Background: Signaling by the IL-36 receptor is poorly characterized.
Results: Activation of IL-36R signaling is coupled with its endocytosis to lysosomes. Tollip mediates IL-1 receptor turnover and increases the accumulation of IL-36R.
Conclusion: IL-36R signaling has differences in signaling from the IL-1R.
Significance: This work defines the requirements for IL-36R signaling and trafficking.
Keywords: endocytosis, immunology, IL-1, signal transduction, trafficking, interleukin 36 receptor, Tollip
Abstract
Improper signaling of the IL-36 receptor (IL-36R), a member of the IL-1 receptor family, has been associated with various inflammation-associated diseases. However, the requirements for IL-36R signal transduction remain poorly characterized. This work seeks to define the requirements for IL-36R signaling and intracellular trafficking. In the absence of cognate agonists, IL-36R was endocytosed and recycled to the plasma membrane. In the presence of IL-36, IL-36R increased accumulation in LAMP1+ lysosomes. Endocytosis predominantly used a clathrin-mediated pathway, and the accumulation of the IL-36R in lysosomes did not result in increased receptor turnover. The ubiquitin-binding Tollip protein contributed to IL-36R signaling and increased the accumulation of both subunits of the IL-36R.
Introduction
The IL-36 receptor (IL-36R)4 is a member of the IL-1 receptor superfamily that plays key roles in the activation and amplification of the immune response (1, 2). Improper receptor signaling has been associated with chronic and acute inflammatory responses and diseases such as rheumatoid arthritis, psoriasis, and lupus erythematosus (2–5). At present, there is little information about the requirements for signal transduction by the IL-36R.
IL-36R signaling is assumed to share common features with signaling by the IL-1R. The IL-1R is composed of two subunits, IL-1R1, the receptor subunit, and IL-1RAcp, the receptor accessory protein. Both IL-1R1 and IL-1RAcp contain an extracellular ligand-binding domain, a single-pass transmembrane sequence, and a cytoplasmic Toll-IL-1 receptor domain. IL-1β binding to IL-1R1 is stabilized by the IL-1RAcp protein, which induces conformational changes in the Toll-IL-1 receptor domains of the two proteins. The protein complex then recruits the adaptor protein MyD88, IL-1 receptor-associated kinase 1 (protein kinase IRAK-1), and the Toll-interacting protein (Tollip), leading to activation of transcription factors such as NF-κβ to induce gene expression (4, 6–9). IL-1β binding to the IL-1R also induces receptor endocytosis, a process facilitated by Tollip (10, 11). Trafficking of IL-1R1 from the cell surface to mature endosomes results in the degradation of the IL-1R in lysosomes. In the absence of ligand, the IL-1 receptor recycles to the cell surface (10, 11).
The IL-36R, like the IL-1R, is composed of two subunits. The receptor subunit is IL-1Rrp2 (also known as IL-1R6 and IL-1RL2). The accessory protein is IL-1RAcp, the same as in the IL-1R (12). The organization of IL-1Rrp2 is similar to that of IL-1R1. Binding to IL-1Rrp2 by any one of the three known IL-36 cytokines (IL-36α, IL-36β, or IL-36γ) induces complex formation with IL-1RAcp that stabilizes agonist binding (13). In addition, a receptor antagonist, IL-36RA, can prevent IL-36R signaling (13, 14).
We examined the requirements for human IL-36R signaling and found it to recycle in the absence of agonists but accumulate in higher abundance in lysosomes in the presence of agonists. Tollip modulates IL-36R trafficking by increasing the accumulation of IL-1Rrp2 and IL-1RAcp, which is different from what is observed with the IL-1R. Therefore, significant differences exist between IL-1R and IL-36R signaling.
Materials and Methods
Reagents and Cell Lines
The antibody that recognizes the IL-1Rrp2 ectodomain was from R&D Systems (catalog no. AF872). The antibody to detect IL-1RAcp was from Pierce (catalog no. PA5-19921). Antibodies to Tollip (catalog no. sc-27315), human MyD88 (catalog no. sc-11356), ubiquitin (P4D1, catalog no. sc-8017), and β-Actin (catalog no. sc-58679) were from Santa Cruz Biotechnology. Antibodies to detect Rab5, Rab7, Rab11, and LAMP1 were from Cell Signaling Technology. Secondary antibodies conjugated to Alexa Fluor 488 or 594 were from Life Technologies. Alexa Fluor-conjugated cholera toxin B subunit (catalog no. C-34777) and human transferrin (catalog no. T-13343) were from Life Technology. Human IL-36α (catalog no. 6995-IL/CF), IL-36β (catalog no. 6834-IL/CF), IL-36γ (catalog no. 6835-ILC/CF), IL-36RA (catalog no. 1275-IL-025/CF), and IL-1β (catalog no. 201-LB-005/CF) were from R&D Systems.
The endocytosis inhibitors methyl-β-cyclodextrin, chloropromazine, bafilomycin A1, and ammonium chloride were from Sigma-Aldrich and were dissolved in either water or dimethyl sulfoxide according to their solubility requirements. Poly(I:C) was from Amersham Biosciences (catalog no. 27-4732-01), and LPS was from Sigma (catalog no. L3024).
Cells and Culture Media
NCI/ADR-RES (NCI) cells were cultured in RPMI medium + l-glutamine (Gibco, catalog no. 11875) and 10% fetal bovine serum. BEAS-2B cells were cultured in bronchial epithelial cell growth medium with its supplements (Lonza Inc. catalog no. CC-3170) as described in Singh et al. (15). Human dermal fibroblasts (catalog no. C-013-5C) were from Life Technologies and grown in medium 106 (catalog no. M-106-500) with low serum growth supplement (catalog no. S-003-10), gentamicin (10 mg/ml), and amphotericin B (0.25 mg/ml) as described by Life Technology. All cells were grown in rat collagen type I-coated flasks (BD Biosciences, catalog no. 354236) and incubated at 37 °C and 5% CO2.
Construction of NCI-KO Cells
NCI cells with an in-frame deletion of residues 91–97 in the first immunoglobulin-like domain of the IL-1Rrp2 gene were made using the CompoZrTM custom zinc finger nuclease targeting kit from Sigma-Aldrich (catalog no. CSTZFN-1KT). 2 ml of NCI cells at 2 × 105 cells/ml were transfected with 5 μl of the zinc finger mRNA using Lipofectamine 2000 (Life Technologies, catalog no. 11668-019). Upon confluence, the cells were tested for lack of IL-36R expression on the cell surface via flow cytometry and detected with the antibody recognizing the IL-36R ectodomain. Cells lacking cell surface IL-36R expression were selected via live-cell staining and sorting. Clonal dilution was used to generate clones for testing of IL-36α and IL-36β-mediated phosphorylation of NF-κB. The cDNA for IL-1Rrp2 from NF-κβ phosphorylation-defective cells was amplified and cloned into a TOPO vector (Clontech), and DNA sequencing was used to confirm the presence of the in-frame deletion in IL-1Rrp2.
Quantification of Cytokine Production in Vitro
Human IL-6 production was quantified by ELISA using the Human OptEIATM kit (BD Biosciences). Cells plated at 2 × 104 cells/well for 24 h in flat-bottom 96-well plates were stimulated with 1 μg/ml of poly(I:C) or LPS. Unless stated otherwise, 1.0 ng/ml of each cytokine was used in all experiments. The media were centrifuged at 2000 × g for 5 min to remove cells that were used for ELISA. All ELISAs were performed in triplicate and in at least three independent experiments.
