Background: Mice with a defect in mitochondrial fatty acid oxidation (FAO) have reduced lung function but the mechanism is not known.
Results: Acylcarnitines accumulate in FAO-deficient lungs and inhibit pulmonary surfactant.
Conclusion: Acylcarnitines contribute to reduced lung function in FAO-deficient mice.
Significance: Humans with FAO deficiencies may be at risk for lung injury due to acylcarnitine inhibition of pulmonary surfactant.
Keywords: fatty acid oxidation, gene knockout, lung, mitochondria, pulmonary surfactant, acyl-CoA dehydrogenase, acylcarnitine, mildronate
Abstract
The role of mitochondrial energy metabolism in maintaining lung function is not understood. We previously observed reduced lung function in mice lacking the fatty acid oxidation enzyme long-chain acyl-CoA dehydrogenase (LCAD). Here, we demonstrate that long-chain acylcarnitines, a class of lipids secreted by mitochondria when metabolism is inhibited, accumulate at the air-fluid interface in LCAD−/− lungs. Acylcarnitine accumulation is exacerbated by stress such as influenza infection or by dietary supplementation with l-carnitine. Long-chain acylcarnitines co-localize with pulmonary surfactant, a unique film of phospholipids and proteins that reduces surface tension and prevents alveolar collapse during breathing. In vitro, the long-chain species palmitoylcarnitine directly inhibits the surface adsorption of pulmonary surfactant as well as its ability to reduce surface tension. Treatment of LCAD−/− mice with mildronate, a drug that inhibits carnitine synthesis, eliminates acylcarnitines and improves lung function. Finally, acylcarnitines are detectable in normal human lavage fluid. Thus, long-chain acylcarnitines may represent a risk factor for lung injury in humans with dysfunctional fatty acid oxidation.
Introduction
Mitochondrial long-chain fatty acid oxidation (FAO)2 is the process by which fatty acids are catabolized for the production of ATP. The pathway begins with carnitine-dependent facilitated transport of fatty acids across the outer mitochondrial membrane, catalyzed by carnitine palmitoyltransferase-1 (CPT1). On the inner membrane, CPT2 converts incoming acylcarnitines back to acyl-CoAs which then undergo repeated cycles of chain-shortening. Each β-oxidation cycle requires four enzymatic steps. The first step is dehydrogenation catalyzed by the acyl-CoA dehydrogenase (ACAD) enzyme family. There are two ACAD family members specific for long-chain substrates known as long-chain acyl-CoA dehydrogenase (LCAD) and very long-chain acyl-CoA dehydrogenase (VLCAD). The second through fourth steps of long-chain FAO are catalyzed by mitochondrial trifunctional protein (TFP). With each round of chain-shortening, TFP produces an acetyl-CoA and an acyl-CoA that is two carbons shorter.
FAO is a critical metabolic pathway in the heart, muscle, and liver, but little is known about its role in other tissues. We previously observed high rates of FAO in mouse lung and showed that LCAD is the dominant long-chain ACAD enzyme in both murine and human lung (1). LCAD is specifically expressed in alveolar type II (ATII) pneumocytes (1). ATII cells, which only comprise 5% of the alveolar surface, fulfill several critical roles in the lung. They produce pulmonary surfactant, a specialized mixture of phospholipids and proteins that controls surface tension and alveolar compression during breathing. ATII cells also maintain active ion transport to prevent edema, and they regenerate the alveolar epithelium upon injury (2). They are rich in mitochondria suggesting a high demand for energy (3). LCAD knock-out mice have reduced lung FAO, reduced levels of active pulmonary surfactant, and altered breathing mechanics characterized by reduced pulmonary compliance, or in other words, stiffer, less elastic lungs (1).
When fatty acid metabolism is blocked, such as in patients with inborn errors of FAO, acyl-CoAs accumulate inside the mitochondria and drive the CPT2 reaction in reverse, producing acylcarnitines, which are subsequently secreted. Increased acylcarnitine levels in the blood are the basis of newborn screening programs for FAO disorders (4). Biologically, acylcarnitines are a poorly understood class of lipids. Short-chain acylcarnitines may be used for energy and can serve as a carbon source for phospholipid synthesis in some tissues (5, 6). They appear to have clinical benefits for neurological disorders although the specific mechanisms are not understood (7, 8). Long-chain acylcarnitines, on the other hand, have been linked to cardiac arrhythmias, insulin resistance, and epithelial barrier dysfunction, although their role in disease pathogenesis is controversial (9–12). Here, we show that acylcarnitines accumulate at high concentrations in the epithelial lining fluid of LCAD−/− lungs. The epithelial lining fluid is an incredibly thin (<0.1 μm) layer of fluid separating alveolar tissue from the air. We further show that acylcarnitines directly inhibit the surface-activity of pulmonary surfactant, which forms a film at the surface of the epithelial lining fluid. Future work will evaluate the FAO pathway and acylcarnitines as risk factors for acute lung injury.
Experimental Procedures
Animals
LCAD+/− mice (B6.129S6-Acadltm1Uab) were purchased from the Mutant Mouse Regional Resource Center (University of Missouri, Columbia, MO). LCAD−/− and LCAD+/+ littermates were used to establish breeding colonies of mutant and control mice according to protocols approved by the University of Pittsburgh Institutional Animal Care and Use Committee. All studies were done in 5–6-week-old male mice. d-Carnitine and l-carnitine (Sigma-Aldrich) were delivered in drinking water at 150 mg/kg/day. Mildronate (Grindex, Latvia) was given in drinking water at 200 mg/kg/day. For the influenza study, mice were infected with 100 plaque-forming units (PFU) of virus in 50 μl of sterile PBS by oropharyngeal aspiration. The influenza A/PR/8/34 (H1N1) virus was propagated in chicken eggs as described (13).
