Abstract
Niemann-Pick type C (NPC) is a progressive neurodegenerative disease characterized by lysosomal/endosomal accumulation of unesterified cholesterol and glycolipids. Recent studies have shown that plasma cholestane-3β,5α,6β-triol (CT) and 7-ketocholesterol (7-KC) could be potential biomarkers for the diagnosis of NPC patients. We aimed to know the sensitivity and specificity of these biomarkers for the diagnosis of NPC compared with other diseases that can potentially lead to oxysterol alterations. We studied 107 controls and 122 patients including 16 with NPC, 3 with lysosomal acid lipase (LAL) deficiency, 8 with other lysosomal diseases, 5 with galactosemia, 11 with cerebrotendinous xanthomatosis (CTX), 3 with Smith-Lemli-Opitz, 14 with peroxisomal biogenesis disorders, 19 with unspecific hepatic diseases, 13 with familial hypercholesterolemia, and 30 with neurological involvement and no evidence of an inherited metabolic disease. CT and 7-KC were analyzed by HPLC-ESI-MS/MS as mono-dimethylglycine derivatives. Levels of 7-KC were high in most of the studied diseases, whereas those of CT were only high in NPC, LAL, and CTX patients. Consequently, although CT is a sensitive biomarker of NPC disease, including those cases with doubtful filipin staining, it is not specific. 7-KC is a very unspecific biomarker.
Keywords: oxysterols; 7-ketocholesterol; high-performance liquid chromatography/electrospray ionization/tandem mass spectrometry; 3α,7α,12α-trihydroxycoprostane
Niemann-Pick type C (NPC; MIM#257220) is a progressive neurodegenerative disease caused by a disorder in the intracellular trafficking of cholesterol that leads to a lysosomal/endosomal accumulation of unesterified cholesterol and glycolipids in many tissues (1–3). The detection of free cholesterol accumulation by filipin staining in fibroblasts has been for many years the gold standard methodology for the biochemical diagnosis of the disease. This method has good sensitivity and specificity. However, juvenile or adult onset forms sometimes present interpretation difficulties (4). In addition, the method involves an invasive skin biopsy and the culture of fibroblasts that requires several weeks for the cellular growth, which delays the diagnosis. Some studies in cellular and animal models showed a correlation between lipid accumulation and cellular oxidative stress that produces an increase of reactive oxygen species and lipid peroxidation (5–8). In the NPC murine model, an increase of two oxysterols, cholestane-3β,5α,6β-triol (CT) and 7-ketocholesterol (7-KC), has been observed (8). This evidence was later confirmed in NPC patients (8–13), showing a correlation between the oxysterol profile and the age of onset and severity of the disease (8, 14). Moreover, CT specifically has been found to be increased in NPC disease compared with other lysosomal and neurodegenerative diseases (8). These results suggest that CT and 7-KC might be good biomarkers for the diagnosis, prognosis, and probably therapeutic monitoring of NPC patients.
Over time different analytical methods have been described for the determination of oxysterols in plasma (9, 15–24). Recently, other authors (12, 13) developed new methods for the analysis of CT and 7-KC and confirmed previous results for the diagnosis of NPC disease. Based on this evidence, we aimed to investigate plasma CT and 7-KC not only in NPC patients but also in other patients that might potentially have an increase of CT and 7-KC in order to know the specificity and sensitivity of these biomarkers, as well as the possible pitfalls for the diagnosis of NPC disease.
MATERIALS AND METHODS
Subjects
A total of 107 EDTA plasma samples obtained from children and adults, which were referred to our center for metabolic study and were not diagnosed with an inborn error of metabolism, were used as age-matched controls. EDTA plasma samples of 122 patients were studied including the following pathologies: 16 patients with NPC [the biochemical, molecular, and clinical data are summarized in supplementary Table 1 (25-28); 3 patients were under treatment with miglustat (patients 13, 15, and 16), and pretreatment samples were not available]; 11 patients with lysosomal storage diseases (LSDs) including Fabry (n = 5), metachromatic leukodystrophy (n = 2), mucolipidosis II/III (n = 1), and lysosomal acid lipase (LAL) deficiency (n = 3); 5 patients with galactosemia; 11 patients with cerebrotendinous xanthomatosis (CTX); 14 patients with peroxisomal disorders (including peroxisomal β-oxidation and biogenesis disorders); and 3 patients with Smith-Lemli-Opitz (SLO). Moreover, we analyzed plasma samples from 19 patients with hepatic disease (jaundice of prematurity, neonatal cholestasis, hepatosplenomegaly, and liver disease), 30 patients with neurological involvement (ataxia, gait disturbance, behavioral disorders, and language disorders) with no evidence of an inherited metabolic disease, and 13 patients with familial hypercholesterolemia with cholesterol in the range of 221–310 mg/dl (adult patients, n = 5) and 308–403 mg/dl (pediatric patients, n = 8). All the samples were collected and stored at −80°C until analysis.
Samples were obtained in accordance with the Helsinki Declaration of 1964, as revised in 2000. Informed consent was obtained from the patients or patients’ parents.
Extraction, derivatization, and HPLC-ESI-MS/MS
The extraction procedure was performed according to Jiang et al. (9) with some modifications. Briefly, 50 μl of plasma was mixed with 150 μl of methanol that included a mixture of internal standards, cholestane-3β,5α,6β-triol-25,26,26,26,27,27,27-d7 (CT-d7) and 7-ketocholesterol-25,26,26,26,27,27,27-d7 (7-KC-d7), at a concentration of 100 ng/ml. The mixture was vortexed for 30 s and centrifuged during 10 min at 16,100 g. The dried supernatant was derivatized using 20 μl 0.5 M of N,N-dimethylglycine hydrochloride (DMG)/2M 4-(dimethylamino)pyridine (DMAP) in chloroform and 20 μl of N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide (EDC) in chloroform during 1 h at 45°C in a shaking water bath. The dimethylglycine derivatives were extracted using a mixture of hexane-water (2:1). The organic phase was transferred to a clean tube, evaporated to dryness under nitrogen, and finally reconstituted with 250 μl of acetonitrile-water (70:30); 50 μl of the mixture was injected into the HPLC-ESI-MS/MS (Waters-Micromass model Quattro micro™ API). To optimize the derivatization reaction, three different concentrations of CT and 7-KC (50, 200, and 2,500 ng/ml) with plasma matrix at different incubation times (30 min, 1 h, 1.5 h, 2 h, 2.5 h, 3 h, and 3.5 h) were studied.
