Abstract
Glucocorticoids are widely used anti-inflammatory medication in diseases like asthma and chronic obstructive pulmonary disease. Glucocorticoids can either activate (transactivation) or inhibit (transrepression) transcription. RU24858 was introduced as a “dissociated” glucocorticoid and it has been reported to transrepress but not to transactivate. The aim of this study was to compare the effects of RU24858 and dexamethasone in human neutrophils. RU24858 delayed spontaneous neutrophil apoptosis and further enhanced GM-CSF- induced neutrophil survival to a similar extent as dexamethasone. Like dexamethasone RU24858 also reduced CXCL8 and MIP-1α. Unexpectedly however, RU24858 increased the expression of the glucocorticoid-inducible genes BLT-1, Annexin-1 and Grb-2 in neutrophils to a similar level as seen with dexamethasone. We have shown here that dexamethasone and RU24858 both increase Grb-2, BLT1 and Annexin-1 expression and inhibit CXCL8 and MIP-1α production. This suggests that RU24858 was not able to dissociate between transactivation and transrepression in human neutrophils but enhanced neutrophil survival.
Keywords: neutrophils, apoptosis, glucocorticoids, transrepression, transactivation, dexamethasone
Introduction
Neutrophils have an important role in the pathogenesis of chronic obstructive pulmonary disease (COPD), severe asthma, rheumatoid arthritis and many other inflammatory diseases.1–3 Glucocorticoids are widely used to treat many inflammatory conditions such as COPD, asthma and rheumatoid arthritis.4 Glucocorticoids act by entering the cell and binding to the glucocorticoid receptor (GR) in the cytoplasm leading to GR activation and nuclear import.4 Inside the nucleus the glucocorticoid-GR complex can induce gene transcription (transactivation) by binding to specific DNA sequences called glucocorticoid response elements (GREs) in the promoter region of steroid responsive genes. The glucocorticoid-GR complex can also inhibit pro-inflammatory transcription factors such as nuclear factor-κB (NF-κB) and activator protein (AP)-1. This interaction results in down-regulation of proinflammatory gene transcription (transrepression).4,5
Glucocorticoids which only have the ability to transrepress but not transactivate, known as dissociated steroids, have been developed based on the hypothesis that the metabolic side-effects of glucocorticoids such as regulation of calcium and glucose metabolism are due to transactivation but the anti-inflammatory effects derive from transrepression.6,7 RU24858 is one such compound and in human and murine cell lines the transrepression capability of RU24858 was shown to be almost comparable to dexamethasone. In contrast to dexamethasone, RU24858 showed almost nonexistent transactivation potency.67,8 Surprisingly when studying in vivo a rat model of lung inflammation, Belvisi et al9 could not see any dissociation between the anti-inflammatory activity and metabolic side effects of RU24858. Furthermore, RU24858 did not dissociate between transactivation and transre-pression in primary human eosinophils.10
Glucocorticoids can suppress inflammation in part via modulation of inflammatory cell apoptosis.11 Apoptosis is a form of programmed cell death and it has been shown to be important in the resolution of pulmonary inflammation.2,12 Dibbert et al13 reported that neutrophil apoptosis is delayed in inflammatory lung diseases such as cystic fibrosis, pneumonia and idiopathic fibrosis. Apoptosis is characterized by shrinkage of the cells, nuclear condensation and formation of apoptotic bodies. These apoptotic cells are then phagocytosed intact by macrophages. This allows the cell death to occur with no leakage of intracellular contents and no accompanying inflammation.12,14 In contrast to the induction of eosinophil15 and lymphocyte16 apoptosis, glucocorticoids decrease neutrophil apoptosis.17–19 This may account for the increased neutrophilia seen in severe asthmatics treated with high doses of glucocorticoids.20,21 If neutrophilia in severe asthma is an iatrogenic phenomenon due to inhibition of neutrophil apoptosis by glucocorticoids, a glucocorticoid able to suppress inflammation, but devoid of ability to suppress neutrophil apoptosis could potentially be beneficial in the treatment of these patients. However, there are no published data as to whether transactivation and/or transrepression events play a role in the regulation of apoptosis in human neutrophils. A gluco-corticoid differentiating between transactivation and transrepression in neutrophils would be an excellent tool to address this question.
The aim of this study was to assess the functional consequences of dexamethasone and RU24858 on protein expression in neutrophil as well as in neutrophil apoptosis. We have shown here that dexam-ethasone and RU24858 both induce BLT1, Annexin 1 and Grb-2 expression as well as inhibit CXCL8 and MIP-1α production. Both steroids also inhibit spontaneous neutrophil apoptosis.
Methods
Neutrophil isolation
Neutrophils were isolated under sterile conditions as previously reported.18,19 After obtaining an informed consent to a study protocol accepted by the ethical committee of Tampere University Hospital, venous blood (50 mL) was collected from normal individuals into 10–20 mL of acid citrate dextrose anticoagulant and hydroxyethyl starch solution. White blood cells were obtained from the upper layer and were overlaid onto Ficoll and centrifuged at 700 g for 30 min at 20°C. The mononuclear cell layer was removed and the remaining pellet containing granulocytes and red blood cells was washed in HBSS (Hank’s Balanced Salt Solution without Phenol Red). Red blood cells were lysed by hypotonic lysis. Neutrophils were washed with RPMI 1640 (2% fetal calf serum and 5 mM EDTA), stained with Kimura and counted using microscopy. Only neutrophil populations with purity of >99% were used accepted for experiments. Cells were incubated in the presence and absence of RU24858 or dexamethasone. Both steroids were dissolved in DMSO. The final concentration of DMSO in the cells was 0.1% and the same concentration of DMSO was used in control experiments.