MLE12 and ATII Cell Studies
Mouse lung epithelial cells (MLE12; American Type Culture Collection, Manassas, VA) were cultured as described (1). Primary human ATII cells were isolated and cultured as described (14). The human lungs, from de-identified organ donors whose lungs were not suitable for transplantation, were obtained through the International Institute for the Advancement of Medicine (Edison, NJ) and the Center for Organ Recovery (Pittsburgh, PA). Both MLE12 and primary ATII cells were grown in 24-well plates and treated with either 80 μm of l-carnitine or 1 mm of d-carnitine. Forty-eight hours later, [3H]palmitate oxidation was measured as described with 125 μm of [3H]palmitate-BSA for 2 h (1). For phospholipid synthesis determination, 1 μCi/well of [3H]choline was added to medium and incubated for 24 h. Total [3H]phospholipids were extracted following Bligh and Dyer and normalized to cellular protein (1).
Bronchoalveolar Lavage
For acylcarnitine profiling, anesthetized, tracheotomized mice were lavaged with two 1-ml aliquots of 50% acetonitrile/0.3% formic acid to aid with solubility of long-chain acylcarnitine species. For isolation of large aggregate surfactant, mouse lungs were washed three times with 1-ml aliquots of 0.9% (w/v) NaCl. The fluid was pooled, centrifuged at 300 × g for 10 min to remove cells, and the supernatant centrifuged at 40,000 × g for 15 min to pellet large aggregate surfactant. Human lavage fluid was collected from organ donor lungs deemed not suitable for transplantation. One lung lobe was perfused to remove blood and then lavaged with 60 ml of HEPES/saline. Cells were removed by centrifugation.
Acylcarnitine Profiling
Acyl-carnitine measurements were made by flow injection tandem mass spectrometry using sample preparation methods described previously (15, 16). The data were acquired using a Waters AcquityTM UPLC system equipped with a TQ (triple quadrupole) detector and a data system controlled by MassLynx 4.1 operating system (Waters, Milford, MA).
Constrained Drop Surfactometry
To measure adsorption, palmitoylcarnitine was dissolved into a buffer of 0.9% NaCl, 1.5 mm CaCl2, and 2.5 mm HEPES, pH 7.0. A ∼10 μl droplet was dispensed onto the drop holder. Measurements were conducted at 37 °C and 100% relative humidity. Drop images were taken at a rate of 10 frames per second. The surface tension, surface area and drop volume were determined with axisymmetric drop shape analysis (17). Infasurf studies were done in the same buffer and under the same conditions. For dynamic cycling, the drop was compressed and expanded at least five times. All drop images were recorded to calculate minimum surface tension at the end of compression and maximum surface tension at the end of expansion. Film compressibility κ; = (1/A) (∂A/∂γ) was calculated as described (18).
Palmitate Oxidation in Mouse Lung Tissues
Minced tissues were incubated in 350 μl of PBS with 5 mm glucose, 1 mm carnitine, and 125 μm final concentration of [3H]palmitate-BSA at 37 °C for 1 h as described (1). After homogenization using a Bullet Blender (Next Advance, Averill Park, NY), the aqueous layer was separated using chloroform/methanol extraction and counted.
Surfactant Phospholipids
Colorimetric determination of phospholipids was performed using the malachite green assay as described (1). Total surfactant phospholipids were calculated from the measured concentrations by multiplying by the volume of fluid recovered during lavage.
Pulmonary Function Testing
Respiratory mechanics were measured as described previously (1). Briefly, mice were anesthetized with pentobarbital sodium (90 mg/kg ip), tracheotomized, and attached to a computer-controlled piston ventilator (FlexiVent, SCIREQ, Montreal, QC, Canada). Pressure-volume analysis was completed in a series of stepwise volume inflation and deflation with a maximum lung pressure of 30 cmH20, and used to calculate pulmonary compliance.
Carnitine Assay
Free carnitine in mouse lung tissues was measured using a carnitine assay kit (Sigma-Aldrich) following the manufacturer's instructions.
Statistical Analyses
All bar graphs depict means and standard deviations. Group comparisons were made using two-tailed Student's t-tests. The level of statistical significance was taken as p < 0.05.
Results
Reducing Mitochondrial FAO Does Not Reduce Surfactant Phospholipid Synthesis or Surfactant Protein Expression
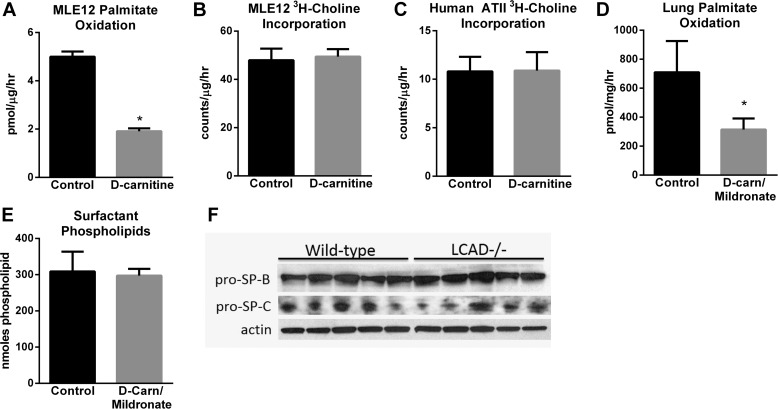
We previously analyzed LCAD−/− mice and observed ∼50% reduction in lung palmitate oxidation and a significant loss of surface-active pulmonary surfactant (1). Explants of LCAD−/− lung showed normal incorporation of [3H]choline into lipids in a short-term incubation (1). To study phospholipid synthesis specifically in ATII cells, we simulated a long-chain FAO deficiency in the murine ATII-like cell line MLE12 through treatment with d-carnitine. Long-chain FAO is l-carnitine-dependent, and d-carnitine effectively inhibits l-carnitine uptake by competition for the carnitine transporter. d-carnitine reduced 3H-palmitate oxidation by 60% in MLE12 cells (Fig. 1A), a reduction similar to what is seen in LCAD−/− lung in vivo (1), but the rate of incorporation of radiolabeled choline into total phospholipids was not affected (Fig. 1B). This experiment was repeated with primary human ATII cells. d-Carnitine reduced [3H]palmitate oxidation by 62% but again did not alter the incorporation of [3H]choline into the cellular lipid fraction (Fig. 1C). The effects of carnitine deficiency were then further tested in vivo by treating wild-type mice with d-carnitine combined with mildronate, a drug known to block carnitine synthesis in the liver (19). The d-carnitine/mildronate treatment resulted in a 55% reduction in lung FAO, but no change was observed in large aggregate surfactant phospholipid levels (Fig. 1, D and E). These data suggest that blocking FAO does not inhibit phospholipid synthesis, and furthermore, that carnitine deficiency does not recapitulate the loss of surfactant quantity seen in LCAD−/− mice.