The chromatographic separation was performed on a Symmetry®C18 column (2.1 mm × 50 mm, 3.5 μm; Thermo Fisher Scientific). The mobile phase consisted of 1 mM ammonium formate in water (mobile phase A) and 1 mM ammonium formate in acetonitrile-water (95:5) (mobile phase B), both adjusted with formic acid to pH 3. The step gradient used increased linearly from 40% B to 80% B in 4 min, then to 100% during the next 1 min, followed by 40% during 1 min. The flow rate was 0.5 ml/min and the column temperature was 45°C. The mass spectrometer was operated in the electrospray positive ion mode using multiple reaction monitoring (MRM) mode. In our hands, the bis-dimethylglycine derivative of CT was not found. The corresponding [M+H]+ ion at m/z 591 and [M+2H]2+ ion at m/z 296 of the bis derivative were observed in negligible amounts. Because both target molecules can be formed ex vivo, we should find a reason to explain the discrepancies. One of the reasons could be the lower sensitivity of ESI compared with the atmospheric pressure chemical ionization (APCI) used by Jiang et al. (9) or the fact that perhaps the bis derivative is formed when using more drastic conditions of dryness of the reagents. However, as a prominent peak of monoderivative CT was detected, we decided to monitor the transitions 506 > 104 and 486 > 104 of the mono-dimethylglycine derivatives of CT and 7-KC, as well as 513 > 104 and 493 > 104 of the corresponding deuterated internal standards. Nitrogen and argon were used as nebulizing and collision gas, respectively. Dwell time for each transition was 200 ms, and the interchannel delay was 20 ms. Run time was 6.1 min. The following instrumental settings were used: source temperature, 150°C; desolvatation temperature, 500°C; and capillary voltage, 3.2 kV. Data acquisition and data analyses were performed using MassLynxTM (V3.2) software. The quantification of the mono-dimethylglycine derivatives of CT and 7-KC was relative to the internal standards (CT-d7 and 7-KC-d7, respectively). External calibration curves were used.
To select the appropriate detection conditions and to optimize the mass spectrometer parameters, 10 μg/ml of CT, CT-d7, 7-KC, and 7-KC-d7 were derivatized with dimethylglycine and monitored in the positive ion mode. To obtain the precursor and product ions, different cone voltage (10, 15, 20, 25, 30, and 35 V) or collision energy (10, 15, 20, 25, and 30 eV) in full scan or in daughter scan mode were tested.
Reagents
CT and CT-d7 were purchased from Toronto Research Chemicals (Toronto, ON, Canada), and 7-KC and 7-KC-d7 from Avanti Polar Lipids (Alabaster, AL). Formic acid, ammonium formate, butylhydroxytoluene (BHT), DMG, EDC, and DMAP were obtained from Sigma-Aldrich (St. Louis, MO). Methanol, acetonitrile, and hexane (LC/MS PAI grade) were obtained from Panreac (Barcelona, Spain), and chloroform from EMD Millipore Corporation (Madrid, Spain).
Selectivity
To know the interferences of the plasma matrix, five independent plasma samples with and without added internal standards were studied.
Linearity
A stock solution of 1 mg/ml of CT and 7-KC in methanol were prepared. Serial dilutions were prepared from a working solution (10 μg/ml) at concentrations of 3, 6, 12.5, 25, 50, 100, 200, 400, and 800 ng/ml with or without added plasma matrix. Standard samples were processed five times and analyzed as samples. Calibration curves were constructed by linear regression analysis of the ratio of CT/CT-d7 and 7-KC/7-KC-d7 areas to the corresponding concentrations.
Limit of detection and quantification
Successive dilutions of CT and 7-KC were measured to estimate the limit of detection (LOD) from a signal-to-noise (S/N) ratio of 3, and the limit of quantification (LOQ) from S/N ratio >10. All the analyses were performed in triplicate.
Imprecision and accuracy
The within-day imprecision (% coefficient of variation [% CV]) and accuracy were evaluated by performing 10 analyses of a spiked plasma matrix with standards at concentrations of 12.5, 100, and 800 ng/ml in the same day. To assess the between-day imprecision and accuracy, plasma matrix spiked with standards (12.5, 100, and 800 ng/ml) were processed in 10 independent preparations in different days. The accuracy was expressed as the percent relative error, calculated as mean concentration subtracting the theoretical concentration, divided by the theoretical amount, and multiplied by 100.
Recovery
Recovery was evaluated by adding known amounts of CT and 7-KC (25, 200, and 400 ng/ml) to a pooled plasma sample. All the analyses were performed in triplicate.
Sample stability
To asses sample stability, we used five different plasma samples from NPC patients. Two aliquots of each were prepared with and without the addition of BHT (0.1 mM). Freeze-thaw effects on CT and 7-KC were studied using three samples of plasma from NPC patients. Samples were frozen at −80°C and thawed at room temperature for ∼2 h. One aliquot of 50 μl was taken from each sample and analyzed, then the initial sample was refrozen at −80°C. This cycle was repeated six times to yield six freeze-thaw samples. Stability of the processed samples was also studied by reinjecting the derivatized extract once a day during 5 days stored both at room temperature and at −20°C.
Statistical analysis
Statistical analysis was performed with GraphPad Prism 6 software and PASW Statistics 18.0. The unpaired nonparametric Mann-Whitney U-test was used to evaluate the significance of differences among different age groups in controls (with the exception of those age-matched groups with size ≥30 samples, for which the Student’s t-test was used) and between patients and controls. Correlations between total cholesterol and CT and 7-KC levels were assessed using Spearman’s correlation analysis. The diagnostic capacity of plasma CT and 7-KC was assessed by 2 × 2 contingency table. Receiver-operating characteristic (ROC) curve was constructed, and the area under ROC curve (AUC) was calculated to evaluate the discriminatory capability of plasma CT and 7-KC. A P value <0.05 was considered statistically significant.
