Background: Little is known about how mammalian cells modulate retrograde sterol transport for cellular cholesterol homeostasis.
Results: ABCA1 deficiency impairs endocytic retrograde sterol transport to the endoplasmic reticulum and activates the SREBP-2 pathway.
Conclusion: ABCA1 participates in bidirectional sterol movement at the plasma membrane to regulate cellular cholesterol homeostasis.
Significance: A novel function of ABCA1 is identified.
Keywords: ABC transporter, cholesterol, cholesterol regulation, endocytosis, lipid trafficking, lipid transport, abca1
Abstract
Cellular cholesterol homeostasis involves sterol sensing at the endoplasmic reticulum (ER) and sterol export from the plasma membrane (PM). Sterol sensing at the ER requires efficient sterol delivery from the PM; however, the macromolecules that facilitate retrograde sterol transport at the PM have not been identified. ATP-binding cassette transporter A1 (ABCA1) mediates cholesterol and phospholipid export to apolipoprotein A-I for the assembly of high density lipoprotein (HDL). Mutations in ABCA1 cause Tangier disease, a familial HDL deficiency. Several lines of clinical and experimental evidence suggest a second function of ABCA1 in cellular cholesterol homeostasis in addition to mediating cholesterol efflux. Here, we report the unexpected finding that ABCA1 also plays a key role in facilitating retrograde sterol transport from the PM to the ER for sterol sensing. Deficiency in ABCA1 delays sterol esterification at the ER and activates the SREBP-2 cleavage pathway. The intrinsic ATPase activity in ABCA1 is required to facilitate retrograde sterol transport. ABCA1 deficiency causes alternation of PM composition and hampers a clathrin-independent endocytic activity that is required for ER sterol sensing. Our finding identifies ABCA1 as a key macromolecule facilitating bidirectional sterol movement at the PM and shows that ABCA1 controls retrograde sterol transport by modulating a certain clathrin-independent endocytic process.
Introduction
Cellular cholesterol homeostasis is maintained by the sterol-sensing system in the endoplasmic reticulum (ER)5 for its biosynthesis and uptake and by the sterol export system in the plasma membrane (PM) (1–3). Most cellular cholesterol is located in the PM, which forms a functional regulatory network with other intracellular compartments, particularly the ER (4). Upon cholesterol supply, ER cholesterol down-regulates sterol regulatory element-binding protein-2 (SREBP-2) through binding to the SREBP cleavage-activating protein (Scap) to suppress cholesterol synthesis and low density lipoprotein (LDL) receptor (LDLR) expression (1). The sterol removal is regulated by cholesterol release from the PM mainly to high density lipoprotein (HDL). It involves the ATP-binding cassette transporters, ABCA1 and ABCG1 (3); ABCA1 plays a predominant role in cholesterol efflux (5). It mediates formation of new HDL particles at the PM with cellular cholesterol, phospholipid (PL), and α-helical apolipoproteins such as apolipoprotein A-I (apoA-I) (6, 7). Excessive PM cholesterol is also esterified in the ER by acyl-CoA:cholesterol acyltransferase 1 (ACAT1) for storage (2). Retrograde sterol transport from the PM to the ER is thus an important part of cellular cholesterol homeostasis; aside from its independence from Niemann-Pick C proteins (8, 9), the mechanisms of this process are largely unknown. Specifically, the macromolecules located at the PM that modulate retrograde sterol transport have not been identified.
In whole body cholesterol homeostasis, sterol must be transported from peripheral cells to the liver for conversion to bile acids, and HDL plays a central role in this “reverse cholesterol transport.” This is perhaps why plasma HDL levels inversely correlate with the incidence of cardiovascular disease. Loss-of-function mutations in ABCA1 cause HDL deficiency known as Tangier disease (TD) (3, 6). ABCA1 deficiency in humans or animals exhibits a risk for premature atherosclerosis. TD patients display cholesterol deposition in various tissues, despite reduced plasma LDL levels (10). In Abca1−/− mice, free cholesterol accumulates in macrophages (11). In addition, deletion of hepatic Abca1 in mice resulted in increases of LDLR expression and of LDL clearance in the liver (12). In a mouse model of hypoxic advanced atherosclerotic plaques, ABCA1 expression and cell cholesterol release are decreased, but HMG-CoA reductase (HMGR) expression and sterol synthesis are increased, resulting in the accumulation of free sterol in macrophages (13). These puzzling observations cannot be satisfactorily explained by the assumption that ABCA1 functions only in cellular sterol release, suggesting that ABCA1 may have additional functions.
ABCA1 is expressed in various tissues and cell types, including hepatocytes, macrophages, and fibroblasts. Since defective apoA-I-mediated cholesterol efflux was first described in fibroblasts isolated from TD patients (14), fibroblasts are widely accepted as a model system to study ABCA1-dependent HDL formation. In this study, we examine cellular sterol trafficking and ER sterol sensing in Abca1+/+ and Abca1−/− mouse embryonic fibroblasts (MEFs) and other mammalian cell models. We report an undisclosed, novel function of ABCA1 in regulation of cellular cholesterol homeostasis.
Experimental Procedures
Materials
ApoA-I was prepared as described previously (15, 16). [3H]Acetic acid was from American Radiochemicals. [1,2-3H]Cholesterol and [14C]oleate were from PerkinElmer Life Sciences. [3H]Cholesteryl oleate was from GE Healthcare. [3H]Cholesteryl oleate-containing LDL was prepared as described (17). [3H]Lanosterol was prepared and purified as described (18). Dynasore was from Cayman Chemical. Dynole 34-2 and dynole 31-2 were from Abcam. Other chemical compounds were described previously (8).
Cell Culture
MEFs from Abca1+/+ and Abca1−/− mice were isolated as described previously (19). MEFs isolated were expanded and stored as stocks at an early passage stage. MEFs less than 15 passages were used in experiments reported here. Wild-type (WT) and 25RA CHO cells were employed as described previously (16). A mouse fibroblast cell line, BALB/3T3, was obtained from Riken Cell Bank. Baby hamster kidney (BHK) cells harboring WT-ABCA1 expression plasmid or empty plasmid were the kind gift of Dr. Oram (20). BHK cells harboring ABCA1-MM were generated using the mifepristone-inducible GeneSwitch system (Invitrogen) as described previously (20). Human ABCA1-MM cDNA was inserted into pGene/V5-HisA(blasticidin), in which the original Zeocin resistance gene was replaced by the blasticidin resistance gene. BHK cells harboring pSwitch plasmid (20) were transfected with pGene/V5-HisA(blasticidin)/ABCA1-MM; stable transfects were selected with hygromycin (350 μg/ml) and blasticidin (5 μg/ml). After the selection, a clone in which ABCA1-MM expression was highly induced by mifepristone was isolated. Mouse and hamster cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and in DMEM/F-12 (DF) supplemented with 7.5% FBS, respectively. All cells were grown in humidified CO2 incubators at 37 °C. Media used were designated as follows: medium A contains 10 or 7.5% FBS as described above; medium B contains 0.1% BSA; medium D contains 5% delipidated FBS; medium F contains no supplements. Incubation of MEFs in medium F for up to 24 h did not cause significant cell death, as demonstrated by flow cytometric measurement of the incorporation of 7-amino-actinomycin D (BD Biosciences) into cells (viability ≥97%).
Intracellular Sterol Transport Assays
Five different assays were employed to study retrograde transport of sterol from PM. Cells were seeded in 6-well plates at 2.5 × 105 cells/well for MEFs or at 2.0 × 105 cells/well for CHO and BHK cells and were treated as indicated in the figure legends before labeling. First, cells were pulse-labeled with [3H]acetate for 1 h at 37 °C and then chased in medium F for various times as indicated to monitor the conversion of precursor sterols to cholesterol by a method employed previously (8). Second, the conversion of [3H]lanosterol to [3H]cholesterol was monitored. Cells were pulse-labeled with 0.1 μCi/ml [3H]lanosterol (added to medium B as 0.1% ethanolic solution) in medium B for 30 min at 37 °C, followed by washing cells with medium B twice and chasing in medium F for various times as indicated. Sterols were extracted, saponified, and analyzed by TLC as above. Third, to monitor retrograde movement of cholesterol, the conversion of [3H]cholesterol to [3H]cholesteryl ester was monitored; cells were pulse-labeled with 0.1 μCi/ml [3H]cholesterol (added to medium B as 0.1% ethanolic solution without adding unlabeled cholesterol as carrier) in medium B for 30 min at 37 °C, followed by chasing as above. Alternatively, cells were incubated with [3H]cholesterol for 8 h in medium A and further incubated for 16 h in medium A in the absence of [3H]cholesterol to reach cellular [3H]cholesterol distribution at the steady-state level. Cellular lipids were separated by TLC; radioactivities in cholesterol and cholesteryl oleate fractions were counted. In certain experiments as indicated, agents were included in medium during the chase period. When using mifepristone-induced ABCA1 expression system, cells were either treated or untreated with mifepristone and then used to perform the pulse/chase experiments; counts in [3H]cholesteryl ester formed in untreated cells were subtracted from those formed in mifepristone-treated cells, to calculate ABCA1-dependent esterification. Fourth, ACAT1 activity in intact cells was monitored by feeding cells with [14C]oleate for 3 h and determined the incorporation of [14C]oleate into CE according to procedures described previously (16). Fifth, intracellular transport of LDL-derived cholesterol (LDL-Chol) was monitored by using [3H]cholesteryl oleate-containing LDL; cells were incubated with [3H]cholesteryl oleate-containing LDL for 4 h at 18 °C and chased in medium D at 37 °C for various times. After the chase period, cells were treated with 4% methyl-β-cyclodextrin (MCD) for 10 min at 4 °C to determine PM-associated [3H]cholesterol. LDL uptake was determined by measuring the sum of 3H counts present in cells and in medium.
Antibodies and Immunoblot
Rabbit anti-human ABCA1 antiserum was described previously (15). The anti-ACAT1 rabbit polyclonal antibodies (DM102) were as described previously (21). Hybridoma cells producing anti-HMG-CoA reductase (IgG-A9), anti-SREBP-1 (IgG-2A4), or anti-SREBP-2 (IgG-7D4) antibodies were obtained from ATCC, and supernatants of hybridoma cell cultured media were used for immunoblotting. Other antibodies were obtained from commercial sources as follows: anti-caveolin-1 polyclonal antibodies (N-20) and anti-Insig-1 polyclonal antibodies from Santa Cruz Biotechnology; monoclonal antibodies to flotillin-1 and calnexin from BD Biosciences; anti-SREBP-2 polyclonal antibodies from Cayman Chemical; anti-β-actin monoclonal antibody (AC74) from Sigma; anti-transferrin receptor monoclonal antibody from Zymed Laboratories Inc.; and anti-p70 S6 kinase (numbers 2708 and 9206) and anti-4E-BP1 (numbers 9644 and 2855) monoclonal antibodies from Cell Signaling Technology. Whole cell lysate was prepared by lysing cells with urea buffer (8 m urea, 50 mm sodium phosphate, pH 8.0, 10 mm Tris-HCl, pH 8.0, 100 mm NaCl, 0.2% protease inhibitor mixture (Sigma)) as described previously (22). To detect mouse SREBP-2, polyclonal anti-SREBP-2 antibodies from Cayman Chemical were employed. Protein concentration was determined by BCA protein assay (Pierce). Equal amounts of proteins were subjected to SDS-PAGE and immunoblot analysis. Expression of a protein was analyzed by using ImageJ software and was normalized to a loading control.
Cell Fractionation
Cells grown in 100-mm dishes were treated with 1% Triton X-100 (Sigma) in TNE buffer (25 mm Tris-HCl, pH 7.5, 150 mm NaCl, 5 mm EDTA) containing protease inhibitor mixture (Sigma) for 30 min at 4 °C. Afterward, cells were homogenized with a stainless homogenizer for 10 strokes. Post-nuclear supernatant was then subjected to density gradient centrifugation as described (22). Eight 0.5-ml fractions were collected from the top. Lipids were extracted from equal amounts of each fraction by chloroform/methanol (2:1, v/v).
Small Interfering RNA (siRNA)
Small interfering RNA experiments were performed as described previously (23). siRNA duplexes specific for mouse Abca1 (5′-AUUUCUUCCUGUCAGAUUCUGAAGG-3′) or control (5′-UAGUGAAGACAGUCACUCGGGAAGC-3′) were obtained from Invitrogen and transfected into BALB/3T3 mouse fibroblast cells using Lipofectamine 2000 (Invitrogen). Seventy two hours after transfection, cells were subjected to further analyses.
