Background: Inorganic pyrophosphate-type phosphoenolpyruvate carboxylase (PPi-PEPCK) was unidentified.
Results: A conserved hypothetical protein was annotated as PPi-PEPCK.
Conclusion: PPi-PEPCK arose independently from the functional homologs ATP/GTP-PEPCKs and PEP carboxylase.
Significance: Identification of PPi-PEPCK reveals the wide distribution of this enzyme and accelerates understanding the diversity of the central metabolism.
Keywords: carbohydrate metabolism, carbon fixation, enzyme, parasite metabolism, phosphoenolpyruvate carboxykinase, central metabolism, novel enzyme, pyrophosphate
Abstract
Phosphoenolpyruvate carboxykinase (PEPCK) is one of the pivotal enzymes that regulates the carbon flow of the central metabolism by fixing CO2 to phosphoenolpyruvate (PEP) to produce oxaloacetate or vice versa. Whereas ATP- and GTP-type PEPCKs have been well studied, and their protein identities are established, inorganic pyrophosphate (PPi)-type PEPCK (PPi-PEPCK) is poorly characterized. Despite extensive enzymological studies, its protein identity and encoding gene remain unknown. In this study, PPi-PEPCK has been identified for the first time from a eukaryotic human parasite, Entamoeba histolytica, by conventional purification and mass spectrometric identification of the native enzyme, followed by demonstration of its enzymatic activity. A homolog of the amebic PPi-PEPCK from an anaerobic bacterium Propionibacterium freudenreichii subsp. shermanii also exhibited PPi-PEPCK activity. The primary structure of PPi-PEPCK has no similarity to the functional homologs ATP/GTP-PEPCKs and PEP carboxylase, strongly suggesting that PPi-PEPCK arose independently from the other functional homologues and very likely has unique catalytic sites. PPi-PEPCK homologs were found in a variety of bacteria and some eukaryotes but not in archaea. The molecular identification of this long forgotten enzyme shows us the diversity and functional redundancy of enzymes involved in the central metabolism and can help us to understand the central metabolism more deeply.
Introduction
Inorganic pyrophosphate (PPi) is composed of two molecules of phosphate (Pi) linked by a phosphoanhydride bond. PPi has been proposed as an evolutionary precursor of ATP and GTP (1) because this structurally simple compound can be formed spontaneously, and the hydrolysis of PPi produces high energy (2, 3). If ancestral organisms could utilize only PPi or polyphosphates as an energy and/or phosphate donor for enzymatic reactions, there should have been an event in which new enzymes utilizing ATP/GTP arose. This further raises the question of how organisms change the major substrate: by accumulation of mutation on the enzyme to change the substrate specificity from PPi to nucleotide triphosphate or the substitution of PPi-utilizing enzymes by an independently emerging functional homolog that utilizes nucleotide triphosphates. To gain insight into the evolutionary transition from PPi to nucleotide triphosphate, the evolutionary relationship between the extant PPi- and ATP/GTP- utilizing enzymes has been examined (4–11).
In glycolytic/gluconeogenic pathways and the closely related reactions in current organisms, three enzymatic reactions that can utilize PPi as the substrate have been reported. The first one is a PPi-dependent phosphofructokinase (PFK)3 (EC 2.7.1.90) reaction. PPi-PFK catalyzes a reversible reaction, whereas ATP-dependent PFK (EC 2.7.1.11) catalyzes an irreversible reaction.
 |
PPi-PFKs from an eukaryotic human parasite, Entamoeba histolytica (12), and an anaerobic bacterium, Propionibacterium freudenreichii subsp. shermanii (13), were proposed to work for fructose 1,6-bisphosphatase synthesis like ATP-PFKs. In higher plants, it has been shown that PPi-PFK works in the opposite direction, at least during internode developmental stages (14). Therefore, PPi-PFK can catalyze both PPi-utilizing and PPi-producing reactions not only in vivo but also in vitro. ATP- and PPi-PFK share a common origin, but the evolutionary history is highly complex. Change of the phosphate donors in PFKs occurred more than once, as suggested by phylogenetic analyses of ATP- and PPi-PFKs (6, 9, 10, 15). The complex evolution of substrate utility in PFK suggests that, at least in PFK, the transition of PPi-utilizing and nucleotide triphosphate-utilizing ability can have occurred relatively easily. Indeed, mutation of just a few amino acids can change the substrate preference from PPi to ATP, based on the ratio of kcat/Km (8).
The second reaction utilizing PPi is catalyzed by pyruvate phosphate dikinase (PPDK; EC 2.7.9.1).
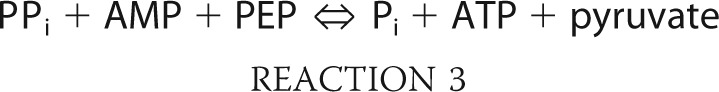 |
PEP-pyruvate conversion is also catalyzed by PPi-independent enzymes, PEP synthase (EC 2.7.9.2; Reaction 4) and pyruvate kinase (EC 2.7.1.40; Reaction 5).
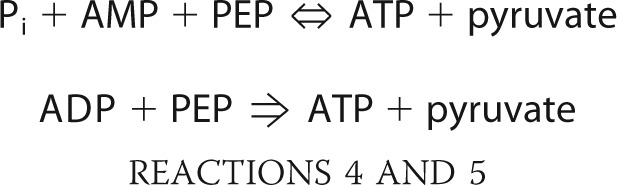 |
PPDK and PEP synthase share conserved domains, whereas overall amino acid sequence similarity is not high (16). On the other hand, neither PPDK nor PEP synthase shows substantial similarity to pyruvate kinase at the primary structure level. This case is apparently distinguishable from that of PFK because transition of substrate utility for the PEP-pyruvate conversion has not occurred by simple amino acid substitutions, as seen in PFK.
The third mechanism is a PEP-oxaloacetate interconverting reaction catalyzed by phosphoenolpyruvate carboxykinase (PEPCK). Because PEP is a key intermediate in a variety of metabolic processes in all living organisms (17, 18), PEPCK works as a major crossroad that connects glycolysis/gluconeogenesis and organic acids metabolisms like the tricarboxylic acid cycle and fumarate fermentation. According to the phosphate donor to oxaloacetate, PEPCK can be divided into three types: GTP-PEPCK (EC 4.1.1.32), ATP-PEPCK (EC 4.1.1.49), and PPi-PEPCK (EC 4.1.1.38). PEPCK reactions are fundamentally reversible; however, ATP- and GTP-PEPCKs prefer a PEP-producing reaction, whereas PPi-PEPCK prefers an oxaloacetate-producing reaction, at least in vitro (19). PEP carboxylase (EC 4.1.1.31) also catalyzes PEP-oxaloacetate interconversion, but this reaction is irreversible and requires HCO3− instead of CO2 (18, 20).
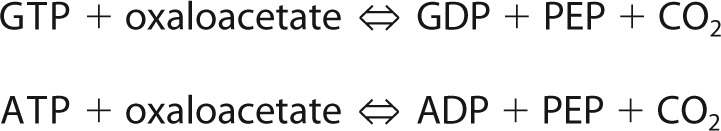 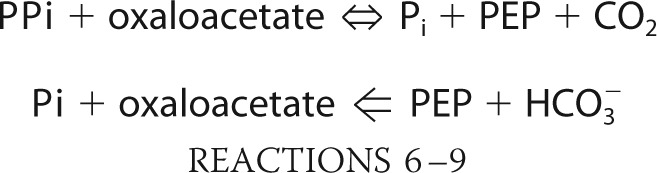
|
ATP- and GTP-PEPCKs and PEP carboxylase have been well characterized. ATP-PEPCKs are mainly present in bacteria, yeast, and plants, whereas GTP-PEPCKs are mostly present in higher eukaryotes, archaea, and some bacteria (21). PEP carboxylase exists in various bacteria and in limited archaea and eukaryotes. Although there is no discernible similarity in the overall structure of these proteins, the residues implicated for the binding to metal, oxaloacetate/PEP, and nucleotides are conserved between ATP- and GTP-PEPCKs (22, 23), whereas conservation of these residues has not been reported in the case of PEP carboxylase. In contrast, the information on PPi-PEPCK, which is also called PEP carboxytransphosphorylase, is limited. Although PPi-PEPCK was previously purified from P. freudenreichii subsp. shermanii (19, 24–26) and E. histolytica (27) and extensively characterized enzymologically, the gene encoding PPi-PEPCK has remained unknown since the first description in 1961 (24). Both the distribution of PPi-PEPCK among the three domains of life and the evolutionary relationship between other PEP-oxaloacetate interconverting enzymes were totally obscure.
In the present study, we identified the protein for PPi-PEPCK activity and its encoding gene from E. histolytica and P. freudenreichii. The identification of PPi-PEPCK finally enabled us to compare the amino acid sequences of all three types of PEPCKs as well as PEP carboxylase. It also allowed us to estimate the distribution of PPi-PEPCK in the tree of life. Last, we described the complex evolutionary history of proteins utilizing different substrates for the same catalytic reaction.
