Abstract
High-Fat Diet (HFD) has emerged as an important risk factor not only for obesity and diabetes but also for urological disorders. Recent research provides ample evidence that HFD is a putative cause for prostatic diseases including prostate cancer. The mechanisms whereby these diseases develop in the prostate have not been fully elucidated. In this review we discuss signaling pathways intricately involved in HFD-induced prostate disease. We performed a search through PUBMED using key words “high fat diet” and “prostate”. Our data and perspectives are included in this review along with research performed by various other groups. HFD is positively associated with an increased risk of benign prostatic hyperplasia (BPH) and prostate cancer. HFD induces oxidative stress and inflammation in the prostate gland, and these adverse influences transform it from a normal to a diseased state. Studies demonstrate that HFD accelerates the generation of reactive oxygen species by driving the NADPH oxidase system, exacerbating oxidative stress in the prostate. HFD also causes a significant increase in the levels of pro-inflammatory cytokines and gene products through activation of two important signaling pathways: the Signal Transducer and Activator of Transcription (STAT)-3 and Nuclear Factor-kappa B (NF-κB). Both these pathways function as transcription factors required for regulating genes involved in proliferation, survival, angiogenesis, invasion and inflammation. The crosstalk between these two pathways enhances their regulatory function. Through its influences on the NF-κB and Stat-3 signaling pathways, it appears likely that HFD increases the risk of development of BPH and prostate cancer.
Keywords: Signal transducer and activator of transcription, Nuclear factor-kappa B, Benign prostatic hyperplasia, NADPH oxidase, prostate cancer
Introduction
The current trend towards an increasingly sedentary lifestyle and increasing consumption of high-caloric ‘Western style’ high-fat diet (HFD) has contributed to the sharply increasing prevalence of metabolic disorders such as obesity and type 2 diabetes in the United States [1]. Presently, obesity is a major health concern, and according to the Center for Disease Control & Prevention, approximately 40% of the adults in the United States are obese and ~20% of children and adolescents are overweight [2]. A closer examination of obesity has revealed that a preferential accumulation of fat in the abdominal region of men is associated with increased risk of urologic complications including urinary incontinence, erectile dysfunction, benign prostatic hyperplasia (BPH) and perhaps cancer [3]. A strong association between fatty acids and prostate diseases has been reported with several intriguing hypothesis. These include mechanisms involving inflammation, oxidative stress, peroxidation of lipids and accumulation of 8-hydroxy-2′ –deoxyguanosine and increased androgen synthesis driving the growth of the prostate. High-fat diet induces a low-grade chronic inflammatory response, a phenomenon designated as ‘metabolically triggered inflammation’ or meta-inflammation [4,5]. The direct effects of HFD on the prostate are still unclear, though it is considered to cause inflammation and oxidative stress through alteration in various signaling pathways that increases the vulnerability of the prostate to numerous diseases [4–6]. In this review we focused on the role of HFD in the genesis of oxidative stress and intraprostatic inflammation and their influences on signaling pathways that orchestrate various prostate diseases, including cancer.
High-fat diet induced intraprostatic inflammation
Prostate inflammation is frequently observed in histological specimens from aging men [7]. Mounting data suggest that intraprostatic inflammation plays a pivotal role in the development of BPH, lower urinary tract symptoms (LUTS) and cancer [5–7]. Accumulating evidence suggests that other than aging, androgens and genetic predisposition, modifiable factors such as obesity, diet, dyslipedimia, hormonal imbalance, hypertension, metabolic syndrome, alcohol and smoking also contribute to the development of BPH and/or LUTS [4,5,6,8]. A previous study from our group reported an association between chronic intraprostatic inflammation and carcinogenesis. In this 5 year follow-up study, initial biopsies from patients with chronic inflammation had a 20% higher incidence of the development of prostate cancer with a significant association between serum prostate specific antigen (PSA) and the degree of inflammation [9]. Clinically, a number of cross sectional studies reveal the association between the presence of inflammatory infiltrates and increased prostate volume. A recent study on Prostate Cancer Prevention Trial patients with inflammation and BPH has higher incidence towards the development of prostate cancer [10]. In another study, Giovannucci et al. observed that consumption of animal fat is a risk factor for prostate cancer [11]. HFD intake has been shown to cause a remarkable downregulation in the gene expression of glutathione peroxidase 3 (GPx3) in the prostate, which is considered to modulate carcinogenesis [12]. Tumor progression is accelerated in genetically-engineered transgenic adenocarcinoma of the mouse prostate (TRAMP) mice that progresses through multiple stages and exhibits both histological and molecular features similar to that of human prostate cancer, upon HFD feeding [13]. In another study, Vykhovanets et al. showed that NF-κB reporter mice consuming HFD with a composition similar to a Western-style diet developed increased intraprostatic inflammation as well as increased cellular proliferation and increased size of the prostate gland [14]. A study by Cai et al. demonstrated increased ventral prostate weight in mice fed with HFD, without any changes in serum or intraprostatic androgen levels [15]. Consumption of a diet rich in animal fat not only causes obesity, impaired glucose tolerance, and insulin resistance, but also elevates ataxin levels in the adipose tissue, causing increased production of lysophosphatidic acid. This chemical in turn has been shown to act directly on the prostate to induce hyperplastic and neoplastic growth contributing to prostate cancer progression and BPH by creating a pro-inflammatory environment [16]. Another recent report has shown that in a HFD setting, prostate cancer cell proliferation is increased through activation of MCP-1/CCR2 signaling [17].
While inflammation of the prostate is believed to cause tissue damage and growth, there is no clear understanding of the mechanisms involved. It has been suggested that inflammation leads to activation of lymphocytes and macrophages which are attracted to the prostate tissue, leading to the secretion of pro-inflammatory cytokines directly affecting prostate growth [18]. Our previous studies revealed that activation of cytokine IL-1β initiates a time-dependent wave of signals that initiates early phase of intraprostatic inflammation and signal inflammatory pathways [19,20]. In adipose tissue, HFD-induced inflammation leads to the production of various pro-inflammatory cytokines such as IL-1, IL-6 and TNFα [21,22]. Reports indicate that pro-inflammatory cytokine TNFα mediates insulin resistance as a result of obesity [23,24]. Feeding HFD to mice caused a macrophage inflammatory response in white adipose tissue that was associated with morbid fat mass expansion and TNFα release that triggered pre-adipocytes to enhance the expression of inflammatory genes [25,26]. This is consistent with studies in obese patients demonstrating an increase in the plasma levels of TNFα that is associated with weight gain [27,28]. Our studies have shown that HFD not only caused a significant increase in the plasma levels of IL-6, IL-17, IL-1β and TNFα but also a marked elevation of intraprostatic IL-6 expression [29]. These pro-inflammatory cytokines affect peripheral tissues, causing inflammation via activation of transcription factors that result in the recruitment and activation of macrophages and lymphocyte infiltration [30].
