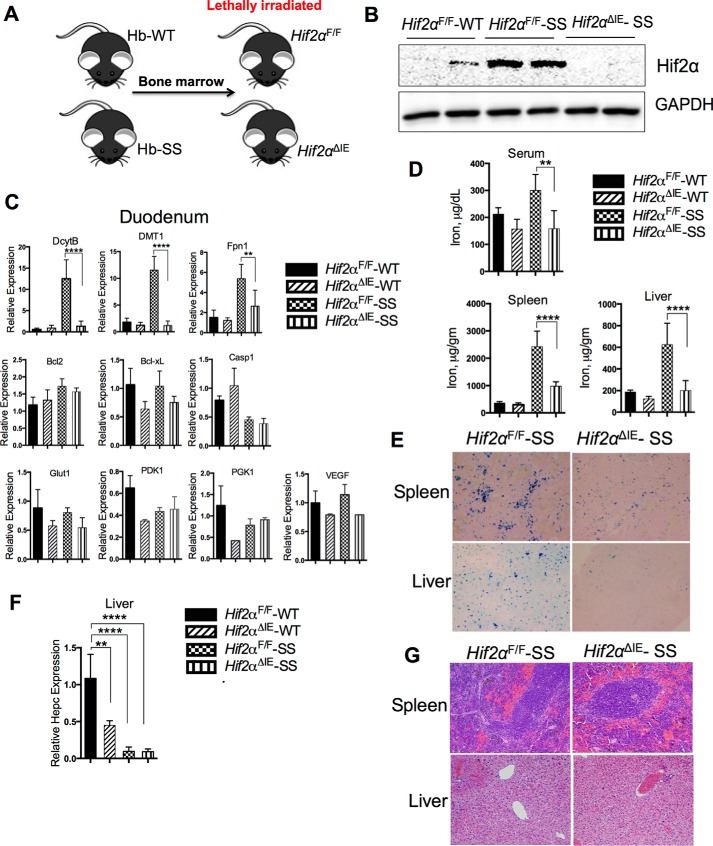
A humanized mouse model of SCD was used in our study. Murine adult α- and β-globin genes were replaced in the germ line by fragments of the human α- and sickle βS-globin loci (14). Heterozygous mating of these mice produced homozygous SCD mice, displaying the characteristic severe form of SCD (14). To investigate the role of intestinal HIF-2α in sickle cell disease, Hif2αΔIE mice, which have a disruption of HIF-2α specifically in intestinal epithelial cells, and wild type littermates (Hif2αF/F) (18) were lethally irradiated and subjected to BMT of sickle cell (SS) or healthy (WT) bone marrow, and were designated as Hif2αΔIE-SS (or -WT) and Hif2αF/F-SS (or -WT) respectively. As shown in Fig. 1B, 6 months following BMT, Hif2αF/F-SS mice led to an increased HIF-2α expression in the small intestine as compared with control mice (Hif2αF/F-WT), which was abrogated in the Hif2αΔIE-SS mice, demonstrating that intestinal epithelial HIF-2α is activated in SCD. Furthermore, gene expressions of HIF-2α-regulated duodenal iron transporters, DcytB, DMT1, and ferroportin (Fpn1) (19, 20), were significantly induced in the Hif2αF/F-SS mice, and their expressions were reduced to near control levels in the Hif2αΔIE-SS group (Fig. 1C). Also, mRNA expression analyses of intestinal glycolytic (glucose transporter 1 (Glut1), pyruvate dehydrogenase kinase isozyme 1 (PDK1), and phosphoglycerate kinase 1(PGK1)), apoptosis-related (Bcl2, Bcl-xL, and Casp1 (caspase 1)), and angiogenic (VEGF) genes were done to evaluate whether intestinal HIF-2α inhibition has a general effect of enterocyte survival and metabolism that could indirectly influence the intestinal iron absorption. Unlike the duodenal iron transporters, the expressions of these genes were not altered in the Hif2αF/F-SS and Hif2αΔIE-SS mice as compared with the corresponding control groups i.e. Hif2αF/F-WT and Hif2αF/F-SS mice (Fig. 1C). This is consistent with previous work demonstrating that HIF-2α inhibition predominantly influences the expression of critical iron transporters, whereas no appreciable alterations in the intestinal villi morphology and enterocyte survival are observed (21). In agreement with the gene expression data, the serum and tissue iron parameters also displayed a characteristic iron overload in Hif2αF/F-SS mice, whereas disruption of intestinal HIF-2α abolished the tissue iron overload in the Hif2αΔIE-SS mice, establishing the role of HIF-2α in heightened intestinal iron uptake in SCD (Fig. 1D). Transplantation of SS bone marrow to the control group (Hif2αF/F-SS) displayed high serum and spleen iron, although the hepatic iron level was only mildly increased. Intestinal HIF-2α disruption in the SCD model (Hif2αΔIE-SS) significantly decreased tissue and serum iron accumulation (Fig. 1D). Consistent with these data, Perls' staining for iron in the liver and the spleen also showed distinctly increased tissue iron level in the Hif2αF/F-SS (Fig. 1E), which in the Hif2αΔIE-SS liver and spleen showed significant improvement (Fig. 1E). Hepatic hepcidin expression in Hif2αΔIE-WT, Hif2αF/F-SS, and Hif2αΔIE-SS mice was significantly reduced as compared with that in Hif2αF/F-WT. However, there was no significant difference between the Hif2αF/F-SS and Hif2αΔIE-SS groups, demonstrating a significant role of intestinal HIF-2α in developing SCD-associated iron overload (Fig. 1F). Although measurement of hepatic hepcidin transcript remains as the standard method for assessing systemic iron status (22), a recent work describes a competitive ELISA method of hepcidin protein detection that is more sensitive and correlative to the systemic iron status in some cases of iron overload disorders (23). We think that assessment of serum hepcidin by this new method could be more informative for the further assessment of systemic iron dysregulation in SCD. Moreover, the data also provide evidence that non-transfusional iron overload may contribute significantly to tissue iron accumulation in SCD. Despite appreciable differences in tissue iron accumulation, spleen and liver histology were similar in the Hif2αF/F-SS and Hif2αΔIE-SS mice. In the liver, there were no signs of cholestasis or fat accumulation; however, vascular occlusions were present. The spleens were enlarged, and occlusions of the splenic cords along with increased fibroconnective tissue were observed (Fig. 1G).
FIGURE 1.
Disruption of intestinal HIF-2α improves SCD-induced tissue iron overload. A–C, Hif2αF/F and Hif2αΔIE mice were lethally irradiated and transplanted with Hb-WT or Hb-SS bone marrow (A) and analyzed 6 months later for HIF-2α protein expression in the duodenal mucosa (B) and gene expression analysis of duodenal Dcytb, Dmt1, Fpn1, Bcl2, Bcl-xL, Casp1, Glut1, PDK1, PGK1, and VEGF1 (normalized to β-actin) (C). D, serum (μg/dl) and tissue iron content (calculated as μg per gm of wet weight). E, Perls' iron stain of the spleen and liver. F, gene expression analysis of liver hepcidin (Hepc) normalized to β-actin. G, H&E staining of spleen and liver. Bar graph represents the mean value ±S.D. ****, p < 0.0001; **, p < 0.01; *, p < 0.05; ns, not significant.