Experimental Procedures
Microorganisms and Cultivation
Trophozoites of E. histolytica clonal strain HM-1:IMSS Cl6 were maintained axenically in Diamond's BI-S-33 medium at 35.5 °C as described previously (28). Cells were grown to the late logarithmic phase (2–3 days after inoculation), harvested by the addition of ice-cold PBS buffer to culture flasks, after discarding the medium, and followed by centrifugation at 300 × g for 5 min at 4 °C. For protein purification, the harvested cell pellets were stored at −80 °C until use.
Enzyme Assays
PPi and oxaloacetate-producing direct PEPCK activity was assayed by measuring the oxidation of NADH by detecting the decrease of absorbance at 340 nm as described previously (27). The reaction mixture contained 10 mm potassium phosphate buffer (pH 6.5), 0.4 mm PEP, 0.1 mm CoCl2, 20 mm KHCO3, 10 mm MgCl2, 2.0 units/ml malate dehydrogenase (from rabbit muscle; Sigma), 0.25 mm NADH, and an enzyme solution. Reaction was started by adding PEP, and the NADH oxidizing activity without the addition of PEP was subtracted as the base line. To obtain the absolute active value, 400 μl of the reaction mixture was added in 1.0-cm path length cuvettes, and absorbance was monitored by a spectrometer. To obtain the relative activity during the purification, 100 μl of the mixture was put in a 96-well plate and incubated at 37 °C, and 340 nm was monitored by a microplate reader. One unit of activity was defined as the amount of enzyme oxidizing 1 μmol of NADH/min.
Purification of Native E. histolytica PEPCK
EhPEPCK was purified from 5 g of wet cells as follows. The cells were suspended in 15 ml of 20 mm Tris-HCl (pH 8.0) containing 0.1 mm CoCl2 (buffer A) and 0.5 mg/ml E-64 and disrupted by freezing and thawing, and cell debris was then removed by centrifugation at 100,000 × g for 1 h. After the supernatant was diluted to 30 ml with buffer A, ammonium sulfate was added to give 30% saturation. The samples were then applied to a Butyl-Toyopearl column (22 × 15 cm; Tosoh, Tokyo, Japan) equilibrated with buffer A supplemented with ammonium sulfate at 30% saturation. All chromatography steps were performed using an ÄKTA purifier system (GE Healthcare) at room temperature. Proteins were eluted with a gradient of ammonium sulfate from 30 to 0% at a flow rate of 4 ml/min. The active fractions were desalted using a PD10 column (GE Healthcare) and then applied to a MonoQ HR 5/5 column (bed volume, 1 ml; GE Healthcare) equilibrated with buffer A. Proteins were eluted with a gradient of NaCl from 0 to 500 mm for 20 column volumes at a flow rate of 1.0 ml/min. The active fractions were pooled and loaded onto a Superdex 200 (10/300) column (GE Healthcare). Proteins were eluted with buffer A supplemented with 150 mm NaCl.
Identification of EhPEPCK by LC-MS/MS Analysis
∼130 kDa bands were excised from silver-stained gel and subjected to LC-MS/MS analysis at the W. M. Keck Biomedical Mass Spectrometry Laboratory (University of Virginia, Charlottesville, VA). The analysis was performed in almost the same manner as before (29); the only difference was that 5 μl of the extract was injected to a reversed-phase capillary column, and peptides were eluted over 0.3 h.
To estimate the relative ratio of EhPEPCK1, -2, and -3 in the analyzed sample, the peak area for the selected ion chromatogram of the monoisotopic mass of the most abundant charge state (±0.02 Da) for each peptide was quantified. Three peptides conserved in all three proteins, specific to EhPEPCK1, and conserved only in EhPEPCK2 and -3, respectively, and one peptide unique to EhPEPCK2 or -3, respectively, were selected for the analysis because these peptides were detected in all three samples: immunoprecipitated samples (see below) using EhPEPCK1-HA, EhPEPCK2-HA, and purified PPi-PEPCK from the wild-type amoeba lysate. The sum of the peak area value of the three peptides conserved in all was used for normalization.
Construction of Plasmids
For expression in Escherichia coli, the protein coding sequences of EhPEPCK1 (XP_654765.1) and EhPEPCK2 (XP_650862.1) genes were PCR-amplified from E. histolytica cDNA using the following pair of forward and reverse primers: 5′-TCGAAGGTAGGCATATGTTTAATCAAGAAAAAGGTACC-3′ (PEPCK_1_pCold_F) and 5′-ATTCGGATCCCTCGATTAATGTTTCATGCATTTGTATG-3′ (PEPCK_1_2_pCold_R) for PEPCK1 and 5′-TCGAAGGTAGGCATATGTTTAATCAAGAACAAGGTA-3′ (PEPCK_2_pCold_F) and PEPCK_1_2_pCold_R for PEPCK2. For expression in E. histolytica trophozoites with a hemagglutinin (HA) tag at the C termini, the protein coding sequences of EhPEPCK1 and -2 genes were PCR-amplified using the following primers: 5′-ACACATTAACAGATCATGTTTAATCAAGAAAAAGGTAC-3′ (PEPCK_1_pEhExHA_F) and 5′-ATGGATACATAGAATGTTTCATGCATTTGTATG-3′ (PEPCK_1_2_pEhExHA_R) and 5′-ACACATTAACAGATCATGTTTAATCAAGAACAAGGTA-3′ (PEPCK_2_pEhExHA_F) and PEPCK_1_2_pEhExHA_R, respectively. pColdI (Takara, Tokyo, Japan) and pEhExHA (30) plasmids were used for expression in E. coli and E. histolytica, respectively. The amplified fragments were inserted into the plasmids cut with NdeI and XhoI (pColdI) or BglII (pEhExHA) using the In-Fusion HD cloning system (Takara).
The PPi-PEPCK gene from P. freudenreichii subsp. freudenreichii (PfPEPCK; LC062511) was PCR-amplified from the genomic DNA (DSM 20271) purchased from the German Collection of Microorganisms and Cell Cultures using the primers 5′-TCGAAGGTAGGCATAATGTCCGTAGTCGAACGC-3′ (Pf_PEPCK_pCold_F) and 5′-ATTCGGATCCCTCGATCAGACGAACCTGGGCTG-3′ (Pf_PEPCK_pCold_R) and cloned into pColdI as described above.
Overexpression and Purification of Recombinant PPi-PEPCKs
The plasmids for bacterial expression were introduced into E. coli BL21 CodonPlus (DE3)-RIL (EhPEPCK1 and -2; Agilent) and E. coli BL21 Star (DE3) (PfPEPCK; Life Technologies, Inc.). The hosts transformed with the expression plasmids were inoculated into 400 ml or Luria-Bertani medium in a 1-liter conical flask containing 50 μg/ml ampicillin and 34 μg/ml chloramphenicol if necessary. After cultivating the cells aerobically at 37 °C until the A600 reached ∼0.5, protein expressions were induced by cooling the culture on ice for 30 min and adding 0.1 mm isopropyl thio-β-d-galactopyranoside to the medium, followed by overnight cultivation at 15 °C. Harvested cells (∼8 g of wet cells from 1.2 liters of culture) were disrupted by adding BugBuster (∼5 ml/g of wet cells; Merck), and cell debris was removed by centrifugation. Imidazole and NaCl concentrations in the supernatant were adjusted to 10 and 300 mm, respectively. The supernatant was then applied to an open column packed with Ni-NTA-agarose (1.5-ml bed volume; Qiagen). After washing the column with 16 bed volumes of 50 mm Tris-HCl, 300 mm NaCl, and 20 mm imidazole-HCl (pH 8.0), the His-tagged protein was eluted with 4 bed volumes of the same buffer containing 250 mm imidazole-HCl. The eluted proteins were further purified using a MonoQ column as described above.
Antibodies
To make anti-EhPEPCK antisera, full-length EhPEPCK1 with a His tag at the N terminus was expressed in E. coli, purified using Ni-NTA and MonoQ columns as described above, and used as the antigen. The anti-EhPEPCK1 antisera from rabbit were commercially raised by Operon Biotechnology (Tokyo, Japan). The specificity of these antibodies was confirmed using E. histolytica lysates by Western blotting (WB) analysis (data not shown).
Amoeba Transformation
The plasmids generated as described above were introduced into E. histolytica trophozoites by lipofection as described previously (31) with minor modifications. Approximately 40 μl of the transfection medium containing 3–5 μg of one of the plasmids was mixed with 10 μl of PLUSTM reagent (Life Technologies) and kept at room temperature for 15 min. This mixture was combined with 20 μl of LipofectamineTM transfection reagent mixed with 30 μl of transfection medium, kept at room temperature for 15 min, diluted with 400 μl of transfection medium, and added to the seeded trophozoites attached to a 12-well plate after removing the medium. The plate was then incubated at 35.5 °C for 5 h, and cells were transferred to a tube containing 5.5 ml of BI-S-33 medium. About 24 h after transfection, BI-S-33 medium was changed into fresh medium with 1 μg/ml G418 and gradually increased for ∼2 weeks until the G418 concentration reached 10 μg/ml.