Flow Cytometry
Immunostaining and flow cytometry analyses were performed as described previously (16). Cells were fixed with flow cytometry fixation buffer (R&D Systems) and permeabilized with flow cytometry permeabilization/wash buffer I (1×, catalog no. FC005, R&D Systems). The cells were stained to detect the ectodomain of the IL-36R for 1 h. The primary antibody to detect IL-1Rrp2 was from R&D Systems, and the secondary antibody was a donkey anti-goat immunoglobulin conjugated to Alexa Fluor 488 (Life Technologies). Single-cell suspensions of 0.5 × 106 cells/tube of NCI cells were analyzed and enumerated using a FACSCalibur flow cytometer (BD Biosciences), and the data were processed using FlowJo software. Background controls were determined using cells stained with only a secondary antibody conjugated to Alexa Fluor 488.
Confocal Microscopy
Cells were grown on coverslips coated with poly-l-lysine. At ∼60% confluency, the cells were treated with the indicated ligand and then fixed with 4% paraformaldehyde for 15 min at room temperature. Permeabilized cells were treated for 30 min on ice in T buffer (0.5% Triton X-100 in PBS) in the presence of 1% normal goat serum. Nonspecific antibody binding was blocked with 2% BSA in Tris-buffered saline (TBS-T (pH 7.4) with 0.5% Triton X-100) for 1 h prior to an overnight 4 °C incubation with primary antibodies diluted in TBS-T amended with 2% BSA. The cells were then washed twice with TBS-T and incubated with secondary antibodies for 1 h at room temperature. After three additional washes with TBS-T, the coverslips were mounted on glass slides with anti-fade mounting medium containing DAPI and dried overnight in the dark. Micrographs were acquired with a Leica TCS SP5 confocal inverted-base microscope with a ×63 oil objective as described by Singh et al. (15). Images were analyzed by Leica LAS AF and ImageJ software. Fluorophore colocalization was quantified using the ImageJ plug-in tool JACoP (17).
siRNA Knockdown
NCI/ADR-RES cells were seeded at 1.5 × 105 cells/well in RPMI 1640 medium amended with 10% FBS (Fisher Scientific) in a 6-well tissue culture plate or 1.0 × 104 cells/well in a 96-well plate. 24 h later, the cells were transfected with 30 nm of a mixture of three siRNAs specific to MyD88 (catalog no. sc-35986), Tollip (catalog no. sc-63332), or nonspecific control siRNA (catalog no. sc-37007) (Santa Cruz Biotechnology). For cell transfection, we used Lipofectamine RNAiMax (Life Technology) according to the protocol of the manufacturer. The cells were typically incubated for 48 h prior to treatment with ligands. The target message was quantified using Western blot and real-time RT-PCR. Sequences of oligonucleotide primers are available upon request.
Western Blot Analysis
For Western blots, we used cells lysed with ice-cold radioimmune precipitation assay buffer (150 mm NaCl, 1 mm EDTA, 50 mm Tris-HCl (pH 7.4), 0.5% Nonidet P-40, and 0.1% SDS) amended with a mixture of phosphatase and protease inhibitors (Sigma-Aldrich). The lysate was clarified from insoluble materials by a brief centrifugation at 12,000 × g for 10 min, suspended in denaturing Laemmli loading buffer, and incubated for 5 min at 70 °C. Western blot analyses were performed by electrophoresis of the lysates in 4–12% NuPAGE BisTris gels followed by transfer to PVDF membranes as described previously by us (15, 18). Signals from the Western blots were developed with Thermo Supersignal Dura substrate solution. The amount of β-Actin in each sample was also quantified from the same samples to allow normalization of the amount of IL-1Rrp2 and IL-1RAcp. The signals were quantified using a ChemiDocTM XRS+ system and ImageLab software (Bio-Rad).
Coimmunoprecipitation Assay
Coimmunoprecipitation assays were performed using the buffers and protocols of Brinkmann et al. (19). Cells were grown on 6-well plates until the cells become 50% confluent. The cells were lysed in radioimmune precipitation assay buffer and incubated with primary antibodies, followed by incubation with protein A/G magnetic beads (Thermo Scientific, catalog no. 88803). After two washes with buffer TBS amended with 0.05% Tween 20 and 0.5 m NaCl, the beads were washed with TBS. The precipitated materials were solubilized with SDS-PAGE loading buffer for 5 min at 70 °C and resolved by running on a 4–12% NuPAGE BisTris gel. Western blot analyses were performed as described above.
Cycloheximide Chase Experiment
The half-lives of IL-1Rrp2 and IL-1RAcp were determined in cells grown in 6-well plates at 1.5 × 106 cells/ml. Cycloheximide was added immediately after the cytokine to a final concentration of 60 μg/ml. The protein content in cell lysates was analyzed by Western blot as described above.
Results
The NCI Cell Line Responds to Human IL-36
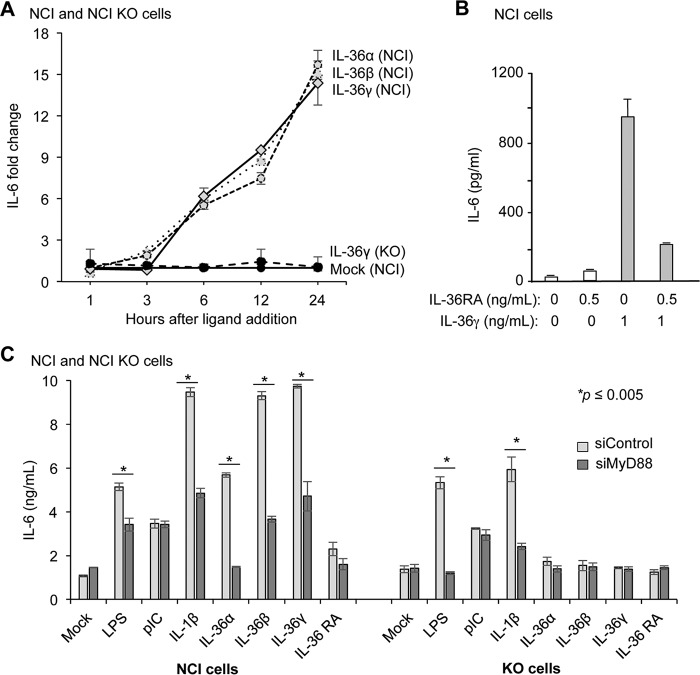
To characterize signal transduction by the human IL-36R, we examined several cell lines for their response to IL-36 agonists. The NCI/ADR-RES cell line (hereafter called NCI cells) derived from an ovarian tumor was found to respond to all three IL-36 agonists and produce robust levels of the proinflammatory human cytokines expected by IL-36 signaling, including IL-6 (Fig. 1A and data not shown). NCI cells treated with IL-36α, IL-36β, and IL-36γ produced similar levels of IL-6 over a 24-h period. As expected, the addition of the recombinant antagonist IL-36RA did not activate IL-36R signaling to result in IL-6 production (Fig. 1B). The addition of both IL-36γ and IL-36RA resulted in reduced IL-6 production relative to cells treated with only IL-36γ, indicating that NCI cells also respond to IL-36RA (Fig. 1B).