RNA Isolation, mRNA Expression Analysis, and Luciferase Reporter Assay
Total RNA was isolated with TRIzol reagent (Invitrogen). mRNA levels of various genes were determined by quantitative real time PCR (qRT-PCR) and quantified by using the ΔΔCT method; Hprt expression was used as an internal control as described previously (24). SRE promoter activity was assessed by a luciferase reporter assay. The 4×SRE tandem repeat region was amplified by the Hmgcs-specific primers using DNA isolated from mouse liver. The resultant fragment was inserted into a pGL4 basic vector (Promega). The sequence was verified. On day 0, cells were plated into 24-well plates and grown for 24 h in medium A. On day 1, the reporter luciferase plasmid (1 μg) and the phRL-TK (Promega) encoding Renilla luciferase (for normalization; 30 ng) were transfected with the indicated siRNA by using Lipofectamine 2000 (Invitrogen). Cells were then incubated in medium D for 2 days. On day 3, the cells were lysed, and the luciferase activity was measured by the Dual-Luciferase Reporter Assay System (Promega).
Lipid Analyses and Visualization
To estimate cell surface cholesterol levels, cells were incubated either with 4% 2-hydroxypropyl-β-cyclodextrin for 10 min at 37 °C (25) or with 3 mm MCD for 5 min at 37 °C (26). Afterward, cholesterol contents in medium were determined as described (16). Alternatively, cell surface cholesterol in living cells was visualized by using mCherry-fused domain 4 of perfringolysin O (also known as θ-toxin) (27) (mCherry-D4), which specifically binds to the cholesterol-rich membrane domain. mCherry-D4 was prepared as described (27) except enhanced GFP was replaced by mCherry. Cells cultured on glass-bottom dishes in medium A for 2 days were washed with phenol red-free DF. Cells were then incubated with mCherry-D4 (16 μg/ml) in phenol red-free DF at room temperature for 10 min. After several washes with phenol red-free DF, cell were further incubated in phenol red-free DF at room temperature. Cell images were acquired within 15 min without fixation by using a Zeiss LSM 700 confocal microscope equipped with PLAN-NEOFLUAR ×20 (0.5 NA) objective (Zeiss). Images were then processed with LSM 700 software Zen (Zeiss) and ImageJ software. Cell surface GM1 levels were examined by flow cytometry. Cells were detached by trypsin and incubated with 1 μg/ml biotin-conjugated cholera toxin B subunit (biotin-CTxB) (List Biological Laboratories) for 60 min at 4 °C. After washing cells with ice-cold PBS, they were incubated with FITC-conjugated avidin for 60 min and washed extensively, followed by fluorescence measurement using a FACSCalibur (BD Biosciences). Gated 10,000 cells were analyzed for each sample, and mean fluorescence intensity was determined. Total cholesterol, free cholesterol, and choline-PLs were measured by colorimetric enzymatic assay systems as described (16). Cholesteryl ester (CE) was determined by subtracting free cholesterol from total cholesterol.
Immunofluorescence Microscopy
Cells were seeded on glass coverslips in 6-well plates and grown for 2 days in medium A. After incubation of the cells in medium F overnight, they were fixed with 4% paraformaldehyde for 10 min and blocked with 5% FBS in PBS for 1 h. Specimens were incubated with biotin-conjugated CTxB (1 μg/ml) and anti-caveolin-1 antibody or with biotin-conjugated CTxB (1 μg/ml) and anti-flotillin-1 antibody for 1 h at room temperature. Specimens were then stained with FITC-conjugated avidin (for biotin-CTxB detection) and Alexa Fluor 555-conjugated anti-rabbit IgG antibody (for caveolin-1) or with Alexa Fluor 555-conjugated anti-mouse IgG antibody (for flotillin-1), respectively. The nuclei were stained with Hoechst 33342. After extensive washes, they were mounted with ProLong Gold Antifade Reagent (Invitrogen). Cell images were acquired by using a confocal laser microscopy FV500 (Olympus) or FV10i (Olympus). Images were processed by a Fluoview software (Olympus).
CTxB Internalization
To analyze internalization of CTxB, cells were seeded into glass-bottom 35-mm dishes at a density of 1 × 105 cells/well and grown for 2–3 days. The cells were then washed twice with ice-cold medium F and incubated with 2 μg/ml Alexa Fluor 555-CTxB (Molecular Probe) either at 4 or 37 °C for the indicated times. The cells incubated at 37 °C were washed three times for 30 s with 0.5 m glycine, pH 2.2, to remove cell surface Alexa Fluor 555-CTxB (28). Cells were then fixed with 4% paraformaldehyde, and nuclei were stained with Hoechst 33342. After extensive washing with PBS, images were acquired by using a confocal laser microscope FV10i (Olympus). Cellular mean fluorescence intensity was analyzed by MetaMorph software (Molecular Devices).
Electron Microscopy
Cells were seeded on glass coverslips and grown for 1 day in medium A. After incubation in medium F for 18 h, they were fixed with 2.5% glutaraldehyde and 2% formaldehyde in 0.1 m sodium cacodylate buffer, pH 7.4, added with 1 mm CaCl2 for 2 h. After rinses, samples were postfixed with 1% osmium tetroxide and 0.1% potassium ferrocyanide in the same buffer, dehydrated in ethanol series, and embedded in Quetol 812. Ultrathin sections cut perpendicular to the substrate were stained with lead citrate and observed under a JEM-1011 electron microscope (JEOL).
Statistical Analysis
Data are presented as means ± S.D. or S.E. as indicated in the figure legends. Statistical analyses of results were performed using the two-tailed, unpaired Student's t test, using Mann-Whitney U test or using one-way ANOVA followed by Tukey-Kramer post hoc test for multiple comparisons.
Results
ABCA1 Deficiency Leads to Hyperactivation of SREBP-2 Pathway
We hypothesize that ABCA1 may have an additional role in cholesterol homeostasis that does not require extracellular cholesterol acceptors. To test this possibility, we incubated Abca1+/+ (wild type, WT) and Abca1−/− MEFs in serum-free medium (medium F), which does not contain any extracellular cholesterol acceptors. Lipid analysis of these cells showed that lack of ABCA1 caused a substantial increase of free (unesterified) cholesterol in cells (Fig. 1A). In contrast, the CE contents in these two cell types did not differ significantly (1.5 ± 0.9 μg/mg cell protein in WT MEFs; n = 8, and 2.0 ± 0.6 μg/mg cell protein in Abca1−/− MEFs; n = 10, means ± S.D.; p = 0.167). As a control experiment, we determined cholesterol release to serum-free medium, after cells were labeled with [3H]cholesterol and its distribution in cells reached steady state. The result of this experiment showed that in both WT and Abca1−/− cells, cholesterol release to serum-free medium in 6 h was at a minimal level (0.85 ± 0.02 and 0.93 ± 0.11%, respectively). Together, these results suggest that ABCA1 may play an undisclosed role in cellular cholesterol homeostasis, independent of extracellular sterol acceptors. Additional results showed that despite an increase in cellular cholesterol content, cleavages of both SREBP-1 and -2, which are known to be sensitive to feedback inhibition by cholesterol (29), were activated in Abca1−/− MEFs (Fig. 1B). Consistent with this finding, mRNA levels of various cholesterol biosynthetic enzymes were elevated in both cholesterol-rich (medium A) and cholesterol-poor (medium D) conditions in Abca1−/− cells (Fig. 1C). Additionally, the sterol biosynthesis rate was also increased in these cells (Fig. 1D). Mechanistic target of rapamycin complex 1 (mTORC1) signaling has been implicated in SREBP regulation (30). We tested the possibility that altered mTORC1 signaling activity might occur in Abca1−/− MEFs, but we did not observe a difference in phosphorylation of the two mTOR substrates p70-S6K1 and 4E-BP1 (Fig. 1E). The results of an additional control experiment showed that when 25-hydroxycholesterol (25HC), a membrane-permeable regulatory oxysterol that enters the cell interior rapidly, was added to the growth medium, it caused efficient down-regulation of various SREBP-2 response genes in both WT and Abca1−/− MEFs, demonstrating that the ER sterol-sensing machinery in the Abca1−/− cells is not impaired (Fig. 1C). These data suggest that ABCA1 deficiency decreases the ER cholesterol levels despite a substantial increase in total cellular cholesterol. We noted that a significant increase in cholesterol content occurred in Abca1−/− cells; this result could in part be due to the consequence of a significant increase in cholesterol synthesis in these cells (Fig. 1D).
FIGURE 1.
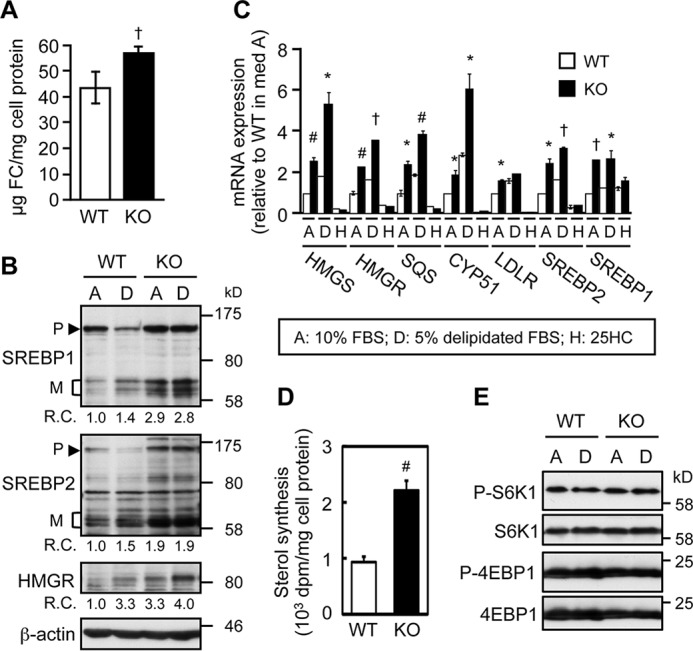
ABCA1 deficiency causes elevated SREBP-2 pathway. A, cellular free cholesterol contents. On day 0, WT and Abca1−/− (KO) MEFs were seeded and grown in medium A (medium containing FBS). On day 3, cells were switched to medium F (serum-free medium) and incubated for 1 day. †, p = 0.00001 (n = 8 for WT, n = 10 for KO). B, SREBP processing. MEFs were set up as in A. On day 2, cells were replaced with medium A (medium with FBS) or D (medium with delipidated-serum) and incubated for 2 days. SREBP-1, SREBP-2, and HMGR expression was examined by immunoblot. β-Actin serves as a loading control. Average relative changes (R.C.) of three to five experiments are shown at the bottom of each blot image. P, precursor form; M, mature form. C, mRNA expression. MEFs were set up as in B. Cells grown in medium D were further treated without or with 10 μm 25-HC (H) for 7 h. mRNA levels of HMG-CoA synthase (HMGS), HMGR, squalene synthase (SQS), CYP51, LDLR, SREBP-2, and SREBP-1 were analyzed by qRT-PCR. Values reported were relative to values in WT MEFs grown in medium A. *, p < 0.05; #, p < 0.01; †, p < 0.005 (n = 3 except 25-HC was n = 2). D, cholesterol synthesis. After incubation in medium D for 24 h, MEFs were incubated with [3H]acetate for 2 h to determine sterol synthesis. #, p < 0.01 (n = 3). E, mTORC1 signaling. MEFs were incubated in medium A or medium D as in B. Phosphorylation of S6K1 and 4E-BP1 was examined by immunoblot. Phospho(P)-S6K1 and phospho-4E-BP1 were detected first. After stripping, membranes were reprobed with anti-S6K1 and anti-4EBP1 antibodies. Error bars represent S.D. Statistical analyses were performed by Student's t test.
To validate these results, we silenced ABCA1 expression in a mouse fibroblast cell line BALB/3T3 using siRNA (Fig. 2A). The result showed that knockdown of ABCA1 markedly increased the expression of various SREBP target genes (Fig. 2B) and the SRE promoter activity (Fig. 2C). ABCA1 knockdown in BALB/3T3 cells also led to a significant increase in unesterified cholesterol content (Fig. 2D). Together, these results demonstrate a crucial role of ABCA1 in the regulation of the SREBP-2 pathway.
FIGURE 2.
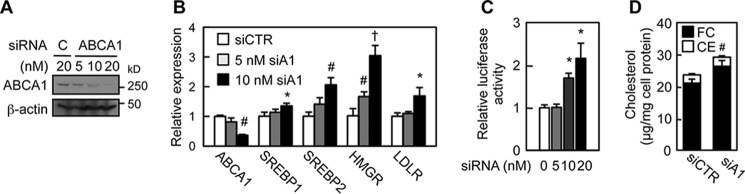
ABCA1 knockdown activates SREBP-2 pathway. A, ABCA1 expression. The indicated amounts of control (C) or ABCA1 siRNA were transfected in BALB/3T3 cells. ABCA1 expression was examined by immunoblot. B, mRNA expression. BALB/3T3 cells were transfected with control (siCTR) or ABCA1 (siA1) siRNA. mRNA levels of the indicated genes were analyzed by qRT-PCR. *, p < 0.05; #, p < 0.01; †, p < 0.001 (n = 3). C, SRE promoter activity. BALB/3T3 cells were transfected with luciferase reporter vector harboring SRE and control or ABCA1 siRNA as indicated. Luciferase activity was measured. *, p < 0.05 (n = 3). D, cellular cholesterol contents in BALB/3T3 cells transfected with 10 nm control (siCTR) or ABCA1 (siA1) siRNA were determined. #, p < 0.01 (n = 3). FC, free cholesterol. Error bars represent S.D. Statistical analyses were performed by Student's t test.