Protein Assay
Protein concentrations were measured using a Bio-Rad protein assay DC dye. Bovine γ-globulin was used as a standard.
WB
Protein samples were subjected to SDS-PAGE after boiling at 95 °C for 3 min with SDS-PAGE loading buffer. Proteins in the gel were transferred to nitrocellulose membrane and then blocked with 5% (w/v) skim milk in Tris-buffered saline with Tween 20 (TBST) overnight at 4 °C. Proteins on the membrane were reacted with antibodies in TBST for 1 h at room temperature. Primary antibodies were used at a 1:5000 dilution for anti-EhPEPCK rabbit antibody and at a 1:1000 dilution for anti-cysteine synthase 1 (32) and anti-Cpn60 (33) rabbit antibodies and anti-HA mouse monoclonal antibody (clone 11MO, Covance (Princeton, NJ)). The blots were visualized using alkaline phosphatase-conjugated anti-rabbit or mouse IgG antibody (1:2000 dilution; Cell Signaling Technology, Danvers, MA) with AP Color Reagent (Bio-Rad) according to the manufacturer's protocol.
Immunoprecipitation
To examine the interaction between EhPEPCKs, 0.08 g of wet cells expressing PEPCK1-HA, PEPCK2-HA, or only HA were lysed with 1 ml of lysis buffer (50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 0.1 mm CoCl2, 0.2% Triton X-100, 0.5 mg/ml E-64) for 10 min on ice. After centrifugation at 20,000 × g for 10 min, the supernatants were precleaned with 150 μl of Protein G-Sepharose (GE Healthcare) for 1 h. After removing the beads by centrifugation, the precleared supernatant samples were mixed and incubated with 50 μl of anti-HA monoclonal antibody-conjugated agarose (Sigma-Aldrich) for 4 h. The agarose beads were washed with 400 μl of lysis buffer without E-64 five times (9300 × g for 1 min each) to remove the unbound proteins, and the bound proteins were eluted by incubating with 200 μl of lysis buffer containing 0.2 mg/ml HA peptide (Sigma-Aldrich) overnight. The eluted samples were collected by centrifugation at 800 × g for 3 min. All of the centrifugations and incubations were done at 4 °C otherwise mentioned.
Phylogenic Analysis
Homologues of PPi-PEPCK were searched by BLASTP analysis against the non-redundant protein sequence database using EhPEPCK1 as a query (May 5, 2015). In addition, we also retrieved the homologues of Mastigamoeba balamuthi and Spironucleus barkhanus by tBLASTN analysis against the whole genome shotgun reads and the expressed sequence tag database, respectively. Other eukaryotic counterparts were retrieved by tBLASTN analysis against the Marine Microbial Eukaryote Transcriptome Sequencing Project database (34). No more than two sequences with 97–100% amino acid sequence similarity were included, except for Entamoeba spp. and Giardia intestinalis (for details, see “Discussion”). Sequences were aligned by MAFFT (35), and ambiguously aligned sites were excluded, resulting in a data set comprising 158 taxa 749 positions. The data set was subjected to the maximum likelihood method with the LG + Γ + F model. The maximum likelihood tree was heuristically searched from 10 distinct maximum parsimony trees. In maximum likelihood bootstrap analyses (100 replicates), a heuristic tree search was performed from a single maximum parsimony tree per replicate. The maximum likelihood phylogenetic analyses described above were conducted by RAxML version 7.2.8 (36).
Results
Identification of PPi-PEPCK from E. histolytica
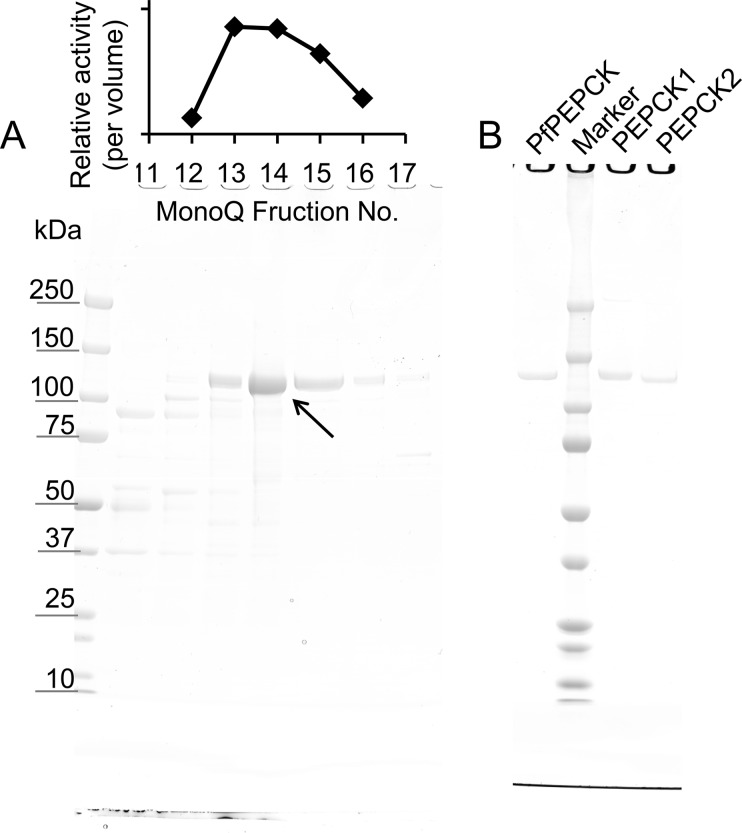
An in silico search of potential PEPCK homologs in the E. histolytica genome (AmoebaDB) using canonical ATP- and GTP-PEPCK from Dictyostelium discoideum (XP_645490.1 and XP_645396.1, respectively) failed to detect any candidate of PEPCK (data not shown), although PPi-PEPCK activity was detected from E. histolytica trophozoite lysates (0.165 units/mg of protein) as previously reported by Reeves (27). PPi-PEPCK activity was sequentially separated using hydrophobic (Butyl-Toyopearl) and anion exchange (MonoQ) columns (Table 1), and a MonoQ fraction containing peak PPi-PEPCK activity gave one major protein band at ∼130 kDa on SDS-PAGE analysis (Fig. 1A). PPi-PEPCK activity detected in this MonoQ fraction indicated 206-fold purification with a specific activity of 34.0 units/mg, which agrees well with the previous results of PPi-PEPCK from P. freudenreichii that showed 12.8 units/mg of activity after 99-fold purification (25). The activity level was also similar to that of ATP-PEPCK from E. coli (24 units/mg) (37). Phosphate, PEP, and MDH dependence of the NADH oxidizing activity in the MonoQ fraction was also confirmed. NADH oxidizing activity was not detected when phosphate was substituted to ADP, indicating that the fraction did not contain ATP-PEPCK activity.
TABLE 1.
Purification of PPi-PEPCK from E. histolytica
1 unit represents the amount of enzyme required to oxidize 1 μmol of NADH/min.
| Activity | Specific activity | Purification | Yield | |
|---|---|---|---|---|
| units | units/mg | -fold | % | |
| 100,000 ×g supernatant | 75.4 | 0.165 | 1.0 | 100 |
| Butyl-Toyopearl | 32.1 | 4.86 | 29.4 | 42.6 |
| MonoQ | 8.06 | 34.0 | 206 | 10.7 |
FIGURE 1.
SDS-PAGE analysis of purified native EhPEPCKs (A) and E. coli-expressed recombinant EhPEPCK1, EhPEPCK2, and PfPEPCK (B). A, relative PPi-PEPCK activities in the selected MonoQ fractions (per volume) are shown at the top. Approximately 15 μl of the MonoQ fractions were applied to SDS-PAGE and stained with Coomassie Brilliant Blue (bottom). The arrow indicates the band corresponding to EhPEPCK. B, homogeneity of purified E. coli-expressed recombinant EhPEPCK1 (PEPCK1), EhPEPCK2 (PEPCK2), and PfPEPCK. One μg of recombinant proteins after the purification by MonoQ column was subjected to SDS-PAGE and Coomassie Brilliant Blue staining.
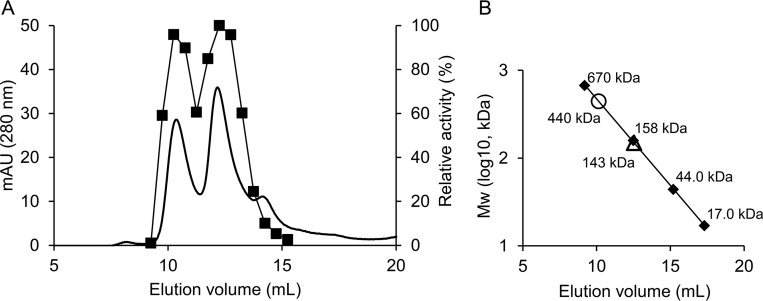
The fraction containing PEPCK activity was further subjected to size exclusion chromatography using Superdex 200. The PPi-PEPCK activity was detected in the fractions corresponding to two major peaks around ∼143 and ∼440 kDa (Fig. 2). The integrated areas of the absorbance at 280 nm corresponding to the 440- and 143-kDa proteins (the first and second peaks, respectively) and their enzymatic activity levels are comparable, suggesting that active PPi-PEPCK is present as both monomer and trimer in E. histolytica (Fig. 2A). PPi-PEPCK from P. freudenreichii has been previously reported to exist as a monomer, dimer, and tetramer with activity (26). Formation of several quaternary structures may be a characteristic of PPi-PEPCK.
