Abstract
DHA (22:6,ω3), but not EPA (20:5,ω3), attenuates Western diet (WD)-induced hepatic fibrosis in a Ldlr−/− mouse model of nonalcoholic steatohepatitis. We examined the molecular basis for the differential effect of dietary EPA and DHA on WD-induced hepatic fibrosis. DHA was more effective than EPA at preventing WD-induced effects on hepatic transcripts linked to fibrosis, including collagen 1A1 (Col1A1), transforming growth factor-β (TGFβ) signaling and proteins involved in remodeling the extracellular matrix, including metalloproteases, tissue inhibitors of metalloproteases, and lysyl oxidase subtypes. Examination of the TGFβ pathway showed that mice fed the WD supplemented with either olive oil or EPA had a significant (≥2.5-fold) increase in hepatic nuclear abundance of phospho-mothers against decapentaplegic homolog (Smad)3 when compared with mice fed the reference diet (RD); Smad3 is a key regulator of Col1A1 expression in stellate cells. In contrast, mice fed the WD supplemented with DHA had no increase in phospho-Smad3 when compared with mice fed the RD. Changes in hepatic phospho-Smad3 nuclear content correlated with proCol1A1 mRNA and protein abundance. Pretreatment of human LX2 stellate cells with DHA, but not other unsaturated fatty acids, blocked TGFβ1-mediated induction of Col1A1. In conclusion, DHA attenuates WD-induced fibrosis by targeting the TGFβ-Smad3-Col1A1 pathway in stellate cells.
Keywords: nonalcoholic steatohepatitis, collagen1A1, stellate cells, inflammation
Nonalcoholic fatty liver disease (NAFLD) is a significant public health burden in Western societies. NAFLD is defined as excess accumulation of liver fat (hepatosteatosis), mainly as neutral lipids consisting of triacylglycerols, cholesterol esters, and diacylglycerols. NAFLD ranges in severity from benign hepatosteatosis to nonalcoholic steatohepatitis (NASH) (1). NASH is defined as hepatosteatosis with inflammation and hepatic injury (2). Although simple hepatosteatosis is considered clinically benign, NASH is the progressive form of the disease that can lead to significant changes in hepatic morphology (hepatocyte ballooning) and injury (cell death and fibrosis).
The incidence of NAFLD has increased in parallel with the obesity epidemic in Western societies. In the general population, the prevalence of NAFLD is estimated to range from 6 to 33% (3), and ∼30–40% of individuals with hepatic steatosis progress to NASH (4). The prevalence of NASH ranges from 3 to 5% in the general population (3). NAFLD and NASH have high prevalence (≥60%) in patients with type 2 diabetes (5). Additionally, NASH patients have higher mortality rates than NAFLD patients (6–8), and over a 10 year period, cirrhosis and liver-related death occurs in 20 and 12% of NASH patients, respectively (9). Because NASH can progress to cirrhosis, hepatocellular cancer, and liver failure (4, 10–14), it is the third most common cause for liver transplantation. NASH is projected to be the leading cause of liver transplantation in the United States by 2020 (15).
Hepatic fibrosis involves the increased production of extracellular matrix (ECM) from activated hepatic stellate cells and myofibroblasts infiltrating the liver. The liver has an underlayment of connective tissue composed of several collagen subtypes (16). Fibrosis resulting from hepatic damage is linked to increased extracellular deposition of type 1 collagen [collagen1A1 (Col1A1)], smooth muscle actin, elastin, fibronectin, and other proteins. Fibrosis is also associated with increased production of proteins from stellate cells and macrophages that are involved in ECM remodeling; these proteins include lysyl oxidase and lysyl oxidase-like subtypes involved in collagen cross-linking, metalloprotease subtypes (MMPs), and tissue inhibitors of metalloproteases (TIMPs). The relative abundance of these proteins affects fibrosis progression and severity.
We previously reported that Ldlr−/− mice fed a Western diet (WD) develop a NASH phenotype that recapitulates human NASH in obese patients, including obesity, hyperlipidemia, hyperglycemia, hepatic damage, hepatosteatosis, induction of multiple markers of inflammation, oxidative stress, and fibrosis (17, 18). The WD is moderately high in saturated fat and trans-fat (43% total calories), and simple sugar (30% total calories), and cholesterol (1.5 g%) and reflects a modern, but unhealthy, diet (19). Dietary (fat, cholesterol, and fructose), metabolic (hepatic or plasma NEFA, hepatic ceramide), endocrine (insulin and leptin), gut (endotoxemia and the microbiome), and genetic (patatin-like phospholipase domain containing three polymorphisms) factors have been implicated in the progression of benign hepatosteatosis to NASH (20–27).
We also reported that adding EPA (20:5,ω3) or DHA (22:6,ω3) to the WD affected diet-induced hepatic fibrosis. DHA was more effective than EPA at attenuating WD-induced hepatic fibrosis (17, 18, 28). Although EPA and DHA attenuated WD-induced dyslipidemia, neither EPA nor DHA affected WD-induced body weight, the percent of body fat, blood glucose, or plasma endotoxin. The effect of C20-22 ω3 PUFA on hepatic fibrosis was established by histology and quantifying the expression of fibrosis markers (i.e., Col1A1 and TIMP1) (17, 18). DHA and EPA are known to attenuate inflammation, decrease fatty acid synthesis, and increase fatty acid oxidation (29). The finding that DHA attenuated WD-induced hepatic fibrosis in obese mice was unexpected.
Several clinical trials have reported that dietary ω3 PUFA supplementation lowered hepatic fat in obese children and adults with NAFLD (30–37), whereas other investigators report that dietary supplementation with fish oil (36) or EPA-ethyl esters (37) does not attenuate the histological features of the disease, like fibrosis. As such, human studies using ω3 PUFA to treat NAFLD/NASH have yielded mixed results. Our studies have established a difference in how specific ω3 PUFAs affect clinical features associated with NASH. Herein, we examined potential mechanisms to explain how DHA and EPA differentially affect WD-induced hepatic fibrosis. Our findings establish that dietary DHA interferes with the TGFβ-mothers against decapentaplegic homolog (Smad)3 signaling pathway in vivo and attenuates TGFβ-mediated induction of Col1A1 expression in human stellate cells. The outcome of these studies has the potential to influence the design of clinical studies in children and adults with NAFLD/NASH.
MATERIALS AND METHODS
Animals and diets
All procedures for the use and care of animals for laboratory research were approved by the Institutional Animal Care and Use Committee at Oregon State University. Male Ldlr−/− mice (C57BL/6J background, Jackson Laboratories) at 2 months of age were fed one of the following four diets ad libitum for 16 weeks; each group consisted of 8 male mice (17). The reference diet (RD) (Purina chow 5053) consisted of 13.5% energy as fat, 58.0% energy as carbohydrates, and 28.5% energy as protein. The WD (D12079B; Research Diets) consisted of 17% energy as protein, 43% energy as carbohydrate, and 41% energy as fat; cholesterol was at 1.5 g%. The WD was supplemented with olive oil (WD + O), EPA (WD + E), or DHA (WD + D). WD supplementation with olive oil, EPA, or DHA increased total fat energy to 44.7% and reduced protein and carbohydrate energy to 15.8% and 39.5%, respectively. Olive oil was added to the WD to ensure a uniform level of energy from fat, protein, and carbohydrate in all WD diets. Preliminary studies established that the addition of olive oil to the WD had no effect on development or progression of diet-induced NAFLD in Ldlr−/− mice. The C20-22 ω3 PUFA in the WD + E or WD + D diets were at 2% total energy. This dose of C20-22 ω3 PUFA is comparable to the dose consumed by patients taking LovazaTM (GlaxoSmithKline) for treating dyslipidemia (38), and this dose increases plasma C20-22 ω3 PUFA in mice to levels seen in humans consuming 4–6 g/d of C20-22 ω3 PUFA (39–41). A description of the diets and diet effects on mouse plasma and liver lipid composition was previously reported (17). At the end of the 16 week feeding period, all mice were fasted overnight (18:00 to 08:00 the next day) and euthanized at 08:00 for the collection of blood and liver (17, 28).
Measurement of plasma adipokines and proinflammatory cytokines
Plasma adipokines (leptin and adiponectin) were quantified by ELISA (R and D Systems). Plasma proinflammatory cytokines were quantified using a cytometric bead array mouse inflammation kit (BD Biosciences, San Jose, CA). Quantitative measurements of IL-12, TNFα, IFNγ, MCP-1, IL10, and IL6 were determined by flow cytometry per the manufacturer’s protocol. Data were acquired using FACSCalibur (BD Biosciences), and data analyses were done using FCAP Array Software version 3.0 (BD Biosciences). Plasma for these assays was obtained from mice described previously (17, 18).
