Background: Quercetin-3-rutinoside is an inhibitor of protein disulfide isomerase, a potential target for antithrombotic therapy.
Results: Quercetin-3-rutinoside induces a compact conformation in PDI and binds to PDI with an IC50 of about 10 μm.
Conclusion: Quercetin-3-rutinoside interacts with the b′x domain of protein disulfide isomerase with a 1:1 stoichiometry.
Significance: The b′x domain reverses the antithrombotic properties of quercetin-3-rutinoside in a thrombosis model in a live mouse.
Keywords: coagulation factor, fibrin, mouse, platelet, protein-disulfide isomerase, thrombosis, quercetin-3-rutinoside, isoquercetin, PDI, thrombus formation
Abstract
Quercetin-3-rutinoside inhibits thrombus formation in a mouse model by inhibiting extracellular protein disulfide isomerase (PDI), an enzyme required for platelet thrombus formation and fibrin generation. Prior studies have identified PDI as a potential target for novel antithrombotic agents. Using a fluorescence enhancement-based assay and isothermal calorimetry, we show that quercetin-3-rutinoside directly binds to the b′ domain of PDI with a 1:1 stoichiometry. The binding of quercetin-3-rutinoside to PDI induces a more compact conformation and restricts the conformational flexibility of PDI, as revealed by small angle x-ray scattering. The binding sites of quercetin-3-rutinoside to PDI were determined by studying its interaction with isolated fragments of PDI. Quercetin-3-rutinoside binds to the b′x domain of PDI. The infusion of the b′x fragment of PDI rescued thrombus formation that was inhibited by quercetin-3-rutinoside in a mouse thrombosis model. This b′x fragment does not possess reductase activity and, in the absence of quercetin-3-rutinoside, does not affect thrombus formation in vivo. The isolated b′ domain of PDI has potential as an antidote to reverse the antithrombotic effect of quercetin-3-rutinoside by binding and neutralizing quercetin-3-rutinoside.
Introduction
Protein disulfide isomerase (PDI)2 is an endoplasmic reticulum-resident enzyme that facilitates post-translational disulfide bond formation and disulfide bond shuffling, and serves as a chaperone during the folding of nascent polypeptide chains (1, 2). This thiol isomerase consists of four domains: a, b, b′, and a′, where a and a′ contain the enzyme active site motifs and are the catalytic domains, whereas b and b′ may be important for substrate recognition. Recently, it has been demonstrated that PDI and other thiol isomerases can escape the endoplasmic reticulum and function extracellularly (3–9). PDI is rapidly secreted from both platelets and endothelial cells during thrombus formation in vivo (4, 5, 10). Inhibition of PDI activity blocks both platelet accumulation and fibrin generation in a mouse thrombosis model (4). We have identified quercetin-3-rutinoside and isoquercetin as inhibitors of PDI reductase activity using a high-throughput screen (11). We further found that quercetin-3-rutinoside inhibits both platelet thrombus formation and fibrin generation in a dose-dependent manner via inhibition of PDI in a mouse thrombosis model, and have raised the possibility that PDI be considered as a target for antithrombotic therapy (11). Most PDI inhibitors interact irreversibly with the active site cysteine(s) within the thioredoxin-like a or a′ domains. However, inhibition of PDI activity by quercetin-3-rutinoside is reversible. Therefore, the mechanism by which quercetin-3-rutinoside blocks PDI activity was unclear and justified further investigation.
Quercetin-3-rutinoside is a naturally occurring phenolic glycoside found in many plants, especially fruits and vegetables. Quercetin-3-rutinoside, as an inhibitor of PDI, is a potential antithrombotic agent that may prove useful for thromboprophylaxis (12). All currently employed anticoagulant and antiplatelet agents, whether administered orally or parenterally, are associated with bleeding complications (13). The ability to quickly reverse their antithrombotic effects in the face of bleeding complications ensures their safe use. Isoquercetin, structurally similar to quercetin-3-rutinoside and with increased oral availability, is being explored in humans as an antithrombotic. For these reasons, we have characterized the molecular interaction of quercetin-3-rutinoside and isoquercetin with PDI and the isolated domains of PDI. We determine that quercetin-3-rutinoside binds directly to the b′ domain of PDI or any PDI fragments that contain the b′ domain. Based on these findings, we demonstrate that fragment b′x of PDI reverses quercetin-3-rutinoside-induced inhibition of thrombus formation in vivo using a mouse thrombosis model.
Experimental Procedures
Animals
C57BL/6J mice were obtained from The Jackson Laboratory. The Beth Israel Deaconess Medical Center Institution Animal and Use Committee approved all animal care and experimental procedures.
Antibodies and Reagents
Anti-platelet antibody DyLight 649 CD42b was purchased from Emfret Analytics. Quercetin-3-rutinoside, isoquercetin, insulin, and DTT were purchased from Sigma-Aldrich. Mouse anti-human fibrin monoclonal antibody was purified over protein G-Sepharose (Invitrogen) from a 59D8 hybridoma cell line (14) and labeled with Alexa Fluor 488 (Invitrogen).
Plasmid Construction and Recombinant Protein Expression
Recombinant His-tagged full-length human PDI (abb′xa′c) and its domain fragments, ERp5, ERp57, and ERp72, were cloned into a pET-15b vector at the NdeI and BamHI sites and transformed into Escherichia coli Origami B (DE3) cells (EMD Chemicals). The recombinant proteins were expressed and isolated by affinity chromatography with cOmplete His-Tag purification resin (Roche Applied Science) and purified on a Superdex 200 (GE Healthcare).