RESULTS
Method validation
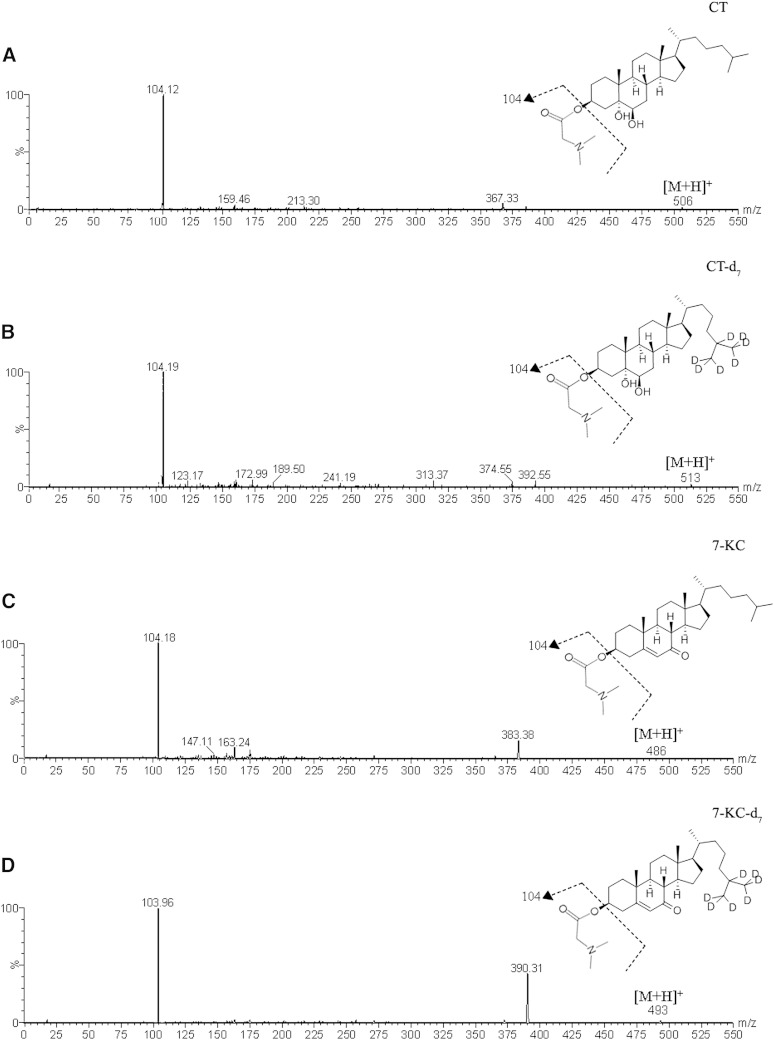
We could not validate the method proposed by Jiang et al. (9). We tried several MS/MS conditions, at different concentrations and several incubation times, but in none of them could we find any daughter ion of the bis-dimethylglycine derivative of CT reported by these authors. We therefore decided to monitor the mono-dimethylglycine derivative of CT, and it was very prominent, as was that for 7-KC and the corresponding deuterated internal standards. Consequently, we modified the methodology of Jiang et al. (9) and validated our own method. The precursor ion peaks of CT, CT-d7, 7-KC, and 7-KC-d7 at m/z 506, 513, 486, and 493 were identified, and the most important daughter for each one was at m/z 104 (Fig. 1). Accordingly, the corresponding MRM modes (m/z 506 > 104, 513 > 104, 486 > 104, 493 > 104) were monitored. The derivatization yield for both CT and 7-KC was constant at different times and at the three different concentrations (supplementary Fig. 1). Consequently, 1 h incubation was considered enough to achieve a good compromise between a complete derivatization and a reduction of the total time of sample preparation.
Fig. 1.
ESI-MS/MS product ion spectra from the corresponding precursor ions. A: CT (m/z 506). B: CT-d7 (m/z 513). C: 7-KC (m/z 486). D: 7-KC-d7 (m/z 493).
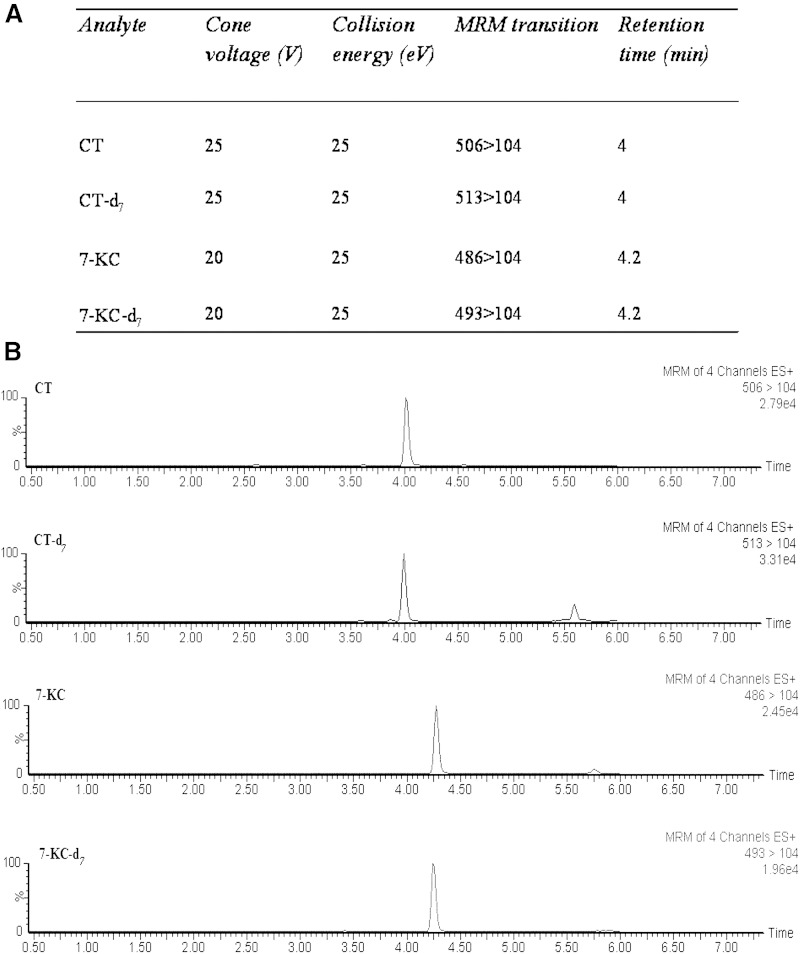
The optimized MS parameters in the MRM mode and the chromatographic separation of the four compounds are shown in Fig. 2. CT and CT-d7 eluted from the column at the retention time of 4 min, and 7-KC and KC-d7 at 4.2 min.
Fig. 2.
Optimized MS parameters in the MRM mode and chromatographic separation of CT and 7-KC. Optimized MS parameters for the detection of each oxysterol (A) and MRM extracted ion chromatograms of CT, CT-d7, 7-KC, and 7-KC-d7 (B).
The selectivity assay was performed both in methanol and plasma, and no significant interferences from endogenous substances at these retention times or at the corresponding MRM channels (supplementary Fig. 2A, B) were found. Interferences from the internal standards were also ruled out (supplementary Fig. 2C, D).
The calibration curve prepared in plasma matrix had similar slope and intercept as that prepared in methanol-water (Table 1). Consequently, plasma matrix was chosen in order to have the best similarity between standards and samples. The calibration curve from 3 to 800 ng/ml for CT and 7-KC showed a linear regression of r2 ≥ 0.9908. The LOD of the assay was 1 ng/ml, and the LOQ was 3 ng/ml for both compounds. The within-day and between-day imprecision was <6% for CT and <8% for 7-KC. The within-day and between-day accuracy was <9% and <6% for CT and <5% and <7% for 7-KC, respectively. The recovery was >91% for CT and >94% for 7-KC. All the validation data are shown in Table 2.
TABLE 1.
Calibration curves prepared both in methanol-water and plasma
| Matrix | Slope | Intercept | r2 |
| CT | |||
| Methanol-water | 0.0076 | 0.048 | 0.998 |
| Plasma | 0.0077 | 0.07 | 0.998 |
| 7-KC | |||
| Methanol-water | 0.0079 | 0.074 | 0.995 |
| Plasma | 0.0081 | 0.079 | 0.996 |
TABLE 2.