FIGURE 1.
Reducing mitochondrial FAO does not reduce surfactant phospholipid synthesis or surfactant protein expression. A, radiolabeled palmitate was used to determine the rate of FAO in MLE12 cells rendered carnitine-deficient by treatment with d-carnitine (n = 4). Phospholipid synthesis in d-carnitine-treated (B) MLE12 cells or (C) primary human ATII cells, measured as radiolabeled choline incorporation into the lipid fraction over 24-hr. D, radiolabeled palmitate oxidation in minced lung tissue from wild-type mice treated for 21 days with a combination of d-carnitine and mildronate to induce carnitine deficiency (n = 6). E, total phospholipids recovered in the large aggregate surfactant fraction of wild-type mice after 21 days of treatment with d-carnitine/mildronate (n = 5–6). F, immunoblots of lung lysates from untreated wild-type and LCAD−/− mice, using 50 μg of protein per lane, with anti-surfactant protein B (SP-B) and surfactant protein C (SP-C) antibodies. Densitometric analysis relative to actin revealed no significant differences between genotypes of mice. All bars depict means and standard deviations. *, p < 0.01, carnitine-deficient versus control.
In addition to phospholipids, surface-active, large-aggregate surfactant requires the presence of surfactant protein-B (SP-B) and surfactant protein-C (SP-C). To determine whether reduced SP-B and SP-C contribute to reduced large aggregate surfactant in LCAD−/− mice, we performed immunoblotting on lung tissue lysates followed by densitometric analysis of the bands. Neither SP-B nor SP-C protein levels were significantly altered in LCAD−/− lungs (Fig. 1F).
LCAD−/− Lungs Accumulate Long-chain Acylcarnitines
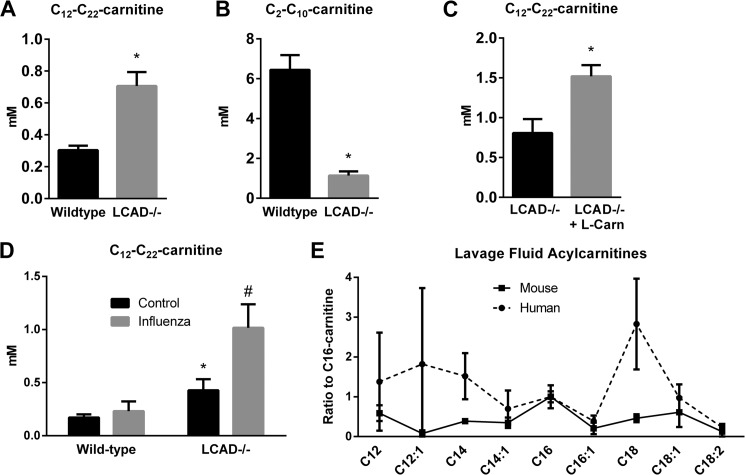
Genetic blockades of FAO are associated with secretion of acylcarnitines to avoid lipid toxicity, while carnitine deficiency would inhibit FAO without acylcarnitine secretion. We reasoned that this difference may account for the lack of a change in surfactant levels in carnitine-deficient mice. Therefore, we performed acylcarnitine profiling on bronchoalveolar lavage fluid from overnight-fasted LCAD−/− and wild-type control mice. During lavage the lungs were washed with 2 ml, which captures the ∼8 μl of epithelial lining fluid (ELF) that is estimated to coat the interior of the mouse lung at the air-tissue interface (20). After accounting for the ∼250 dilution factor, we estimate that the total concentration of long-chain acylcarnitines (C12-C22) in wild-type mouse ELF is about 0.3 mm, which is 200X higher than what has been reported in normal mouse blood (21). In LCAD−/− mice the concentration of long-chain species was more than doubled (Fig. 2A), and some species such as the unsaturated 14-carbon acylcarnitines C14:1 and C14:2 were elevated as much as 14-fold. At the same time, short-chain acylcarnitine species were severely reduced in LCAD−/− mice, indicating that LCAD−/− lungs are unable to catabolize fatty acids beyond C12 (Fig. 2B).
FIGURE 2.
LCAD−/− lungs accumulate long-chain acylcarnitines. Wild-type and LCAD−/− mice (n = 5) were fasted overnight and then lavaged with 50% acetonitrile/0.3% formic acid. The BALF was analyzed for 66 acylcarnitine species using mass spectrometry. LCAD−/− mice have (A) significantly increased total long-chain acylcarnitine species, and (B) significantly decreased short-chain species. C, carnitine supplementation increases long-chain acylcarnitine secretion even further in LCAD−/− mice, indicating that free carnitine is rate-limiting in the lung. D, stressing the lung with infection (influenza) also stimulates acylcarnitine secretion. E, five human lungs and three mice were lavaged with saline and acylcarnitine profiling performed. *, p < 0.01, LCAD−/− versus control.