Fluorescence-based Binding Assay
Recombinant PDI and its fragments, ERp5, ERp57, and ERp72, were incubated with quercetin-3-rutinoside or isoquercetin in 20 mm Tris-HCl, 100 mm NaCl, pH 8.0, for 30 min, and the fluorescence emission spectra were measured with excitation at 430 nm at 25 °C on a BioTek Synergy microplate reader.
Isothermal Calorimetry Measurements
Microcalorimetric titrations of quercetin-3-rutinoside with PDI were performed with a MicroCal ITC200 microcalorimeter (GE Healthcare) using PDI (300 μl; 480 μm) and quercetin-3-rutinoside (7.2 mm) at 25 °C. The initial delay time was 60 s. The reference power and the filter were set to 11.2 μcal/s and 2.5 s, respectively. The titration experiment consisted of 20 injections of 1.5 μl of quercetin-3-rutinoside with a duration of 3 s, and the time interval between two consecutive injections was set to 150 s. Data were analyzed with MicroCal Origin 7.0 (MicroCal) and Prism (GraphPad).
Insulin Reduction Assay
Reductase activity was assayed by measuring the thiol isomerase-catalyzed reduction of insulin in the presence of DTT. The aggregation of reduced insulin chains was measured by absorption at 650 nm. The reductase activity was measured in 100 μl in the presence of 104 μm insulin, 0.8 μm PDI fragments, 0.75 mm DTT, and 2 mm EDTA in 100 mm potassium phosphate, pH 7.4, at 25 °C over 100 min. For inhibition assays, 100 μm quercetin-3-rutinoside or control buffer was added to the reaction system.
Small Angle X-ray Scattering (SAXS)
Human PDI was further purified by gel filtration. PDI (4.5, 3.0, 1.5 mg/ml) was prepared by dialysis against 20 mm Tris, 150 mm NaCl, pH 8.0, 5% glycerol with or without 0.5 mm quercetin-3-rutinoside. Samples were evaluated where the SAXS data were collected on the SIBYLS beamline in the Advanced Light Source using high-throughput data collection mode (15). Each sample was exposed four times to x-rays with 0.5-, 1-, 2-, and 5-s exposure at λ = 1.0 Å. Buffer blanks were subtracted from the protein signal. Data were merged to produce a final scattering curve for further analysis using PRIMUS (16) within ATSAS and used to generate the molecular envelope (17).
Intravital Microscopy in a Mouse Model of Laser-induced Arteriolar Thrombosis
Intravital video microscopy of the cremaster muscle microcirculation was performed as described previously (11, 18). Digital images were captured with a Cooke Sensicam CCD camera (The Cooke Corp., Auburn Hills, MI) connected to a VS4-1845 Image Intensifier GEN III (Video Scope International, Dulles, VA). Alexa Fluor- or DyLight-labeled antibodies were infused into mice prior to arteriolar wall injury. Image analysis was performed using SlideBook v5.5 (Intelligent Imaging Innovations). Representative images are presented, but the median curves include the full data. The kinetics of fibrin generation and platelet thrombus formation were analyzed by determining median fluorescence values over time in ∼30–35 thrombi in three or four mice.
Laser-induced Injury
Injury to a cremaster arteriolar (30–50-μm diameter) vessel wall was induced with a MicroPoint laser system (Photonics Instruments, Chicago, IL) focused through the microscope objective, parfocal with the focal plane and tuned to 440 nm through the dye cell containing 5 mm coumarin in methanol (13). Data were captured digitally from two fluorescence channels, 488/520 nm and 647/670 nm. Data acquisition was initiated both prior to and following a single laser pulse for each injury. The microscope system was controlled and images were analyzed using SlideBook (Intelligent Imaging Innovations, Denver, CO).
Image Analysis
For each thrombus generated by laser injury, a rectangular mask was defined that included a portion of the vessel upstream of the site of injury. The maximum fluorescence intensity of the pixels contained in this mask was extracted for all frames (pre- and post-injury) for each thrombus. The mean value calculated from the maximal intensity values in the mask for each frame was determined and used as the background value. Finally, for each frame, the integrated fluorescence intensity was calculated as per the following equation.
 |
This calculation was performed for all frames in each thrombus and plotted versus time to provide the kinetics of thrombus formation (5, 19–21). For multiple fluorescence channels, calculations of background were made independently for each channel.
Statistics
A value representing the area under the curve was calculated for each curve generated by measurement of fluorescence after laser injury of an arteriole. A Mann-Whitney test was used for statistical comparison of data sets composed of all thrombi formed under each indicated condition. Statistical analyses were performed using Prism software (version 5, GraphPad).
