Abstract
HDL is the primary mediator of cholesterol mobilization from the periphery to the liver via reverse cholesterol transport (RCT). A critical first step in this process is the uptake of cholesterol from lipid-loaded macrophages by HDL, a function of HDL inversely associated with prevalent and incident cardiovascular disease. We hypothesized that the dynamic ability of HDL to undergo remodeling and exchange of apoA-I is an important and potentially rate-limiting aspect of RCT. In this study, we investigated the relationship between HDL-apoA-I exchange (HAE) and serum HDL cholesterol (HDL-C) efflux capacity. We compared HAE to the total and ABCA1-specific cholesterol efflux capacity of 77 subjects. We found that HAE was highly correlated with both total (r = 0.69, P < 0.0001) and ABCA1-specific (r = 0.47, P < 0.0001) efflux, and this relationship remained significant after adjustment for HDL-C or apoA-I. Multivariate models of sterol efflux capacity indicated that HAE accounted for approximately 25% of the model variance for both total and ABCA1-specific efflux. We conclude that the ability of HDL to exchange apoA-I and remodel, as measured by HAE, is a significant contributor to serum HDL efflux capacity, independent of HDL-C and apoA-I, indicating that HDL dynamics are an important factor in cholesterol efflux capacity and likely RCT.
Keywords: high density lipoprotein, high density lipoprotein/metabolism, lipoproteins, ATP binding cassette transporter A1, high density lipoprotein remodeling/exchange
Over 40 years ago, Ross and Glomset (1) postulated that cholesterol mobilization via reverse cholesterol transport (RCT) is critical for preventing atherosclerosis. HDL has since been identified as the primary mediator of RCT, along with additional potentially anti-atherogenic properties, including antioxidant, anti-inflammatory, and endothelial maintenance activities (2). Numerous clinical studies have demonstrated the inverse association between plasma HDL cholesterol (HDL-C) levels and CVD risk in populations (3–6), and HDL-C concentration has long been utilized as a proxy measure of HDL’s aggregate anti-atherogenic functions (7). More recently, however, the causal relationship between HDL-C level and reduced CVD risk has been questioned in light of human genetic variations that reduce HDL-C yet do not increase CVD risk, such as LCAT deficiency and apoA-IMilano (8, 9), and clinical studies of compounds that increase HDL-C but do not improve cardiovascular outcomes (10–12). These observations underscore the complexities of HDL and the importance of direct measurement of HDL function (biological activity), in particular, its ability to facilitate RCT.
The most widely utilized measure of HDL function is cholesterol efflux capacity (13–15), quantified by cell-based assays employing cultured macrophages or genetically modified cells (16, 17). Recent studies demonstrated an inverse relationship of sterol efflux capacity with prevalent CVD, as well as incident CVD (13, 14), independent of HDL-C and apoA-I levels. Although multiple mechanisms mediate macrophage cholesterol efflux to HDL, cell-based cholesterol efflux assays have principally focused on ABCA1-mediated efflux due to the accumulation of cholesterol in macrophages when ABCA1 is absent or impaired (18, 19), as well as its ability to measure serum efflux capacity (20).
apoA-I, the major protein component of HDL, plays a key role in RCT, and is integral to the interaction between HDL and the various receptors and HDL remodeling enzymes (21). apoA-I is a highly dynamic protein (22), and this property is important for both the remodeling of HDL particles (23–26) and the promotion of cholesterol efflux (27). apoA-I structure and function are critical to its anti-atherogenic molecular processes (28), largely due to its ability to promote cholesterol efflux from ABCA1 (29). In vitro studies suggest that the latter may be impaired by oxidative modification of apoA-I, which we hypothesized to act by inhibiting HDL remodeling/exchange of apoA-I (24, 30).
We recently developed a rapid and precise assay employing electron paramagnetic resonance (EPR) spectroscopy that measures the relative rate of HDL-apoA-I exchange (HAE). HAE provides a measure of the ability of HDL to remodel and release lipid-poor apoA-I (24, 31). The assay capitalizes on the high specificity of apoA-I for HDL and the exchangeable nature of HDL-associated apoA-I, which can be released upon the addition of exogenous lipid-free apoA-I (31). As spin-labeled apoA-I associates with HDL, the EPR spectra’s peak amplitude increases due to structural changes in apoA-I from a lipid-free to a lipid-bound conformation (31, 32). The ratio of lipid-free to lipid-bound apoA-I provides a measure of the relative exchangeability of endogenous apoA-I and the dynamic nature of HDL particles. Our early investigation into the clinical relevance of HDL dynamics suggests that HAE is impaired in people with prevalent CVD, and as such it may be a CVD-relevant measure of HDL function (31).
Despite the widely held conjecture that HDL dynamics are important in RCT, there are limited data on the role of HDL remodeling and dynamics in cholesterol efflux. In macrophages, cholesterol efflux is thought to be facilitated by ABCA1, with contributions from ABCG1, scavenger receptor class B type I (SR-BI), and passive diffusion (33). In turn, the prevailing understanding is that ABCA1-mediated cholesterol efflux is most efficiently driven by small dense HDL and/or lipid-poor apoA-I (29, 34). In this study, we investigated the relationship between HDL dynamics and cholesterol efflux capacity in human subjects. We quantified efflux in both J774 macrophages and ABCA1-transfected baby hamster kidney (BHK) cells (ABCA1-BHK) to assess the relative contribution of HAE to total efflux and ABCA1-mediated efflux, respectively. We found that HAE was significantly correlated (P < 0.0001) with both measures of HDL efflux capacity, independent of HDL-C and apoA-I, supporting the conclusion that HDL dynamics/remodeling is an important factor in HDL’s sterol efflux capacity.