FIGURE 1.
NCI cells respond to IL-36 ligands. A, IL-36γ induction of IL-6 production in NCI cells was inhibited by IL-36RA. IL-36RA was added 5 min before the addition of IL-36γ. The amount of IL-6 secreted into the cell culture medium was quantified using ELISA. NCI cells produce IL-6 at comparable levels in response to the three IL-36 cytokines. KO denotes results from the NCI-KO cell line, which has an in-frame deletion of the IL-1Rrp2 gene. B, IL-36RA can inhibit signaling by the IL-36R. Shown is IL-6 production in NCI cells treated with mock IL-36RA. IL-6 production in NCI-KO cells served as controls. All data represent the means ± S.E. for three independent experiments. C, signal transduction by the IL-36R requires MyD88. Shown is IL-6 production by NCI or NCI-KO cells knocked down for MyD88 in response to IL-36 agonists. LPS, poly(I:C), and IL-1β, which activate signal transduction by, respectively, TLR4, TLR3, and the IL-1R, served as controls for the specific effects of the IL-36 agonists in inducing IL-6 production. All data represent the mean of three independent experiments. The p values were calculated using Student's t test.
To ensure that the cytokine produced by NCI cells was specific to the activation of the IL-36R, nucleotides encoding residues 91–97 in the first immunoglobulin-like domain of IL-1Rrp2 were deleted using zinc finger technology (20), resulting in the cell line NCI-KO. When treated with IL-36 agonists, NCI-KO was defective in IL-6 production (Fig. 1A). All of these results demonstrate that NCI cells can serve as a model cell line to analyze human IL-36R function.
IL-36R Signaling Requires MyD88
IL-1R signals through the adaptor protein MyD88, which recruits the kinase IRAK1 and Tollip to up-regulate signaling (6). To examine whether MyD88 functioned in IL-36R signaling in NCI cells, siRNA was used to knock down MyD88. The knockdown decreased the level of MyD88 to less than 50% that of cells treated with a control siRNA (data not shown). Addition of either IL-36 or IL-1β in cells knocked down for MyD88 resulted in significantly reduced IL-6 production compared with cells treated with control siRNAs (Fig. 1C). In NCI-KO cells, knockdown of MyD88 reduced the response to IL-1β as expected but did not affect signaling by IL-36 as expected. In addition, cells induced with poly(I:C), which activates Toll-like receptor 3 signaling through a MyD88-independent pathway (21), was unaffected by the knockdown of MyD88 (Fig. 1C). Therefore, MyD88 acts in both IL-1R and IL-36R signaling.
Human IL-36R Traffics between the Cell Surface and Endosomes upon Agonist Addition
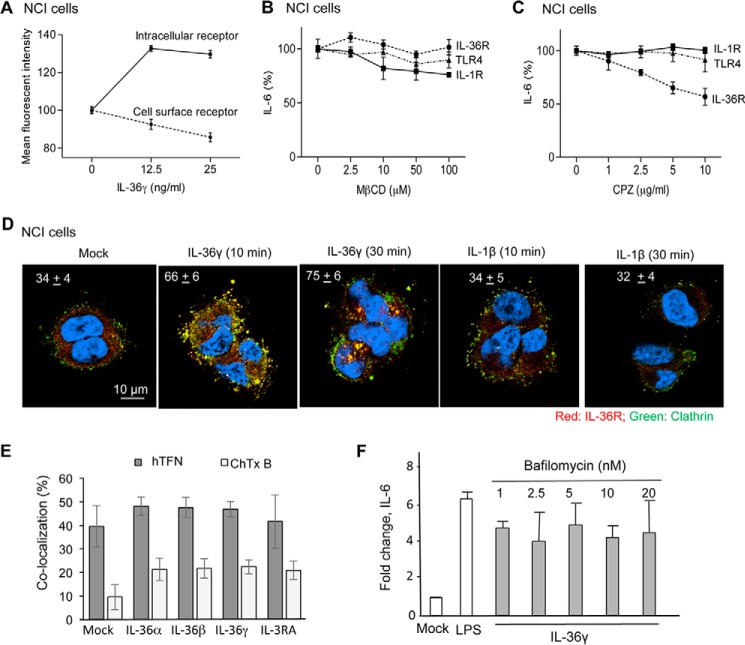
IL-1R expressed on the cell surface endocytoses in response to IL-1β (10). Using flow cytometry, we examined the cellular localization of IL-36R and whether its localization changes in response to agonist using flow cytometry. A polyclonal antibody that recognizes the IL-1Rrp2 ectodomain revealed an abundance of signal on the cell surface of unpermeabilized NCI cells (Fig. 2A). The addition of IL-36γ reduced the abundance of cell surface IL-36γ. Correspondingly, the amount of intracellular IL-1Rrp2 increased along with the decreased cell surface IL-1Rrp2 (Fig. 2A).
FIGURE 2.
IL-36 treatment alters the localization of the IL-36R. A, flow cytometry analysis of the IL-1Rrp2 present on the cell surface and within cells. NCI cells were treated or mock-treated with IL-36γ for 30 min. Cell surface-localized IL-1Rrp2 was determined in fixed NCI cells that were not permeabilized. Intracellular IL-1Rrp2 was determined by the difference in the total signal for IL-1Rrp2 subtracted from the signal present on the cell surface. All samples were tested in triplicates, with the signal from 10,000 cells enumerated. B and C, effects of endocytosis inhibitors on IL-36R signaling. Shown is IL-6 production in NCI cells treated with IL-36γ in the presence of increasing concentrations of the clathrin-independent endocytosis inhibitor MβCD or the clathrin-dependent endocytosis inhibitor chloropromazine. IL-6 production by NCI cells was used as a control. D, IL-1Rrp2 colocalized with clathrin after the addition of IL-36γ. NCI cells were fixed and stained with antibodies to detect IL-1Rrp2 (red), clathrin (green), or DNA (blue). Colocalization of IL-36R and clathrin is pseudocolored yellow. The percent colocalization is shown in the top left corners of the micrographs, which are representative for the analyzed conditions. A minimum of 20 cells were quantified in each sample. E, quantified colocalization of IL-1Rrp2 with Alexa Fluor 594-labeled human transferrin receptor or cholera toxin B (CTxB). The hTFN receptor trafficked into cells using clathrin-mediated endocytosis, whereas cholera toxin B traffics into cells using a clathrin-independent endocytosis marker. Percent colocalization was quantified from more than 20 independent cells from three independently prepared samples. F, IL-36R signaling does not require endosome acidification. IL-36γ induced IL-6 production in NCI cells in the presence of increasing concentrations of the endosomal acidification inhibitor bafilomycin A1. All data are the mean of three independent experiments where the IL-6 levels secreted by the cells into the medium were normalized for the mock-treated NCI cells.
Immunoconfocal microscopy also revealed that IL-1Rrp2 was on the cell surface and that the addition of IL-36γ increased the abundance of intracellular IL-1Rrp2 (data not shown). Notably, the intracellular IL-1Rrp2 appeared in punctate forms that resemble endosomes (data not shown).