Analysis of plasma Toll-like receptor-2 and -4 activators
Plasma levels of Toll-like receptor (TLR)-2 and TLR-4 activators used Hek-BlueTM mTLR2 and Hek-blueTM mTLR4 cells (Invivogen), respectively. Cells were grown in 96 well cell culture plates overnight in 200 μl of Hek-Blue Detection mediumTM (Invivogen) and 10 μl of plasma from the previous study (17). The formation of a blue color, reflecting activation of the TLR-NFκB pathway, was quantified by absorbance at 620 nm. Standard curves with authentic TLR2 (PAM3SK4) and TLR4 (LPS-B5) agonists are shown in supplementary Fig. 1.
RNA extraction and quantitative RT-PCR
Total RNA was extracted from liver, and specific transcripts were quantified by quantitative RT-PCR (qRT-PCR) (17, 42). Primers for each transcript are listed in supplementary Table 1. Cyclophilin was used as the internal control for all liver transcripts, and hydroxymethyl bilane synthase was used as the internal control of RNAs isolated from LX2 cells in culture. Transcripts were also quantified by qRT-PCR using the mouse fibrosis array (PAMM-120ZE; SABioscience-Qiagen). Hsp90ab1 was used as the internal control for array analysis. Transcripts and their acronyms are provided in supplementary Table 2. Results from these studies were analyzed online using RT2 Profiler PCR Array Data Analysis version 3.5 (SABioscience).
Immunoblot analysis
The quantitation of hepatic proteins by immunoblotting was previously described (17). The antibodies used to detect Smad2/3, Smad4, phospho-Smad2/3, and GAPDH were obtained from Cell Signaling Technologies. The antibody for procollagen 1A was obtained from Santa Cruz Biotechnologies.
Hydroxyproline
Hepatic hydroxyproline was quantified using the Hydroxyproline Kit (Sigma, MAK008). The colorimetric assay was carried out according to the manufacturer’s instructions.
LX2 cells
LX2 cells were obtained from SL Friedman (Mount Sani Medical School) (43, 44). LX2 cells are activated human hepatic stellate cells; they are maintained in DMEM with 5% FCS containing penicillin, streptomycin, and normocin. Cells were plated on 100 mm plastic petri dishes at ∼100,000 cells/plate and grown to confluence. Cells were treated with fatty acids (at 25 μM) in endotoxin-free BSA (at 10 μM) during the growth phase. Fatty acids [oleic acid (18:1,ω9), arachidonic acid (20:4,ω6), and DHA (22:6,ω3)] were obtained from Nu-Chek Prep. Cells were treated with 100 pM recombinant-human TGF-β1 (R and D Systems) in media containing no fatty acids or BSA for 24 h. RNA was extracted for qRT-PCR as previously described (18). Human PCR primers are listed in supplementary Table 1.
For fatty acid analysis, confluent cells were washed twice with cold PBS. Cells were extracted for fatty acids and quantified by gas chromatography analysis (17, 45) or applied to a reverse-phase HPLC system for the quantitation of 14C-labeled fatty acids as described (46).
Statistical analysis
Statistical analysis of data used one-way ANOVA and Tukey-HSD to detect significant differences between groups. Data were analyzed for homogenous variances by the Levine test. If unequal variances were detected, data were log-transformed. ANOVA analysis was performed on both transformed and untransformed data. The hydroxyproline data were analyzed by Student’s t-test. Values are reported as mean ± SD. A P value ≤0.05 was considered significantly different.
RESULTS
Effect of the WD and ω3 PUFA on plasma factors linked to hepatic inflammation and fibrosis
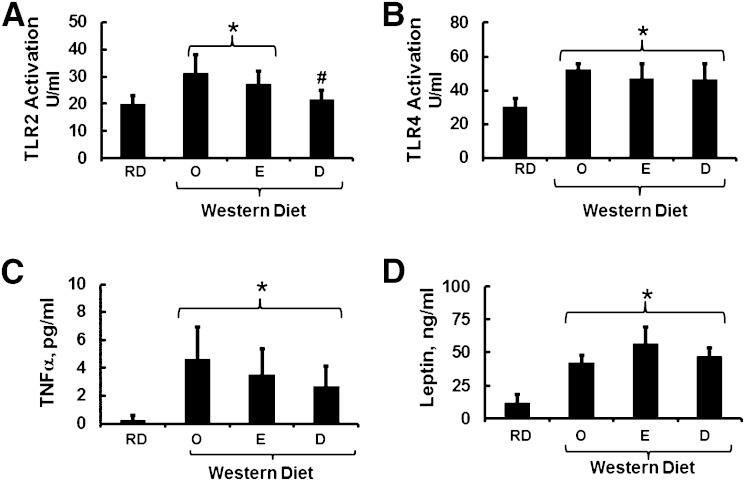
Toll-like receptors (TLR2 and TLR4) have been linked to hepatic inflammation and fibrosis (47–50). We previously reported that feeding Ldlr−/− mice the WD increased plasma endotoxin (18), a gut-derived microbial factor that activates the TLR4 pathway (51). WD-induced endotoxinemia was associated with increased hepatic nuclear content of NFκB as well as multiple NFκB-regulated transcripts linked to hepatic inflammation (17, 18). EPA or DHA supplementation of the WD did not attenuate WD-induced plasma endotoxin (18). Herein, we use a cell-based approach to quantify plasma levels of bioactive TLR2 and TLR4 agonist. Feeding Ldlr−/− mice the WD + O diet increased TLR2 and TLR4 agonist activity by ∼60% (Fig. 1). Although the WD + D diet significantly lowered TLR2 agonists in plasma, WD + E and WD + D failed to lower plasma TLR4 agonists. Failure of the ω3 PUFA-containing diets to suppress TLR4 agonist is consistent with our previous finding, which showed that dietary ω3 PUFAs do not attenuate WD-induced endotoxinemia (18).
Fig. 1.
Inflammatory and endocrine factors affecting liver fibrosis. Plasma levels of TLR2 (A) and TLR4 (B) activators were quantified using a cell-based assay as described in Materials and Methods. Results are represented as TLR activation units (U)/ml (n = 8; mean ± SD). The feeding groups were RD, WD + O, WD + E, and WD + D. Plasma TNFα (C) and leptin (D) were quantified as described in Materials and Methods. Results are expressed as pg/ml and ng/ml, respectively (mean ± SD; n = 8). *P ≤ 0.05 versus RD; #P ≤ 0.05 versus WD + O (ANOVA).
Hepatic fibrosis is also linked to products derived from adipose tissue and cells involved in innate immunity, including adipokines and cytokines, respectively (52, 53). We quantified plasma leptin, adiponectin, IFNγ, interleukins (IL6, IL10, and IL12), monocyte chemoattractant protein-1 (MCP1), and TNFα. Of these, WD + O feeding significantly increased plasma leptin and TNFα (Fig. 1). Addition of EPA or DHA to the WD had no significant effect on plasma levels of TNFα or leptin. The effect on leptin was anticipated because plasma leptin levels parallel adiposity. WD-fed mice are obese, and including EPA or DHA in the WD had no effect on adiposity (17).
Effects of WD and ω3 PUFA on the expression of genes involved in fibrosis
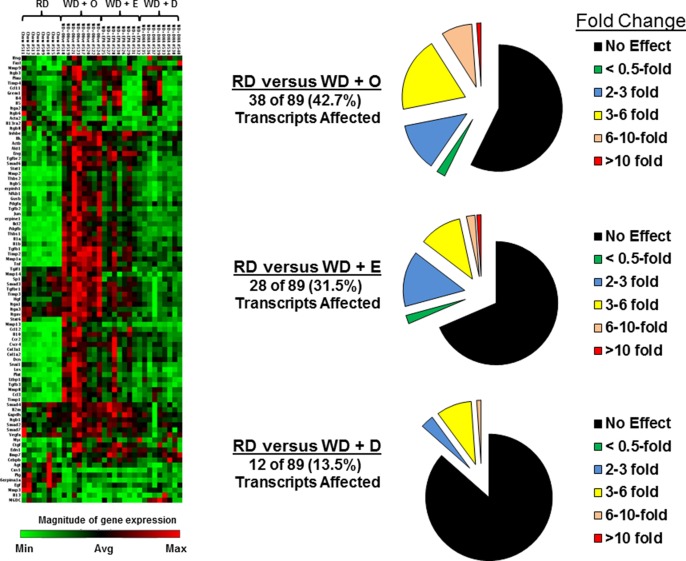
Feeding Ldlr−/− mice the WD elevated hepatic expression of Col1A1, TGFβ1, and Timp1, and dietary C20-22 ω3 PUFA attenuated this effect (17). Herein, we used the SABioscience qRT-PCR fibrosis array to get a broad assessment of the impact of diet on hepatic fibrosis. The heat map (Fig. 2) illustrates the results with samples from livers of mice fed the RD, WD + O, WD + E, or WD + D (8 mice/group). The pie charts represent quantitation of transcripts that changed significantly. When compared with the RD group, 38, 28, and 12 transcripts were significantly induced by the WD + O, WD + E, and WD + D diets, respectively. Only two transcripts [metalloprotease-3 (Mmp3) and caveolin1] were significantly suppressed by the WD + O diet when compared with RD-fed mice. As illustrated in the heat map and in detailed quantitation of transcripts described below, the WD + D diet was more effective than the WD + E diet at attenuating WD effects on the expression of genes linked to fibrosis.
