Abstract
It has recently become clear that obstructive sleep apnea (OSA) is an independent risk factor for the development of metabolic syndrome, a disorder of defective energy storage and use. Several mechanisms have been proposed to explain this finding, drawing upon the characteristics that define OSA. In particular, intermittent hypoxia, sleep fragmentation, elevated sympathetic tone, and oxidative stress – all consequences of OSA – have been implicated in the progression of poor metabolic outcomes in OSA. In this review we examine the evidence to support each of these disease manifestations of OSA as a unique risk for metabolic dysfunction. Tissue hypoxia and sleep fragmentation are each directly connected to insulin resistance and hypertension, and each of these also may increase sympathetic tone, resulting in defective glucose homeostasis, excessive lipolysis, and elevated blood pressure. Oxidative stress further worsens insulin resistance and in turn, metabolic dysfunction also increases oxidative stress. However, despite many studies linking each of these individual components of OSA to the development of metabolic syndrome, there are very few reports that actually provide a coherent narrative about the mechanism underlying metabolic dysfunction in OSA.
Keywords: hypoxia, oxidative stress, sleep fragmentation, sympathetic nervous system
INTRODUCTION
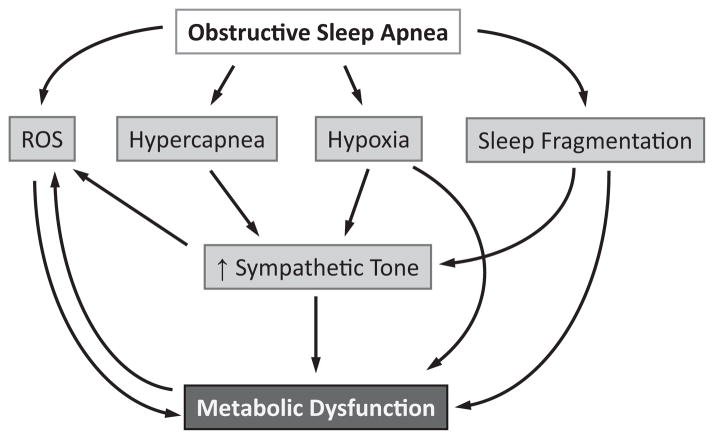
Obstructive sleep apnea (OSA) is a common syndrome, affecting approximately 20–30% of men, and 10–15% of women.1–3 In the last 15 years, many studies have shown that OSA is a risk factor for the development of metabolic syndrome, independent of obesity.4,5 Metabolic syndrome is marked by the presence of at least three of the following: abdominal obesity, hypertriglyceridemia, low plasma high-density lipoprotein (HDL) levels, hyperglycemia, and elevated blood pressure.6 Insulin resistance is a primary manifestation of metabolic syndrome,7 and metabolic syndrome is a major risk factor for cardiovascular morbidity and mortality.6 The most commonly cited pathways by which OSA is believed to affect metabolism include oxidative stress, sympathetic nervous system activation, tissue hypoxia, and sleep fragmentation (Fig. 1). The purpose of this review is to critically examine these theories and the literature supporting their validity in linking OSA to metabolic syndrome.
Figure 1.
Proposed mechanisms that may underlie metabolic dysfunction in obstructive sleep apnea. ROS, reactive oxygen species.
OXIDATIVE STRESS
Links between OSA and oxidative stress
Reactive oxygen species (ROS) are molecules possessing one or more unpaired electrons, such as superoxide (O2·−), hydrogen peroxide (H2O2), or hydroxyl radicals (HO·). Reactive nitrogen species (RNS) describe nitric oxide synthase products, including nitric oxide (NO) or peroxynitrate (OONO−). The odd number of electrons in these molecules renders them highly reactive, which is why both ROS and RNS have important roles in both health and disease. ROS are formed from the reduction of oxygen during respiration, or may be generated by ROS-generating enzyme systems. ROS may also enter biological systems from the environment in the form of pollutants, UV radiation, carcinogens, smoking, and infection. They play critical physiological roles in signal transduction, anti-microbial defense, and cell proliferation. However, when present in high levels, ROS interfere with cell structure and/or function, leading to “oxidative stress.”
Some manifestations of oxidative stress include modification of nucleic acids, leading to genetic mutations; oxidation of lipids, which can foster atherosclerosis; or oxidation of proteins, which can alter enzyme function and signaling pathways. To limit excessive ROS, anti-oxidant systems have evolved, including superoxide dismutase and glutathione reductase. In 1956, Harman proposed the free radical theory of aging.8 Since then, oxidative stress has been implicated to varying degrees in the development and progression of atherosclerosis, neurodegenerative disorders, diabetes, and cancer.
Given the transient existence of most ROS/RNS, quantitative assessment of oxidative stress is difficult. It is often easier to measure the impact of ROS on their cellular environment. For instance, ROS interact with glutathione, a free radical scavenging protein, changing it from reduced (GSH) to oxidized (GSSG) gluthathione in the process. Inferences about the redox state of a biological system can also be drawn by measuring levels or types of oxidized cellular lipids, protein, or nucleic acids. In addition, investigators may analyze the activity of ROS-generating enzyme systems such as xanthine oxidase, lipooxgenase, or nicotinamide adenine dinucleotide phosphate-oxidase (NADPH) oxidase.
Several studies have demonstrated that OSA patients exhibit elevated markers of oxidative stress. These biomarkers include modified lipids,9–13 protein,14,15 nucleic acids,16 and reduction in antioxidant capacity.17 In addition, OSA is associated with increased ROS production from leukocytes,18,19 with attenuation of this phenomenon after CPAP treatment. A thorough cataloguing of these studies appears in expert reviews.20,21
The mechanism by which OSA induces oxidative stress is unknown, but is commonly ascribed to hypoxia. OSA is characterized by transient falls in hemoglobin saturation during obstructed or flow-limited breathing, followed by recovery of oxygen levels during resumption of effective breathing. This cyclic pattern of hypoxia and re-oxygenation is termed intermittent hypoxia (IH). Chronic IH in rats increases brain oxidative stress22 and NADPH oxidase activity.23 Chronic IH in mice induces cardiac lipid peroxidation24 in association with left ventricular dysfunction, as well as increased nuclear factor (NF-κB) expression in the aorta, heart and lungs.24 However, other studies either show no significant oxidative stress from IH exposure25 or selective changes in oxidative stress only in certain tissues such as the liver.26 The leading theory of how IH causes oxidative stress contends that “the oscillation of O2 concentrations during chronic IH remarkably mimics the processes of ischemia/re-oxygenation and could therefore increase cellular production of ROS”.22 Ischemia-reperfusion (I-R) injury is a devastating form of tissue damage often occurring in the setting of thrombosis or transplantation,27 where ROS mediate reperfusion capillary leak and organ dysfunction.28 During the ischemic phase of I-R injury, ATP depletion causes toxic accumulations of metabolites and buildup of intracellular calcium. The high calcium level may be a stimulus for protease activation of xanthine oxidase,29 an enzyme that catabolizes purines and in so doing, generates ROS. During reperfusion, oxygen becomes available as a substrate for xanthine oxidase H2O2 production. Influx of activated neutrophils may cause further oxidative injury from NADPH oxidase.30
It is not known whether the IH of OSA in patients with preserved blood flow is sufficient to induce the same pathophysiology as I-R injury. Inadequacy of oxygen to sustain usual metabolic activity (i.e. ischemia) is not evident even when breathing severely hypoxic gases, which has led to a critical view of the term “hypoxia”.27 In addition, sustained hypoxia (i.e. from high altitude exposure) without reoxygenation induces oxidative stress.31–34 This oxidative stress may be due to a paradoxical increase in the amount of superoxide normally generated by mitochrondria during respiration. This phenomenon has been termed “reductive stress”.35 It might be argued that the distinctions between ROS generation from I-R theory and reductive stress are merely academic. However, there may be practical implications for how sleep-related hypoxemia is addressed, depending upon the nature and origin of ROS in OSA.