Pro-inflammatory cytokines induce inflammatory mediators such as cyclooxygenase -2 (COX-2) and inducible nitric oxide (iNOS) that contribute to prostate growth [7]. It has been shown that IL-17 can exert a direct influence on COX-2, thus stabilizing and preventing its degradation, increasing its enzymatic activity [31]. Vykhovanets et al. have demonstrated increased production of IL-17 by macrophages and neutrophils in proliferative inflammatory atrophy (PIA) lesions [32]. Levels of IL-17 producing cells were similar in zones of benign prostate tissue and areas of prostate cancer. Increased COX-2 expression has been reported in BPH with moderate to severe T lymphocyte and macrophage infiltration. The increased COX-2 expression was noted in areas where the epithelium was highly proliferative [33]. The presence of COX-2 has been reported in inflammatory cells in the epithelium and interstitial spaces in proliferative inflammatory atrophy lesions, generating pro-inflammatory prostaglandins [34,35]. Inflammation is reported to induce expression and enzyme activity of iNOS and COX-2, which produces pro-inflammatory mediators such as prostaglandin E2 (PGE2) and nitric oxide [36–39]. We reported that HFD intake by C57BL/6 mice caused a significant increase in the levels of COX-2 and iNOS in the prostate [14]. The levels of iNOS increases in epithelial cells identified with conditions of BPH, high-grade prostatic intraepithelial neoplasia (PIN) and prostate cancer [40]. Long term HFD intake has been shown to decrease excretion of nitrite in the urine as a result of oxidative stress [41].
High-fat diet induced oxidative stress in the prostate
It is hypothesized that inflammation of the prostate, through the generation of reactive oxygen species (ROS), causes repeated tissue damage and post-translational DNA modifications, thereby inducing neoplasia in the prostate [42]. The major sources of ROS are the mitochondrial respiratory chain, an uncontrolled arachidonic acid cascade, and NADPH oxidase. These processes make use of molecular oxygen and produce ROS which include superoxide anion and hydrogen peroxide [43]. Elevated ROS production has a deleterious effect and is associated with tissue injury, DNA damage, neoplastic transformation and aberrant growth and proliferation. Thus, disproportionate formation of ROS can result in oxidative stress and might play an important role in the pathogenesis of several prostatic diseases including cancer [44]. The NADPH oxidase system has been reported as a contributor to the genesis of prostate diseases, including prostate cancer [45]. Several recent studies have shown a substantial generation of ROS and NADPH oxidase activity, which are thought to play a critical role in the growth and maintenance of prostate cancer [45–50]. We found that HFD feeding to NF-ҡB reporter mice caused an increase in the expression of NADPH oxidase subunits such as gp91phox, p47phox and p22phox in the prostate [14]. We hypothesize that HFD accelerates the generation of ROS by driving the NADPH oxidase system, thereby enhancing several signaling pathways that are involved in inflammation. This is in concurrence with the findings of other investigators who have shown that generation of ROS either through the NADPH oxidase system or arachidonic acid pathway leads to the activation of inflammatory signaling pathways, primarily the NF-ҡB pathway, that orchestrate the gene expression of several pro-inflammatory mediators [43]. Sustained oxidative stress and inflammatory mediators activate two important signaling pathways that have a role as core transcription factors in diverse immune responses; specifically, the signal transducer and activator of transcription (STAT)-3 and nuclear factor-kappa B (NF-ҡB) pathways. Both these pathways are activated by external stimuli through cytosolic signals and function as transcription factors required for regulating genes involved in proliferation, survival, angiogenesis, invasion and inflammation. Pro-inflammatory cytokines have been shown to activate both Stat-3 and NF-ҡB and their transcriptional targets establishes a feedback loop [51].
Nuclear factor-kappa-B (NF-ҡB)
NF-ҡB encompass a family of pleiotropic transcription factors that integrate a complex network of extracellular perturbations and signaling pathways, resulting in the transcriptional regulation of hundreds of genes related to inflammation, immunity, apoptosis, cell proliferation, and differentiation [52,53]. NF-ҡB activation is orchestrated either through classical/canonical or alternative/non-canonical pathways resulting in its nuclear translocation. Activating stimuli involve phosphorylation and engagement of the inhibitor of the ҡB kinase (IKK) signalosome, which is composed of two catalytic subunits, IKKα (IKK1) and IKKβ (IKK2), and a regulatory subunit, IKKγ (NF-ҡB essential modulator) and inhibitors of ҡB (IҡB) proteins, IҡBα, IҡBβ, IҡBɛ that regulate nuclear translocation and DNA binding of NF-ҡB. Normally NF-ҡB dimers are either bound to IҡBα, IҡBβ, IҡBɛ, or the precursor proteins p100 and p105 that maintain these dimers in the cytoplasm in an inactive state. The activation of canonical pathway occurs as a result of interaction of ligands to their corresponding receptors that result in activation of the β subunit of IҡB kinase (IKK) complex IKKβ that phosphorylates and degrades IҡBα. This causes the NF-ҡB heterodimers (comprising of p65, p50 and c-Rel) to translocate into the nucleus and induce or repress genes by binding to discrete DNA sequences known as ҡB elements in promoter and enhancer elements of target genes [52–54]. In the case of the alternative pathway activation results from the transformation of NF-ҡB2/p100 to p52 that is activated by phosphorylation of NF-ҡB2/p100 by IKKα, allowing the translocation of p52 along with RelB into the nucleus.
Constitutive activation of NF-ҡB has been implicated not only in prostate cancer but also in a wide range of human diseases like rheumatoid arthritis, inflammatory bowel diseases, neurodegenerative conditions, asthma and chronic obstructive pulmonary disease [55–60]. Since NF-ҡB is the key mediator of inflammation, we and others have investigated whether or not HFD can cause its activation. Carlson et al. reported that HFD caused elevated NF-ҡB activation in mice and there was a regional difference between males and females: in females the activity was localized to the thoracic region, whereas in males the activity was observed in the abdominal region. We reported that HFD feeding to NF-ҡB reporter mice caused increased activation of NF-ҡB in the whole body and remarkably extended activation was observed in the prostate [61]. A notable elevation in the levels of Rel A/p65, phosphorylation of IKKα/β and IҡBα in the prostate suggested that HFD activates NF-ҡB. Overexpression of NF-ҡB and its association with increased presence of ROS and NADPH oxidase activity has been reported in various inflammatory diseases [62]. Upregulation of NF-ҡB during inflammation also results in the recruitment of inflammatory cells leading to the production of various pro-inflammatory cytokines, such as IL-1, IL-6, IL-8 at the site of inflammation [63,64]. Earlier reports from our group suggest that NF-ҡB is constitutively activated in prostate adenocarcinoma [59]. Constitutive activation of NF-ҡB is associated with upregulation of pro-survival molecules including Bcl-2 family members such as Bcl-2, Bcl-XL, and Mcl-1 that helps evade apoptosis. Transcriptional regulation of Bcl-2 by NF-ҡB as well as strong association between NF-ҡB activation and Bcl-XL/Mcl-1 expression has been documented in prostate cancer [65]; but not in HFD environment. Detailed studies are required to establish the role of Bcl-2 family members in the etiology of BPH/LUTS and HFD environment. In the kidney, consequence of HFD caused a contemporaneous increase in the levels of TNFα, resulting in increased phosphorylation of p-IKK/β and NF-ҡB activation, causing oxidative stress [66]. In the mouse intestine, HFD-induced increase in TNFα and NF-ҡB activation promotes inflammation through interaction with enteric bacteria which precedes obesity and insulin resistance [67]. In our study with HFD we observed increased plasma levels of TNFα and IL-6, suggesting that HFD feeding may cause sustained activation of NF-ҡB in the prostate [29]. Previously we had reported that cytokines such as TNFα, IL-6 and IL-1β could activate NF-ҡB, and that IL-1β initiates a time depended wave of signals that could induce the initial phase of intraprostatic inflammation [19,20].
