Abstract
PUFAs, which account for 25–30% of the total fatty acids in the human brain, are important for normal brain development and cognitive function. However, it remains unclear how PUFAs are delivered to neurons and exert their effects. In this study, we demonstrated that n-3 and n-6 PUFAs added to the medium are incorporated into membrane phospholipids of primary glial cells from rat cortices, and then secreted as the fatty acid moiety of phospholipids in apoE-containing lipoproteins (LpEs). Tandem mass spectrometry analysis further showed that LpEs secreted from glial cells contain a variety of metabolites of PUFAs produced in glial cells by elongation and unsaturation. LpEs are absorbed by endocytosis into neurons via LDL receptor-related protein 1. LpE-containing n-3 and n-6 PUFAs exhibit a strong effect on neurite outgrowth of hippocampal neurons by increasing the number of branches. This study sheds light on the novel role of LpEs in the central nervous system and also a novel pathway in which PUFAs act on neurons.
Keywords: polyunsaturated fatty acid, apolipoprotein E-containing lipoprotein, neuron, apolipoprotein E, low density lipoprotein receptor-related protein 1
PUFAs account for 25–30% of total fatty acids in human brain tissue (1). PUFAs affect neurotransmission, gene expression, and synaptic plasticity (2–4) and are involved in cognition, learning, and memory; in addition, they are involved in neurological disorders including Alzheimer’s disease and schizophrenia (5). PUFAs are primarily delivered from plasma, whereas saturated and monounsaturated fatty acids are synthesized de novo in the brain. The major PUFAs are n-6 PUFAs such as arachidonic acid (20:4n-6, AA) and n-3 PUFAs such as DHA (22:6n-3) and EPA (20:5n-3); DHA is the most abundant in neural membranes. In vitro, the addition of DHA in free fatty acid form to neurons increases neurite outgrowth, which is critical for synaptic formation in developing and adult brain (6–8). However, it remains unclear whether DHA is primarily delivered to neurons in vivo in the unesterified free form or the phospholipid-esterified form.
The blood-brain barrier prevents plasma lipoproteins, such as LDL and HDL, from entering the brain freely; moreover, apoE secreted from glial cells is the major apolipoprotein in the brain. Therefore, apoE-containing lipoproteins (LpEs) are the major lipoproteins in the central nervous system (9). LpEs are HDL-like in density (10), and glial cells, primarily astrocytes, supply cholesterol and phospholipids to neurons via LpEs (11). LpEs bind to receptors of the LDL receptor (LDLR) family expressed in neurons and are internalized via endocytosis (12, 13). Cholesterol derived from LpE promotes synaptogenesis (14). Furthermore, LpEs stimulate axon growth of retinal ganglion cells and neurons in the central nervous system and protect retinal ganglion cells from apoptosis (15–17). Thus, LpEs play a variety of important roles in the brain.
Because LpEs deliver lipids to neurons, it is possible that PUFAs are first incorporated into membrane phospholipids of glial cells and secreted as LpEs, and that PUFAs in LpE phospholipids impact neuronal function or structure. In this study, we determined the impacts of fatty acid composition of LpE phospholipids on neurite outgrowth. We demonstrate that the fatty acid composition in LpE is modified by n-3 and n-6 PUFAs provided to glial cells, and that n-3 and n-6 PUFAs in LpEs are incorporated by LDL receptor-related protein 1 (LRP1)-mediated endocytosis and enhance neurite outgrowth of hippocampal neurons.
MATERIALS AND METHODS
Materials
Recombinant rat receptor-associated protein (RAP) was purchased from PROGEN. Goat polyclonal antibody (sc-16166) raised against the N terminus of human LRP1 and normal goat IgG (sc-2028) were purchased from Santa Cruz Biotechnology. α2-macroglobulin (α2M) and cytochalasin D were purchased from Sigma. Stearic acid (18:0, SA), oleic acid (18:1n-9, OA), AA, EPA, and DHA were purchased from Nu-Chek-Prep.
Primary culture of rat hippocampal neurons
Hippocampal neurons were isolated from 2-day-old Sprague-Dawley rats according to the procedure of Kaech et al. (18) with minor modifications. In brief, hippocampi were dissected from rat brain and incubated in phosphate-buffered saline containing 0.25% trypsin (Invitrogen) at 37°C for 20 min. After the tissue was washed, it was passed through a fire-polished Pasteur pipette. Cells were suspended in Neurobasal medium (Invitrogen) supplemented with B-27 supplement (Invitrogen) and GlutaMAX (Invitrogen) and plated at a density of 13,000 cells/cm2 on coverslips coated with poly-d-lysine (Sigma) in 12-well plates. After 2 h, the culture medium was changed to either N2 medium [Neurobasal medium with N2 supplement (Invitrogen) and GlutaMAX], with or without LpE, or glial cell-conditioned medium (GCM) (see below). Cells were then cultured for 3 days. In some experiments, recombinant rat RAP, antibodies, or methylamine-activated α2M were added to the medium. α2M was activated by treatment with 100 mM methylamine for 1 h at room temperature (19). For the experiment on inhibition of LpE uptake, cells were washed 18 h after plating and cultured for 1 h in N2 medium with 20 μM cytochalasin D, after which the medium was replaced with N2 medium + LpE in the presence of cytochalasin D. After 24 h, cells were further cultured for 28 h in N2 medium without cytochalasin D. Animal experiments were conducted in accordance with the institutional policies following approval from the Animal Experimentation Committee of Institute for integrated Cell-Material Sciences and the Graduate School of Agriculture, Kyoto University.
Primary culture of glial cells
Glial cells were isolated from the cerebral cortex of 2-day-old Sprague-Dawley rats (20). Glial cells were maintained in DMEM supplemented with 10% heat-inactivated fetal bovine serum.
Isolation of LpE from GCM
Glial cells were washed with DMEM containing 2.5 mg/ml (38 μM) fatty acid free BSA (Sigma) and incubated for 24 h with DMEM with or without 50 μM of various fatty acids conjugated to 2.5 mg/ml BSA. Cells were washed with DMEM or Neurobasal medium supplemented with GlutaMAX and then cultured for 3 days with DMEM for preparation of LpE or Neurobasal medium supplemented with GlutaMAX for culturing neurons. As fatty acid dissolved in ethanol was added to BSA for conjugation, the media contained 0.2% (v/v) ethanol. Ethanol (0.2%) was added to DMEM/BSA without fatty acid. The conditioned medium was collected and centrifuged for 5 min at 180 g. The supernatant was designated GCM. When neurons were cultured in GCM, GCM was supplemented with N2 supplement. LpEs were isolated from GCM by ultracentrifugation on a discontinuous sucrose density gradient (12, 17) consisting of the following sucrose solutions: 6 ml of density 1.30 g/ml; 9 ml of density 1.20 g/ml; 9 ml of density 1.10 g/ml; and 12 ml of GCM. The gradient was centrifuged in a SW42-Ti rotor (Beckmann) at 4°C for 48 h at 160,000 g. Twelve fractions were sequentially removed from the top of the tube. Fractions containing apoE (i.e., LpE, typically fractions 5–8, density 1.07–1.12 g/ml) were identified by immunoblotting, combined, washed four times, and concentrated in phosphate-buffered saline using an Amicon Ultra filter (100 kDa molecular mass cutoff; Millipore). The protein concentration of LpE was adjusted to 10 μg/ml, when neurons were cultured with LpE. To prepare Alexa Fluor 488-labeled LpE, the succinimidyl ester of Alexa Fluor 488 (Molecular Probes) was conjugated to LpE according to the manufacturer’s instructions.