Flow cytometry
Unless otherwise stated the percentage of apoptotic cells was measured using a relative DNA fragmentation assay in propidium iodide stained cells by flow cytometry as previously described18,19 after culture for 16 h. The cells showing decreased relative DNA content were considered as apoptotic.
To determine BLT1 and Annexin-1 expression, neutrophils were cultured for 20 h in the presence or absence of RU24858 or dexamethasone. BLT1 and Annexin-1 expression was determined by using anti-BLT1-PE or anti-Annexin-1 antibodies with the corresponding PE labelled anti-mouse secondary antibody. For comparison an isotype control antibody was used. Briefly, after culture cells were washed and incubated for 30 min with primary antibody on ice. After incubation cells were washed 3 times. Anti-Annexin-1 labelled cells were further labelled with 1:100 secondary antibody for 30 min on ice. After incubation cells were washed once more with PBS containing 1% BSA and analysed by flow cytometry.
For determination of Grb-2 expression, neutrophils were cultured for 6 h in the presence or absence of RU24858 or dexamethasone. Grb-2 expression was determined by using anti-Grb-2 antibody. A PE-conjugated anti-mouse IgG secondary antibody was used for detection and an isotype-control antibody used to confirm specificity. Briefly, after culture cells were centrifuged and incubated in PBS containing 0.25% formaldehyde for 30 min at room temperature. After incubation 1 mL PBS with 10% fetal calf serum was added and cells were centrifuged. Cells were then incubated for 10 min at 4°C in 200 μL PBS with 10% fetal calf serum and 0.025% saponin. Thereafter the cells were centrifuged (400 g for 5 min) and suspended in PBS solution containing 10% fetal calf serum and 0.025% saponin and the antibody (1:100). After 1 h incubation samples were washed twice with PBS containing 10% fetal calf serum and 0.025% saponin and finally the secondary antibody was added and cells were incubated for 1 h. After incubation cells were once more washed with PBS containing 10% fetal calf serum and analysed with flow cytometry.
Cytokine measurement
Neutrophils were cultured in the presence or absence of a range of concentrations of RU24858 or dexamethasone (both 0.01–1 μM) and the appropriate stimulus. After culture for 18 h supernatants were collected and stored at −20°C. Cytokines were measured by ELISA-based assays.
Morphological analysis
Cells were centrifuged onto cytospin slides. After fixation in methanol slides were stained with May-Grünwald-Giemsa. Cells showing typical features of apoptosis such as condensation of chromatin, nuclear coalescence and shrinkage of the cell were considered as apoptotic.
Materials
RU24858 was obtained from Aventis Pharma, Romainville Cedex, France. Dexamethasone and propidium iodide and anti-Grb-2 antibody and PE-conjugated secondary antibody were purchased from Sigma Chemical Co. (St. Louis, MO). Anti-BLT1-PE, anti-Annexin-1 and PE-conjugated Rat anti-mouse secondary were purchased from BD Biosciences (Franklin Lakes, NJ). Other reagents were obtained as follows: antibiotics, fetal calf serum, RPMI 1640 (Gibco BRL, Paisley, Scotland, UK), GM-CSF and DuoSet ELISA Development System for IL-8/CXCL8 and MIP-1α (R&D system Europe, Abingdon, UK), May-Grünwald (Merck, Darmstadt, Germany), and Giemsa (J.T. Baker, Deventer, Holland).
Statistics
Data is expressed as mean ± SEM. Differences were analyzed by analysis of variance supported by Student-Newman-Keuls test and they were considered significant when P < 0.05.
Results
Effects of dexamethasone and RU24858 on spontaneous neutrophil survival
Glucocorticoids such as dexamethasone are known to enhance neutrophil survival by inhibiting apoptosis.18,22,23 As expected dexamethasone inhibited constitutive neutrophil apoptosis (Fig. 1; Table 1). RU24858 (0.1–1 μM) also enhanced spontaneous neutrophil survival in a concentration-dependent manner by inhibiting apoptosis (Fig. 1). Analysis of morphological features of apoptosis also confirmed that dexamethasone and RU24858 (both at 1 μM) inhibit spontaneous neutrophil apoptosis to a similar extent (Table 2). No statistically significant differences between corresponding concentrations of RU24858 and Dexamethasone could be seen.
Figure 1.
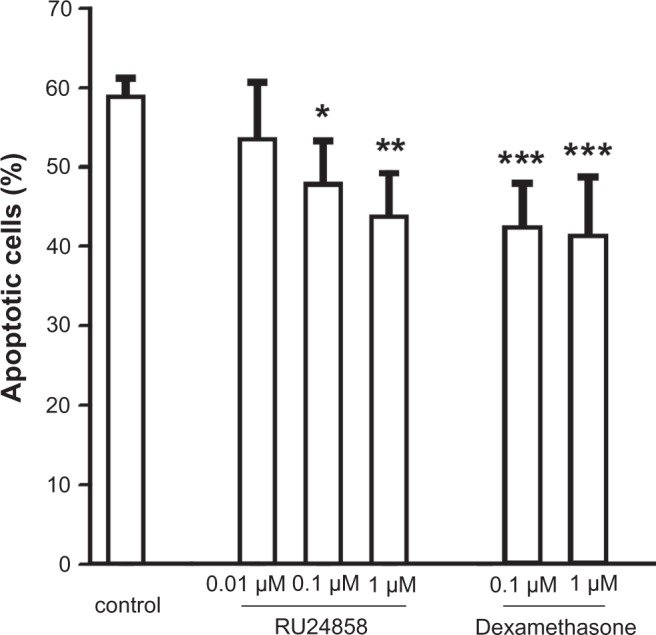
The effects of RU24858 and dexamethasone on spontaneous neutrophil apoptosis. Apoptosis was determined by the relative DNA fragmentation assay and flow cytometry in propidium iodide-stainded neutrophils.