FIGURE 2.
Molecular weight of purified native EhPEPCKs. A, chromatograph of native EhPEPCKs on Superdex200. The optical absorbance at 280 nm is shown with a thick line, and the relative activity per volume of each fraction is shown with squares and a thin line. The highest activity per volume in all fractions was defined as 100%. B, estimation of molecular weight of the proteins corresponding to the two peaks in A (circle and triangle). Standard proteins are shown with diamonds.
Because both of the fractions seemed to contain an identical ∼130-kDa protein band on SDS-PAGE, the protein band from the fractions corresponding to the first peak (∼440 kDa) was excised and subjected to TOF MS/MS analysis. Peptides detected from the band correspond to a “cluster of hypothetical proteins,” which consist of three homologous proteins: EHI_166920 (XP_654765.1), EHI_030750 (XP_650862.1), and EHI_198620 (XP_655201.1) (Table 2). The calculated molecular mass of these proteins (131 kDa) agreed well with that expected from the SDS-PAGE result, and the detected peptides covered 63–67% of the three proteins. Hereafter these proteins are referred to as EhPEPCK1, -2, and -3, respectively. EhPEPCK1 and EhPEPCK2 or -3 showed 91% amino acid identity, whereas EhPEPCK2 and -3 showed 98% identity. Amino acid variations between EhPEPCK1 and EhPEPCK2/3 were mainly found in the first 60 residues at the N-terminal region, showing only 45% identity between EhPEPCK1 and EhPEPCK2/3. Neither of these proteins show any similarity to known proteins or domains as predicted by Pfam, indicating that the purified proteins were novel proteins.
TABLE 2.
Proteins detected by MS/MS analysis
Peptide thresholds were standard, and protein thresholds were 90.0% minimum and 1 peptide minimum.
| Identified proteins | Accession numbers | Molecular mass | Quantitative value |
|---|---|---|---|
| kDa | |||
| Hypothetical protein (EhPEPCK1) | EHI_166920; XP_654765 | 131 | 214 |
| Hypothetical protein (EhPEPCK2) | EHI_030750; XP_650862 | 131 | 209 |
| Hypothetical protein (EhPEPCK3) | EHI_198620; XP_655201 | 131 | 198 |
| Hypothetical protein | EHI_192430; XP_656829 | 29 | 1 |
| Hypothetical protein | EHI_189920; XP_652326 | 62 | 1 |
Confirmation of PPi-PEPCK Activity of the Amebic Enzymes Expressed in E. coli
EhPEPCK1, EhPEPCK2, and PfPEPCK were expressed as soluble recombinant proteins with the histidine tag at the N terminus in E. coli. A Coomassie Brilliant Blue-stained SDS-polyacrylamide gel of the proteins purified using Ni-NTA and MonoQ chromatography showed that the full-length proteins of the expected size were purified to homogeneity (Fig. 1B). PPi-PEPCK activity of the purified EhPEPCK1, EhPEPCK2, and PfPEPCK was 8.9, 3.9, and 23.0 units/mg, respectively, confirming that the identified genes indeed encode proteins with PPi-PEPCK activity.
Expression of EhPEPCK1 and -2 with HA Tag in E. histolytica
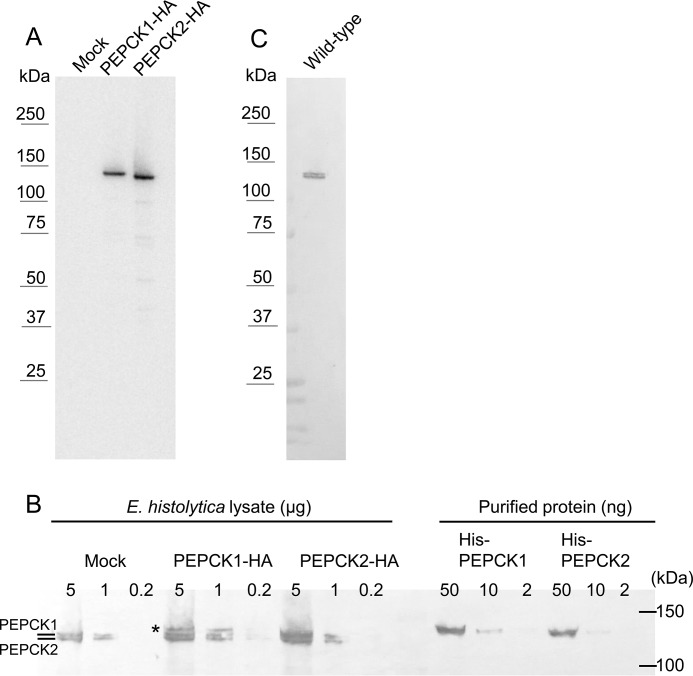
Amebic transformants expressing either EhPEPCK1 or EhPEPCK2 with the HA tag at the carboxyl terminus (EhPEPCK1-HA or EhPEPCK2-HA) using the episomal plasmid were established. Expression of the epitope-tagged proteins were confirmed by WB using anti-HA antibody (Fig. 3A). The molecular mass estimated from the band corresponding to EhPEPCK1-HA was slightly larger than that of EhPEPCK2-HA. We hypothesize that this subtle difference in the mobility between EhPEPCK1 and -2 is attributable to the difference in the isoelectric point between the two proteins. The isoelectric point values of EhPEPCK1 and -2 are calculated as 6.15 and 6.06, respectively. At this stage, we do not know whether the slight difference in the isoelectric point and the molecular size between EhPEPCK1 and -2 is physiologically important.
FIGURE 3.
WB analysis of EhPEPCKs with anti-HA (A) and anti-EhPEPCK (B and C) antibodies. A, expression of EhPEPCK1-HA and EhPEPCK2-HA was confirmed. Note that proteins with the expected size (∼130 kDa) were expressed. Amebic lysates containing 5 μg of protein were applied per lane on SDS-PAGE. B, expression of EhPEPCKs in EhPEPCK1-HA- or EhPEPCK2-HA-expressing and mock control E. histolytica transformants. Total lysates of the above mentioned transformants (0.2–5 μg/lane) and purified histidine-tagged PEPCK1 and -2 (2–50 ng/lane) were electrophoresed on SDS-PAGE and subjected to WB analysis with anti-EhPEPCK antiserum. Note that lysates from mock transformant showed two bands, whereas EhPEPCK1-HA-expressing transformant showed one additional band (shown with an asterisk) corresponding to PEPCK1 with the HA tag. Recombinant PEPCKs possess the His tag at the N terminus and were purified to homogeneity using Ni-NTA and MonoQ columns. C, specificity of anti-EhPEPCK antibody. Approximately 1 μg of lysate from the wild-type E. histolytica was reacted with anti-EhPEPCK antibody.
Heteromeric Configuration of EhPEPCKs
To investigate whether EhPEPCKs form homo- or heterotrimer, EhPEPCK1 and -2 were immunoprecipitated using lysates from transformants expressing corresponding EhPEPCK with the HA tag using anti-HA antibody. Both of the immunoprecipitated samples contained peptides from all of the three EhPEPCKs (Table 3). When proteins were immunoprecipitated using EhPEPCK1-HA, EhPEPCK2/3-specific peptides were also detected, whereas the relative ratio decreased about 1.7-fold compared with that of trimetric EhPEPCK purified from the wild-type E. histolytica. Similarly, the immunoprecipitated sample using EhPEPCK2-HA also contained peptides unique to EhPEPCK1, whereas the relative ratio decreased 2.0-fold. These data suggest that some proportion of the trimer consists of a mixture of EhPEPCK1, -2, and -3. The detailed composition of the trimeric complex should be analyzed in the future.
TABLE 3.