Immunoblotting
Equal amounts of protein were separated by 12% polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membrane, and probed with goat anti-human apoE antibody (Academy Bio-Medical, catalog no. 50A-G1b). For dot-blot analysis, 4 μl of each fraction by ultracentrifugation was applied to a nitrocellulose membrane, and apoE in the fraction was proved by anti-human apoE antibody. Immunoreactive proteins were detected by peroxidase-conjugated rabbit anti-goat IgG (Abcam). The intensities of the bands were quantitated using the Fujifilm MultiGauge software.
Lipid analysis
The cholesterol content in LpE was determined using a fluorescent enzyme assay (21). The content of choline phospholipids in LpE was determined as described (22). Briefly, lipids in LpE were extracted with chloroform-methanol (2:1). The lipid-containing solution was dried and resuspended in 2-propanol, and the levels of choline phospholipids were determined using enzymatic assay kit purchased from Wako.
LC/MS/MS
The LC/MS/MS analysis was performed on a Shimadzu Nexera ultra-high-performance liquid chromatography (UHPLC) system (Shimadzu, Kyoto, Japan) coupled with QTRAP 4500 hybrid triple quadrupole linear ion trap mass spectrometer (AB SCIEX, Framingham, MA). Chromatographic separation was performed on an Acquity UPLC HSS T3 column (100 mm × 2.1 mm, 1.8 μm; Waters, Milford, MA) maintained at 40°C using mobile phase A [water-methanol, 50:50 (v/v) containing 10 mM ammonium acetate and 0.2% acetic acid] and mobile phase B [isopropanol-acetone, 50:50 (v/v)] in a gradient program (0–20 min: 30% B→70% B; 20–24 min: 90% B; 24–28 min: 30% B) with a flow of 0.5 ml/min. Neutral loss scans of 74 and 87 Da in the negative ion mode and 141 Da in the positive ion mode were used for detecting choline-containing phospholipids [phosphatidylcholine (PC) and sphingomyelin (SM)], phosphatidylserine, and phosphatidylethanolamine, respectively. Precursor ion scans of m/z 241 and 153 in the negative ion mode were used for detecting phosphatidylinositol and phosphatidic acid, respectively. The instrument parameters were as follows (arbitrary units if not specified): curtain gas = 10 psi; collision gas = 7; ionspray voltage = −4,500 V; temperature = 700°C; ion source gas 1 = 40 psi; ion source gas 2 = 80 psi; declustering potential = −105 V; entrance potential = −10 V; collision energy = −32 V; collision cell exit potential = −19 V. Product ion analysis in the negative ion mode was performed to determine the fatty acid composition of each PC species.
Immunostaining
Cells ware fixed with 4% paraformaldehyde for 30 min, and permeabilized with 0.4% Triton X-100 for 5 min at room temperature, and then incubated with 10% goat serum to reduce nonspecific binding of antibodies. Cells were incubated with the first antibody against neuron-specific class III β-tubulin (Millipore, catalog no. MAB1637) overnight at 4°C, and then incubated with fluorescent Alexa Fluor 488-conjugated anti-mouse IgG (Molecular Probes) for 1 h.
Neurite measurements
Immunostained cells were photographed from randomly selected fields with a confocal microscope (LSM 700; Carl Zeiss). Neurons were photographed blindly (with the name of the sample covered). The length of neurite was analyzed by tracing neurites using the National Institutes of Health (NIH) ImageJ software. The number of neurites and branches was determined by counting neurites in the drawings. At least 45 neurons were measured in every case.
Quantitative RT-PCR
Total RNA was extracted using the RNeasy Mini Kit (QIAGEN). cDNA was synthesized from total RNA (1.3 μg) using the High-capacity cDNA Reverse Transcription Kit (Applied Biosystems). Quantitative RT-PCR was performed on the StepOnePlus Real-Time PCR System with Fast SYBR Green Master Mix (Applied Biosystems). Target gene expression was normalized against glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Statistical analysis
Multiple comparisons were performed using ANOVA with Tukey honestly significant difference test. A value of P < 0.05 was considered statistically significant.
RESULTS
Effect of conditioned medium from glial cells nourished with PUFAs on neurite outgrowth of hippocampal neurons
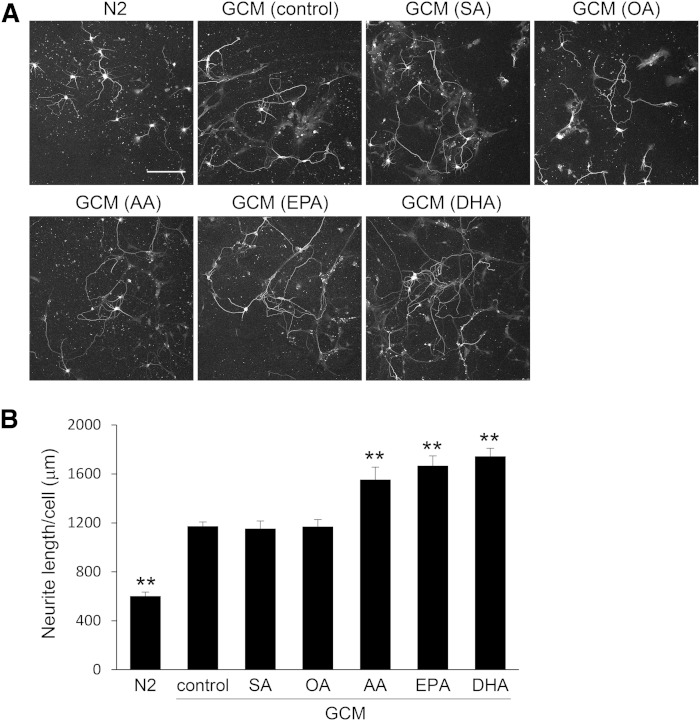
Because glial cells supply lipids to neurons in the central nervous system, we first cultured glial cells with medium containing PUFAs to examine the effects of PUFAs on neurite outgrowth of hippocampal neurons. Primary glial cells prepared from 2-day-old Sprague-Dawley rat cortices were cultured for 24 h with medium containing 50 μM of various BSA-conjugated fatty acids, such as SA (18:0), OA (18:1n-9), AA (20:4n-6), EPA (20:5n-3), and DHA (22:6n-3). After the medium was replaced with fresh medium, the glial cells were cultured for an additional 3 days in the absence of serum, and GCM was collected. Next, primary hippocampal neurons were cultured for 3 days in N2 medium or GCM, collected as described previously, and immunostained for class III β-tubulin (Fig. 1A). Total neurite length per hippocampal neuron was determined by tracing neurites using NIH ImageJ (Fig. 1B). Consistent with a previous report (23), GCM increased neurite outgrowth. GCM (AA), GCM (EPA), and GCM (DHA), all of which were prepared from glial cells nourished with PUFAs, enhanced neurite outgrowth more efficiently than GCM (control). However, GCM (SA) and GCM (OA) prepared from glial cells nourished with saturated or monounsaturated fatty acids did not result in any enhancement relative to GCM (control).