Genetic FAO disorders as well as complex disease states such as obesity can be associated with a secondary carnitine deficiency due to the enlarged acylcarnitine pool sequestering free carnitine. Carnitine given as a supplement to patients with FAO disorders typically enhances acylcarnitine secretion even further, which some have proposed is beneficial due to clearing of intra-mitochondrial lipids (22). To determine whether carnitine is rate-limiting in LCAD−/− lung and whether acylcarnitine secretion into ELF would respond to carnitine therapy, we treated LCAD−/− mice for 21 days with l-carnitine. Acylcarnitine profiling showed an approximate doubling of acylcarnitines (Fig. 2C). This indicates that acylcarnitine secretion into ELF is dynamic and that the amount of free carnitine can become rate-limiting in the context of an FAO disorder. We next asked whether stressing the lung may also alter acylcarnitine secretion. Wild-type and LCAD−/− mice were infected with influenza and lavaged for acylcarnitine analysis 7 days later. Influenza increased long-chain acylcarnitines by more than 2-fold in LCAD−/− mice but had no effect on acylcarnitines in wild-type mice (Fig. 2D).
Finally, we investigated whether acylcarnitine secretion into ELF is a mouse-specific phenomenon. Five samples of human lung wash fluids were subjected to acylcarnitine profiling. Interestingly, among the long-chain species relevant to mitochondrial FAO metabolism and LCAD activity (C12-C18), human lungs showed a different profile than mice: C18 was the most abundant species, compared with C16 for mice (Fig. 2E). These data demonstrate the utility of profiling acylcarnitines as biomarkers in human bronchoalveolar wash fluid.
Long-chain Acylcarnitines Inhibit Pulmonary Surfactant
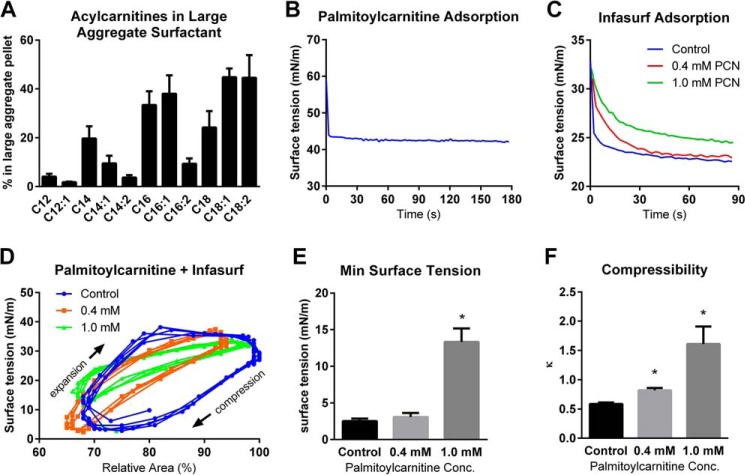
Short-chain acylcarnitines are water-soluble and readily diffusible, while long-chain species have limited solubility. Hydrophobic interactions have been observed between long-chain acylcarnitines and lipid bilayers (23). To determine whether long-chain acylcarnitines interact with pulmonary surfactant, LCAD−/− lavage fluid was subjected to centrifugation to pellet the surface-active large-aggregate surfactant. The pellet and supernatant were both profiled for acylcarnitines and the relative distribution between fractions was calculated. Short-chain species <C12 were found almost exclusively in the supernatant, while as much as 40% of the C12-C18 species were found associated with the active fraction of pulmonary surfactant (Fig. 3A). This ability to associate with surfactant was further studied in vitro using the commercially available species C16-carnitine (palmitoylcarnitine). 14C-labeled C16-carnitine was added to freshly collected wild-type lavage fluid. The fluid was centrifuged to isolate the large aggregate surfactant pellet and both the pellet and supernatant were used for scintillation counting. In keeping with the in vivo data, 44.6 ± 2.2% of the radiolabel was recovered in the surfactant pellet fraction.
FIGURE 3.
Long-chain acylcarnitines inhibit pulmonary surfactant. A, LCAD−/− mice (n = 6) were fasted overnight and lavaged with saline. The BALF was centrifuged to pellet large-aggregate surfactant. The large aggregate pellet and the supernatant were then analyzed for acylcarnitines by mass spectrometry, and the data used to calculate the % of the total amount of each species appearing in the pellet fraction. Shown are several relevant long-chain species. B, palmitoylcarnitine in solution (1 mg/ml) was observed to rapidly adsorb to the air-water interface and lower surface tension of the air-water interface in a constrained drop surfactometer. C, palmitoylcarnitine was added (0.4 or 1.0 mm) to Infasurf, a clinical formulation of pulmonary surfactant. The rate of adsorption to the air-water surface, revealed by surface tension decrease, was monitored as a function of time using the constrained drop surfactometer. D, infasurf with and without palmitoylcarnitine was allowed to reach surface equilibrium on the constrained drop surfactometer, and then was subjected to cycles of compression/expansion “breathing” using a motor-driven syringe to deflate/inflate the droplet. Surface tension (Y-axis) and surface area (X-axis) were measured and plotted. The data in these cycles were used to calculate (E) minimum surface tension reached during cycling, and (F) compressibility of the surfactant film. All bars depict means and standard deviations. *, p < 0.01, palmitoylcarnitine-treated versus control.
For surfactant to properly function in vivo, it must adsorb rapidly to the air-water interface and reduce surface tension, which facilitates breathing by making the alveoli easier to inflate (pulmonary compliance) (24). The surface tension of a pure air-water interface is ∼70 mN/m at body temperature. Using a constrained-drop surfactometer, the adsorption isotherm of C16-carnitine was determined (Fig. 3B). It was found that C16-carnitine rapidly adsorbed to the air-water interface where it significantly reduced surface tension to an equilibrium value of 42 mN/m. Pulmonary surfactant adsorbed onto a static air-water interface will reduce surface tension to about 25 mN/m (24). We next asked whether the surface-activity of C16-carnitine would compete with surfactant for the air-water interface and reduce its ability to lower surface tension to 25 mN/m. Indeed, when mixed with the clinical surfactant preparation Infasurf (1 mg/ml) and introduced onto the surfactometer, 0.4 mm C16-carnitine delayed the adsorption of the surfactant to the air-water interface (Fig. 3C). At a concentration of 1.0 mm, C16-carnitine was sufficient to both delay adsorption and prevent maximal lowering of surface tension (Fig. 3C).