Validation results of CT and 7-KC by HPLC-ESI-MS/MS
| CT | 7-KC | |||
| Calibration curve, n = 5 | ||||
| Mean slope (range; SD) | 0.0080 (0.0074–0.0089; 0.0006) | 0.0044 (0.0037–0.0049; 0.0005) | ||
| Mean intercept (range; SD) | 0.39 (0.29–0.44; 0.061) | 0.10 (0.09–0.12; 0.013) | ||
| Mean coefficient of linear regression, r2 (range; SD) | 0.9908 (0.9830–0.999; 0.006) | 0.9926 (0.9817–0.9975; 0.0058) | ||
| Imprecision (% CV) | Accuracy (%) | Imprecision (% CV) | Accuracy (%) | |
| Within day, n = 10 | ||||
| 12.5 ng/ml | 5.5 | 3.8 | 5.5 | 0.3 |
| 100 ng/ml | 3.6 | 1.7 | 4.4 | 3.7 |
| 800 ng/ml | 2.4 | 8.9 | 3.9 | 4.4 |
| Between day, n = 10 | ||||
| 12.5 ng/ml | 5.8 | 1.5 | 5.8 | 4 |
| 100 ng/ml | 5.8 | 5.3 | 7.9 | 6.4 |
| 800 ng/ml | 2.9 | 0.5 | 7.7 | 5.4 |
| Recovery (%), n = 3 | ||||
| 25 ng/ml | 94.4 | 94 | ||
| 200 ng/ml | 98.2 | 107 | ||
| 400 ng/ml | 91.8 | 100.7 | ||
| LOD (ng/ml), n = 3 | 1 | 1 | ||
| LOQ (ng/ml), n = 3 | 3 | 3 | ||
To avoid autoxidation of cholesterol during sample preparation, we added the antioxidant BHT to the plasma samples of NPC patients, but the addition of this antioxidant did not show remarkable changes of both CT and 7-KC as compared with samples lacking BHT (supplementary Fig. 3A). NPC samples kept at −80°C under six freeze-thaw cycles were reasonably stable showing a % CV <6% and <11% for CT and 7-KC, respectively (supplementary Fig. 3B). The derivatized samples were stable at −20°C for 44 days (supplementary Fig. 3C), and they were stable at least during 5 days if maintained at room temperature (supplementary Fig. 3D).
Subjects’ study
Controls.
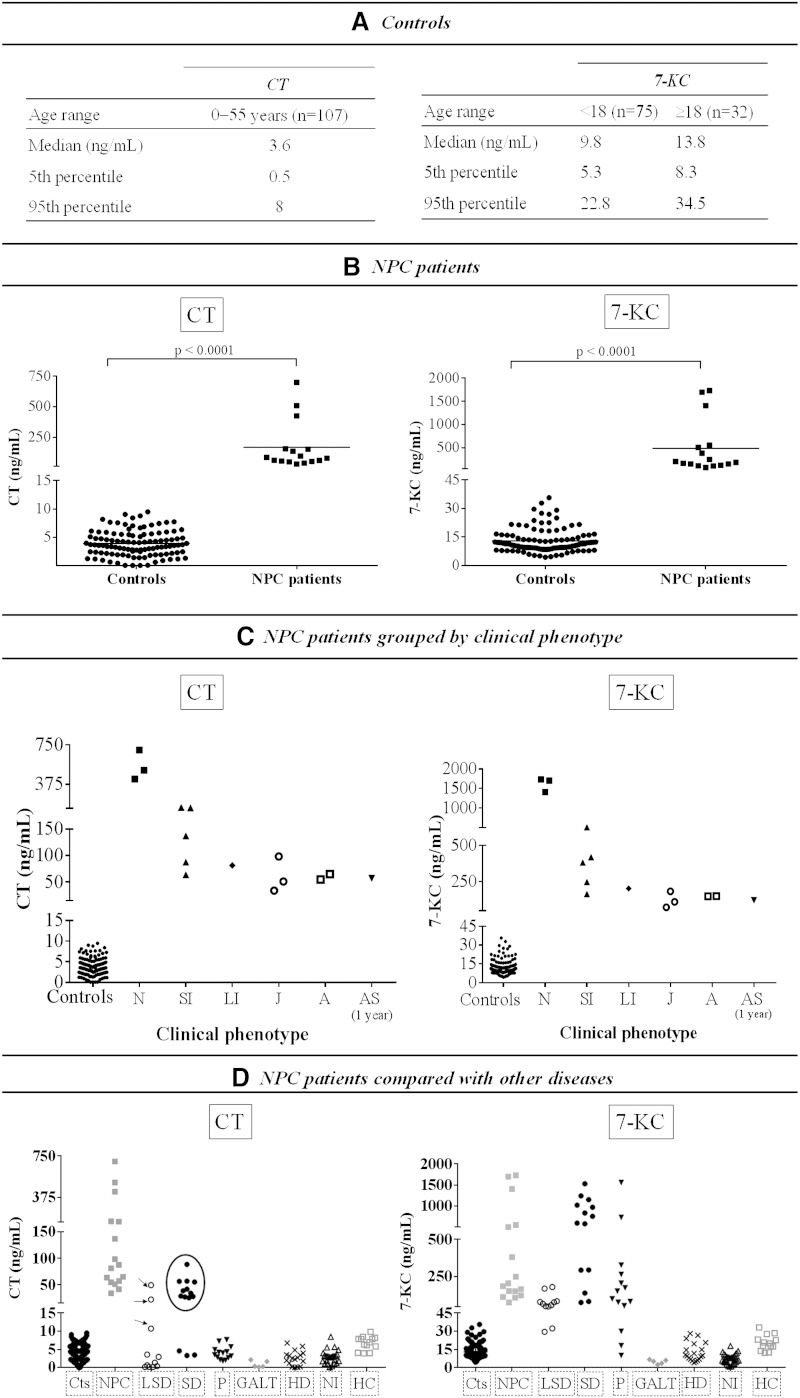
We studied 107 control samples from donors aged 1 day to 55 years (mean of age, 9.7 years). Concerning CT concentration, no significant differences with age were found (95% confidence interval [CI], 3.5–4.4 ng/ml), whereas 7-KC concentration was significantly lower in children than in the adult group (95% CI, 10.2–12.4 ng/ml vs. 14–19.5 ng/ml, respectively; P < 0.0001; Fig. 3A).
Fig. 3.