ABCA1 Facilitates PM-to-ER Retrograde Sterol Movement
To further test whether the ER cholesterol level is reduced in Abca1−/− MEFs, we labeled WT and Abca1−/− MEFs with [3H]cholesterol for 8 h, removed the [3H]cholesterol present in the medium, and allowed the cellular [3H]cholesterol to equilibrate within cells for 16 h and then measured percent esterification of [3H]cholesterol. Under this condition, we found a marked reduction in esterification of [3H]cholesterol in the Abca1−/− MEFs (Fig. 3A), which supports the notion that under this condition the ER cholesterol level is reduced in Abca1−/− MEFs. The majority of cellular cholesterol is located at the PM. Partial defects in PM-to-ER cholesterol transport are known to increase cholesterol synthesis (31). To directly explore whether aberrant activation of the SREBP-2 pathway in Abca1−/− cells is due to impaired sterol delivery to the ER, we assessed PM-to-ER sterol movement by using multiple intracellular sterol trafficking assays. We pulse-labeled the WT and Abca1−/− cells with trace amounts of [3H]cholesterol (with high specific radioactivity but without unlabeled cholesterol as carrier, to prevent minimal membrane disturbance), and we monitored its esterification by the ER resident enzyme ACAT1 at the ER. In WT MEFs, about 3.5% of the cellular surface [3H]cholesterol was esterified within 3 h (Fig. 3B). In contrast, this esterification was substantially attenuated in Abca1−/− MEFs. Despite the differences in esterification efficiency, ACAT1 protein levels were found to be the same between the two cells (Fig. 3C). Treatment of cells with sphingomyelinase (SMase) causes hydrolysis of PM sphingomyelin and accelerates cholesterol movement from the PM to the ER (32). We utilized this method to further examine retrograde cholesterol transport. In WT MEFs, adding exogenous SMase enhanced esterification of PM-derived cholesterol 3.5-fold. In contrast, in Abca1−/− MEFs, the SMase-enhanced esterification was reduced by ∼50% of values found in treated WT cells (Fig. 3D). A previous work showed that the SMase treatment increases the PM cholesterol pool available for ABCA1-dependent cholesterol efflux to apoA-I (33). The results presented in Fig. 3D show that the SMase treatment also increases the PM cholesterol pool, which is available for ABCA1-dependent inward movement of cholesterol. Similar to SMase, 25HC is also known to facilitate PM-to-ER cholesterol transport, in part by altering PM composition (34). However, the action of 25HC on cholesterol esterification in intact cells may be complicated because, in addition to its ability to mobilize cholesterol from the PM, it may disrupt composition of internal membranes; 25HC can also serve as an allosteric activator for ACAT1, when cholesterol is used as the ACAT1 substrate (35). To serve as a control experiment for the SMase treatment, we assessed the effect of 25HC on esterification of PM cholesterol in WT and Abca1−/− MEFs. The result showed that when used at 1 μm, 25HC failed to significantly affect the esterification of PM cholesterol in either WT or Abca1−/− MEFs; at a very high concentration (10 μm), 25HC caused a modest increase in esterification; the increase occurred more in the Abca1−/− cells than in the WT cells (Fig. 3E). In the presence of 10 μm 25HC, retardation in the esterification of PM cholesterol was observed in Abca1−/− cells. These results implicate the 25HC-dependent activation of cholesterol esterification is not a sensitive assay to monitor the cholesterol movement from the PM to the ER in intact cells.
FIGURE 3.
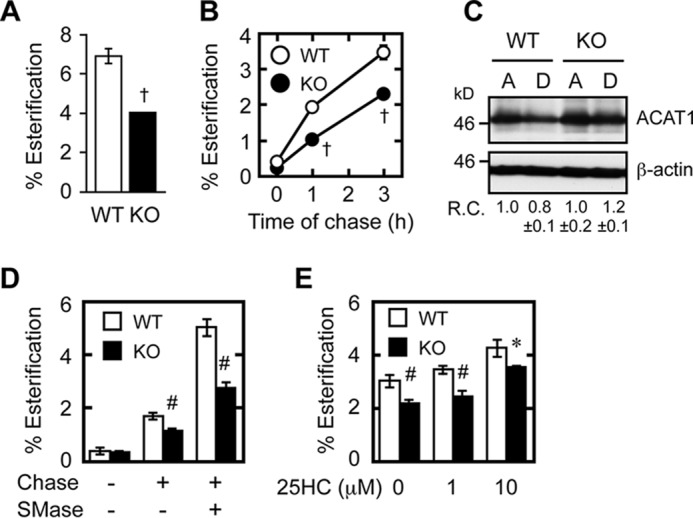
Retrograde cholesterol transport is impaired in Abca1−/− cells. A, esterification of [3H]cholesterol at steady-state level. MEFs were set up as described in Fig. 1. On day 2, cells were labeled with [3H]cholesterol for 8 h in medium A. After washing [3H]cholesterol present in the medium, cells were incubated in medium A for 16 h for equilibration, and further incubated for 6 h in medium F. [3H]Cholesterol and [3H]CE in cells were analyzed. †, p < 0.001 (n = 3). B, esterification of cell surface cholesterol. MEFs were set up as described in Fig. 1. On day 3, cells were pulse-labeled with [3H]cholesterol for 30 min in medium B (medium containing 0.1% BSA) at 37 °C, washed twice, and chased in medium F (serum-free medium) for various times as indicated. [3H]Cholesterol and [3H]CE in cells were analyzed. †, p ≤ 0.001 (n = 3). C, ACAT1 protein expression. MEFs were incubated in medium A or D for 2 days. ACAT1 and β-actin expressions were analyzed by immunoblots. Relative changes in ACAT1 expression (ACAT1/β-actin) (R.C.) are indicated at the bottom. D, effect of SMase on retrograde cholesterol transport. MEFs were set up and pulse-labeled with [3H]cholesterol as in B and chased or not chased for 1 h with or without SMase (0.1 unit/ml). Cellular [3H]cholesterol and [3H]CE were analyzed. #, p < 0.003 (n = 3). E, effect of 25HC on the esterification of PM cholesterol. MEFs were set up and pulse-labeled with [3H]cholesterol as in B and chased or not chased for 3 h with or without 25HC (1 or 10 μm as indicated). Cellular [3H]cholesterol and [3H]CE were analyzed. *, p < 0.05; #, p < 0.01 (n = 3). Error bars represent S.D. Statistical analyses were performed by one-way ANOVA with Tukey-Kramer post hoc test.
We next looked at whether retrograde movement of biosynthetic precursor sterols was also retarded in Abca1−/− cells. Lanosterol, the first sterol synthesized in the cholesterol biosynthetic pathway, is immediately transported to the PM upon its synthesis at the ER. After arriving at the PM, it is rapidly transported back to and converted to cholesterol at the ER (8), where the enzymes involved in the conversion of lanosterol to cholesterol are located. One of the key enzymes involved in converting lanosterol to cholesterol is CYP51 (lanosterol 14-demethylase); CYP51 is transcriptionally up-regulated by SREBP-2; in our cell system, Cyp51 gene expression is higher in Abca1−/− MEFs than in WT MEFs (Fig. 1C). If the retrograde movement of lanosterol was normal in Abca1−/− MEFs, one would predict a more rapid conversion of lanosterol to cholesterol in Abca1−/− MEFs. We monitored the conversion of [3H]lanosterol biosynthesized de novo to [3H]cholesterol, and we found that in contrast, the rate of conversion of lanosterol to cholesterol is clearly impaired in Abca1−/− MEFs (Fig. 4A). As an additional approach, the same assay was used to monitor the conversion of [3H]lanosterol added directly to the growth medium of cells (without adding unlabeled lanosterol as carrier). The results showed that the conversion of [3H]lanosterol to [3H]cholesterol was also markedly retarded in Abca1−/− MEFs (Fig. 4B). Together, these results demonstrated an important role of ABCA1 in the PM-to-ER transport of both cholesterol and lanosterol. A previous study suggested that ABCB1 (also known as multidrug-resistant protein or P-glycoprotein) might be involved in the retrograde movement of precursor sterols (36). Verapamil inhibits ABCB1 but not ABCA1 (37). We found that verapamil only partially inhibited the conversion of lanosterol to cholesterol in both WT and Abca1−/− cells and that ABCA1 deficiency caused a much more significant retardation in the conversion than verapamil did (Fig. 4C), indicating that ABCA1 plays the predominant role in retrograde lanosterol transport.
FIGURE 4.
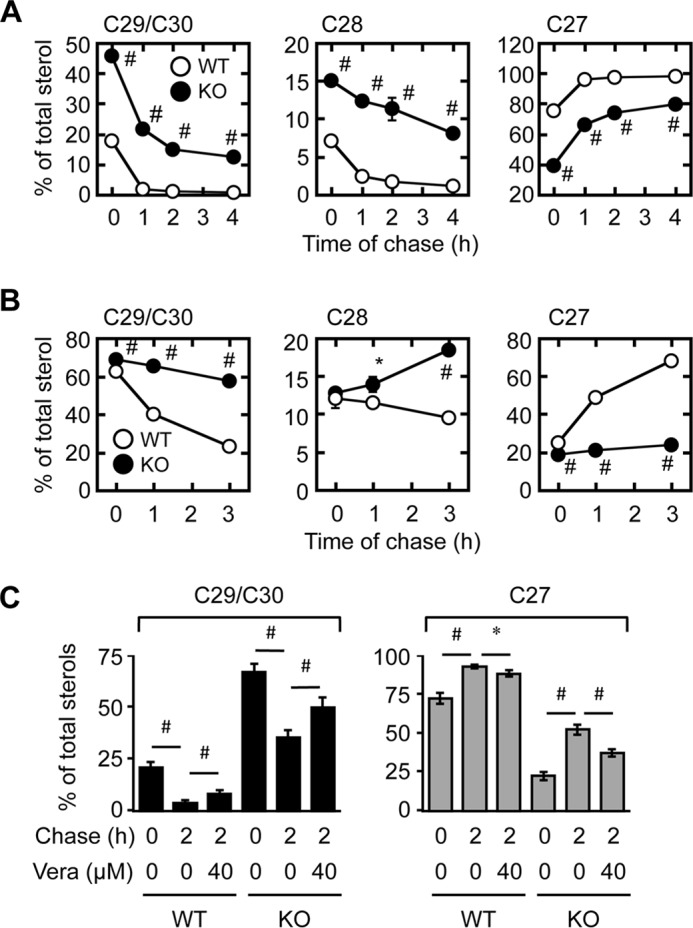
Retrograde transport of precursor sterols is retarded in Abca1−/− cells. A and B, conversion of lanosterol to cholesterol. MEFs incubated in medium D for 2 days were pulse-labeled with [3H]acetate for 1 h in medium F (A), or with [3H]lanosterol for 30 min in medium B (B). Afterward, cells were chased in medium F for various times. Cellular radioactive sterols were analyzed. Lanosterol and cholesterol are major components of C29/C30 sterols and C27 sterols, respectively. *, p < 0.03; #, p < 0.005 (n = 3). C, effect of verapamil on retrograde sterol transport. WT and KO MEFs were incubated in medium D for 2 days. Cells were pretreated with or without 40 μm verapamil (Vera) for 60 min and pulse-labeled with [3H]acetate for 60 min. The cells were then chased for 2 h in medium F. Verapamil was present throughout the experiments where indicated. Results of C29/C30 sterols (left) and C27 sterols (right) are shown. *, p < 0.05; #, p < 0.01 (n = 3). Error bars represent S.D. Statistical analyses were performed by Student's t test.
We next tested whether ABCA1 is involved in the retrograde movement of LDL-Chol. Cells were incubated with [3H]cholesteryl oleate-containing LDL at 18 °C for 4 h and then chased with the label at 37 °C. At the indicated chase time, cells were briefly incubated with the soluble cholesterol acceptor MCD. As expected, uptake of [3H]cholesteryl oleate-labeled LDL was significantly increased in Abca1−/− cells (Fig. 5A). After a 30-min chase, the MCD extractable counts (considered as [3H]cholesterol associated with the PM) were the same in these two cell types (Fig. 5B). However, after 2 h of chase, the MCD extractable counts decreased much less in Abca1−/− MEFs than in WT MEFs, indicating that the disappearance of PM-arrived [3H]cholesterol from the PM to cell interior is slower in Abca1−/− cells. A control experiment showed that the hydrolysis of LDL-derived [3H]cholesteryl oleate was the same between these two cell types (Fig. 5C). Consequently, an increase in [14C]oleate incorporation into CE upon LDL addition was markedly attenuated in Abca1−/− cells (Fig. 5D). Together, these results indicate that although the transport of LDL-Chol to the PM is not impaired in Abca1−/− cells, its delivery from the PM to the ER is. The results described in Figs. 3–5 show that, in addition to mediating lipid efflux, ABCA1 expresses influx-like activity for sterols located at the PM.