MATERIALS AND METHODS
Study subjects
Plasma samples (n = 77) were collected from subjects undergoing coronary angiography at the University of Washington Medical Center. Coronary angiography was clinically indicated either for ischemic chest pain or as a part of preoperation evaluation for other surgeries in subjects with known coronary heart disease. The cohort included subjects who reported to the emergency room with chest pain, but did not have angiographically diagnosed coronary artery disease, subjects with diagnosed coronary artery disease (medical history of myocardial infarction (MI), percutaneous coronary intervention (PCI), or coronary artery bypass graft surgery (CABG)) but not acute coronary syndrome, and subjects suffering from acute coronary syndrome [confirmed MI, both ST-elevation myocardial infarction (STEMI) and nonSTEMI]. All subjects gave written informed consent that was approved by the University of Washington Institutional Review Board.
Plasma lipid analysis
The lipid panel (total cholesterol, HDL-C, and triglycerides) was measured in fasted EDTA-plasma by standard validated clinical assays on a Beckman DXC, and LDL cholesterol (LDL-C) was calculated using the Friedewald formula. Plasma apoA-I levels were determined by validated nephelometric method.
Cholesterol efflux assays
Cholesterol efflux assays were performed essentially as described by Khera et al. (13). The apoB-containing particles were precipitated from serum using polyethylene glycol and the resulting LDL/VLDL-free serum, representing serum HDL (at 2.8%), was used in the assays. J774 macrophages were labeled with [3H]cholesterol (1 μCi/ml) for 24 h at 37°C in DMEM. The cells were then washed with DMEM and incubated for an additional 24 h in DMEM containing cAMP (0.5 mM). Efflux of [3H]cholesterol was measured after a 4 h incubation with DMEM supplemented with 0.1% BSA without or with 2.8% of serum HDL. Cholesterol efflux mediated by HDL was calculated as the percentage of total [3H]cholesterol (medium plus cells) released into the medium after the value obtained with DMEM/BSA alone was subtracted.
ABCA1-specific sterol efflux was performed in BHK cells overexpressing human ABCA1 under mifepristone control. The ABCA1-BHK cells were labeled for 24 h at 37°C in DMEM containing [3H]cholesterol (1 μCi/ml). Cells were then incubated with or without mifepristone (10 nM) for 24 h, followed by 4 h incubation with serum HDL (2.8%) in DMEM supplemented with 0.1% BSA. Cholesterol efflux was calculated in mifepristone-treated and untreated cells as described for J774 cells, and the ABCA1-specific efflux was determined as the difference between efflux in cells treated with and without mifepristone. Each sample was analyzed in duplicate and an average is reported. Both assays were performed on the same day and a control sample was included on each plate. The coefficient of variability across all plates was 3.7% for J774 and 4.5% for ABCA1 efflux.
HAE assay
HAE assay methods were performed with apoB-depleted plasma as described in Borja, et al. (31), except that the EPR spectrum collection at 6°C was omitted, as analysis of previous data revealed the assay gave the same results when this step was not performed. Spin labeling of apoA-I is described in Oda et al. (32). Plasma was thawed and mixed 1:4 with PBS [20 mM phosphate, 150 mM NaCl (pH 7.4)] and 24% w/v PEG 6000 (Sigma) was added to a final concentration of 4%. Samples were centrifuged at 13,000 rpm for 10 min at 4°C to remove apoB-containing lipoproteins. The clarified plasma was mixed with 3 mg/ml spin-labeled apoA-I in a 3:1 ratio. Samples were incubated for 15 min at 37°C. EPR measurements were performed with a Bruker eScan spectrometer outfitted with a temperature controller (Noxygen). The peak amplitude of the nitroxide signal was compared with the peak amplitude of a proprietary internal standard provided by Bruker. HAE was determined by subtracting the sample/internal standard ratio of EPR intensities obtained from spin-labeled apoA-I in PBS from the ratio obtained from the plasma sample and dividing by the maximum sample/internal standard ratio obtained from spin-labeled apoA-I in a solution of SDS. The inter-assay coefficient of variability was 5.3%.
Statistical analysis
All HAE and cholesterol efflux assays were performed in duplicate and the mean was computed and used for analysis. Associations between different parameters were established by using linear regression and Pearson’s correlation coefficient. Multivariate regression was used to investigate the predictors of cholesterol efflux and HAE. Initial multivariate models were developed including the following variables: age, gender, plasma HDL-C, plasma apoA-I, plasma LDL-C, plasma triglycerides (log transformed), and presence of diabetes, hypertension, statins, and smoking. For the model building, all variables except gender, age, and binary variables (diabetes, hypertension, statins, and smoking) were standardized as a z-score. Models were optimized using a backward and forward stepwise approach and selecting the model with a minimum Akaike information criterion (AIC). Models for both J774 and ABCA1-BHK efflux were assessed for relative importance of individual variables [R, lmg algorithm in relaimpo package (35)]. The relative importance of each variable is presented as percent of the dependent variable (efflux) variance explained by the given model. Statistical significance was determined for P < 0.05 for all tests. Statistical analyses were performed using SAS (version 9.3) and R software (version. 3.1).
RESULTS
The clinical characteristics of 77 subjects, along with cholesterol efflux capacity and HAE, are summarized in Table 1. Subjects exhibited a spectrum of cardiovascular risk factors (see Materials and Methods), HAE, and efflux capacities. Due to the small size of the study population, our aim was to use the whole population with the assumption that the disease state would introduce physiologically relevant variance in the measured HDL properties, facilitating modeling of the relationship between HAE and sterol efflux capacity.
TABLE 1.