Obstructive sleep apnea may also induce oxidative stress by other pathways that have received comparatively little attention. First, OSA dynamically increases circulating substrate levels during sleep, including glucose36 and free fatty acids (FFA).37 These elevations may be related to hypoxia-induced stimulation of the autonomic nervous system and lead to ROS generation through the aforementioned pathways. In particular, elevated FFA induce endothelial dysfunction via vascular ROS38 and induce skeletal muscle insulin resistance and inflammation.39 Second, OSA40,41 as well as experimental IH exposure in humans42 and rodents43 activates the renin-angiotensin system. Angiotensin II has potent effects on blood pressure, inflammation and oxidative stress,44 in part through its action on endothelial NADPH oxidase.45 ACE inhibitors have been shown to improve insulin signaling in skeletal muscle.46 In addition, the fluid retention from elevated renin-angiotensin could be a unifying factor behind the development of OSA and hypertension.47 Third, OSA can lead to sleep loss. Sleep deprivation, through as yet poorly understood pathways, induces production of the pro-inflammatory cytokines tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), and increases appetite, which can lead to systemic oxidative stress through excessive consumption of calorie-rich foods.48 Fourth, obesity is a common risk factor for both OSA and oxidative stress49 and may therefore drive the association between the two. In summary, OSA may induce oxidative stress through several possible pathways, although I-R injury is the most widely cited theory.
Links between oxidative stress and metabolism
Oxidative stress could play a role in the development or progression of diabetes or metabolic syndrome.50 Hypertension, a key component of metabolic syndrome, may be mediated by suppressed levels of the arterial vasodilator nitric oxide. Superoxide, generated by the stimulation of NADPH oxidase by Angiotensin II,51 degrades endothelial nitric oxide.52 Diets rich in fruits and vegetables53,54 and antioxidant vitamins55 have been shown to reduce blood pressure and oxidative stress. Another key element of metabolic syndrome is obesity. While it is not clear whether oxidative stress induces obesity, it is abundantly clear that obesity is as a pro-inflammatory state. Obese mice exhibit high levels of adipose tissue NADPH oxidase activity, in association with reduced expression of adiponectin and PPAR-γ and elevated TNF-α, reflecting an inflammatory phenotype. Inhibition of NADPH oxidase decreased inflammation and attenuated hyperlipidemia and hyperglycemia as well as liver steatosis.56 This study illustrates that ROS can directly alter metabolism through-cross talk with inflammatory pathways in adipose tissue.
One of the primary insults leading to diabetes mellitus is insulin resistance, which is also considered the core defect in metabolic syndrome.57 Insulin stimulates glucose uptake in muscle and adipose tissues, inhibits hepatic glucose production, and suppresses lipolysis. Circulating insulin binds its receptor on the surface of insulin-responsive cells. Upon insulin binding, the receptor undergoes auto-phosphorylation of tyrosine residues, which in turn leads to phosphorylation of intracellular insulin receptor substrates (IRS). Tyrosine-phosphorylated IRS proteins bind to Src-homology-2 (SH2) domains including phosphatidylinositol 3′-kinase (PI3K). PI3K in turn phosphorylates downstream messengers, which leads to GLUT-4 translocation (glucose transport), GSK-3 activation (glycogen synthesis), and fatty acid synthesis. The insulin signaling cascade can be interrupted by inflammatory stimuli. For example, TNF-α or increased FFA levels58 cause phosphorylation of IRS-1 on serine, instead of tyrosine residues, effectively preventing downstream insulin signal transduction, and IRS-1 binding to the insulin receptor.59
Several clinical studies show an association between oxidative stress and insulin resistance.60 Interestingly, physiologic levels of ROS (H2O2) are generated during insulin stimulation of target cells, and are actually necessary to propagate insulin signaling.60,61 Paradoxically, high levels of exogenous H2O2 inhibit insulin signaling62–64 and these levels of ROS may occur during in vivo insults such as hyperglycemia.60 In fact, chronically elevated glucose and FFA have been proposed as pathways leading to excessive mitochondrial ROS generation and insulin resistance.65 However, the more precise mechanisms by which ROS impair insulin signaling are unclear. Some of the proposed pathways are reviewed elsewhere66 and include (a) activation of Ser/Thr “stress” kinases such as NF-κB or p38-MAPK, which cause inappropriate phosphorylation of the insulin receptor or IRS; (b) disruption of cellular compartmentalization of insulin signaling elements; (c) reduced GLUT-4 transcription; and (d) impaired mitochondrial oxidation of fatty acids.67 A second “hit” in the pathogenesis of diabetes mellitus is pancreatic β-cell failure. ROS have been proposed as mediators of β-cell failure from excessive glucose and fatty acid loads.68 The hyperglycemia of diabetes induces oxidative stress, which may mediate devastating neurovascular consequences.69,70 Hence, a vicious cycle may be operant in which oxidative stress leads to diabetes, which in turn causes further oxidative stress.
A practical question is whether treating oxidative stress can improve glucose homeostasis. The data are limited, but small studies involving short-term administration of antioxidants have shown improved glucose disposal and/or insulin action.71–74 However, no long-term, large-scale studies have been published that demonstrate the efficacy of antioxidants for metabolic syndrome. Nutrition guidelines by the American Diabetes Association do not recommend antioxidant supplements75 and enthusiasm for the use of antioxidants has been tempered by disappointing trials for the primary and secondary prevention of cardiovascular diseases.76 Whether this lack of evidence should be construed as proof against the role of ROS in diabetes is controversial,77 reflecting the larger debate about the free radical theory of aging and disease.78
Evidence that metabolic dysfunction is mediated by oxidative stress in OSA
Several studies demonstrate that elements of metabolic syndrome, such as hypertension and insulin resistance, improve with CPAP treatment79 while other studies show that CPAP treatment attenuates oxidative stress.20 One month after CPAP treatment, Murri et al. showed a decrease in blood pressure that correlated with improvements in oxidative stress.80 However, these studies do not directly prove a functional role of oxidative stress in OSA.
One approach to demonstrate that oxidative stress is a mediator of OSA consequences is to examine the physiological or metabolic impact of antioxidant therapies in OSA. Intravenous administration of vitamin C to patients with OSA acutely normalized vascular responses to acetylcholine81 and improved flow-mediated dilation (FMD) of the brachial artery.82 Allopurinol, a xanthine oxidase inhibitor, also improved FMD compared to placebo in patients with OSA, in association with a lowering of plasma lipid peroxides.83 A diet enriched in fruits and vegetables led to weight loss and reductions of blood pressure in patients with OSA, albeit without a detectable change in antioxidant levels measured using a ferric-reducing/antioxidant power (FRAP) assay.84 Interestingly, the antioxidant N-acetylcysteine (NAC) improved lipid peroxidation, and improved OSA itself.85
Overall, there is compelling evidence that ROS may play a role in OSA-mediated endothelial dysfunction, but the role of oxidative stress in mediating other OSA consequences is decidedly less clear.