During exhalation, an alveolus is contracted and the relative concentration of surfactant at the alveolar surface becomes greater, which causes surface tension to drop from ∼25 mN/m to near-zero (24). Pulmonary surfactant at a high surface concentration, achieved during exhalation, also has low compressibility which limits compression (shrinking) of the alveolus to ∼20%, thereby preventing alveolar collapse. To mimic human breathing, we allowed Infasurf to adsorb to the surface on the constrained drop surfactometer and then dynamically cycled the droplet with twenty compression/expansion cycles (“breaths”) per minute using a motor-driven syringe. This was done in the presence and absence of C16-carnitine, while measuring the correlation between the surface tension and surface area in real-time (Fig. 3D). As the adsorbed surfactant film was compressed (by withdrawing liquid from the droplet), the surface tension was reduced to 2 mN/m for both Infasurf alone and Infasurf plus 0.4 mm C16-carnitine, but did not go below 12 mN/m in the presence of 1.0 mm C16-carnitine (Fig. 3E). Compressibility constants calculated from the cycling data in Fig. 3F showed that C16-carnitine significantly increases film compressibility, which in vivo would translate into a softer surfactant film less able to resist alveolar collapse upon exhalation.
Eliminating Lung Acylcarnitines Improves Surfactant Levels and Lung Function in LCAD−/− Mice
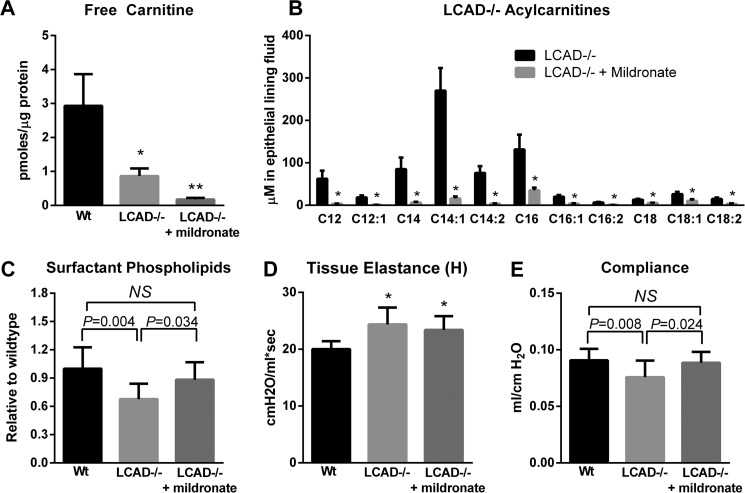
The total concentration of long-chain acylcarnitines in LCAD knock-out lung is greater than 0.7 mm (Fig. 2A), which is well within the range demonstrated to inhibit surfactant in vitro. To determine the contribution of long-chain acylcarnitines to lung function in vivo we treated LCAD−/− mice with the drug mildronate for 21 days. Mildronate, an inhibitor of hepatic carnitine synthesis, is used clinically in Eastern Europe to treat ischemia (25). Free carnitine in LCAD−/− lung tissue was already very low compared with wild type mice, due to greater partitioning of carnitine into acylcarnitines, and a high dose of mildronate (200 mg/kg/day) nearly eliminated lung free carnitine (Fig. 4A). Consequently, acylcarnitines recovered in lavage fluids decreased by ∼85% across all measured species (Fig. 4B). Functionally, mildronate increased large aggregate surfactant phospholipid levels significantly over untreated LCAD−/− mice (Fig. 4C).
FIGURE 4.
Mildronate treatment eliminates acylcarnitines and improves lung function in LCAD−/− mice. LCAD−/− mice were treated with 200 mg/kg/day mildronate to induce carnitine deficiency. A, free carnitine concentration in mouse lung tissue (n = 4–5). B, changes in key species of long-chain acylcarnitines in the epithelial lining fluid. C, mildronate improved the levels of large aggregate surfactant phospholipids in LCAD−/− lavage fluids (n = 9–10). D, mildronate did not improve the tissue elastance (H) parameter of lung function as determined with a FlexiVent small animal ventilator, but (E) did normalize pulmonary compliance determined by analysis of pressure-volume loops (n = 9–10). *, p < 0.01; **, p < 0.001; NS, not significant between indicated groups. All bars depict means and standard deviations.
We next measured pulmonary function in mildronate-treated LCAD−/− mice using a small-animal ventilator (FlexiVent). FlexiVent measures three tissue-related parameters termed elastance (H), damping (G), and central airway resistance (Rn). LCAD−/− mice were observed to have a significant increase in tissue elastance (H) compared with wild-type controls (Fig. 4D), and mildronate treatment did not correct this defect in lung mechanics. The G and Rn parameters were not significantly different between groups (not shown). In addition to the tissue parameters H, G, and Rn, pressure-volume curves were measured and analyzed to produce the parameter of compliance. Compliance is an index of overall lung mechanics, representing the elasticity of the lungs during breathing. In the context of surfactant deficiencies or inflammation-induced lung injury, lungs must work harder to achieve the same inflation (low compliance). In models of severe lung injury such as those induced by lipopolysaccharide (LPS) or bleomycin, compliance may decrease by 25–35% (26, 27). In comparison, unstressed LCAD knock-out mice were observed to have a ∼16% reduction in compliance compared with wild-type mice, which was corrected back to near-normal levels by mildronate treatment (Fig. 4D). As a control, we evaluated the effects of mildronate in a separate cohort of wild-type animals and observed that the drug had no effect on compliance or the tissue parameters H, G, and Rn. Together, these experiments show that mildronate improves some aspects of lung function (compliance) but not others (tissue elastance). This is consistent with an improvement in surfactant, which directly contributes to pulmonary compliance but not to tissue elastance.