Clinical characteristics of study subjects
| Mean (±SD) for All Subjects [Range] (n = 77) | |
| Age (years) | 63 ± 11 [41–87] |
| Sex (male/female) | 61/16 (79% males) |
| Diabetes | 26 (34%) |
| Total cholesterol (mg/dl) | 147 ± 38 [81–298] |
| HDL-C (mg/dl) | 42 ± 12 [20–69] |
| LDL-C (mg/dl)a | 78 ± 35 [8–215] |
| Triglycerides (mg/dl) | 137 ± 103 [31–559] |
| apoA-I (mg/dl)b | 123 ± 24 [70–188] |
| Statins | 49 (64%) |
| Smoking | 42 (55%) |
| Hypertension | 60 (78%) |
| HAE (%) | 46.8 ± 7.3 [32.4–65.8] |
| Efflux, J774 cells (%) | 6.7 ± 1.2 [4.3–9.6] |
| Efflux, ABCA1-BHK cells (%) | 7.5 ± 1.3 [5.2–11.5] |
LDL-C data for 76 subjects.
apoA-I data for 68 subjects.
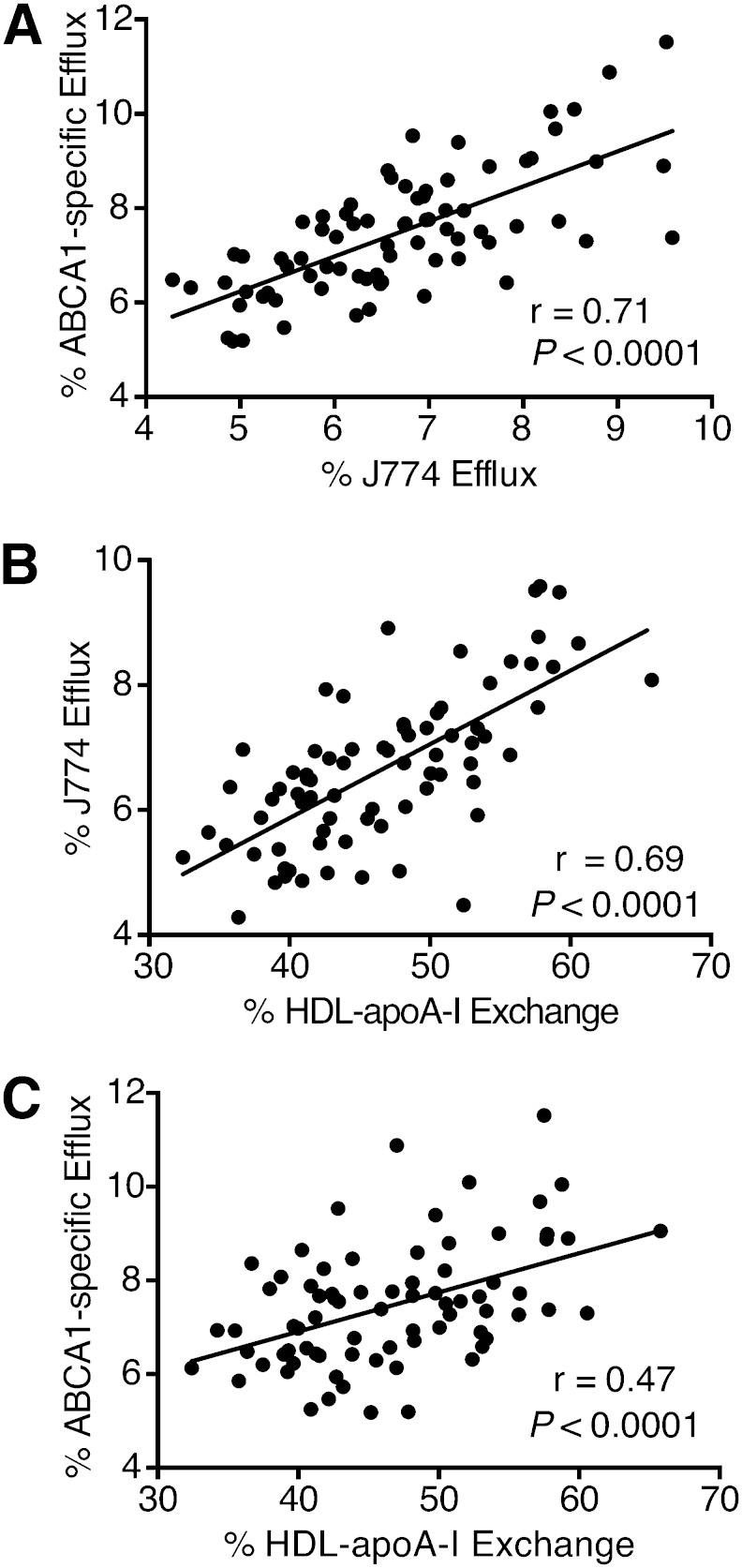
We investigated the effect of apoB depletion on HAE. Plasma from healthy volunteers (n = 10) was tested directly or after PEG-induced precipitation of apoB particles. There was no difference between the two sample preparations, indicating that apoB precipitation does not affect this measure of HDL function (Fig. 1). Additionally, we examined the relationship between the two cell-based efflux assays (J774 versus ABCA1-BHK). They exhibited a high positive correlation with each other (Fig. 2A; r = 0.71, P < 0.0001), confirming ABCA1 as the major contributor to mobilization of cholesterol from J774 cells. HAE had a similarly high positive correlation with efflux from J774 cells (Fig. 2B; r = 0.69, P < 0.0001) and was also correlated to efflux from ABCA1-BHK cells (Fig. 2C; r = 0.47, P < 0.0001). Further, HAE, J774 efflux, and ABCA1-BHK efflux all demonstrated significant positive correlations with apoA-I and HDL-C levels (Fig. 3). The significant correlations between the three assays suggest that they represent interdependent aspects of HDL function. To ascertain the degree to which apoA-I and HDL-C determine HAE and cholesterol efflux, we adjusted the correlations between HAE, J774 efflux, and ABCA1-BHK efflux for apoA-I or HDL-C levels. Adjusting for both HDL-C and apoA-I attenuated the relationship between HAE and J774 cholesterol efflux, but the correlation remained significant (Table 2). The relationship between HAE and ABCA1-BHK efflux was similarly attenuated, but remained significant following adjustment for HDL-C, and nearly reached significance after adjustment for apoA-I (Table 2; P = 0.068). Taken together, these results suggest that total HDL efflux capacity, as measured by J774 efflux, is in part associated with HAE, independent of apoA-I and HDL-C levels; whereas, the relationship between ABCA1-specific efflux capacity and HDL dynamics is independent of HDL-C, but dependent on apoA-I.