Plasma CT and 7-KC concentration in controls, NPC patients, and other diseases. A: CT and 7-KC concentration in controls: reference values by age range. B: CT and 7-KC in NPC patients. C: NPC patients grouped by clinical phenotype. D: NPC patients compared with other diseases: LSD [metachromatic leukodystrophy (n = 2), mucolipidosis II/III (n = 1), and LAL-deficient patients (n = 3)]; sterol disorders [CTX (n = 11) and SLO (n = 3)]; peroxisomal β-oxidation or biogenesis disorders (n = 14); galactosemia (n = 5); hepatic diseases (n = 19); patients with neurological involvement (n = 30); and patients with familial hypercholesterolemia (n = 13). Black arrow indicates LAL-deficient patients. Black circle indicates CTX patients. A, adult; AS, asymptomatic; Cts, controls; GALT, galactosemia; HC, hypercholesterolemia; HD, hepatic diseases; J, juvenile; LI, late infantile; N, neonatal; NI, neurological involvement; P, peroxisomal diseases; SD, sterol disorders; SI, severe infantile. Significant differences were considered when P < 0.05.
NPC patients.
Plasma samples of all patients showed significant differences for both CT and 7-KC compared with controls (95% CI for CT, 62–275 ng/ml vs. 3.5–4.4 ng/ml, P < 0.0001; 95% CI for 7-KC, 178–795 ng/ml vs. 11.8–14, P < 0.0001; Fig. 3B). A negative correlation between CT and 7-KC levels and the age of disease onset was observed (Fig. 3C). The highest levels of CT and 7-KC (>400 ng/ml and >1,400 ng/ml, respectively) were found in patient 1, patient 2, and patient 3 presenting the neonatal form of the disease (supplementary Table 1), followed by patients presenting the severe infantile form (CT range, 64–158 ng/ml, and 7-KC range, 164–505 ng/ml) (Fig. 3C). Patients with late infantile, juvenile, and adult forms present lower CT and 7-KC concentrations (range, 34–98 ng/ml and 73–203 ng/ml, respectively) (Fig. 3C; supplementary Table 1). All the patients but patients 11 and 13 presented the classical biochemical phenotype. Patients 11 and 13, with the juvenile form of the disease, presented a variant biochemical phenotype that was difficult to diagnose with the classical filipin staining, but CT and 7-KC concentrations were significantly higher (CT, 51 and 98 ng/ml; 7-KC, 111 and 183 ng/ml) compared with age-matched controls (supplementary Table 1). In addition, it is interesting to mention the case of one asymptomatic patient (patient 7) presenting the classical filipin staining and significantly high CT and 7-KC concentrations (CT, 57 ng/ml, and 7-KC, 122 ng/ml; supplementary Table 1).
Other LSDs.
With the aim to know the possible overlapping of both compounds with other LSDs we analyzed samples of Fabry, metachromatic leukodystrophy, mucolipidosis II/III, and LAL deficiency. Plasma CT concentration in these patients was in the control range except for patients with LAL deficiency showing CT levels overlapping with those observed in different clinical phenotypes of NPC except the neonatal form (10.7, 22.2, and 49.3 ng/ml, respectively); 7-KC was high in all LSDs tested (mean, 77.7 ng/ml; range, 29.6–178; Fig. 3D).
Peroxisomal disorders.
Patients with peroxisomal β-oxidation and biogenesis disorders showed normal CT, but some of the patients presented high levels of 7-KC (Fig. 3D).
Sterol disorders.
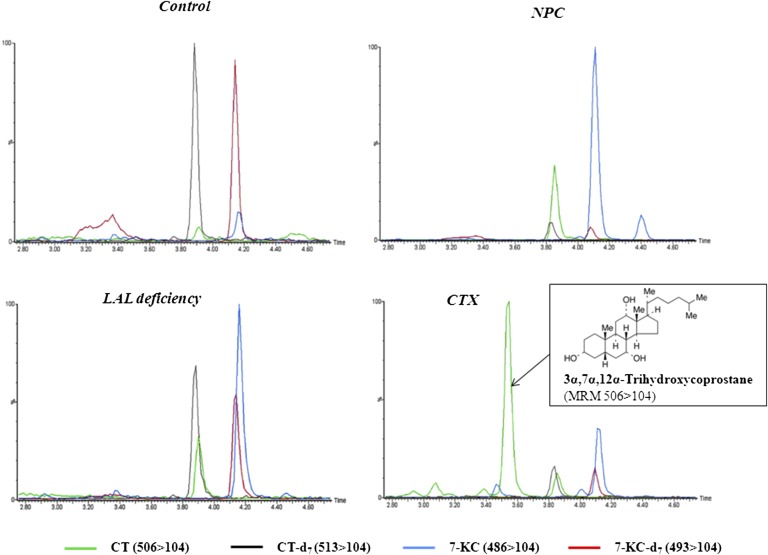
Patient with SLO syndrome showed normal CT, but the levels of 7-KC were high in all three patients (Fig. 3D). All CTX patients showed high levels of CT and 7-KC (CT mean, 43.7 ng/ml, and range, 25.4–88.6; 7-KC mean, 830 ng/ml, and range, 137–1,529) clearly overlapping with NPC patients (Fig. 3D). It is interesting to remark that a big peak eluting prior CT was detected in all CTX patients (Fig. 4). CT and this peak share the same molecular ion (m/z 506) and the same prominent daughter ion (m/z 104) (Fig. 4) and fit well with the triol (3α,7α,12α-trihydroxycoprostane) already described to be found in CTX (29). Interestingly, we have evaluated four CTX patients before and after treatment with chenodeoxy-cholic acid, and as expected, 3α,7α,12α-trihydroxycoprostane and 7-KC showed an impressive decrease, but the levels of CT remained unchanged (Table 3).
Fig. 4.
Plasma MRM of control, NPC, CTX, and LAL deficiency. MRM-extracted ion chromatograms of CT (506 > 104), CT-d7 (513 > 104), 7-KC (486 > 104), and 7-KC-d7 (493 > 104) in a control, LAL-deficient patient, NPC patient, and CTX patient. CTX patient showed a peak with the same 7-KC transition that eluted at 3.46 min (blue line) and is probably 7-α-hydroxycholest-4-en-3-one.
TABLE 3.
Levels of CT, 3α,7α,12α-trihydroxycoprostane, and 7-KC before and after chenodeoxy-cholic treatment in CTX patients
| CT | 3α,7α,12α-Trihydroxycoprostane | 7-KC | ||||
| CTX Patient | Pre-T | Post-T | Pre-T | Post-T | Pre-T | Post-T |
| CTX-1 | 28 | 35 | 1,251 | 24 | 596 | 38 |
| CTX-2 | 57 | 88 | 1,732 | 363 | 838 | 112 |
| CTX-3 | 57 | 47 | 3,101 | 29 | 1,021 | 51 |
| CTX-4 | 55 | 48 | 2,588 | 121 | 1,529 | 56 |
T, treatment. Values are expressed as ng/ml.
Patients with other disorders.
Patients with galactosemia, unspecific hepatic diseases, or different neurological affectation and patients with familial hypercholesterolemia all showed CT and 7-KC within the control range (Fig. 3D).
Correlation between total cholesterol and high levels of CT and 7-KC in plasma
Spearman’s correlation analysis including all the patients available (NPC, CTX, and LAL deficiency) was assessed and showed a moderate negative correlation between total cholesterol and CT (r = −0.679, P < 0.0001), and between total cholesterol and 7-KC (r = −0.687, P < 0.0001; supplementary Fig. 4).