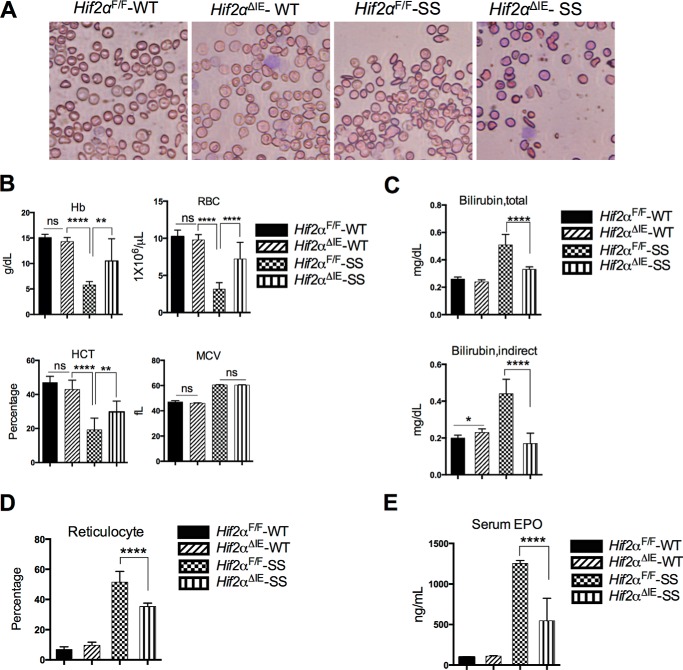
Blood smear analysis shows that Hif2αF/F-SS mice developed sickling of RBC characteristic of typical SCD, whereas no significant difference in sickling was observed between Hif2αF/F-SS and Hif2αΔIE-SS mice (Fig. 2A). Hif2αF/F-SS exhibited severe anemia, showing significant reduction in RBC, hemoglobin, and hematocrit values (Fig. 2B). However, Hif2αΔIE-SS mice exhibited an improvement in hematological parameters as compared with Hif2αF/F-SS mice. RBC, Hb, and hematocrits, although lower than the normal values, were significantly elevated in Hif2αF/F-SS mice (Fig. 2B). To further understand the mechanism, serum bilirubin was assessed as a marker of hemolysis. Hif2αF/F-SS mice exhibited a significant increase in total and indirect bilirubin, suggesting enhanced hemolysis (Fig. 2C). In the Hif2αΔIE-SS mice, there was a reduction in hemolysis as evidenced by a decrease in serum bilirubin level, both total and indirect (Fig. 2C). Furthermore, reticulocyte counts and serum erythropoietin levels were high in the Hif2αF/F-SS mice, and significantly improved in the Hif2αΔIE-SS, providing further evidence of decreased hemolysis following disruption of intestinal HIF-2α (Fig. 2, D and E). We propose, similar to the benefits of hepcidin overexpression in β-thalassemic iron overload (13), that intestinal HIF-2α disruption in SCD mice reduces the RBC hemoglobinization because of the diminished intestinal iron absorption. As a consequence, there is reduced density of Hb-SS in the individual RBCs, decreasing their vulnerability to deformity and thereby hemolysis. The resultant reduction in hemolysis would thus reduce free heme-induced toxicity and reactive oxygen species formation to ultimately improve both RBC lifespan and anemia.
FIGURE 2.
Disruption of intestinal HIF-2α improves Sickle cell anemia. A, representative blood smear of Hif2αF/F-SS and Hif2αΔIE-SS mice. B, hematological analyses (Hb, RBC number, hematocrit value (HCT), and mean corpuscular volume (MCV)). C and D, total and indirect bilirubin measurement (C) and reticulocyte count (D). E, serum erythropoietin (EPO). Each bar graph represents the mean value ±S.D. ****, p < 0.0001; **, p < 0.01; *, p < 0.05; ns, not significant. The data represent 10 mice per group.
Improvement in the hematologic and iron parameters of sickle cell anemia following intestine-specific HIF-2α deletion offers a significant promise in therapy for SCD, either alone or in conjunction with the existing regimens. Targeting intestinal HIF-2α may reduce the need of repeated blood transfusions or pharmacologic iron chelation. With a recent discovery of orally bioavailable HIF-2α-specific inhibitors (24), the present data suggest that use of these inhibitors could be a novel and effective treatment strategy for SCD.