Signal transducer and activator of transcription (STAT-3)
STAT-3 is a transcription factor located in the cytosol during inactive state. Several types of receptor tyrosine kinases activate STAT-3. They are epidermal growth factor receptor (EGFR), fibroblast growth factor receptor (FGFR), platelet-derived growth factor receptor and Janus kinase (JAK) family members that are constitutively bound to the cytoplasmic tails of cytokine receptors, or non-receptor-associated tyrosine kinases. Growth factors like EGF and FGF, initiate STAT-3 signal transduction when they bind to their receptors and activate intracellular kinases. JAK proteins or receptor tyrosine kinases recruit inactive STAT-3 monomers and phosphorylate them on tyrosine-705, leading to homo- or heterodimer formation. STAT-3 dimers then translocate to the cell nucleus, where they act as transcription activators [68,69]. Discovered as an acute-phase response protein, STAT-3 has been implicated in driving inflammation. Several pro-inflammatory cytokines have been shown to activate STAT-3 especially IL-6. Activation by IL-6 has been shown to be dependent on COX-2 [68,70,71]. It has been shown that IL-11 and its glycoprotein 130 (gp130) receptor activation in inflammation-associated gastric epithelial cell oncogenic transformation is mediated by and dependent on increased activation of STAT-3 [72].
Several infectious and non-infectious agents are known to cause inflammation and involve STAT-3 activation by various distinct mechanisms [70,73,74]. Case control studies draw a link between STAT-3 polymorphism and metabolic syndrome and these studies demonstrate that common genetic variants in the locus of STAT-3 is associated with abdominal obesity, a key factor of metabolic syndrome [75]. In fact, dietary saturated fat has been reported to activate STAT-3, which is involved in body weight regulation. Park et al. demonstrate that dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNFα expression [76]. In the prostate, HFD feeding resulted in increased STAT-3 activation and DNA binding that might be IL-6 mediated, as there was a significant increase in the plasma levels [29]. HFD may create an environment that potentiates the IL-6-STAT-3 axis, thereby sustaining persistent chronic inflammation.
Crosstalk between NF-ҡB and STAT3
NF-ҡB and STAT3 are ubiquitously expressed transcription factors that are thought to play critical roles in inflammation and tumorigenesis, and their association occurs in a direct and an indirect manner [51,69,77]. These proteins interact directly with one another via a physical interaction; indirectly, these pathways activate one another to sustain inflammation and tumor progression. Although these pathways transcribe their own sets of genes, induction of certain gene subsets requires cooperation between the STAT-3 and NF-ҡB pathways [51]. The crosstalk between these two pathways has become a hallmark during inflammation driven carcinogenesis [51,78,79]. The persistently activated STAT-3 retains NF-ҡB activity in the nucleus by causing its acetylation at Lysine 310 [77]. The association or crosstalk requires cytokines or other signaling pathways such as the release of IL-6 by NF-ҡB that causes the stimulation of STAT-3 by an autocrine/paracrine mechanism [79]. In colitis associated cancer, IL-6 not only bridges NF-ҡB and Stat-3 but also is responsible for tumor growth and tumor initiation [51]. NF-ҡB and STAT-3 are interconnected and require phosphoinositide 3-kinase (PI3K) and Myc expression, and the physical association between NF-ҡB and STAT-3 is important in the development of Myc-driven B-cell neoplasia [80].
The functions of NF-ҡB and STAT-3 broaden beyond carcinogenesis, as both of these transcriptional factors play a pivotal role in immune and inflammatory responses [52,81]. STAT-3 creates a pro-inflammatory condition and simultaneously suppresses anti-tumor immune response [82]. In intestinal epithelial cells, STAT-3 and NF-ҡB have been reported to be required in tumor promotion and NF-ҡB is reported to control STAT-3 activation in a dual manner by recruiting myeloid cells that secrete STAT-3 induced cytokines, and transcribing these cytokines that activate STAT-3 [73]. Other notable feature of STAT-3-NF-ҡB interaction is the induction of inflammatory mediators like IL-6, COX-2, IL-17 and IL-23, which require STAT-3 as a co-transcription factor with RelA for potentiating their expression and thus causing immune suppression [71,81]. We have recently demonstrated a physical association between STAT-3 and NF-ҡB and their binding to the promoter regions of inflammatory genes in C57BL/6 mouse fed with high-fat diet [29]. Together, these two transcription factors require association for persistent induction of their activators. It is unclear whether this association is only present in tumor microenvironment. Could potential risk factors such as HFD drive the association between STAT-3 and NF-ҡB in cells that are in a state of inflammation? In our studies we observed that HFD not only activated STAT-3 and NF-ҡB but also their association caused persistent chronic inflammation in the mouse prostate. The uniqueness of this association is that it happens in a non-cancerous environment induced by HFD that drives a wide range of pro-survival, proliferative and inflammatory genes [29]. Another function of STAT-3 is to suppress immune response as it inhibits the expression of NF-ҡB target genes involved in TH1 innate immunity and adaptive immune responses that is required for controlling microbial infections and tumor growth [81]. Thus, interaction of STAT-3 with NF-ҡB occurs at multiple levels and the outcome of this interaction depends on the cellular environment. In the prostate, HFD driven STAT-3/NF-ҡB interaction may be one of the underlying causes of chronic prostatic inflammation which could be the initiator for the development of common prostatic diseases, such as BPH and prostate cancer. Theoretically, HFD initiated STAT-3/NF-ҡB association should cease once the dietary pattern changes and normalcy could be restored. Unremitting HFD consumption may set the stage for persistent activation of STAT-3 and NF-ҡB, leading to their association, which may exacerbate prostate inflammation, leading to prostate diseases including cancer.
Protein kinase C epsilon (PKCε) and Akt
In our recent studies we observed that HFD caused increased activation of upstream kinases such as Protein Kinase C epsilon (PKCε) and Akt which orchestrates signaling via STAT-3 and NF-ҡB in the prostate [29]. While PKCε has been reported to activate STAT-3, Akt activates NF-ҡB and increases pro-survival signals through upregulation of Bcl-2, Bcl-XL and Mcl-1 [65,83,84]. In a HFD environment, PKCε is reported to play a critical role in the liver by mediating fat-induced hepatic insulin resistance [85]. Leptin has also been associated with PKCε and PI3K/Akt, while it has been shown to cause inhibition of lysophosphatidic acid-induced intracellular calcium rise in a PKCε dependent manner, its resistance has been reported to cause impairment in the PI3K pathway [86,87]. We reported that HFD feeding could induce a repertoire of pathways which could lead to the activation of the inflammatory signals [29]. The scenario seems to be complex as these upstream kinases also activate pro-inflammatory cytokines. In adipocytes PKCε has been implicated in the activation of IL-6 via a MAPK pathway thereby contributing to the pathogenesis of type 2 diabetes [88]. In a HFD prone milieu we reported that PKCε activation in the prostate could serve as an impetus not only for STAT-3 activation but also IL-6. More systematic studies are needed to evaluate this mechanism.