Signaling and endocytosis could be coupled functionally (22). Two principal pathways are primarily responsible for endocytosis: clathrin-mediated and caveolae/raft (23). To analyze the role for endocytosis and IL-36R signaling, we first examined the effects of the addition of methyl-β-cyclodextrin (MβCD), which extracts cholesterol and can affect endocytosis (24). The amount of IL-6 made by NCI cells in response to IL-36γ was determined to assess signaling. To ensure that the production and secretion of IL-6 was not affected, the signaling by TLR4, which does not require endocytosis, was also determined. The addition of MβCD did not inhibit IL-6 production in response to the TLR4 agonist LPS (Fig. 2B). MβCD also did not affect signaling in response to IL-36γ, suggesting that endocytosis via the caveolae/lipid raft does not significantly contribute to IL-36R signaling. Cells treated with MβCD did modestly reduce IL-6 production in response to IL-1β. Blanco et al. (23) have reported previously that caveolae contributed to the endocytosis for the IL-1R.
Chlorpromazine, an inhibitor of clathrin-mediated endocytosis (25, 26), inhibited IL-6 production in IL-36γ-treated NCI cells (Fig. 2C). Notably, the production of IL-6 from induction of IL-1R1 and TLR4 signaling was not affected by chlorpromazine, demonstrating that IL-6 production and secretion were not affected by chlorpromazine. These results suggest that IL-36R signaling is linked to endocytosis by a clathrin-mediated pathway.
To confirm that a clathrin-mediated pathway was responsible for IL-36R endocytosis, we used immunoconfocal microscopy to examine the colocalization of intracellular IL-1Rrp2 with clathrin. Within 10 min of the addition of IL-36γ, IL-1Rrp2 and clathrin were found to colocalize, and the colocalization increased by 30 min (Fig. 2D). As expected, IL-1Rrp2 did not colocalize with clathrin above the background level after the addition of IL-1β. Finally, we determined whether IL-1Rrp2 can colocalize with the transferrin receptor, which is endocytosed using clathrin cages (27, 28), and the cholera toxin B subunit, which enters cells using a caveola-mediated pathway (29). IL-1Rrp2 was detected using a fluorescently labeled antibody, and the transferrin and cholera toxin B receptors were labeled with Alexa Fluor. In the absence of IL-36, IL-1Rrp2 preferentially colocalized with the transferrin receptor (Fig. 2E). A smaller fraction of IL-1Rrp2 did colocalize with cholera toxin (Fig. 2E). In cells stimulated with IL-36, colocalization of IL-36R with the transferrin receptor increased slightly. Together, these results demonstrate that clathrin-mediated endocytosis is the primary means by which IL-36R enters cells.
We examined whether signal transduction by the IL-36R requires endosome acidification. Increasing the concentration of the ATPase pump inhibitor bafilomycin A1 did not affect IL-6 production by NCI cells treated with IL-36γ (Fig. 2F). Similar results were observed in cells treated with the endosomal acidification inhibitors ammonium chloride and chloroquine (data not shown). These results suggest that, unlike the case with Toll-like receptor signaling (30, 31), endosome acidification is not required for signal transduction by the IL-36R.
Activated IL-36R Traffics to LAMP1+ Lysosomes
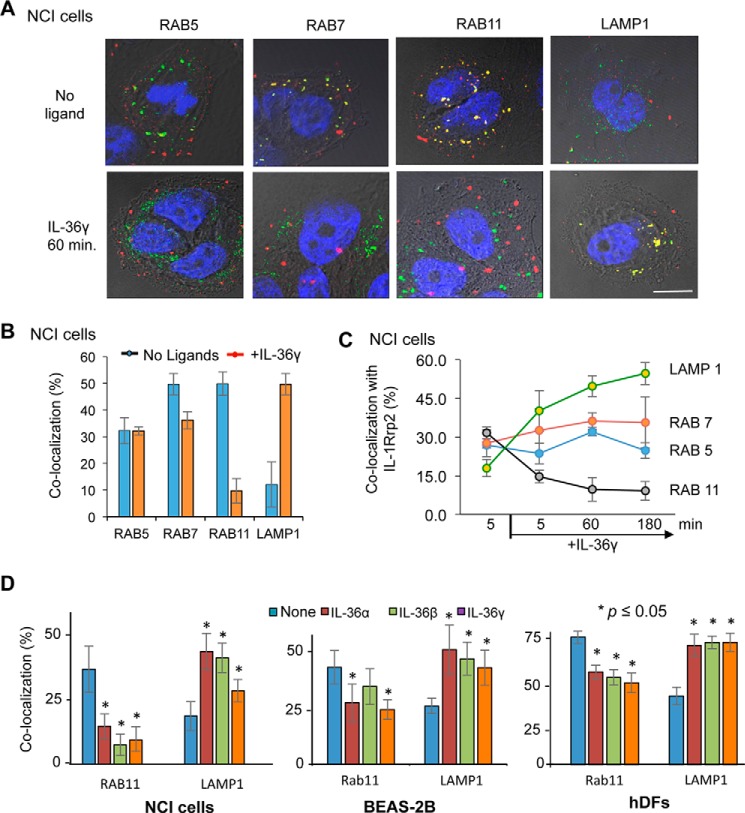
We used immunoconfocal microscopy to identify the endosomes with which IL-1Rrp2 colocalizes after treatment with IL-36. The Rab GTPases are diagnostic for the function of endosomes (15, 32). In the absence of agonists, significant proportions of IL-1Rrp2 were associated with Rab7 and Rab11 (Fig. 3, A and B), the latter of which is a marker for recycling endosomes. 1 h after the addition of the IL-36γ, IL-1Rrp2 colocalization with LAMP1+ lysosomes increased, whereas colocalization with Rab11 endosomes decreased (Fig. 3B). A similar change was also seen with IL-36α and IL-36β (data not shown). This change in the marker for the endosome-associated IL-1Rrp2 was observed within 5 min after the addition of ligand.
FIGURE 3.
IL-1Rrp2 localizes to different endosome subpopulations in response to IL-36 agonists. A, sample micrographs of NCI cells stained to identify IL-1Rrp2 (red), endosome markers (green), and DNA (blue). Colocalization of IL-1Rrp2 and the endosomal marker is shown in yellow. B, quantification of the colocalization of IL-1Rrp2 with endosomal markers in NCI cells after the addition of ligands. The percent colocalization of IL-1Rrp2 with endosome subpopulations identified with specific markers was quantified from confocal microscopy and plotted. All data points were from a minimum of 20 cells analyzed for colocalization. C, IL-6 production by the human bronchial epithelial BEAS-2B cell line and hDF primary cells in response to IL-36 and IL-36RA. IL-6 production was determined using an ELISA assay. The levels of IL-6 produced by the cells in response to different ligands were compared with the mock-treated samples using Student's t test. All data shown were quantified from more than 20 individual cells from three independently prepared samples.
We examined whether IL-36 agonist addition affects IL-1Rrp2 localization in two additional cell lines, the immortalized lung epithelial cell line BEAS-2B and primary human dermal fibroblasts (hDFs). The addition of IL-36 resulted in IL-6 production in both cell lines at least 5-fold over background, demonstrating that the cells are competent for IL-36R signaling. The addition of any of the three IL-36 ligands to BEAS-2B and hDFs resulted in a decrease of IL-1Rrp2 localization in Rab11 endosomes and increased colocalization with LAMP1 lysosomes (Fig. 3D). Together, these results indicate that IL-36R activation is associated with changes in endosomal localization and increased trafficking to lysosomes.