Author Contributions
S. R. and Y. M. S conceived and designed the study. N. D., L. X, S. K. R., A. C., S. R., and Y. M. S. developed the methodology. N. D. and L. X. acquired data. N. D. L. X., A. C., S. R., and Y. M. S. analyzed and interpreted data. N. D., L. X., S. K. R., A. C., S. R., and Y. M. S. wrote, reviewed, and/or revised the manuscript. Y. M. S. supervised the study.
Acknowledgments
We thank the staff of the University of Michigan Gastrointestinal Peptide Research Center (supported by National Institutes of Health Grant P30 DK034933).
This work was supported, in whole or in part, by National Institutes of Health Grants CA148828 and DK095201 (to Y. M. S) and DK095112 and DK090554 (to S. R.) N. Das, L. Xie, S. Ramakrishnan, and Y. M. Shah have no disclosures to present. S. Rivella is a consultant for Isis, Bayer AG, and Medgenics and Novartis Pharmaceuticals and is supported by Merganser Biotech. He also holds restricted stocks in Merganser Biotech, Inc. In addition, he is a co-inventor for the patents US8058061 B2 C12N 20111115 and US7541179 B2 C12N 20090602. The consulting work and intellectual property of Stefano Rivella did not affect in any way the design, conduct, or reporting of this research. A. Campbell is a Co-Investigator for an Iron Overload Treatment Trial with Apopharma Pharmaceuticals, and this did not affect in any way the design, conduct, or reporting of this research.
- SCD
- sickle cell disease
- BMT
- bone marrow transplantation
- SS
- sickle cell
- qPCR
- quantitative PCR.
References
- 1. Weatherall D. J. (2008) Hemoglobinopathies worldwide: present and future. Curr. Mol. Med. 8, 592–599 [DOI] [PubMed] [Google Scholar]
- 2. Williams T. N., Weatherall D. J. (2012) World distribution, population genetics, and health burden of the hemoglobinopathies. Cold Spring Harb. Perspect. Med. 2, a011692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rees D. C., Williams T. N., Gladwin M. T. (2010) Sickle-cell disease. Lancet 376, 2018–2031 [DOI] [PubMed] [Google Scholar]
- 4. Chakravorty S., Williams T. N. (2015) Sickle cell disease: a neglected chronic disease of increasing global health importance. Arch. Dis. Child. 100, 48–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kato G. J., Hebbel R. P., Steinberg M. H., Gladwin M. T. (2009) Vasculopathy in sickle cell disease: Biology, pathophysiology, genetics, translational medicine, and new research directions. Am. J. Hematol. 84, 618–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Manwani D., Frenette P. S. (2013) Vaso-occlusion in sickle cell disease: pathophysiology and novel targeted therapies. Blood 122, 3892–3898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Felfly H., Haddad G. G. (2014) Hematopoietic stem cells: potential new applications for translational medicine. J. Stem Cells 9, 163–197 [PubMed] [Google Scholar]
- 8. Inati A. (2009) Recent advances in improving the management of sickle cell disease. Blood Rev. 23, Suppl. 1, S9–S13, 10.1016/S0268-960X(09)70004-9 [DOI] [PubMed] [Google Scholar]
- 9. Raghupathy R., Manwani D., Little J. A. (2010) Iron overload in sickle cell disease. Adv. Hematol. 2010, 272940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Drasar E., Vasavda N., Igbineweka N., Awogbade M., Allman M., Thein S. L. (2012) Serum ferritin and total units transfused for assessing iron overload in adults with sickle cell disease. Br. J. Haematol. 157, 645–647 [DOI] [PubMed] [Google Scholar]