Relative quantification of EhPEPCK isotypes
| Peptide | Peak area of monoisotopic ion |
Normalized relative ratio against common peptides |
||||
|---|---|---|---|---|---|---|
| EhPEPCK1-HA IP | EhPEPCK2-HA IP | Purification from cells | EhPEPCK1-HA IP | EhPEPCK2-HA IP | Purification from cells | |
| Unique to EhPEPCK1 | ||||||
| GTDYPILNIQELEALADLK | 4.54E+07 | 3.96E+06 | 4.61E+06 | |||
| SDAIDVVAPLVDIIAEGDQESTAPIDAR | 4.09E+07 | 6.45E+06 | 2.17E+06 | |||
| NISDAIFEGK | 2.41E+09 | 3.91E+08 | 6.71E+08 | |||
| Sum | 2.50E+09 | 4.01E+08 | 6.78E+08 | 3.0E-01 | 5.5E-02 | 1.1E-01 |
| Specific to EhPEPCK2 and -3 | ||||||
| ITTAFANHFLR | 9.22E+07 | 6.07E+08 | 1.68E+08 | |||
| KLESLANLK | 3.81E+08 | 1.76E+09 | 8.54E+08 | |||
| LAGPLLEEVEESEVNHTTAPIDAR | 2.62E+08 | 1.03E+09 | 2.54E+08 | |||
| Sum | 7.35E+08 | 3.40E+09 | 1.28E+09 | 8.8E-02 | 4.6E-01 | 2.1E-01 |
| Unique to EhPEPCK2 | ||||||
| AVQEIFDHDFSKR | 5.79E+06 | 2.38E+07 | 2.05E+07 | 6.9E-04 | 3.3E-03 | 3.3E-03 |
| Unique to EhPEPCK3 | ||||||
| GANLSSQYLR | 2.14E+08 | 9.04E+06 | 3.37E+08 | 2.6E-02 | 1.2E-03 | 5.5E-02 |
| Common to all | ||||||
| YLIEHGYLEPCPDVTYNGK | 2.15E+08 | 1.87E+08 | 3.52E+08 | |||
| LFQRPDDAVFR | 4.41E+09 | 4.39E+09 | 2.91E+09 | |||
| DFFPAAK | 3.71E+09 | 2.74E+09 | 2.92E+09 | |||
| Sum | 8.34E+09 | 7.32E+09 | 6.18E+09 | 1.0 | 1.0 | 1.0 |
Detection of PPi-PEPCKs by WB Using Anti-EhPEPCK Antiserum
The anti-EhPEPCK antiserum reacted with recombinant PEPCK1 and -2 with comparable efficiency (Fig. 3B). Major bands of the predicted molecular mass (∼130 kDa) were observed in the lysate of E. histolytica (Fig. 3C), confirming the specificity of this anti-EhPEPCK antiserum. We estimated that EhPEPCKs account for ∼1% of the total protein of the amebic lysate by comparing the band intensity of serially diluted recombinant PEPCKs and amebic lysates in WB analysis (Fig. 3B). This estimation also agrees well with the finding that about 200-fold purification of E. histolytica lysate yields highly purified PPi-PEPCK (Table 1 and Fig. 1A).
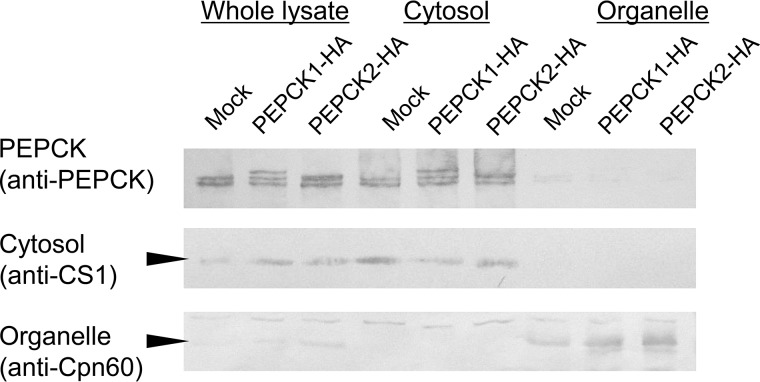
Localization of PPi-PEPCKs in E. histolytica Trophozoites
E. histolytica trophozoites were mechanically disrupted in sucrose-MOPS buffer and centrifuged to separate the cytosol and organelle/membrane fractions. The cytosolic marker, cysteine synthase 1 and the organelle marker, Cpn60, were demonstrated in the corresponding fractions (Fig. 4). When these fractions were reacted with anti-EhPEPCK antiserum, clear bands were detected from the supernatant fraction. Therefore, major parts of both EhPEPCK1 and EhPEPCK2/3 localize in the cytosol (Fig. 4), whereas two isoforms of GTP-PEPCK exist in the cytosol and mitochondria in the case of animals (38). In silico analysis to find organelle targeting signals (TargetP version 1.1 and SignalP 4.1) or transmembrane regions (TMHMM Server version 2.0) did not detect any targeting signals and thus was consistent with the result from the wet experiment described above.
FIGURE 4.
Distribution of E. histolytica PEPCKs by fractionation and WB analysis. Trophozoites of mock, EhPEPCK1-HA-expressing, or EhPEPCK2-HA-expressing transformants were mechanically disrupted and centrifuged to separate into cytosolic and organelle fractions. Those fractions were reacted with anti-EhPEPCK, cysteine synthase 1 (CS1, a cytosolic marker), or Cpn60 (a mitosomal matrix protein, used as an organellar marker) antibodies. Whereas ∼5 μg of lysates were used for anti-PEPCK antibody, 20 μg of lysates were reacted with anti-CS1 and anti-Cpn60 antibodies.
Distribution of PPi-PEPCK in the Tree of Life
Homologs of EhPEPCKs were found in limited lineages of eukaryotes and bacteria but not in archaea (Tables 4 and 5). PPi-PEPCK genes were found in all of the three genomes of Entamoeba species that were currently present in GenBankTM, although the copy numbers are different among the three. The PPi-PEPCK gene was also found in the genome data of a free living amoebozoan, M. balamuthi, whereas the other amoebozoans Acanthamoeba and Dictyostelium lacked the gene. In addition to the two amoebozoan genera bearing PPi-PEPCK, PPi-PEPCK homologs were also present in some eukaryotes, such as excavates, dinoflagellates, diatoms, and cryptophytes, which are distantly related to each other (39). In bacteria, a limited number of species in Actinobacteria, including P. freudenreichii used in this study, Verrucomicrobia, Bacteroidetes, Planctomycetes, Proteobacteria, Firmicutes, and Spirochates, possessed PPi-PEPCK homologs (Table 5); many species were found to lack PPi-PEPCK even if many of them were closely related to PPi-PEPCK-bearing species.
TABLE 4.
Distribution of PPi-PEPCK and functional homologues in 10 represented genomes
One organism from every phylum that has a PPi-PEPCK homolog with the highest identity to EhPEPCK in a BLASTP search was listed. For bacteria, only phyla in which more than two organisms possess a PPi-PEPCK homolog were shown. +, ATP, and GTP, the organism has a protein that shows 35% or higher identity to biochemically analyzed PEP carboxylase (from E. coli, UniProtKB; P00864), ATP-PEPCK (from E. coli, UniProtKB; P22259), or GTP-PEPCK (from G. intestinalis, XP_001709869.1), respectively.
| Organism | Phylum | Identitya | PEPCK ATP/GTP | PEP carboxylase |
|---|---|---|---|---|
| % | ||||
| Eukaryote | ||||
| E. histolytica HM-1:IMSS | Amoebozoa | 100 | ||
| Naegleria gruberi strain ATCC 30224 | Heterolobosea | 45 | ATP | |
| Guillardia theta CCMP2712 | Cryptophyta | 44 | ATP | + |
| Thalassiosira pseudonana CCMP1335 | Heterokontophyta | 44 | ATP | + |
| G. intestinalis strain ATCC 50581 | Metamonada | 39 | GTP | |
| Bacteria | ||||
| Pedosphaera parvula Ellin514 | Verrucomicrobia | 48 | GTP | |
| Planctomyces maris DSM 8797 | Planctomycetes | 46 | ATP | |
| Thioalkalivibrio nitratireducens DSM 14787 | Proteobacteria | 46 | GTP | + |
| Actinomyces graevenitzii C83 | Actinobacteria | 45 | GTPb | |
| Formosa agariphila KMM 3901 | Bacteroidetes | 42 | ATP | +b |
a Identity against EhPEPCK1.
b A protein with significant homology to the query, although the identity is lower than 35%.
TABLE 5.
Distribution of PPi-PEPCK in bacteria
| Phylum | No. of genomesa | No. of PPi-PEPCK-containing genomesa |
|---|---|---|
| Actinobacteria | 910 | 104 |
| Aquificae | 16 | 0 |
| Armatimonadetes | 3 | 0 |
| Bacteroidetes/Chlorobi group | 470 | 19 |
| Caldiserica | 2 | 0 |
| Chlamydiae/Verrucomicrobia group | 59 | 15 |
| Chloroflexi | 25 | 0 |
| Chrysiogenetes | 2 | 0 |
| Cyanobacteria | 104 | 0 |
| Deferribacteres | 6 | 0 |
| Deinococcus-Thermus | 43 | 0 |
| Dictyoglomi | 2 | 0 |
| Elusimicrobia | 2 | 0 |
| Fibrobacteres/Acidobacteria group | 46 | 1 |
| Firmicutes | 1129 | 1 |
| Fusobacteria | 25 | 0 |
| Gemmatimonadetes | 5 | 0 |
| Nitrospinae | 2 | 0 |
| Nitrospirae | 10 | 0 |
| Planctomycetes | 22 | 18 |
| Proteobacteria | 2200 | 33 |
| Spirochaetes | 80 | 2 |
| Synergistetes | 18 | 0 |
| Tenericutes | 140 | 0 |
| Thermodesulfobacteria | 7 | 0 |
| Thermotogae | 24 | 0 |
| Unclassified bacterium | 210 | 1 |
a In GenBankTM, accessed May 5, 2015.