Sensitivity and specificity
To assess the performance of plasma CT as a potential biomarker for patients with NPC, we calculated test sensitivity and specificity. The contingency table for the diagnostic capacity showed high sensitivity (100%) and an acceptable specificity (88.7%) (cutoff threshold of ≥8 ng/dl). These results indicate an absence of false negatives and a mild percentage of false positives (11.3%). ROC curve used to determine the discriminatory capability of CT between NPC patients and healthy subjects showed that plasma CT has a high detection capability (AUC = 0.992, P < 0.05). Concerning 7-KC, sensitivity and specificity were 100% and 79.8%, respectively (cutoff threshold of ≥22.8 ng/dl for age <18 years and ≥34.5 ng/ml for age ≥18 years), without a false-negatives percentage, but with a mild rate (20.2%) of false positives (AUC = 0.932, P < 0.05).
DISCUSSION
The first approach for the diagnosis of NPC is clinical suspicion, and the gold standard methodology for the biochemical diagnosis is filipin staining in cultured fibroblasts (1–3). However, this method is time consuming, and some forms of the disease present interpretation difficulties. Therefore, it is desirable to have the concurrence of other methods or other biomarkers to help the diagnosis of this disease. Recent studies have shown that plasma CT and 7-KC could be potential biomarkers for the diagnosis of NPC patients (8–13). We aimed to determine the sensitivity and specificity of these biomarkers for the diagnosis of NPC compared with patients suffering from other lysosomal, peroxisomal, or sterol disorders, or other patients with hepatic or neurological alterations, as well as patients with familial hypercholesterolemia.
To this aim, we initially followed the method described by Jiang et al. (9), but we could not validate it. We tried several MS/MS conditions, at different concentrations and several incubation times, but in none of them could we find appreciable amounts of the bis-dimethylglycine derivative of CT reported by these authors. On the contrary, we detected predominantly the mono-dimethylglycine derivative of CT. In agreement with our findings, Boenzi et al. (12) also showed that the bis-derivative of CT was not formed, although the derivatization reagent was different. Our method showed a good analytical performance with the capability to discriminate between patients and controls. We have demonstrated that CT monoderivative and 7-KC monoderivative are stable compounds in a wide range of concentrations and temperatures. Therefore, both compounds in their monoderivative form were good analytes to be used for the purpose of this study. In addition, HPLC-MS/MS with ESI is an instrument available in most laboratories, whereas APCI (9) is not. Therefore, our method and also the method described by Boenzi et al. (12) represent an advantage as they facilitate the general use of laboratories working in the diagnosis of inherited metabolic diseases. We would like to emphasize that we participate in the external quality control program “Special Assays in Serum” managed by ERNDIM (www.erndimqa.nl), which includes CT and 7-KC. The mean value obtained with the present method fits very well with the mean value obtained by other laboratories using other methods (data not shown).
Concerning NPC patients, a positive correlation between CT and 7-KC profile and the clinical severity of the disease was found. These results are in agreement with those reported by other authors (8, 14). The neonatal form presented with the highest levels of both oxysterols, whereas these levels were intermediate in the severe infantile form and were much lower in the late infantile, juvenile, and adult forms (Fig. 3C). Moreover, two patients with the variant biochemical phenotype (patients 11 and 13) included in our study showed high CT and 7-KC, close to the levels of patients with the classical phenotype. Consequently, the method appears useful to identify those cases with doubtful filipin staining, including the adult presentation and the asymptomatic form of the disease. These data are remarkable because few correlations between CT and 7-KC and the biochemical and clinical phenotypes have been previously published (14).
To determine the specificity of these biomarkers, we studied other diseases that might potentially have high levels of CT and 7-KC. In fact, high levels of 7-KC were found in all lysosomal diseases tested. These results are in agreement with a previous study showing elevated 7-KC in patients with Gaucher and infantile neuronal ceroid pipofuscinosis (8). However, CT was only high in patients with LAL deficiency, overlapping with the adult and the infantile form of NPC (Fig. 3D). These results support the data previously reported at the Society for the Study of Inborn Errors of Metabolism (SSIEM) meeting in one patient (30) with LAL deficiency and those reported many years ago (31, 32) showing accumulation of oxygenated esteryl esters, including 7-ketocholesteryl esters, 5,6-α-epoxycholesteryl esters, and 5,6-β-epoxycholesteryl esters in liver, spleen, and adrenal samples of patients with LAL deficiency. In addition, Amraoui et al. (30) and Klinke et al. (13) also reported an increase of CT and 7-KC in Niemann-Pick type A and B (NPA/NPB) patients. Therefore, levels of CT and 7-KC were higher than normal not only in NPC but also in other lysosomal lipidosis. The primary product of cholesterol autoxidation is cholesterol-5,6-epoxide (5,6-EC), which is then cleaved either enzymatically or nonenzymatically to give CT (15, 33–35). Moreover, a recent study suggested that CT would be a substrate for ABCG1 (36) involved in cellular export of oxysterols, sterols, phospholipids, and sphingomyelin, suggesting that ABCG1 would act as an oxysterol detoxification system removing CT from cells. All these findings could explain the high plasma levels of CT in these patients, which would result from intracellular 5,6-EC accumulation, their hydrolysis into CT, and their efflux to plasma via ABCG1. However, it remains to be known how 5,6-epoxycholesteryl esters could be transesterified.
Moreover, 7-KC was also high in patients with CTX and SLO syndrome, as has been previously described (37–39), but it is interesting to note that all CTX patients showed high plasma levels not only of 7-KC but also of CT (Fig. 3D), data not previously described, and a very big peak of another triol presumably of 3α,7α,12α-trihydroxycoprostane (Fig. 4). The MS/MS data of this compound fit very well with the identity of 3α,7α,12α-trihydroxycoprostane, in the metabolic pathway of cholic acid (40, 41); in addition, it is a substrate for sterol 27-hydroxylase activity that is deficient in CTX. On the other hand, this compound and 7-KC decreased dramatically after chenodeoxy-cholic treatment (Table 3), whereas CT remained unchanged. Therefore, it is quite clear that it is an intermediate in cholic acid biosynthesis, and the most likely possibility is 3α,7α,12α-trihydroxycoprostane. This peculiar profile could be useful to distinguish CTX from NPC and other lipidosis. Moreover, our results suggest that 3α,7α,12α-trihydroxycoprostane would be a good biomarker for treatment monitoring.
Other patients with liver disease including cholestasis, galactosemia, neurologic symptoms, or familial hypercholesterolemia showed CT and 7-KC within the control range, which therefore will not interfere with the diagnosis of the diseases previously mentioned.