SYMPATHETIC NERVOUS SYSTEM
Links between OSA and sympathetic activation
The sympathetic nervous system (SNS) is responsible for eliciting adaptive responses to stressful stimuli. Physiologic stress stimulates the locus coeruleus in the hypothalamus,86,87 which instigates a variety of “fight-or-flight” reflexes, such as an increase in cardiac output, and redistribution of blood flow to skeletal muscles. In terms of metabolism, the SNS orchestrates a host of tissue signals that result in a net efflux of glucose and FFA into the bloodstream. Some of these signals arise from sympathetic efferent fibers acting upon adipose tissue, liver, and skeletal muscle. In addition, the adrenal medulla secretes catecholamines into the plasma, which are carried to target tissues. In contrast to these rapid effects of SNS stimulation, other responses to stress are activated more gradually, including the hypothalamic-pituitary-adrenal (HPA) axis, which mediates the secretion of corticotropin-releasing hormone, pituitary adrenocorticotropic hormone, and ultimately corticosteroids produced in the adrenal cortex.88,89
OSA has been firmly linked to SNS activation, confirmed by measurements of muscle sympathetic nerve activity and elevated urine and plasma catecholamines90–94 with normalization after CPAP.90,95 One of the hallmarks of OSA that increases SNS activity is IH. IH stimulates chemoreflexes in the carotid body that in turn stimulate the SNS,96,97 with evidence of increased efferent stimulation in cervical, renal, splanchnic, thoracic, and lumbar sympathetic nerves.96,98–101 In rodents, IH activates the sympathetic nervous system and increases catecholamine efflux by the adrenal medulla.96,97,102–113 Besides IH, OSA also can cause arousals from sleep and hypercapnia, and these stimuli have interactive effects on sympathetic signaling. For example, hypoxia and hypercapnia augment SNS activity in a synergistic manner.114,115 In experimentally-induced apneas in sleeping dogs, hypoxia and sleep arousals showed additive effects on SNS activity and systemic blood pressure.116
What are the functional consequences of IH-induced SNS activation? Healthy humans exposed to acute IH develop insulin resistance.117 Qualitatively similar findings occur in subjects exposed to acute sustained hypoxia.118 Moreover, acute hypoxia-induced insulin resistance is attenuated by the sympatholytic drug clonidine.119 It is not yet clear whether long-term IH also induces glucose intolerance or insulin resistance via SNS activity. In rats, IH causes a time-dependent increase in blood pressure. This elevation in blood pressure can be abolished by sympathetic denervation with 6-hydroxydopamine, denervation of the carotid bodies or sympathetic nerves, or adrenal medullectomy.110,120 However, it remains unclear whether IH is the primary driver of SNS activation and hypertension in human OSA. Exposure of humans to 2 weeks of IH increases daytime blood pressure121 in association with increased SNS activity, but preventing hypoxemia in OSA with supplemental oxygen does not lower blood pressure, although CPAP does.122
Links between the sympathetic nervous system and metabolism
Catecholamines are considered counter-regulatory hormones since they oppose the anabolic, energy-conserving, and glucose utilizing role of insulin. Catecholamines stimulate glucagon secretion, activate glycogenolysis and gluconeogenesis in the liver, and cause breakdown of muscle glycogen and adipose tissue triglycerides. They also inhibit insulin secretion and insulin-mediated glucose uptake by skeletal muscle.123–125 The tight coupling of SNS and metabolism is particularly evident in the anatomy of liver tissue. In the liver, sympathetic nerve fibers travel with the hepatic artery and portal vein, branching into smooth muscle layers to reach hepatic lobules.126–130
Sympathetic nervous system activation is known to rapidly alter glucose homeostasis. For decades it has been recognized that epinephrine, the prototypical stress hormone, causes insulin resistance in humans.131 Norepinephrine and epinephrine also inhibit insulin secretion from pancreatic islets132–136 and blunt the usual stimulation of insulin secretion by glucose.134–137 At least in some species, insulin secretion may be under tonic inhibition by this pathway: Mice lacking α2A-adrenoreceptors have elevated plasma insulin and reduced blood glucose levels, as well as improved glucose tolerance.136 SNS signaling, predominantly via α-adrenoreceptors, controls hepatic glucose output.128,138–140 SNS activation also has potent effects on lipid metabolism. Lipolysis is chiefly activated by stimulation of β-adrenoreceptors located on the surface of adipocytes. A signaling cascade leads to an elevation of intracellular cAMP, followed by hydrolysis of intracellular triglycerides and release of FFA, which serve as an important source of fuel for oxidation, particularly in the skeletal muscle and heart.
Although the SNS is critical for survival during life-threatening stress, excessive SNS activity may have deleterious consequences. For example, the SNS was implicated in the development of hypertension many decades ago, which led to the development of sympatholytic drugs for lowering blood pressure. Recently, catheter-based renal sympathetic denervation has been offered to treat poorly controlled hypertension.141,142 Brotman and Girod envision a duel between insulin and counter-regulatory hormones as a “tug of war with no winner,” culminating in metabolic syndrome.143 It is theoretically plausible that repetitive episodes of insulin resistance could ultimately progress towards pancreatic β-cell failure and type 2 diabetes. In particular, excessive lipolysis with an over-abundance of FFA can lead to systemic “lipotoxicity,” which refers to the ectopic accumulation of lipids in skeletal muscle and liver.144–147 Norepinephrine infused into dogs caused acute insulin resistance, high plasma FFA levels, and acute fatty degeneration of the liver, which “was yellow and cut like butter” within 48 h.148 More physiologic elevations of FFA have been implicated in skeletal muscle insulin resistance, where FFA and their metabolic intermediates inhibit tyrosine phosphorylation of insulin receptor substrate 1 (IRS-1), which in turn decreases insulin-stimulated glucose transport.149,150 In the liver, increased FFA flux stimulates gluconeogenesis151 and the assembly of triglyceride rich very low density lipoproteins (VLDL) and VLDL secretion to the bloodstream, resulting in dyslipidemia.152
In summary, acute stimulation of the SNS can induce multiple physiologic changes that resemble metabolic syndrome. Chronic stress and elevated SNS activity have been proposed as causal factors in the development of metabolic syndrome.89,153,154
Evidence that metabolic dysfunction is mediated by SNS activity in OSA
Evidence for the role of the SNS in mediating OSA consequences is mostly circumstantial. For example, CPAP decreases both sympathetic activity and blood pressure.90,95,122,155 Exposure of healthy humans to IH causes insulin resistance with increased sympathetic activity.117 Patients with OSA have elevated plasma FFA levels, perhaps via SNS-mediated lipolysis.37,156
TISSUE HYPOXIA
Links between OSA and tissue hypoxia
Intermittent hypoxia is a defining characteristic of OSA.157 The nadir arterial oxyhemoglobin saturations seen in OSA may be quite low, and are typically lower than in other pulmonary illnesses. In some cases, the hypoxemia of OSA can lead to cor pulmonale. As such, considerable importance has also been attached to tissue hypoxia as a mechanism that may underlie metabolic dysfunction in OSA.
No studies have directly measured tissue oxygen tension in patients with OSA. Rather, inferences about inadequate tissue oxygenation have been made using indirect evidence. For example, two case reports describe acute elevation of liver enzymes in OSA; in these cases, severe nocturnal desaturations in OSA were thought to have caused ischemic liver damage.158,159 Another study reported higher morning creatine phosphokinase (CPK) levels in OSA patients, which were then lowered with CPAP treatment.160 These results suggest that patients with OSA may experience liver and muscle damage during sleep, but do not specifically prove that tissue ischemia is the underlying mechanism. Morning lactate levels are modestly elevated in OSA.161,162 During exercise, subjects with OSA show impaired aerobic and glycolytic capacity, a finding similar to that seen at high altitude.163 In addition, OSA severity correlates modestly with hematocrit level,164 although the disease rarely leads to polycythemia.165,166 Taken together, these findings suggest that there may be patients with OSA who have inadequate tissue oxygenation but more invasive assessments would be needed to confirm this hypothesis.
Translational models of OSA using IH exposure in rodents have provided some insight about the potential burden of tissue hypoxia in OSA. Reinke et al. measured tissue oxygen levels during exposure of mice to IH. As arterial oxygen levels fluctuated between baseline and a nadir saturation of 60%, oxygen tension in fat and liver also varied with the same periodicity.167 Interestingly, obese mice showed an even greater degree of tissue hypoxia, suggesting possible interactions of hypoxia and obesity. Similar reductions in oxygen tension were reported during extrinsic airway obstruction in rats.168 From these animal studies, it is apparent that IH can rapidly decrease oxygen tension in tissues, and a similar phenomenon might occur in human OSA. Whether these transient reductions in tissue oxygen are sufficient to cause anoxic or ischemic injury is unclear.
Tissue hypoxia can also be assessed by examining pathways of cellular adaptation to hypoxia. One might surmise that tissue hypoxia could be accompanied by activation of mediators of a global cellular hypoxic response, such as hypoxia inducible factor-1 (HIF-1). HIF-1 is a heterodimer that consists of a constitutively expressed β subunit and an O2-regulated α subunit.169 HIF-1α activation by sustained hypoxia occurs due to the inhibition of O2-dependent prolyl hydroxylation,170 thereby preventing ubiquitination and proteasomal degradation. An alternative pathway of ROS-mediated accumulation of HIF-1α has been demonstrated in adrenal cells during in vitro IH.171 HIF-1 regulates a multitude of cellular functions, including metabolism, angiogenesis, and cellular growth.172 Few studies have actually examined HIF-1 activation in OSA. One such study examined gene expression profiles of skin biopsy samples from OSA patients, mouse aortas from animals exposed to 4 weeks of IH, and human dermal microvascular and coronary artery endothelial cells cultured in IH.173 Severely hypoxic OSA patients had increased skin HIF-1α expression relative to less hypoxic patients, but there was no increase in mouse aorta HIF-1α expression in IH, and of the two cell types examined, only coronary artery endothelial cells showed an increase in HIF-1α. Another study reported no increase in HIF-1 activity in OSA patients relative to a control population, as measured by DNA binding assay.174 However, HIF-1α expression increased in the liver and lung tissues of mice after chronic IH,175 and in vitro exposure of cells to IH has mirrored this finding.176 Whether these experimental paradigms are realistic simulations of cellular hypoxia in OSA remains to be shown. Still, these results do suggest that HIF-1α activation depends upon factors including tissue, species, as well as the nature and severity of hypoxia.