Notes: Values are the mean ± S.E.M., n = 7. Results are expressed as percentage of apoptotic cells. *Indicates P < 0.05, **P < 0.01, ***P < 0.001 as compared with the respective control in the absence of glucocorticoids.
Table 1.
The effects of dexamethasone and RU24858 on apoptosis of human neutrophils in the presence and absence of GM-CSF as analyzed by the relative DNA fragmentation assay.
| Apoptotic neutrophils %
|
||||
|---|---|---|---|---|
| Control | GM-CSF (7 pM) | GM-CSF (70 pM) | GM-CSF (700 pM) | |
| Control | 58.9 ± 2.3 | 38.1 ± 4.1 | 35.8 ± 4.1 | 33.0 ± 3.3 |
| RU24858 (1 μM) | 43.8 ± 5.4** | 24.5 ± 2.8* | 24.0 ±1.8** | 26.3 ± 2.3** |
| Dexamethasone (1 μM) | 41.2 ± 7.3*** | 29.5 ± 5.8 | 26.8 ± 3.4* | 27.1 ± 4.9* |
Notes: Apoptosis was analyzed by the relative DNA fragmentation assay. Results are expressed as percentage of apoptotic neutrophils. Values are the mean ± S.E.M., n = 7.
Indicates P < 0.05,
P < 0.01,
P < 0.001 as compared with the respective control in the absence of glucocorticoids.
Table 2.
The effects of dexamethasone and RU24858 on apoptosis of human neutrophils in the presence and absence of GM-CSF as analyzed by morphological criteria.
| Apoptotic neutrophils %
|
|||
|---|---|---|---|
| Control | GM-CSF (70 pM) | GM-CSF (700 pM) | |
| Control | 50.5 ± 6.2 | 30.4 ± 3.7 | 26.5 ± 2.8 |
| RU24858 (1 μM) | 28.8 ± 4.6*** | 17.2 ± 3.0** | 16.5 ± 2.3** |
| Dexamethasone (1 μM) | 25.4 ± 3.3*** | 13.8 ± 1.8** | 14.5 ± 2.2** |
Notes: Apoptosis was analyzed by morphological criteria. All results are expressed as percentage of apoptotic neutrophils. Values are the mean ± S.E.M., n = 6
Indicates P < 0.05,
P < 0.01,
P < 0.001 as compared with the respective control in the absence of glucocorticoids.
Effects of dexamethasone and RU24858 on GM-CSF-afforded neutrophil survival
As expected, dexamethasone further enhanced the survival of GM-CSF treated human neutrophils as evidenced by reduced number of cells showing hypo-diploid DNA content (Table 1) or morphological features of apoptosis (Table 2). RU24858 had a similar effect on neutrophil apoptosis as dexamethasone (Tables 1 and 2). RU24858 further enhanced GM-CSF-induced neutrophil survival independently of the concentration of GM-CSF (7–700 pM) (Table 1). Similar results were obtained using morphological signs of apoptosis as a readout (Table 2). No statistically significant differences between corresponding concentrations of RU24858 and Dexamethasone were observed. Taken together, the effect of RU24858 on spontaneous human neutrophil apoptosis and GM-CSF-induced neutrophil survival is similar to that of the classical glucocorticoid dexamethasone.
Effects of dexamethasone and RU24858 on cytokine production
Glucocorticoids have previously been reported to inhibit the NF-κB regulated CXCL8 and MIP-1α expression in neutrophils24,25 and therefore we compared the effects of dexamethasone and RU24858 on the production of those inflammatory factors. Spontaneous CXCL8 and MIP-1α production was low and LPS (100 ng/mL) and TNF-α (1 ng/mL) increased CXCL8 (Fig. 2A and B) and MIP-1α (Fig. 3A and B) production 6–20-fold. Both dexamethasone (0.1–1 μM) and RU24858 (0.1–1 μM) inhibited CXCL8 (Fig. 2A and B) and MIP-1α (Fig. 3A and B) generation in a concentration-dependent manner and there were no statistically significant differences between corresponding concentrations of RU24858 and Dexamethasone.
Figure 2.