Tollip Colocalizes with and Affects IL-36R Trafficking
Tollip is required for IL-1R1 signaling and trafficking (10). To determine whether Tollip also participates in IL-36R signaling, we used siRNA to knock down Tollip expression. NCI cells treated with siRNA specific to Tollip reduced Tollip accumulation by 50% (data not shown). The cells also showed a reduction in IL-6 production in response to stimulation with IL-36 by a corresponding amount (Fig. 4A). IL-1β signaling was also reduced with Tollip knockdown, demonstrating that Tollip functions in signaling of both the IL-1R and IL-36R. TLR4 signaling that does not require Tollip was unaffected by Tollip knockdown (Fig. 4B).
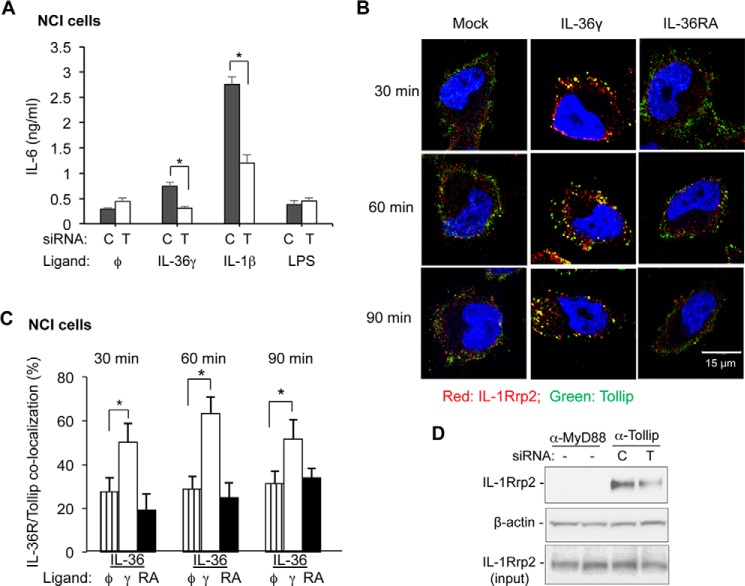
FIGURE 4.
Tollip participates in IL-36R signaling and endocytosis. A, the effect of Tollip knockdown on IL-6 production by the IL-36R and the IL-1R in NCI and NCI-KO cells. siRNA knockdown used control siRNAs (C) or siRNAs specific to Tollip (T). IL-6 produced in response to LPS served as a control for the responsiveness of the cells. *, p < 0.05. B, IL-1Rrp2 colocalizes with Tollip after IL-36γ addition. The cells were fixed and processed for immunofluorescence confocal microscopy 30 min after the addition of IL-36γ or IL-36RA. C, quantification of the colocalization of Tollip and IL-1Rrp2 in NCI cells after addition of IL-36γ and IL-36RA at different time points. The data were derived from immunoconfocal microscopy of cells stained with antibodies to detect IL-1Rrp2 or Tollip. Student's t test was used to calculate the degree of colocalization in cells treated with different ligands. *, p < 0.05. D, IL-1Rrp2 coimmunoprecipitates with Tollip. Shown are images from Western blots probed with antibodies to detect IL-1Rrp2, the loading control β-actin, and the amount of IL-1Rrp2. The cells were also treated with siRNAs to either controls or Tollip. Immunoprecipitations used a monoclonal antibody specific to Tollip.
Intracellular colocalization of Tollip and IL-1Rrp2 were examined using immunoconfocal microscopy. A basal level of Tollip and IL-1Rrp2 colocalization was observed in NCI cells, but colocalization increased ∼2-fold after the cells were treated with IL-36γ (Fig. 4, B and C). Treatment with IL-36RA did not increase the presence of IL-1Rrp2 and Tollip in endosomes (Fig. 4B). Colocalization of IL-Rrp2 and Tollip also did not increase in NCI-KO cells, likely because of the lack of agonist binding by IL-1Rrp2 (data not shown). These results show that, in addition to having an effect on signaling, Tollip has a role in IL-36R trafficking.
Tollip Forms a Complex with the IL-36R
The effect of Tollip on IL-36R trafficking could be indirect or through the formation of a complex. To distinguish these two possibilities, we investigated whether the IL-36R could be coimmunoprecipitated with Tollip. Cells treated with IL-36γ were solubilized with detergent, and the clarified extract was immunoprecipitated with an antibody to either MyD88 or Tollip. The proteins were then probed to detect IL-1Rrp2 in Western blots. IL-1Rrp2 coimmunoprecipitated with Tollip but not with MyD88 (Fig. 6D). Furthermore, cells knocked down for Tollip had reduced amounts of coprecipitated IL-1Rrp2. These results demonstrate that Tollip forms a complex with IL-1Rrp2.
FIGURE 6.
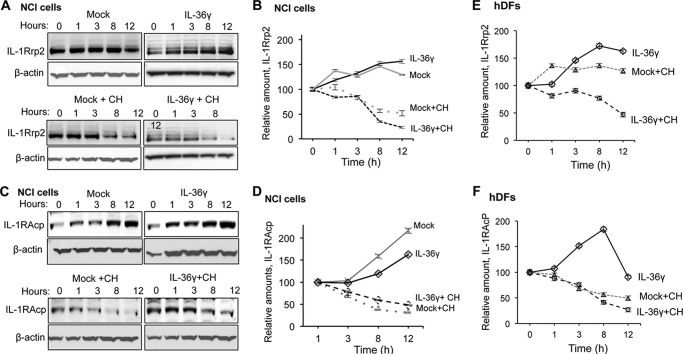
Effects of IL-36 induction on IL-1Rrp2 and IL-1RAcp accumulation. A, Western blot analysis examining the accumulation of IL-1Rrp2 in NCI cells treated with IL-36g and/or cycloheximide. The cells were collected at the indicated time after mock treatment or treatment with IL-36γ. β-actin accumulation served as a loading control in all samples. Cycloheximide (CH) was added to the cells to inhibit new rounds of translation to allow an estimate of the half-life of IL-1Rrp2 without additional protein synthesis. B, the half-life of IL-1Rrp2 after normalization to β-actin. NCI cells after mock treatment or treatment with IL-36γ or IL-1β in the presence or absence of cycloheximide over a 12-h time course. C, accumulation of IL-1RAcp increased over time and was not degraded rapidly after agonist addition. D, the half-life of IL-1RAcp in the presence of IL-36γ or cycloheximide in NCI cells. E, accumulation of IL-1Rrp2 in human dermal fibroblasts after mock treatment or treatment with IL-36γ. F, accumulation of IL-1RAcp in human dermal fibroblasts after mock treatment or treatment with IL-36γ. The data in B and D–F represent mean ± S.D. of three independent samples per time point.