Links between tissue hypoxia and metabolism
Hypoxia has complex effects on metabolism, many of which are mediated by the central nervous system. Hypobaric hypoxia encountered at high altitude leads to anorexia, weight loss, and SNS activation with associated insulin resistance.177 In many mammals, hypoxia causes a decrease in the thermoregulatory set point, leading to a fall in body temperature.178 Therefore, hypoxia at relatively cool ambient temperatures can lead to a dramatic fall in metabolic rate, deceleration of tissue lipid uptake, and the development of hyperlipidemia179 – a finding not seen at warmer ambient temperature.180 Besides eliciting these systemic responses, hypoxia also can directly affect cellular metabolism at the tissue level. For the purposes of this review, we will focus on tissue-level hypoxia, since this is a commonly cited hypothesis connecting OSA to metabolic dysfunction.
There is mounting evidence that oxygen tension in adipose tissue may have important metabolic consequences. It has been proposed that HIF-1 plays a role in the development and progression of metabolic syndrome, since the oxygen tension of adipose tissue in obese humans181,182 and mice183 is decreased. This reduced oxygen tension may be sufficient to induce HIF-1 expression. However, HIF-1 may also be upregulated in obesity by high levels of insulin.184 Regardless of whether adipose HIF-1 is stimulated by hypoxia or insulin, the consequences of HIF-1 activation have been demonstrated by both gain-of-function and loss-of-function studies: Overexpression of HIF-1α in adipose tissues causes weight gain,185 while inhibition of HIF-1 leads to weight loss in mice fed a high-fat diet.186 Adipose tissue hypoxia may also have other consequences. In vitro exposure of cultured adipocytes to IH significantly reduced secretion of adiponectin,187 a hormone that fosters insulin sensitivity and weight loss. Additionally, cultured adipocytes increase expression of the glucose transporter GLUT-1 in hypoxia188 and decrease expression of the insulin-sensitive transporter GLUT-4,189 suggesting that hypoxia, via HIF-1 activation, may cause impaired glucose handling in adipocytes.
Hypoxia in the liver may play a role in the progression of non-alcoholic fatty liver disease (NAFLD), the hepatic manifestation of metabolic syndrome.190 Baze et al. examined hepatic gene expression in mice by microarray, in response to chronic continuous hypoxia of increasing severity. Their team found a difference in the expression profile in hypoxia in genes associated with immune response, angiogenesis, oxygen transport, glycolysis, and carbohydrate, fatty acid and lipid metabolism.191 Hypoxia led to impaired glucose metabolism and induced liver injury in PTEN knockout mice, a transgenic animal that develops steatohepatitis.192 Hypoxia was also found to increase the expression of several lipogenic genes, such as PPAR-γ (peroxisome proliferator activated receptor-gamma), SREBP-1c (sterol regulatory element binding protein-1c), and ACC1/2 (acetyl CoA carboxylase 1 and 2); and was associated with decreased expression of mitochondrial beta oxidation genes. Hepatic HIF-1 activation is necessary for the development of liver fibrosis in a mouse model of NAFLD using bile duct ligation,193 and is involved in the activation of hepatic Kupffer cells194 and stellate cells195 in the development of hepatic fibrosis.
Hypoxia may be involved in the development of atherosclerosis. Atherosclerosis was accelerated in rabbits exposed to sustained chronic hypoxia.196 In fact, arterial wall hypoxia has been hypothesized as an inciting event in early atheroma formation.197 In humans, OSA is associated with early signs of atherosclerosis, and OSA severity correlated with the severity of vascular abnormalities.198 Moreover, CPAP reverses early signs of atherosclerosis in patients with OSA.199 Chronic IH also accelerates atherosclerosis in mice. This finding was evident at early stages of plaque formation200 and at advanced stages, assessed in mice deficient in ApoE (a lipoprotein necessary for catabolism of cholesterol).201 The increased atherosclerosis was associated with hypertension, vascular stiffness, and dyslipidemia, and could be attenuated by correcting the lipid transport defect caused by IH.202 It remains to be seen whether human OSA exacerbates atherosclerosis by these pathways examined in translational IH models.
However, not all studies suggest that hypoxia is harmful for metabolic health. In fact, hypoxic exposures have been used to treat obesity and to improve body composition.203 Hypoxia was found to improve glucose metabolism in some studies,204,205 suggesting that tissue-level hypoxia may actually have insulin-sensitizing effects that prevail over SNS-mediated insulin resistance. Mice exposed to hypoxia for 4 weeks displayed marked improvement in insulin sensitivity, contrasting with the acute effects of hypoxia.206 Authors speculated that improvements in skeletal muscle blood flow, perhaps induced by endothelial nitric oxide, mediated the observed improvements in glucose disposal. Acute hypoxia was also shown to improve, rather than worsen, the plasma lipid profile in mice housed at thermoneutrality.180 This reduction in plasma lipids was ascribed to hypoxia lowering hepatic VLDL secretion, and increasing fatty acid uptake in the heart.
Evidence that metabolic dysfunction is mediated by tissue hypoxia in OSA
Several studies have shown an association between metrics of hypoxia during sleep and metabolism. For example, in the Sleep Heart Health Study, sleep related hypoxemia was associated with glucose intolerance after controlling for multiple confounding variables.207 However, it is difficult to study the effects of OSA-related hypoxia in complete isolation from other aspects of the disorder. Some of this information can be gleaned from studies where OSA was treated with supplemental oxygen rather than CPAP. Several studies have shown that supplemental oxygen alone is effective in reducing the magnitude of nocturnal desaturations in OSA, although it does not correct the syndrome itself (reviewed in Mehta et al.208). A few studies have examined the effect of oxygen therapy on blood pressure;209–211 two of these studies showed no effect,210,211 and the third showed a modest reduction in diastolic blood pressure relative to patients placed on supplemental air.209 One larger recent study has examined the effect of CPAP or supplemental oxygen on blood pressure in patients with moderate to severe OSA (AHI 15–50 events/h).122 Supplemental oxygen did not alter mean arterial pressure. CPAP therapy, however, caused a 2.4 mm Hg decrease in mean arterial pressure relative to untreated patients – a finding replicated in several other studies.212 These findings call into question whether hypoxia is the major factor that leads to hypertension in OSA. Other features of OSA, such as repetitive arousals, hypercapnia, and shifts in intrathoracic pressure, may be more important. Currently, there are no studies that have examined any other metabolic outcomes in OSA when comparing supplemental oxygen to CPAP.
SLEEP FRAGMENTATION
Links between sleep fragmentation and metabolism
Fragmented sleep is another of the cardinal manifestations – indeed, one of the defining characteristics – of sleep apnea.157 There is ample evidence, both in animal models and in human subjects, that sleep fragmentation results in insulin resistance and hypertension.
A few studies have examined the role of acute sleep fragmentation in the development of metabolic dysfunction in humans. One study used mechanical or auditory stimuli to fragment sleep over two nights in healthy, nonobese volunteers.213 After sleep fragmentation, the volunteers showed worsened glucose handling and insulin sensitivity, as measured by intravenous glucose tolerance test. Similar results were seen in patients after slow wave sleep deprivation.214 Auditory sleep fragmentation also blunted the physiologic decrease in blood pressure during sleep.215 Mechanisms by which sleep fragmentation induces these effects are unknown but are likely related to physiologic changes in autonomic tone that occur between sleep and wake states.216
The role of chronic sleep fragmentation in the development of metabolic syndrome is unclear. One cross-sectional study of an elderly population found that each standard deviation increase in fragmented sleep, assessed with actigraphic recording, was associated with an increase in body mass index of 0.59 kg/m2.217 Frequent arousals from sleep in snorers without OSA was also found to be associated with hypertension.218
Animal models of sleep fragmentation have been developed, often using mechanical disruption to prevent REM sleep219–221 or manipulation of the airway or environment to simulate obstructive apneas.222–224 In one study, rats were subjected to either restricted sleep,” or “disturbed sleep,” imposed by timed revolutions of a wheel in which the animals were housed. Both protocols caused hyperglycemia and decreased insulin levels during an IV glucose tolerance test.225 Longer durations of sleep fragmentation (up to 20 weeks) have also been shown to induce insulin resistance, in association with activation adipose tissue inflammation and oxidative stress.226 Sleep fragmentation in mice also caused glucose intolerance and increased food intake after 2 weeks,227 and obesity after 8 weeks.228
Evidence that metabolic dysfunction is mediated by sleep fragmentation in OSA
There is little evidence to support a direct link between sleep fragmentation and the development of specific manifestations of metabolic syndrome in patients with OSA. As noted above, CPAP therapy appears to improve some metabolic outcomes in OSA,79 and CPAP clearly decreases fragmented sleep, but the effects of CPAP are myriad, so ascribing the metabolic improvements to less fragmented sleep specifically is impossible.