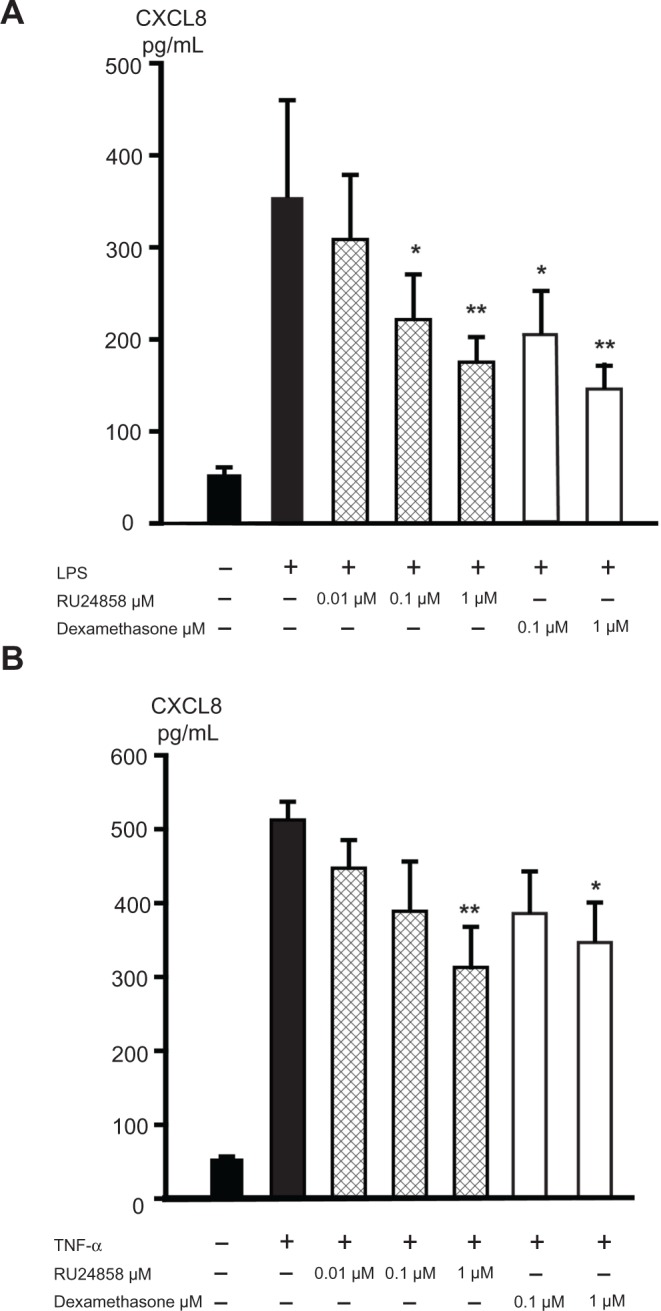
The effects of RU24858 and dexamethasone on CXCL8 production in human neutrophils. LPS (100 ng/mL; A) and TNF-α (1 ng/mL; B) were used to stimulate CXCL8 production. CXCL8 production was analyzed by ELISA.
Notes: Values are the mean ± S.E.M., n = 6. Results: *Indicates P < 0.05, **P < 0.01 and ***P < 0.001 as compared with the respective control in the presence of LPS or TNF-α but in the absence of glucocorticoids.
Figure 3.
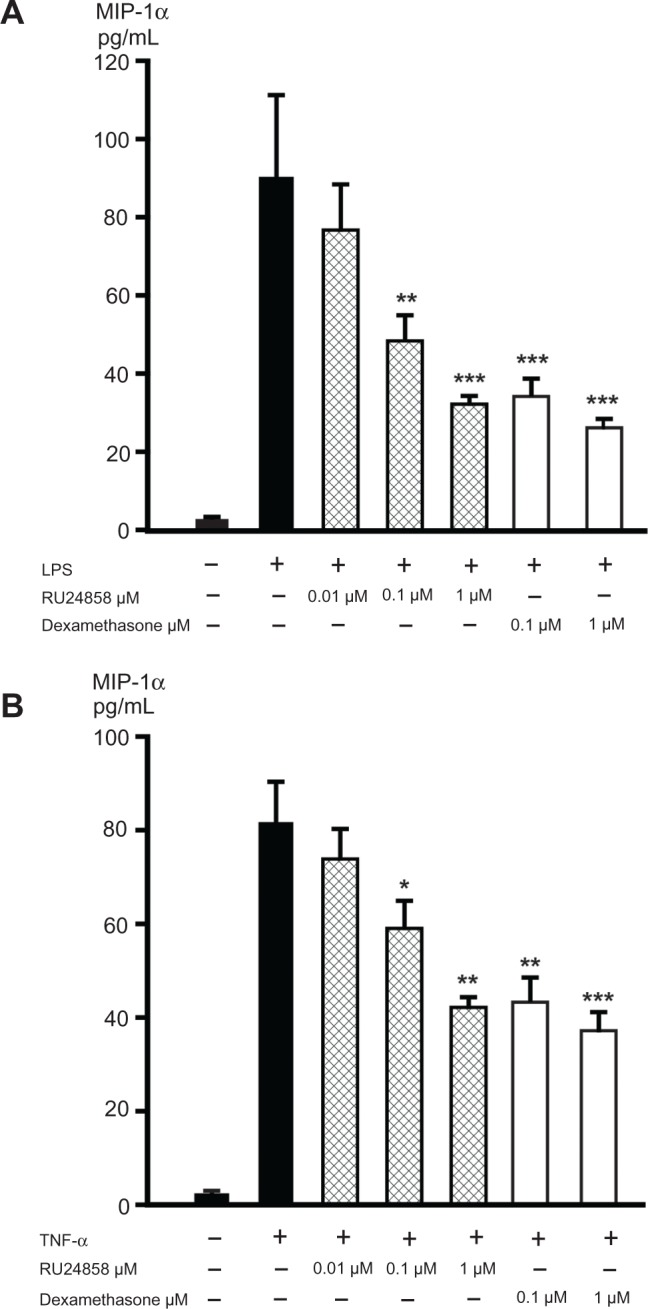
The effects of RU24858 and dexamethasone on MIP-1α production in human neutrophils. LPS (100 ng/mL; A) and TNF-α (1 ng/mL; B) were used to stimulate MIP-1α production in cells. MIP-1α production was analyzed by ELISA.