Fig. 1.
Neurite measurements of neurons cultured in GCM derived from glial cells nourished with fatty acids. A: Glial cells were cultured in medium containing BSA (38 μM) and 50 μM of indicated fatty acids or 0.2% ethanol (control). After 24 h, cells were washed once and the medium was changed, the glial cells were cultured for 3 days, and GCM was collected. Two hours after hippocampal neurons prepared from P2 rats were plated, the medium was replaced with GCM prepared from glial cells nourished with various PUFAs or N2 medium, and hippocampal neurons were cultured for 3 days. Neurons were immunostained for class III β-tubulin. Scale bar, 150 μm. B: Total neurite length per neuron was quantitated using ImageJ; data are expressed as means ± SEM (n = 45). ** P < 0.01 compared with GCM (control).
Effect of LpE prepared from GCM (PUFA) on neurite outgrowth of hippocampal neurons
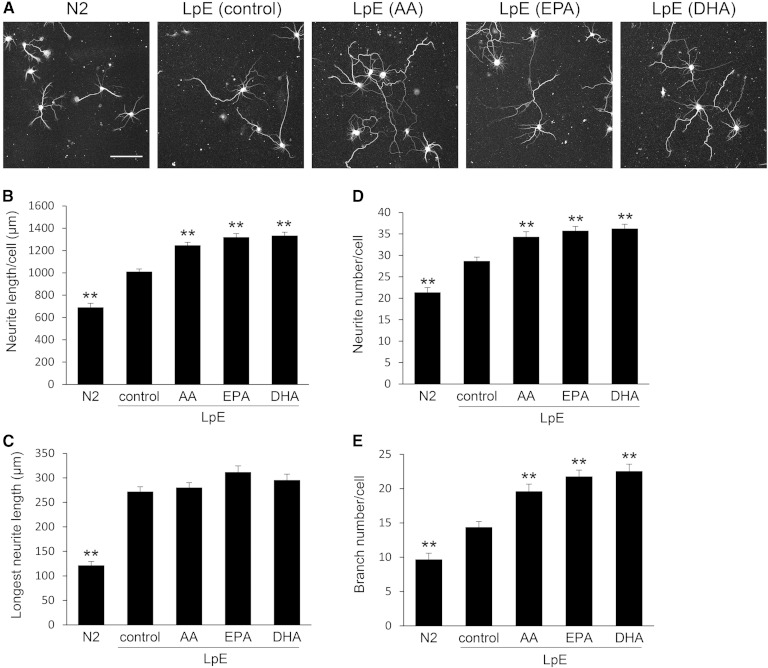
In order to supply lipids to neurons, glial cells secrete LpEs consisting of apoE and lipids such as cholesterol and phospholipids (10). Furthermore, glial cells secrete many types of neurotrophic factors such as glial cell line-derived neurotrophic factor and nerve growth factor (24, 25). We speculated that n-3 and n-6 PUFAs added to the medium are first incorporated into membrane phospholipids of glial cells, and then secreted in association with apoE as LpE, and that the PUFAs incorporated in phospholipids in LpE would enhance neurite outgrowth. To test this idea and exclude the possibility that neurotrophic factors secreted from glial cells are responsible for enhanced neurite outgrowth, we isolated LpE from GCM (AA), GCM (EPA), and GCM (DHA) by sucrose density gradient ultracentrifugation (12, 15, 17) and examined their effects on neurite outgrowth. Dot-blot analysis revealed that apoE was primarily present in fractions 5–8 (supplementary Fig. 1A). The density of these fractions was 1.07–1.12 g/ml, in the range of the density of LpE. These LpE fractions were combined and adjusted to 10 μg protein/ml, and the amounts of cholesterol and choline phospholipids in LpE were measured (Table 1). The amounts of cholesterol and choline phospholipids did not differ among LpE samples, indicating that fatty acids added to glial cells did not affect the lipid content of LpE. Immunoblotting revealed that the amounts of apoE in LpE samples were comparable (supplementary Fig. 1B, C). When N2 medium, supplemented with LpE (control), was added to hippocampal neurons, total neurite length per neuron was increased (Fig. 2A, B). LpE (AA), LpE (EPA), and LpE (DHA), prepared from GCM (AA), GCM (EPA), and GCM (DHA), respectively, enhanced neurite outgrowth more efficiently than LpE (control).
TABLE 1.
The amounts of cholesterol and choline phospholipids in LpE
| LpE (Control) | LpE (AA) | LpE (EPA) | LpE (DHA) | |
| Protein (μg) | 10 | 10 | 10 | 10 |
| Cholesterol (μg) | 1.03 ± 0.09 | 0.88 ± 0.08 | 1.24 ± 0.24 | 0.87 ± 0.13 |
| Choline phospholipids (μg) | 1.81 ± 0.21 | 1.60 ± 0.20 | 1.96 ± 0.08 | 1.60 ± 0.19 |
Cholesterol and choline phospholipids in LpE were quantitated using a fluorescence assay and a colorimetric assay, respectively. Values are means ± SD (n = 3).
Fig. 2.
