Abstract
Purpose
To evaluate whether the addition of two biological markers (MYC and BCL-2 protein overexpression) improves the stratification of high-risk patients with diffuse large B-cell lymphoma (DLBCL).
Method
Seven risk factors were identified at diagnosis, and a maximum of 7 points were assigned to each patient. The patients were classified according to four risk groups: low (0–1), low-intermediate (2–3), high-intermediate (4), and high (5–7). Only high-risk patients with DLBCL were included in this analysis. We retrospectively examined 20 cases from 2008 to 2013 at the Nanjing Drum Tower Hospital.
Results
The median expression of MYC protein was 60%, and 17 of 20 (65%) evaluable cases overexpressed MYC. The median expression of BCL-2 protein was also 60%. Eighteen of 20 (90%) evaluable cases showed BCL-2 overexpression. Additionally, 12 out of 20 cases (60%) demonstrated coexpression of MYC and BCL-2 proteins. The percentages of overall survival and progression-free survival at the median follow-up time (36 months) were 33.3%±16.1% and 16.9%±13.5%, respectively. By comparison, nine, four, and 20 patients were classified as high risk based on the International Prognostic Index (IPI), National Comprehensive Cancer Network(NCCN)-IPI, and revised IPI criteria, respectively. According to the IPI and NCCN-IPI stratification, the risk groups demonstrated closely overlapping survival curves. In addition, four out of 20 cases were identified as low-intermediate risk according to the NCCN-IPI criteria.
Conclusion
The addition of MYC and BCL-2 protein expression to the IPI could identify a subset of DLBCL patients with high-risk clinicopathological characteristics and poor clinical outcome.
Keywords: diffuse large B-cell lymphoma, MYC, BCL-2, International Prognostic Index
Introduction
Diffuse large B-cell lymphoma (DLBCL) is a heterogeneous group of B-cell lymphomas with various immunophenotypes, different molecular and genetic abnormalities, and a wide range of clinical presentations and outcomes.1,2 The International Prognostic Index (IPI) has been the primary clinical tool used to predict the prognoses of patients with DLBCL.3 Its predictive capacity has been validated in the past two decades based on the clinical characteristics of patients treated with CHOP-like chemotherapy. The introduction of the anti-CD20 monoclonal antibody rituximab in chemotherapies has transformed the treatment practices for aggressive lymphoma and led to a marked improvement in outcome.4,5 As a result, the capacity of the IPI to discriminate between risk groups has declined in the era of immunochemotherapy, particularly among the high-risk patients.6–8 An increasing number of studies have indicated that MYC and/or BCL-2 protein detected by immunohistochemistry could be used to identify distinct outcomes of patients with DLBCL.9–14 We have also created a novel clinicopathologic prognostic model to predict the prognoses of DLBCL patients.10 On the basis of the combination of MYC expression and IPI at the time of diagnosis, three discrete outcome groups have been identified with a 3-year overall survival (OS), ranging from 39.1% in the high-risk group to 100% in the low-risk group.10 Most studies agree that patients with DLBCL expressing both MYC and BCL-2 proteins have an aggressive clinical course and a poor outcome.11,15–17 The addition of the biological markers MYC and BCL-2 to the IPI might enhance its capacity to prognosticate.18 The aim of this study was to evaluate whether the addition of the biological markers MYC and BCL-2 protein overexpression could improve the stratification of high-risk patients with DLBCL, compared with the IPI, NCCN-IPI,19 and revised IPI alone.7
Materials and methods
Patient selection
This study is a retrospective analysis of patients with de novo DLBCL. Seven risk factors (age >60 years, stage III/IV disease, elevated lactate dehydrogenase level, Eastern Cooperative Oncology Group [ECOG] performance status ≥2, more than one extranodal site of disease, high MYC expression, and high BCL-2 expression) of patients at diagnosis were identified, and a maximum of 7 points were assigned to each patient. The patients were classified according to four risk groups: low (0–1), low-intermediate (2–3), high-intermediate (4), and high (5–7). Only high-risk patients with DLBCL were included in this analysis. We retrospectively examined 20 cases from 2008 to 2013 at the Nanjing Drum Tower Hospital. All cases were diagnosed according to the World Health Organization classification criteria. All patients were treated with an R-CHOP regimen. This study was approved by the institutional review board, and all patients gave written informed consent.