Fig. 2.
Diet effects on the expression of genes involved in fibrosis. The mouse fibrosis array was used to quantify expression of 84 transcripts linked to fibrosis. The method uses a qRT-PCR approach. The heat map was generated using Qiagen online software and represents the relative abundance of transcripts. Each group had eight mice per group, and the groups were RD, WD + O, WD + E, and WD + D. The magnitude of gene expression was represented by green and red bars, indicating decreased and increased expression, respectively. The pie plots represent the number of transcripts affected by diet and the relative change in transcript abundance (fold change). Detailed analysis of transcripts is provided in Figs. 3–6 and supplementary Fig. 3.
Collagen subtypes
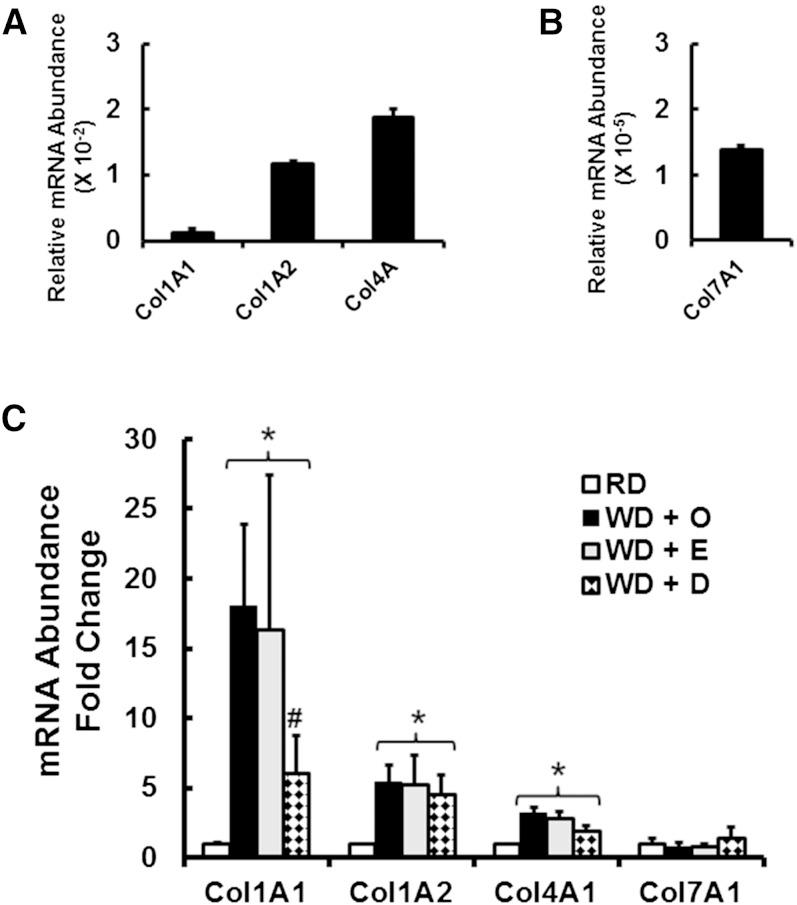
Although the liver expresses several collagen subtypes (Fig. 3), Col1A1 is the major collagen subtype associated with hepatic fibrosis in WD-fed mice (17) and humans with NASH (16, 52). In normal mouse liver, Col1A1 and Col7A1 are expressed at low levels when compared with Col1A2 or Col4A. Using the qRT-PCR fibrosis array data (Fig. 2) as well as independent qRT-PCR analysis, WD feeding induced the expression of Col1A1, Col1A2, and Col4A1 by 17-, 5-, and 3-fold, respectively. Of these transcripts, only Col1A1 expression was significantly attenuated by dietary DHA. Dietary EPA did not attenuate WD-mediated induction of any collagen subtype.
Fig. 3.
Diet effects on hepatic collagen subtype expression. Expression of hepatic collagen subtypes used qRT-PCR and primers listed in supplementary Table 1. The upper panels represent relative mRNA abundance of the collagen subtypes in mice maintained on the RD; cyclophilin was the reference transcript. The lower panel represents the fold change in hepatic expression of the collagen subtypes of mice fed the RD or the WD + O, WD + E, or WD + D diets. Results are expressed as fold change (mean ± SD; n = 8). *P ≤ 0.05 versus RD; #P ≤ 0.05 versus WD + O.
ECM remodeling proteins
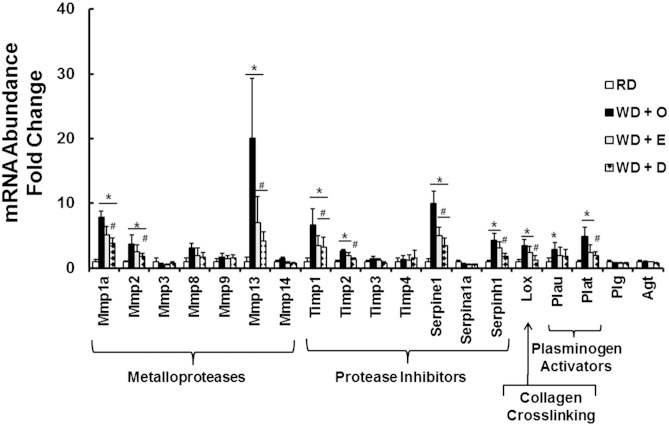
Fibrosis is associated with the induction of multiple proteins involved in remodeling the ECM, including metalloproteases, protease inhibitors, and enzymes involved in collagen cross-linking (Fig. 4). The mRNA abundance of three metalloproteases (Mmp1a, Mmp2, and Mmp13), four protease inhibitors (Timp1, Timp2, Serpine1, and Serpinh1), and the cross-linking enzyme lysyl oxidase (Lox) was significantly induced by WD + O. Although both WD + E and WD + E significantly attenuated the WD + O effect on the hepatic abundance of these transcripts, the WD + D diet was more effective than WD + E at attenuating expression of Mmp1a, Mmp2, Timp2, Serpinh1, and Lox.
Fig. 4.
Diet effects on expression of remodeling enzymes. Transcript abundance of proteins involved in extracellular matrix remodeling was quantified using data from the qRT-PCR array described in Fig. 2. Results are represented as mRNA abundance-fold change (mean ± SD; n = 8). *P ≤ 0.05 versus RD; #P ≤ 0.05 versus WD + O.
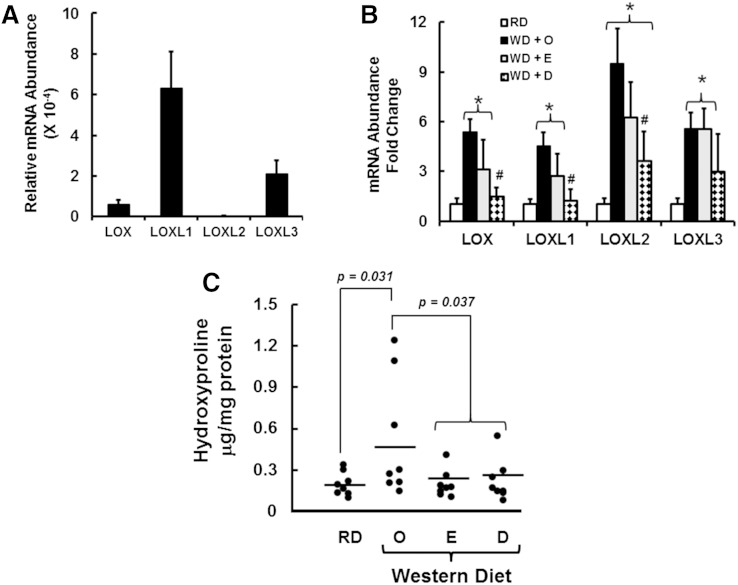
Collagen crosslinking
Lox mRNA was induced by WD + O feeding and attenuated by WD + D > WD + E (Figs. 4 and 5). Lox is one of several enzymes involved in collagen crosslinking. Lox and the lysyl oxidase-like (LoxL) family of enzymes are expressed in stellate cells and portal fibroblast (54). Accordingly, we quantified the effects of diet on the expression of Lox and LoxL subtypes (LoxL1, LoxL2, and LoxL3). In RD-fed mice, hepatic LoxL1 and LoxL3 are more abundant than Lox and LoxL2. Lox and all LoxL subtypes were induced (≥4-fold) by WD + O feeding. Feeding mice the WD + D diet attenuated the expression of Lox, LoxL1, and Loxl2 but not LoxL3. Expression levels of Lox and all LoxL subtypes in WD + O- and WD + E-fed mice were not different.
Fig. 5.