Notes: Values are the mean ± S.E.M., n = 6. Results; *Indicates P < 0.05, **P < 0.01 and ***P < 0.001 as compared with the respective control in the presence of LPS or TNF-α but in the absence of glucocorticoids.
Effects of dexamethasone and RU24858 on Leukotriene B4 receptor 1 (BLT-1), Annexin-1 and Growth Factor receptor bound protein 2 expression
BLT-1 and Annexin-1 expression is upregulated by GR via a GRE-mediated mechanism in human neutrophils.26,27 Both dexamethasone (1 μM) and RU24858 (1 μM) both enhanced both BLT-1 and Annexin-1 expression (Fig. 4A and B). We also measured Grb-2 expression. Grb-2 is an adaptor protein involved in signal transduction and glucocorticoids have been shown to reduce its recruitment to receptor complexes by an indirect mechanism.28 Again both dexamethasone (1 μM) and RU24858 (1 μM) enhanced Grb-2 expression (Fig. 4C). Differences between corresponding concentrations of RU24858 and Dexamethasone were not statistically significant. To exclude the possibility that RU24858 affects the fluorescence properties of the FITC-conjugated secondary antibody, its effects were studied on cells labelled with a FITC-conjugated isotype control antibody only. RU24858 (0.01 μM–1 μM) and dexamethasone (1 μM) had no effect on the fluorescence of isotype control (n = 6, data not shown).
Figure 4.
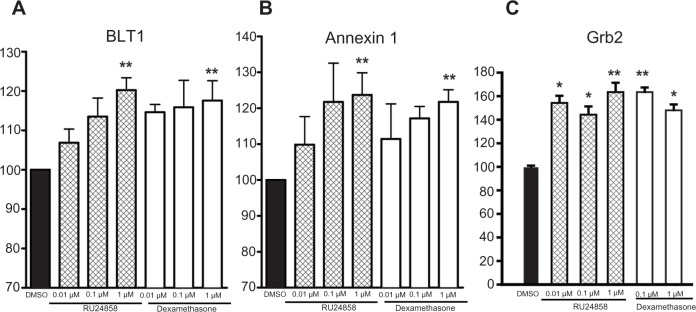
The effects of RU24858 and dexamethasone on BLT-1 (A) and Annexin-1 (B) and Grb-2 (C) expression in human neutrophils. Cells were stained and analyzed by using flow cytometry.
Notes: Results are shown as mean fluorescence intensity following treatment of cells with dexamethasone (0.01–1 μM) and RU24858 (0.01–1 μM). Values are the mean ± S.E.M., n = 4 (A and B) or n = 6 (C). *Indicates P < 0.05, **Indicates P < 0.01 as compared with the respective control in the absence of glucocorticoids.
Discussion
We report here the effects of the putative “dissociated steroid” RU24858 in primary human neutrophils. Our results show that the dissociated glucocorticoid RU24858 induces constitutive neutrophil survival as well as GM-CSF-induced neutrophil survival. RU24858 acts as a transrepressor in human neutro-phils but in contrast to our expectations, RU24858 had a similar ability as dexamethasone to induced transactivation in human neutrophils as evidenced by increased BLT-1, Annexin-1 and Grb-2 expression. Thus, the present results suggest that RU24858 does not act as a dissociated steroid in primary human neutrophils.
Many of the anti-inflammatory effects of glucocorticoids are believed to be due to inhibition of activated pro-inflammatory transcription factors, whereas the metabolic and other adverse effects may derive from transactivation via GREs. It was hypothesised, therefore, that it would be possible to separate positive anti-inflammatory effects from the side effects of glucocorticoids, by developing novel compounds which could transrepress but not transactivate. RU24858 is such a dissociated gluco-corticoid. In human and murine cells, RU24858 is almost as effective as dexamethasone in inducing tran-srepression but showed little or no transactivation.6,7 In this study, as expected, we demonstrated that both dexamethasone and RU24858 repressed CXCL8 and MIP-1α production to a similar extent indicating that RU24858 possess a transrepression capacity.
The transactivation profile of RU24858 in rat HTC cells, murine L929 fibroblast, human THP 1, Hela and A549 cells has been reported to be almost negligible.6,7,29 However, an equivalent transactivation activity as compared with prednisolone was reported in the African green monkey kidney fibroblast cell line, CV-1.8 Furthermore, no dissociation between anti-inflammatory activity and side effects was reported in in vivo animal models.9 Therefore, the activity profile of RU24858 has been proposed to be divergent and with some species and cell selectivity. Only one study to date has reported the activity profile of RU24858 in primary human cells and this reports that RU24858 is not dissociated in primary human eosinophils.10 In the present study we demonstrate that RU24858 also does not dissociate between transrepression and transactivation in human neutro-phils supporting the evidence in in vivo animal models and primary human eosinophils. Taken together, these results suggest that studies in transfected cell lines can lead to anomalous conclusions. This may be due to abnormal expression and or integration of key receptors and GR- and inflammation-associated signalling pathways.30
In human eosinophils RU24858 was less potent than dexamethasone and mometasone in suppressing cytokine production and inducing apoptosis.10 In contrast, RU24848 had a similar efficacy to dexamethasone in repressing transcription in mouse macrophages.31 Furthermore, RU24858 inhibited the expression of tristetraprolin at the level of mRNA and protein expression in J774 mouse macrophages at concentrations used in the present study.32 In the present study dexamethasone and RU2485 suppressed cytokine production in human neutrophils to a similar extent. A possible explanation for the differences in potency and the divergent dissociation abilities of this compound is that RU24858 works as a partial agonist at GR. In cells where the numbers of receptors or signal transduction efficiency is high it works as a full agonist. In contrast, in cells where the number of receptors is low or the efficiency of signal transduction is poor it may produce a maximal response that is less than that of the full agonist or it may even be an antagonist. Use of cells from different species may also lead to variability in results, as different species demonstrate divergent sensitivities to glucocorticoids.