Results
Direct Binding of Quercetin-3-rutinoside and Isoquercetin to PDI
Quercetin-3-rutinoside and isoquercetin inhibit both platelet thrombus formation and fibrin generation in a mouse model of thrombosis (11). Impaired thrombus formation by quercetin-3-rutinoside was mediated by the inhibition of PDI activity. We developed a direct binding assay based upon the enhancement of the intrinsic fluorescence of the quercetins to allow measurement of the affinity of these ligands to PDI. Both quercetin-3-rutinoside (Fig. 1A) and isoquercetin (Fig. 1C) showed peak emission at about 550 nm when irradiated at 430 nm and, upon binding to PDI, the magnitude of the fluorescence peak increased significantly and was blue-shifted to 530 nm. Using this fluorescence perturbation, we measured the binding isotherms of these quercetin species interacting with PDI by monitoring the PDI-induced fluorescence enhancement at different concentrations. Binding between PDI and quercetin-3-rutinoside or isoquercetin was characterized by IC50 values of 12.3 μm ± 4.2 and 14 μm ± 6.7, respectively (Fig. 1, B and D). Within the limits of detection, quercetin-3-rutinoside emission was not perturbed in the presence of increasing amounts of ERp5, ERp57, or ERp72 (Fig. 1E). Thus, PDI binds to quercetin-3-rutinoside, whereas these other vascular thiol isomerases do not, a conclusion consistent with previous studies (11). We generated a PDI active site mutant where both CGHC motifs located in the a and a′ domains were mutated to AGHA, and then measured the IC50 of quercetin-3-rutinoside binding to this mutant. The binding curves of quercetin-3-rutinoside to wild type PDI (IC50 12.3 μm ± 4.2) or the active site-mutated PDI (IC50 15.4 μm) were nearly identical, thus confirming that the PDI active site CGHC motifs are not involved in quercetin-3-rutinoside binding (Fig. 1F).
FIGURE 1.
Interaction of quercetin-3-rutinoside and isoquercetin with thiol isomerases. A and C, direct molecular interaction of PDI with quercetin-3-rutinoside (A) and isoquercetin (C) monitored by the fluorescence emission spectra of the complex. In the presence of PDI (18 μm), quercetin-3-rutinoside (55 μm) and isoquercetin (55 μm) emission maxima are enhanced and blue-shifted from 550 to 530 nm when excited at 430 nm. ····, quercetin-3-rutinoside or isoquercetin alone; – – –, PDI alone; ——, complex. B, the binding affinity, IC50, of PDI and quercetin-3-rutinoside (Q-3-R), measured by monitoring the perturbation of the emission fluorescence, was 12.3 μm. D, the binding affinity, IC50, of PDI and isoquercetin, measured by monitoring the perturbation of the emission fluorescence, was 14 μm. E, quercetin-3-rutinoside binds directly to PDI (●), but not to ERp5 (■), ERp57 (▴), or ERp72 (▾), as detected by monitoring the emission fluorescence of quercetin-3-rutinoside. TI, thiol isomerase. F, binding isotherm of quercetin-3-rutinoside to PDI-AGHA, an enzymatically inactive mutant. The IC50 was 15.5 μm. G, analysis of a mixture of PDI and quercetin-3-rutinoside at a concentration of 100 μm at a 1:1 molar ratio by gel filtration on Superdex 200. Black, PDI and quercetin-3-rutinoside; gray, PDI alone; light gray, quercetin-3-rutinoside alone. H, isothermal calorimetry measurement of the binding between quercetin-3-rutinoside and PDI. A dissociation constant of 18.3 μm, ΔH = −9.6 kcal/mol, and ΔS = −10.7 cal/mol/K were determined. Error bars indicate ± S.E.
To test the reversibility of binding of quercetin-3-rutinoside to PDI, we mixed PDI and quercetin-3-rutinoside at a 1:1 molar ratio (100 μm:100 μm) and a 1:20 molar ratio (100 μm:2000 μm) and analyzed the mixture by gel filtration. We observed separation of the components of the complex, thus demonstrating that quercetin-3-rutinoside binds reversibly to PDI (Fig. 1G). The stoichiometry between PDI and quercetin-3-rutinoside was measured by isothermal calorimetry. PDI was titrated with increasing amounts of quercetin-3-rutinoside, and the heat dissipated from complex formation was measured (Fig. 1H). The results yielded a stoichiometric ratio of ∼1:1 for the PDI·quercetin-3-rutinoside complex, with a dissociation constant of 18.3 μm ± 2.1. The isothermal calorimetry measurement also yielded a ΔH of −9.6 kcal/mol and ΔS of −10.7 cal/mol/K for PDI·quercetin-3-rutinoside complex formation. These values for enthalpy and entropy show that quercetin-3-rutinoside binding to PDI is mainly driven by the enthalpy factor.
Preparation of Recombinant PDI and PDI Fragments
We generated recombinant PDI fragments containing different domain(s) of PDI. PDI contains four domains, a, b, b′, and a′, with a linker region (x) between the b′ and a′ domains, and an extension (c) after the a′ domain (Fig. 2A). We generated 12 recombinant PDI fragments (Fig. 2B). These PDI fragments and full-length PDI were expressed in E. coli, and purified to near homogeneity as defined by a single band on SDS-PAGE (Fig. 2C). We were unable to express the b′ domain alone, but it was prepared linked to the x linker, b′x.
FIGURE 2.
Recombinant PDI and PDI fragments: purification and characterization.