Diet effects on hepatic expression of Lox and LoxL subtypes and hydroxyproline abundance. A: The relative expression of hepatic LOX and LOXL subtypes was quantified by qRT-PCR using liver RNA from mice fed the RD; cyclophilin was the reference transcript. The qRT-PCR primers are listed in supplementary Table 1. B: Effect of diet on LOX and LOXL subtype mRNA abundance. Results are expressed as mRNA abundance-fold change (mean ± SD; n = 8). *P ≤ 0.05 versus RD; #P ≤ 0.05 versus WD + O. C: Hepatic hydroxyproline abundance was quantified as described in Materials and Methods and represented as μg hydroxyproline/mg protein (mean ± SD; n = 8). P values were calculated using Student’s t-test.
We also quantified hepatic levels of the product of Lox and LoxL1-3 activity (i.e., hydroxyproline) (Fig. 5C). Hydroxyproline levels were highly variable in livers from WD + O-fed mice. Hydroxyproline increased in three of the eight mice to levels >6-fold above the mean value quantified in RD-fed mice and mice fed WD + E or WD + D. WD + E and WD + D attenuated hepatic hydroxyproline content when compared with mice fed WD + O.
TGF-β Superfamily
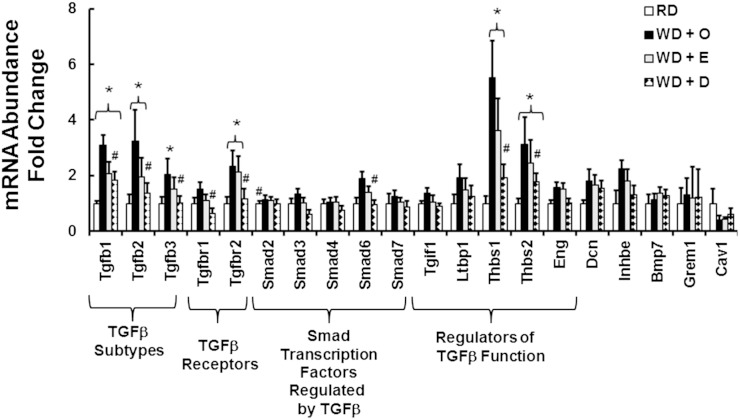
TGF-β plays a major role in promoting fibrosis (48). Accordingly, we examined the TGFβ superfamily for diet effects on gene expression (Fig. 6). Mice fed the RD express three TGFβ subtypes in liver; the relative abundance of the three transcripts is: TGFβ1, 1.0 ± 0.1; TGFβ2, 0.031 ± 0.01; TGFβ3, 0.06 ± 0.013. Of these, TGFβ1 is the predominant hepatic TGFβ subtype. Feeding mice the WD + O diet induced all three TGFβ subtypes (2- to 3-fold). The WD + O diet also increased expression of the TGFβ receptor subunit-2 (Tfgbr2, 2-fold), Smad 6 (Smads are transcription factors and downstream targets of TGFβ signaling) (∼2-fold), and two proteins that regulate TGFβ function [thrombospondins (Thbs1, Thbs2), 3- to 5-fold]. No significant difference was detected in the expression of these transcripts in mice fed the WD + O and the WD + E diets. Feeding mice the WD + D diet, however, significantly attenuated expression of all TGFβ subtypes, both TGFβ receptor subunits, Smad6, Thbs1, and Thbs2 when compared with WD + O-fed mice. As such, DHA, but not EPA, attenuates multiple transcripts associated with TGFβ signaling.
Fig. 6.
Diet effects on expression of the TGFβ superfamily. Transcript abundance of proteins involved in TGFβ signaling was quantified using data from the qRT-PCR fibrosis array (Fig. 2). Results are represented as mRNA abundance-fold change (mean ± SD; n = 8). *P ≤ 0.05 versus RD; #P ≤ 0.05 versus WD + O.
TGFβ Signaling
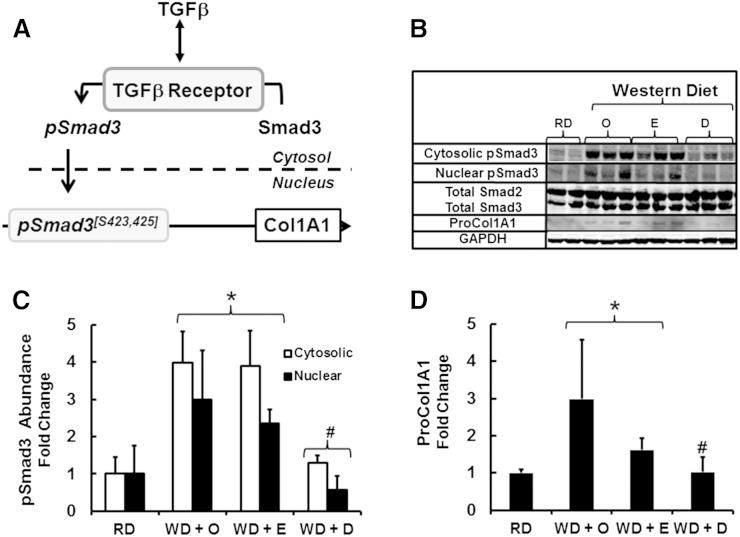
To further explore the impact of the WD and ω3 PUFA on TGFβ signaling, we examined the pathway for TGFβ induction of Col1A1 expression. TGFβ binds to type 1 and type II TGFβ receptors; TGFβ binding promotes recruitment of Smad2 and Smad3 to the TGFβ receptor complex where Smad2 and Smad3 are phosphorylated (55–60) (Fig. 7A). Smad2 is phosphorylated at S465 and S467, whereas Smad3 is phosphorylated at S423 and S425. Phospho-Smads move from the cytosol to the nucleus in association with Smad4. Binding of phospho-Smad3 to the Col1A1 promoter is associated with increased Col1A1 gene transcription and the accumulation of Col1A1 mRNA and protein (59).
Fig. 7.
Diet effects on Smad3 phosphorylation and Col1A1 precursor protein. Mouse liver cytosolic and nuclear extracts were prepared for immunoblotting as described (17). Antibodies used to detect total and phosphorylated Smad2 and -3 are listed in the Materials and Methods section. A: The pathway for TGFβ regulation of Col1A1 gene transcription (56–59). B: Representative immunoblot of total Smad 2, Smad3, phospho-Smad3, and proCol1A1. The loading control for phospho-Smad3 was total Smad3; while the loading control for proCol1A1 was GAPDH. The number of independent samples for each group was: RD, 2; WD + O, 3; WD + E, 3; WD + D, 3. C and D: Results are quantified for phospho-Smad3 (C) and proCol1A1 (D) and expressed as fold change (mean ± SD). *P ≤ 0.05 versus RD; #P ≤ 0.05 versus WD + O.
Total and phosphorylated Smad2 and Smad3 were quantified in cytosol and nuclear extracts from livers of mice fed the RD or the WD + O, WD + E, or WD + D diets. Total hepatic Smad2 and Smad3 levels remained unchanged by diet. Although the antibody for phospho-Smads recognized both phospho-Smad 2 and phospho-Smad3, the position of the phosphorylated protein in the immunoblot corresponded to phospho-Smad3. Feeding mice the WD + O and WD + E diets significantly increased cytosolic and nuclear phospho-Smad3, reflecting activation of the TGF-β pathway. Feeding mice the WD + D diet, however, showed no increase in cytosolic or nuclear phospho-Smad3. Changes in nuclear phospho-Smad3 paralleled changes in Col1A mRNA (Fig. 3C) and proCol1A protein abundance (Fig. 7B and D). Although the WD + O and WD + E diets activate the TGFβ-Smad3 pathway and promote fibrosis, feeding mice the WD + D diet attenuates this pathway.
DHA attenuates TGFβ induction of Col1A1 in LX2 cells
The induction of Col1A1 expression in stellate cells is in response to increased production of TGFβ and cytokines (e.g., IL-1β) from hepatocytes, Kupffer cells, macrophages, and T-cells (53, 56–59). Herein, we determine whether DHA can act directly on stellate cells to regulate TGFβ1 control of Col1A1. LX2 cells are a human activated stellate cell line (43, 44). We first determined if LX2 cells assimilate and metabolize exogenous fatty acids. Accordingly, LX2 cells were treated with 25 μM fatty acids for 96 h to enrich cellular lipids (Fig. 8A). Treatment of cells with fatty acids (at 25 μM) had no adverse effects on cell growth or morphology. Fatty acid analysis shows that treatment of cells with fatty acids increased the cellular content of fatty acids used to treat cells; 18:1,ω9, 20:4,ω6, and 22:6,ω3 increased ∼2-, 3-, and 5-fold, respectively. Treatment of cells with 20:4,ω6 increased LX2 cell levels of 22:4,ω6 and 22:5,ω6, whereas treatment of cells with DHA increased 20:5,ω3 and 22:5,ω3. The appearance of 22:4,ω6 and 22:5,ω6 in cells treated with 20:4,ω6 is due to elongation, desaturation, and peroxisomal β-oxidation activities in these cells. The appearance of 20:5,ω3 and 22:5,ω3 in DHA-treated cells is due to retro-conversion of 22:6,ω3 to 20:5,ω3, a peroxisome-dependent mechanism, and the elongation of 20:5,ω3 to 22:5,ω3 (61). We also show that LX2 cells express key enzymes involved in fatty acid synthesis and are capable of de novo lipogenesis and fatty acid elongation and desaturation of linoleic acid (18:2,ω6) and α-linolenic acid (18:3,ω3) to generate docosapentaenoic acid (22:5,ω6) and DHA (22:6,ω3), respectively (supplementary Fig. 2). In addition, other investigators have reported that LX2 and isolated primary rat stellate cells assimilate exogenous fatty acids into phospho- and neutral lipids (62). As such, LX2 cells have the capacity to synthesize fatty acids de novo and to transport exogenous fatty acids from the media into cells and metabolize these fatty acids.