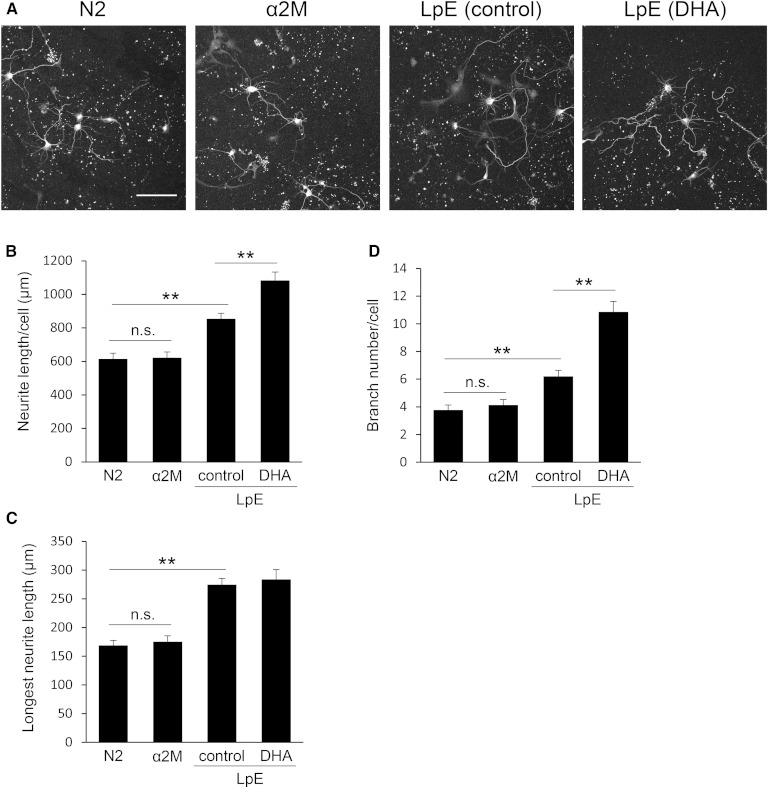
Neurite measurements of neurons cultured with LpE secreted from glial cells nourished with PUFAs. A: Two hours after hippocampal neurons were plated, the medium was replaced with N2 medium with or without LpE isolated from GCM. Then, the neurons were cultured for 3 days and immunostained for class III β-tubulin. Scale bar, 100 μm. B–E: Total neurite length, the longest neurite length, neurite number, and branch number per neuron were quantitated using ImageJ; data are expressed as means ± SEM (n = 60). ** P < 0.01 compared with LpE (control).
Next, we examined whether the increase of total neurite length was due to an increase in the length of neurites, the number of neurites, or both. LpE (control), LpE (AA), LpE (EPA), and LpE (DHA) increased the longest neurite length of neurons, but there was no significant difference between the effects of the LpE samples (Fig. 2C). LpE samples increased the number of neurites and the number of branches per neuron, and LpE (AA), LpE (EPA), and LpE (DHA) caused a greater increase than LpE (control) in both cases (Fig. 2D, E). On the other hand, LpE (SA) and LpE (OA) caused no significant increase in the number of neurites or the number of branches per neuron relative to LpE (control) (supplementary Fig. 2). These results suggest that LpEs secreted from glial cells nourished with n-3 and n-6 PUFAs promote neurite outgrowth of hippocampal neurons, by increasing the number of branches, more strongly than LpE (control).
MS analysis of phospholipids in LpE (PUFA)
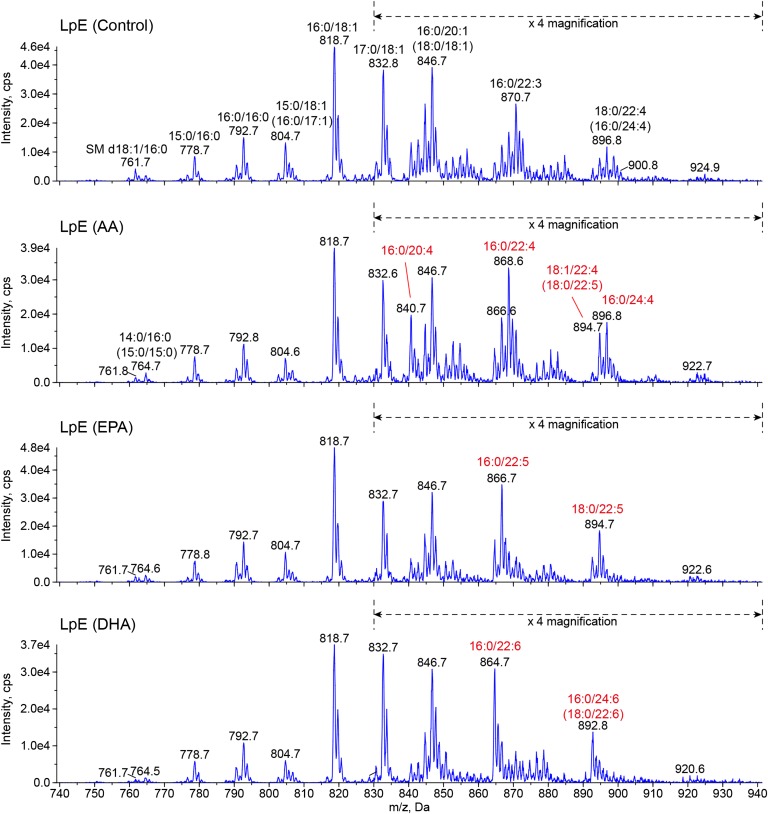
To determine whether PUFAs are incorporated into phospholipids in LpE, we analyzed the fatty acid composition of phospholipids in LpE by LC/MS/MS (Fig. 3). PC was the most abundant phospholipid in LpE, and SM was also detected. However, the levels of lysoPC, phosphatidylethanolamine, phosphatidylinositol, phosphatidylserine, and phosphatidic acids were quite low. LpE (DHA) contained high levels of PC 38:6 (16:0/22:6) and PC 40:6 (18:0/22:6 and 16:0/24:6) relative to LpE (control). LpE (AA) contained high levels of PC 36:4 (16:0/20:4), PC 38:4 (16:0/22:4), PC 40:4 (16:0/24:4), and PC 40:5 (18:0/22:5 and 18:1/22:4). LpE (EPA) contained high levels of PC 38:5 (16:0/22:5) and PC 40:5 (18:0/22:5). We also performed quantitative measurements of actual PUFA enrichment by GC/MS (data not shown) and found that DHA, AA, and EPA, and their metabolites were enriched about 5.5-, 2.1-, and 18-fold in phospholipids in LpE, respectively. These results suggest that n-3 and n-6 PUFAs added to the culture medium and their metabolites are incorporated into the phospholipids in LpE secreted from glial cells.
Fig. 3.
Lipidomic analysis of LpE. Mass spectra of [M+CH3COO]− ions of PC and SM by scanning for neutral loss of 74 Da. Lipid extracts (0.7 nmol of total phospholipids) were subjected to LC/MS/MS analysis. Assigning specific phospholipid species to m/z values was based on their calculated theoretical monoisotopic masses and verified by product ion scanning.