In conclusion, we have demonstrated that CT is a sensitive but not a specific biomarker of NPC disease. Moreover, the present procedure is quick and sensitive for the diagnosis of all NPC variants. Based on this evidence, we have included this approach in the first-line diagnostic algorithm of NPC disease in our laboratory. Therefore, we expect this inclusion will be of help to uncover new patients with not only NPC but also other diseases such as CTX, SLO, LAL deficiency, or NPA/NPB.
Supplementary Material
Acknowledgments
The authors thank Dr. Gregori Casals and Dr. Nayra Rico from Servicio de Bioquímica y Genética Molecular, Hospital Clinic, and Dr. Rafael Artuch from Hospital Sant Joan de Deu who provided plasma samples from hypercholesterolemic patients.
Footnotes
Abbreviations:
- 7-KC
- 7-ketocholesterol
- 7-KC-d7
- 7-ketocholesterol-25,26,26,26,27,27,27-d7
- AUC
- area under receiver-operating characteristic curve
- BHT
- butylhydroxytoluene
- CI
- confidence interval
- CT
- cholestane-3β,5α,6β-triol
- CT-d7
- cholestane-3β,5α,6β-triol-25,26,26,26,27,27,27-d7
- CTX
- cerebrotendinous xanthomatosis
- % CV
- percentage coefficient of variation
- LAL
- lysosomal acid lipase
- LOD
- limit of detection
- LOQ
- limit of quantification
- LSD
- lysosomal storage disease
- MRM
- multiple reaction monitoring
- NPC
- Niemann-Pick type C
- ROC
- receiver-operating characteristic
- SLO
- Smith-Lemli-Opitz
This work was supported by DG-SANCO from the European Union (EU) (No. 308901, an EU rare diseases registry for Niemann-Pick disease type A, B, and C) and Fondo de Investigaciones Sanitarias Grant PI10/00936. A. Arias was supported by the CIBER of Rare Diseases (CIBERER). J. Macías-Vidal is a recipient of the Juan Girón Fellowship from the Fundación Niemann-Pick de España. This study was supported in part by Actelion Pharmaceuticals Spain. CIBERER and CIBEROBN are initiatives of Instituto de Salud Carlos III, Spain.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Vanier M. T. 2010. Niemann-Pick disease type C. Orphanet J. Rare Dis. 5: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patterson M. C., Hendriksz C. J., Walterfang M., Sedel F., Vanier M. T., Wijburg F.; NP-C Guidelines Working Group. 2012. Recommendations for the diagnosis and management of Niemann-Pick disease type C: an update. Mol. Genet. Metab. 106: 330–344. [DOI] [PubMed] [Google Scholar]
- 3.Vanier M. T. 2015. Complex lipid trafficking in Niemann-Pick disease type C. J. Inherit. Metab. Dis. 38: 187–199. [DOI] [PubMed] [Google Scholar]
- 4.Vanier M. T., Rodriguez-Lafrasse C., Rousson R., Gazzah N., Juge M. C., Pentchev P. G., Revol A., Louisot P. 1991. Type C Niemann-Pick disease: spectrum of phenotypic variation in disruption of intracellular LDL-derived cholesterol processing. Biochim. Biophys. Acta. 1096: 328–337. [DOI] [PubMed] [Google Scholar]
- 5.Tint G. S., Pentchev P., Xu G., Batta A. K., Shefer S., Salen G., Honda A. 1998. Cholesterol and oxygenated cholesterol concentrations are markedly elevated in peripheral tissue but not in brain from mice with the Niemann-Pick type C phenotype. J. Inherit. Metab. Dis. 21: 853–863. [DOI] [PubMed] [Google Scholar]
- 6.Reddy J. V., Ganley I. G., Pfeffer S. R. 2006. Clues to neuro-degeneration in Niemann-Pick type C disease from global gene expression profiling. PLoS One. 1: e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zampieri S., Mellon S. H., Butters T. D. 2009. Oxidative stress in NPC1 deficient cells: protective effect of allopregnanolone. J. Cell. Mol. Med. 13: 3786–3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porter F. D., Scherrer D. E., Lanier M. H., Langmade S. J., Molugu V., Gale S. E., Olzeski D., Sidhu R., Dietzen D. J., Fu R., et al. 2010. Cholesterol oxidation products are sensitive and specific blood-based biomarkers for Niemann-Pick C1 disease. Sci. Transl. Med. 2: 56ra81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang X., Sidhu R., Porter F. D., Yanjanin N. M., Speak A. O., te Vruchte D. T., Platt F. M., Fujiwara H., Scherrer D. E., Zhang J., et al. 2011. A sensitive and specific LC-MS/MS method for rapid diagnosis of Niemann-Pick C1 disease from human plasma. J. Lipid Res. 52: 1435–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin N., Zhang H., Qiu W., Ye J., Han L., Wang Y., Gu X. 2014. Determination of 7-ketocholesterol in plasma by LC-MS for rapid diagnosis of acid SMase-deficient Niemann-Pick disease. J. Lipid Res. 55: 338–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H., Wang Y., Lin N., Yang R., Qiu W., Han L., Ye J., Gu X. 2014. Diagnosis of Niemann-Pick disease type C with 7-ketocholesterol screening followed by NPC1/NPC2 gene mutation confirmation in Chinese patients. Orphanet J. Rare Dis. 9: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boenzi S., Deodato F., Taurisano R., Martinelli D., Verrigni D., Carrozzo R., Bertini E., Pastore A., Dionisi-Vici C., Johnson D. W. 2014. A new simple and rapid LC-ESI-MS/MS method for quantification of plasma oxysterols as dimethylaminobutyrate esters. Its successful use for the diagnosis of Niemann-Pick type C disease. Clin. Chim. Acta. 437: 93–100. [DOI] [PubMed] [Google Scholar]
- 13.Klinke G., Rohrbach M., Giugliani R., Burda P., Baumgartner M. R., Tran C., Gautschi M., Mathis D., Hersberger M. 2015. LC-MS/MS based assay and reference intervals in children and adolescents for oxysterols elevated in Niemann-Pick diseases. Clin Biochem. [DOI] [PubMed] [Google Scholar]
- 14.Stampfer M., Theiss S., Amraoui Y., Jiang X., Keller S., Ory D. S., Mengel E., Fischer C., Runz H. 2013. Niemann-Pick disease type C clinical database: cognitive and coordination deficits are early disease indicators. Orphanet J. Rare Dis. 8: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kudo K., Emmons G. T., Casserly E. W., Via D. P., Smith L. C., St Pyrek J., Schroepfer G. J., Jr 1989. Inhibitors of sterol synthesis. Chromatography of acetate derivatives of oxygenated sterols. J. Lipid Res. 30: 1097–1111. [PubMed] [Google Scholar]