FIGURE 5.
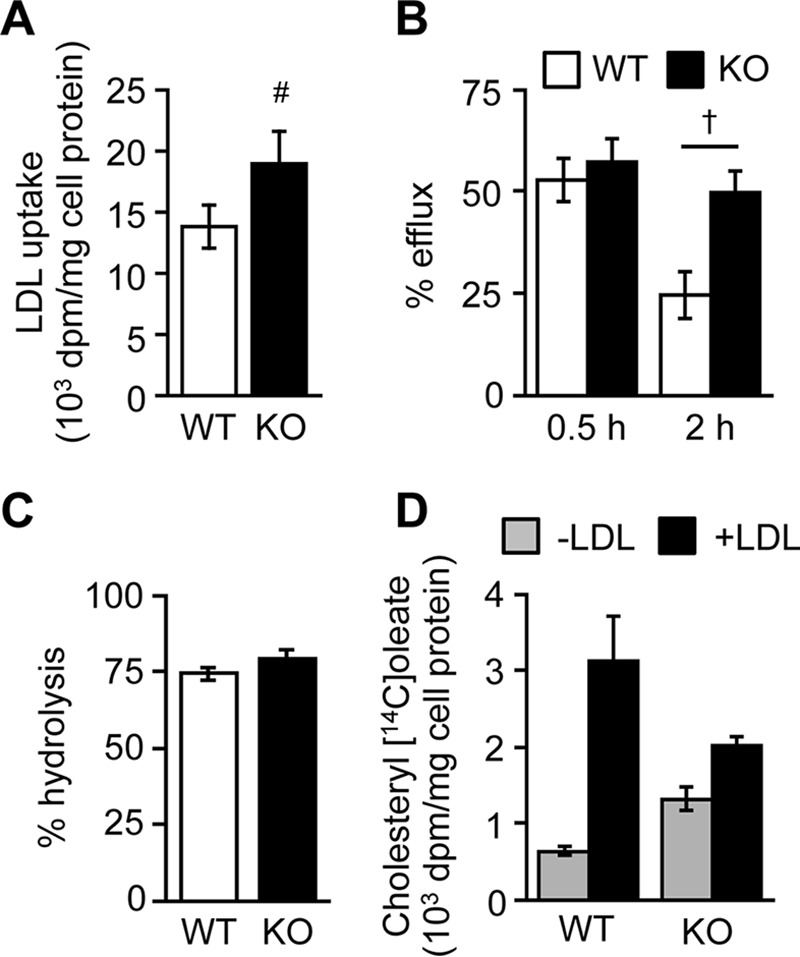
Retrograde transport of LDL-derived cholesterol is retarded in Abca1−/− cells. A–C, LDL uptake and retrograde movement of LDL-Chol. MEFs set up as in Fig. 4A were fed with [3H]cholesteryl oleate-containing LDL (100 μg/ml) for 4 h at 18 °C. Cells were then chased in medium D for 0.5 or 2 h at 37 °C. Afterward, LDL uptake (A), PM-associated [3H]cholesterol (B), and hydrolysis of [3H]cholesteryl oleate (C) were determined. #, p = 0.003; †, p < 0.001 (n = 6). D, cholesterol esterification. MEFs were incubated with or without LDL (150 μg/ml) for 5 h in medium D. Afterward, cells were incubated with [14C]oleate for 3 h (n = 3). Error bars represent S.D. Statistical analyses were performed by Student's t test.
ABCA1 Deficiency Impairs Sterol Sensing at the ER
Both cholesterol and lanosterol are biologically important regulatory sterols; but they differ in mode of action. Lanosterol is first converted to dihydrolanosterol by the enzyme 3β-hydroxysterol Δ24-reductase located at the ER; dihydrolanosterol enhances ubiquitination of HMGR, causing HMGR to be rapidly degraded (38, 39). Instead, cholesterol interacts with Scap and inhibits the ER exit of the precursor form of SREBP-2 and subsequently its proteolytic cleavage, thus preventing it from acting as an active transcription factor (1). In Abca1−/− MEFs, the impaired retrograde transport of the two regulatory sterols may lead to a sluggish response to regulatory sterols at the ER. To test this possibility, we first showed that in WT CHO cells, SMase treatment caused a rapid reduction in the mature form of SREBP-2, as reported previously (40), but not in a mutant CHO cell line 25RA that is resistant to cholesterol-dependent regulation due to a mutation (D443N) within the sterol-sensing domain of Scap (Fig. 6A) (41, 42). These results demonstrate the specificity of the SMase assay to assess cholesterol sensing at the ER. We next showed that in cells maintained without sterol acceptors, SMase treatment resulted in an efficient reduction of SREBP-2 cleavage in WT MEFs; in contrast, in Abca1−/− MEFs, the cleavage of SREBP-2 was highly resistant to the SMase treatment (Fig. 6B). As a control experiment, we treated these cells with various concentrations of 25HC and examined SREBP-2 processing. As expected, 25HC suppressed SREBP-2 cleavage in both cell types at various concentrations (from 1 to 10 μm) (Fig. 6C), indicating that the impaired cholesterol delivery to the ER is a major cause of the resistance to the SMase treatment observed in Abca1−/− cells. To test whether ABCA1 also plays important roles in delivering lanosterol as a regulatory sterol, we monitored the turnover rates of HMGR in the WT and Abca1−/− MEFs, by treating these cells with cycloheximide, an inhibitor of protein synthesis. The results showed that the apparent half-life of HMGR in the WT cells was ∼1 h (Fig. 6, D and E), similar to the value reported previously (43). In contrast, its half-life was markedly prolonged to more than 4 h in Abca1−/− cells. These results show that ABCA1 mediates sterol sensing at the ER.
FIGURE 6.
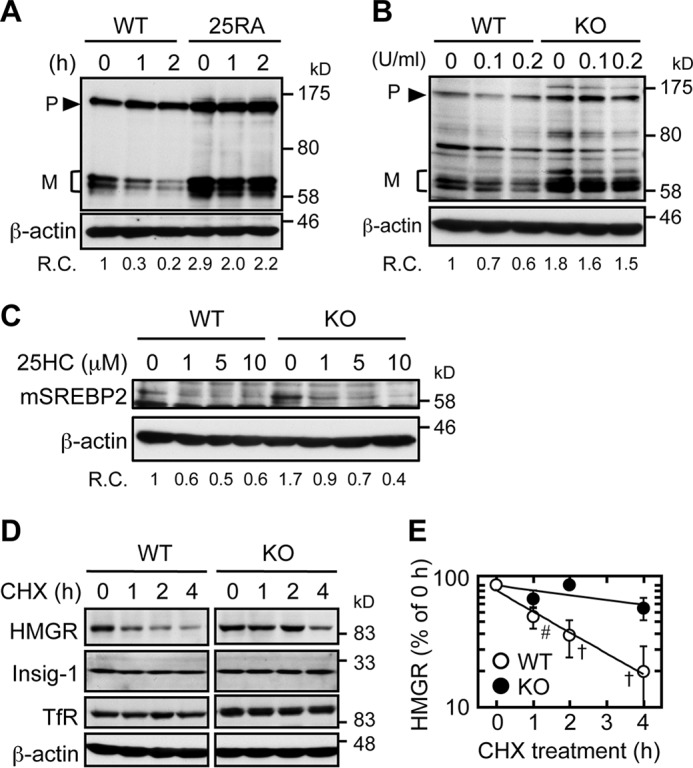
Sterol sensing at the ER is impaired in Abca1−/− cells. A, SREBP-2 cleavage in response to SMase treatment in WT CHO cells and in the sterol-resistant mutant CHO cells 25RA (containing a gain-of-function mutation in Scap). On day 0, cells (2 × 105 cells/well) were set up in medium A in 6-well plates. On day 1, cells were switched to medium D. On day 3, cells were treated with or without SMase (0.1 unit/ml) in medium F for 1 or 2 h. Afterward, whole cell lysates were subjected to immunoblot analysis with anti-SREBP-2 antibody (IgG-7D4). Relative changes (R.C.) in mature form/β-actin ratios are indicated at the bottom (n = 2). B, SREBP-2 cleavage in response to SMase treatment in WT and KO MEFs. Cells were seeded as described in Fig. 1. On day 2, cells were switched to medium D for 2 days. Cells were then treated with the indicated concentration of SMase for 2 h in medium F. Whole cell lysates were subjected to immunoblot analysis with the indicated antibodies. Relative changes (R.C.) in mature form/β-actin ratio are indicated at the bottom (n = 2). C, SREBP-2 cleavage in response to 25HC treatment in WT and KO MEFs. MEFs set up as above were treated without or with 1, 5, or 10 μm 25HC for 2 h. Whole cell lysates were subjected to immunoblot analysis with the indicated antibodies. Relative changes (R.C.) in mature form/β-actin ratio are indicated at the bottom. D and E, half-lives of HMGR in WT and KO MEFs. MEFs were set up as described in B. Cells were then treated with cycloheximide (CHX) (20 μg/ml) for various times as indicated. Afterward, whole cell lysates were subjected to immunoblot analysis with antibodies as indicated. Transferrin receptor (TfR) and β-actin were used as controls. A representative result was shown (C). The results of three experiments were plotted (D). Data represent means ± S.D. #, p = 0.0017; †, p < 0.0001 by Student's t test.
Intrinsic ATPase Activity of ABCA1 Is Required to Facilitate Retrograde Cholesterol Transport
ABCA1 possesses intrinsic ATPase activity, which is essential for its role in lipid efflux (44). We sought to determine whether this activity is also required for the ABCA1-dependent retrograde sterol movement. We used cells that express WT ABCA1 or mutant ABCA1 (ABCA1-MM) lacking ATPase activity by replacement of the two lysine residues (Lys-939 and Lys-1952) crucial for ATP hydrolysis by methionine (45), under the control of mifepristone (Fig. 7A). The results showed that, in the absence of mifepristone, neither WT-ABCA1 nor ABCA1-MM cells exported cholesterol to apoA-I (Fig. 7B). When mifepristone was added to the medium, only cells expressing WT-ABCA1 but not cells expressing ABCA1-MM exported cholesterol and PL to apoA-I (Fig. 7, B and C). We next used the same cell systems to perform the retrograde cholesterol transport assay. Cells were treated with or without mifepristone for 18 h and then were labeled with [3H]cholesterol. The arrival of [3H]cholesterol at the ER was monitored by measuring the amount of [3H]CE formed. The results showed that WT-ABCA1 markedly increased PM-to-ER cholesterol transport but ABCA1-MM did not exhibit an increase (Fig. 7D). These results indicate that the ATPase activity intrinsically associated with ABCA1 is required to facilitate retrograde cholesterol transport.
FIGURE 7.
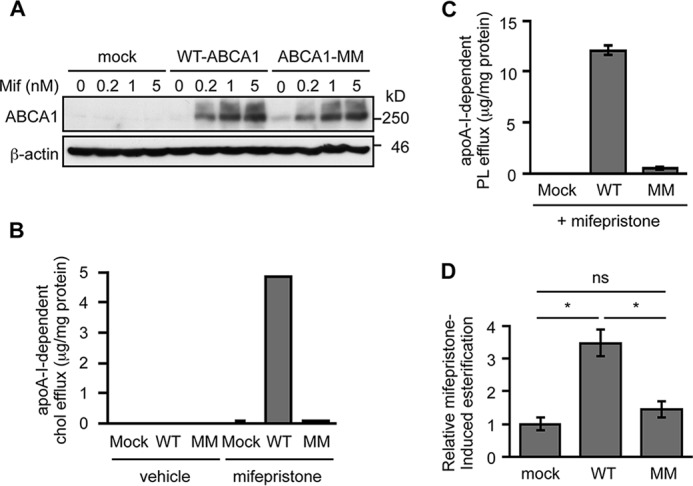
ATPase activity of ABCA1 is required for its ability to facilitate retrograde cholesterol transport. A, induction of ABCA1 expression by mifepristone. BHK cells stably transfected with vector or vector harboring WT-ABCA1 or ABCA1-MM cDNA (BHK/mock, BHK/WT-ABCA1, and BHK/ABCA1-MM respectively) cells were incubated with the indicated concentrations of mifepristone for 18 h. ABCA1 expression was examined by immunoblot. B and C, apoA-I-dependent cholesterol and PL efflux. BHK/mock, BHK/WT-ABCA1, and BHK/ABCA1-MM cells were incubated with or without apoA-I (5 μg/ml) in the absence or presence of 5 nm mifepristone for 18 h, as indicated. Amounts of cholesterol (B) and PL (C) released into medium were measured. Subtracted values are shown. Data represent means ± S.D. (n = 3). D, PM-to-ER cholesterol transport. BHK cells treated without or with 5 nm mifepristone were pulse-labeled with [3H]cholesterol and then chased for 3 h. ABCA1-specific esterification was calculated by subtracting counts of mifepristone-untreated cells. Data represent means ± S.D. (n = 3). #, p < 0.01 by one-way ANOVA with Tukey-Kramer post hoc test. ns, not significant.