CONCLUSION
In conclusion, OSA is believed to affect metabolism by several pathways including oxidative stress, SNS activation, tissue hypoxia, and sleep fragmentation. However, the relative contribution of each of these mechanisms to metabolic dysfunction in OSA is unclear and probably varies considerably from patient to patient depending on a disease phenotype. Future investigation may focus on specific disease phenotypes, which lead to the development of poor metabolic outcomes, and on utilizing these phenotypic markers to optimize future therapeutic strategies and outcomes.
References
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–14. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM. Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ. 2009;108:246–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Coughlin SR, Mawdsley L, Mugarza JA, Calverley PM, Wilding JP. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25:735–41. doi: 10.1016/j.ehj.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 5.Bonsignore MR, Borel AL, Machan E, Grunstein R. Sleep apnoea and metabolic dysfunction. Eur Respir Rev. 2013;22 (129):353–64. doi: 10.1183/09059180.00003413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). . Executive summary of the third report of the National Cholesterol Education Program (NCEP) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 7.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 8.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 9.Lavie L, Vishnevsky A, Lavie P. Evidence for lipid peroxidation in obstructive sleep apnea. Sleep. 2004;27:123–8. [PubMed] [Google Scholar]
- 10.Barcelo A, Miralles C, Barbe F, Vila M, Pons S, Agusti AG. Abnormal lipid peroxidation in patients with sleep apnoea. Eur Respir J. 2000;16:644–7. doi: 10.1034/j.1399-3003.2000.16d13.x. [DOI] [PubMed] [Google Scholar]
- 11.Tóthová L, Hodosy J, Mucska I, Celec P. Salivary markers of oxidative stress in patients with obstructive sleep apnea treated with continuous positive airway pressure. Sleep Breath. 2013 doi: 10.1007/s11325-013-0919-z. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ. 8-Isoprostane, a marker of oxidative stress, is increased in exhaled breath condensate of patients with obstructive sleep apnea after night and is reduced by continuous positive airway pressure therapy. Chest. 2003;124:1386–92. doi: 10.1378/chest.124.4.1386. [DOI] [PubMed] [Google Scholar]
- 13.Itzhaki S, Dorchin H, Clark G, Lavie L, Lavie P, Pillar G. The effects of 1-year treatment with a herbst mandibular advancement splint on obstructive sleep apnea, oxidative stress, and endothelial function. Chest. 2007;131:740–9. doi: 10.1378/chest.06-0965. [DOI] [PubMed] [Google Scholar]
- 14.Yang XH, Liu X, Shang J, Liu HG, Xu YJ. Correlation between the serum level of advanced oxidation protein products and the cognitive function in patients with obstructive sleep apnea hypopnea syndrome. Zhonghua Jie He He Hu Xi Za Zhi. 2013;36:274–9. [PubMed] [Google Scholar]
- 15.Celec P, Hodosy J, Behuliak M, et al. Oxidative and carbonyl stress in patients with obstructive sleep apnea treated with continuous positive airway pressure. Sleep Breath. 2012;16:393–8. doi: 10.1007/s11325-011-0510-4. [DOI] [PubMed] [Google Scholar]
- 16.Yamauchi M, Nakano H, Maekawa J, et al. Oxidative stress in obstructive sleep apnea. Chest. 2005;127:1674–9. doi: 10.1378/chest.127.5.1674. [DOI] [PubMed] [Google Scholar]
- 17.Christou K, Moulas AN, Pastaka C, Gourgoulianis KI. Antioxidant capacity in obstructive sleep apnea patients. Sleep Med. 2003;4:225–8. doi: 10.1016/s1389-9457(02)00253-8. [DOI] [PubMed] [Google Scholar]
- 18.Schulz R, Mahmoudi S, Hattar K, et al. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Impact of continuous positive airway pressure therapy. Am J Respir Crit Care Med. 2000;162 (2 Pt 1):566–70. doi: 10.1164/ajrccm.162.2.9908091. [DOI] [PubMed] [Google Scholar]
- 19.Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med. 2002;165:934–9. doi: 10.1164/ajrccm.165.7.2104126. [DOI] [PubMed] [Google Scholar]
- 20.Lavie L, Lavie P. Molecular mechanisms of cardiovascular disease in OSAHS: the oxidative stress link. Eur Respir J. 2009;33:1467–84. doi: 10.1183/09031936.00086608. [DOI] [PubMed] [Google Scholar]
- 21.Dumitrascu R, Heitmann J, Seeger W, Weissmann N, Schulz R. Obstructive sleep apnea, oxidative stress and cardiovascular disease: lessons from animal studies. Oxid Med Cell Longev. 2013;2013:234631. doi: 10.1155/2013/234631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu W, Chi L, Row BW, et al. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience. 2004;126:313–23. doi: 10.1016/j.neuroscience.2004.03.055. [DOI] [PubMed] [Google Scholar]
- 23.Zhan G, Serrano F, Fenik P, et al. NADPH oxidase mediates hypersomnolence and brain oxidative injury in a murine model of sleep apnea. Am J Respir Crit Care Med. 2005;172:921–9. doi: 10.1164/rccm.200504-581OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenberg H, Ye X, Wilson D, Htoo AK, Hendersen T, Liu SF. Chronic intermittent hypoxia activates nuclear factor-kappaB in cardiovascular tissues in vivo. Biochem Biophys Res Commun. 2006;343:591–6. doi: 10.1016/j.bbrc.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 25.Esteva S, Pedret R, Fort N, Torrella JR, Pages T, Viscor G. Oxidative stress status in rats after intermittent exposure to hypobaric hypoxia. Wilderness Environ Med. 2010;21:325–31. doi: 10.1016/j.wem.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Jun J, Savransky V, Nanayakkara A, et al. Intermittent hypoxia has organ-specific effects on oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1274–81. doi: 10.1152/ajpregu.90346.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huckabee WE. Relationships of pyruvate and lactate during anaerobic metabolism. III. Effect of breathing low-oxygen gases. J Clin Invest. 1958;37:264–71. doi: 10.1172/JCI103605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buckley L, Beres L. Studies on the stability of tritium-labeled polyuridylic acid and polyadenylic acid. Anal Biochem. 1978;84:319–23. doi: 10.1016/0003-2697(78)90516-x. [DOI] [PubMed] [Google Scholar]
- 29.McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159–63. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 30.Kerrigan CL, Stotland MA. Ischemia reperfusion injury: a review. Microsurgery. 1993;14:165–75. doi: 10.1002/micr.1920140307. [DOI] [PubMed] [Google Scholar]
- 31.Jefferson JA, Escudero E, Johnson RJ, Swenson ER, Hurtado A. Increased oxidative stress at altitude. Chest. 2014;145:423. doi: 10.1378/chest.13-2062. [DOI] [PubMed] [Google Scholar]
- 32.Siervo M, Riley HL, Fernandez BO, et al. Effects of prolonged exposure to hypobaric hypoxia on oxidative stress, inflammation and gluco-insular regulation: the not-so-sweet price for good regulation. PLoS One. 2014;9:e94915. doi: 10.1371/journal.pone.0094915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pichler Hefti J, Sonntag D, Hefti U, et al. Oxidative stress in hypobaric hypoxia and influence on vessel-tone modifying mediators. High Alt Med Biol. 2013;14:273–9. doi: 10.1089/ham.2012.1110. [DOI] [PubMed] [Google Scholar]