Fig. 1.
Plasma from healthy individuals (n = 10) was used to confirm that HAE values are similar whether obtained in apoB-depleted or whole plasma. ApoB depletion was performed as described in the Materials and Methods. Samples with whole plasma were diluted 1:5 in PBS prior to EPR. Each apoB-depleted and non-apoB-depleted pair was measured by EPR, and the data was compared using a two-tailed t-test.
Fig. 2.
Correlation of cell-based cholesterol efflux assays with each other and with HAE assay. Relationships between parameters were established using Pearson’s correlation coefficient. A: Correlation of cholesterol efflux from ABCA1-BHK cells with cholesterol efflux from J774 macrophage cells. B: Correlation of cholesterol efflux from J774 macrophages to apoB-depleted serum with HAE. C: Correlation of cholesterol efflux from human ABCA1-transfected BHK cells to apoB-depleted serum with HAE.
Fig. 3.
Correlation of HDL functional measures with apoA-I and HDL-C levels. Relationships between parameters were established using Pearson’s correlation coefficient. Correlations of HDL function data to apoA-I are in the left column, and correlations to HDL-C are in the right column. A: HAE. B: Cholesterol efflux to serum HDL from J774 macrophage cells. C: ABCA1-specific cholesterol efflux to serum HDL from BHK cells expressing human ABCA1.
TABLE 2.
Pearson correlation coefficients for full and partial correlation of HAE and cholesterol efflux data, adjusted for apoA-I or HDL-C
| Comparison | Unadjusted (n = 76) | Adjusted for ApoA-I (n = 67)a | Adjusted for HDL-C (n = 76) |
| HAE (%) versus efflux (%) (J774) | 0.69 | 0.29c | 0.36d |
| HAE (%) versus efflux (%) (ABCA1-BHK) | 0.47 | 0.22b | 0.30d |
| Efflux (%) J774 versus efflux (%) ABCA1-BHK | 0.71 | 0.60e | 0.74e |
Only 67 subjects were included in the data adjusted for apoA-I due to insufficient sample for nine subjects for apoA-I quantitation and one subject missing cholesterol efflux data.
P = 0.068.
P < 0.05.
P < 0.01.
P < 0.001.
To further investigate the factors driving the relationship between sterol efflux capacity and HAE, we employed multivariate linear regression modeling. We built models predicting sterol efflux capacity using HAE, controlling for the traditional CVD risk factors of gender, age, HDL-C, apoA-I, LDL-C, triglycerides, diabetes, hypertension, statins, and smoking. We applied step-wise model optimization that systematically eliminated variables that did not contribute to the prediction. We estimated the contribution of individual variables to the model by the lmg algorithm (36, 37). The optimized model for J774 sterol efflux capacity included HAE, gender, age, HDL-C, triglycerides, and statin use, and explained over 80% of the J774 efflux variance, with HDL-C (β = 0.884, P < 0.0001, 59% model variance explained) and HAE (β = 0.202, P = 0.031, 26% model variance explained) as the most significant contributors to the model (Table 3). The model for ABCA1-specific efflux capacity explained over 50% of the variance, with HAE as the strongest predictor (β = 0.342, P = 0.015, 24% explained) followed by HDL-C (β = 0.390, P = 0.01, 23% explained). In both models, male gender was a significant negative predictor of sterol efflux capacity (ABCA1-BHK sterol efflux, β = −0.754, P = 0.006; J774 sterol efflux β = −0.493, P = 0.007). As expected in both J774 and ABCA1-BHK models, apoA-I and HDL-C were highly correlated and, therefore, only one of them was included in the final model. As confirmation, models containing either apoA-I or HDL-C were nearly identical. Because statin use and diabetes were significant contributors to the sterol efflux prediction model, we also tested whether they might affect HAE in particular. Logistic regression was used to evaluate the effect of smoking, statin use, and diabetic status on HAE. Neither statin use nor smoking was associated with HAE with or without adjustment for HDL-C, age, and sex. While diabetic status was associated with significantly lower HAE (P = 0.008), this association disappeared after adjustment for HDL-C (P = 0.31), indicating that HAE is lower in diabetics due to reduced HDL-C. Adjustment for age and sex did not alter this relationship. In summary, multivariate models reveal that HDL dynamics, as measured by HAE, are a significant predictor of cholesterol efflux capacity from both J774 and ABCA1-BHK cells.
TABLE 3.
Multivariate models of sterol efflux with HAE
| J774 (R2 = 0.81) | ABCA1-BHK (R2 = 0.53) | |||||
| β Coefficienta | P | Relative Importanceb (%) | β Coefficienta | P | Relative Importanceb (%) | |
| Sex [F (reference)] | −0.493 | 0.007 | 7.9 | −0.754 | 0.006 | 22.7 |
| Age | 0.011 | 0.134 | 3.5 | — | — | — |
| HAE | 0.202 | 0.031 | 25.6 | 0.342 | 0.015 | 24.0 |
| HDL-C | 0.884 | <0.0001 | 58.5 | 0.390 | 0.010 | 22.9 |
| Triglycerides | 0.126 | 0.097 | 1.9 | 0.271 | 0.018 | 9.1 |
| Diabetes | — | — | — | 0.796 | 0.001 | 13.9 |
| Statins | −0.240 | 0.101 | 2.6 | −0.483 | 0.028 | 7.4 |
Standardized β coefficient; change of 1 SD in independent variable corresponding to β standard deviations of change in dependent variable.