Immunohistochemistry
FFPE sections of 3 μm were placed on adhesive-coated slides. Immunohistochemistry for BCL-2 and MYC protein expression was performed as previously reported.10,11 Cutoff values of 30% for BCL-2 staining and 50% for MYC staining were considered to be high.10,11
Statistical analysis
This analysis is based on patient follow-up data obtained through to August 5, 2014. Progression-free survival (PFS) was calculated from the date of diagnosis to the date of documented disease progression; observations were censored on the date the patient was last known to be alive or for patients dying as a result of causes unrelated to lymphoma or treatment. OS was calculated from the date of diagnosis until death as a result of any cause or the date last known alive. PFS and OS were assessed using the Kaplan–Meier method and compared among risk groups using the log-rank test. The data were analyzed using SPSS version 11.0.
Results
Patients
A summary of the major clinical characteristics of the patients included in this study is listed in Table 1. The median age was 63 years old (range: 30–75 years). The expression of MYC and BCL-2 proteins was quantified according to previously published cutoff values.10,11 The median expression of MYC protein was 60% and 17 out of 20 (65%) evaluable cases overexpressed MYC (Figure S1A). The median expression of BCL-2 protein was 60%. Eighteen out of 20 (90%) evaluable cases showed BCL-2 overexpression (Figure S1B). Additionally, 12 out of 20 cases (60%) had coexpression of MYC and BCL-2 proteins.
Table 1.
Patient characteristics
| Patient characteristics | Value |
|---|---|
| Number | 20 |
| Median age, years (range) | 59 (30–75) |
| Male sex (%) | 50 |
| IPI factors | |
| Age greater than 60 years (%) | 65 |
| ECOG greater than 2 (%) | 30 |
| Elevated LDH (%) | 75 |
| More than 1 extranodal site (%) | 75 |
| Stage III/IV (%) | 75 |
| Tumor characteristics | |
| MYC overexpression (%) | 65 |
| BCL-2 overexpression (%) | 90 |
| MYC and BCL-2 overexpression (%) | 60 |
Abbreviations: IPI, International Prognostic Index; ECOG, Eastern Cooperative Oncology Group.
Outcome according to standard IPI, NCCN-IPI, and revised IPI
The percentages of OS and PFS at the median follow-up time (36 months) were 33.3%±16.1% and 16.9%±13.5%, respectively. The median survival time for OS was 24.0±15.49 months and the median PFS was 13.0±1.49 months. The patient outcomes according to the IPI, NCCN-IPI, and revised IPI are listed in Table 2. According to the IPI, NCCN-IPI, and revised IPI stratification, nine, four, and 20 patients were classified as high risk, respectively. The risk groups demonstrated closely overlapping survival curves (Figures 1 and 2). Additionally, four out of 20 cases were identified as low-intermediate risk according to the NCCN-IPI criteria. The clinicopathological characteristics of the four patients are listed in Table 3.
Table 2.
Risk stratification and outcomes of 4-year OS and PFS
| Risk group | Number of prognostic factors | Patients | 4-yr PFS (%) | 4-yr OS (%) |
|---|---|---|---|---|
| IPI | ||||
| Low | 0, 1 | 0 | – | – |
| Low-intermediate | 2 | 0 | – | – |
| High-intermediate | 3 | 11/20 | 0 | 23.9 |
| High | 4, 5 | 9/20 | 27.8 | 55.6 |
| NCCN-IPI | ||||
| Low | 0, 1 | 0 | – | – |
| Low-intermediate | 2, 3 | 4/20 | 0 | 0 |
| High-intermediate | 4, 5 | 12/20 | 25.0 | 50.0 |
| High | 6, 7, 8 | 4/20 | 0 | 50.0 |
| Revised IPI | ||||
| Low | 0 | 0 | – | – |
| Intermediate | 1, 2 | 0 | – | – |
| High | 3, 4, 5 | 20/20 | 16.9 | 33.3 |
Abbreviations: OS, overall survival; PFS, progression-free survival; IPI, International Prognostic Index; NCCN, National Comprehensive Cancer Network.