A, PDI contains four domains (a, b, b′, a′), with a linker region (x) between b′ and a′ domains, and an extension c right after the a′ domain. Residues 1–117 (a domain), 118–218 (b domain), 219–331 (b′ domain), 332–351 (x′ linker), 342–462 (a′ domain), and 463–491 (c extension) comprise the mature PDI protein. B, His-tagged full-length human PDI (abb′xa′c) and each of its fragments (a, b, b′x, a′, ab, bb′, b′xa′, abb′, abb′x, bb′xa′, and abb′xa′) were cloned into a pET-15b vector at the restriction site of NedI and BamHI and transformed into E. coli Origami B (DE3) cells. The recombinant proteins were isolated by affinity chromatography with the cOmplete His-Tag purification resin (Roche Applied Science) and further purified by gel filtration chromatography on a Superdex 200. C, the purified proteins appeared as single bands on reduced SDS-PAGE. D, thiol reductase activity was measured in each of these expressed proteins using the insulin reductase assay. a, ▾; b, □; b′x,  ; a′, ▵; ab, ♦; bb′, ■; b′xa′, *; abb′, ▴; abb′x, +; bb′xa′, ♢; abb′xa′, ○; abb′xa′c, ●; buffer control, −. Error bars indicate ± S.E. A650, optical density at 650 nm.
; a′, ▵; ab, ♦; bb′, ■; b′xa′, *; abb′, ▴; abb′x, +; bb′xa′, ♢; abb′xa′, ○; abb′xa′c, ●; buffer control, −. Error bars indicate ± S.E. A650, optical density at 650 nm.
We evaluated these fragments for PDI activity using the insulin reductase assay (Fig. 2D). The isolated catalytic domains (a or a′) showed lower reductase activities when compared with the full-length PDI. The b and b′x fragments did not manifest any catalytic activity. However, their adjacency increased the catalytic activities of the a domain modestly and the a′ domain substantially. The b′ domain is effective in increasing the activities of the a′ or the ab domains, suggesting that the b′ domain plays an important role in enzymatic function. All of the three-domain fragments had comparable reductase activities, whereas the one-domain or two-domain fragments had lower activities. All four domains were required to approximate full PDI reductase activity.
The b′ Domain of PDI Contains the Major Binding Site of Quercetin-3-rutinoside
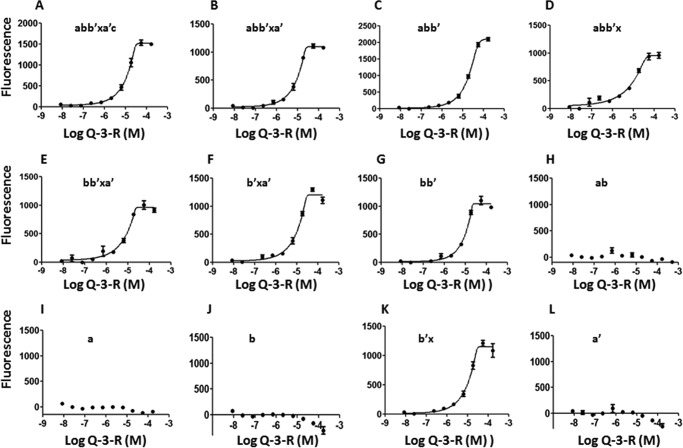
We examined quercetin-3-rutinoside binding to the PDI fragments using the fluorescence enhancement binding assay (Fig. 3). Quercetin-3-rutinoside did not bind to the catalytic a and a′ domains, further confirming that the active site does not play a role in this interaction. Quercetin-3-rutinoside selectively binds to the b′x domain, but not the b domain. Indeed, quercetin-3-rutinoside binds to any fragment that contains the b′ domain. The IC50 values that characterize the binding of quercetin-3-rutinoside to these b′-containing fragments are within a range of 8.6–20.8 μm when compared with the IC50 of 12.3 μm for the full-length PDI. These results demonstrate that any fragment containing the b′ domain binds to quercetin-3-rutinoside with similar affinity.
FIGURE 3.
Direct binding measurements of quercetin-3-rutinoside with different recombinant PDI fragments by fluorescence assay. The fragments a, a′, b, and ab do not bind to quercetin-3-rutinoside (Q-3-R). All fragments that contain the b′ domain have a similar binding affinity for quercetin-3-rutinoside. A–L: IC50 values for PDI or fragments of PDI binding to quercetin-3-rutinoside were: abb′xa′c, 12.3 μm (A); abb′xa′, 10.1 μm (B); abb′, 20.8 μm (C); abb′x, 10.2 μm (D); bb′xa′, 8.6 μm (E); b′xa′, 11.6 μm (F); bb′, 10.4 μm (G); ab, N. M. (H); a, N. M. (I); b, N. M. (J); b′x, 11.5 μm (K); and a′, N. M. (L). N. M. = not measurable. Error bars indicate ± S.E.
Effect of Quercetin-3-rutinoside on Reductase Activity of PDI Fragments
PDI fragments containing either the a domain or the a′ domain expressed reductase activity at a lower level than full-length PDI (Fig. 4). When the a′ domain was adjacent to the b′ domain, reductase activity was increased. The progress curves of the reductase activities of the PDI domain fragments containing the a and a′ domains were measured in the presence and absence of quercetin-3-rutinoside (Fig. 4). Quercetin-3-rutinoside did not influence the activities of the PDI single catalytic domain fragments, a and a′, nor the two-domain fragment, ab (Fig. 4, A–C). However, any fragment that contained an a or a′ domain and the b′ domain was inhibited by quercetin-3-rutinoside (Fig. 4, D–I). Taken together, we conclude that quercetin-3-rutinoside inhibits PDI reductase activities by binding to the b′ domain of PDI.