ABCA1 Deficiency Alters PM Composition
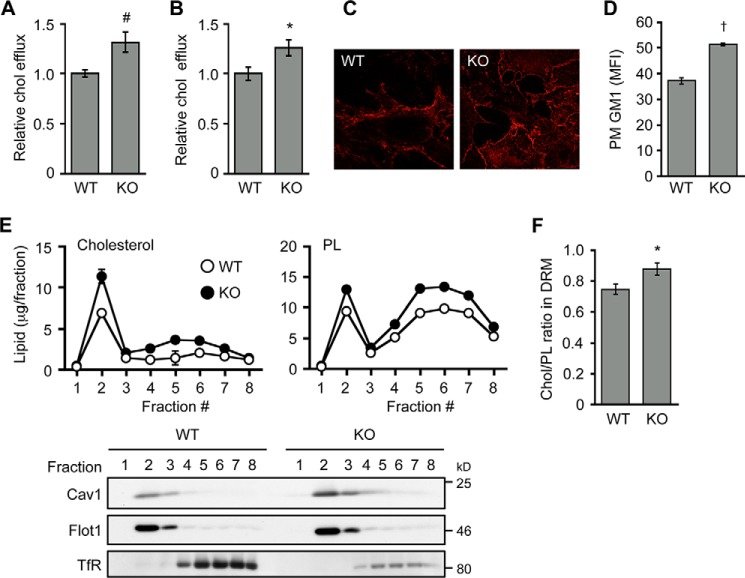
To explain how ABCA1 acts to control retrograde sterol movement at the PM, we examined possible changes in PM caused by lack of ABCA1. We found that in live cells, PM from Abca1−/− MEFs contained elevated levels of cholesterol as probed by using 2-hydroxypropyl-β-cyclodextrin- or MCD-mediated removal of cell surface cholesterol (Fig. 8, A and B). Similarly, an increase of PM cholesterol in Abca1−/− cells was observed (Fig. 8C) when cell surface cholesterol was assessed by using mCherry-D4, a nontoxic, cholesterol-binding domain of perfringolysin O that recognizes cholesterol-rich membrane domains but has no pore-forming activity (27). We also found an increase in cell surface ganglioside GM1, as detected by using the specific GM1 probe CTxB, in Abca1−/− MEFs (Fig. 8D). Cholesterol content in detergent-resistant membrane (DRM, fraction 2) domains, where caveolin-1 (Cav1) and flotillin-1 were enriched, was markedly increased in Abca1−/− cells (Fig. 8, E and F). In addition to DRM, however, a modest increase of cholesterol content in non-DRM (fractions 4–8) was also observed in Abca1−/− cells, suggesting an overall increase in PM cholesterol. Furthermore, Cav1 expression was increased (Fig. 9A), and the cell surface colocalization between Cav1 and GM1 was more frequent in Abca1−/− MEFs (Fig. 9B). The difference in flotillin-1 expression between the two cell types was less significant (Fig. 9A). Cav1, cholesterol, and ganglioside are essential components to form caveolae (46). We looked for possible changes in caveolae in Abca1−/− cells and found that the number of cell surface-associated caveolae is increased in Abca1−/− cells (Fig. 9C). Overall, our results suggest that ABCA1 regulates membrane composition at the PM. This interpretation is in agreement with previous observations made in macrophages (11, 47).
FIGURE 8.
ABCA1 deficiency causes cholesterol accumulation at the PM. A and B, cell surface cholesterol levels. WT and KO MEFs were seeded as described in Fig. 1. On day 2, cells were switched to medium F (medium with no supplement). After incubation for 18 h, cells were washed twice with ice-cold PBS and incubated with 4% 2-hydroxypropyl-β-cyclodextrin for 10 min at 37 °C (A) or 3 mm (∼ 0.4%) MCD for 5 min at 37 °C (B). Afterward, medium was collected to determine cholesterol released from cells. *, p = 0.013; #, p = 0.007 (n = 3). C, cell surface cholesterol staining in live cells. WT and KO MEFs were treated with mCherry-D4 to visualize cell surface cholesterol in live cells. D, cell surface GM1 levels. Cells were set up as described in A. Binding of the GM1 probe CTxB to the cell surface was measured by FACS. Mean fluorescent intensity (MFI) was plotted. †, p < 0.0001 (n = 3). E and F, DRM analysis. WT and KO MEFs were treated with 1% Triton X-100 for 30 min at 4 °C. Post-nuclear supernatant was subjected to density gradient ultracentrifugation. Amounts of cholesterol and phospholipid in each fraction were determined and normalized to cellular protein (upper panels). Equal amount of each fraction was subjected to immunoblot analysis to determine the distribution of caveolin-1 (Cav1), flotillin-1 (Flot1), and transferrin receptor (TfR) (lower panels). Fraction 2 contained the majority of DRM domains. Cholesterol/phospholipid ratio in DRM fraction (fraction 2) is shown in F. Data represent means ± S.D. (n = 3). *, p = 0.016. Error bars represent S.D. Statistical analyses were performed by Student's t test.
FIGURE 9.
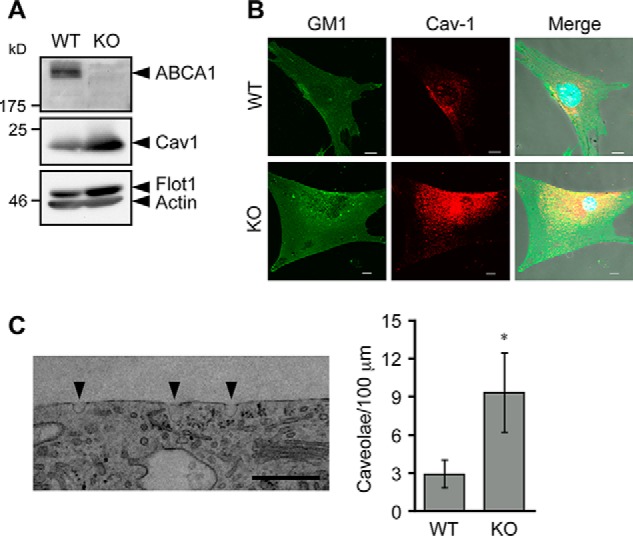
ABCA1 deficiency increases caveolae. A, expression of caveolin-1. MEFs were set up as described in A, and whole cell lysates were prepared. Expressions of ABCA1, caveolin-1 (Cav1), Flotillin-1 (Flot1), and β-actin were analyzed by immunoblot. B, colocalization of GM1 and Cav1. MEFs set up as in A were fixed. Cells were incubated with biotin-CTxB and with anti-Cav1 antibody followed by appropriate secondary antibodies (FITC-avidin for biotin-CTxB and Alexa Fluor 555-anti-rabbit IgG for anti-Cav1). Images were taken under a confocal laser microscope. Nuclei were stained with Hoechst 33342. Bar, 10 μm. C, number of caveolae. MEFs set up as described in A were subjected to EM analysis, and cell surface-associated caveolae were counted. Left panel shows typical picture of caveolae in KO MEFs. Arrowheads indicate caveolae. Right panel shows the number of cell surface-associated caveolae per 100-μm cell surface length. Data represent mean ± S.E. (WT; n = 29, KO; n = 36). *, p = 0.036 by Mann-Whitney U test. Bar, 0.5 μm.
ABCA1 Is Involved in Certain Clathrin-independent Endocytoses
Changes in PM composition are expected to affect PM functionality. Endocytosis is a cellular process that internalizes extracellular and membrane-associated molecules into the cell interior and requires dynamic membrane reorganization. Endocytosis is largely categorized into two pathways as follows: clathrin-dependent and clathrin-independent. Clathrin-independent endocytosis (CIE) plays an important role in internalization of proteins and lipid molecules located to lipid rafts. It has been reported that Cav1 acts as a negative regulator of CIE (28, 48). We asked whether ABCA1 deficiency alters CIE, and we tested this possibility by assessing internalization of CTxB, an established marker of CIE (49). Our results showed that when compared with WT cells, CTxB internalization was significantly retarded in Abca1−/− MEFs, despite significant increases in cell surface binding of CTxB in these cells (Fig. 10, A and B, see also Fig. 8D). The decrease in CTxB internalization in Abca1−/− cells was observed as early as 10 min after incubation with CTxB (Fig. 10, C and D). These results show that ABCA1 deficiency causes a reduction in a certain CIE activity that internalizes CTxB.
FIGURE 10.
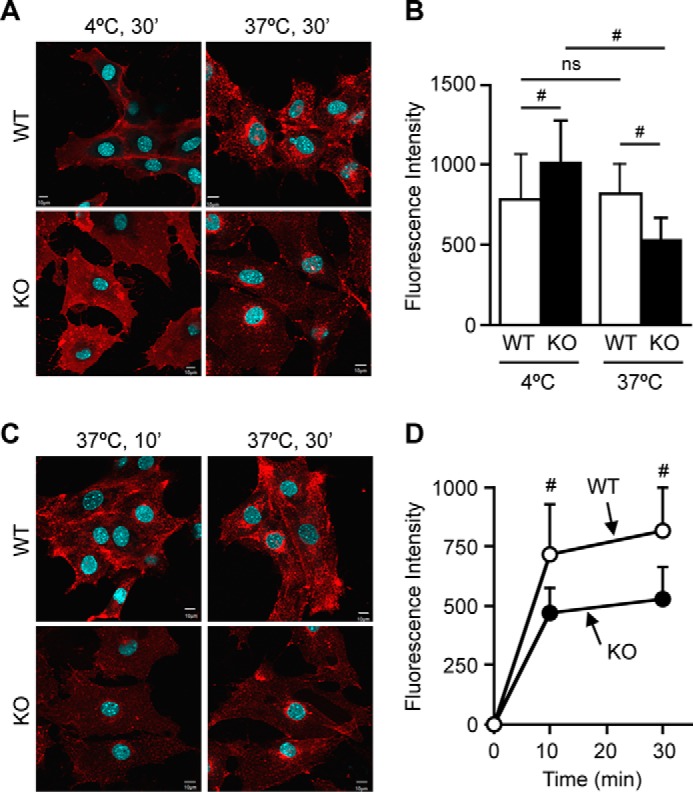
CTxB internalization is impaired in Abca1−/− cells. Internalization of CTxB is shown. WT and KO MEFs were incubated with AF555-CTxB for 30 min either at 4 or at 37 °C (A and B) or for 10 or 30 min at 37 °C (C and D). Cellular mean fluorescent intensity of 30–60 cells was plotted in B and D. CTxB was found at cell periphery at 10 min, but at a later time (30 min) it was localized to peri-nuclear regions, most likely Golgi. Data represent means ± S.D. Statistical analyses were performed by one-way ANOVA with Tukey-Kramer post hoc test. #, p < 0.01; ns, not significant. Bar, 10 μm.