Post-transcriptional mechanisms as well as interactions and synergy with other transcription factors can affect the transactivation and transrepression profile of glucocorticoids, giving a possible reason for noncanonical effects.33 Indeed Chivers et al29 showed a RU24858 unable to induce classical GRE mediated transactivation while being able to activate non-traditional GC mediated gene expression. To be able to asses GRE- mediated traditional activation of transcription we studied Annexin-1 and BLT-1 expression. They both have GREs in their promoters and have been shown to be induced in GRE-mediated manner in human neutrophils.26,27 Additionally, we also studied Grb2 expression, which was also increased by glucocorticoid treatment. Grb2 is not known to have GRE in its promoter region and as such is a non-traditional glucocorticoid dependent protein. Unlike previously reported in adenocarcinomic human alveolar basal epithelial cells A549,29 in human neutrophils RU24858 was able to activate both traditional GRE-dependent as well as nontraditional gene transcription further supporting that the transactivation effects of “dissociated glucocorticoids” are cell type dependent. GR exists as at least 8 functional isoforms.34 These GR isoforms display diverse transcriptional activities and the levels of these isoforms vary among different cell types. Whether this cell type-specific isoform profile of GR activation can explain why RU24858 has divergent transcription profile depending on cell type remains to be investigated.
Glucocorticoids are widely used to treat inflammatory diseases such as rheumatoid arthritis, COPD and severe asthma that are characterised with a marked neutrophilic inflammatory component. Apoptosis of neutrophils and its regulation by cytokines such as GM-CSF and by glucocorticoids is considered to be an important pathogenic event in several diseases.13,15,35 Glucocorticoids can either up- or down-regulate the expression of a large number of genes. In peripheral blood monocytes, Galon et al36 found that 12% of the 9182 genes examined were up-regulated and 9% were down-regulated by glucocorticoids. However, the role of glucocorticoid-mediated transactivation or transrepression events in the regulation of neutrophil apoptosis is unclear.
Neutrophils are terminally differentiated, very short-lived non-dividing cells. Thus, molecular techniques are difficult to perform in these cells. For this reason, a pharmacological approach to dissecting out GR mechanisms is the only viable approach. The main aim of our study was to determine whether the “dissociated steroid” RU24858 could be a useful pharmacological tool to further enable elucidation of the GR mechanisms involved in neutrophil survival. Unfortunately, RU24858 does not dissociate between transactivation and transrepression in human neutrophils and thus is not suitable to evaluating GR mechanisms in primary human neutrophils. Functionally, RU24858 has similar effects to dexamethasone and inhibits spontaneous neutrophil apoptosis and further enhances cytokine-induced survival of human neutrophils. Clinically, RU24858 would be expected to have a similar profile as inhaled corticosteroids which in human may result in the tissue neutrophilia seen in severe asthma and may balance sensitivity to infection during glucocorticoid treatment.20,21
Conclusions
In conclusion, we report here that RU24858 and dexamethasone both induce Grb-2, BLT1 and Annexin-1 expression and inhibit CXCL8 and MIP1α production. RU24858 does not dissociate between transactivation and transrepression in non-malignant primary human neutrophils but enhances neutrophil survival similarly to dexamethasone.
Acknowledgments
We appreciate the skilful technical assistance of Mrs Marja-Leena Lampen and Mrs Elina Jaakkola and secretarial help of Mrs Heli Määttä. The financial support was obtained from Tampere Tuberculosis Foundation (Tampere, Finland), the Academy of Finland, Finnish Anti-Tuberculosis Association Foundation (Helsinki, Finland), Jalmari and Rauha Ahokas Foundation (Finland) and the Competitive Research Funding of Seinäjoki Central Hospital and Tampere University Hospital (Grants 9N023, 9K048, 9H031 and 9M059) and is gracefully acknowledged.
Abbreviations
- AP-1
Activator protein 1
- BLT1
Leukotriene B4 receptor 1
- COPD
Chronic obstructive pulmonary disease
- CXCL8
Chemokine (C-X-C motif) ligand 8 (also known as interleukin-8)
- GM-CSF
Granulocyte macrophage colony stimulating factor
- GR
Glucocorticoid receptor
- Grb-2
Growth Factor receptor bound protein 2
- GRE
Glucocorticoid response element
- NF-κB
Nuclear factor κB
- MIP-1α
macrophage inflammatory protein 1 alpha
Footnotes
Disclosures
Author(s) have provided signed confirmations to the publisher of their compliance with all applicable legal and ethical obligations in respect to declaration of conflicts of interest, funding, authorship and contributor-ship, and compliance with ethical requirements in respect to treatment of human and animal test subjects. If this article contains identifiable human subject(s) author(s) were required to supply signed patient consent prior to publication. Author(s) have confirmed that the published article is unique and not under consideration nor published by any other publication and that they have consent to reproduce any copyrighted material. The peer reviewers declared no conflicts of interest.