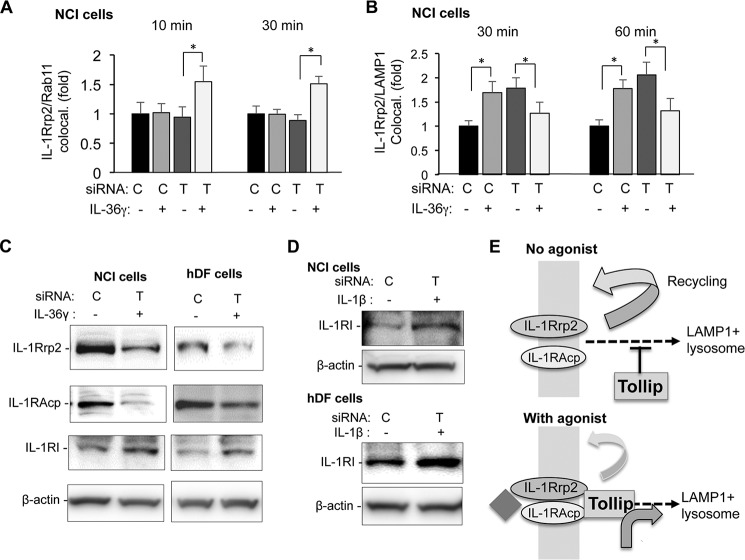
To identify whether Tollip affects IL-36R colocalization with subsets of endosomes, we knocked down Tollip in NCI cells and examined IL-1Rrp2 colocalization with Rab11, which identifies recycling endosomes. Tollip knockdown did not affect IL-1Rrp2 colocalization with Rab11 in the absence of IL-36R agonist. However, cells treated with IL-36γ and knocked down for Tollip increased IL-1Rrp2 colocalization with Rab11 (Fig. 5A). These results suggest that Tollip plays a role in directing endosomes containing the IL-36R to recycle after agonist binding has occurred.
FIGURE 5.
Tollip contributes to IL-1Rrp2 localization to lysosomes after agonist addition. A, the effects of Tollip in the colocalization to Rab11 endosomes. B, the effects of Tollip on the colocalization of IL-1Rrp2 to LAMP1 lysosomes. The results in A and B had NCI cells treated with siRNA to Tollip (T) or to a nonspecific control (C). Tollip and LAMP1 colocalization was determined by immunoconfocal microscopy. *, p < 0.05. All data shown were quantified from over 20 cells from three independently prepared samples. C, Western blot showing the accumulation of IL-1Rrp2, IL-1RAcp, and IL-1R1 in NCI or hDF cells 3 h after IL-36γ addition. All images are from Western blots performed with the same set of cell lysates. The abundance of β-actin was used as an internal control. These results for Tollip knockdown decreasing IL-1Rrp2 and IL-1RAcp accumulation were reproduced in more than three independent experiments. The decrease in the Tollip knockdown was ∼50% in these experiments. D, Western blot showing that IL-1β-treated NCI and hDF cells also exhibit an increase in IL-1R1 accumulation. Cell lysates used for the analysis were from 3 h after the addition of IL-1β. E, model for the role of Tollip in IL-36R trafficking in the absence or presence of IL-36R agonists.
Does Tollip play a role in increasing IL-1Rrp2 localization in the LAMP1+ lysosome? Consistent with our previous results, cells treated with a control siRNA increased IL-1Rrp2 colocalization with LAMP1+ lysosomes (Fig. 5E). Unexpectedly, however, cells knocked down for Tollip increased IL-1Rrp2 colocalization with the LAMP1+ lysosomes even in the absence of IL-36γ (Fig. 5A). Furthermore, IL-1Rrp2 colocalization with LAMP1 increased only when the cells were treated with IL-36γ. This result was consistent at three different time points and with six preparations of NCI cells. Therefore, Tollip has two distinct roles with the IL-36 agonist-activated IL-36R and the steady-state unactivated IL-36R. Tollip facilitates the agonist-activated IL-36R to stably associate with LAMP+ lysosomes, and it prevents the unactivated IL-36R from locating to lysosomes.
Tollip Modulates the Accumulation of the IL-36R
We examined whether the knockdown of Tollip affected the accumulation of IL-1Rrp2 and IL-1RAcp in NCI cells treated with IL-36γ. Tollip knockdown decreased the levels of both IL-1Rrp2 and IL-1RAcp (Fig. 5C), suggesting that Tollip can stabilize the IL-36R even though the receptor accumulates in lysosomes in association with signaling. The same result was found in hDFs (Fig. 5C, right column). The model for the role of Tollip in IL-36R trafficking is shown in Fig. 5D.
Tollip stabilization of the IL-36R was unexpected because it has been reported by Brissoni et al. (10) to increase the degradation of the IL-1R. Consistent with their report, we observed that NCI cells knocked down for Tollip had increased IL-1R1 accumulation in both NCI cells and hDFs (Fig. 5E).
The differential effects of Tollip on IL-1R1 and IL-1Rrp2 accumulation could be due to treatment of the cells with IL-36γ and not IL-1β. To examine whether this is the case, NCI cells knocked down for Tollip were treated with IL-1β. The addition of IL-1β increased IL-1R1 accumulation in both NCI cells and hDFs (Fig. 5E). Therefore, although Tollip is required for signaling by both the IL-36R and IL-1R1 (Fig. 4A), it increases the stability of the IL-36R while decreasing the stability of the IL-1R.
The IL-36R Half-life Is Not Affected by Agonist Addition
The presence of Tollip stabilizes activated IL-36R even though IL-36R preferentially accumulates in lysosomes. To document this further, we assessed the half-life of IL-1Rrp2 in the absence and presence of IL-36γ in NCI cells. A complication in the analysis was that NCI cells treated with IL-36γ expressed higher levels of IL-1Rrp2 compared with mock-treated cells (Fig. 6A). RT-PCR analysis of IL-36γ-treated cells also revealed an increase in the abundance of the IL-1Rrp2 message.5 These results suggest that activation of the IL-36R for signal transduction also activates IL-36R gene expression. This phenomenon was not examined further in this work. However, to determine the half-life of the IL-36R upon agonist addition, we needed to inhibit subsequent rounds of protein synthesis.
NCI cells were treated or mock-treated with IL-36γ and cycloheximide to inhibit subsequent translation to allow quantification of the IL-1Rrp2 molecules that were induced by the agonist (Fig. 5A). In the absence of IL-36γ, IL-1Rrp2 had a half-life of ∼8 h. Cells treated with IL-36γ also had a half-life of ∼8 h (Fig. 5, A and B). This result was reproducible in four independent experiments. Furthermore, a similar half-life for IL-1Rrp2 was observed with hDFs treated with IL-36γ (Fig. 5C and data not shown). These results suggest that trafficking of the IL-36R to the LAMP1+ lysosomes does not facilitate the degradation of IL-1Rrp2.
We examined whether the half-life of IL-1RAcp was affected by the addition of IL-36γ in NCI cells. Unlike IL-1Rrp2, the level of IL-1RAcP increased with cell density even in the absence of IL-36 (Fig. 6D). In the presence of cycloheximide, IL-1RAcp had a half-life of ∼5 h in three independent experiments (Fig. 6, D and E). Similar half-lives for the IL-1RAcp were observed in hDFs (Fig. 6F and data not shown). In conclusion, IL-36γ, which increased IL-36R localization into the LAMP1 lysosomes, did not significantly accelerate the degradation of IL-1RAcp.