Fig. 8.
TGFβ1 and DHA regulation of proCol1A1 expression in human LX2 stellate cells. LX2 cells were plated at ∼10% confluence on plastic petri dishes in DMEM + 5% FCS and antibiotics. The next day, cells were treated with vehicle (Veh: fatty acid- and endotoxin-free BSA at 10 μM) or 25 μM fatty acids in BSA-containing media. Media was change every third day. This treatment enriches membrane lipids with exogenous fatty acids. A: Cells were harvested for fatty acid extraction and gas chromatographic quantitation. The results are presented as fatty acid, Mol% (mean ± range of two separate studies). B: Cells were treated with oleic acid (OA), arachidonic acid (ARA), or DHA as described above. After fatty acid pretreatment, cells were treated without or with 100 pM TGFβ1 overnight in the absence of fatty acids. Cells were harvested for RNA for qRT-PCR quantitation of proCol1A1 mRNA; hydroxymethylbilane synthase was the reference RNA. Results are presented as Col1A1 mRNA-fold change (mean ± SD; n = 3). *P ≤ 0.05 versus Veh; #P ≤ 0.05 versus 18:1,ω9 + TGFβ.
To assess the impact of fatty acids on stellate cell function, LX2 cells were treated with TGFβ1 (100 pM, overnight) after prior fatty acid treatment, as described above. TGFβ treatment of LX2 cells induced Col1A1 expression 4-fold in vehicle-treated cells and cells pretreated with oleic acid (18:1,ω9) or arachidonic acid (20:4,ω6). DHA pretreatment, however, blocked TGFβ1 induction of Col1A1 mRNA. Thus, DHA has the capacity to act directly on human hepatic stellate cells to attenuate TGFβ1-mediated induction of Col1A1.
DISCUSSION
The goal of this report was to identify mechanisms that explain the differential effect of EPA and DHA on WD-induced hepatic fibrosis. This is highly relevant because dietary ω3 PUFAs are being evaluated as treatment strategies for NAFLD/NASH in children and adults (30–35, 63). Although these clinical studies show that dietary ω3 PUFAs reduce liver fat (30–37), some clinical trials have reported that ω3 PUFAs, like fish oil (36) or EPA-ethyl esters (37), fail to improve fibrosis scores associated with NASH. Our previous report established that DHA was more effective than EPA at attenuating WD-induced hepatic fat, inflammation, and fibrosis (17). Hepatic fibrosis in that study examined hepatic expression of three fibrosis gene expression markers (Col1A1, Timp1, and TGF-β1) and histology (i.e., trichrome staining of liver).
In this report, we have expanded our analysis of diet effects on hepatic fibrosis by quantifying 84 gene expression markers of fibrosis, four plasma markers linked to fibrosis, hepatic TGFβ-Smad signaling, and fatty acid effects on TGFβ signaling in human stellate (LX2) cells. Feeding Ldlr−/− mice the WD supplemented with DHA (WD + D), but not the WD + E diet, abrogated WD + O-induced accumulation of phospho-Smad3 in hepatic nuclei (Fig. 7). TGFβ is a major regulator of hepatic fibrosis (48). TGFβ binding to the TGFβ receptor increases Smad3 phosphorylation, and phospho-Smad3 migrates from the cytosol to the nucleus where it binds the Col1A promoter and induces transcription of the Col1A1 gene (55, 59). Although TGFβ signaling is operative in multiple hepatic cell types, including hepatocytes (64), the effect of WD and DHA on phospho-Smad3 correlates with changes in hepatic stellate cell expression of Col1A1 mRNA and proCol1A1 (Fig. 7). Moreover, we show that DHA acts directly on human LX2 stellate cells to block TGFβ1 induction of Col1A1 gene expression (Fig. 8). Taken together, these findings establish DHA as a regulator of TGFβ signaling and Col1A1 expression.
Our analysis revealed broad effects of the WD and C20-22 ω3 PUFA on the expression of genes involved in fibrosis and TGFβ signaling (Fig. 2–6). Feeding mice the WD + O diet induced multiple components associated with TGFβ signaling, including several TGFβ subtypes (TGFβ1–3), a TGFβ receptor subunit (TGFβ-R2), and two thrombospondins (Thbs1 and Thbs2). Thbs1 and Thbs2 are expressed in macrophage, whereas Thbs2 is also expressed in endothelial cells (65, 66). Thbs1 induces TGFβ−dependent and TGFβ−independent fibrosis. DHA has been reported to suppress Thbs1 expression in adipose tissue (65). Thbs2, in contrast, attenuates fibrosis (67, 68). We are unaware of reports documenting effects of dietary PUFA on Thbs2 expression. The WD + D diet was more effective than the WD + E diet in attenuating WD + O induction of all TGFβ subtypes, both TGFβ subunits (R1 and R2), Smad6, and Thbs1 and Thbs2.
Our findings go beyond an earlier report by Chen et al. (69), who showed that DHA attenuated fibrosis in a rat bile duct ligation model for cholestatic liver injury. Although these investigators showed that DHA targeted TGFβ, NFκB, and Erk signaling, the authors did not assess effects of other fatty acids, Smad phosphorylation, and cytosolic-nuclear trafficking or TGFβ control in human hepatic stellate cells. Although cholestatic liver injury has a different etiology than diet-induced NASH in obese patients, these and other studies (70) suggest that DHA may have antifibrotic effects in liver and other tissues by controlling TGFβ signaling.
Regulators of hepatic fibrosis
Factors promoting diet-induced hepatic fibrosis are derived from multiple sources, including the gut (51) and multiple cell types, such as adipocytes (leptin), macrophages (TGFβ, PDGF, and IL1β), and T-cells (IL4, IL13, and TGFβ) that secret adipokines, growth factors, cytokines, and chemokines (52, 53). Hyperleptinemia, endotoxinemia, and increased plasma TNFα and TLR agonists are associated with hepatic fibrosis (49, 52). Supplementing the WD with EPA or DHA had no effect on WD-induced obesity or plasma levels of glucose, leptin, endotoxin, TNFα or TLR-4 agonist. The WD + D diet, however, attenuated WD-induced dyslipidemia (17) and TLR2 agonist (Fig. 1).
Although ω3 PUFA-containing WD diets reduce blood lipids, these diets significantly change the blood fatty acid profiles, leading to enrichment in C20-22 ω3 PUFA and a decline in C20-22 ω6 PUFA. The C20-22 ω6 and the C20-22 ω6 PUFA are precursors to proinflammatory and antiinflammatory bioactive lipids, respectively (71). Our studies establish that DHA can act directly on LX2 cells to regulate TGFβ1 control of Col1A1 expression (Fig. 8). This outcome does not exclude a role for other factors affecting stellate cell function. In fact, our previous (17) and current studies (supplementary Fig. 3) show WD + O effects on the expression of multiple hepatic chemokines [monocyte chemoattractant protein-1/Ccl2, Ccl3, Ccl12, and Cxcr4 (Ccl12 receptor)], cytokines (TNFα, IL1α, IL1β, and IL10), growth factors (PDGF-A, PDGF-B, and HGF), and transcription factors (Jun, Myc, and Stat1) linked to fibrosis. Moreover, we previously reported that the WD + O diet induced hepatic nuclear abundance of NFκB-p50 and NFκB-p65, whereas the WD + D (but not the WD + E) diet significantly lowered hepatic nuclear content of NFκB-p50 (17). Diet-induced changes in hepatic NFκB-p50 correlated with the hepatic abundance of downstream targets of TLR and NFκB, including cytokines and chemokines.