CIE Inhibitors and Dynamin Inhibitors Abolish Retrograde Cholesterol Movement
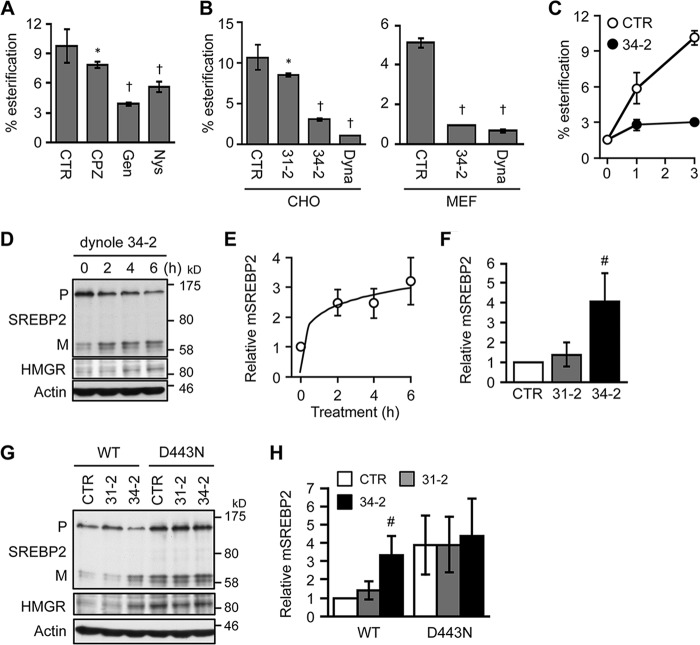
Next, we used various small molecule inhibitors to further test whether the CIE activity is involved in PM-to-ER cholesterol transport. First, we performed pulse labeling of cells with [3H]cholesterol and chased the cells without or with chlorpromazine, a clathrin-mediated endocytosis (CME) inhibitor, or with genistein and nystatin, the CIE inhibitors. Genistein and nystatin are known to block cholesterol-dependent endocytic internalization such as caveolar endocytosis, without affecting CME (50). The result showed that genistein and nystatin significantly suppressed the delivery of [3H]cholesterol to the ER (Fig. 11A), whereas chlorpromazine only had little effect, suggesting that CIE but not CME may play a major role in delivering PM cholesterol to the ER. This result is consistent with our previous work reporting that genistein or nystatin suppressed PM-to-ER transport of precursor sterols (8). It is also important to point out that LDLR-mediated LDL uptake, which requires CME, was not impaired in Abca1−/− MEFs (Fig. 5A), indicating that CME is not disrupted in Abca1−/− cells. The CIE activity can mainly be classified into two categories based on its dependence on dynamin, a GTPase that plays a key role in scission of endocytic vesicles from the PM (51). CTxB is internalized by a CIE pathway that requires dynamin and endophilin (49). To test whether dynamin is involved in retrograde cholesterol transport, we used two structurally distinct specific dynamin inhibitors, dynasore (52) and dynole 34-2 (53), with dynole 34-2 being more potent than dynasore. The results showed that both dynole 34-2 and dynasore markedly retarded PM-to-ER cholesterol transport within 1 h of treatment in both CHO cells and WT MEFs (Fig. 11, B and C). The results of a control experiment showed that dynole 31-2, an inactive analog of dynole 34-2, only slightly affected retrograde cholesterol transport. Additional results showed that dynole 34-2, but not its inactive analog, rapidly activated SREBP-2 processing and up-regulated HMGR expression in CHO cells (Fig. 11, D and E) and WT MEFs (Fig. 11F). To further test the potential role of dynamin in ER sterol sensing, we examined the effect of dynole 34-2 in the mutant CHO cell line 25RA that expresses sterol-resistant ScapD443 (41, 42), and we showed that the dynamin inhibitor had no effect on SREBP-2 processing in this mutant cell (Fig. 11, G and H). Together, these results are consistent with the interpretation that a dynamin-mediated endocytic pathway may play a key role in the delivery of PM cholesterol to the ER for sterol sensing.
FIGURE 11.
Effects of small molecule inhibitors for endocytosis on PM-to-ER cholesterol transport and ER sterol sensing. A and B, effects of inhibitors for CIE and for dynamin in PM-to-ER cholesterol transport. CHO cells (A and B, left) or WT MEFs (B, right) were pulse-labeled with [3H]cholesterol for 30 min. Afterward, cells were incubated for 3 h with vehicle (CTR), 10 μm chlorpromazine (CPZ), 50 μm genistein (Gen), 50 μm nystatin (Nys), 10 μm dynole 31-2 (31-2), 10 μm dynole 34-2 (34-2), or 50 μΜ dynasore (Dyna). Esterification of [3H]cholesterol was assessed to determine its delivery to the ER (n = 3). C, CHO cells were pulse-labeled as above and chased for 0, 1, or 3 h in the presence of vehicle (CTR) or 10 μm dynole 34-2. Esterification of [3H]cholesterol was determined (n = 3). D–F, effect of dynole 34-2 on SREBP-2 processing. D and E, CHO cells grown in medium A were switched to medium F for 6 h. Dynole 34-2 (10 μm) was added to medium 2, 4, or 6 h before harvesting cells. F, WT MEFs were treated with vehicle (CTR), dynole 31-2 (10 μm), and dynole 34-2 (10 μm) for 6 h (n = 3). SREBP-2 processing and HMGR expression were analyzed by immunoblot. Relative changes in mature form presented in D are shown in E (n = 3). G and H, effects of dynamin inhibitors on cholesterol sensing by Scap. CHO cells expressing WT and gain-of-function (D443N) Scap were treated with vehicle (CTR), dynole 31-2 (10 μm), or dynole 34-2 (10 μm) for 6 h in medium F. SREBP-2 processing and HMGR expression were assessed. Relative changes in mature form are shown in H (n = 3–5). Error bars represent S.D. Statistical analyses were performed by Student's t test. *, p < 0.05; #, p < 0.01; †, p < 0.001 (versus respective control).
Discussion
Our current work reveals a new function of ABCA1; in addition to the well established role in mediating cellular cholesterol release, it plays a crucial role in the retrograde movements of both biosynthesized sterols and LDL-Chol arriving at the PM, to be sensed by regulatory enzymes/proteins located at the ER. We propose a model to depict how ABCA1 acts at the PM to regulate cellular cholesterol homeostasis (Fig. 12A); the increase in cellular cholesterol induces ABCA1 gene expression by the liver X receptor-mediated mechanism (54). Elevated ABCA1 expression leads to the increase in sterol release from the PM and also leads to the increase in retrograde sterol movement from the PM. The retrograde sterol movement results in an increase in cholesterol esterification and decreases in LDL uptake and sterol synthesis by inhibiting SREBP-2 processing. The net effect of induced ABCA1 expression is to reduce toxic accumulation of free cholesterol in cells. Through its ability to facilitate bidirectional sterol flux at the PM, ABCA1 participates in controlling cholesterol homeostasis.
FIGURE 12.
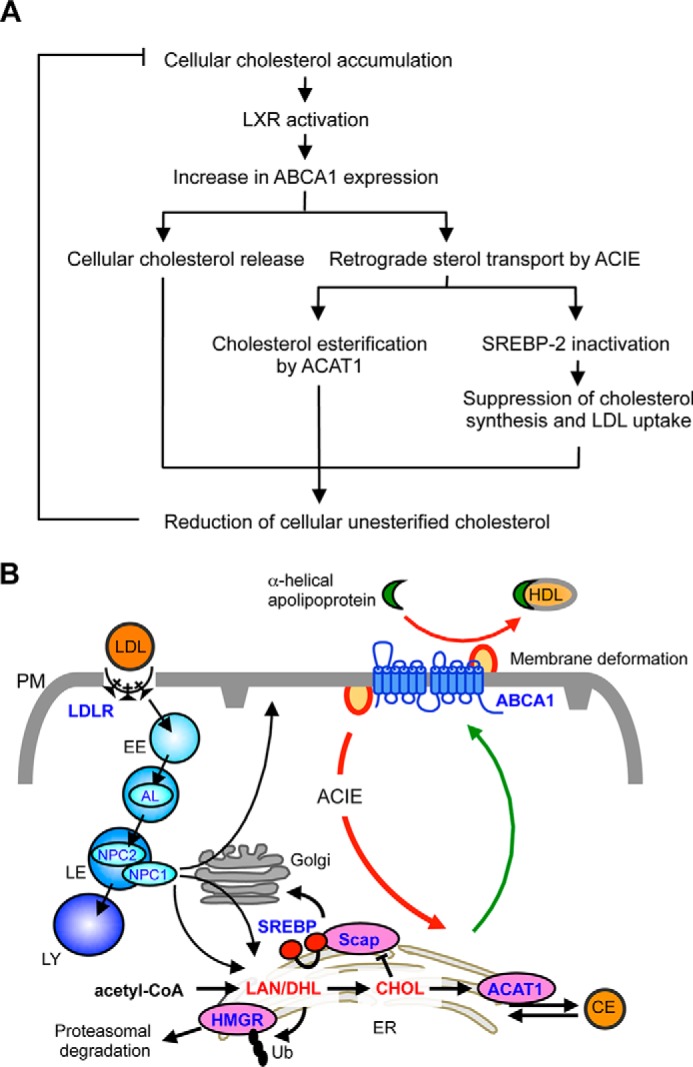
Model to describe the roles of ABCA1 in cholesterol homeostasis. A, ABCA1 participates in bidirectional sterol movement at the PM to prevent excess cholesterol accumulation in cells. See text for more detail. B, newly synthesized sterols (lanosterol, cholesterol, etc.) are rapidly transported from the ER to the PM (green line). LDL-Chol exiting the LE moves to the PM or to the ER. In the presence of ABCA1, the ABCA1-regulated, clathrin-independent (ACIE) pathway delivers cholesterol from the PM to the ER (red line) as a major trafficking route, or it releases cholesterol to apoA-I (red line). However, in the absence of ABCA1, such retrograde sterol transport pathway and sterol efflux are reduced or defective, causing cholesterol to be accumulated at the PM and ER sterol sensing to be disrupted. See text for more details. AL, acid lipase; DHL, dihydrolanosterol; EE, early endosome; LAN, lanosterol; LE, late endosome; LY, lysosome; Ub, ubiquitin.
How does ABCA1 participate in both cholesterol efflux and retrograde sterol transport? Most ATP-binding cassette transporters act as substrate exporters (55), and only a few of them show substrate influx activity (56, 57). ABCA1, as reported here, is the first ATP-binding cassette transporter to be shown to mediate substrate flux bidirectionally. It has previously been shown that overexpression of ABCA1 in cells alters lipid packing at PM (58, 59) and creates outward membrane curvatures where apoA-I binds (7). Here we show that in the absence of sterol acceptors, ABCA1 deficiency causes cholesterol accumulation at the PM. Cholesterol forms stoichiometric complexes with PLs, including SM at the PM; the cholesterol pool that exceeds the binding capacity of PLs can be described as “active cholesterol” (60, 61). Based on the active cholesterol hypothesis, ABCA1 may act by producing local lipid packing deformations at the PM, which in turn generates more active cholesterol. The active cholesterol at the membrane deformation sites created by ABCA1 may serve as a source for sterol release for apoA-I-mediated HDL biogenesis or may undergo retrograde movement to exert regulatory actions at the ER (Fig. 12B). “Activation” of cholesterol can therefore be considered as local destabilization of PM. Depletion of SM by SMase also generates more active cholesterol to become readily accessible for sterol sensing at the ER (32, 40) and for ABCA1-dependent efflux out of cells (33). A recent proteoliposome study showed that ABCA1 directly exports select PLs, including phosphatidylcholine, phosphatidylserine, and SM, across the membrane and suggested that cholesterol efflux occurs in an indirect manner (62). Through its PL floppase activity, ABCA1 may change PL composition in the PM, which causes alterations in membrane-cholesterol interaction and cholesterol activation threshold; these changes lead to an increase in the availability of cholesterol in membranes (63). Consistent with this view, a study with a mammalian cell mutant suggested that altered PL composition at the PM affects PM-to-ER cholesterol transport (31). Thus, it is conceivable that in the absence of ABCA1, reduced PL movement within the PM leads to stabilization of cholesterol-membrane complexes, causing a decrease in cholesterol movement at the PM. Caveolae are highly stable, immobile membrane domains, and they may serve as cholesterol storage sites at the PM (46). Consistent with this view, we found that ABCA1 deficiency causes increases in Cav1 expression and in caveola number, in addition to increases in PM cholesterol. Cholesterol associated with caveolae may not be capable of acting as active cholesterol. In this study, by assessing CTxB internalization and retrograde cholesterol transport, we show that a certain endocytic activity, likely CIE, becomes defective in ABCA1-deficient cells. We designate this endocytic pathway as ABCA1-regulated, clathrin-independent endocytosis (ACIE). We also showed that this pathway is probably dynamin-dependent. Caveolar endocytosis is known to be a dynamin-mediated CIE process; this process internalizes PM glycosphingolipids (50, 64). We speculate that the caveolar endocytosis pathway may play an important role in internalization of PM cholesterol in a dynamin-dependent manner, and this pathway may be controlled by ABCA1. Further studies are required to determine the functional significance and molecular nature of ACIE. Endocytic internalization involves recognition of membrane deformation sites by membrane curvature-sensing proteins, which is a key endocytic process (51, 65). Endophilin was recently identified as a membrane curvature-sensing protein for dynamin-mediated CIE (49, 66). Interestingly, intracellular curvature-sensing proteins and extracellular apolipoproteins (lipid acceptors) such as apoA-I share a similar structural feature, namely amphipathic α-helices. As such, certain membrane curvature-sensing proteins may be involved in ABCA1-mediated retrograde sterol transport. In addition to its effect on ACIE, ABCA1 deficiency may also affect nonvesicular retrograde sterol transport in a certain manner.