Discussion
We previously identified a lung phenotype in LCAD−/− mice characterized by reduced surfactant levels and altered surfactant biophysical properties (1). These changes were correlated with a significant reduction in pulmonary compliance. Here, we have established that blocking FAO by deleting an intramitochondrial FAO enzyme (LCAD) causes long-chain acylcarnitines to accumulate in the epithelial lining fluid. Long-chain acylcarnitines compete with and integrate into pulmonary surfactant at the air-fluid interface and inhibit its ability to reduce surface tension. We propose that the presence of acylcarnitines in pulmonary surfactant is a risk factor for enhanced lung injury in patients with FAO disorders in response to a “second hit” such as a respiratory infection, sepsis, or inhaled toxins. In keeping with this, there have been reports of respiratory distress syndrome among patients with inborn errors of long-chain FAO (28–30). In one longitudinal study of 13 TFP-deficient patients, four died from pneumonia and two died from respiratory failure (31). While no LCAD-deficient patients have been described, we previously identified two cases of infant death where no LCAD antigen was detected in post-mortem lung (1). Both cases were homozygous for a polymorphism that may reduce LCAD function, and we speculate that acylcarnitine accumulation may have sensitized their lungs to injury from infection or hypoxia. A final consideration is the observed effect of acylcarnitines on Infasurf, a surfactant formulation which is used clinically to treat respiratory distress syndrome. Infasurf may have compromised utility in patients with elevated lung acylcarnitines.
This work has direct implications for managing inborn errors of FAO. Additionally, complex polygenic diseases may also affect lung function via the proposed acylcarnitine mechanism. Acylcarnitines are known biomarkers of obesity and the metabolic syndrome (32, 33). They are elevated in people with hypertension and hemodialysis patients (34). Cardiac patients taking drugs like trimetazidine and ranolazine, which are partial FAO inhibitors, may have increased acylcarnitines (35, 36). Chronic ethanol consumption also reduces FAO and thus the mechanism uncovered here may be relevant to the reduced lung function seen among alcoholics (37). In short, our findings are not relevant merely to rare genetic diseases of metabolism but to many other patient populations and diseases.
Our data suggest that using l-carnitine as a dietary supplement may exacerbate the effects of acylcarnitines in the lung of individuals with compromised FAO. At present time there are few treatment options for patients with genetic FAO disorders. l-Carnitine is given under the assumption that it will help clear the mitochondria of accumulated lipid, which would prevent toxicity. However, experimental evidence to support the therapeutic benefit of l-carnitine supplementation is generally lacking (8). On the other hand, several studies have suggested that acylcarnitine accumulation may be toxic, particularly amphiphilic long-chain acylcarnitines which have been shown to inhibit ion channels, disrupt calcium signaling, impair ATP production, alter membrane fluidity, and trigger the opening of tight junctions (10, 38–41). These published studies and our current data with mildronate would indicate that strategies to reduce acylcarnitine secretion may be clinically useful, while l-carnitine should be avoided.
Mildronate works by suppressing hepatic carnitine synthesis, therefore reducing carnitine availability to tissues that cannot synthesize carnitine such as the lung. While mildronate is widely used as an anti-ischemic drug in Eastern Europe, and was shown to be safe and well-tolerated (42, 43), to our knowledge it has not been tested in the context of FAO defects. Here, we have used a high dose of mildronate to dramatically manipulate acylcarnitine levels as “proof of principle” to reveal a link between acylcarnitines and lung function; however, we did not investigate the consequences of such severe carnitine deficiency on the liver, muscle, or heart, which are organs that are known to rely heavily upon fatty acids for energy. Future studies will investigate the use of lung-specific strategies for lowering acylcarnitines. For example, CPT-1 inhibitors such as etomoxir may be applied as inhalants. Blocking CPT-1 could prevent fatty acid entry into the mitochondria, and thus prevent acylcarnitine formation within the mitochondria, while at the same time not inducing carnitine deficiency which may have adverse effects in other tissues. The accessibility of the air-fluid interface in the lung makes alveolar mitochondrial metabolism a promising therapeutic target.
Author Contributions
E. S. G. and C. O. designed the studies and wrote the paper, with subsequent editing by Y. Y. Z., J. F. A., J. W., and M. D. H. C. O. executed all mouse and MLE12 cell culture experiments with assistance from E. S. G., S. B., and R. U.. O. R. I., D. W., and M. D. H. performed acylcarnitine profiling and related data analysis. Y. Z. and Y. Y. Z. conducted constrained drop surfactometry and data analysis. K. M. and J. F. A. infected mice with influenza, performed lung function testing, and analyzed data. J. W. isolated and cultured human type II pneumocytes and lavaged human lungs. All authors reviewed the results and approved the final version of the manuscript.
The authors declare that they have no conflicts of interest with the contents of this article.
- FAO
- mitochondrial fatty acid oxidation
- LCAD
- long-chain acyl-CoA dehydrogenase
- CPT
- carnitine palmitoyltransferase
- TFP
- mitochondrial trifunctional protein
- PFU
- plaque-forming units
- SP
- surfactant protein.