Because TLRs and cytokines are expressed in multiple hepatic cell types, we predict that the in vivo mechanism for DHA control of Col1A1 expression likely involves both direct effects of DHA on stellate cells (Fig. 8) and indirect effects mediated by changes in the function of Kupffer cells and macrophages and T-cells infiltrating the liver. Two factors support this hypothesis. First, the level of TGFβ1-mediated induction of Col1A1 in LX2 stellate cells is modest (4-fold) when compared with the nearly 20-fold induction of Col1A1 seen in vivo. Second, DHA is a pleiotropic mediator of cell function affecting multiple pathways, such as membrane composition as well as multiple cell signaling and transcription regulatory networks (29). In addition to stellate-macrophage interaction, hepatocyte-stellate cell interaction has been reported (72). As such, the LX2 cells can serve as a model to investigate how ω3 PUFAs regulate factors derived from hepatocytes and Kupffer cell/macrophage to control stellate cell function.
ECM remodeling enzymes
The progression and remission of fibrosis requires a balance in expression of ECM genes and enzymes that remodel the ECM. The WD induces expression of several hepatic collagen subtypes (Fig. 3) as well as enzymes involved in ECM remodeling, including metalloproteases (Mmp1a, Mmp2, and Mmp13), protease inhibitors [Timp1 and Timp2, serine protease inhibitors (serpine1 and serpinh1)], and enzymes involved in collagen crosslinking (Lox and LoxL1-3) (Figs. 4 and 5). Mmps and Timps are products of hepatocytes, leukocytes, Kupffer cells, and stellate/myofibrillar cells (16). A response favoring ECM removal will involve increased expression of Mmps, decreased Timp expression, decreased interaction of Timps with Mmps, and increased IL-10 and IL-13Ra2 (decoy receptor for IL13) expression (supplementary Fig. 3) (53). The WD, however, induced both metalloproteases and protease inhibitors involved in ECM remodeling, whereas C20-22 ω3 PUFA attenuated this response. As such, the impact of ω3 PUFA on Mmp1a, Mmp2, and Mmp13 expression raises a concern for the capacity of these dietary lipids to promote remission of fibrosis in livers with preexisting fibrosis. Moreover, the WD + O diet induced IL-10, whereas the WD + D diet attenuated this response (supplementary Fig. 3). Although our study design was on prevention of diet-induced fibrosis, these concerted effects of DHA on factors involved in ECM removal raise the possibility that DHA may not be an effective NASH therapy that promotes remission of fibrosis. Further studies are required to quantify protein levels of Timps and Mmps, assess their activity, establish their subcellular localization, and examine protein-protein interaction. Such studies may reveal that C20-22 ω3 PUFAs change the balance of ECM remodeling enzymes to favor removal of ECM.
Can ω3 PUFA be used to treat human NASH?
Our mouse studies provide strong evidence in support of the use of DHA in the prevention of NAFLD/NASH. Omega-3 PUFAs have well-defined effects on hepatic lipid metabolism and inflammation (29, 73), and more recently their effects on hepatic fibrosis have been noted (17, 28, 69). Although several human studies have provided evidence in support of using supplemental ω3 PUFAs to treat NAFLD (30–37), other studies suggest there may be limitations for their use in NASH treatment (36, 37). In trials using either fish oil or EPA-ethyl ester supplements, investigators report that ω3 PUFA supplements failed to improve fibrosis scores. Because DHA attenuates fibrosis in two separate rodent models of liver injury and fibrosis (i.e., WD-fed mice and rats with bile duct ligation) (17, 28, 69), failure of ω3 PUFAs to attenuate fibrosis in human clinical studies may be due to the type and dose of ω3 PUFAs used in the study. The finding that DHA suppressed TGF-β signaling and Col1A1 expression in mouse liver (Figs. 3 and 7) and human stellate cells (Fig. 8) suggests that DHA likely functions in humans.
DHA is the main ω3-PUFA accumulating in human and rodent tissues. Although α-linolenic acid and EPA are converted to DHA in humans, this process is insufficient for the accumulation of tissue levels of DHA needed to affect disease processes (74, 75). Humans and rodents retro-convert ingested DHA to EPA. In Ldlr−/− mice, for example, WD supplementation with DHA leads to a 30% and 500% increase in hepatic phospholipid DHA and EPA content, respectively (18). In contrast, phospholipid DHA levels do not increase in mice fed the WD supplemented with EPA. Moreover, the JELIS trial, a primary prevention trial for cardiovascular disease, clearly showed that feeding humans EPA did not increase blood DHA (74). In rodents, EPA and DHA suppress the expression of enzymes involved in PUFA synthesis (17). This mechanism involves the suppression of sterol regulatory element binding protein nuclear abundance (28), a key transcription factor regulating de novo lipogenesis and PUFA synthesis (29). As such, this mechanism lowers the capacity of cells to convert dietary C18-PUFA precursors to C20-22 ω3 and ω6 PUFAs. Because dietary DHA is retroconverted to EPA through a peroxisomal mechanism (61) and EPA is not well converted to DHA in vivo, DHA is a logical choice for usage in clinical trials.
In a recent double-blind, placebo-controlled trial, NAFLD patients received placebo or LovazaTM at 4 g/d (∼50:50 mix of EPA- and DHA-ethyl esters) for 15–18 months (35). When compared with the placebo-treated group, the LovazaTM-treated group showed a significant reduction in liver fat without a significant reduction in fibrosis scores. In our studies, C20-22 ω3 PUFA in the WD + E or WD + D diets was used at 2% total energy. This dose of C20-22 ω3 PUFA is comparable to the dose consumed by patients taking LovazaTM to treat dyslipidemia (38). The dose used in our studies increased plasma C20-22 ω3 PUFA in mice to levels seen in humans consuming 4–6 g/d of C20-22 ω3 PUFA (39–41). We also used a combination of EPA and DHA to mimic LovazaTM treatment (17). Whereas the overall C20-22 ω3 PUFA dose was at 2% total energy, EPA and DHA were at 1% total energy each. The combination of EPA + DHA was less effective than using DHA alone at suppressing Col1A1 expression. As such, the preclinical prevention studies with Ldlr−/− mice suggest that DHA (at 4–6 g/d) may be more effective than EPA or fish oil at preventing diet-induced NASH and fibrosis in humans. A search of clinical trials (www.clinicaltrials.gov; query “NASH and DHA”) yields three trials that are recruiting or active. The outcome of these studies may provide evidence in support of the use of dietary DHA in the prevention or treatment of diet-induced fibrosis.
Summary
This study identified mechanisms to explain the differential effects of EPA and DHA on WD-induced hepatic fibrosis. Feeding Ldlr−/− mice the WD induced changes in expression of multiple genes associated with hepatic inflammation and fibrosis. DHA, but not EPA, attenuated WD-induced hepatic fibrosis by targeting the TGFβ-Smad3-Col1A1 pathway. Moreover, DHA acted directly on human LX2 stellate cells to block TGFβ1-mediated induction of Col1A1. These outcomes establish DHA as a key regulator of TGFβ signaling and hepatic fibrosis.