High-fat diet induced increase in leptin
Accumulation of fat occurs primarily in the adipose tissue and previous studies delineate a clear link between obesity, insulin resistance, and inflammation as a result of inflammatory cytokines, adipokines and leptin [89–91]. The end result of HFD intake is an increase in the amount of adipose tissue which is a large repository of cholesterol serving as precursor for testosterone, androstenedione and triglycerides that may stimulate prostate epithelial cell growth through upregulation of androgen receptor [92]. Leptin is a pivotal hormone of the adipose tissue that regulates appetite and body weight and transduces the signaling of STAT-3 by activating the JAK-STAT pathway [93]. HFD induces resistance to the effect of leptin causing impairment in the PI3K pathway precedes that of the STAT-3 pathway [87]. High expression of leptin receptor has been observed in the prostate and circulating leptin levels increase in parallel with prostate growth at puberty in rats [94,95]. Elevated immunoreactivity for leptin receptors in prostatic cancer specimens, with a strong expression in high-grade PIN lesion has been reported [96]. Stattin et al. observed that moderately increased leptin levels are associated with the development of prostate cancer [97]. HFD feeding has been shown to increase circulating levels of leptin in normal mice and mice with transgene-induced ablation of brown adipose tissue making them obese without increasing their caloric intake [98]. We have observed that intake of HFD to C57BL/6 male mice in a time-dependent manner caused increased levels of inflammatory molecules related to leptin and estrogen signaling in the prostate [99]. Thus, excessive leptin may be a possible key link between Western lifestyle and prostate diseases.
Immune cell response
As mediators of low-grade chronic inflammation associated with obesity, several reports implicate the involvement of immune cells [100,101]. HFD feeding has been shown to cause depletion of hepatic Natural Killer T (NKT) cells resulting in obesity and insulin resistance [102]. This study demonstrate that NKT cells are the key mediators of HFD-induced metabolic abnormalities. HFD feeding of C57BL6 mice has also shown to elevate CD4, CD8 and macrophages levels in the inguinal adipose tissue suspected to be a cause for low-grade inflammation [103]. Significant recruitment of peripheral immune cells was observed in the central nervous system of obese mice fed with HFD contributing to inflammatory response during obesity [104]. In clinical studies, nodules of BPH patients contain infiltrates of T-lymphocytes, macrophages and B-lymphocytes that are chronically activated [105]. These infiltrating cells produce cytokines viz. IL-2, IFN-γ and TGF-β which may support fibromuscular growth in BPH. Other in situ studies have demonstrated that elevated expression of pro-inflammatory cytokines in BPH, IL-6, IL-8 and IL-17, may perpetuate chronic immune response in BPH and induce persistent intraprostatic inflammation and fibromuscular growth by an autocrine or paracrine loop [106,107]. Further studies are needed to establish association between obesity and the role of invading immune cells in causing BPH/LUTS symptoms in the prostate.
Conclusions and future directions
Accumulated evidence suggests that HFD influences prostatic inflammation and plays a significant role in the development of prostatitis, BPH and prostate cancer. Though the precise molecular mechanisms potentiating prostate growth or inflammation mediated by HFD is unclear, current studies offer evidence supporting the involvement of the above described pathways in the development of these diseases. A schematic presentation of these pathways and disease development is shown in Figure 1. HFD-induced chronic inflammation plays a key role in the induction of prostate growth and BPH progression, while potentiation of oxidative stress may give rise to PIA lesions making the prostate vulnerable to cancer initiation. Our work in this area suggests that HFD-induced association between NF-ҡB and STAT-3 is possibly one such signaling mechanism that drives inflammation in the prostate. We speculate that HFD may also orchestrate other pathways that may trigger inflammation of the prostate leading to BPH or cancer. Dietary fat has been reported to affect the secretion and metabolism of androgens [108]. While HFD may deregulate androgen metabolism, future work may shed light on the signaling pathways that crosstalk or activate NF-ҡB, STAT-3 and AR, promoting the onset of BPH/LUTS or prostate cancer. The pro-survival members of Bcl-2 family of proteins including Bcl-2, Bcl-XL and Mcl-1 have been shown to play a definitive role during prostate cancer progression and resistance to apoptosis [65]; however the role of these proteins in HFD-induced intraprostatic inflammation and its association with BPH/LUTS is lacking. Although evidences in the literature reveals transcriptional regulation of Bcl-2 by NF-ҡB and synergy between Akt and Bcl-XL, but these associations are not demonstrated in BPH/LUTS or in the HFD setting. Other potential pathways that may drive inflammation or proliferation in the prostate leading to BPH include but are not limited to IGF-1, estrogen or aromatase signaling [99]. These signaling pathways may lead to BPH in many ways: i) induce proliferation or inflammation independently, or ii) induce proliferation that may be a consequence of inflammation, and iii) crosstalk between proliferation and inflammation. It will be worthwhile to explore these pathways induced by HFD that may potentate BPH and other prostatic diseases.
Figure 1.
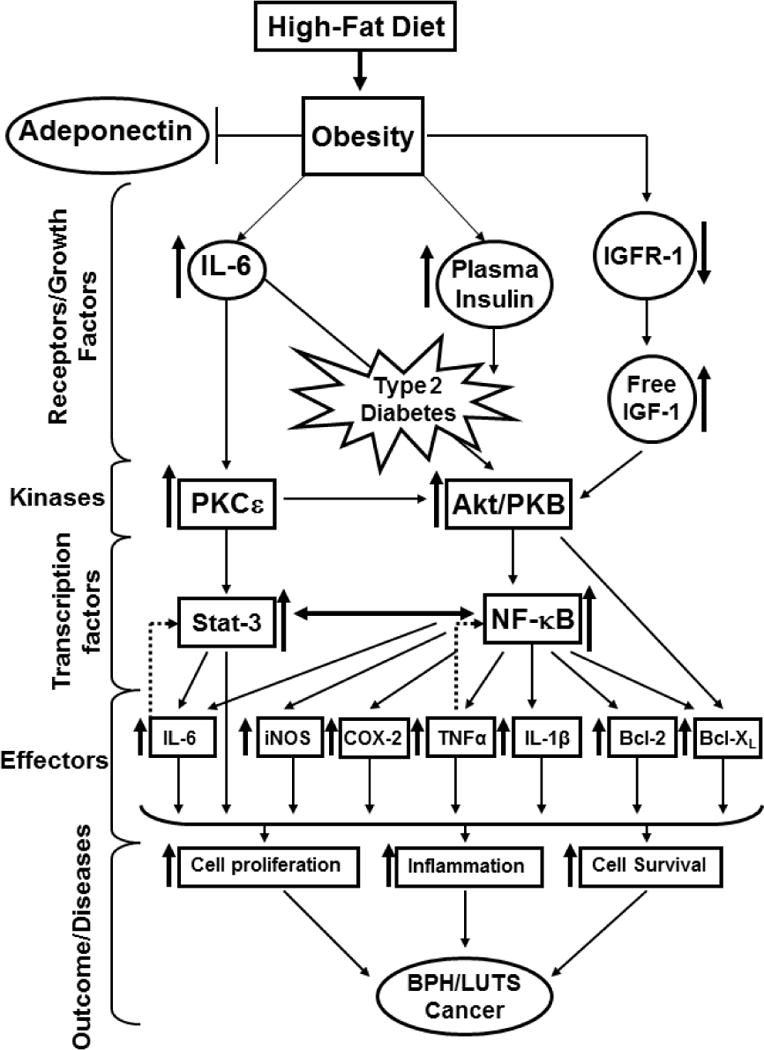
High-fat diet (HFD) is a key cause for benign prostatic hyperplasia, lower urinary tract symptom and cancer in men. The consequence of HFD-induced obesity leads to prostate diseases observed at various levels. 1) Adeponectin, involved in the regulation of glucose levels and fatty acid breakdown is deregulated by HFD contributing to obesity. 2) HFD causes alterations of receptors and growth factors such as IGFR-1, IL-6 and plasma insulin leading to type 2 diabetes. 3) Upstream kinases such as PKCε and Akt/PKB are constitutively activated by these receptors and growth factors that in turn activate transcription factors, Stat-3 and NF-ҡB initiating pro-inflammatory signaling pathways. 4) These transcription factors translocate into the nucleus and initiate transcription of several genes (iNOS, COX-2, TNFα, Bcl-XL, Bcl-2, IL-1β, IL-6) responsible for cell proliferation, inflammation and cell survival. The crosstalk between Stat-3 and NF-ҡB sustain pro-inflammatory signals and enhance disease progression. ↑ denotes upregulation, ↓ denotes downregulation, ↔ denotes crosstalk, and dotted arrow denotes feedback regulation.