Relative importance of individual independent variables as a fraction of the explained variance of dependent variable (lmg algorithm, see Materials and Methods).
DISCUSSION
Cell-based cholesterol efflux studies have been instrumental in demonstrating that the ability of HDL to accept cholesterol from lipid-loaded macrophages is linked to prevalent and incident CVD, and that HDL-C and apoA-I levels do not fully reflect HDL’s anti-atherogenic properties (13, 14, 20). However, factors controlling HDL sterol efflux capacity are largely unknown. Our previous work indicated that the ability of HDL to remodel and/or exchange apoA-I is attenuated in CVD subjects with similar apoA-I and HDL-C levels, suggesting that direct measurement of HDL dynamics measures a HDL property distinct from apoA-I or cholesterol concentration (31). To establish whether HAE and cholesterol efflux capacity assays measure independent HDL properties, we investigated the relationship between HDL dynamics, as measured by the HAE assay, and cholesterol efflux capacity, as measured by the cell-based assays. We found that HDL dynamics (HAE) and cholesterol efflux capacity are strongly associated and are, in part, independent of circulating levels of HDL-C and apoA-I. Multivariate models predicting serum HDL-C efflux capacity in J774 and ABCA1-BHK cell systems revealed that while HDL-C (and apoA-I) contributes to a large proportion of the sterol efflux capacity of serum HDL, HAE accounts for as much as 26% of model variance, indicating that HDL dynamics are a major factor in HDL sterol efflux capacity.
The capacity of HDL to remodel and exchange apoA-I is a critical aspect of HDL quality (24, 31), which is linked to HDL’s ability to interact with proteins involved in RCT, including ABC transporters, HDL remodeling proteins [including LCAT, cholesteryl ester transfer protein (CETP), phospholipid transfer protein (PLTP), hepatic lipase, and endothelial lipase] (38), and potentially SR-BI (33). In vitro oxidation studies have demonstrated that protein-protein interactions and dynamics of apoA-I are critical elements in HDL’s interaction with these factors (39–41). HDL remodeling and exchange of apoA-I can be induced when the equilibrium between lipid-associated and lipid-free apoA-I is altered, as when exogenous lipid-free apoA-I is added during the HAE assay (24, 31). The HAE assay is not appreciably affected by apoB depletion of plasma (Fig. 1), supporting the notion that lipid transfer with apoB-containing lipoproteins does not significantly influence HDL to apoA-I exchange under the conditions of the HAE assay. We have previously shown that, within the time frame of the HAE assay, lipid-free apoA-I predominantly exchanges onto smaller HDL particles (31). Furthermore, in vitro studies of HDL remodeling in the absence of apoB particles catalyzed by LCAT (42, 43), PLTP (44, 45), or CETP (46) suggest that HDL remodeling by these enzymes occurs on the time-scale of hours rather than minutes and, thus, is unlikely to affect HAE. While we cannot rule out the possibility that these factors may influence HDL’s propensity to exchange apoA-I in vivo, within the context of the HAE analyses reported here, any relationship between HAE and these remodeling enzymes is unlikely. Future studies will be necessary to examine the likely subtle interplay between HDL remodeling factors and HAE.
During RCT, apoA-I transitions through multiple lipidation states, during which it assumes an array of conformations (47). RCT is thus mediated by a spectrum of diverse HDL particle subspecies (21). Structural transitions in apoA-I and HDL are therefore important to the process of cholesterol efflux. In ABCA1-specific cholesterol efflux, for instance, lipid-poor preβ-1 HDL is associated with enhanced cholesterol efflux from ABCA1 (20), but high circulating levels of preβ-1 HDL are also associated with CVD (48), which may be indicative of dysfunctional HDL maturation or remodeling (49). A potential mechanism responsible for the latter is oxidative modification of HDL by myeloperoxidase, which inhibits desorption of lipid-poor apoA-I from HDL and its interaction with ABCA1, resulting in reduced HAE and cholesterol efflux capacity (24, 31, 50).
We observed that while HAE is correlated with ABCA1-specific efflux capacity independent of circulating levels of HDL-C, the relationship appears to be dependent on apoA-I (Table 2). This likely reflects the dependence of ABCA1-specific sterol efflux on the release of lipid-poor preβ-1 particles from HDL (29). Total efflux capacity measured from J774 macrophages was associated with HAE independent of both HDL-C and apoA-I, suggesting that HAE is more reflective of total efflux capacity and represents more than the mere release of endogenous apoA-I from HDL particles. In addition to the ABCA1-mediated pathway, cholesterol efflux from J774 cells is also driven by ABCG1, SR-BI, and passive diffusion (33). Cholesterol mobilization via these processes is predominantly through larger HDL particles (33). The ABCG1 and SR-BI components of efflux are subtracted from the ABCA1-BHK assay to provide the ABCA1-specific component of cholesterol efflux (see Materials and Methods). Correlation between the ABCA1-BHK and J774 macrophage assays (Fig. 1A; r = 0.71) suggests that about 50% of the cholesterol efflux from J774 cells is mediated by the ABCA1 pathway, consistent with previous observations (51). The binding of exogenous apoA-I to HDL involves both the release of lipid-poor apoA-I and remodeling of HDL to both larger and smaller particles (24). Thus, the full repertoire of cholesterol-accepting HDL particles is better described by the J774 efflux assay, as total serum HDL or purified HDL3 yields a greater cholesterol efflux capacity versus lipid-free apoA-I alone (52).