Supplementary Material
Footnotes
Abbreviations:
- Col1A1
- collagen 1A1
- DAG
- diacylglycerol
- ECM
- extracellular matrix
- Elovl
- fatty acid elongase
- IL1β
- interleukin-1β Lox, lysyl oxidase
- LoxL
- lysyl oxidase-like
- NAFLD
- nonalcoholic fatty liver disease
- NASH
- nonalcoholic steatohepatitis
- NFκB
- nuclear factor κB
- qRT-PCR
- quantitative RT-PCR
- RD
- reference diet
- Smad
- mothers against decapentaplegic homolog
- TIMP
- tissue inhibitor of metalloprotease
- TGFβ
- transforming growth factor-β TLR, toll-like receptor
- WD
- Western diet
This work was supported by USDA, National Institute of Food and Agriculture grant 2009-65200-05846 and by National Institutes of Health grants DK 43220 and DK 094600.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Angulo P. 2002. Nonalcoholic fatty liver disease. N. Engl. J. Med. 346: 1221–1231. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N., Younossi Z., Lavine J. E., Diehl A. M., Brunt E. M., Cusi K., Charlton M., Sanyal A. J. 2012. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 142: 1592–1609. [DOI] [PubMed] [Google Scholar]
- 3.Vernon G., Baranova A., Younossi Z. M. 2011. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 34: 274–285. [DOI] [PubMed] [Google Scholar]
- 4.McCullough A. J. 2006. Pathophysiology of nonalcoholic steatohepatitis. J. Clin. Gastroenterol. 40(Suppl 1): S17–S29. [DOI] [PubMed] [Google Scholar]
- 5.Prashanth M., Ganesh H. K., Vima M. V., John M., Bandgar T., Joshi S. R., Shah S. R., Rathi P. M., Joshi A. S., Thakkar H., et al. 2009. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. J. Assoc. Physicians India. 57: 205–210. [PubMed] [Google Scholar]
- 6.Soderberg C., Stal P., Askling J., Glaumann H., Lindberg G., Marmur J., Hultcrantz R. 2010. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 51: 595–602. [DOI] [PubMed] [Google Scholar]
- 7.Ekstedt M., Franzen L. E., Mathiesen U. L., Thorelius L., Holmqvist M., Bodemar G., Kechagias S. 2006. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 44: 865–873. [DOI] [PubMed] [Google Scholar]
- 8.Adams L. A., Lymp J. F., St Sauver J., Sanderson S. O., Lindor K. D., Feldstein A., Angulo P. 2005. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 129: 113–121. [DOI] [PubMed] [Google Scholar]
- 9.McCullough A. J. 2004. The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease. Clin. Liver Dis. 8: 521–533. (viii.). [DOI] [PubMed] [Google Scholar]
- 10.Sanyal A. J., Yoon S. K., Lencioni R. 2010. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist. 15(Suppl 4): 14–22. [DOI] [PubMed] [Google Scholar]
- 11.Cohen J. C., Horton J. D., Hobbs H. H. 2011. Human fatty liver disease: old questions and new insights. Science. 332: 1519–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang C. M., Pu C. W., Hou Y. H., Chen Z., Alanazy M., Hebbard L. 2014. Non alcoholic steatohepatitis a precursor for hepatocellular carcinoma development. World J. Gastroenterol. 20: 16464–16473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oda K., Uto H., Mawaari S., Ido A. 2015. Clinical features of hepatocellular carcinoma associated with nonalcoholic fatty liver disease: a review of human studies. Clin. J. Gastroenterol. In press. [DOI] [PubMed] [Google Scholar]
- 14.Scalera A., Tarantino G. 2014. Could metabolic syndrome lead to hepatocellular carcinoma via non-alcoholic fatty liver disease? World J. Gastroenterol. 20: 9217–9228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCullough A. J. 2011. Epidemiology of the metabolic syndrome in the USA. J. Dig. Dis. 12: 333–340. [DOI] [PubMed] [Google Scholar]
- 16.Duarte S., Baber J., Fujii T., Coito A.J. 2015. Matrix metalloproteases in liver injury, repair and fibrosis. Matrix Biol. 44–66C: 147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Depner C. M., Philbrick K. A., Jump D. B. 2013. Docosahexaenoic acid attenuates hepatic inflammation, oxidative stress, and fibrosis without decreasing hepatosteatosis in a Ldlr(−/−) mouse model of western diet-induced nonalcoholic steatohepatitis. J. Nutr. 143: 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Depner C. M., Traber M. G., Bobe G., Bohren K. M., Morin-Kensicki E., Milne G., Jump D. B. 2013. A metabolomic analysis of omega-3 fatty acid mediated attenuation of western diet-induced non-alcoholic steatohepatitis in LDLR−/− mice. PLoS One. 8: e83756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cordain L., Eaton S. B., Sebastian A., Mann N., Lindeberg S., Watkins B. A., O’Keefe J. H., Brand-Miller J. 2005. Orgins and evolution of the western diet: health implications for the 21st century. Am. J. Clin. Nutr. 81: 341–354. [DOI] [PubMed] [Google Scholar]
- 20.Abdelmalek M. F., Suzuki A., Guy C., Unalp-Arida A., Colvin R., Johnson R. J., Diehl A. M. 2010. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 51: 1961–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guturu P., Duchini A. 2012. Etiopathogenesis of nonalcoholic steatohepatitis: role of obesity, insulin resistance and mechanisms of hepatotoxicity. Int. J. Hepatol. 2012: 212865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wouters K., van Gorp P. J., Bieghs V., Gijbels M. J., Duimel H., Lutjohann D., Kerksiek A., van Kruchten R., Maeda N., Staels B., et al. 2008. Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatology. 48: 474–486. [DOI] [PubMed] [Google Scholar]
- 23.Pagadala M., Kasumov T., McCullough A. J., Zein N. N., Kirwan J. P. 2012. Role of ceramides in nonalcoholic fatty liver disease. Trends Endocrinol. Metab. 23: 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harte A. L., da Silva N. F., Creely S. J., McGee K. C., Billyard T., Youssef-Elabd E. M., Tripathi G., Ashour E., Abdalla M. S., Sharada H. M., et al. 2010. Elevated endotoxin levels in non-alcoholic fatty liver disease. J. Inflamm. (Lond). 7: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hooper A. J., Adams L. A., Burnett J. R. 2011. Genetic determinants of hepatic steatosis in man. J. Lipid Res. 52: 593–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dapito D. H., Mencin A., Gwak G. Y., Pradere J. P., Jang M. K., Mederacke I., Caviglia J. M., Khiabanian H., Adeyemi A., Bataller R., et al. 2012. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 21: 504–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henao-Mejia J., Elinav E., Jin C., Hao L., Mehal W. Z., Strowig T., Thaiss C. A., Kau A. L., Eisenbarth S. C., Jurczak M. J., et al. 2012. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 482: 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Depner C. M., Torres-Gonzalez M., Tripathy S., Milne G., Jump D. B. 2012. Menhaden oil decreases high-fat diet-induced markers of hepatic damage, steatosis, inflammation, and fibrosis in obese Ldlr−/− mice. J. Nutr. 142: 1495–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jump D. B., Tripathy S., Depner C. M. 2013. Fatty acid-regulated transcription factors in the liver. Annu. Rev. Nutr. 33: 249–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nobili V., Bedogni G., Alisi A., Pietrobattista A., Rise P., Galli C., Agostoni C. 2011. Docosahexaenoic acid supplementation decreases liver fat content in children with non-alcoholic fatty liver disease: double-blind randomised controlled clinical trial. Arch. Dis. Child. 96: 350–353. [DOI] [PubMed] [Google Scholar]
- 31.Sofi F., Giangrandi I., Cesari F., Corsani I., Abbate R., Gensini G. F., Casini A. 2010. Effects of a 1-year dietary intervention with n-3 polyunsaturated fatty acid-enriched olive oil on non-alcoholic fatty liver disease patients: a preliminary study. Int. J. Food Sci. Nutr. 61: 792–802. [DOI] [PubMed] [Google Scholar]
- 32.Bulchandani D. G., Nachnani J. S., Nookala A., Naumovitch C., Herndon B., Molteni A., Quinn T., Alba L. M. 2010. Treatment with omega-3 fatty acids but not exendin-4 improves hepatic steatosis. Eur. J. Gastroenterol. Hepatol. 22: 1245–1252. [DOI] [PubMed] [Google Scholar]
- 33.Ishikawa Y., Yokoyama M., Saito Y., Matsuzaki M., Origasa H., Oikawa S., Sasaki J., Hishida H., Itakura H., Kita T., et al. 2010. Preventive effects of eicosapentaenoic acid on coronary artery disease in patients with peripheral artery disease. Circ. J. 74: 1451–1457. [DOI] [PubMed] [Google Scholar]
- 34.Kishino T., Ohnishi H., Ohtsuka K., Matsushima S., Urata T., Watanebe K., Honda Y., Mine Y., Matsumoto M., Nishikawa K., et al. 2011. Low concentrations of serum n-3 polyunsaturated fatty acids in non-alcoholic fatty liver disease patients with liver injury. Clin. Chem. Lab. Med. 49: 159–162. [DOI] [PubMed] [Google Scholar]
- 35.Scorletti E., Bhatia L., McCormick K. G., Clough G. F., Nash K., Hodson L., Moyses H. E., Calder P. C., Byrne C. D.; WELCOME study. 2014. Effects of purified eicosapentaenoic and docosahexaenoic acids in non-alcoholic fatty liver disease: results from the WELCOME study. Hepatology. 60: 1211–1221. [DOI] [PubMed] [Google Scholar]
- 36.Argo C. K., Patrie J. T., Lackner C., Henry T. D., de Lang E. E., Weltman A. L., Shah N. L., Al-Osaimi A. M., Pramoonjago P., Jayakumar S., et al. 2015. Effects of n-3 fish oil on metabolic and histological parameters in NASH: a double-blind, randomized, placebo-controlled trial. J. Hepatol. 62: 190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanyal A. J., Abdelmalek M. F., Suzuki A., Cummings O. W., Chojkier M. 2014. No significant effects of ethyl-eicosapentaenoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology. 147: 377–384. [DOI] [PubMed] [Google Scholar]