References
- 1. Goetzman E. S., Alcorn J. F., Bharathi S. S., Uppala R., McHugh K. J., Kosmider B., Chen R., Zuo Y. Y., Beck M. E., McKinney R. W., Skilling H., Suhrie K. R., Karunanidhi A., Yeasted R., Otsubo C., Ellis B., Tyurina Y. Y., Kagan V. E., Mallampalli R. K., Vockley J. (2014) Long-chain Acyl-CoA dehydrogenase deficiency as a cause of pulmonary surfactant dysfunction. J. Biol. Chem. 289, 10668–10679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guillot L., Nathan N., Tabary O., Thouvenin G., Le Rouzic P., Corvol H., Amselem S., Clement A. (2013) Alveolar epithelial cells: master regulators of lung homeostasis. Int. J. Biochem. Cell Biol. 45, 2568–2573 [DOI] [PubMed] [Google Scholar]
- 3. Miller M. L., Andringa A., Hastings L. (1995) Relationships between the nuclear membrane, nuclear pore complexes, and organelles in the type II pneumocyte. Tissue Cell 27, 613–619 [DOI] [PubMed] [Google Scholar]
- 4. Millington D. S., Stevens R. D. (2011) Acylcarnitines: analysis in plasma and whole blood using tandem mass spectrometry. Methods Mol. Biol. 708, 55–72 [DOI] [PubMed] [Google Scholar]
- 5. Jones L. L., McDonald D. A., Borum P. R. (2010) Acylcarnitines: role in brain. Progr. Lipid Res. 49, 61–75 [DOI] [PubMed] [Google Scholar]
- 6. Kurth L., Fraker P., Bieber L. (1994) Utilization of intracellular acylcarnitine pools by mononuclear phagocytes. Biochim. Biophys. Acta 1201, 321–327 [DOI] [PubMed] [Google Scholar]
- 7. Onofrj M., Ciccocioppo F., Varanese S., di Muzio A., Calvani M., Chiechio S., Osio M., Thomas A. (2013) Acetyl-L-carnitine: from a biological curiosity to a drug for the peripheral nervous system and beyond. Exp. Rev. Neurotherap. 13, 925–936 [DOI] [PubMed] [Google Scholar]
- 8. Reuter S. E., Evans A. M. (2012) Carnitine and acylcarnitines: pharmacokinetic, pharmacological and clinical aspects. Clinical Pharmacokinetics 51, 553–572 [DOI] [PubMed] [Google Scholar]
- 9. Schooneman M. G., Vaz F. M., Houten S. M., Soeters M. R. (2013) Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes 62, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Doi N., Tomita M., Hayashi M. (2011) Absorption enhancement effect of acylcarnitines through changes in tight junction protein in Caco-2 cell monolayers. Drug Metab. Pharmacokinetics 26, 162–170 [DOI] [PubMed] [Google Scholar]
- 11. Adams S. H., Hoppel C. L., Lok K. H., Zhao L., Wong S. W., Minkler P. E., Hwang D. H., Newman J. W., Garvey W. T. (2009) Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J. Nutr. 139, 1073–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. DaTorre S. D., Creer M. H., Pogwizd S. M., Corr P. B. (1991) Amphipathic lipid metabolites and their relation to arrhythmogenesis in the ischemic heart. J. Mol. Cell Cardiol. 23, 11–22 [DOI] [PubMed] [Google Scholar]
- 13. Braciale T. J. (1977) Immunologic recognition of influenza virus-infected cells. I. Generation of a virus-strain specific and a cross-reactive subpopulation of cytotoxic T cells in the response to type A influenza viruses of different subtypes. Cell. Immunol. 33, 423–436 [DOI] [PubMed] [Google Scholar]
- 14. Wang J., Nikrad M. P., Phang T., Gao B., Alford T., Ito Y., Edeen K., Travanty E. A., Kosmider B., Hartshorn K., Mason R. J. (2011) Innate immune response to influenza A virus in differentiated human alveolar type II cells. Am. J. Respir. Cell Mol. Biol. 45, 582–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kao H. J., Cheng C. F., Chen Y. H., Hung S. I., Huang C. C., Millington D., Kikuchi T., Wu J. Y., Chen Y. T. (2006) ENU mutagenesis identifies mice with cardiac fibrosis and hepatic steatosis caused by a mutation in the mitochondrial trifunctional protein beta-subunit. Hum. Mol. Genet. 15, 3569–3577 [DOI] [PubMed] [Google Scholar]
- 16. An J., Muoio D. M., Shiota M., Fujimoto Y., Cline G. W., Shulman G. I., Koves T. R., Stevens R., Millington D., Newgard C. B. (2004) Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat. Med. 10, 268–274 [DOI] [PubMed] [Google Scholar]
- 17. Zuo Y. Y., Ding M., Li D., Neumann A. W. (2004) Further development of Axisymmetric Drop Shape Analysis-captive bubble for pulmonary surfactant related studies. Biochim. Biophys. Acta 1675, 12–20 [DOI] [PubMed] [Google Scholar]
- 18. Valle R. P., Liwen-Huang C., Loo J. S., Zuo Y. Y. (2014) Increasing Hydrophobicity of Nanoparticles Intensifies Lung Surfactant Film Inhibition and Particle Retention. ACS Sustainable Chemistry and Engineering 2, 6 [Google Scholar]
- 19. Liepinsh E., Konrade I., Skapare E., Pugovics O., Grinberga S., Kuka J., Kalvinsh I., Dambrova M. (2011) Mildronate treatment alters gamma-butyrobetaine and l-carnitine concentrations in healthy volunteers. J. Pharmacy Pharmacol. 63, 1195–1201 [DOI] [PubMed] [Google Scholar]
- 20. Icard P., Saumon G. (1999) Alveolar sodium and liquid transport in mice. Am. J. Physiol. 277, L1232–1238 [DOI] [PubMed] [Google Scholar]
- 21. Bakermans A. J., van Weeghel M., Denis S., Nicolay K., Prompers J. J., Houten S. M. (2013) Carnitine supplementation attenuates myocardial lipid accumulation in long-chain acyl-CoA dehydrogenase knockout mice. J. Inherit. Metab. Dis. 36, 973–981 [DOI] [PubMed] [Google Scholar]
- 22. Nasser M., Javaheri H., Fedorowicz Z., Noorani Z. (2012) Carnitine supplementation for inborn errors of metabolism. Cochrane Database System Review 2, 1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Veiga M. P., Requero M. A., Goñi F. M., Alonso A. (1996) Effect of long-chain acyl-CoAs and acylcarnitines on gel-fluid and lamellar-hexagonal phospholipid phase transitions. Molecular Membrane Biology 13, 165–172 [DOI] [PubMed] [Google Scholar]