Multivariate regression modeling of cholesterol efflux capacity describes HDL dynamics, as measured by HAE, as a significant independent predictor of cholesterol efflux capacity from both J774 and ABCA1-BHK cells. The optimized J774 model explained more than 80% of the observed variance in efflux capacity, with HDL-C and HAE having the greatest contribution to the model (over 80% combined). The ABCA1-BHK model explained more than 50% of the variance in efflux, with HAE, HDL-C, and gender as the strongest predictors (∼75% combined). In both models, gender contributed significantly to the efflux capacity prediction, in agreement with previous data suggesting that efflux capacity has a gender-specific component (53, 54). The finding that HAE is a significant predictor of both J774 and ABCA1-BHK efflux provides a mechanistic rationale as to why cholesterol efflux capacity is a superior predictor of CVD risk compared with HDL-C levels. The J774 macrophage efflux assay has been used to demonstrate the inverse association between cholesterol efflux capacity and prevalent CVD, as well as incident CVD (13, 14, 55). Our finding that HAE strongly associates with sterol efflux capacity suggests that it may have similar prognostic value, although this will need to be validated by cross-sectional and longitudinal studies with large clinical cohorts. Given the highly heterogeneous distribution of HDL with respect to particle size, charge, protein, and lipid composition, HAE offers a facile means of investigating the effects of these properties on HDL dynamics and their relationship to cholesterol efflux capacity and CVD risk.
In conclusion, HDL dynamics, as measured by HAE, are positively correlated with serum HDL efflux capacity, measured in J774 macrophages and ABCA1-BHK cells. This association is independent of both HDL-C and apoA-I levels in the total efflux (J774) assay and HDL-C in the ABCA1-specific efflux assay. Our findings demonstrate that HDL dynamics are an important factor in HDL’s ability to efflux cholesterol from cells and ultimately its facilitation of RCT.
Footnotes
Abbreviations:
- BHK
- baby hamster kidney
- CETP
- cholesteryl ester transfer protein
- EPR
- electron paramagnetic resonance
- HAE
- HDL-apoA-I exchange
- HDL-C
- HDL cholesterol
- LDL-C
- LDL cholesterol
- PLTP
- phospholipid transfer protein
- RCT
- reverse cholesterol transport
- SR-BI
- scavenger receptor class B type I
This work was supported by California Tobacco-Related Disease Research Program (TRDRP) Grant 22RT-0095 to M.N.O. and American Heart Association Western States Affiliate postdoctoral fellowship 14POST1833018 to M.S.B. A.I., J.H., X.W., D.I., X-Q.Z., B.P. and T.V. were supported by National Institutes of Health Grant HL083578.
REFERENCES
- 1.Ross R., Glomset J. A. 1973. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 180: 1332–1339. [DOI] [PubMed] [Google Scholar]
- 2.Rosenson R. S., Brewer H. B., Jr, Chapman M. J., Fazio S., Hussain M. M., Kontush A., Krauss R. M., Otvos J. D., Remaley A. T., Schaefer E. J. 2011. HDL measures, particle heterogeneity, proposed nomenclature, and relation to atherosclerotic cardiovascular events. Clin. Chem. 57: 392–410. [DOI] [PubMed] [Google Scholar]
- 3.Di Angelantonio E., Sarwar N., Perry P., Kaptoge S., Ray K. K., Thompson A., Wood A. M., Lewington S., Sattar N., Packard C. J., et al. 2009. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. JAMA. 302: 1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon D. J., Probstfield J. L., Garrison R. J., Neaton J. D., Castelli W. P., Knoke J. D., Jacobs D. R., Jr, Bangdiwala S., Tyroler H. A. 1989. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 79: 8–15. [DOI] [PubMed] [Google Scholar]
- 5.Castelli W. P., Garrison R. J., Wilson P. W., Abbott R. D., Kalousdian S., Kannel W. B. 1986. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA. 256: 2835–2838. [PubMed] [Google Scholar]
- 6.Gordon T., Castelli W. P., Hjortland M. C., Kannel W. B., Dawber T. R. 1977. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am. J. Med. 62: 707–714. [DOI] [PubMed] [Google Scholar]
- 7.Glomset J. A. 1968. The plasma lecithins:cholesterol acyltransferase reaction. J. Lipid Res. 9: 155–167. [PubMed] [Google Scholar]
- 8.Calabresi L., Baldassarre D., Simonelli S., Gomaraschi M., Amato M., Castelnuovo S., Frigerio B., Ravani A., Sansaro D., Kauhanen J., et al. 2011. Plasma lecithin:cholesterol acyltransferase and carotid intima-media thickness in European individuals at high cardiovascular risk. J. Lipid Res. 52: 1569–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sirtori C. R., Calabresi L., Franceschini G., Baldassarre D., Amato M., Johansson J., Salvetti M., Monteduro C., Zulli R., Muiesan M. L., et al. 2001. Cardiovascular status of carriers of the apolipoprotein A-I(Milano) mutant: the Limone sul Garda study. Circulation. 103: 1949–1954. [DOI] [PubMed] [Google Scholar]
- 10.Barter P. J., Caulfield M., Eriksson M., Grundy S. M., Kastelein J. J., Komajda M., Lopez-Sendon J., Mosca L., Tardif J. C., Waters D. D., et al. ; ILLUMINATE Investigators. 2007. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357: 2109–2122. [DOI] [PubMed] [Google Scholar]
- 11.Boden W. E., Probstfield J. L., Anderson T., Chaitman B. R., Desvignes-Nickens P., Koprowicz K., McBride R., Teo K., Weintraub W. 2011. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 365: 2255–2267. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz G. G., Olsson A. G., Abt M., Ballantyne C. M., Barter P. J., Brumm J., Chaitman B. R., Holme I. M., Kallend D., Leiter L. A., et al. ; dal-OUTCOMES Investigators. 2012. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N. Engl. J. Med. 367: 2089–2099. [DOI] [PubMed] [Google Scholar]