- 38.Barter P., Ginsberg H. N. 2008. Effectiveness of combined statin plus omega-3 fatty acid therapy for mixed dyslipidemia. Am. J. Cardiol. 102: 1040–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Stasi D., Bernasconi R., Marchioli R., Marfisi R. M., Rossi G., Tognoni G., Tacconi M. T. 2004. Early modification of fatty acid composition in plasma phospholipids, platelets and mononucleates of healthy volunteers after low doses of n-3 PUFA. Eur. J. Clin. Pharmacol. 60: 183–190. [DOI] [PubMed] [Google Scholar]
- 40.Superko H. R., Superko S. M., Nasir K., Agatston A., Garrett B. C. 2013. Omega-3 fatty acid blood levels. Clinical significance and controversy. Circulation. 128: 2154–2161. [DOI] [PubMed] [Google Scholar]
- 41.Lockyer S., Tzanetou M., Carvalho-Wells A. L., Jackson J. G., Minihane A. M., Lovegrove J. A. 2012. STAT gene dietary model to implement diets of differing fat composition in prospectively genotyped groups (apoE) using commercially available foods. Br. J. Nutr. 108: 1705–1713. [DOI] [PubMed] [Google Scholar]
- 42.Tripathy S., Torres-Gonzalez M., Jump D. B. 2010. Elevated hepatic fatty acid elongase-5 activity corrects dietary fat-induced hyperglycemia in obese C57BL/6J mice. J. Lipid Res. 51: 2642–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu L., Hui A. Y., Albanis E., Arthur M. J., O’Byrne S. M., Blaner W. S., Mukherjee P., Friedman S. L., Eng F. J. 2005. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for the analysis of hepatic fibrosis. Gut. 54: 142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiskirchen R., Weimer J., Meurer S.K., Kron A., Seipel B., Vater I., Arnold N., Siebert R., Xu L., Friedman S. L., Bergmann C., C 2013. Genetic characteristics of the human hepatic stellate cell line LX-2. PLoS One. 8: e75692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tripathy S., Lytle K. A., Stevens R. D., Bain J. R., Newgard C. B., Greenberg A. S., Huang L-S., Jump D. B. 2014. Fatty acid elongase-5 (Elovl5) regulates hepatic triglyceride catabolism in obese C57BL/6J mice. J. Lipid Res. 55: 1448–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jump D. B., Torres-Gonzalez M., Olson L. K. 2011. Soraphen A, an inhibitor of acetyl CoA carboxylase activity, interferes with fatty acid elongation. Biochem. Pharmacol. 81: 649–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seki E., De Minicis S., Osterreicher C. H., Kluwe J., Osawa Y., Brenner D. A., Schwabe R. F. 2007. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat. Med. 13: 1324–1332. [DOI] [PubMed] [Google Scholar]
- 48.Schuppan D., Y. O. Kim. 2013. Evolving therapies for liver fibrosis. J. Clin. Invest. 123: 1887–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cengiz M., Ozenirler S., Elbeg S. 2015. Role of serum toll-like receptors 2 and 4 in non-alcoholic steatohepatitis and liver fibrosis. J. Gastroenterol. Hepatol. 30: 1190–1196. [DOI] [PubMed] [Google Scholar]
- 50.Roh Y. S., Seki E. 2013. Toll-like receptors in alcoholic liver disease, non-alcoholic steatohepatitis and carcinogenesis. J. Gastroenterol. Hepatol. 28(Suppl 1): 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beutler B. 2002. TLR4 as the mammalian endotoxin sensor. Curr. Top. Microbiol. Immunol. 270: 109–120. [DOI] [PubMed] [Google Scholar]
- 52.Saxena N. K., Anania F. A. 2015. Adipocytokines and hepatic fibrosis. Trends Endocrinol. Metab. 26: 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barron L., Wynn T. A. 2011. Fibrosis is regulated by Th2 and Th17 responses and by dynamic interactions between fibroblasts and macrophage. Am. J. Physiol. Gastrointest. Liver Physiol. 300: G723–G728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perepelyuk M., Terajima M., Wang A. Y., Georges P. C., Janmey P. A., Yamauchi M., Wells R. G. 2013. Hepatic stellate cells and portal fibrosis are the major cellular sources of collagens and lysyl oxidases in normal liver and early after injury. Am. J. Physiol. Gastrointest. Liver Physiol. 304: G605–G614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Massague J. 2012. TGF-β signalling in context. Nat. Rev. Mol. Cell Biol. 13: 616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Akhurst R. J., Hata A. 2012. Targeting the TGF-β signalling pathway in disease. Nat. Rev. Drug Discov. 11: 790–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Han J., Alverez-Breckenridge C. A., Wang Q-E., Yu J. 2015. TGF-β signaling and its targeting for glioma treatment. Am. J. Cancer Res. 5: 945–955. [PMC free article] [PubMed] [Google Scholar]
- 58.Murata M., Yoshida K., Yamaguchi T., Matsuzaki K. 2014. Linker phosphorylation of Smad3 promotes fibro-carcinogenesis in chronic viral hepatitis of hepacellular carcinoma. World J. Gastroenterol. 20: 15018–15027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Verrecchia F., Chu M-L., Mauviel A. 2001. Identification of novel TGF-β/Smad gene targets in dermal fibroblast using a combined cRNA microarray/promoter transactivation approach. J. Biol. Chem. 276: 17058–17062. [DOI] [PubMed] [Google Scholar]
- 60.Nyati S., Schinske-Sebolt K., Pitchiaya S., K Chekhovskiy, Chator A., Chaudhry N., Dosch J., Van Dort M. E., Varambally S., Kumar-Sinha C., et al. 2015. The kinase activity of the Ser/Thr kinase Bub1 promotes TGF-β signaling. Sci. Signal. 8: ra1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sprecher H. 2000. Metabolism of highly unsaturated n-3 and n-6 fatty acids. Biochim. Biophys. Acta. 1486: 219–231. [DOI] [PubMed] [Google Scholar]
- 62.Tuohetahuntila M., Spee B., Kruitwagen H. S., Wubbolts R., Brouwers J. F., van de Lest C. H., Molenaar M. R., Houweling M., Helms J. B., Vaandrager A. B. 2015. Role of long-chain acyl-CoA synthetase 4 in formation of polyunsaturated lipid species in hepatic stellate cells. Biochim. Biophys. Acta. 1851: 220–230. [DOI] [PubMed] [Google Scholar]
- 63.Parker H. M., Johnson N. A., Burdon C. A., Cohn J. S., O’Connor H. T., George J. 2012. Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J. Hepatol. 56: 944–951. [DOI] [PubMed] [Google Scholar]
- 64.Moreno-Caceres J., Caja L., Mainez J., Mayoral R., Martin-Sanz P., Moreno-VIcente R., del Pozo M. A., Dooley S., Egea G., Fabregat I. 2014. Caveolin-1 is required for TGFβ-induced transactivation of the EGF receptor pathway in hepatocytes through the activation of the metalloprotease TACD/ADAM17. Cell Death Dis. 5: e1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Finlin B. S., Zhu B., Starnes C. P., McGehee R. E., Jr, Peterson C. A., Kern P. A. 2013. Regulation of thrombospondin-1 expression in alternatively activated macrophage and adipocytes: role of cellular crosstalk and omega-3 fatty acids. J. Nutr. Biochem. 24: 1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park Y. W., Kang Y. M., Butterfield J., Detmar M., Goronzy J. J., Weyand C. M. 2004. Thrombospondin 2 functions as an endogenous regulator of angiogenesis and inflammation in rheumatoid arthritis. Am. J. Pathol. 165: 2087–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Masli S., Sheibani N., Cursiefen C., Zieske J. 2014. Matricellular protein thrombospondins: influence on ocular angiogenesis, wound healing and immuneregulation. Curr. Eye Res. 39: 759–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim D. J., Christofidou E. D., Keene D. R., Milde M. H., Adams J. C. 2015. Inter-molecular interactions of thrombospondins drive their accumulation in extracellular matrix. Mol. Biol. Cell.In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen W-Y., Lin S-Y., Pan H-C., Liao S-L., Chuang Y-H., Yen Y-J., Lin S-Y., Chen C-J. 2012. Beneficial effect of docosahexaenoic acid on cholestatic liver injury in rats. J. Nutr. Biochem. 23: 252–264. [DOI] [PubMed] [Google Scholar]
- 70.Zhao H., Chan-Li Y., Collins S. L., Zhang Y., Hallowell R. W., Mitzner W., Horton M. R. 2014. Pulmonary delivery of docosahexaenoic acid mitigates bleomycin-induced pulmonary fibrosis. BMC Pulm Med 14: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Calder P. C. 2015. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim. Biophys. Acta. 1851: 469–484. [DOI] [PubMed] [Google Scholar]
- 72.Giraudi P. J., Barbero Becerra V. J., Marin V., Chavez-Tapia N. C., Tiribelli C., Rosso N. 2015. The importance of the interaction between hepatocyte and hepatic stellate cells in fibrogenesis induced fatty accumulation. Exp. Mol. Pathol. 98: 85–92. [DOI] [PubMed] [Google Scholar]
- 73.Calder P. C. 2012. Mechanisms of action of (n-3) fatty acids. J. Nutr. 142: 592S–599S. [DOI] [PubMed] [Google Scholar]
- 74.Itakura H., Yokoyama M., Matsuzaki M., Saito Y., Origasa H., Ishikawa Y., Oikawa S., Sasaki J., Hishida H., Kita T., et al. 2011. Relationships between plasma fatty acid composition and coronary artery disease. J. Atheroscler. Thromb. 18: 99–107. [DOI] [PubMed] [Google Scholar]
- 75.Jump D. B., Depner C. M., Tripathy S. 2012. Omega-3 fatty acid supplementation and cardiovascular disease. J. Lipid Res. 53: 2525–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.