Stimulation of neurite outgrowth is mediated by LRP1
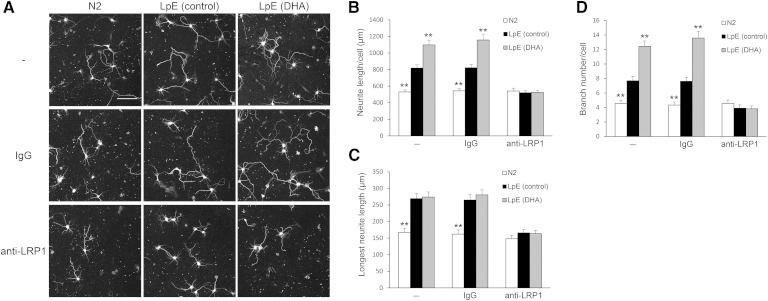
LpEs bind to the LDLR (13) and LRP family (26). To determine whether LpE increase neurite outgrowth via one of the receptors of the LDLR family, we incubated neurons with RAP, which inhibits ligand binding to the LDLR family. Addition of RAP itself did not alter neurite outgrowth but abolished the effects of LpE (control) and LpE (DHA) on neurite outgrowth (Fig. 4). LRP1, a member of the LDLR family, is highly expressed in neurons (27, 28), and LpE protect neurons from apoptosis via LRP1 (29). To determine whether LRP1 is involved in the stimulation of neurite outgrowth by LpE, we incubated neurons with LpE in the presence or absence of a polyclonal antibody against the N-terminus peptide of human LRP1 (Fig. 5A). This anti-LRP1 antibody blocked the stimulation of neurite outgrowth by LpE (control) and LpE (DHA) but did not alter neurite outgrowth in the absence of LpE (Fig. 5B–D). Goat IgG did not affect neurite outgrowth in response to LpE (control) or LpE (DHA). These results suggest that LpEs increase neurite outgrowth via LRP1.
Fig. 4.
Measurements of neurites of neurons cultured with LpE in the presence of RAP. Two hours after hippocampal neurons were plated, the medium was replaced with N2 medium with or without LpE in the presence or absence of 300 nM RAP. Then, hippocampal neurons were cultured for 3 days and immunostained for class III β-tubulin. Total neurite length, longest neurite length, and branch number per neuron were quantitated using ImageJ and shown as means ± SEM (n = 45). ** P < 0.01 compared with LpE (control) in the presence or absence of RAP.
Fig. 5.
Neurite measurements of neurons cultured with LpE in the presence of anti-LRP1 antibody. A: Two hours after hippocampal neurons were plated, the medium was changed to N2 medium with or without LpE in the presence or absence of 20 μg/ml of goat IgG or anti-LRP1 antibody. Then, neurons were cultured for 3 days and immunostained for class III β-tubulin. Scale bar, 100 μm. B–D: Total neurite length, longest neurite length, and branch number per neuron were quantitated using ImageJ; data are expressed as means ± SEM (n = 45). ** P < 0.01 compared with LpE (control) in the presence or absence of IgG or anti-LRP1 antibody.
Uptake of LpE via endocytosis is involved in neurite outgrowth enhancement
LpEs bind to LRP1 and initiate a signaling pathway that protects neurons from apoptosis (12), and LRP1 internalizes various ligands including LpE (30, 31). Therefore, we investigated whether either a signaling pathway initiated by LRP1 or LpE uptake is important for the stimulation of neurite outgrowth. Both LpE and methylamine-activated α2M, a ligand of LRP1, protect neurons from apoptosis by initiating a signaling pathway that is activated by LRP1 (12, 29). To determine whether an LRP1-mediated signaling pathway is involved in the stimulation of neurite outgrowth, we added α2M to neurons (Fig. 6A). The addition of α2M did not affect neurite outgrowth (Fig. 6B–D), whereas α2M protected hippocampal neurons from apoptosis induced by withdrawal of growth factors (data not shown). These results suggest that α2M, which initiates a signaling pathway by binding to LRP1, does not stimulate neurite outgrowth.
Fig. 6.
Neurite measurements of neurons cultured with α2M. A: Two hours after hippocampal neurons were plated, the medium was changed to N2 medium with or without α2M or LpE. Then, neurons were cultured for 3 days and immunostained for class III β-tubulin. Scale bar, 100 μm. B–D: Total neurite length, longest neurite length, and branch number per neuron were quantitated using ImageJ; data are expressed as means ± SEM (n = 5). ** P < 0.01.
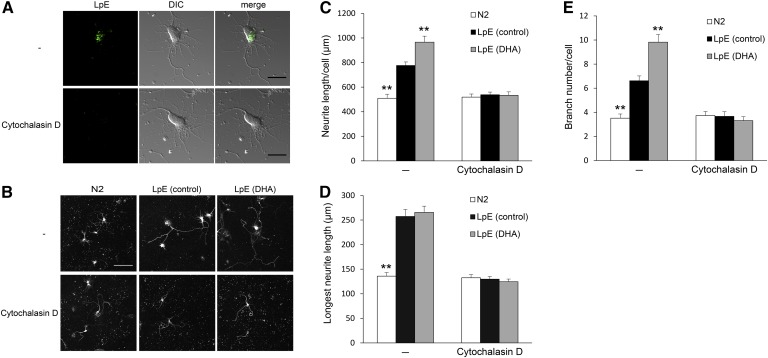
Glial cells supply cholesterol to neurons via LpE and promote synaptogenesis (14). In order to determine whether uptake of LpE is required for stimulation of neurite outgrowth, neurons were incubated with cytochalasin D, which induces actin depolymerization and blocks the uptake of LpE via endocytosis (12). Fluorescence microscopy showed that the uptake of Alexa Fluor 488-labeled LpE was blocked in the presence of cytochalasin D (Fig. 7A). To determine whether inhibition of LpE uptake abrogates the effects of LpE on neurite outgrowth, we incubated neurons with LpE for 24 h in the presence or absence of cytochalasin D (Fig. 7B). The addition of cytochalasin D abolished the stimulation of neurite outgrowth by LpE (control) and LpE (DHA), whereas cytochalasin D did not affect neurite outgrowth in the absence of LpE (Fig. 7C–E). These results suggest that the uptake of LpE via endocytosis is involved in the stimulation of neurite outgrowth.
Fig. 7.
Neurite measurements of neurons cultured with LpE in the presence of cytochalasin D. A: Hippocampal neurons were cultured for 2 h in N2 medium in the presence or absence of 20 μM cytochalasin D. Then, the cells were cultured with Alexa Fluor 488-labeled LpE in the presence or absence of cytochalasin D for 3 h. Alexa Fluor 488-labeled LpE was visualized by fluorescence microscopy. Scale bars, 20 μm. B: Eighteen hours after neurons were plated, the medium was replaced with N2 medium in the presence or absence of 20 μM cytochalasin D, and then neurons were cultured for 1 h. The medium was then replaced with N2 medium with or without LpE in the presence or absence of cytochalasin D, and neurons were cultured for 24 h. After the medium was replaced with N2 medium, neurons were further cultured for 28 h and immunostained for class III β-tubulin. Scale bar, 100 μm. C–E: Total neurite length, longest neurite length, and branch number per neuron were quantitated using ImageJ; data are expressed as means ± SEM (n = 45). ** P < 0.01 compared with LpE (control) in the presence or absence of cytochalasin D.