- 13.Khera A. V., Cuchel M., de la Llera-Moya M., Rodrigues A., Burke M. F., Jafri K., French B. C., Phillips J. A., Mucksavage M. L., Wilensky R. L., et al. 2011. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 364: 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rohatgi A., Khera A., Berry J. D., Givens E. G., Ayers C. R., Wedin K. E., Neeland I. J., Yuhanna I. S., Rader D. R., de Lemos J. A., et al. 2014. HDL cholesterol efflux capacity and incident cardiovascular events. N. Engl. J. Med. 371: 2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X. M., Tang W. H., Mosior M. K., Huang Y., Wu Y., Matter W., Gao V., Schmitt D., Didonato J. A., Fisher E. A., et al. 2013. Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arterioscler. Thromb. Vasc. Biol. 33: 1696–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothblat G. H., de la Llera-Moya M., Favari E., Yancey P. G., Kellner-Weibel G. 2002. Cellular cholesterol flux studies: methodological considerations. Atherosclerosis. 163: 1–8. [DOI] [PubMed] [Google Scholar]
- 17.Vaughan A. M., Oram J. F. 2003. ABCA1 redistributes membrane cholesterol independent of apolipoprotein interactions. J. Lipid Res. 44: 1373–1380. [DOI] [PubMed] [Google Scholar]
- 18.Oram J. F. 2000. Tangier disease and ABCA1. Biochim. Biophys. Acta. 1529: 321–330. [DOI] [PubMed] [Google Scholar]
- 19.Francone O. L., Royer L., Boucher G., Haghpassand M., Freeman A., Brees D., Aiello R. J. 2005. Increased cholesterol deposition, expression of scavenger receptors, and response to chemotactic factors in Abca1-deficient macrophages. Arterioscler. Thromb. Vasc. Biol. 25: 1198–1205. [DOI] [PubMed] [Google Scholar]
- 20.de la Llera-Moya M., Drazul-Schrader D., Asztalos B. F., Cuchel M., Rader D. J., Rothblat G. H. 2010. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler. Thromb. Vasc. Biol. 30: 796–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenson R. S., Brewer H. B., Jr, Davidson W. S., Fayad Z. A., Fuster V., Goldstein J., Hellerstein M., Jiang X. C., Phillips M. C., Rader D. J., et al. 2012. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 125: 1905–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chetty P. S., Nguyen D., Nickel M., Lund-Katz S., Mayne L., Englander S. W., Phillips M. C. 2013. Comparison of apoA-I helical structure and stability in discoidal and spherical HDL particles by HX and mass spectrometry. J. Lipid Res. 54: 1589–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cavigiolio G., Shao B., Geier E. G., Ren G., Heinecke J. W., Oda M. N. 2008. The interplay between size, morphology, stability, and functionality of high-density lipoprotein subclasses. Biochemistry. 47: 4770–4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cavigiolio G., Geier E. G., Shao B., Heinecke J. W., Oda M. N. 2010. Exchange of apolipoprotein A-I between lipid-associated and lipid-free states: a potential target for oxidative generation of dysfunctional high density lipoproteins. J. Biol. Chem. 285: 18847–18857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pownall H. J., Hosken B. D., Gillard B. K., Higgins C. L., Lin H. Y., Massey J. B. 2007. Speciation of human plasma high-density lipoprotein (HDL): HDL stability and apolipoprotein A-I partitioning. Biochemistry. 46: 7449–7459. [DOI] [PubMed] [Google Scholar]
- 26.Lee J. Y., Lanningham-Foster L., Boudyguina E. Y., Smith T. L., Young E. R., Colvin P. L., Thomas M. J., Parks J. S. 2004. Prebeta high density lipoprotein has two metabolic fates in human apolipoprotein A-I transgenic mice. J. Lipid Res. 45: 716–728. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen S. D., Oorni K., Lee-Rueckert M., Pihlajamaa T., Metso J., Jauhiainen M., Kovanen P. T. 2012. Spontaneous remodeling of HDL particles at acidic pH enhances their capacity to induce cholesterol efflux from human macrophage foam cells. J. Lipid Res. 53: 2115–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher E. A., Feig J. E., Hewing B., Hazen S. L., Smith J. D. 2012. High-density lipoprotein function, dysfunction, and reverse cholesterol transport. Arterioscler. Thromb. Vasc. Biol. 32: 2813–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mulya A., Lee J. Y., Gebre A. K., Thomas M. J., Colvin P. L., Parks J. S. 2007. Minimal lipidation of pre-beta HDL by ABCA1 results in reduced ability to interact with ABCA1. Arterioscler. Thromb. Vasc. Biol. 27: 1828–1836. [DOI] [PubMed] [Google Scholar]
- 30.Shao B., Tang C., Sinha A., Mayer P. S., Davenport G. D., Brot N., Oda M. N., Zhao X. Q., Heinecke J. W. 2014. Humans with atherosclerosis have impaired ABCA1 cholesterol efflux and enhanced high-density lipoprotein oxidation by myeloperoxidase. Circ. Res. 114: 1733–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borja M. S., Zhao L., Hammerson B., Tang C., Yang R., Carson N., Fernando G., Liu X., Budamagunta M. S., Genest J., et al. 2013. HDL-apoA-I exchange: rapid detection and association with atherosclerosis. PLoS One. 8: e71541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oda M. N., Forte T. M., Ryan R. O., Voss J. C. 2003. The C-terminal domain of apolipoprotein A-I contains a lipid-sensitive conformational trigger. Nat. Struct. Biol. 10: 455–460. [DOI] [PubMed] [Google Scholar]