In summary, substantial research has demonstrated that lifestyle factors, especially natural and fiber-rich diet provides an opportunity for prevention of prostate diseases. It becomes potentially beneficial and important to promote healthy diets that would lower the risk for various prostate diseases and help in reducing the costs of medical treatment.
Acknowledgments
The original work cited in this review was supported by grants from United States Public Health Services RO1 CA108512, RO1 AT002709, to S.G. and P20DK090871 to F.D. We apologize to those investigators whose original work could not be cited owing to the space limitation.
Abbreviations
- BPH
benign prostatic hyperplasia
- COX-2
cyclooxgynase-2
- EGFR
epidermal growth factor receptor
- FGFR
fibroblast growth factor receptor
- gp130
glycoprotein 130
- GPx3
glutathione peroxidase 3
- HFD
high-fat diet
- HGPIN
high-grade prostatic intraepithelial neoplasia
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- IKK
inhibitor of the ҡB kinase
- JAK
janus kinase
- LUTS
lower urinary tract symptom
- NF-ҡB
nuclear factor-kappa B
- PIA
proliferative inflammatory atrophy
- PI3K
phosphoinositide 3-kinase
- PKCε
protein kinase C epsilon
- PGE2
prostaglandin E2
- ROS
reactive oxygen species
- STAT-3
signal transducer and activator of transcription
- TRAMP
transgenic adenocarcinoma of the mouse prostate
Footnotes
Conflict of interest: All authors disclose no financial or commercial conflict of interest.
References
- 1.Shah B, Sha D, Xie D, Mohler ER, 3rd, Berger JS. The relationship between diabetes, metabolic syndrome, and platelet activity as measured by mean platelet volume: the National Health And Nutrition Examination Survey, 1999–2004. Diabetes Care. 2012;35:1074–1078. doi: 10.2337/dc11-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang S, Mao Q, Lin Y, Wu J, Wang X, Zheng X, Xie L. Body mass index and risk of BPH: a meta-analysis. Prostate Cancer Prostatic Dis. 2012;15:265–272. doi: 10.1038/pcan.2011.65. [DOI] [PubMed] [Google Scholar]
- 3.Nandeesha H. Benign prostatic hyperplasia: dietary and metabolic risk factors. Int Urol Nephrol. 2008;40:649–656. doi: 10.1007/s11255-008-9333-z. [DOI] [PubMed] [Google Scholar]
- 4.Powell K. Obesity: the two faces of fat. Nature. 2007;447:525–527. doi: 10.1038/447525a. [DOI] [PubMed] [Google Scholar]
- 5.Christensen JH, Fabrin K, Borup K, Barber N, Poulsen J. Prostate tissue and leukocyte levels of n-3 polyunsaturated fatty acids in men with benign prostate hyperplasia or prostate cancer. BJU Int. 2006;97:270–273. doi: 10.1111/j.1464-410X.2006.05951.x. [DOI] [PubMed] [Google Scholar]
- 6.Savas M, Verit A, Ciftci H, Yeni E, Aktan E, Topal U, Erel O. Oxidative stress in BPH. J Nepal Med Assoc. 2009;48:41–45. [PubMed] [Google Scholar]
- 7.Sciarra A, Mariotti G, Salciccia S, Gomez AA, Monti S, Toscano V, Di Silverio F. Prostate growth and inflammation. J Steroid Biochem Mol Biol. 2008;108:254–260. doi: 10.1016/j.jsbmb.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Montie JE, Pienta KJ. Review of the role of androgenic hormones in the epidemiology of benign prostatic hyperplasia and prostate cancer. Urology. 1994;43:892–899. doi: 10.1016/0090-4295(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 9.MacLennan GT, Eisenberg R, Fleshman RL, Taylor JM, Fu P, Resnick MI, Gupta S. The influence of chronic inflammation in prostatic carcinogenesis: a 5-year follow up study. J Urol. 2006;176:1012–1016. doi: 10.1016/j.juro.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 10.Gurel B, Lucia MS, Thompson IM, Jr, Goodman PJ, Tangen CM, Kristal AR, et al. Chronic inflammation in benign prostate tissue is associated with high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2014;23:847–856. doi: 10.1158/1055-9965.EPI-13-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giovannucci E, Rimm EB, Colditz GA, Stampfer MJ, Ascherio A, Chute CC, Willett WC. A prospective study of dietary fat and risk of prostate cancer. J Natl Cancer Inst. 1993;85:1571–1579. doi: 10.1093/jnci/85.19.1571. [DOI] [PubMed] [Google Scholar]
- 12.Sekine Y, Osei-Hwedieh D, Matsuda K, Raghavachari N, Liu D, Furuya Y, et al. High fat diet reduces the expression of glutathione peroxidase 3 in mouse prostate. Prostate. 2011;71:1499–1509. doi: 10.1002/pros.21365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llaverias G, Danilo C, Wang Y, Witkiewicz AK, Daumer K, Lisanti MP, et al. A Western-type diet accelerates tumor progression in an autochthonous mouse model of prostate cancer. Am J Pathol. 2010;177:3180–3191. doi: 10.2353/ajpath.2010.100568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vykhovanets EV, Shankar E, Vykhovanets OV, Shukla S, Gupta S. High-fat diet increases NF-κB signaling in the prostate of reporter mice. Prostate. 2011;71:147–156. doi: 10.1002/pros.21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai X, Haleem R, Oram S, Cyriac J, Jiang F, Grayhack JT, et al. High fat diet increases the weight of rat ventral prostate. Prostate. 2001;49:1–8. doi: 10.1002/pros.1112. [DOI] [PubMed] [Google Scholar]
- 16.Kulkarni P, Getzenberg RH. High-fat diet, obesity and prostate disease: the ATX-LPA axis? Nat Clin Pract Urol. 2009;6:128–131. doi: 10.1038/ncpuro1311. [DOI] [PubMed] [Google Scholar]
- 17.Mingguo H, Shintaro N, Kazuyuki N, Hiroshi T, Mitsuru S, Takamitsu I, et al. A high-fat diet enhances proliferation of prostate cancer cells and activates MCP-1/CCR2 signaling. Prostate. 2012;72:1779–1788. doi: 10.1002/pros.22531. [DOI] [PubMed] [Google Scholar]
- 18.St Sauver JL, Jacobsen SJ. Inflammatory Mechanisms Associated with Prostatic Inflammation and Lower Urinary Tract Symptoms. Curr Prostate Rep. 2008;6:67–73. doi: 10.1007/s11918-008-0011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vykhovanets EV, Shukla S, MacLennan GT, Resnick MI, Carlsen H, Blomhoff R, Gupta S. Molecular imaging of NF-kappaB in prostate tissue after systemic administration of IL-1 beta. Prostate. 2008;68:34–41. doi: 10.1002/pros.20666. [DOI] [PubMed] [Google Scholar]