Fatty acid composition of phospholipids in LpE affects gene expression involved in neurite outgrowth
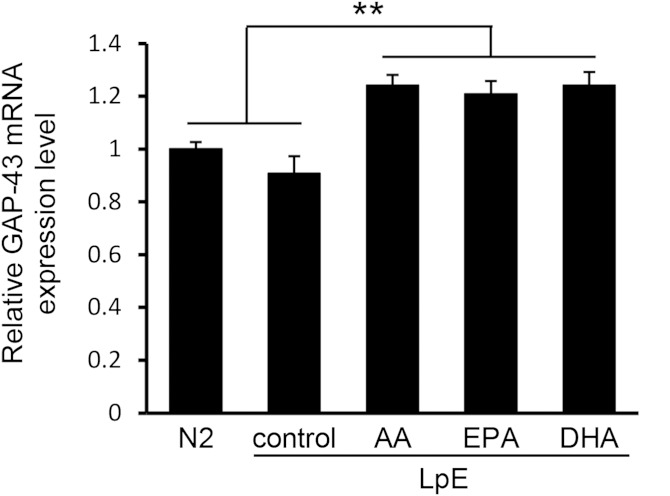
Growth-associated protein 43 (GAP-43) is mainly localized in neurites and induces neurite outgrowth (32, 33). EPA and DHA in free fatty acid form increase GAP-43 mRNA expression in the SH-SY5Y human neuroblastoma cells (34), and neurons take up lipids contained in LpE via internalization of LpE secreted from glial cells (10). We used quantitative RT-PCR to examine GAP-43 mRNA expression in neurons incubated with or without LpE. LpE (AA), LpE (EPA), and LpE (DHA) significantly increased GAP-43 mRNA expression in neurons, whereas LpE (control) did not (Fig. 8). This result suggests that the fatty acid composition of phospholipids in LpE affects GAP-43 expression levels.
Fig. 8.
Relative expression of GAP-43. Hippocampal neurons were cultured for 3 days in N2 medium with or without LpE. GAP-43 mRNA expression in neurons was determined by quantitative RT-PCR and shown as means ± SD (n = 3). ** P < 0.01.
DISCUSSION
PUFAs, which account for 25–30% of the total fatty acids in human brain, are important for normal brain development and cognitive function (1, 5). However, it remains unclear how PUFAs are delivered to neurons and exert their effects. In this study, we found that n-3 and n-6 PUFAs were secreted as the fatty acid moiety of phospholipids in LpE produced from glial cells, and then absorbed by endocytosis into neurons via LRP1. LpE containing n-3 and n-6 PUFAs exerted strong effect on neurite outgrowth of hippocampal neurons by increasing the number of branches.
LpEs, major lipoproteins in the central nervous system, exert various activities on neurons. LpEs protect neurons from apoptosis and promote synaptogenesis (14, 29). Here, we showed that LpEs secreted from glial cells promote neurite outgrowth of hippocampal neurons. In previous studies, various lipoproteins (β-very low density lipoprotein, HDL, and cerebrospinal fluid HDL) increased neurite outgrowth only when exogenous apoE was added simultaneously (35, 36). Even though the major apolipoprotein of cerebrospinal fluid HDL is apoE, cerebrospinal fluid HDL failed to stimulate neurite outgrowth without exogenous apoE; previous studies did not determine whether LpEs promote neurite outgrowth. Our results indicated that physiologically significant LpE secretion from glial cells stimulates neurite outgrowth. The antiapoptotic effect of LpE is induced by a signaling pathway via LRP1, and this pathway is also initiated by α2M, another LRP ligand (29). We showed that the stimulation of neurite outgrowth by LpE is mediated by LRP1 (Fig. 5). However, α2M did not affect neurite outgrowth (Fig. 6). Furthermore, in contrast to the antiapoptotic effect that does not require uptake of LpE, cytochalasin D, which blocks the uptake of LpE, abolished the stimulation of neurite outgrowth by LpE (Fig. 7). These results suggest that endocytosis of LpE via LRP1, but not a signaling pathway via LRP1, is involved in the stimulation of neurite outgrowth.
MS analysis showed that PC is a major phospholipid in LpE. This finding suggests that the phospholipid composition of LpE secreted from rat cortical glial cells is similar to that of peripheral HDL. MS analysis also revealed that n-3 and n-6 PUFAs added to the culture medium were incorporated into phospholipids in LpE secreted from glial cells. Moreover, LpE (EPA) contained docosapentaenoic acid (22:5n-3, DPA), rather than EPA. This result is consistent with a previous report showing that EPA is rapidly catabolized to DPA in the brain (37, 38). Astrocytes, but not neurons, primarily synthesize a variety of n-3 and n-6 PUFAs by elongation or unsaturation of PUFAs (39, 40). Consistent with this report, our results showed that metabolites of PUFAs provided to glial cells were incorporated into LpE phospholipids. These results suggest that glial cells, mainly astrocytes, supply various n-3 and n-6 PUFAs to neurons via LpE.
It is controversial whether AA and EPA, in addition to DHA, stimulate neurite outgrowth. It was reported that EPA and AA, as well as DHA, stimulated neurite outgrowth (41, 42), while Calderon et al. (7) reported that DHA specifically showed the effect. In this study, we demonstrated that EPA and AA, as well as DHA, added to the medium were incorporated into the fatty acid moiety of LpE phospholipids and stimulated neurite outgrowth. We analyzed the free fatty acid contents in the isolated LpE by GC/MS (data not shown) and found that the media containing the isolated LpE contained low amounts of DHA (0.08 μM), AA (0.09 μM), and EPA (0.003 μM). In this study, we added the isolated LpE at the same concentration with that in the conditioned medium and examined the effect. The isolated LpE increased total neurite length as efficiently as GCM, and LpE (AA), LpE (EPA), and LpE (DHA) enhanced neurite outgrowth more efficiently than LpE (control). These results suggest that LpEs in GCM largely contribute to the neurite outgrowth. Furthermore because the concentrations of PUFAs in LpE are much lower than those (1.5–100 μM) of fatty acids used in the previous reports (7, 41, 42) to show their effects on neurite outgrowth, it is conceivable that PUFAs in LpE phospholipids, rather than the free fatty acids, stimulated neurite outgrowth of hippocampal neurons in this study. The metabolism of PUFAs in LpE phospholipids could be different from that of PUFAs absorbed as free fatty acids, which are efficiently incorporated into the plasma membrane (8). We did not find any significant changes in phospholipid species in the total membrane lipid of hippocampal neurons (supplementary Fig. 3). These results suggest that PUFAs in LpE exert their effects after endocytosed via LRP1 and metabolized, while the possibility that PUFAs in LpE are incorporated into phospholipids of specific membrane microdomains and exert their effects cannot be ruled out.
PUFAs influence expression of multiple genes in neurons. GAP-43 is mainly localized in neurites and induces neurite outgrowth (32, 33), and EPA and DHA in free fatty acid form stimulate GAP-43 mRNA expression in neuroblastoma cells (34). LpE (AA), LpE (EPA), and LpE (DHA) increased GAP-43 mRNA expression in neurons (Fig. 8), suggesting that n-3 and n-6 PUFAs in LpE phospholipids affect gene expression involved in neurite outgrowth. However, comprehensive analysis of gene expression is required to elucidate the molecular mechanisms underlying the effect of n-3 and n-6 PUFAs on neurite outgrowth. n-3 and n-6 PUFAs stimulate neurite outgrowth by acting on syntaxin 3, which is mainly localized in neuronal growth cones, in order to allow membrane expansion (41, 42). As components of cell membranes, PUFAs in phospholipids increase membrane fluidity, altering the function of membrane proteins and vesicle budding and fusion, which are important for neurite outgrowth (43). It is likely that PUFAs exert their effects through multiple mechanisms.