- 33.Phillips M. C. 2014. Molecular mechanisms of cellular cholesterol efflux. J. Biol. Chem. 289: 24020–24029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Du X. M., Kim M. J., Hou L., Le Goff W., Chapman M. J., van Eck M., Curtiss L. K., Burnett J., Cartland S. P., Quinn C. M., et al. 2015. HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export. Circ. Res. 116: 1133–1142. [DOI] [PubMed] [Google Scholar]
- 35.Grömping U. 2006. Relative importance for linear regression in R: the package relaimpo. J. Stat. Softw. 17: 1–27. [Google Scholar]
- 36.Lindeman R. H., Merenda P. F., Gold R. Z. 1980. Introduction to Bivariate and Multivariate Analysis. Scott, Foresman, Glenview, IL. [Google Scholar]
- 37.Johnson J. W., LeBreton J. M. 2004. History and use of relative importance indices in organizational research. Organ. Res. Methods. 7: 238–257. [Google Scholar]
- 38.Lund-Katz S., Phillips M. C. 2010. High density lipoprotein structure-function and role in reverse cholesterol transport. Subcell. Biochem. 51: 183–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shao B., Cavigiolio G., Brot N., Oda M. N., Heinecke J. W. 2008. Methionine oxidation impairs reverse cholesterol transport by apolipoprotein A-I. Proc. Natl. Acad. Sci. USA. 105: 12224–12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shao B., Tang C., Heinecke J. W., Oram J. F. 2010. Oxidation of apolipoprotein A-I by myeloperoxidase impairs the initial interactions with ABCA1 required for signaling and cholesterol export. J. Lipid Res. 51: 1849–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pussinen P. J., Metso J., Keva R., Hirschmugl B., Sattler W., Jauhiainen M., Malle E. 2003. Plasma phospholipid transfer protein-mediated reactions are impaired by hypochlorite-modification of high density lipoprotein. Int. J. Biochem. Cell Biol. 35: 192–202. [DOI] [PubMed] [Google Scholar]
- 42.Clay M. A., Pyle D. H., Rye K. A., Barter P. J. 2000. Formation of spherical, reconstituted high density lipoproteins containing both apoA-I and A-II is mediated by lecithin:cholesterol acyltransferase. J. Biol. Chem. 275: 9019–9025. [DOI] [PubMed] [Google Scholar]
- 43.Liang H. Q., Rye K. A., Barter P. J. 1996. Remodelling of reconstituted high density lipoproteins by lecithin:cholesterol acyltransferase. J. Lipid Res. 37: 1962–1970. [PubMed] [Google Scholar]
- 44.Lusa S., Jauhiainen M., Metso J., Somerharju P., Ehnholm C. 1996. The mechanism of human plasma phospholipid transfer protein-induced enlargement of high-density lipoprotein particles: evidence for particle fusion. Biochem. J. 313: 275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ji A., Wroblewski J. M., Webb N. R., van der Westhuyzen D. R. 2014. Impact of phospholipid transfer protein on nascent high-density lipoprotein formation and remodeling. Arterioscler. Thromb. Vasc. Biol. 34: 1910–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rye K. A., Hime N. J., Barter P. J. 1997. Evidence that cholesteryl ester transfer protein-mediated reductions in reconstituted high density lipoprotein size involve particle fusion. J. Biol. Chem. 272: 3953–3960. [DOI] [PubMed] [Google Scholar]
- 47.Wang L., Mei X., Atkinson D., Small D. M. 2014. Surface behavior of apolipoprotein A-I and its deletion mutants at model lipoprotein interfaces. J. Lipid Res. 55: 478–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Asztalos B. F., Collins D., Cupples L. A., Demissie S., Horvath K. V., Bloomfield H. E., Robins S. J., Schaefer E. J. 2005. Value of high-density lipoprotein (HDL) subpopulations in predicting recurrent cardiovascular events in the Veterans Affairs HDL Intervention Trial. Arterioscler. Thromb. Vasc. Biol. 25: 2185–2191. [DOI] [PubMed] [Google Scholar]
- 49.Asztalos B. F., Tani M., Schaefer E. J. 2011. Metabolic and functional relevance of HDL subspecies. Curr. Opin. Lipidol. 22: 176–185. [DOI] [PubMed] [Google Scholar]
- 50.Shao B., Heinecke J. W. 2011. Impact of HDL oxidation by the myeloperoxidase system on sterol efflux by the ABCA1 pathway. J. Proteomics. 74: 2289–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adorni M. P., Zimetti F., Billheimer J. T., Wang N., Rader D. J., Phillips M. C., Rothblat G. H. 2007. The roles of different pathways in the release of cholesterol from macrophages. J. Lipid Res. 48: 2453–2462. [DOI] [PubMed] [Google Scholar]
- 52.Sankaranarayanan S., Kellner-Weibel G., de la Llera-Moya M., Phillips M. C., Asztalos B. F., Bittman R., Rothblat G. H. 2011. A sensitive assay for ABCA1-mediated cholesterol efflux using BODIPY-cholesterol. J. Lipid Res. 52: 2332–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Catalano G., Duchene E., Julia Z., Le Goff W., Bruckert E., Chapman M. J., Guerin M. 2008. Cellular SR-BI and ABCA1-mediated cholesterol efflux are gender-specific in healthy subjects. J. Lipid Res. 49: 635–643. [DOI] [PubMed] [Google Scholar]
- 54.Villard E. F., El Khoury P., Frisdal E., Bruckert E., Clement K., Bonnefont-Rousselot D., Bittar R., Le Goff W., Guerin M. 2013. Genetic determination of plasma cholesterol efflux capacity is gender-specific and independent of HDL-cholesterol levels. Arterioscler. Thromb. Vasc. Biol. 33: 822–828. [DOI] [PubMed] [Google Scholar]
- 55.Doonan R. J., Hafiane A., Lai C., Veinot J. P., Genest J., Daskalopoulou S. S. 2014. Cholesterol efflux capacity, carotid atherosclerosis, and cerebrovascular symptomatology. Arterioscler. Thromb. Vasc. Biol. 34: 921–926. [DOI] [PubMed] [Google Scholar]