FIGURE 4.
Reductase activity of PDI fragments in the presence and absence of quercetin-3-rutinoside. A–I, reduction of insulin by PDI fragments in the absence (gray) and presence (black) of quercetin-3-rutinoside (100 μm): a′ (A); a (B); ab (C); b′xa′ (D); abb′ (E); abb′x (F); bb′xa′ (G); abb′xa′ (H); and abb′xa′c (I). Error bars indicate ± S.E.
Binding of Quercetin-3-rutinoside Induces Conformational Changes in PDI
We further characterized recombinant PDI and its complex with quercetin-3-rutinoside using SAXS (Fig. 5A). This method measures the gyration radius (Rg) of a protein in aqueous solution and can derive the overall molecular envelope of the protein. Our SAXS results show that the gyration radius for the full-length human PDI was 39.6 Å, and, upon quercetin-3-rutinoside binding, the gyration radius decreased to 34.4 Å (Table 1, Fig. 5). This demonstrates that in the presence of quercetin-3-rutinoside, PDI adopts a more compact conformation. This result illustrates that quercetin-3-rutinoside likely decreases the interdomain flexibility between the b′ and a′ domains. The calculated SAXS molecular envelop shows that PDI appears as an elliptical disk (Fig. 5B), and then becomes a more circular disk upon quercetin-3-rutinoside binding. The current SAXS PDI data are consistent with previous SAXS results (22, 23), and both showed that the derived SAXS molecular envelope of PDI is larger than the crystal structure of PDI (PDB 4EL1 and 4EKZ), demonstrating that PDI is flexible in solution.
FIGURE 5.
PDI·quercetin-3-rutinoside structure. A, comparison of the SAXS molecular envelopes of PDI (magenta) and PDI·quercetin-3-rutinoside (PDI·Q-3-R) complex (yellow), overlapped with the crystal structure of PDI (Protein Data Bank code 4KEZ), in two orthogonal orientations (top view and side view). B, SAXS profiles of PDI (yellow) and the PDI·quercetin-3-rutinoside complex (blue) show that the scattering profile of PDI·quercetin-3-rutinoside complex is shifted upward (blue), from free PDI (taupe). The observed profiles were compared with those calculated from the restored molecular shapes (red). C, the P(r) plot showing that the size of PDI indicates contraction of PDI (taupe) when complexed with quercetin-3-rutinoside (blue).
TABLE 1.
Results of SAXS measurement on PDI and its complex with quercetin-3-rutinoside
| Protein | Rg | I(0) | Dmax |
|---|---|---|---|
| nm | |||
| PDI | 39.6 | 2722.4 | 130.6 |
| PDI: Q-3-R | 34.4 | 2301.0 | 120.2 |
Rescue of Thrombus Formation Blockade from PDI Inhibition; PDI b′x Fragment Reverses Quercetin-3-rutinoside Inhibition of Thrombus Formation
Quercetin-3-rutinoside inhibits thrombus formation induced by laser injury in the cremaster arterioles (11). Based upon the ability of quercetin-3-rutinoside and isoquercetin to block both platelet thrombus formation and fibrin generation in vivo in the mouse thrombosis model, these agents are being evaluated in mice and humans in ongoing preclinical and clinical trials as possible new antithrombotics targeting PDI. Because all current antithrombotics have the potential to lead to hemorrhage in recipients of these agents, we have worked concurrently to develop an antidote for quercetin-related antithrombotics. Because the b′x fragment of PDI is functionally inert but binds to quercetin-3-rutinoside, we examined the reversal of inhibition of laser-induced thrombus formation with infusion of the b′x fragment of PDI (18).
Platelets were monitored in vivo using an anti-CD42b antibody conjugated to DyLight 649, and fibrin generation was monitored using a fibrin-specific monoclonal antibody conjugated to Alexa Fluor 488. Thrombus formation was imaged by intravital microscopy (18); representative images of thrombus formation at various time points are shown in Fig. 6A (upper panel), whereas the kinetics of platelet thrombus formation and fibrin generation determined as the median fluorescence values of 30–35 separate thrombi are shown in Fig. 6A (lower panel). Initially, platelet accumulation and fibrin generation were monitored and quantitated during thrombus formation over 120 s following laser injury to the vessel wall (Fig. 6A, lane 1). Quercetin-3-rutinoside (0.5 μg/g of body weight) was subsequently infused into the mouse to an estimated plasma concentration of 10 μm, and thrombus formation was triggered by vessel wall injury. Inhibition of thrombus formation by quercetin-3-rutinoside was observed. Full inhibition, reached 30 min following quercetin-3-rutinoside infusion, is shown in Fig. 6A, lane 2, and Fig, 7A. About 45 min after quercetin-3-rutinoside infusion, the b′x fragment of PDI was infused (48 μg/g of body weight), the vessel wall was injured, and platelet accumulation and fibrin generation were measured. This concentration of b′x corresponded to an estimated plasma concentration of 40 μm. Fig. 6, lane 3, and Fig. 7B show that both platelet aggregation and fibrin deposition were fully restored by infusion of the b′x fragment of PDI to an extent similar to that of wild type mice.
FIGURE 6.