- 34.Behn C, Araneda OF, Llanos AJ, Celedon G, Gonzalez G. Hypoxia-related lipid peroxidation: evidences, implications and approaches. Respir Physiol Neurobiol. 2007;158:143–50. doi: 10.1016/j.resp.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Kehrer JP, Lund LG. Cellular reducing equivalents and oxidative stress. Free Radic Biol Med. 1994;17:65–75. doi: 10.1016/0891-5849(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 36.Mesarwi O, Polak J, Jun J, Polotsky VY. Sleep disorders and the development of insulin resistance and obesity. Endocrinol Metab Clin North Am. 2013;42:617–34. doi: 10.1016/j.ecl.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jun JC, Drager LF, Najjar SS, et al. Effects of sleep apnea on nocturnal free fatty acids in subjects with heart failure. Sleep. 2011;34:1207–13. doi: 10.5665/SLEEP.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Egan BM, Lu G, Greene EL. Vascular effects of non-esterified fatty acids: implications for the cardiovascular risk factor cluster. Prostaglandins Leukot Essent Fatty Acids. 1999;60:411–20. doi: 10.1016/s0952-3278(99)80022-2. [DOI] [PubMed] [Google Scholar]
- 39.Boden G. Fatty acid-induced inflammation and insulin resistance in skeletal muscle and liver. Curr Diab Rep. 2006;6:177–81. doi: 10.1007/s11892-006-0031-x. [DOI] [PubMed] [Google Scholar]
- 40.Wang HL, Wang Y, Zhang Y, et al. Changes in plasma angiotensin II and circadian rhythm of blood pressure in hypertensive patients with sleep apnea syndrome before and after treatment. Chin Med Sci J. 2011;26:9–13. doi: 10.1016/s1001-9294(11)60013-8. [DOI] [PubMed] [Google Scholar]
- 41.Kizawa T, Nakamura Y, Takahashi S, Sakurai S, Yamauchi K, Inoue H. Pathogenic role of angiotensin II and oxidised LDL in obstructive sleep apnoea. Eur Respir J. 2009;34:1390–8. doi: 10.1183/09031936.00009709. [DOI] [PubMed] [Google Scholar]
- 42.Foster GE, Hanly PJ, Ahmed SB, Beaudin AE, Pialoux V, Poulin MJ. Intermittent hypoxia increases arterial blood pressure in humans through a Renin-Angiotensin system-dependent mechanism. Hypertension. 2010;56:369–77. doi: 10.1161/HYPERTENSIONAHA.110.152108. [DOI] [PubMed] [Google Scholar]
- 43.Fletcher EC. Invited review: physiological consequences of intermittent hypoxia: systemic blood pressure. J Appl Physiol. 2001;90:1600–5. doi: 10.1152/jappl.2001.90.4.1600. [DOI] [PubMed] [Google Scholar]
- 44.Dandona P, Dhindsa S, Ghanim H, Chaudhuri A. Angiotensin II and inflammation: the effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockade. J Hum Hypertens. 2007;21:20–7. doi: 10.1038/sj.jhh.1002101. [DOI] [PubMed] [Google Scholar]
- 45.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–8. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 46.Henriksen EJ, Jacob S. Modulation of metabolic control by angiotensin converting enzyme (ACE) inhibition. J Cell Physiol. 2003;196:171–9. doi: 10.1002/jcp.10294. [DOI] [PubMed] [Google Scholar]
- 47.Egan BM. Overnight rostral fluid shift and obstructive sleep apnea in treatment resistant hypertension: connecting the dots clarifies the picture. Hypertension. 2010;56:1040–1. doi: 10.1161/HYPERTENSIONAHA.110.161422. [DOI] [PubMed] [Google Scholar]
- 48.Lacroix S, Rosiers CD, Tardif JC, Nigam A. The role of oxidative stress in postprandial endothelial dysfunction. Nutr Res. 2012;25:288–301. doi: 10.1017/S0954422412000182. [DOI] [PubMed] [Google Scholar]
- 49.Vincent HK, Taylor AG. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int J Obes (Lond) 2006;30:400–18. doi: 10.1038/sj.ijo.0803177. [DOI] [PubMed] [Google Scholar]
- 50.Pitocco D, Tesauro M, Alessandro R, Ghirlanda G, Cardillo C. Oxidative stress in diabetes: implications for vascular and other complications. Int J Mol Sci. 2013;14:21525–50. doi: 10.3390/ijms141121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Touyz RM. Reactive oxygen species and angiotensin II signaling in vascular cells – implications in cardiovascular disease. Braz J Med Biol Res. 2004;37:1263–73. doi: 10.1590/s0100-879x2004000800018. [DOI] [PubMed] [Google Scholar]
- 52.Ceriello A. Possible role of oxidative stress in the pathogenesis of hypertension. Diabetes Care. 2008;31 (Suppl 2):S181–4. doi: 10.2337/dc08-s245. [DOI] [PubMed] [Google Scholar]
- 53.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–24. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 54.John JH, Ziebland S, Yudkin P, Roe LS, Neil HA. Effects of fruit and vegetable consumption on plasma antioxidant concentrations and blood pressure: a randomised controlled trial. Lancet. 2002;359:1969–74. doi: 10.1016/s0140-6736(02)98858-6. [DOI] [PubMed] [Google Scholar]
- 55.Galley HF, Thornton J, Howdle PD, Walker BE, Webster NR. Combination oral antioxidant supplementation reduces blood pressure. Clin Sci (Lond) 1997;92:361–5. doi: 10.1042/cs0920361. [DOI] [PubMed] [Google Scholar]
- 56.Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–61. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Groop LC, Saloranta C, Shank M, Bonadonna RC, Ferrannini E, DeFronzo RA. The role of free fatty acid metabolism in the pathogenesis of insulin resistance in obesity and noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1991;72:96–107. doi: 10.1210/jcem-72-1-96. [DOI] [PubMed] [Google Scholar]
- 58.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha-and obesity-induced insulin resistance. Science. 1996;271:665–8. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 59.Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem. 2002;277:1531–7. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- 60.Evans JL, Maddux BA, Goldfine ID. The molecular basis for oxidative stress-induced insulin resistance. Antioxid Redox Signal. 2005;7:1040–52. doi: 10.1089/ars.2005.7.1040. [DOI] [PubMed] [Google Scholar]
- 61.Mahadev K, Motoshima H, Wu X, et al. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol. 2004;24:1844–54. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gardner CD, Eguchi S, Reynolds CM, Eguchi K, Frank GD, Motley ED. Hydrogen peroxide inhibits insulin signaling in vascular smooth muscle cells. Exp Biol Med (Maywood) 2003;228:836–42. doi: 10.1177/15353702-0322807-09. [DOI] [PubMed] [Google Scholar]
- 63.Maddux BA, See W, Lawrence JC, Jr, Goldfine AL, Goldfine ID, Evans JL. Protection against oxidative stress-induced insulin resistance in rat L6 muscle cells by mircomolar concentrations of alpha-lipoic acid. Diabetes. 2001;50:404–10. doi: 10.2337/diabetes.50.2.404. [DOI] [PubMed] [Google Scholar]
- 64.Patel S, Van Der Kaay J, Sutherland C. Insulin regulation of hepatic insulin-like growth factor-binding protein-1 (IGFBP-1) gene expression and mammalian target of rapamycin (mTOR) signalling is impaired by the presence of hydrogen peroxide. Biochem J. 2002;365 (Pt 2):537–45. doi: 10.1042/BJ20020266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eriksson JW. Metabolic stress in insulin’s target cells leads to ROS accumulation – a hypothetical common pathway causing insulin resistance. FEBS Lett. 2007;581:3734–42. doi: 10.1016/j.febslet.2007.06.044. [DOI] [PubMed] [Google Scholar]
- 66.Bloch-Damti A, Bashan N. Proposed mechanisms for the induction of insulin resistance by oxidative stress. Antioxid Redox Signal. 2005;7:1553–67. doi: 10.1089/ars.2005.7.1553. [DOI] [PubMed] [Google Scholar]
- 67.Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 2006;55 (Suppl 2):S9–S15. doi: 10.2337/db06-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351–66. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Du X, Stocklauser-Farber K, Rosen P. Generation of reactive oxygen intermediates, activation of NF-kappaB, and induction of apoptosis in human endothelial cells by glucose: role of nitric oxide synthase? Free Radic Biol Med. 1999;27:752–63. doi: 10.1016/s0891-5849(99)00079-9. [DOI] [PubMed] [Google Scholar]
- 70.Gupta S, Chough E, Daley J, et al. Hyperglycemia increases endothelial superoxide that impairs smooth muscle cell Na+-K+-ATPase activity. Am J Physiol Cell Physiol. 2002;282:C560–6. doi: 10.1152/ajpcell.00343.2001. [DOI] [PubMed] [Google Scholar]