- 20.Vykhovanets EV, Shukla S, MacLennan GT, Vykhovanets OV, Bodner DR, Gupta S. Il-1 beta-induced post-transition effect of NF-kappa B provides time-dependent wave of signals for initial phase of intrapostatic inflammation. Prostate. 2009;69:633–643. doi: 10.1002/pros.20916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146:4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- 22.Oh-I S, Thaler JP, Ogimoto K, Wisse BE, Morton GJ, Schwartz MW. Central administration of interleukin-4 exacerbates hypothalamic inflammation and weight gain during high-fat feeding. Am J Physiol Endocrinol Metab. 2010;299:E47–53. doi: 10.1152/ajpendo.00026.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 24.Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994;43:1271–1278. doi: 10.2337/diab.43.11.1271. [DOI] [PubMed] [Google Scholar]
- 25.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiao P, Xu H. Adipose inflammation: cause or consequence of obesity-related insulin resistance. Diabetes Metab Syndr Obes. 2008;1:25–31. doi: 10.2147/dmso.s4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schreyer SA, Chua SC, Jr, LeBoeuf RC. Obesity and diabetes in TNF-alpha receptor-deficient mice. J Clin Invest. 1998;102:402–411. doi: 10.1172/JCI2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 29.Shankar E, Vykhovanets EV, Vykhovanets OV, Maclennan GT, Singh R, Bhaskaran N, et al. High-fat diet activates pro-inflammatory response in the prostate through association of Stat-3 and NF-κB. Prostate. 2012;72:233–243. doi: 10.1002/pros.21425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Alcaide P, Liu L, Sun J, He A, Luscinskas FW, et al. Regulation of endothelial cell adhesion molecule expression by mast cells, macrophages, and neutrophils. PLoS One. 2011;6:e14525. doi: 10.1371/journal.pone.0014525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faour WH, Mancini A, He QW, Di Battista JA. T-cell-derived interleukin-17 regulates the level and stability of cyclooxygenase-2 (COX-2) mRNA through restricted activation of the p38 mitogen-activated protein kinase cascade: role of distal sequences in the 3′-untranslated region of COX-2 mRNA. J Biol Chem. 2003;278:26897–26907. doi: 10.1074/jbc.M212790200. [DOI] [PubMed] [Google Scholar]
- 32.Vykhovanets EV, Maclennan GT, Vykhovanets OV, Gupta S. IL-17 Expression by macrophages is associated with proliferative inflammatory atrophy lesions in prostate cancer patients. Int J Clin Exp Pathol. 2011;4:552–565. [PMC free article] [PubMed] [Google Scholar]
- 33.Wang W, Bergh A, Damber JE. Chronic inflammation in benign prostate hyperplasia is associated with focal upregulation of cyclooxygenase-2, Bcl-2, and cell proliferation in the glandular epithelium. Prostate. 2004;61:60–72. doi: 10.1002/pros.20061. [DOI] [PubMed] [Google Scholar]
- 34.Palapattu GS, Sutcliffe S, Bastain PJ, Platz EA. Prostate carcinogenesis and inflammation: emerging insights. Carcinogenesis. 2004;26:1170–1181. doi: 10.1093/carcin/bgh317. [DOI] [PubMed] [Google Scholar]
- 35.Sugar LM. Inflammation and prostate cancer. Can J Urol. 2006;13:46–47. [PubMed] [Google Scholar]
- 36.Buskens CJ, Van Rees BP, Sivula A, Reitsma JB, Haglund C, Bosma PJ, et al. Prognostic significance of elevated cyclooxygenase 2 expression in patients with adenocarcinoma of the esophagus. Gastroenterology. 2002;122:1800–1807. doi: 10.1053/gast.2002.33580. [DOI] [PubMed] [Google Scholar]
- 37.Hur GM, Ryu YS, Yun HY, Jeon BH, Kim YM, Seok JH, et al. Hepatic ischemia/reperfusion in rats induces iNOS gene expression by activation of NF-κB. Biochem Biophys Res Commun. 1999;261:917–922. doi: 10.1006/bbrc.1999.1143. [DOI] [PubMed] [Google Scholar]
- 38.Hussain T, Gupta S, Mukhtar H. Cyclooxygenase-2 and prostate carcinogenesis. Cancer Lett. 2003;191:125–135. doi: 10.1016/s0304-3835(02)00524-4. [DOI] [PubMed] [Google Scholar]
- 39.Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, Lee SS. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: Down-regulation of COX-2 and iNOS through suppression of NF-kappaB activation. Mutat Res. 2001:480–481. 243–268. doi: 10.1016/s0027-5107(01)00183-x. [DOI] [PubMed] [Google Scholar]
- 40.Gradini R, Realacci M, Petrangeli E, Di Silverio F, Russo M. Nitric oxide synthases in normal and benign hyperplastic human prostate: immunohistochemistry and molecular biology. J Pathog. 1999;189:224–229. doi: 10.1002/(SICI)1096-9896(199910)189:2<224::AID-PATH422>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 41.Dobrian AD, Davies MJ, Schriver SD, Lauterio TJ, Prewitt RL. Oxidative stress in a rat model of obesity-induced hypertension. Hypertension. 2001;37:554–560. doi: 10.1161/01.hyp.37.2.554. [DOI] [PubMed] [Google Scholar]
- 42.Naber KG, Weidner W. Chronic prostatitis-an infectious disease? J Antimicrob Chemother. 2000;46:157–161. doi: 10.1093/jac/46.2.157. [DOI] [PubMed] [Google Scholar]
- 43.Farooqui T, Farooqui AA. Lipid-mediated oxidative stress and inflammation in the pathogenesis of Parkinson’s disease. Parkinsons Dis. 2011;2011:247–467. doi: 10.4061/2011/247467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khandrika L, Kumar B, Koul S, Maroni P, Koul HK. Oxidative stress in prostate cancer. Cancer Lett. 2009;282:125–136. doi: 10.1016/j.canlet.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar B, Koul S, Khandrika L, Meacham RB, Koul HK. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008;68:1777–1785. doi: 10.1158/0008-5472.CAN-07-5259. [DOI] [PubMed] [Google Scholar]
- 46.Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 47.Lim SD, Sun C, Lambeth JD, Marshall F, Amin M, Chung L, Petros JA, Arnold RS. Increased Nox1 and hydrogen peroxide in prostate cancer. Prostate. 2005;62:200–207. doi: 10.1002/pros.20137. [DOI] [PubMed] [Google Scholar]
- 48.Brar SS, Corbin Z, Kennedy TP, Hemendinger R, Thornton L, Bommarius B. NOX5 NAD(P)H oxidase regulates growth and apoptosis in DU 145 prostate cancer cells. Am J Physiol Cell Physiol. 2003;285:C353–C369. doi: 10.1152/ajpcell.00525.2002. [DOI] [PubMed] [Google Scholar]
- 49.Block K, Ricono JM, Lee DY, Bhandari B, Choudhury GG, Abboud HE, Gorin Y. Arachidonic acid-dependent activation of a p22(phox)-based NAD(P)H oxidase mediates angiotensin II-induced mesangial cell protein synthesis and fibronectin expression via Akt/PKB. Antioxid Redox Signal. 2006;8:1497–1508. doi: 10.1089/ars.2006.8.1497. [DOI] [PubMed] [Google Scholar]
- 50.Arbiser JL, Petros J, Klafter R, Govindajaran B, McLaughlin ER, Brown LF, et al. Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc Natl Acad Sci USA. 2002;99:715–720. doi: 10.1073/pnas.022630199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappa B collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21:11–19. doi: 10.1016/j.cytogfr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karin M. Nuclear factor-B in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 53.Richmond A. NF-kappa B, chemokine gene transcription and tumor growth. Nat Rev Immunol. 2002;2:664–674. doi: 10.1038/nri887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanz AB, Sanchez-Niño MD, Ramos AM, Moreno JA, Santamaria B, Ruiz-Ortega M, et al. NF-kappaB in renal inflammation. J Am Soc Nephrol. 2010;21:1254–1262. doi: 10.1681/ASN.2010020218. [DOI] [PubMed] [Google Scholar]