Several lines of clinical and experimental observations in TD patients and Abca1−/− mice (10–13) are not fully explained by assuming that ABCA1 functions only in sterol release. Our current findings provide a plausible mechanism to explain these paradoxical observations. ABCA1 deficiency causes abnormal cholesterol accumulation at the PM and a deficiency in the delivery of regulatory sterols to the ER. Abnormalities at the PM disrupt PM function, including ACIE, whereas the lack of regulatory sterols at the ER causes enhanced SREBP cleavage. It is therefore conceivable that in local tissues and in macrophages, the enhanced SREBP cleavage leads to elevated LDLR expression and higher cholesterol biosynthesis, which accelerates tissue cholesterol deposition, despite concomitant plasma LDL reduction. In addition to TD, HDL levels are often associated with rare mutant alleles in the ABCA1 gene (67, 68). Patients with low HDL have a higher risk of coronary heart disease (68, 69). The bidirectional sterol movement driven by ABCA1 demonstrated in this work may provide a plausible explanation for the higher risk of coronary heart disease observed in patients with low HDL due to mutations in the ABCA1 gene; in addition to a defect in cholesterol efflux, the defect in ACIE described here could accelerate cholesterol accumulation in macrophages and in local tissues, causing premature atherosclerosis in the patients.
In summary, we have shown that ABCA1 affects cellular sterol influx; lack of ABCA1 diminishes sterol influx and disrupts sterol sensing at the ER. We also showed that ABCA1 is involved in CIE. To our knowledge, we are the first to report that lack of ABCA1 diminishes both sterol influx and CIE. We provided additional evidence to suggest that dynamin-mediated endocytosis may be involved in the retrograde sterol movement from the PM. At this point, we do not know whether the effect of ABCA1 on sterol influx and on CIE is direct or indirect. It is conceivable that ABCA1 mainly acts by fine-tuning the lipid composition at the PM, which in turn affects cellular lipid efflux, cellular lipid influx, as well as CIE. Future investigations are needed to test this interpretation.
Author Contributions
Y. Y., N. I., T. Y. C., and S. Y. conceived and designed the study. Y. Y. and T. Y. C. wrote the paper with help of M. A. R. and S. Y. Y. Y. performed most of the experiments. N. I. performed Figs. 2 and 5. A portion of the initial findings described in Figs. 1, 3, and 8 were made by Y. Y. and by N. I. independently. M. A. R. performed qRT-PCR experiments. T. F. performed EM. T. Kishimoto and T. Kobayashi performed live cell cholesterol staining (Fig. 8C). M. I. and K.U. established BHK/ABCA1-MM cells used in Fig. 7. S. A. D. and N. I. isolated MEFs. C. C. Y. C. and K. F. contributed reagents. Y. Y., N. I., T. Y. C., and S. Y. analyzed data with the help from the other authors. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We thank Jinglei Cheng for electron microscopy, Haruka Hayashi for mouse genotyping, and Stephanie Murphy for expert editing of this manuscript.
Addendum
Very recently, Frechin et al. (70) employed novel techniques to monitor expressions of various genes at the single cell level in response to local cell crowding. The authors made the surprise finding that among the more than 1000 genes examined, ABCA1 stands out as one of the few genes with the most profound changes. Additional analyses by Frechin et al. (70) suggest that ABCA1 participates in local cell crowding by controlling cell membrane lipid composition through a mechanism yet to be clarified. The model proposed for the action of ABCA1 (Fig. 12) is consistent with this interpretation.
This work was supported, in whole or in part, by National Institutes of Health Grants AG037609 and HL060306 (to T. Y. C. and C. C. Y. C.). This work was also supported by a grant-in-aid for young scientists from the Japan Society for the Promotion of Sciences (to Y. Y.), grants-in-aid for scientific research from the Ministry of Education, Sciences, Technology, Culture, and Sports of Japan (to Y. Y., to S. A.-D., to T. F., to K. U, and to S. Y.), and a MEXT-supported program for the Strategic Research Foundation at Private Universities (to S. Y.). The authors declare that they have no conflicts of interest with the contents of this article.
This article was selected as a Paper of the Week.
- ER
- endoplasmic reticulum
- ACAT
- acylCoA:cholesterol acyltransferase
- apoA-I
- apolipoprotein A-I
- Cav1
- caveolin-1
- CIE
- clathrin-independent endocytosis
- CME
- clathrin-mediated endocytosis
- CTxB
- cholera toxin B subunit
- CE
- cholesteryl ester
- HMGR
- HMG-CoA reductase
- LDLR
- LDL receptor
- MEF
- mouse embryonic fibroblast
- mTORC1
- mechanistic target of rapamycin complex 1
- PL
- phospholipid
- PM
- plasma membrane
- SM
- sphingomyelin
- SMase
- sphingomyelinase
- Scap
- SREBP cleavage activating protein
- SREBP
- sterol regulatory element-binding protein
- TD
- Tangier disease
- qRT
- quantitative RT
- MCD
- methyl-β-cyclodextrin
- ANOVA
- analysis of variance
- ACIE
- ABCA1-regulated, clathrin-independent endocytosis
- CE
- cholesteryl ester
- LDL-Chol
- LDL-derived cholesterol
- DF
- DMEM/F-12
- DRM
- detergent-resistant membrane
- BHK
- baby hamster kidney
- SRE
- sterol regulatory element
- 25HC
- 25-hydroxycholesterol.
References
- 1. Brown M. S., Goldstein J. L. (2009) Cholesterol feedback: from Schoenheimer's bottle to Scap's MELADL. J. Lipid Res. 50, S15–S27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chang T. Y., Chang C. C., Ohgami N., Yamauchi Y. (2006) Cholesterol sensing, trafficking, and esterification. Annu. Rev. Cell Dev. Biol. 22, 129–157 [DOI] [PubMed] [Google Scholar]
- 3. Tall A. R., Yvan-Charvet L., Terasaka N., Pagler T., Wang N. (2008) HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab. 7, 365–375 [DOI] [PubMed] [Google Scholar]
- 4. Ikonen E. (2008) Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 9, 125–138 [DOI] [PubMed] [Google Scholar]
- 5. Du X. M., Kim M. J., Hou L., Le Goff W., Chapman M. J., Van Eck M., Curtiss L. K., Burnett J. R., Cartland S. P., Quinn C. M., Kockx M., Kontush A., Rye K. A., Kritharides L., Jessup W. (2015) HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export. Circ. Res. 116, 1133–1142 [DOI] [PubMed] [Google Scholar]
- 6. Yokoyama S. (2006) Assembly of high density lipoprotein. Arterioscler. Thromb. Vasc. Biol. 26, 20–27 [DOI] [PubMed] [Google Scholar]
- 7. Vedhachalam C., Duong P. T., Nickel M., Nguyen D., Dhanasekaran P., Saito H., Rothblat G. H., Lund-Katz S., Phillips M. C. (2007) Mechanism of ATP-binding cassette transporter A1-mediated cellular lipid efflux to apolipoprotein A-I and formation of high density lipoprotein particles. J. Biol. Chem. 282, 25123–25130 [DOI] [PubMed] [Google Scholar]
- 8. Yamauchi Y., Reid P. C., Sperry J. B., Furukawa K., Takeya M., Chang C. C., Chang T. Y. (2007) Plasma membrane rafts complete cholesterol synthesis by participating in retrograde movement of precursor sterols. J. Biol. Chem. 282, 34994–35004 [DOI] [PubMed] [Google Scholar]
- 9. Abi-Mosleh L., Infante R. E., Radhakrishnan A., Goldstein J. L., Brown M. S. (2009) Cyclodextrin overcomes deficient lysosome-to-endoplasmic reticulum transport of cholesterol in Niemann-Pick type C cells. Proc. Natl. Acad. Sci. U.S.A. 106, 19316–19321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Assmann G., von Eckardstein A., Brewer H. B. (2001) in The Metabolic & Molecular Bases of Inherited Disease (Scriver R. C., Beaydet L. A., Sly S. W., Valle D., eds) pp. 2937–2960, 8th Ed., McGraw-Hill, New York [Google Scholar]
- 11. Zhu X., Lee J. Y., Timmins J. M., Brown J. M., Boudyguina E., Mulya A., Gebre A. K., Willingham M. C., Hiltbold E. M., Mishra N., Maeda N., Parks J. S. (2008) Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J. Biol. Chem. 283, 22930–22941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chung S., Timmins J. M., Duong M., Degirolamo C., Rong S., Sawyer J. K., Singaraja R. R., Hayden M. R., Maeda N., Rudel L. L., Shelness G. S., Parks J. S. (2010) Targeted deletion of hepatocyte ABCA1 leads to very low density lipoprotein triglyceride overproduction and low density lipoprotein hypercatabolism. J. Biol. Chem. 285, 12197–12209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parathath S., Mick S. L., Feig J. E., Joaquin V., Grauer L., Habiel D. M., Gassmann M., Gardner L. B., Fisher E. A. (2011) Hypoxia is present in murine atherosclerotic plaques and has multiple adverse effects on macrophage lipid metabolism. Circ. Res. 109, 1141–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Francis G. A., Knopp R. H., Oram J. F. (1995) Defective removal of cellular cholesterol and phospholipids by apolipoprotein A-I in Tangier disease. J. Clin. Invest. 96, 78–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arakawa R., Yokoyama S. (2002) Helical apolipoproteins stabilize ATP-binding cassette transporter A1 by protecting it from thiol protease-mediated degradation. J. Biol. Chem. 277, 22426–22429 [DOI] [PubMed] [Google Scholar]
- 16. Yamauchi Y., Chang C. C., Hayashi M., Abe-Dohmae S., Reid P. C., Chang T. Y., Yokoyama S. (2004) Intracellular cholesterol mobilization involved in the ABCA1/apolipoprotein-mediated assembly of high density lipoprotein in fibroblasts. J. Lipid Res. 45, 1943–1951 [DOI] [PubMed] [Google Scholar]
- 17. Nishikawa O., Yokoyama S., Kurasawa T., Yamamoto A. (1986) A method for direct incorporation of radiolabeled cholesteryl ester into human plasma low-density-lipoproteins: preparation of tracer substrate for cholesteryl ester transfer reaction between lipoproteins. J. Biochem. 99, 295–301 [DOI] [PubMed] [Google Scholar]
- 18. Moller M. L., Tchen T. T. (1961) Inhibition of cholesterol biogenesis by arsenite: preparation of labeled lanosterol. J. Lipid Res. 2, 342–343 [Google Scholar]
- 19. Hu W., Abe-Dohmae S., Tsujita M., Iwamoto N., Ogikubo O., Otsuka T., Kumon Y., Yokoyama S. (2008) Biogenesis of HDL by SAA is dependent on ABCA1 in the liver in vivo. J. Lipid Res. 49, 386–393 [DOI] [PubMed] [Google Scholar]
- 20. Oram J. F., Vaughan A. M., Stocker R. (2001) ATP-binding cassette transporter A1 mediates cellular secretion of α-tocopherol. J. Biol. Chem. 276, 39898–39902 [DOI] [PubMed] [Google Scholar]
- 21. Chang C. C., Miyazaki A., Dong R., Kheirollah A., Yu C., Geng Y., Higgs H. N., Chang T. Y. (2010) Purification of recombinant acyl-coenzyme A:cholesterol acyltransferase 1 (ACAT1) from H293 cells and binding studies between the enzyme and substrates using difference intrinsic fluorescence spectroscopy. Biochemistry 49, 9957–9963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamauchi Y., Furukawa K., Hamamura K., Furukawa K. (2011) Positive feedback loop between PI3K-Akt-mTORC1 signaling and the lipogenic pathway boosts Akt signaling: induction of the lipogenic pathway by a melanoma antigen. Cancer Res. 71, 4989–4997 [DOI] [PubMed] [Google Scholar]
- 23. Tanaka N., Abe-Dohmae S., Iwamoto N., Fitzgerald M. L., Yokoyama S. (2010) Helical apolipoproteins of high density lipoprotein enhance phagocytosis by stabilizing ATP-binding cassette transporter A7. J. Lipid Res. 51, 2591–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bryleva E. Y., Rogers M. A., Chang C. C., Buen F., Harris B. T., Rousselet E., Seidah N. G., Oddo S., LaFerla F. M., Spencer T. A., Hickey W. F., Chang T. Y. (2010) ACAT1 gene ablation increases 24(S)-hydroxycholesterol content in the brain and ameliorates amyloid pathology in mice with AD. Proc. Natl. Acad. Sci. U.S.A. 107, 3081–3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sugii S., Reid P. C., Ohgami N., Du H., Chang T. (2003) Distinct endosomal compartments in early trafficking of low density lipoprotein-derived cholesterol. J. Biol. Chem. 278, 27180–27189 [DOI] [PubMed] [Google Scholar]