Rescue of thrombus formation following quercetin-3-rutinoside-mediated PDI inhibition using the b′x fragment of PDI. A–C, top panels: representative images as a function of time of platelet thrombus formation and fibrin generation as monitored by intravital microscopy over 120 s using platelet-specific anti-CD42b antibody conjugated to DyLight 649 and a mouse monoclonal anti-fibrin-specific antibody conjugated to Alexa Fluor 488. A, rescue with b′x fragment of PDI. Lane 1, thrombus formation following vessel wall injury. Lane 2, after infusion of quercetin-3-rutinoside to a final plasma concentration of ∼10 μm (0.5 μg/g of body weight), vessel wall injury does not lead to thrombus formation. Lane 3, if PDI fragment b′x is infused to an estimated final plasma concentration of 40 μm, there is full recovery of thrombus formation following vessel wall injury. RFU, relative fluorescence units. B, infusion of b fragment of PDI has no effect on thrombus formation. Lane 1, thrombus formation following vessel wall injury. Lane 2, after infusion of quercetin-3-rutinoside to a final plasma concentration of ∼10 μm (0.5 μg/g of body weight), vessel wall injury does not lead to thrombus formation. Lane 3, if fragment b of PDI is infused to an estimated final plasma concentration of 40 μm, there is no recovery of thrombus formation following vessel wall injury. C, infusion of b′x fragment of PDI, in the absence of quercetin-3-rutinoside, has no effect on thrombus formation. Lane 1, thrombus formation after infusion of PDI fragment b′x to an estimated plasma concentration of 40 μm. A–C, bottom panels: kinetics of thrombus formation for the median relative fluorescence units of ∼30 thrombi. Red, platelets,; green, fibrin. The results of aggregate thrombi generation experiments from three or four mice for each set of experiments are depicted.
FIGURE 7.

Statistical analysis of the rescue of thrombus inhibition induced by quercetin-3-rutinoside using the b′x fragment of PDI. A, thrombus formation was fully inhibited following quercetin-3-rutinoside (Q-3-R) infusion when compared with untreated control mice. Platelets, p < 0.0001; fibrin, p < 0.0001. Gray, control (no quercetin-3-rutinoside or PDI fragment); black, quercetin-3-rutinoside plus no PDI fragment, b′x fragment of PDI or b fragment of PDI. RFU, relative fluorescence units. B, infusion of the b′x fragment of PDI rescued thrombus formation in mice treated with quercetin-3-rutinoside. Platelets, p = 0.77; fibrin, p = 0.93. C, infusion of the b domain of PDI did not rescue thrombus formation in mice treated with quercetin-3-rutinoside. Platelets, p < 0.0001; fibrin, p < 0.0001. In contrast to the representative images of a single experiment shown in Fig. 6, these analyses are based on the results from 30–34 vascular injuries.
In parallel experiments in a new cohort of mice, the b domain of PDI was infused instead of the b′x fragment (Fig. 6B). The b domain fragment failed to rescue quercetin-3-rutinoside inhibition of platelet accumulation and fibrin generation following laser-induced vessel wall injury (Fig. 6B, lane 3, and Fig. 7C). Because the first phases of the experiments performed in Fig. 6, A and B, are the same, the kinetic results for the experiments depicted in lanes 1 in both A and B were combined, as were those in lanes 2 in both A and B, to obtain greater statistical significance.
In control experiments, thrombus formation was unaffected by the b′x fragment of PDI in the absence of quercetin-3-rutinoside (Fig. 6C). These in vivo results further support that the b′x fragment of PDI contains the major binding site for quercetin-3-rutinoside and demonstrate that the b′x fragment of PDI can reverse the inhibition of thrombus formation and can be used as an antidote for quercetin-3-rutinoside or other inhibitors targeting the b′ domain of PDI.
Discussion
Following vascular injury, PDI is secreted from platelets and the endothelium to initiate thrombus formation (4, 5). Although biologically active PDI is required, the mechanism by which PDI plays an essential role in platelet thrombus formation and fibrin generation is presently unknown. PDI, after vascular injury, is localized to the area of vascular injury bound to αvβ3 on the activated endothelium and αIIbβ3 on the activated platelets associated with the thrombus (24). Platelet microparticles are incorporated into the thrombus, but whether they bind PDI is unknown.
In the current study, we characterized the binding properties of quercetin-3-rutinoside to PDI by exploiting the intrinsic fluorescence properties of quercetin-3-rutinoside. Upon binding of quercetin-3-rutinoside to PDI, the fluorescence emission of quercetin-3-rutinoside is enhanced, and this property can be used to develop binding isotherms. Quercetin-3-rutinoside binds to PDI with an IC50 of ∼10 μm and with a 1:1 stoichiometry. This interaction requires the b′ domain of PDI. Although model building gives a suggestion of its binding site at the interface of the b′ and a′ domains, x-ray crystallographic studies of the binary complex will be required to define the binding site. To attempt to resolve whether quercetin-3-rutinoside requires the native, folded form of the b′x domain of PDI or whether a small b′x-derived peptide might suffice to interact with quercetin-3-rutinoside, we examined the proteolysis products of trypsin-digested and chymotrypsin-digested PDI b′x to determine whether any peptide(s) retained the ability to bind to quercetin-3-rutinoside. We observed no binding of quercetin-3-rutinoside. However, this does not rule out that a larger peptide fragment might bind to quercetin-3-rutinoside, nor does it demonstrate that the tertiary structure of b′x is required for quercetin-3-rutinoside binding. The b′ domain, which has only about 20% sequence homology with ERp57, plays a critical role in substrate recognition (25–27), whereas the conserved a and a′ domains, which show about 80% homology with those domains of ERp57, contain the active site motifs critical for catalysis.