- 55.Caramori G, Adcock IM, Ito K. Anti-inflammatory inhibitors of IkappaB kinase in asthma and COPD. Curr Opin Investig Drugs. 2004;5:1141–1147. [PubMed] [Google Scholar]
- 56.Kaltschmidt B, Widera D, Kaltschmidt C. Signaling via NF-kappaB in the nervous system. Biochim Biophys Acta. 2005;1745:287–299. doi: 10.1016/j.bbamcr.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 57.Okamoto T. NF-kappaB and rheumatic diseases. Endocr Metab Immune Disord Drug Targets. 2006;6:359–372. doi: 10.2174/187153006779025685. [DOI] [PubMed] [Google Scholar]
- 58.Schottelius AJ, Dinter H. Cytokines, NF-kappa-B, microenvironment, intestinal inflammation and cancer. Cancer Treat Res. 2006;130:67–87. doi: 10.1007/0-387-26283-0_3. [DOI] [PubMed] [Google Scholar]
- 59.Shukla S, MacLennan GT, Fu P, Patel J, Marengo SR, Resnick MI, Gupta S. Nuclear factor-kappaB/p65 (Rel A) is constitutively activated in human prostate adenocarcinoma and correlates with disease progression. Neoplasia. 2004;6:390–400. doi: 10.1593/neo.04112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sweeney C, Li L, Shanmugam R, Bhat-Nakshatri P, Jayaprakasan V, Baldridge LA, Gardner T, et al. Nuclear factor-B is constitutively activated in prostate cancer in vitro and is overexpressed in prostatic intraepithelial neoplasia and adenocarcinoma of the prostate. Clin Cancer Res. 2004;10:5501–5507. doi: 10.1158/1078-0432.CCR-0571-03. [DOI] [PubMed] [Google Scholar]
- 61.Carlsen H, Haugen F, Zadelaar S, Kleemann R, Kooistra T, Drevon CA, Blomhoff R. Diet-induced obesity increases NF-kappaB signaling in reporter mice. Genes Nutr. 2009;4:215–222. doi: 10.1007/s12263-009-0133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maloney E, Sweet IR, Hockenbery DM, Pham M, Rizzo NO, Tateya S, et al. Activation of NF-kappaB by palmitate in endothelial cells: a key role for NADPH oxidase-derived superoxide in response to TLR4 activation. Arterioscler Thromb Vasc Biol. 2009;29:1370–1375. doi: 10.1161/ATVBAHA.109.188813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pasparakis M. Regulation of tissue homeostasis by NF-kappaB signalling: implications for inflammatory diseases. Nat Rev Immunol. 2009;9:778–788. doi: 10.1038/nri2655. [DOI] [PubMed] [Google Scholar]
- 64.Blackwell TS, Christman JW. The role of nuclear factor-kappa B in cytokine gene regulation. Am J Respir Cell Mol Biol. 1997;17:3–9. doi: 10.1165/ajrcmb.17.1.f132. [DOI] [PubMed] [Google Scholar]
- 65.Catz SD, Johnson JL. Transcriptional regulation of bcl-2 by nuclear factor kappaB and its significance in prostate cancer. Oncogene. 2001;20:7342–7351. doi: 10.1038/sj.onc.1204926. [DOI] [PubMed] [Google Scholar]
- 66.Elmarakby AA, Imig JD. Obesity is the major contributor to vascular dysfunction and inflammation in high-fat diet hypertensive rats. Clin Sci (Lond) 2010;118:291–301. doi: 10.1042/CS20090395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock NM, Magness S, et al. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS One. 2010;5:e12191. doi: 10.1371/journal.pone.0012191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brantley EC, Benveniste EN. Signal transducer and activator of transcription-3: a molecular hub for signaling pathways in gliomas. Mol Cancer Res. 2008;6:675–684. doi: 10.1158/1541-7786.MCR-07-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aggarwal BB, Kunnumakkara AB, Harikumar KB, Gupta SR, Tharakan ST, Koca C, et al. Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann N Y Acad Sci. 2009;1171:59–76. doi: 10.1111/j.1749-6632.2009.04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Naugler WE, Karin M. The wolf in sheep’s clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008;14:109–119. doi: 10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 72.Mahboubi K, Li F, Plescia J, Kirkiles-Smith NC, Mesri M, Du Y, et al. Interleukin-11 up-regulates survivin expression in endothelial cells through a signal transducer and activator of transcription-3 pathway. Lab Invest. 2001;81:327–334. doi: 10.1038/labinvest.3780241. [DOI] [PubMed] [Google Scholar]
- 73.Bollrath J, Greten FR. IKK/NF-kappa B and STAT3 pathways: central signalling hubs in inflammation-mediated tumour promotion and metastasis. EMBO Rep. 2009;10:1314–1319. doi: 10.1038/embor.2009.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jarnicki A, Putoczki T, Ernst M. Stat3: linking inflammation to epithelial cancer – more than a “gut” feeling? Cell Div. 2010;5:14. doi: 10.1186/1747-1028-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Phillips CM, Goumidi L, Bertrais S, Field MR, Peloso GM, Shen J, et al. Dietary saturated fat modulates the association between STAT3 polymorphisms and abdominal obesity in adults. J Nutr. 2009;139:2011–2017. doi: 10.3945/jn.109.110635. [DOI] [PubMed] [Google Scholar]
- 76.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee H, Herrmann A, Deng JH, Kujawski M, Niu G, Li Z, et al. Persistently activated Stat3 maintains constitutive NF-kappa B activity in tumors. Cancer Cell. 2009;15:283–293. doi: 10.1016/j.ccr.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Macha MA, Matta A, Chauhan SS, Siu KW, Ralhan R. Guggulsterone (GS) inhibits smokeless tobacco and nicotine-induced NF-κB and STAT3 pathways in head and neck cancer cells. Carcinogenesis. 2011;32:368–380. doi: 10.1093/carcin/bgq278. [DOI] [PubMed] [Google Scholar]
- 79.Squarize CH, Castilho RM, Sriuranpong V, Pinto DS, Jr, Gutkind JS. Molecular cross-talk between the NF-kappaB and STAT3 signaling pathways in head and neck squamous cell carcinoma. Neoplasia. 2006;8:733–746. doi: 10.1593/neo.06274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Han SS, Yun H, Son DJ, Tompkins VS, Peng L, Chung ST, et al. NF-kappaB/STAT3/PI3K signaling crosstalk in iMyc E mu B lymphoma. Mol Cancer. 2010;9:97. doi: 10.1186/1476-4598-9-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nature Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 82.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 83.Aziz MH, Manoharan HT, Church DR, Dreckschmidt NE, Zhong W, Oberley TD, et al. Protein kinase Cepsilon interacts with signal transducers and activators of transcription 3 (Stat3), phosphorylates Stat3Ser727, and regulates its constitutive activation in prostate cancer. Cancer Res. 2007;67:8828–8838. doi: 10.1158/0008-5472.CAN-07-1604. [DOI] [PubMed] [Google Scholar]