Figure 1.
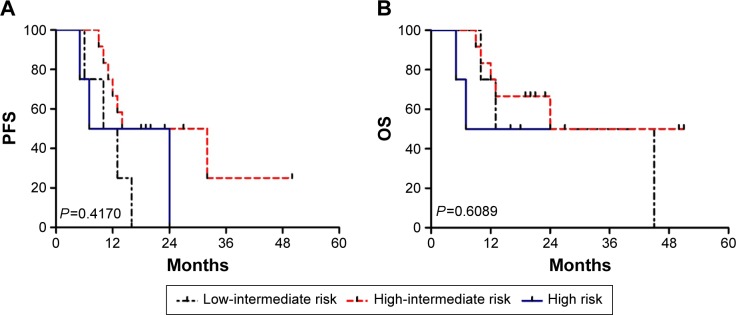
Survival according to the NCCN-IPI.
Notes: (A) Progression-free survival. (B) Overall survival.
Abbreviations: OS, overall survival; PFS, progression-free survival; IPI, International Prognostic Index; NCCN, National Comprehensive Cancer Network.
Figure 2.
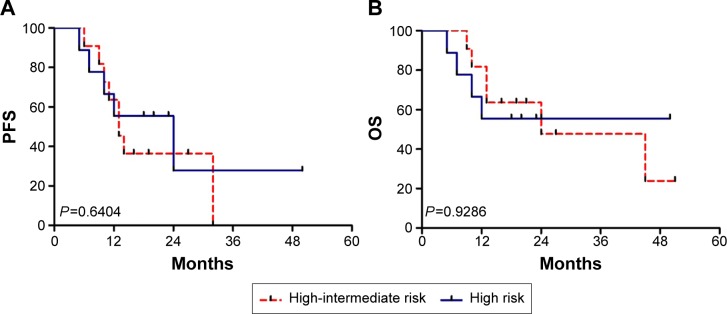
Survival according to the IPI.
Notes: (A) Progression-free survival. (B) Overall survival.
Abbreviations: OS, overall survival; PFS, progression-free survival; IPI, International Prognostic Index; NCCN, National Comprehensive Cancer Network.
Table 3.
Four high-risk patients’ characteristics
| Patient number 1 | Patient number 2 | Patient number 3 | Patient number 4 | |
|---|---|---|---|---|
| Age (years) | 50 | 30 | 51 | 53 |
| Sex | Male | Male | Male | Male |
| ECOG | 1 | 1 | 1 | 1 |
| LDH (220 U/L) | 493 | 404 | 350 | 107 |
| Extranodal site of disease | – | Bone marrow | – | Bone marrow |
| Stage | III | IV | III | IV |
| MYC expression (%) | 95 | 65 | 85 | 60 |
| BCL-2 expression (%) | 85 | 85 | 75 | 50 |
| Replase (months) | 6 | 12 | 10 | 15 |
| Survival (months) | 45 | 13 | 10 | 16 |
| NCCN-IPI score | 3 | 3 | 3 | 3 |
Abbreviations: IPI, International Prognostic Index; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; NCCN, National Comprehensive Cancer Network.
Discussion
DLBCL remains a heterogeneous hematological disease with biologic indicators of treatment response and outcome that are not fully elucidated.1,2 The IPI was originally devised in the era before the introduction of rituximab.3 The use of rituximab has not only improved the survival of patients with DLBCL across all risk groups,4,5 but has also narrowed the prognostic differences between the IPI risk groups.6–8 Thus, a revision of the IPI for patients with DLBCL in the rituximab era using well-standardized clinical variables is needed.