This article is available from http://www.la-press.com.
Competing Interests
The authors declare that they have no competing interest.
Authors’ Contributions
MJ carried out the neutrophil isolation, flow cytometric assays, morphological analyses, statistics and drafted the manuscript. HH gave valuable collaboration in laboratory and in apoptosis studies. IA and EM participated in the design of the study, interpretation of the data and helped to draft the manuscript. HK conceived the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
References
- 1.Stockley RA. Neutrophils and the pathogenesis of COPD. Chest. 2002;121(5 Suppl):151S–5. doi: 10.1378/chest.121.5_suppl.151s. [DOI] [PubMed] [Google Scholar]
- 2.Witko-Sarsat V, Pederzoli-Ribeil M, Hirsh E, Sozzani S, Cassatella MA. Regulating neutrophil apoptosis: New players enter the game. Trends Immunol. 2011;32(3):117–24. doi: 10.1016/j.it.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Tsoumakidou M, Papadopouli E, Tzanakis N, Siafakas NM. Airway inflammation and cellular stress in noneosinophilic atopic asthma*. Chest. 2006;129(5):1194. doi: 10.1378/chest.129.5.1194. [DOI] [PubMed] [Google Scholar]
- 4.Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. The Lancet. 2009;373(9678):1905–17. doi: 10.1016/S0140-6736(09)60326-3. [DOI] [PubMed] [Google Scholar]
- 5.Newton R. Molecular mechanisms of glucocorticoid action: What is important? Thorax. 2000;55(7):603–13. doi: 10.1136/thorax.55.7.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vayssiere BM, Dupont S, Choquart A, et al. Synthetic glucocorticoids that dissociate transactivation and AP-1 transrepression exhibit antiinflammatory activity in vivo. Mol Endocrinol. 1997;11(9):1245–55. doi: 10.1210/mend.11.9.9979. [DOI] [PubMed] [Google Scholar]
- 7.Vanden Berghe W, Francesconi E, De Bosscher K, Resche-Rigon M, Haegeman G. Dissociated glucocorticoids with anti-inflammatory potential repress interleukin-6 gene expression by a nuclear factor-kappaB-dependent mechanism. Mol Pharmacol. 1999;56(4):797–806. [PubMed] [Google Scholar]
- 8.Tanigawa K, Tanaka K, Nagase H, Miyake H, Kiniwa M, Ikizawa K. Cell type-dependent divergence of transactivation by glucocorticoid receptor ligand. Biol Pharm Bull. 2002;25(12):1619–22. doi: 10.1248/bpb.25.1619. [DOI] [PubMed] [Google Scholar]
- 9.Belvisi MG, Wicks SL, Battram CH, et al. Therapeutic benefit of a dissociated glucocorticoid and the relevance of in vitro separation of transrepression from transactivation activity. J Immunol. 2001;166(3):1975–82. doi: 10.4049/jimmunol.166.3.1975. [DOI] [PubMed] [Google Scholar]
- 10.Janka-Junttila M, Moilanen E, Hasala H, Zhang X, Adcock I, Kankaanranta H. The glucocorticoid RU24858 does not distinguish between transrepression and transactivation in primary human eosinophils. J Inflamm (Lond) 2006;3:10. doi: 10.1186/1476-9255-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walsh G, Sexton D, Blaylock M. Corticosteroids, eosinophils and bronchial epithelial cells: New insights into the resolution of inflammation in asthma. J Endocrinol. 2003;178(1):37. doi: 10.1677/joe.0.1780037. [DOI] [PubMed] [Google Scholar]
- 12.Luo HR, Loison F. Constitutive neutrophil apoptosis: Mechanisms and regulation. Am J Hematol. 2008;83(4):288–95. doi: 10.1002/ajh.21078. [DOI] [PubMed] [Google Scholar]
- 13.Dibbert B, Weber M, Nikolaizik WH, et al. Cytokine-mediated bax deficiency and consequent delayed neutrophil apoptosis: A general mechanism to accumulate effector cells in inflammation. P Natl Acad Sci U S A. 1999;96(23):13330–5. doi: 10.1073/pnas.96.23.13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cabrini M, Nahmod K, Geffner J. New insights into the mechanisms controlling neutrophil survival. Curr Opin Hematol. 2010;17(1):31–5. doi: 10.1097/MOH.0b013e3283333b29. [DOI] [PubMed] [Google Scholar]
- 15.Kankaanranta H, Moilanen E, Zhang X. Pharmacological regulation of human eosinophil apoptosis. Curr Drug Targets. 2005;4:433–45. doi: 10.2174/1568010054526395. [DOI] [PubMed] [Google Scholar]
- 16.Herold M, McPherson K, Reichardt H. Glucocorticoids in T cell apoptosis and function. Cellular and molecular life sciences. 2006;63(1):60–72. doi: 10.1007/s00018-005-5390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liles WC, Dale DC, Klebanoff SJ. Glucocorticoids inhibit apoptosis of human neutrophils. Blood. 1995;86(8):3181–8. [PubMed] [Google Scholar]