Discussion
In the present study, we successfully identified the gene encoding PPi-PEPCK, which is one of the key enzymes in the central metabolism and connects sugar and organic acids metabolisms, from E. histolytica and P. freudenreichii. We also found that the homologs of EhPEPCK were distributed in limited but diverse lineages of unicellular eukaryotes and bacteria but not in archaea.
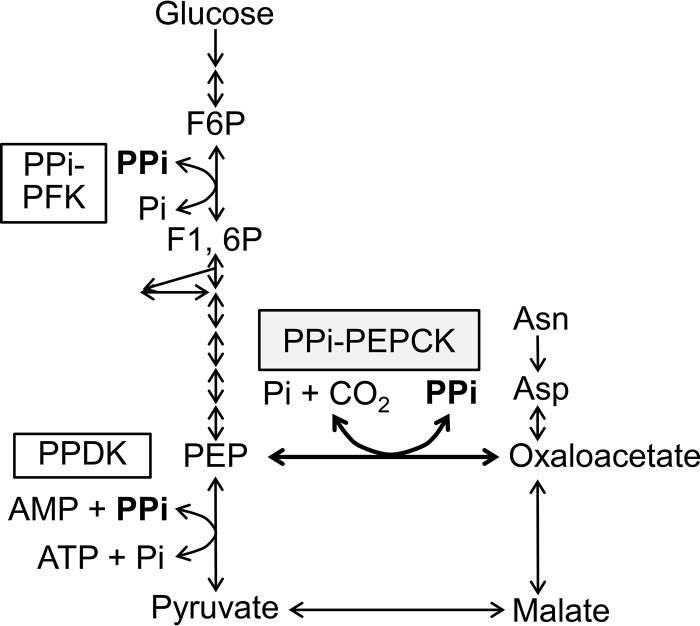
In E. histolytica, which lacks ATP/GTP-PEPCK and PEP carboxylase, PPi-PEPCK has been believed to work in the PPi- and oxaloacetate-producing direction (27, 40). This is because a supply of PPi is required to replenish PPi consumed by the PPi-utilizing enzymes in glycolysis, PPi-PFK and PPDK (4, 12, 41). However, it is uncertain whether PPi produced by PPi-PEPCK is truly essential because PPi is supplied as a by-product of many reactions in vivo (3, 42). Alternatively, it would be possible that PPi-PEPCK works for the PPi-utilizing direction when there is enough supply of PPi because of the following reason. E. histolytica is able to consume asparagine and aspartate from media (43), and these amino acids can be converted to oxaloacetate by aminotransferase (Fig. 5) (44). Although the oxaloacetate catabolic pathway has not been experimentally confirmed in this organism, which lacks the tricarboxylic acid cycle, the amoeba may be capable of producing ATP from these amino acids by using the energy of PPi if oxaloacetate is further converted to PEP by PPi-PEPCK and then pyruvate by PPDK. Future experiments to investigate which direction is critical (or whether both directions are important) for viability in E. histolytica would give us deeper insight into the central metabolic pathway in this human parasite.
FIGURE 5.
Glycolytic pathway and oxaloacetate-malate node of E. histolytica. Arrows, reaction steps and their directions conducted by the enzymes in E. histolytica (46, 47). F6P, fructose 6-phosphate; F1,6P, fructose 1,6-bisphosphate.
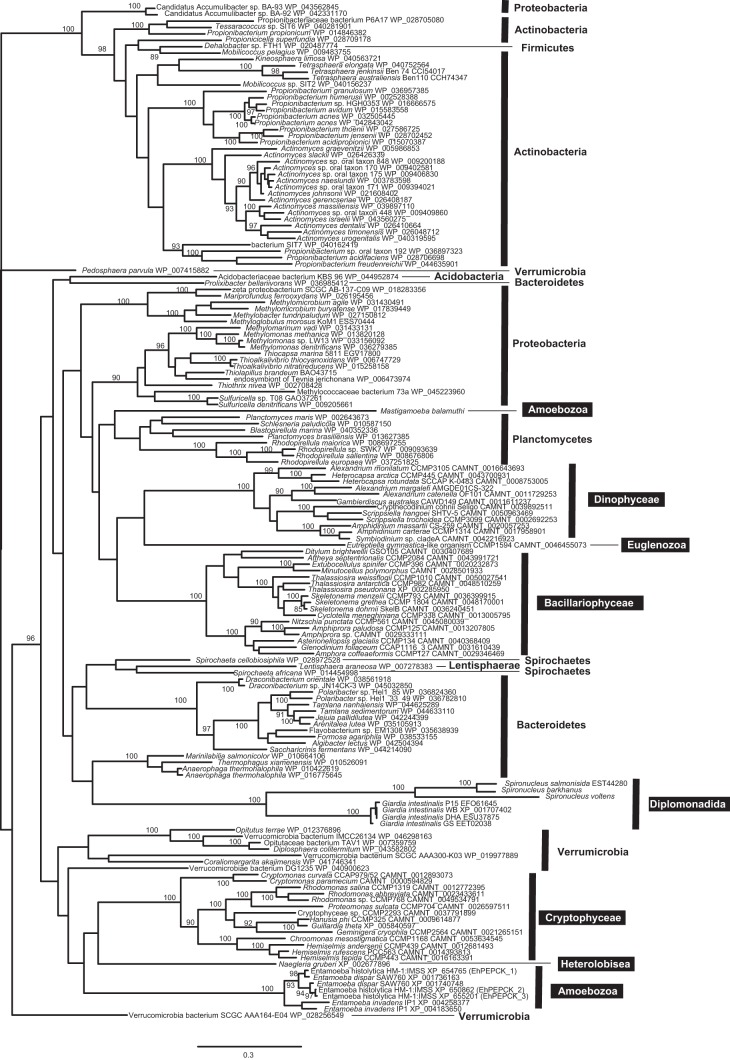
E. histolytica has three isoforms of PPi-PEPCK, which are strongly suggested to form heterotrimers among the isoforms. This may be worthy of mention because the two other species of Entamoeba have two isoforms, and the four strains of the other eukaryotic human gut parasite Giardia intestinalis, for example, possess the single copy of this gene in the genomes (Fig. 6). Thus, it is still a key question whether each isoform of PPi-PEPCK from E. histolytica has different roles in the heterotrimer and whether their roles are, if different, also shared among Entamoeba species. These issues should be examined in the future to consider the roles of PPi-PEPCK in the human parasite E. histolytica.
FIGURE 6.
Phylogenic tree of PPi-PEPCKs and their homologs. Sequences were aligned by MAFFT, and the data set was subjected to maximum likelihood analysis with RAxML version 7.2.8. Only bootstrap supports equal to or more than 85 are shown on each node. White letters in black boxes and black letters indicate eukaryotic and bacterial phyla, respectively.
Most of the organisms possessing PPi-PEPCK homologs have canonical ATP/GTP-PEPCK genes, and some of them have PEP carboxylase as well (Table 4), although E. histolytica possesses the three cytosolic PPi-PEPCKs but lacks the others. How these functional homologs are utilized/regulated in each organism is an open question. They might work in different directions from each other, or they might work in different environmental conditions. Especially, in some eukaryotes, these functional homologs might be utilized in different cellular compartments. For instance, a potential PPi-PEPCK from the unicellular alga Thalassiosira pseudonana (XP_002285950.1) is predicted to be cytosolic according to the in silico predictions (data not shown) as well as those of E. histolytica, whereas its ATP-PEPCK was confirmed to exist in mitochondria, and two types of PEP carboxylase localize in mitochondria and the chloroplast, respectively (45). Although the localization of ATP-PEPCK and PEP carboxylase indicates that PEP-oxaloacetate conversion occurs only in organelles of the diatom, our findings suggest that this reaction important for the central carbon metabolism can also occur in the cytosol of T. pseudonana. Our findings in this study could further open a new era for deeper understanding the central metabolism of various organisms.
Identification of PPi-PEPCK also allows us to compare the amino acid sequences between PPi-PEPCK and the functional homologs, ATP/GTP-PEPCK and PEP carboxylase. PPi-PEPCK bears none of the known catalytic domains, such as oxaloacetate binding sites, kinase 1a/P-loop, kinase-2 region, and nucleotide binding sites conserved in ATP- and GTP-PEPCK (22). In addition, no similarity in primary structure was observed between EhPEPCK and the functional homologs. Therefore, it is unlikely that PPi-PEPCK shares a common origin with ATP- and GTP-PEPCK/PEP carboxylase. This is similar to the case between pyruvate kinase and PPDK/PEP synthase but different from the cases of ATP-PFK/PPi-PFK and PPDK/PEP synthase that have most probably emerged from single origins. Our findings further illuminate the complexity of evolution among the proteins, which are functionally redundant but utilize different phosphate donors (e.g. PPi and nucleotide triphosphates).