- 24. Lopez-Rodriguez E., Perez-Gil J. (2014) Structure-function relationships in pulmonary surfactant membranes: From biophysics to therapy. Biochim. Biophys. Acta [DOI] [PubMed] [Google Scholar]
- 25. Sjakste N., Gutcaits A., Kalvinsh I. (2005) Mildronate: an antiischemic drug for neurological indications. CNS Drug Reviews 11, 151–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Duan Y., Learoyd J., Meliton A. Y., Leff A. R., Zhu X. (2012) Inhibition of Pyk2 blocks lung inflammation and injury in a mouse model of acute lung injury. Respir. Res. 13, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Madala S. K., Maxfield M. D., Davidson C. R., Schmidt S. M., Garry D., Ikegami M., Hardie W. D., Glasser S. W. (2011) Rapamycin Regulates Bleomycin-Induced Lung Damage in SP-C-Deficient Mice. Pulmonary Medicine 2011, 653524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lundy C. T., Shield J. P., Kvittingen E. A., Vinorum O. J., Trimble E. R., Morris A. A. (2003) Acute respiratory distress syndrome in long-chain 3-hydroxyacyl-CoA dehydrogenase and mitochondrial trifunctional protein deficiencies. Journal of inherited metabolic disease 26, 537–541 [DOI] [PubMed] [Google Scholar]
- 29. Gentili A., Iannella E., Masciopinto F., Latrofa M. E., Giuntoli L., Baroncini S. (2008) Rhabdomyolysis and respiratory failure: rare presentation of carnitine palmityl-transferase II deficiency. Minerva Anestesiologica 74, 205–208 [PubMed] [Google Scholar]
- 30. Spiekerkoetter U., Bennett M. J., Ben-Zeev B., Strauss A. W., Tein I. (2004) Peripheral neuropathy, episodic myoglobinuria, and respiratory failure in deficiency of the mitochondrial trifunctional protein. Muscle Nerve 29, 66–72 [DOI] [PubMed] [Google Scholar]
- 31. Tyni T., Palotie A., Viinikka L., Valanne L., Salo M. K., von Döbeln U., Jackson S., Wanders R., Venizelos N., Pihko H. (1997) Long-chain 3-hydroxyacyl-coenzyme A dehydrogenase deficiency with the G1528C mutation: clinical presentation of thirteen patients. J. Pediatr. 130, 67–76 [DOI] [PubMed] [Google Scholar]
- 32. Sampey B. P., Freemerman A. J., Zhang J., Kuan P. F., Galanko J. A., O'Connell T. M., Ilkayeva O. R., Muehlbauer M. J., Stevens R. D., Newgard C. B., Brauer H. A., Troester M. A., Makowski L. (2012) Metabolomic profiling reveals mitochondrial-derived lipid biomarkers that drive obesity-associated inflammation. PLoS ONE 7, e38812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mihalik S. J., Goodpaster B. H., Kelley D. E., Chace D. H., Vockley J., Toledo F. G., DeLany J. P. (2010) Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity 18, 1695–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kalim S., Clish C. B., Wenger J., Elmariah S., Yeh R. W., Deferio J. J., Pierce K., Deik A., Gerszten R. E., Thadhani R., Rhee E. P. (2013) A plasma long-chain acylcarnitine predicts cardiovascular mortality in incident dialysis patients. J. Am. Heart Assoc. 2, e000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ussher J. R., Keung W., Fillmore N., Koves T. R., Mori J., Zhang L., Lopaschuk D. G., Ilkayeva O. R., Wagg C. S., Jaswal J. S., Muoio D. M., Lopaschuk G. D. (2014) Treatment with the 3-Ketoacyl-CoA Thiolase Inhibitor Trimetazidine Does Not Exacerbate Whole-Body Insulin Resistance in Obese Mice. J. Pharmacol. Exp. Ther. 349, 487–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lionetti V., Stanley W. C., Recchia F. A. (2011) Modulating fatty acid oxidation in heart failure. Cardiovasc. Res. 90, 202–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Grunnet N., Kondrup J. (1986) The effect of ethanol on the beta-oxidation of fatty acids. Alcohol. Clin. Exp. Res. 10, 64S–68S [DOI] [PubMed] [Google Scholar]
- 38. Requero M. A., Goñi F. M., Alonso A. (1995) The membrane-perturbing properties of palmitoyl-coenzyme A and palmitoylcarnitine. A comparative study. Biochemistry 34, 10400–10405 [DOI] [PubMed] [Google Scholar]
- 39. Duizer E., van der Wulp C., Versantvoort C. H., Groten J. P. (1998) Absorption enhancement, structural changes in tight junctions and cytotoxicity caused by palmitoyl carnitine in Caco-2 and IEC-18 cells. J. Pharmacol. Exp. Ther. 287, 395–402 [PubMed] [Google Scholar]
- 40. Goñi F. M., Requero M. A., Alonso A. (1996) Palmitoylcarnitine, a surface-active metabolite. FEBS Lett. 390, 1–5 [DOI] [PubMed] [Google Scholar]
- 41. Hayashi M., Sakai T., Hasegawa Y., Nishikawahara T., Tomioka H., Iida A., Shimizu N., Tomita M., Awazu S. (1999) Physiological mechanism for enhancement of paracellular drug transport. Journal of Controlled Release 62, 141–148 [DOI] [PubMed] [Google Scholar]
- 42. Zhu Y., Zhang G., Zhao J., Li D., Yan X., Liu J., Liu X., Zhao H., Xia J., Zhang X., Li Z., Zhang B., Guo Z., Feng L., Zhang Z., Qu F., Zhao G. (2013) Efficacy and safety of mildronate for acute ischemic stroke: a randomized, double-blind, active-controlled phase II multicenter trial. Clin. Drug Investig. 33, 755–760 [DOI] [PubMed] [Google Scholar]
- 43. Dzerve V., and MILSS I Study Group (2011) A dose-dependent improvement in exercise tolerance in patients with stable angina treated with mildronate: a clinical trial “MILSS I”. Medicina 47, 544–551 [PubMed] [Google Scholar]