Based upon the discovery that thrombus formation in vivo requires protein disulfide isomerase (4), we are exploring PDI as a potential target for antithrombotic therapy (11). We have identified quercetin-3-rutinoside and other structurally related flavonoids via high-throughput screening of a library of bioactive compounds as inhibitors of PDI, and thus potential antithrombotic agents against PDI. Furthermore, quercetin-3-rutinoside is a flavonoid found in fruits and vegetables, and is widely used as a food additive and alternative medicine. A unique feature of the inhibition of extracellular PDI is that it inhibits both platelet thrombus formation and fibrin generation. Furthermore, this inhibition is reversible. Biochemical and cellular mechanisms for the role of PDI or other thiol isomerases in thrombus formation are unknown. Thiol isomerases have four potential enzymatic activities, including oxidoreductase activity, isomerase activity, nitrosylase-denitrosylase activity, and chaperone activity, but which are critical in this physiologic process is unclear.
In the characterization of quercetin-3-rutinoside binding to PDI and the identification of its binding site, we posited that the b′x domain of PDI might have the properties to serve as an antidote to quercetin-3-rutinoside-inhibited thrombus formation. Indeed, quercetin-3-rutinoside binds to both b′x and bb′ with approximately the same affinity as to full-length PDI, and b′x and bb′ are inactive enzymatically. For these reasons, we do not believe that the x linker contributes to quercetin-3-rutinoside binding. Because we were unable to express adequate amounts of b′, we examined the b′x fragment as an agent to reverse the inhibition of thrombus formation in vivo by quercetin-3-rutinoside. We have demonstrated that quercetin-3-rutinoside, a reversible inhibitor of thrombus formation, binds sufficiently tightly to the b′x fragment to prevent it from blocking the enzymatic activity of PDI.
Author Contributions
L. L. and M. H. initiated the studies, designed and performed experiments, analyzed data, and contributed to writing the manuscript; S. G., A. S., F. P., S. R. B., and J. S. designed and performed experiments, analyzed data, and contributed to the development of the manuscript; L. L., C. Y., G. X., and M. H. performed the SAXS experiments; B. C. F. and R. F. analyzed data and edited the manuscript; and B. F. analyzed data and wrote the manuscript.
Acknowledgments
The SAXS experiments were conducted at the Advanced Light Source, a national user facility operated by Lawrence Berkeley National Laboratory on behalf of the Department of Energy, Office of Basic Energy Sciences, through the Integrated Diffraction Analysis Technologies (IDAT) program, supported by the Department of Energy Office of Biological and Environmental Research.
This work was supported by National Institutes of Health Grants U54 HL112302 and T32 HL007917 (to B. F.) and by the National Institute of Health project MINOS (R01GM105404) (to M. H.). The authors declare that they have no conflicts of interest with the contents of this article.
- PDI
- protein disulfide isomerase
- SAXS
- small angle x-ray scattering.
References
- 1. Gething M. J., Sambrook J. (1992) Protein folding in the cell. Nature 355, 33–45 [DOI] [PubMed] [Google Scholar]
- 2. Ali Khan H., Mutus B. (2014) Protein disulfide isomerase a multifunctional protein with multiple physiological roles. Front. Chem. 2, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Furie B., Flaumenhaft R. (2014) Thiol isomerases in thrombus formation. Circ. Res. 114, 1162–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cho J., Furie B. C., Coughlin S. R., Furie B. (2008) A critical role for extracellular protein disulfide isomerase during thrombus formation in mice. J. Clin. Invest. 118, 1123–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jasuja R., Furie B., Furie B. C. (2010) Endothelium-derived but not platelet-derived protein disulfide isomerase is required for thrombus formation in vivo. Blood 116, 4665–4674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cho J. (2013) Protein disulfide isomerase in thrombosis and vascular inflammation. J. Thromb. Haemost. 11, 2084–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu Y., Ahmad S. S., Zhou J., Wang L., Cully M. P., Essex D. W. (2012) The disulfide isomerase ERp57 mediates platelet aggregation, hemostasis, and thrombosis. Blood 119, 1737–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Holbrook L. M., Sasikumar P., Stanley R. G., Simmonds A. D., Bicknell A. B., Gibbins J. M. (2012) The platelet-surface thiol isomerase enzyme ERp57 modulates platelet function. J. Thromb. Haemost. 