- 11. van Beers E. J., Yang Y., Raghavachari N., Tian X., Allen D. T., Nichols J. S., Mendelsohn L., Nekhai S., Gordeuk V. R., Taylor J. G. 6th, Kato G. J. (2015) Iron, inflammation, and early death in adults with sickle cell disease. Circ. Res. 116, 298–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anderson E. R., Taylor M., Xue X., Ramakrishnan S. K., Martin A., Xie L., Bredell B. X., Gardenghi S., Rivella S., Shah Y. M. (2013) Intestinal HIF2α promotes tissue-iron accumulation in disorders of iron overload with anemia. Proc. Natl. Acad. Sci. U.S.A. 110, E4922–E4930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gardenghi S., Ramos P., Marongiu M. F., Melchiori L., Breda L., Guy E., Muirhead K., Rao N., Roy C. N., Andrews N. C., Nemeth E., Follenzi A., An X., Mohandas N., Ginzburg Y., Rachmilewitz E. A., Giardina P. J., Grady R. W., Rivella S. (2010) Hepcidin as a therapeutic tool to limit iron overload and improve anemia in β-thalassemic mice. J. Clin. Invest. 120, 4466–4477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ryan T. M., Ciavatta D. J., Townes T. M. (1997) Knockout-transgenic mouse model of sickle cell disease. Science 278, 873–876 [DOI] [PubMed] [Google Scholar]
- 15. Campbell A. D., Cui S., Shi L., Urbonya R., Mathias A., Bradley K., Bonsu K. O., Douglas R. R., Halford B., Schmidt L., Harro D., Giacherio D., Tanimoto K., Tanabe O., Engel J. D. (2011) Forced TR2/TR4 expression in sickle cell disease mice confers enhanced fetal hemoglobin synthesis and alleviated disease phenotypes. Proc. Natl. Acad. Sci. U.S.A. 108, 18808–18813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xue X., Taylor M., Anderson E., Hao C., Qu A., Greenson J. K., Zimmermann E. M., Gonzalez F. J., Shah Y. M. (2012) Hypoxia-inducible factor-2α activation promotes colorectal cancer progression by dysregulating iron homeostasis. Cancer Res. 72, 2285–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anderson E. R., Xue X., Shah Y. M. (2011) Intestinal hypoxia-inducible factor-2α (HIF-2α) is critical for efficient erythropoiesis. J. Biol. Chem. 286, 19533–19540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xue X., Ramakrishnan S., Anderson E., Taylor M., Zimmermann E. M., Spence J. R., Huang S., Greenson J. K., Shah Y. M. (2013) Endothelial PAS domain protein 1 activates the inflammatory response in the intestinal epithelium to promote colitis in mice. Gastroenterology 145, 831–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shah Y. M., Matsubara T., Ito S., Yim S. H., Gonzalez F. J. (2009) Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab. 9, 152–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taylor M., Qu A., Anderson E. R., Matsubara T., Martin A., Gonzalez F. J., Shah Y. M. (2011) Hypoxia-inducible factor-2α mediates the adaptive increase of intestinal ferroportin during iron deficiency in mice. Gastroenterology 140, 2044–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mastrogiannaki M., Matak P., Peyssonnaux C. (2013) The gut in iron homeostasis: role of HIF-2 under normal and pathological conditions. Blood 122, 885–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ilyin G., Courselaud B., Troadec M. B., Pigeon C., Alizadeh M., Leroyer P., Brissot P., Loréal O. (2003) Comparative analysis of mouse hepcidin 1 and 2 genes: evidence for different patterns of expression and co-inducibility during iron overload. FEBS Lett. 542, 22–26 [DOI] [PubMed] [Google Scholar]
- 23. Gutschow P., Schmidt P. J., Han H., Ostland V., Bartnikas T. B., Pettiglio M. A., Herrera C., Butler J. S., Nemeth E., Ganz T., Fleming M. D., Westerman M. (2015) A competitive enzyme-linked immunosorbent assay specific for murine hepcidin-1: correlation with hepatic mRNA expression in established and novel models of dysregulated iron homeostasis. Haematologica 100, 167–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scheuermann T. H., Li Q., Ma H. W., Key J., Zhang L., Chen R., Garcia J. A., Naidoo J., Longgood J., Frantz D. E., Tambar U. K., Gardner K. H., Bruick R. K. (2013) Allosteric inhibition of hypoxia inducible factor-2 with small molecules. Nat. Chem. Biol. 9, 271–276 [DOI] [PMC free article] [PubMed] [Google Scholar]