Zhou et al19 presented a refined prognostic index (NCCN-IPI) for patients with de novo DLBCL, using widely available clinical parameters.8 This model is straightforward and looks similar in many ways to the standard IPI. Zhou et al stated that the NCCN-IPI was easy to apply and more powerful than the standard IPI for predicting patients’ prognosis in the rituximab era.19 Many hematologists have sought to identify new prognostic tools using molecular markers or gene expression, but none are ready to be broadly adopted into clinical practice. There has been a shift toward incorporating molecular marker profiling into prognostication. It is agreed that patients with DLBCL expressing both MYC and BCL-2 proteins have an aggressive clinical course and a poor outcome.9,11 We have created a novel clinicopathologic prognostic model (biological marker-adjusted IPI, B-IPI) to identify a high-risk subgroup of DLBCL patients. On the basis of the number of negative prognostic factors present at the time of diagnosis, 20 patients were classified as high risk. These patients have high-risk clinicopathological features and poor outcomes. Was it an exaggerated prognosis to identify more high-risk patients in our study? Among these 20 high-risk patients, according to NCCN-IPI, four patients were assigned to the low-intermediate group. Three out of four patients were deceased within 16 months. Although one patient survived 45 months, his disease relapsed within 6 months. All four patients were found to overexpress both MYC and BCL-2 proteins. Obviously, these patients with a poor prognosis should be classified as high risk. In contrast to the IPI and NCCN-IPI, B-IPI has shown an improved capacity to discriminate high-risk patients. Our results were consistent with the revised-IPI. The revised-IPI was developed in a cohort of 365 patients.7 Our study showed that the revised-IPI and the B-IPI are substantially better for determining high-risk patients. High-risk groups have the most to gain from receiving novel agents as first-line therapy in place of less-effective conventional options, and these patients should be referred for clinical trials.
Eight predictors at diagnosis were identified in NCCN-IPI, which looks similar to the standard IPI. It is generally agreed upon that patients with MYC/BCL-2 double-hit lymphomas (DHLs) have an extremely aggressive clinical course. Treatment is usually tailored according to the individual risk profile of a DLBCL patient. Petrich et al20 developed a novel risk score for DHL, which divides patients into high-, intermediate-, and low-risk groups. This DHL prognostic index resulted in excellent discrimination of a low-risk group with 2-year estimated OS rates of 91%. Thus, elucidating a better predictor of prognosis without consideration of these biological markers would be inappropriate. The addition of the expression of MYC and BCL-2 proteins to the IPI would be helpful in identifying patients with DHL who carry a particularly favorable prognosis. Immunohistochemistry is a rapid and inexpensive method for detecting these biological markers. Immunohistochemistry can be easily applied in academic and community-based hospitals. While our study is a retrospective study with a small number of patients, whether the B-IPI can serve as a prognostic guide for risk-adapted therapy remains to be seen and will depend on whether our results can be confirmed in prospective trials.
In summary, biological marker-adjusted IPI could identify a subset of DLBCL patients with high-risk clinicopathological features and poor clinical outcome.
Supplementary material
(A) MYC protein expression was exclusively brown nuclear (×400). (B) BCL-2 protein expression was exclusively brown cytoplasm (×400).