10, 278–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Passam F. H., Lin L., Gopal S., Stopa J. D., Bellido-Martin L., Huang M., Furie B. C., Furie B. (2015) Both platelet- and endothelial cell-derived ERp5 support thrombus formation in a laser-induced mouse model of thrombosis. Blood 125, 2276–2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim K., Hahm E., Li J., Holbrook L. M., Sasikumar P., Stanley R. G., Ushio-Fukai M., Gibbins J. M., Cho J. (2013) Platelet protein disulfide isomerase is required for thrombus formation but not for hemostasis in mice. Blood 122, 1052–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jasuja R., Passam F. H., Kennedy D. R., Kim S. H., van Hessem L., Lin L., Bowley S. R., Joshi S. S., Dilks J. R., Furie B., Furie B. C., Flaumenhaft R. (2012) Protein disulfide isomerase inhibitors constitute a new class of antithrombotic agents. J. Clin. Invest. 122, 2104–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Flaumenhaft R., Furie B., Zwicker J. I. (2015) Therapeutic implications of protein disulfide isomerase inhibition in thrombotic disease. Arterioscler. Thromb. Vasc. Biol. 35, 16–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Castellucci L. A., Cameron C., Le Gal G., Rodger M. A., Coyle D., Wells P. S., Clifford T., Gandara E., Wells G., Carrier M. (2014) Clinical and safety outcomes associated with treatment of acute venous thromboembolism: a systematic review and meta-analysis. JAMA 312, 1122–1135 [DOI] [PubMed] [Google Scholar]
- 14. Hui K. Y., Haber E., Matsueda G. R. (1983) Monoclonal antibodies to a synthetic fibrin-like peptide bind to human fibrin but not fibrinogen. Science 222, 1129–1132 [DOI] [PubMed] [Google Scholar]
- 15. Hura G. L., Menon A. L., Hammel M., Rambo R. P., Poole F. L. 2nd, Tsutakawa S. E., Jenney F. E. Jr., Classen S., Frankel K. A., Hopkins R. C., Yang S. J., Scott J. W., Dillard B. D., Adams M. W., Tainer J. A. (2009) Robust, high-throughput solution structural analyses by small angle x-ray scattering (SAXS). Nat. Methods 6, 606–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Konarev P. V., Volkov V. V., Sokolova A. V., Koch M. H. J., Svergun D. I. (2003) PRIMUS: a Windows PC-based system for small-angle scattering data analysis. J. Appl. Crystallogr. 36, 1277–1282 [Google Scholar]
- 17. Petoukhov M. V., Konarev P. V., Kikhney A. G., Svergun D. I. (2007) ATSAS 2.1: towards automated and web-supported small-angle scattering data analysis. J. Appl. Crystallogr. 40, s223–s228 [Google Scholar]
- 18. Falati S., Gross P., Merrill-Skoloff G., Furie B. C., Furie B. (2002) Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nat. Med. 8, 1175–1181 [DOI] [PubMed] [Google Scholar]
- 19. Celi A., Merrill-Skoloff G., Gross P., Falati S., Sim D. S., Flaumenhaft R., Furie B. C., Furie B. (2003) Thrombus formation: direct real-time observation and digital analysis of thrombus assembly in a living mouse by confocal and widefield intravital microscopy. J. Thromb. Haemost. 1, 60–68 [DOI] [PubMed] [Google Scholar]
- 20. Bellido-Martín L., Chen V., Jasuja R., Furie B., Furie B. C. (2011) Imaging fibrin formation and platelet and endothelial cell activation in vivo. Thromb. Haemost. 105, 776–782 [DOI] [PubMed] [Google Scholar]
- 21. Dubois C., Atkinson B., Furie B. C., Furie B. (2007) Real-time in vivo imaging of platelets during thrombus formation. in Platelets (Michelson A. D., ed), pp. 611–626, Academic Press/Elsevier, Amsterdam [Google Scholar]
- 22. Li S. J., Hong X. G., Shi Y. Y., Li H., Wang C. C. (2006) Annular arrangement and collaborative actions of four domains of protein-disulfide isomerase: a small angle x-ray scattering study in solution. J. Biol. Chem. 281, 6581–6588 [DOI] [PubMed] [Google Scholar]
- 23. Nakasako M., Maeno A., Kurimoto E., Harada T., Yamaguchi Y., Oka T., Takayama Y., Iwata A., Kato K. (2010) Redox-dependent domain rearrangement of protein disulfide isomerase from a thermophilic fungus. Biochemistry 49, 6953–6962 [DOI] [PubMed] [Google Scholar]
- 24. Cho J., Kennedy D. R., Lin L., Huang M., Merrill-Skoloff G., Furie B. C., Furie B. (2012) Protein disulfide isomerase capture during thrombus formation in vivo depends on the presence of β3 integrins. Blood 120, 647–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klappa P., Stromer T., Zimmermann R., Ruddock L. W., Freedman R. B. (1998) A pancreas-specific glycosylated protein disulphide-isomerase binds to misfolded proteins and peptides with an interaction inhibited by oestrogens. Eur. J. Biochem. 254, 63–69 [DOI] [PubMed] [Google Scholar]
- 26. Nguyen V. D., Wallis K., Howard M. J., Haapalainen A. M., Salo K. E., Saaranen M. J., Sidhu A., Wierenga R. K., Freedman R. B., Ruddock L. W., Williamson R. A. (2008) Alternative conformations of the x region of human protein disulphide-isomerase modulate exposure of the substrate binding b′ domain. J. Mol. Biol. 383, 1144–1155 [DOI] [PubMed] [Google Scholar]
- 27. Byrne L. J., Sidhu A., Wallis A. K., Ruddock L. W., Freedman R. B., Howard M. J., Williamson R. A. (2009) Mapping of the ligand-binding site on the b′ domain of human PDI: interaction with peptide ligands and the x-linker region. Biochem. J. 423, 209–217 [DOI] [PubMed] [Google Scholar]