The origins and evolutionary histories of PPi-PEPCKs were one of the most interesting aspects for us. However, it was very difficult to reconstruct a rooted tree of PPi-PEPCK because we could not find any proteins with sister relationships to PPi-PEPCK. In addition, whether the current distribution of PPi-PEPCK in the tree of life has been shaped by lateral gene transfer or by vertical inheritance followed by independent gene loss basically remains unclear because of the low resolution of the PPi-PEPCK tree (Fig. 6) with one exception for PPi-PEPCK in Dehalobacter sp.; the Dehalobacter homologue was nested in the clade of Actinobacteria, although Dehalobacter belongs to Firmicutes, strongly suggesting actinobacterium-to-Dehalobacter lateral gene transfer.
In summary, PPi-PEPCK was identified at the molecular level for the first time since the activity was first demonstrated more than 50 years ago (24). It was also demonstrated that PPi-PEPCK was distributed in phylogenetically diverse but limited unicellular eukaryotes and bacteria, which would remodel the central metabolic pathways in various organisms. PPi-PEPCK has no clear evolutionary relationship with ATP- or GTP-PEPCK, in sharp contrast to the case of PPi- and ATP-PFK and that of PPDK and PEP synthase, which have evolved from single origins. These data suggest that, in order to substitute the energy and phosphate donors from PPi to nucleotide triphosphates, proteins have employed various strategies.
Author Contributions
Y. C. and T. N. designed this study. Y. C. performed biochemical analysis. K. N.-T. and Y. S.-N. designed molecular experiments. R. K. and Y. C. performed the phylogenetic analysis. Y. C., R. K., and T. N. wrote the manuscript. All of the authors reviewed the results and approved the final version of the manuscript.
Acknowledgment
We thank Dr. Nicholas E. Sherman (W. M. Keck Biomedical Mass Spectrometry Laboratory, University of Virginia, Charlottesville, VA) for mass spectrometric analysis.
This work was supported in part by Grants-in-Aid for Scientific Research on Innovative Areas 23117001 and 23117005 and Grants-in-Aid for Scientific Research (B) 26293093 from the Ministry of Education, Culture, Sports, Science, and Technology, the Research Program on Emerging and Re-emerging Infectious Diseases of the Japan Agency for Medical Research and Development (AMED), the Science and Technology Research Partnership for Sustainable Development (SATREPS), and the Japan International Cooperation Agency (JICA) and AMED (to T. N.); Grant-in-Aid for Young Scientists (B) 26860275 from the Japan Society for the Promotion of Science (JSPS) (to Y. C.); and Grant-in-Aid for Scientific Research on Innovative Areas 23117006, for Young Scientists (A) 15H05606, and for Challenging Exploratory Research 15K14591 from JSPS (to R. K.). The authors declare that they have no conflicts of interest with the contents of this article.
- PFK
- phosphofructokinase
- PEP
- phosphoenolpyruvate
- PPDK
- pyruvate phosphate dikinase
- PEPCK
- phosphoenolpyruvate carboxylase
- Ni-NTA
- nickel-nitrilotriacetic acid
- WB
- Western blot.
References
- 1. Liu C. L., Hart N., Peck H. D. (1982) Inorganic pyrophosphate: energy source for sulfate-reducing bacteria of the genus Desulfotomaculum. Science 217, 363–364 [DOI] [PubMed] [Google Scholar]
- 2. Gest H. (1972) Energy conversion and generation of reducing power in bacterial photosynthesis. Adv. Microb. Physiol. 7, 243–282 [Google Scholar]
- 3. Baykov A. A., Malinen A. M., Luoto H. H., Lahti R. (2013) Pyrophosphate-fueled Na+ and H+ transport in prokaryotes. Microbiol. Mol. Biol. Rev. 77, 267–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mertens E. (1991) Pyrophosphate-dependent phosphofructokinase, an anaerobic glycolytic enzyme? FEBS Lett. 285, 1–5 [DOI] [PubMed] [Google Scholar]
- 5. Alves A. M., Meijer W. G., Vrijbloed J. W., Dijkhuizen L. (1996) Characterization and phylogeny of the pfp gene of Amycolatopsis methanolica encoding PPi-dependent phosphofructokinase. J. Bacteriol. 178, 149–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Siebers B., Klenk H. P., Hensel R. (1998) PPi-dependent phosphofructokinase from Thermoproteus tenax, an archaeal descendant of an ancient line in phosphofructokinase evolution. J. Bacteriol. 180, 2137–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mertens E., Ladror U. S., Lee J. A., Miretsky A., Morris A., Rozario C., Kemp R. G., Müller M. (1998) The pyrophosphate-dependent phosphofructokinase of the protist, Trichomonas vaginalis, and the evolutionary relationships of protist phosphofructokinases. J. Mol. Evol. 47, 739–750 [DOI] [PubMed] [Google Scholar]
- 8. Chi A., Kemp R. G. (2000) The primordial high energy compound: ATP or inorganic pyrophosphate? J. Biol. Chem. 275, 35677–35679 [DOI] [PubMed] [Google Scholar]
- 9. Müller M., Lee J. A., Gordon P., Gaasterland T., Sensen C. W. (2001) Presence of prokaryotic and eukaryotic species in all subgroups of the PPi-dependent group II phosphofructokinase protein family. J. Bacteriol. 183, 6714–6716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bapteste E., Moreira D., Philippe H. (2003) Rampant horizontal gene transfer and phospho-donor change in the evolution of the phosphofructokinase. Gene 318, 185–191 [DOI] [PubMed] [Google Scholar]
- 11. Pocalyko D. J., Carroll L. J., Martin B. M., Babbitt P. C., Dunaway-Mariano D. (1990) Analysis of sequence homologies in plant and bacterial pyruvate phosphate dikinase, enzyme I of the bacterial phospoenolpyruvate: sugar phosphotransferase system and other PEP-utilizing enzymes: identification of potential catalytic and regulatory motifs. Biochemistry 29, 10757–10765 [DOI] [PubMed] [Google Scholar]
- 12. Reeves R. E., South D. J., Blytt H. J., Warren L. G. (1974) Pyrophosphate:d-fructose 6-phosphate 1-phosphotransferase: a new enzyme with the glycolytic function of 6-phosphofructokinase. J. Biol. Chem. 249, 7737–7741 [PubMed] [Google Scholar]
- 13. O'Brien W. E., Bowien S., Wood H. G. (1975) Isolation and characterization of a pyrophosphate-dependent phosphofructokinase from Propionibacterium shermanii. J. Biol. Chem. 250, 8690–8695 [PubMed] [Google Scholar]
- 14. van der Merwe M. J., Groenewald J.-H., Stitt M., Kossmann J., Botha F. C. (2010) Downregulation of pyrophosphate:d-fructose-6-phosphate 1-phosphotransferase activity in sugarcane culms enhances sucrose accumulation due to elevated hexose-phosphate levels. Planta 231, 595–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Michels P. A., Chevalier N., Opperdoes F. R., Rider M. H., Rigden D. J. (1997) The glycosomal ATP-dependent phosphofructokinase of Trypanosoma brucei must have evolved from an ancestral pyrophosphate-dependent enzyme. Eur. J. Biochem. 250, 698–704 [DOI] [PubMed] [Google Scholar]
- 16. Niersbach M., Kreuzaler F., Geerse R. H., Postma P. W., Hirsch H. J. (1992) Cloning and nucleotide sequence of the Escherichia coli K-12 ppsA gene, encoding PEP synthase. Mol. Gen. Genet. 231, 332–336 [DOI] [PubMed] [Google Scholar]
- 17. Davies D. (1979) The central role of phosphoenolpyruvate in plant metabolism. Annu. Rev. Plant Physiol. 30, 131–158 [Google Scholar]
- 18. Sauer U., Eikmanns B. J. (2005) The PEP-pyruvate-oxaloacetate node as the switch point for carbon flux distribution in bacteria. FEMS Microbiol. Rev. 29, 765–794 [DOI] [PubMed] [Google Scholar]
- 19. Siu P. M., Wood H. G. (1962) Phosphoenolpyruvic carboxytransphosphorylase, a CO2 fixation enzyme from propionic acid bacteria. J. Biol. Chem. 237, 3044–3051 [PubMed] [Google Scholar]
- 20. Bandurski R. S., Greiner C. M. (1953) The enzymatic synthesis of oxaloacetate from phosphoryl-enolpyruvate and dioxide. J. Biol. Chem. 204, 781–786 [PubMed] [Google Scholar]