Astrocytes and endothelial cells, major cellular components of the blood-brain barrier, may play important roles in the delivery of PUFAs to neurons (40, 43). Recently, Nguyen et al. (44) reported that Mfsd2a, a transmembrane protein specific to the endothelium of the blood-brain barrier, transfers DHA in the form of lysoPC into endothelial cells. Here, we demonstrated that n-3 and n-6 PUFAs are first incorporated into glial cells, and subsequently secreted as the fatty acid moiety of phospholipids in LpE. LpE containing n-3 and n-6 PUFAs exerted a strong effect on neurite outgrowth of hippocampal neurons by increasing the number of branches. There would be other pathways in which PUFAs are delivered to neurons as free fatty acids. Further studies are necessary to assess the physiological significance and roles of each pathway in the delivery of PUFAs to neurons. This study sheds light on a novel role of LpE in the central nervous system, as well as a novel pathway by which PUFAs act on neurons.
Supplementary Material
Footnotes
Abbreviations:
- α2M
- α2-macroglobulin
- AA
- arachidonic acid (20:4n-6)
- GCM
- glial cell-conditioned medium
- LDLR
- LDL receptor
- LpE
- apoE-containing lipoprotein
- LRP
- LDL receptor-related protein
- OA
- oleic acid (18:1n-9)
- PC
- phosphatidylcholine
- RAP
- receptor-associated protein
- SA
- stearic acid (18:0)
This work was supported by Grant-in-Aid for Scientific Research 25221203 (K.U.) and 24580139 and 26660071 (M.M.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT); by the Bio-Oriented Technology Research Advancement Institution of Japan; and by Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries and Food Industry from the Ministry of Agriculture, Forestry and Fisheries of Japan. This work was also supported by the World Premier International Research Center Initiative, MEXT.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Martin D. D., Robbins M. E., Spector A. A., Wen B. C., Hussey D. H. 1996. The fatty acid composition of human gliomas differs from that found in nonmalignant brain tissue. Lipids. 31: 1283–1288. [DOI] [PubMed] [Google Scholar]
- 2.Aveldaño M. I., Bazán N. G. 1973. Fatty acid composition and level of diacylglycerols and phosphoglycerides in brain and retina. Biochim. Biophys. Acta. 296: 1–9. [DOI] [PubMed] [Google Scholar]
- 3.Treen M., Uauy R. D., Jameson D. M., Thomas V. L., Hoffman D. R. 1992. Effect of docosahexaenoic acid on membrane fluidity and function in intact cultured Y-79 retinoblastoma cells. Arch. Biochem. Biophys. 294: 564–570. [DOI] [PubMed] [Google Scholar]
- 4.Horrocks L. A., Farooqui A. A. 2004. Docosahexaenoic acid in the diet: its importance in maintenance and restoration of neural membrane function. Prostaglandins Leukot. Essent. Fatty Acids. 70: 361–372. [DOI] [PubMed] [Google Scholar]
- 5.Bazinet R. P., Layé S. 2014. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 15: 771–785. [DOI] [PubMed] [Google Scholar]
- 6.Poirazi P., Mel B. W. 2001. Impact of active dendrites and structural plasticity on the memory capacity of neural tissue. Neuron. 29: 779–796. [DOI] [PubMed] [Google Scholar]
- 7.Calderon F., Kim H. Y. 2004. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J. Neurochem. 90: 979–988. [DOI] [PubMed] [Google Scholar]
- 8.Cao D., Xue R., Xu J., Liu Z. 2005. Effects of docosahexaenoic acid on the survival and neurite outgrowth of rat cortical neurons in primary cultures. J. Nutr. Biochem. 16: 538–546. [DOI] [PubMed] [Google Scholar]
- 9.Koch S., Donarski N., Goetze K., Kreckel M., Stuerenburg H. J., Buhmann C., Beisiegel U. 2001. Characterization of four lipoprotein classes in human cerebrospinal fluid. J. Lipid Res. 42: 1143–1151. [PubMed] [Google Scholar]
- 10.Vance J. E., Hayashi H. 2010. Formation and function of apolipoprotein E-containing lipoproteins in the nervous system. Biochim. Biophys. Acta. 1801: 806–818. [DOI] [PubMed] [Google Scholar]
- 11.Pitas R. E., Boyles J. K., Lee S. H., Foss D., Mahley R. W. 1987. Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim. Biophys. Acta. 917: 148–161. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi H., Campenot R. B., Vance D. E., Vance J. E. 2009. Protection of neurons from apoptosis by apolipoprotein E-containing lipoproteins does not require lipoprotein uptake and involves activation of phospholipase Cgamma1 and inhibition of calcineurin. J. Biol. Chem. 284: 29605–29613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Innerarity T. L., Pitas R. E., Mahley R. W. 1979. Binding of arginine-rich (E) apoprotein after recombination with phospholipid vesicles to the low density lipoprotein receptors of fibroblasts. J. Biol. Chem. 254: 4186–4190. [PubMed] [Google Scholar]
- 14.Mauch D. H., Nagler K., Schumacher S., Goritz C., Muller E. C., Otto A., Pfrieger F. W. 2001. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 294: 1354–1357. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi H., Campenot R. B., Vance D. E., Vance J. E. 2004. Glial lipoproteins stimulate axon growth of central nervous system neurons in compartmented cultures. J. Biol. Chem. 279: 14009–14015. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi H., Eguchi Y., Fukuchi-Nakaishi Y., Takeya M., Nakagata N., Tanaka K., Vance J. E., Tanihara H. 2012. A potential neuroprotective role of apolipoprotein E-containing lipoproteins through low density lipoprotein receptor-related protein 1 in normal tension glaucoma. J. Biol. Chem. 287: 25395–25406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuo M., Campenot R. B., Vance D. E., Ueda K., Vance J. E. 2011. Involvement of low-density lipoprotein receptor-related protein and ABCG1 in stimulation of axonal extension by apoE-containing lipoproteins. Biochim. Biophys. Acta. 1811: 31–38. [DOI] [PubMed] [Google Scholar]
- 18.Kaech S., Banker G. 2006. Culturing hippocampal neurons. Nat. Protoc. 1: 2406–2415. [DOI] [PubMed] [Google Scholar]