- 71.Jacob S, Henriksen EJ, Tritschler HJ, Augustin HJ, Dietze GJ. Improvement of insulin-stimulated glucose-disposal in type 2 diabetes after repeated parenteral administration of thioctic acid. Exp Clin Endocrinol Diabetes. 1996;104:284–8. doi: 10.1055/s-0029-1211455. [DOI] [PubMed] [Google Scholar]
- 72.Jacob S, Ruus P, Hermann R, et al. Oral administration of RAC-alpha-lipoic acid modulates insulin sensitivity in patients with type-2 diabetes mellitus: a placebo-controlled pilot trial. Free Radic Biol Med. 1999;27:309–14. doi: 10.1016/s0891-5849(99)00089-1. [DOI] [PubMed] [Google Scholar]
- 73.Fulghesu AM, Ciampelli M, Muzj G, et al. N-acetylcysteine treatment improves insulin sensitivity in women with polycystic ovary syndrome. Fertil Steril. 2002;77:1128–35. doi: 10.1016/s0015-0282(02)03133-3. [DOI] [PubMed] [Google Scholar]
- 74.Walden DL, McCutchan HJ, Enquist EG, et al. Neutrophils accumulate and contribute to skeletal muscle dysfunction after ischemia-reperfusion. Am J Physiol. 1990;259 (6 Pt 2):H1809–12. doi: 10.1152/ajpheart.1990.259.6.H1809. [DOI] [PubMed] [Google Scholar]
- 75.American Diabetes Association. Nutrition Recommendations and Interventions for Diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2007;30 (Suppl 1):S48–65. doi: 10.2337/dc07-S048. [DOI] [PubMed] [Google Scholar]
- 76.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–57. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 77.Haidara MA, Yassin HZ, Zakula Z, Mikhailidis DP, Isenovic ER. Diabetes and antioxidants: myth or reality? Curr Vasc Pharmacol. 2010;8:661–72. doi: 10.2174/157016110792006941. [DOI] [PubMed] [Google Scholar]
- 78.Moyer MW. The myth of antioxidants. Sci Am. 2013;308:62–7. doi: 10.1038/scientificamerican0213-62. [DOI] [PubMed] [Google Scholar]
- 79.Steiropoulos P, Papanas N, Nena E, Maltezos E, Bouros D. Continuous positive airway pressure treatment in patients with sleep apnoea: does it really improve glucose metabolism? Curr Diabetes Rev. 2010;6:156–66. doi: 10.2174/157339910791162943. [DOI] [PubMed] [Google Scholar]
- 80.Murri M, Garcia-Delgado R, Alcazar-Ramirez J, et al. Continuous positive airway pressure therapy reduces oxidative stress markers and blood pressure in sleep apnea-hypopnea syndrome patients. Biol Trace Elem Res. 2011;143:1289–301. doi: 10.1007/s12011-011-8969-1. [DOI] [PubMed] [Google Scholar]
- 81.Buchner NJ, Quack I, Woznowski M, Stahle C, Wenzel U, Rump LC. Microvascular endothelial dysfunction in obstructive sleep apnea is caused by oxidative stress and improved by continuous positive airway pressure therapy. Respiration. 2011;82:409–17. doi: 10.1159/000323266. [DOI] [PubMed] [Google Scholar]
- 82.Grebe M, Eisele HJ, Weissmann N, et al. Antioxidant vitamin C improves endothelial function in obstructive sleep apnea. Am J Respir Crit Care Med. 2006;173:897–901. doi: 10.1164/rccm.200508-1223OC. [DOI] [PubMed] [Google Scholar]
- 83.El Solh AA, Saliba R, Bosinski T, Grant BJ, Berbary E, Miller N. Allopurinol improves endothelial function in sleep apnoea: a randomised controlled study. Eur Respir J. 2006;27:997–1002. doi: 10.1183/09031936.06.00101005. [DOI] [PubMed] [Google Scholar]
- 84.Svendsen M, Blomhoff R, Holme I, Tonstad S. The effect of an increased intake of vegetables and fruit on weight loss, blood pressure and antioxidant defense in subjects with sleep related breathing disorders. Eur J Clin Nutr. 2007;61:1301–11. doi: 10.1038/sj.ejcn.1602652. [DOI] [PubMed] [Google Scholar]
- 85.Sadasivam K, Patial K, Vijayan VK, Ravi K. Anti-oxidant treatment in obstructive sleep apnoea syndrome. Indian J Chest Dis Allied Sci. 2011;53:153–62. [PubMed] [Google Scholar]
- 86.Tovar S, Paeger L, Hess S, et al. K(ATP)-channel-dependent regulation of catecholaminergic neurons controls BAT sympathetic nerve activity and energy homeostasis. Cell Metab. 2013;18:445–55. doi: 10.1016/j.cmet.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gompf HS, Mathai C, Fuller PM, et al. Locus ceruleus and anterior cingulate cortex sustain wakefulness in a novel environment. J Neurosci. 2010;30:14543–51. doi: 10.1523/JNEUROSCI.3037-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Patterson ZR, Abizaid A. Stress induced obesity: lessons from rodent models of stress. Front Neurosci. 2013;7:130. doi: 10.3389/fnins.2013.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kyrou I, Tsigos C. Stress hormones: physiological stress and regulation of metabolism. Curr Opin Pharmacol. 2009;9:787–93. doi: 10.1016/j.coph.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 90.Narkiewicz K, van de Borne PJ, Cooley RL, Dyken ME, Somers VK. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation. 1998;98:772–6. doi: 10.1161/01.cir.98.8.772. [DOI] [PubMed] [Google Scholar]
- 91.Somers VK, Mark AL, Zavala DC, Abboud FM. Contrasting effects of hypoxia and hypercapnia on ventilation and sympathetic activity in humans. J Appl Physiol. 1989;67:2101–6. doi: 10.1152/jappl.1989.67.5.2101. [DOI] [PubMed] [Google Scholar]
- 92.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Baruzzi A, Riva R, Cirignotta F, Zucconi M, Cappelli M, Lugaresi E. Atrial natriuretic peptide and catecholamines in obstructive sleep apnea syndrome. Sleep. 1991;14:83–6. doi: 10.1093/sleep/14.1.83. [DOI] [PubMed] [Google Scholar]
- 94.Marrone O, Riccobono L, Salvaggio A, Mirabella A, Bonanno A, Bonsignore MR. Catecholamines and blood pressure in obstructive sleep apnea syndrome. Chest. 1993;103:722–7. doi: 10.1378/chest.103.3.722. [DOI] [PubMed] [Google Scholar]
- 95.Ziegler MG, Mills PJ, Loredo JS, Ancoli-Israel S, Dimsdale JE. Effect of continuous positive airway pressure and placebo treatment on sympathetic nervous activity in patients with obstructive sleep apnea. Chest. 2001;120:887–93. doi: 10.1378/chest.120.3.887. [DOI] [PubMed] [Google Scholar]
- 96.Prabhakar NR, Kumar GK, Peng YJ. Sympatho-adrenal activation by chronic intermittent hypoxia. J Appl Physiol. 2012;113:1304–10. doi: 10.1152/japplphysiol.00444.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gonzalez-Martin MC, Vega-Agapito MV, Conde SV, et al. Carotid body function and ventilatory responses in intermittent hypoxia. Evidence for anomalous brainstem integration of arterial chemoreceptor input. J Cell Physiol. 2011;226:1961–9. doi: 10.1002/jcp.22528. [DOI] [PubMed] [Google Scholar]
- 98.Dick TE, Hsieh YH, Wang N, Prabhakar N. Acute intermittent hypoxia increases both phrenic and sympathetic nerve activities in the rat. Exp Physiol. 2007;92:87–97. doi: 10.1113/expphysiol.2006.035758. [DOI] [PubMed] [Google Scholar]
- 99.Greenberg HE, Sica A, Batson D, Scharf SM. Chronic intermittent hypoxia increases sympathetic responsiveness to hypoxia and hypercapnia. J Appl Physiol. 1999;86:298–305. doi: 10.1152/jappl.1999.86.1.298. [DOI] [PubMed] [Google Scholar]
- 100.Huang J, Lusina S, Xie T, et al. Sympathetic response to chemostimulation in conscious rats exposed to chronic intermittent hypoxia. Respir Physiol Neurobiol. 2009;166:102–6. doi: 10.1016/j.resp.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 101.Marcus NJ, Li YL, Bird CE, Schultz HD, Morgan BJ. Chronic intermittent hypoxia augments chemoreflex control of sympathetic activity: role of the angiotensin II type 1 receptor. Respir Physiol Neurobiol. 2010;171:36–45. doi: 10.1016/j.resp.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Narkiewicz K, Kato M, Phillips BG, Pesek CA, Davison DE, Somers VK. Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation. 1999;100:2332–5. doi: 10.1161/01.cir.100.23.2332. [DOI] [PubMed] [Google Scholar]