- 84.Kane LP, Shapiro VS, Stokoe D, Weiss A. Induction of NF-kappaB by the Akt/PKB kinase. Curr Biol. 1999;9:601–604. doi: 10.1016/s0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- 85.Samuel VT, Liu ZX, Wang A, Beddow SA, Geisler JG, Kahn M, et al. Inhibition of protein kinase Cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest. 2007;117:739–745. doi: 10.1172/JCI30400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eiras S, Camiña JP, Diaz-Rodriguez E, Gualillo O, Casanueva FF. Leptin inhibits lysophosphatidic acid-induced intracellular calcium rise by a protein kinase C-dependent mechanism. J Cell Physiol. 2004;201:214–226. doi: 10.1002/jcp.20046. [DOI] [PubMed] [Google Scholar]
- 87.Metlakunta AS, Sahu M, Sahu A. Hypothalamic phosphatidylinositol 3-kinase pathway of leptin signaling is impaired during the development of diet-induced obesity in FVB/N mice. Endocrinology. 2008;149:1121–1128. doi: 10.1210/en.2007-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ohashi K, Kanazawa A, Tsukada S, Maeda S. PKCepsilon induces interleukin-6 expression through the MAPK pathway in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2005;327:707–712. doi: 10.1016/j.bbrc.2004.12.072. [DOI] [PubMed] [Google Scholar]
- 89.Knight SF, Quigley JE, Yuan J, Roy SS, Elmarakby A, Imig JD. Endothelial dysfunction and the development of renal injury in spontaneously hypertensive rats fed a high-fat diet. Hypertension. 2008;51:352–359. doi: 10.1161/HYPERTENSIONAHA.107.099499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Koh KK, Han SH, Quon MJ. Inflammatory markers and the metabolic syndrome: insights from therapeutic interventions. J Am Coll Cardiol. 2005;46:1978–1985. doi: 10.1016/j.jacc.2005.06.082. [DOI] [PubMed] [Google Scholar]
- 91.Neels JG, Olefsky JM. Inflamed fat: what starts the fire? J Clin Invest. 2006;16:33–35. doi: 10.1172/JCI27280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Deslypere JP, Verdonck L, Vermeulen A. Fat tissue: a steroid reservoir and site of steroid metabolism. J Clin Endo Metab. 1985;61:564–570. doi: 10.1210/jcem-61-3-564. [DOI] [PubMed] [Google Scholar]
- 93.Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab. 2009;297:1247–1259. doi: 10.1152/ajpendo.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Colli S, Silveira Cavalcante F, Peixoto Martins M, Sampaio FJ, da Fonte Ramos C. Leptin role in the rat prostate ventral lobe. Fertil Steril. 2011;95:1490–1493. doi: 10.1016/j.fertnstert.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 95.Nazian SJ, Cameron DF. Temporal relation between leptin and various indices of sexual maturation in the male rat. J Androl. 1999;20:487–491. [PubMed] [Google Scholar]
- 96.Sakr WA, Grignon DJ, Haas GP, Heilbrun LK, Pontes JE, Crissman JD. Age and racial distribution of prostatic intraepithelial neoplasia. Eur Urol. 1996;30:138. doi: 10.1159/000474163. [DOI] [PubMed] [Google Scholar]
- 97.Stattin P, Söderberg S, Hallmans G, Bylund A, Kaaks R, Stenman UH, et al. Leptin is associated with increased prostate cancer risk: a nested case-referent study. J Clin Endocrinol Metab. 2001;86:1341–1345. doi: 10.1210/jcem.86.3.7328. [DOI] [PubMed] [Google Scholar]
- 98.Frederich RC, Hamann A, Anderson S, Löllmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;1:1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 99.Bhaskaran N, Shukla S, Thakur VS, Babcook MA, MacLennan GT, Liu G, Daneshgari F, Gupta S. High-fat diet induces inflammation by increasing estrogen levels through Stat3, estrogen receptor alpha and aromatase in the mouse prostate. Cancer Res. 2013;73:5452. [Google Scholar]
- 100.Ghigliotti G, Barisione C, Garibaldi S, Fabbi P, Brunelli C, Spallarossa P, Altieri P, Rosa G, Spinella G, Palombo D, Arsenescu R, Arsenescu V. Adipose tissue immune response: novel triggers and consequences for chronic inflammatory conditions. Inflammation. 2014;37:1337–1353. doi: 10.1007/s10753-014-9914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105:141–150. doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 102.Ma X, Hua J, Li Z. Probiotics improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. J Hepatol. 2008;49:821–830. doi: 10.1016/j.jhep.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wasinski F, Bacurau RF, Moraes MR, Haro AS, Moraes-Vieira PM, Estrela GR, Paredes-Gamero EJ, Barros CC, Almeida SS, Câmara NO, Araujo RC. Exercise and caloric restriction alter the immune system of mice submitted to a high-fat diet. Mediators Inflamm. 2013;2013:395672. doi: 10.1155/2013/395672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Buckman LB, Hasty AH, Flaherty DK, Buckman CT, Thompson MM, Matlock BK, Weller K, Ellacott KL. Obesity induced by a high-fat diet is associated with increased immune cell entry into the central nervous system. Brain, Behavior, and Immunity. 2014;35:33–42. doi: 10.1016/j.bbi.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kramer G, Steiner GE, Handisurya A, Stix U, Haitel A, Knerer B, Gessl A, Lee C, Marberger M. Increased expression of lymphocyte-derived cytokines in benign hyperplastic prostate tissue, identification of the producing cell types, and effect of differentially expressed cytokines on stromal cell proliferation. Prostate. 2002;52:43–58. doi: 10.1002/pros.10084. [DOI] [PubMed] [Google Scholar]
- 106.Steiner GE, Stix U, Handisurya A, Willheim M, Haitel A, Reithmayr F, Paikl D, Ecker RC, Hrachowitz K, Kramer G, Lee C, Marberger M. Cytokine expression pattern in benign prostatic hyperplasia infiltrating T cells and impact of lymphocytic infiltration on cytokine mRNA profile in prostatic tissue. Lab Invest. 2003;83:1131–1146. doi: 10.1097/01.lab.0000081388.40145.65. [DOI] [PubMed] [Google Scholar]
- 107.König JE, Senge T, Allhoff EP, König W. Analysis of the inflammatory network in benign prostate hyperplasia and prostate cancer. Prostate. 2004;58:121–129. doi: 10.1002/pros.10317. [DOI] [PubMed] [Google Scholar]
- 108.Gromadzka-Ostrowska J. Effects of dietary fat on androgen secretion and metabolism. Reprod Biol. 2006;6:13–20. [PubMed] [Google Scholar]