- 19.Bacskai B. J., Xia M. Q., Strickland D. K., Rebeck G. W., Hyman B. T. 2000. The endocytic receptor protein LRP also mediates neuronal calcium signaling via N-methyl-D-aspartate receptors. Proc. Natl. Acad. Sci. USA. 97: 11551–11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gong J. S., Kobayashi M., Hayashi H., Zou K., Sawamura N., Fujita S. C., Yanagisawa K., Michikawa M. 2002. Apolipoprotein E (ApoE) isoform-dependent lipid release from astrocytes prepared from human ApoE3 and ApoE4 knock-in mice. J. Biol. Chem. 277: 29919–29926. [DOI] [PubMed] [Google Scholar]
- 21.Amundson D. M., Zhou M. 1999. Fluorometric method for the enzymatic determination of cholesterol. J. Biochem. Biophys. Methods. 38: 43–52. [DOI] [PubMed] [Google Scholar]
- 22.Morita S. Y., Kobayashi A., Takanezawa Y., Kioka N., Handa T., Arai H., Matsuo M., Ueda K. 2007. Bile salt-dependent efflux of cellular phospholipids mediated by ATP binding cassette protein B4. Hepatology. 46: 188–199. [DOI] [PubMed] [Google Scholar]
- 23.Monard D., Solomon F., Rentsch M., Gysin R. 1973. Glia-induced morphological differentiation in neuroblastoma cells. Proc. Natl. Acad. Sci. USA. 70: 1894–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin L. F., Doherty D. H., Lile J. D., Bektesh S., Collins F. 1993. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 260: 1130–1132. [DOI] [PubMed] [Google Scholar]
- 25.Houlgatte R., Mallat M., Brachet P., Prochiantz A. 1989. Secretion of nerve growth factor in cultures of glial cells and neurons derived from different regions of the mouse brain. J. Neurosci. Res. 24: 143–152. [DOI] [PubMed] [Google Scholar]
- 26.Ruiz J., Kouiavskaia D., Migliorini M., Robinson S., Saenko E. L., Gorlatova N., Li D., Lawrence D., Hyman B. T., Weisgraber K. H., et al. 2005. The apoE isoform binding properties of the VLDL receptor reveal marked differences from LRP and the LDL receptor. J. Lipid Res. 46: 1721–1731. [DOI] [PubMed] [Google Scholar]
- 27.Tooyama I., Kawamata T., Akiyama H., Kimura H., Moestrup S. K., Gliemann J., Matsuo A., McGeer P. L. 1995. Subcellular localization of the low density lipoprotein receptor-related protein (alpha 2-macroglobulin receptor) in human brain. Brain Res. 691: 235–238. [DOI] [PubMed] [Google Scholar]
- 28.Bu G., Maksymovitch E. A., Nerbonne J. M., Schwartz A. L. 1994. Expression and function of the low density lipoprotein receptor-related protein (LRP) in mammalian central neurons. J. Biol. Chem. 269: 18521–18528. [PubMed] [Google Scholar]
- 29.Hayashi H., Campenot R. B., Vance D. E., Vance J. E. 2007. Apolipoprotein E-containing lipoproteins protect neurons from apoptosis via a signaling pathway involving low-density lipoprotein receptor-related protein-1. J. Neurosci. 27: 1933–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herz J., Strickland D. K. 2001. LRP: a multifunctional scavenger and signaling receptor. J. Clin. Invest. 108: 779–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herz J., Clouthier D. E., Hammer R. E. 1992. LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell. 71: 411–421. [DOI] [PubMed] [Google Scholar]
- 32.Aigner L., Arber S., Kapfhammer J. P., Laux T., Schneider C., Botteri F., Brenner H. R., Caroni P. 1995. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell. 83: 269–278. [DOI] [PubMed] [Google Scholar]
- 33.Teo J. L., Ma H., Nguyen C., Lam C., Kahn M. 2005. Specific inhibition of CBP/beta-catenin interaction rescues defects in neuronal differentiation caused by a presenilin-1 mutation. Proc. Natl. Acad. Sci. USA. 102: 12171–12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu H., Ichikawa S., Tani C., Zhu B., Tada M., Shimoishi Y., Murata Y., Nakamura Y. 2009. Docosahexaenoic acid induces ERK1/2 activation and neuritogenesis via intracellular reactive oxygen species production in human neuroblastoma SH-SY5Y cells. Biochim. Biophys. Acta. 1791: 8–16. [DOI] [PubMed] [Google Scholar]
- 35.Fagan A. M., Bu G., Sun Y., Daugherty A., Holtzman D. M. 1996. Apolipoprotein E-containing high density lipoprotein promotes neurite outgrowth and is a ligand for the low density lipoprotein receptor-related protein. J. Biol. Chem. 271: 30121–30125. [DOI] [PubMed] [Google Scholar]
- 36.Holtzman D. M., Pitas R. E., Kilbridge J., Nathan B., Mahley R. W., Bu G., Schwartz A. L. 1995. Low density lipoprotein receptor-related protein mediates apolipoprotein E-dependent neurite outgrowth in a central nervous system-derived neuronal cell line. Proc. Natl. Acad. Sci. USA. 92: 9480–9484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen C. T., Domenichiello A. F., Trépanier M. O., Liu Z., Masoodi M., Bazinet R. P. 2013. The low levels of eicosapentaenoic acid in rat brain phospholipids are maintained via multiple redundant mechanisms. J. Lipid Res. 54: 2410–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen C. T., Bazinet R. P. 2015. β-oxidation and rapid metabolism, but not uptake regulate brain eicosapentaenoic acid levels. Prostaglandins Leukot. Essent. Fatty Acids. 92: 33–40. [DOI] [PubMed] [Google Scholar]
- 39.Moore S. A., Yoder E., Murphy S., Dutton G. R., Spector A. A. 1991. Astrocytes, not neurons, produce docosahexaenoic acid (22:6 omega-3) and arachidonic acid (20:4 omega-6). J. Neurochem. 56: 518–524. [DOI] [PubMed] [Google Scholar]
- 40.Moore S. A. 2001. Polyunsaturated fatty acid synthesis and release by brain-derived cells in vitro. J. Mol. Neurosci. 16: 195–200. [DOI] [PubMed] [Google Scholar]
- 41.Robson L. G., Dyall S., Sidloff D., Michael-Titus A. T. 2010. Omega-3 polyunsaturated fatty acids increase the neurite outgrowth of rat sensory neurones throughout development and in aged animals. Neurobiol. Aging. 31: 678–687. [DOI] [PubMed] [Google Scholar]
- 42.Darios F., Davletov B. 2006. Omega-3 and omega-6 fatty acids stimulate cell membrane expansion by acting on syntaxin 3. Nature. 440: 813–817. [DOI] [PubMed] [Google Scholar]
- 43.Zhang W., Li P., Hu X., Zhang F., Chen J., Gao Y. 2011. Omega-3 polyunsaturated fatty acids in the brain: metabolism and neuroprotection. Front. Biosci. (Landmark Ed.). 16: 2653–2670. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen L. N., Ma D., Shui G., Wong P., Cazenave-Gassiot A., Zhang X., Wenk M. R., Goh E. L., Silver D. L. 2014. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 509: 503–506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.