- 26. Heino S., Lusa S., Somerharju P., Ehnholm C., Olkkonen V. M., Ikonen E. (2000) Dissecting the role of the Golgi complex and lipid rafts in biosynthetic transport of cholesterol to the cell surface. Proc. Natl. Acad. Sci. U.S.A. 97, 8375–8380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abe M., Makino A., Hullin-Matsuda F., Kamijo K., Ohno-Iwashita Y., Hanada K., Mizuno H., Miyawaki A., Kobayashi T. (2012) A role for sphingomyelin-rich lipid domains in the accumulation of phosphatidylinositol-4,5-bisphosphate to the cleavage furrow during cytokinesis. Mol. Cell. Biol. 32, 1396–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chaudhary N., Gomez G. A., Howes M. T., Lo H. P., McMahon K. A., Rae J. A., Schieber N. L., Hill M. M., Gaus K., Yap A. S., Parton R. G. (2014) Endocytic crosstalk: cavins, caveolins, and caveolae regulate clathrin-independent endocytosis. PLos Biol. 12, e1001832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Adams C. M., Reitz J., De Brabander J. K., Feramisco J. D., Li L., Brown M. S., Goldstein J. L. (2004) Cholesterol and 25-hydroxycholesterol inhibit activation of SREBPs by different mechanisms, both involving SCAP and Insigs. J. Biol. Chem. 279, 52772–52780 [DOI] [PubMed] [Google Scholar]
- 30. Düvel K., Yecies J. L., Menon S., Raman P., Lipovsky A. I., Souza A. L., Triantafellow E., Ma Q., Gorski R., Cleaver S., Vander Heiden M. G., MacKeigan J. P., Finan P. M., Clish C. B., Murphy L. O., Manning B. D. (2010) Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell 39, 171–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brandis K. A., Gale S., Jinn S., Langmade S. J., Dudley-Rucker N., Jiang H., Sidhu R., Ren A., Goldberg A., Schaffer J. E., Ory D. S. (2013) Box C/D small nucleolar RNA (snoRNA) U60 regulates intracellular cholesterol trafficking. J. Biol. Chem. 288, 35703–35713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Slotte J. P., Bierman E. L. (1988) Depletion of plasma-membrane sphingomyelin rapidly alters the distribution of cholesterol between plasma membranes and intracellular cholesterol pools in cultured fibroblasts. Biochem. J. 250, 653–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ito J., Nagayasu Y., Yokoyama S. (2000) Cholesterol-sphingomyelin interaction in membrane and apolipoprotein-mediated cellular cholesterol efflux. J. Lipid Res. 41, 894–904 [PubMed] [Google Scholar]
- 34. Gale S. E., Westover E. J., Dudley N., Krishnan K., Merlin S., Scherrer D. E., Han X., Zhai X., Brockman H. L., Brown R. E., Covey D. F., Schaffer J. E., Schlesinger P., Ory D. S. (2009) Side chain oxygenated cholesterol regulates cellular cholesterol homeostasis through direct sterol-membrane interactions. J. Biol. Chem. 284, 1755–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cheng D., Chang C. C., Qu X., Chang T. Y. (1995) Activation of acyl-coenzyme A:cholesterol acyltransferase by cholesterol or by oxysterol in a cell-free system. J. Biol. Chem. 270, 685–695 [DOI] [PubMed] [Google Scholar]
- 36. Metherall J. E., Li H., Waugh K. (1996) Role of multidrug resistance P-glycoproteins in cholesterol biosynthesis. J. Biol. Chem. 271, 2634-2 640. [DOI] [PubMed] [Google Scholar]
- 37. Suzuki S., Nishimaki-Mogami T., Tamehiro N., Inoue K., Arakawa R., Abe-Dohmae S., Tanaka A. R., Ueda K., Yokoyama S. (2004) Verapamil increases the apolipoprotein-mediated release of cellular cholesterol by induction of ABCA1 expression via liver X receptor-independent mechanism. Arterioscler. Thromb. Vasc. Biol. 24, 519–525 [DOI] [PubMed] [Google Scholar]
- 38. Lange Y., Ory D. S., Ye J., Lanier M. H., Hsu F. F., Steck T. L. (2008) Effectors of rapid homeostatic responses of endoplasmic reticulum cholesterol and 3-hydroxy-3-methylglutaryl-CoA reductase. J. Biol. Chem. 283, 1445–1455 [DOI] [PubMed] [Google Scholar]
- 39. Song B. L., Javitt N. B., DeBose-Boyd R. A. (2005) Insig-mediated degradation of HM-CoA reductase stimulated by lanosterol, an intermediate in the synthesis of cholesterol. Cell Metab. 1, 179–189 [DOI] [PubMed] [Google Scholar]
- 40. Scheek S., Brown M. S., Goldstein J. L. (1997) Sphingomyelin depletion in cultured cells blocks proteolysis of sterol regulatory element binding proteins at site 1. Proc. Natl. Acad. Sci. U.S.A. 94, 11179–11183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chang T. Y., Limanek J. S. (1980) Regulation of cytosolic acetoacetyl coenzyme A thiolase, 3-hydroxy-3-methylglutaryl coenzyme A synthase, 3-hydroxy-3-methylglutaryl coenzyme A reductase, and mevalonate kinase by low density lipoprotein and by 25-hydroxycholesterol in Chinese hamster ovary cells. J. Biol. Chem. 255, 7787–7795 [PubMed] [Google Scholar]
- 42. Hua X., Nohturfft A., Goldstein J. L., Brown M. S. (1996) Sterol resistance in CHO cells traced to point mutation in SREBP cleavage-activating protein. Cell 87, 415–426 [DOI] [PubMed] [Google Scholar]
- 43. Gil G., Faust J. R., Chin D. J., Goldstein J. L., Brown M. S. (1985) Membrane-bound domain of HMG CoA reductase is required for sterol-enhanced degradation of the enzyme. Cell 41, 249–258 [DOI] [PubMed] [Google Scholar]
- 44. Hamon Y., Broccardo C., Chambenoit O., Luciani M. F., Toti F., Chaslin S., Freyssinet J. M., Devaux P. F., McNeish J., Marguet D., Chimini G. (2000) ABC1 promotes engulfment of apoptotic cells and transbilayer redistribution of phosphatidylserine. Nat. Cell Biol. 2, 399–406 [DOI] [PubMed] [Google Scholar]
- 45. Takahashi K., Kimura Y., Kioka N., Matsuo M., Ueda K. (2006) Purification and ATPase activity of human ABCA1. J. Biol. Chem. 281, 10760–10768 [DOI] [PubMed] [Google Scholar]
- 46. Parton R. G., Simons K. (2007) The multiple faces of caveolae. Nat. Rev. Mol. Cell Biol. 8, 185–194 [DOI] [PubMed] [Google Scholar]
- 47. Koseki M., Hirano K., Masuda D., Ikegami C., Tanaka M., Ota A., Sandoval J. C., Nakagawa-Toyama Y., Sato S. B., Kobayashi T., Shimada Y., Ohno-Iwashita Y., Matsuura F., Shimomura I., Yamashita S. (2007) Increased lipid rafts and accelerated lipopolysaccharide-induced tumor necrosis factor-α secretion in Abca1-deficient macrophages. J. Lipid Res. 48, 299–306 [DOI] [PubMed] [Google Scholar]
- 48. Le P. U., Guay G., Altschuler Y., Nabi I. R. (2002) Caveolin-1 is a negative regulator of caveolae-mediated endocytosis to the endoplasmic reticulum. J. Biol. Chem. 277, 3371–3379 [DOI] [PubMed] [Google Scholar]
- 49. Renard H. F., Simunovic M., Lemière J., Boucrot E., Garcia-Castillo M. D., Arumugam S., Chambon V., Lamaze C., Wunder C., Kenworthy A. K., Schmidt A. A., McMahon H. T., Sykes C., Bassereau P., Johannes L. (2015) Endophilin-A2 functions in membrane scission in clathrin-independent endocytosis. Nature 517, 493–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Puri V., Watanabe R., Singh R. D., Dominguez M., Brown J. C., Wheatley C. L., Marks D. L., Pagano R. E. (2001) Clathrin-dependent and -independent internalization of plasma membrane sphingolipids initiates two Golgi targeting pathways. J. Cell Biol. 154, 535–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Doherty G. J., McMahon H. T. (2009) Mechanisms of endocytosis. Annu. Rev. Biochem. 78, 857–902 [DOI] [PubMed] [Google Scholar]
- 52. Macia E., Ehrlich M., Massol R., Boucrot E., Brunner C., Kirchhausen T. (2006) Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell 10, 839–850 [DOI] [PubMed] [Google Scholar]
- 53. Hill T. A., Gordon C. P., McGeachie A. B., Venn-Brown B., Odell L. R., Chau N., Quan A., Mariana A., Sakoff J. A., Chircop M., Robinson P. J., McCluskey A. (2009) Inhibition of dynamin mediated endocytosis by the dynoles–synthesis and functional activity of a family of indoles. J. Med. Chem. 52, 3762–3773 [DOI] [PubMed] [Google Scholar]
- 54. Beaven S. W., Tontonoz P. (2006) Nuclear receptors in lipid metabolism: targeting the heart of dyslipidemia. Annu. Rev. Med. 57, 313–329 [DOI] [PubMed] [Google Scholar]
- 55. van Meer G., Voelker D. R., Feigenson G. W. (2008) Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 9, 112–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Quazi F., Lenevich S., Molday R. S. (2012) ABCA4 is an N-retinylidene-phosphatidylethanolamine and phosphatidylethanolamine importer. Nat. Commun. 3, 925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li Y., Prinz W. (2004) ATP-binding cassette (ABC) transporters mediate nonvesicular, raft-modulated sterol movement from the plasma membrane to the endoplasmic reticulum. J. Biol. Chem. 279, 45226–45234 [DOI] [PubMed] [Google Scholar]
- 58. Landry Y. D., Denis M., Nandi S., Bell S., Vaughan A. M., Zha X. (2006) ATP-binding cassette transporter A1 expression disrupts raft membrane microdomains through its ATPase-related functions. J. Biol. Chem. 281, 36091–36101 [DOI] [PubMed] [Google Scholar]
- 59. Zarubica A., Plazzo A. P., Stöckl M., Trombik T., Hamon Y., Müller P., Pomorski T., Herrmann A., Chimini G. (2009) Functional implications of the influence of ABCA1 on lipid microenvironment at the plasma membrane: a biophysical study. FASEB J. 23, 1775–1785 [DOI] [PubMed] [Google Scholar]
- 60. Radhakrishnan A., Anderson T. G., McConnell H. M. (2000) Condensed complexes, rafts, and the chemical activity of cholesterol in membranes. Proc. Natl. Acad. Sci. U.S.A. 97, 12422–12427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Steck T. L., Lange Y. (2010) Cell cholesterol homeostasis: mediation by active cholesterol. Trends Cell Biol. 20, 680–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Quazi F., Molday R. S. (2013) Differential phospholipid substrates and directional transport by ATP-binding cassette proteins ABCA1, ABCA7, and ABCA4 and disease-causing mutants. J. Biol. Chem. 288, 34414–34426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Olsen B. N., Bielska A. A., Lee T., Daily M. D., Covey D. F., Schlesinger P. H., Baker N. A., Ory D. S. (2013) The structural basis of cholesterol accessibility in membranes. Biophys. J. 105, 1838–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shvets E., Bitsikas V., Howard G., Hansen C. G., Nichols B. J. (2015) Dynamic caveolae exclude bulk membrane proteins and are required for sorting of excess glycosphingolipids. Nat. Commun. 6, 6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Antonny B. (2011) Mechanisms of membrane curvature sensing. Annu. Rev. Biochem. 80, 101–123 [DOI] [PubMed] [Google Scholar]
- 66. Boucrot E., Ferreira A. P., Almeida-Souza L., Debard S., Vallis Y., Howard G., Bertot L., Sauvonnet N., McMahon H. T. (2015) Endophilin marks and controls a clathrin-independent endocytic pathway. Nature 517, 460–465 [DOI] [PubMed] [Google Scholar]
- 67. Cohen J. C., Kiss R. S., Pertsemlidis A., Marcel Y. L., McPherson R., Hobbs H. H. (2004) Multiple rare alleles contribute to low plasma levels of HDL cholesterol. Science 305, 869–872 [DOI] [PubMed] [Google Scholar]
- 68. Clee S. M., Kastelein J. J., van Dam M., Marcil M., Roomp K., Zwarts K. Y., Collins J. A., Roelants R., Tamasawa N., Stulc T., Suda T., Ceska R., Boucher B., Rondeau C., DeSouich C., et al. (2000) Age and residual cholesterol efflux affect HDL cholesterol levels and coronary artery disease in ABCA1 heterozygotes. J. Clin. Invest. 106, 1263–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schaefer E. J., Zech L. A., Schwartz D. E., Brewer H. B. (1980) Coronary heart disease prevalence and other clinical features in familial high density lipoprotein deficiency (Tangier disease). Ann. Intern. Med. 93, 261–266 [DOI] [PubMed] [Google Scholar]
- 70. Frechin M., Stoeger T., Daetwyler S., Gehin C., Battich N., Damm E. M., Stergiou L., Riezman H., Pelkmans L. (2015) Cell-intrinsic adaptation of lipid composition to local crowding drives social behaviour. Nature 523, 88–91 [DOI] [PubMed] [Google Scholar]