- 16.Teng J. I., Smith L. L. 1995. High-performance liquid chromatographic analysis of human erythrocyte oxysterols as delta 4–3-ketone derivatives. J. Chromatogr. A. 691: 247–254. [DOI] [PubMed] [Google Scholar]
- 17.Dzeletovic S., Breuer O., Lund E., Diczfalusy U. 1995. Determination of cholesterol oxidation products in human plasma by isotope dilution-mass spectrometry. Anal. Biochem. 225: 73–80. [DOI] [PubMed] [Google Scholar]
- 18.Schroepfer G. J., Jr 2000. Oxysterols: modulators of cholesterol metabolism and other processes. Physiol. Rev. 80: 361–554. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Z., Li D., Blanchard D. E., Lear S. R., Erickson S. K., Spencer T. A. 2001. Key regulatory oxysterols in liver: analysis as delta4–3-ketone derivatives by HPLC and response to physiological perturbations. J. Lipid Res. 42: 649–658. [PubMed] [Google Scholar]
- 20.Griffiths W. J., Wang Y., Alvelius G., Liu S., Bodin K., Sjövall J. 2006. Analysis of oxysterols by electrospray tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 17: 341–362. [DOI] [PubMed] [Google Scholar]
- 21.Jiang X., Ory D. S., Han X. 2007. Characterization of oxysterols by electrospray ionization tandem mass spectrometry after one-step derivatization with dimethylglycine. Rapid Commun. Mass Spectrom. 21: 141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Honda A., Yamashita K., Miyazaki H., Shirai M., Ikegami T., Xu G., Numazawa M., Hara T., Matsuzaki Y. 2008. Highly sensitive analysis of sterol profiles in human serum by LC-ESI-MS/MS. J. Lipid Res. 49: 2063–2073. [DOI] [PubMed] [Google Scholar]
- 23.Matysik S., Schmitz G. 2013. Application of gas chromatography-triple quadrupole mass spectrometry to the determination of sterol components in biological samples in consideration of the ionization mode. Biochimie. 95: 489–495. [DOI] [PubMed] [Google Scholar]
- 24.Griffiths W. J., Crick P. J., Wang Y. 2013. Methods for oxysterol analysis: past, present and future. Biochem. Pharmacol. 86: 3–14. [DOI] [PubMed] [Google Scholar]
- 25.Macías J. 2012. Molecular Aspects of Both Lysosomal Transport Diseases: Cystinosis and Niemann-Pick Disease Type. MS Thesis. University of Barcelona, Barcelona, Spain.
- 26.Macías-Vidal J., Rodríguez-Pascau L., Sánchez-Ollé G., Lluch M., Vilageliu L., Grinberg D., Coll M. J.; Spanish NPC Working Group. 2011. Molecular analysis of 30 Niemann-Pick type C patients from Spain. Clin. Genet. 80: 39–49. [DOI] [PubMed] [Google Scholar]
- 27.Macías-Vidal J., Gort L., Lluch M., Pineda M., Coll M. J. 2009. Nonsense-mediated mRNA decay process in nine alleles of Niemann-Pick type C patients from Spain. Mol. Genet. Metab. 97: 60–64. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez-Valero E. M., Ballart A., Iturriaga C., Lluch M., Macias J., Vanier M. T., Pineda M., Coll M. J. 2005. Identification of 25 new mutations in 40 unrelated Spanish Niemann -Pick type C patients: genotype-phenotype correlations. Clin. Genet. 68: 245–254. [DOI] [PubMed] [Google Scholar]
- 29.Oftebro H., Björkhem I., Skrede S., Schreiner A., Pederson J. I. 1980. Cerebrotendinous xanthomatosis: a defect in mitochondrial 26-hydroxylation required for normal biosynthesis of cholic acid. J. Clin. Invest. 65: 1418–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amraoui Y., Mengel E., Hennermann J. B. 2014. Oxysterols in Niemann-Pick type C: limitations of sensitivity and specificity (Abstract). J. Inherit. Metab. Dis. 37 (Suppl. 1): S150. [Google Scholar]
- 31.Assmann G., Fredrickson D. S., Sloan H. R., Fales H. M., Highet R. J. 1975. Accumulation of oxygenated steryl esters in Wolman’s disease. J. Lipid Res. 16: 28–38. [PubMed] [Google Scholar]
- 32.Fitoussi G., Nègre-Salvayre A., Pieraggi M. T., Salvayre R. 1994. New pathogenetic hypothesis for Wolman disease: possible role of oxidized low-density lipoproteins in adrenal necrosis and calcification. Biochem. J. 301: 267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Björkhem I. 2013. Five decades with oxysterols. Biochimie. 95: 448–454. [DOI] [PubMed] [Google Scholar]
- 34.Sevanian A., McLeod L. L. 1986. Catalytic properties and inhibition of hepatic cholesterol-epoxide hydrolase. J. Biol. Chem. 261: 54–59. [PubMed] [Google Scholar]
- 35.Silvente-Poirot S., Poirot M. 2012. Cholesterol epoxide hydrolase and cancer. Curr. Opin. Pharmacol. 12: 696–703. [DOI] [PubMed] [Google Scholar]
- 36.Engel T., Fobker M., Buchmann J., Kannenberg F., Rust S., Nofer J. R., Schürmann A., Seedorf U. 2014. 3β,5α,6β-Cholestanetriol and 25-hydroxycholesterol accumulate in ATP-binding cassette transporter G1 (ABCG1)-deficiency. Atherosclerosis. 235: 122–129. [DOI] [PubMed] [Google Scholar]
- 37.Björkhem I. 1986. Assay of unesterified 7-oxocholesterol in human serum by isotope dilution-mass spectrometry. Anal. Biochem. 154: 497–501. [DOI] [PubMed] [Google Scholar]
- 38.Burnett J. R., Moses E. A., Croft K. D., Brown A. J., Grainger K., Vasikaran S. D., Leitersdorf E., Watts G. F. 2001. Clinical and biochemical features, molecular diagnosis and long-term management of a case of cerebrotendinous xanthomatosis. Clin. Chim. Acta. 306: 63–69. [DOI] [PubMed] [Google Scholar]
- 39.Björkhem I., Diczfalusy U., Lövgren-Sandblom A., Starck L., Jonsson M., Tallman K., Schirmer H., Ousager L. B., Crick P. J., Wang Y., et al. 2014. On the formation of 7-ketocholesterol from 7-dehydrocholesterol in patients with CTX and SLO. J. Lipid Res. 55: 1165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Björkhem I., Oftebro H., Skrede S., Pedersen J. I. 1981. Assay of intermediates in bile acid biosynthesis using isotope dilution–mass spectrometry: hepatic levels in the normal state and in cerebrotendinous xanthomatosis. J. Lipid Res. 22: 191–200. [PubMed] [Google Scholar]
- 41.Honda A., Salen G., Matsuzaki Y., Batta A. K., Xu G., Leitersdorf E., Tint G. S., Erickson S. K., Tanaka N., Shefer S. 2001. Differences in hepatic levels of intermediates in bile acid biosynthesis between Cyp27(−/−) mice and CTX. J. Lipid Res. 42: 291–300. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.