Discussion
The IL-1R family contains a number of receptors that regulate the inflammatory responses in different tissues. Although signal transduction and the functionally coupled intracellular trafficking have been characterized for the IL-1R, relatively little information is known about the IL-36R. We sought to establish the requirements for IL-36R signaling and trafficking and compare the requirements to those exhibited by the IL-1R. The activities of the IL-36R were examined primarily in an ovarian cancer cell line, but the key features we elucidated were shared with other non-cancer cell lines, including primary adult human dermal fibroblasts. We found that IL-36R signaling is linked to endocytosis, likely through a clathrin-mediated pathway. In NCI cells, a population of the IL-36R exists on the plasma membrane, and these receptors cycle into endosomes and back to the plasma membrane in the absence of agonists. Exposure to IL-36 will alter the dynamics of intracellular trafficking so that an increased proportion of the IL-36R will enter LAMP1 lysosomes (Fig. 6A). Inhibition of endosome acidification did not dramatically affect IL-36R signaling. Furthermore, MyD88 and Tollip participate in signal transduction by both the IL-1R and IL-36R.
Tollip is involved in both IL-36R and IL-1R signaling and trafficking, but it has distinct effects on the two receptors. Burns et al. (34) reported that Tollip was incorporated into the activated receptor signaling complex by binding to IRAK and IL-1RAcp, thus stabilizing the complex. Brissoni et al. (10) reported that Tollip acts as an endosomal adaptor to direct the IL-1R to the endosomal degradation complex via Tom1 (10). This likely takes place through Tollip binding to polyubiquitinated cargo proteins that then modulate Tollip binding phosphatidylinositol 3-phosphate to allow targeting to endosomes (35). With regard to the IL-1R, this retargeting results in degradation.
For the IL-36R, increased Tollip colocalization with the IL-36R in endosomes occurred after agonist addition. Immunoconfocal microscopic examination of the colocalization of IL-36R with the lysosomal marker LAMP1 and the recycling endosomal marker Rab11 revealed that, in the absence of Tollip, the IL-36R localized in higher quantities to the lysosome. On the other hand, ligand treatment increased IL-36R localization to recycling endosomes, as observed by colocalization of IL-1Rrp2 with Rab11 in cells knocked down for Tollip. These results suggest that Tollip is involved in IL-36R trafficking and that the final destination of the IL-36R depends on the presence of the IL-36R agonist. Consistent with clathrin-mediated endocytosis, Tollip can modulate the function of endosomes through the recruitment of clathrin to the endosomes (36). Although we did not directly addressed the ubiquitination of the IL-36R, we observed frequently that IL-1Rrp2 separate in denaturing gels as a ladder of bands that are suggestive of polyubiquitination (Figs. 5C and 6).
Importantly, a decreased level of Tollip resulted in a decrease in the abundance of both subunits of the IL-36R while increasing the abundance of the IL-1R1 (Fig. 5). These results suggest that Tollip functions to prevent IL-36R turnover, whereas it increases IL-1R turnover. In the presence of normal levels of Tollip, however, IL-36 increases localization of the IL-36R into LAMP1 lysosomes (Fig. 4B), and both subunits of the IL-36R are not turned over to a greater extent (Fig. 6). These results suggest that the lysosome may not be the final destination of the IL-36R. The lysosome has been found to function in nonclassical secretion (37). In fact, the IL-1β cytokine can be processed and secreted from lysosomes into cells that have an activated inflammasome (38). Importantly, the accumulation of IL-36R in lysosomes is different from reports of the IL-1R, which is degraded upon trafficking to lysosomes.
Members of the IL-1R family have important roles as mediators for the innate immune response and inflammation (1–4). With the complex regulation of different agonists and receptor antagonists, myriad ways to regulate receptor signaling appear to have evolved. Our observation is that the fate of the IL-36R differs from that of the IL-1R after trafficking to lysosomes. In NCI cells, IL-1R signal transduction was not affected as significantly by endocytosis inhibitors (Fig. 2, B and C). In fact, the activated IL-36R colocalizes with clathrin and with other receptors that use clathrin-mediated endocytosis. The IL-1R has been reported to endocytose in astroglial cells using caveolae (23). Tollip helps to target the ligand-bound IL-1R for degradation, whereas it stabilized the agonist-activated IL-36R. It is likely that cellular kinases could contribute to the different roles for Tollip because previous studies have revealed that kinases can exert opposite effects on two endocytic pathways to modulate different receptor signaling (39). The differences in the trafficking and accumulation of the IL-1R and IL-36R could contribute to the level or timing of signaling by the receptors.
Author Contributions
C. C. K. and L. M. B. conceived and coordinated the study, analyzed all results, and wrote the paper. S. S. S. acquired the results in Figs, 2, A–C, and 4–6. D. S. acquired and analyzed the results in Figs. 1, 2, D–F, and 3 and contributed to the writing of the paper. E. L. R., G. C., S. E. B., and C. G. identified cells for analysis and characterized the effects of agonists on signaling. R. G., D. W., S. E. B., and J. R. W. identified and provided critical reagents for the analyses.
Acknowledgments
We thank Archita Agrawal for discussions and Laura Kao for editing the manuscript.
The authors declare that they have no conflicts of interest with the contents of this article.
G. Caviness et al., unpublished results.
- IL-36R
- IL-36 receptor
- MβCD
- methyl-β-cyclodextrin
- hDF
- human dermal fibroblast
- hTFN
- human transferrin.
References
- 1. Garlanda C., Dinarello C. A., Mantovani A. (2013) The interleukin-1 family: back to the future. Immunity 39, 1003–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boraschi D., Tagliabue A. (2013) The interleukin-1 receptor family. Semin. Immunol. 25, 394–407 [DOI] [PubMed] [Google Scholar]
- 3. van de Veerdonk F. L., Netea M. G. (2013) New insights in the immunobiology of IL-1 family members. Front. Immunol. 4, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dinarello C. A. (2013) Overview of the interleukin-1 family of ligands and receptors. Semin. Immunol. 25, 389–393 [DOI] [PubMed] [Google Scholar]
- 5. Towne J. E., Sims J. E. (2012) IL-36 in psoriasis. Curr. Opin. Pharmacol. 12, 486–490 [DOI] [PubMed] [Google Scholar]
- 6. Dunne A., O'Neill L. A. (2003) The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Science STKE 2003, re3. [DOI] [PubMed] [Google Scholar]
- 7. Subramaniam S., Stansberg C., Cunningham C. (2004) The interleukin 1 receptor family. Dev. Comp. Immunol. 28, 415–428 [DOI] [PubMed] [Google Scholar]
- 8. Medzhitov R., Preston-Hurlburt P., Kopp E., Stadlen A., Chen C., Ghosh S., Janeway C. A. Jr. (1998) MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell 2, 253–258 [DOI] [PubMed] [Google Scholar]