Acknowledgments of grant support
Medical Science and Technology Key Project of Nanjing, NJGL-2011196. Jiangsu Provincial Special Program of Medical Science, BL2012005. Medical Science and Technology Project of Nanjing, ZKX10012. Medical Science and Technology Key Project of Nanjing, ZKX14015. Peak of Six Talent in Jiangsu Province, 2014-WSN-049.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Geneva, Switzerland: WHO Press; 2008. [Google Scholar]
- 2.Smith A, Roman E, Howell D, et al. The Haematological Malignancy Research Network (HMRN): a new information strategy for population based epidemiology and health service research. Br J Haematol. 2010;148(5):739–753. doi: 10.1111/j.1365-2141.2009.08010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Project TIN-HsLPF A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329(14):987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 4.Feugier P, Van Hoof A, Sebban C, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 5.Hauptrock B, Hess G. Rituximab in the treatment of non-Hodgkin’s lymphoma. Biologics. 2008;2(4):619–633. doi: 10.2147/btt.s3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sehn LH. Paramount prognostic factors that guide therapeutic strategies in diffuse large B-cell lymphoma. Hematology Am Soc Hematol Educ Program. 2012;2012:402–409. doi: 10.1182/asheducation-2012.1.402. [DOI] [PubMed] [Google Scholar]
- 7.Sehn LH, Berry B, Chhanabhai M, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109(5):1857–1861. doi: 10.1182/blood-2006-08-038257. [DOI] [PubMed] [Google Scholar]
- 8.Sengar M, Menon H, Dangi H, et al. Comparison of treatment outcomes with EPOCH-Rituximab versus CHOP-Rituximab in patients with de-novo intermediate and high risk international prognostic index (IPI) diffuse large B cell lymphoma (DLBCL): a single center retrospective analysis. Blood. 2013;122:e5115. [Google Scholar]
- 9.Xia B, Zhang L, Guo SQ, et al. Coexpression of MYC and BCL-2 predicts prognosis in primary gastrointestinal diffuse large B-cell lymphoma. World J Gastroenterol. 2015;21(8):2433–2442. doi: 10.3748/wjg.v21.i8.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou M, Wang J, Ouyang J, et al. MYC protein expression is associated with poor prognosis in diffuse large B cell lymphoma patients treated with RCHOP chemotherapy. Tumour Biol. 2014;35(7):6757–6762. doi: 10.1007/s13277-014-1907-z. [DOI] [PubMed] [Google Scholar]
- 11.Perry AM, Alvarado-Bernal Y, Laurini JA, et al. MYC and BCL2 protein expression predicts survival in patients with diffuse large B-cell lymphoma treated with rituximab. Br J Haematol. 2014;165(3):382–391. doi: 10.1111/bjh.12763. [DOI] [PubMed] [Google Scholar]
- 12.Kluk MJ, Chapuy B, Sinha P, et al. Immunohistochemical detection of MYC-driven diffuse large B-cell lymphomas. PLoS One. 2012;7(4):e33813. doi: 10.1371/journal.pone.0033813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tapia G, Lopez R, Muñoz-Mármol AM, et al. Immunohistochemical detection of MYC protein correlates with MYC gene status in aggressive B cell lymphomas. Histopathology. 2011;59(4):672–678. doi: 10.1111/j.1365-2559.2011.03978.x. [DOI] [PubMed] [Google Scholar]
- 14.Maeshima AM, Taniguchi H, Fukuhara S, et al. Bcl-2, Bcl-6, and the International Prognostic Index are prognostic indicators in patients with diffuse large B-cell lymphoma treated with rituximab-containing chemotherapy. Cancer Sci. 2012;103(10):1898–1904. doi: 10.1111/j.1349-7006.2012.02382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green TM, Young KH, Visco C, et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30(28):3460–3467. doi: 10.1200/JCO.2011.41.4342. [DOI] [PubMed] [Google Scholar]
- 16.Hu S, Xu-Monette ZY, Tzankov A, et al. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from the International DLBCL Rituximab-CHOP Consortium Program. Blood. 2013;121(20):4021–4031. doi: 10.1182/blood-2012-10-460063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL-2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30(28):3452–3459. doi: 10.1200/JCO.2011.41.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Zhou M, Xu JY, et al. MYC and BCL-2 adjusted-International Prognostic Index (A-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Histol Histopathol. 2015 doi: 10.14670/HH-11-673. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Z, Sehn LH, Rademaker AW, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014;123(6):837–842. doi: 10.1182/blood-2013-09-524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrich AM, Gandhi M, Jovanovic B, et al. Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: a multicenter retrospective analysis. Blood. 2014;124(15):2354–2361. doi: 10.1182/blood-2014-05-578963. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) MYC protein expression was exclusively brown nuclear (×400). (B) BCL-2 protein expression was exclusively brown cytoplasm (×400).