- 18.Zhang X, Moilanen E, Kankaanranta H. Beclomethasone, budesonide and fluticasone propionate inhibit human neutrophil apoptosis. Eur J Pharmacol. 2001;431(3):365–71. doi: 10.1016/s0014-2999(01)01437-6. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Moilanen E, Adcock IM, Lindsay MA, Kankaanranta H. Divergent effect of mometasone on human eosinophil and neutrophil apoptosis. Life Sci. 2002;71(13):1523–34. doi: 10.1016/s0024-3205(02)01921-5. [DOI] [PubMed] [Google Scholar]
- 20.Jatakanon A, Uasuf C, Maziak W, Lim S, Chung KF, Barnes PJ. Neutrophilic inflammation in severe persistent asthma. Am J Resp Crit Care. 1999;160(5 Pt 1):1532–9. doi: 10.1164/ajrccm.160.5.9806170. [DOI] [PubMed] [Google Scholar]
- 21.Wenzel SE, Szefler SJ, Leung DY, Sloan SI, Rex MD, Martin RJ. Bronchoscopic evaluation of severe asthma. persistent inflammation associated with high dose glucocorticoids. Am J Resp Crit Care. 1997;156(3 Pt 1):737–43. doi: 10.1164/ajrccm.156.3.9610046. [DOI] [PubMed] [Google Scholar]
- 22.Cox G. Glucocorticoid treatment inhibits apoptosis in human neutrophils. separation of survival and activation outcomes. J Immunol. 1995;154(9):4719–25. [PubMed] [Google Scholar]
- 23.Meagher LC, Cousin JM, Seckl JR, Haslett C. Opposing effects of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytes. J Immunol. 1996;156(11):4422–8. [PubMed] [Google Scholar]
- 24.Ethuin F, Delarche C, Benslama S, Gougerot-Pocidalo MA, Jacob L, Chollet-Martin S. Interleukin-12 increases interleukin 8 production and release by human polymorphonuclear neutrophils. J Leukoc Biol. 2001;70(3):439–46. [PubMed] [Google Scholar]
- 25.Irakam A, Miskolci V, Vancurova I, Davidson D. Dose-related inhibition of proinflammatory cytokine release from neutrophils of the newborn by dexamethasone, betamethasone, and hydrocortisone. Biol Neonate. 2002;82(2):89–95. doi: 10.1159/000063094. [DOI] [PubMed] [Google Scholar]
- 26.Stankova J, Turcotte S, Harris J, Rola-Pleszczynski M. Modulation of leukotriene B4 receptor-1 expression by dexamethasone: Potential mechanism for enhanced neutrophil survival. The Journal of Immunology. 2002;168(7):3570. doi: 10.4049/jimmunol.168.7.3570. [DOI] [PubMed] [Google Scholar]
- 27.Fradin A, Rothhut B, Poincelot-Canton B, Errasfa M, Russo-Marie F. Inhibition of eicosanoid and PAF formation by dexamethasone in rat inflammatory polymorphonuclear neutrophils may implicate lipocortin ‘s’. Biochimica et Biophysica Acta (BBA)-Lipids and Lipid Metabolism. 1988;963(2):248–57. doi: 10.1016/0005-2760(88)90288-3. [DOI] [PubMed] [Google Scholar]
- 28.Croxtall JD, Choudhury Q, Flower RJ. Glucocorticoids act within minutes to inhibit recruitment of signalling factors to activated EGF receptors through a receptor—dependent, transcription—independent mechanism. Br J Pharmacol. 2000;130(2):289–98. doi: 10.1038/sj.bjp.0703272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chivers JE, Gong W, King EM, et al. Analysis of the dissociated steroid RU24858 does not exclude a role for inducible genes in the anti-inflammatory actions of glucocorticoids. Mol Pharmacol. 2006;70(6):2084. doi: 10.1124/mol.106.025841. [DOI] [PubMed] [Google Scholar]
- 30.Kenakin TP, Bond RA, Bonner TI. Definition of pharmacological receptors. Pharmacol Rev. 1992;44(3):351–62. [PubMed] [Google Scholar]
- 31.Korhonen R, Lahti A, Hamalainen M, Kankaanranta H, Moilanen E. Dexamethasone inhibits inducible nitric-oxide synthase expression and nitric oxide production by destabilizing mRNA in lipopolysaccharide-treated macrophages. Mol Pharmacol. 2002;62(3):698–704. doi: 10.1124/mol.62.3.698. [DOI] [PubMed] [Google Scholar]
- 32.Jalonen U, Lahti A, Korhonen R, Kankaanranta H, Moilanen E. Inhibition of tristetraprolin expression by dexamethasone in activated macrophages. Biochem Pharmacol. 2005;69(5):733–40. doi: 10.1016/j.bcp.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 33.Newton R, Holden NS. Separating transrepression and transactivation: A distressing divorce for the glucocorticoid receptor? Mol Pharmacol. 2007;72(4):799. doi: 10.1124/mol.107.038794. [DOI] [PubMed] [Google Scholar]
- 34.Lu NZ, Cidlowski JA. Translational regulatory mechanisms generate N- terminal glucocorticoid receptor isoforms with unique transcriptional target genes. Mol Cell. 2005;18(3):331–42. doi: 10.1016/j.molcel.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 35.Walker A, Ward C, Taylor EL, et al. Regulation of neutrophil apoptosis and removal of apoptotic cells. Current Drug Targets-Inflammation and Allergy. 2005;4(4):447–54. doi: 10.2174/1568010054526278. [DOI] [PubMed] [Google Scholar]
- 36.Galon J, Franchimont D, Hiroi N, et al. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB Journal. 2002;16(1):61–71. doi: 10.1096/fj.01-0245com. [DOI] [PubMed] [Google Scholar]