- 9. Tiwari R. L., Singh V., Singh A., Barthwal M. K. (2011) IL-1R-associated kinase-1 mediates protein kinase Cδ-induced IL-1β production in monocytes. J. Immunol. 187, 2632–2645 [DOI] [PubMed] [Google Scholar]
- 10. Brissoni B., Agostini L., Kropf M., Martinon F., Swoboda V., Lippens S., Everett H., Aebi N., Janssens S., Meylan E., Felberbaum-Corti M., Hirling H., Gruenberg J., Tschopp J., Burns K. (2006) Intracellular trafficking of interleukin-1 receptor I requires Tollip. Curr. Biol. 16, 2265–2270 [DOI] [PubMed] [Google Scholar]
- 11. Bourke E., Cassetti A., Villa A., Fadlon E., Colotta F., Mantovani A. (2003) IL-1 β scavenging by the type II IL-1 decoy receptor in human neutrophils. J. Immunol. 170, 5999–6005 [DOI] [PubMed] [Google Scholar]
- 12. Gresnigt M. S., van de Veerdonk F. L. (2013) Biology of IL-36 cytokines and their role in disease. Semin. Immunol. 25, 458–465 [DOI] [PubMed] [Google Scholar]
- 13. Towne J. E., Renshaw B. R., Douangpanya J., Lipsky B. P., Shen M., Gabel C. A., Sims J. E. (2011) Interleukin-36 (IL-36) ligands require processing for full agonist (IL-36α, IL-36β, and IL-36γ) or antagonist (IL-36Ra) activity. J. Biol. Chem. 286, 42594–42602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Towne J. E., Garka K. E., Renshaw B. R., Virca G. D., Sims J. E. (2004) Interleukin (IL)-1F6, IL-1F8, and IL-1F9 signal through IL-1Rrp2 and IL-1RAcP to activate the pathway leading to NF-κB and MAPKs. J. Biol. Chem. 279, 13677–13688 [DOI] [PubMed] [Google Scholar]
- 15. Singh D., Qi R., Jordan J. L., San Mateo L., Kao C. C. (2013) The human antimicrobial peptide LL-37, but not the mouse ortholog, mCRAMP, can stimulate signaling by poly(I:C) through a FPRL1-dependent pathway. J. Biol. Chem. 288, 8258–8268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ranjith-Kumar C. T., Miller W., Sun J., Xiong J., Santos J., Yarbrough I., Lamb R. J., Mills J., Duffy K. E., Hoose S., Cunningham M., Holzenburg A., Mbow M. L., Sarisky R. T., Kao C. C. (2007) Effects of single nucleotide polymorphisms on Toll-like receptor 3 activity and expression in cultured cells. J. Biol. Chem. 282, 17696–17705 [DOI] [PubMed] [Google Scholar]
- 17. Bolte S., Cordelières F. P. (2006) A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224, 213–232 [DOI] [PubMed] [Google Scholar]
- 18. Qi R., Singh D., Kao C. C. (2012) Proteolytic processing regulates Toll-like receptor 3 stability and endosomal localization. J. Biol. Chem. 287, 32617–32629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brinkmann M. M., Spooner E., Hoebe K., Beutler B., Ploegh H. L., Kim Y. M. (2007) The interaction between the ER membrane protein UNC93B and TLR3, 7, and 9 is crucial for TLR signaling. J. Cell Biol. 177, 265–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sander J. D., Dahlborg E. J., Goodwin M. J., Cade L., Zhang F., Cifuentes D., Curtin S. J., Blackburn J. S., Thibodeau-Beganny S., Qi Y., Pierick C. J., Hoffman E., Maeder M. L., Khayter C., Reyon D., Dobbs D., Langenau D. M., Stupar R. M., Giraldez A. J., Voytas D. F., Peterson R. T., Yeh J. R., Joung J. K. (2011) Selection-free zinc-finger-nuclease engineering by context-dependent assembly (CoDA). Nat. Methods 8, 67–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ahmed S., Maratha A., Butt A. Q., Shevlin E., Miggin S. M. (2013) TRIF-mediated TLR3 and TLR4 signaling is negatively regulated by ADAM15. J. Immunol. 190, 2217–2228 [DOI] [PubMed] [Google Scholar]
- 22. Sorkin A., von Zastrow M. (2009) Endocytosis and signalling: intertwining molecular networks. Nat. Rev. Mol. Cell Biol. 10, 609–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blanco A. M., Perez-Arago A., Fernandez-Lizarbe S., Guerri C. (2008) Ethanol mimics ligand-mediated activation and endocytosis of IL-1RI/TLR4 receptors via lipid rafts caveolae in astroglial cells. J. Neurochem. 106, 625–639 [DOI] [PubMed] [Google Scholar]
- 24. Doherty G. J., McMahon H. T. (2009) Mechanisms of endocytosis. Annu. Rev. Biochem. 78, 857–902 [DOI] [PubMed] [Google Scholar]
- 25. Wang L. H., Rothberg K. G., Anderson R. G. (1993) Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 123, 1107–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ares G. R., Ortiz P. A. (2012) Dynamin2, clathrin, and lipid rafts mediate endocytosis of the apical Na/K/2Cl cotransporter NKCC2 in thick ascending limbs. J. Biol. Chem. 287, 37824–37834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Janes P. W., Ley S. C., Magee A. I. (1999) Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J. Cell Biol. 147, 447–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stoorvogel W., Oorschot V., Geuze H. J. (1996) A novel class of clathrin-coated vesicles budding from endosomes. J. Cell Biol. 132, 21–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nabi I. R., Le P. U. (2003) Caveolae/raft-dependent endocytosis. J. Cell Biol. 161, 673–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun J., Duffy K. E., Ranjith-Kumar C. T., Xiong J., Lamb R. J., Santos J., Masarapu H., Cunningham M., Holzenburg A., Sarisky R. T., Mbow M. L., Kao C. (2006) Structural and functional analyses of the human Toll-like receptor 3: role of glycosylation. J. Biol. Chem. 281, 11144–11151 [DOI] [PubMed] [Google Scholar]
- 31. Leonard J. N., Ghirlando R., Askins J., Bell J. K., Margulies D. H., Davies D. R., Segal D. M. (2008) The TLR3 signaling complex forms by cooperative receptor dimerization. Proc. Natl. Acad. Sci. U.S.A. 105, 258–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Waterman H., Yarden Y. (2001) Molecular mechanisms underlying endocytosis and sorting of ErbB receptor tyrosine kinases. FEBS Lett. 490, 142–152 [DOI] [PubMed] [Google Scholar]
- 33. Zhang G., Ghosh S. (2002) Negative regulation of toll-like receptor-mediated signaling by Tollip. J. Biol. Chem. 277, 7059–7065 [DOI] [PubMed] [Google Scholar]
- 34. Burns K., Janssens S., Brissoni B., Olivos N., Beyaert R., Tschopp J. (2003) Inhibition of interleukin 1 receptor/Toll-like receptor signaling through the alternatively spliced, short form of MyD88 is due to its failure to recruit IRAK-4. J. Exp. Med. 197, 263–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mitra S., Traughber C. A., Brannon M. K., Gomez S., Capelluto D. G. (2013) Ubiquitin interacts with the Tollip C2 and CUE domains and inhibits binding of Tollip to phosphoinositides. J. Biol. Chem. 288, 25780–25791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Katoh Y., Imakagura H., Futatsumori M., Nakayama K. (2006) Recruitment of clathrin onto endosomes by the Tom1-Tollip complex. Biochem. Biophys. Res. Comm. 341, 143–149 [DOI] [PubMed] [Google Scholar]
- 37. Mambula S. S., Calderwood S. K. (2006) Heat shock protein 70 is secreted from tumor cells by a nonclassical pathway involving lysosomal endosomes. J. Immunol. 177, 7849–7857 [DOI] [PubMed] [Google Scholar]
- 38. Qu Y., Franchi L., Nunez G., Dubyak G. R. (2007) Nonclassical IL-1 β secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J. Immunol. 179, 1913–1925 [DOI] [PubMed] [Google Scholar]
- 39. Pelkmans L., Fava E., Grabner H., Hannus M., Habermann B., Krausz E., Zerial M. (2005) Genome-wide analysis of human kinases in clathrin- and caveolae/raft-mediated endocytosis. Nature 436, 78–86 [DOI] [PubMed] [Google Scholar]