- 21. Fukuda W., Fukui T., Atomi H., Imanaka T. (2004) First characterization of an archaeal GTP-dependent phosphoenolpyruvate carboxykinase from the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1. J. Bacteriol. 186, 4620–4627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aich S., Delbaere L. T. (2007) Phylogenetic study of the evolution of PEP-carboxykinase. Evol. Bioinform. Online 3, 333–340 [PMC free article] [PubMed] [Google Scholar]
- 23. Matte A., Tari L. W., Goldie H., Delbaere L. T. (1997) Structure and mechanism of phosphoenolpyruvate carboxykinase. J. Biol. Chem. 272, 8105–8108 [DOI] [PubMed] [Google Scholar]
- 24. Siu P. M., Wood H. G., Stjernholm R. L. (1961) Fixation of CO2 by phosphoenolpyruvic carboxytransphosphorylase. J. Biol. Chem. 236, PC21–PC22 [PubMed] [Google Scholar]
- 25. Lochmüller H., Wood H. G., Davis J. J. (1966) Phosphoenolpyruvate carboxytransphosphorylase II: crystallization and properties. J. Biol. Chem. 241, 5678–5691 [PubMed] [Google Scholar]
- 26. Haberland M. E., Willard J. M., Wood H. G. (1972) Phosphoenolpyruvate carboxytransphosphorylase: study of the catalytic and physical structures. Biochemistry 11, 712–722 [DOI] [PubMed] [Google Scholar]
- 27. Reeves R. E. (1970) Phosphopyruvate carboxylase from Entamoeba histolytica. Biochim. Biophys. Acta 220, 346–349 [DOI] [PubMed] [Google Scholar]
- 28. Diamond L. S., Harlow D. R., Cunnick C. C. (1978) A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans. R. Soc. Trop. Med. Hyg. 72, 431–432 [DOI] [PubMed] [Google Scholar]
- 29. Marumo K., Nakada-Tsukui K., Tomii K., Nozaki T. (2014) Ligand heterogeneity of the cysteine protease binding protein family in the parasitic protist Entamoeba histolytica. Int. J. Parasitol. 44, 625–635 [DOI] [PubMed] [Google Scholar]
- 30. Nakada-Tsukui K., Okada H., Mitra B. N., Nozaki T. (2009) Phosphatidylinositol-phosphates mediate cytoskeletal reorganization during phagocytosis via a unique modular protein consisting of RhoGEF/DH and FYVE domains in the parasitic protozoon Entamoeba histolytica. Cell. Microbiol. 11, 1471–1491 [DOI] [PubMed] [Google Scholar]
- 31. Nozaki T., Asai T., Sanchez L. B., Kobayashi S., Nakazawa M., Takeuchi T. (1999) Characterization of the gene encoding serine acetyltransferase, a regulated enzyme of cysteine biosynthesis from the protist parasites Entamoeba histolytica and Entamoeba dispar: regulation and possible function of the cysteine biosynthetic pathway in Entamoeba. J. Biol. Chem. 274, 32445–32452 [DOI] [PubMed] [Google Scholar]
- 32. Nozaki T., Asai T., Kobayashi S., Ikegami F., Noji M., Saito K., Takeuchi T. (1998) Molecular cloning and characterization of the genes encoding two isoforms of cysteine synthase in the enteric protozoan parasite Entamoeba histolytica. Mol. Biochem. Parasitol. 97, 33–44 [DOI] [PubMed] [Google Scholar]
- 33. Mi-ichi F., Abu Yousuf M., Nakada-Tsukui K., Nozaki T. (2009) Mitosomes in Entamoeba histolytica contain a sulfate activation pathway. Proc. Natl. Acad. Sci. U.S.A. 106, 21731–21736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Keeling P. J., Burki F., Wilcox H. M., Allam B., Allen E. E., Amaral-Zettler L. A., Armbrust E. V., Archibald J. M., Bharti A. K., Bell C. J., Beszteri B., Bidle K. D., Cameron C. T., Campbell L., Caron D. A., Cattolico R. A., Collier J. L., Coyne K., Davy S. K., Deschamps P., Dyhrman S. T., Edvardsen B., Gates R. D., Gobler C. J., Greenwood S. J., Guida S. M., Jacobi J. L., Jakobsen K. S., James E. R., Jenkins B., John U., Johnson M. D., Juhl A. R., Kamp A., Katz L. A., Kiene R., Kudryavtsev A., Leander B. S., Lin S., Lovejoy C., Lynn D., Marchetti A., McManus G., Nedelcu A. M., Menden-Deuer S., Miceli C., Mock T., Montresor M., Moran M. A., Murray S., Nadathur G., Nagai S., Ngam P. B., Palenik B., Pawlowski J., Petroni G., Piganeau G., Posewitz M. C., Rengefors K., Romano G., Rumpho M. E., Rynearson T., Schilling K. B., Schroeder D. C., Simpson A. G., Slamovits C. H., Smith D. R., Smith G. J., Smith S. R., Sosik H. M., Stief P., Theriot E., Twary S. N., Umale P. E., Vaulot D., Wawrik B., Wheeler G. L., Wilson W. H., Xu Y., Zingone A., Worden A. Z. (2014) The marine microbial eukaryote transcriptome sequencing project (MMETSP): illuminating the functional diversity of eukaryotic life in the oceans through transcriptome sequencing. PLoS Biol. 12, e1001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Katoh K., Misawa K., Kuma K., Miyata T. (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30, 3059–3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stamatakis A. (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690 [DOI] [PubMed] [Google Scholar]
- 37. Medina V., Pontarollo R., Glaeske D., Tabel H., Goldie H. (1990) Sequence of the pckA gene of Escherichia coli K-12: relevance to genetic and allosteric regulation and homology of E. coli phosphoenolpyruvate carboxykinase with the enzymes from Trypanosoma brucei and Saccharomyces cerevisiae. J. Bacteriol. 172, 7151–7156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Croniger C. M., Olswang Y., Reshef L., Kalhan S. C., Tilghman S. M., Hanson R. W. (2002) Phosphoenolpyruvate carboxykinase revisited: insights into its metabolic role. Biochem. Mol. Biol. Educ. 30, 14–20 [Google Scholar]
- 39. Adl S. M., Simpson A. G., Lane C. E., Lukeš J., Bass D., Bowser S. S., Brown M. W., Burki F., Dunthorn M., Hampl V., Heiss A., Hoppenrath M., Lara E., Le Gall L., Lynn D. H., McManus H., Mitchell E. A., Mozley-Stanridge S. E., Parfrey L. W., Pawlowski J., Rueckert S., Shadwick R. S., Shadwick L., Schoch C. L., Smirnov A., Spiegel F. W. (2012) The revised classification of eukaryotes. J. Eukaryot. Microbiol. 59, 429–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Müller M., Mentel M., van Hellemond J. J., Henze K., Woehle C., Gould S. B., Yu R.-Y., van der Giezen M., Tielens A. G., Martin W. F. (2012) Biochemistry and evolution of anaerobic energy metabolism in eukaryotes. Microbiol. Mol. Biol. Rev. 76, 444–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Reeves R. E. (1968) A new enzyme with the glycolytic function of pyruvate kinase. J. Biol. Chem. 243, 3202–3204 [PubMed] [Google Scholar]
- 42. Kulaev I. S., Vagabov V. M. (1983) Polyphosphate metabolism in microorganisms. Adv. Microb. Physiol. 24, 83–171 [DOI] [PubMed] [Google Scholar]
- 43. Zuo X., Coombs G. H. (1995) Amino acid consumption by the parasitic, amoeboid protists Entamoeba histolytica and E. invadens. FEMS Microbiol. Lett. 130, 253–258 [DOI] [PubMed] [Google Scholar]
- 44. Clark C. G., Alsmark U. C., Tazreiter M., Saito-Nakano Y., Ali V., Marion S., Weber C., Mukherjee C., Bruchhaus I., Tannich E., Leippe M., Sicheritz-Ponten T., Foster P. G., Samuelson J., Noël C. J., Hirt R. P., Embley T. M., Gilchrist C. A., Mann B. J., Singh U., Ackers J. P., Bhattacharya S., Bhattacharya A., Lohia A., Guillén N., Duchêne M., Nozaki T., Hall N. (2007) Structure and content of the Entamoeba histolytica genome. Adv. Parasitol. 65, 51–190 [DOI] [PubMed] [Google Scholar]
- 45. Tanaka R., Kikutani S., Mahardika A., Matsuda Y. (2014) Localization of enzymes relating to C4 organic acid metabolisms in the marine diatom, Thalassiosira pseudonana. Photosynth. Res. 121, 251–263 [DOI] [PubMed] [Google Scholar]
- 46. Pineda E., Encalada R., Vázquez C., González Z., Moreno-Sánchez R., Saavedra E. (2015) in Amebiasis: Biology and Pathogenesis of Entamoeba (Nozaki T., Bhattacharya A., eds) pp. 351–372, Springer, Berlin [Google Scholar]
- 47. Jeelani G., Nozaki T. (2014) Metabolomic analysis of Entamoeba: applications and implications. Curr. Opin. Microbiol 20, 118–124 [DOI] [PubMed] [Google Scholar]