- 103.Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci US A. 2003;100:10073–8. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Peng YJ, Rennison J, Prabhakar NR. Intermittent hypoxia augments carotid body and ventilatory response to hypoxia in neonatal rat pups. J Appl Physiol. 2004;97:2020–5. doi: 10.1152/japplphysiol.00876.2003. [DOI] [PubMed] [Google Scholar]
- 105.Peng YJ, Yuan G, Ramakrishnan D, et al. Heterozygous HIF-1alpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol. 2006;577 (Pt 2):705–16. doi: 10.1113/jphysiol.2006.114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Peng YJ, Yuan G, Jacono FJ, Kumar GK, Prabhakar NR. 5-HT evokes sensory long-term facilitation of rodent carotid body via activation of NADPH oxidase. J Physiol. 2006;576 (Pt 1):289–95. doi: 10.1113/jphysiol.2006.116020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peng YJ, Nanduri J, Yuan G, et al. NADPH oxidase is required for the sensory plasticity of the carotid body by chronic intermittent hypoxia. J Neurosci. 2009;29:4903–10. doi: 10.1523/JNEUROSCI.4768-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Prabhakar NR, Dick TE, Nanduri J, Kumar GK. Systemic, cellular and molecular analysis of chemoreflex-mediated sympathoexcitation by chronic intermittent hypoxia. Exp Physiol. 2007;92:39–44. doi: 10.1113/expphysiol.2006.036434. [DOI] [PubMed] [Google Scholar]
- 109.Prabhakar NR, Kumar GK, Nanduri J. Intermittent hypoxia augments acute hypoxic sensing via HIF-mediated ROS. Respir Physiol Neurobiol. 2010;174:230–4. doi: 10.1016/j.resp.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bao G, Metreveli N, Li R, Taylor A, Fletcher EC. Blood pressure response to chronic episodic hypoxia: role of the sympathetic nervous system. J Appl Physiol. 1997;83:95–101. doi: 10.1152/jappl.1997.83.1.95. [DOI] [PubMed] [Google Scholar]
- 111.Kumar GK, Rai V, Sharma SD, et al. Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress. J Physiol. 2006;575 (Pt 1):229–39. doi: 10.1113/jphysiol.2006.112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fletcher EC, Lesske J, Behm R, Miller CC, 3rd, Stauss H, Unger T. Carotid chemoreceptors, systemic blood pressure, and chronic episodic hypoxia mimicking sleep apnea. J Appl Physiol. 1992;72:1978–84. doi: 10.1152/jappl.1992.72.5.1978. [DOI] [PubMed] [Google Scholar]
- 113.Knight WD, Little JT, Carreno FR, Toney GM, Mifflin SW, Cunningham JT. Chronic intermittent hypoxia increases blood pressure and expression of FosB/DeltaFosB in central autonomic regions. Am J Physiol Regul Integr Comp Physiol. 2011;301:R131–9. doi: 10.1152/ajpregu.00830.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Somers VK, Mark AL, Abboud FM. Sympathetic activation by hypoxia and hypercapnia–implications for sleep apnea. Clin Exp Hypertens A. 1988;10 (Suppl 1):413–22. doi: 10.3109/10641968809075998. [DOI] [PubMed] [Google Scholar]
- 115.Narkiewicz K, Somers VK. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol Scand. 2003;177:385–90. doi: 10.1046/j.1365-201X.2003.01091.x. [DOI] [PubMed] [Google Scholar]
- 116.Schneider H, Schaub CD, Chen CA, et al. Neural and local effects of hypoxia on cardiovascular responses to obstructive apnea. J Appl Physiol. 2000;88:1093–102. doi: 10.1152/jappl.2000.88.3.1093. [DOI] [PubMed] [Google Scholar]
- 117.Louis M, Punjabi NM. Effects of acute intermittent hypoxia on glucose metabolism in awake healthy volunteers. J Appl Physiol. 2009;106:1538–44. doi: 10.1152/japplphysiol.91523.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Oltmanns KM, Gehring H, Rudolf S, et al. Hypoxia causes glucose intolerance in humans. Am J Respir Crit Care Med. 2004;169:1231–7. doi: 10.1164/rccm.200308-1200OC. [DOI] [PubMed] [Google Scholar]
- 119.Peltonen GL, Scalzo RL, Schweder MM, et al. Sympathetic inhibition attenuates hypoxia induced insulin resistance in healthy adult humans. J Physiol. 2012;590 (Pt 11):2801–9. doi: 10.1113/jphysiol.2011.227090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lesske J, Fletcher EC, Bao G, Unger T. Hypertension caused by chronic intermittent hypoxia–influence of chemoreceptors and sympathetic nervous system. J Hypertens. 1997;15 (12 Pt 2):1593–603. doi: 10.1097/00004872-199715120-00060. [DOI] [PubMed] [Google Scholar]
- 121.Tamisier R, Pepin JL, Remy J, et al. 14 nights of intermittent hypoxia elevate daytime blood pressure and sympathetic activity in healthy humans. Eur Respir J. 2011;37:119–28. doi: 10.1183/09031936.00204209. [DOI] [PubMed] [Google Scholar]
- 122.Gottlieb DJ, Punjabi NM, Mehra R, et al. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med. 2014;370:2276–85. doi: 10.1056/NEJMoa1306766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Barth E, Albuszies G, Baumgart K, et al. Glucose metabolism and catecholamines. Crit Care Med. 2007;35 (9 Suppl):S508–18. doi: 10.1097/01.CCM.0000278047.06965.20. [DOI] [PubMed] [Google Scholar]
- 124.Ziegler MG, Elayan H, Milic M, Sun P, Gharaibeh M. Epinephrine and the metabolic syndrome. Curr Hypertens Rep. 2012;14:1–7. doi: 10.1007/s11906-011-0243-6. [DOI] [PubMed] [Google Scholar]
- 125.Dungan KM, Braithwaite SS, Preiser JC. Stress hyper-glycaemia. Lancet. 2009;373:1798–807. doi: 10.1016/S0140-6736(09)60553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nobin A, Baumgarten HG, Falck B, Ingemansson S, Moghimzadeh E, Rosengren E. Organization of the sympathetic innervation in liver tissue from monkey and man. Cell Tissue Res. 1978;195:371–80. doi: 10.1007/BF00233883. [DOI] [PubMed] [Google Scholar]
- 127.Metz W, Forssmann WG. Innervation of the liver in guinea pig and rat. Anat Embryol. 1980;160:239–52. doi: 10.1007/BF00305105. [DOI] [PubMed] [Google Scholar]
- 128.Hartmann H, Beckh K, Jungermann K. Direct control of glycogen metabolism in the perfused rat liver by the sympathetic innervation. Eur J Biochem. 1982;123:521–6. doi: 10.1111/j.1432-1033.1982.tb06562.x. [DOI] [PubMed] [Google Scholar]
- 129.Henriksen JH, Moller S, Ring-Larsen H, Christensen NJ. The sympathetic nervous system in liver disease. J Hepatol. 1998;29:328–41. doi: 10.1016/s0168-8278(98)80022-6. [DOI] [PubMed] [Google Scholar]
- 130.Oben JA, Roskams T, Yang S, et al. Hepatic fibrogenesis requires sympathetic neurotransmitters. Gut. 2004;53:438–45. doi: 10.1136/gut.2003.026658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Deibert DC, DeFronzo RA. Epinephrine-induced insulin resistance in man. J Clin Invest. 1980;65:717–21. doi: 10.1172/JCI109718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Szollosi A, Nenquin M, Henquin JC. Pharmacological stimulation and inhibition of insulin secretion in mouse islets lacking ATP-sensitive K+ channels. Br J Pharmacol. 2010;159:669–77. doi: 10.1111/j.1476-5381.2009.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Skoglund G, Lundquist I, Ahren B. Effects of alpha 1-and alpha 2-adrenoceptor stimulation and blockade on plasma insulin levels in the mouse. Pancreas. 1986;1:415–20. doi: 10.1097/00006676-198609000-00005. [DOI] [PubMed] [Google Scholar]
- 134.Ahren B, Lundquist I. Effects of alpha-adrenoceptor blockade by phentolamine on basal and stimulated insulin secretion in the mouse. Acta Physiol Scand. 1985;125:211–17. doi: 10.1111/j.1748-1716.1985.tb07709.x. [DOI] [PubMed] [Google Scholar]
- 135.Liggett SB. alpha2A-adrenergic receptors in the genetics, pathogenesis, and treatment of type 2 diabetes. Sci Transl Med. 2009;1:12ps5. doi: 10.1126/scitranslmed.3000606. [DOI] [PubMed] [Google Scholar]
- 136.Ruohonen ST, Ruohonen S, Gilsbach R, Savontaus E, Scheinin M, Hein L. Involvement of alpha2-adrenoceptor subtypes A and C in glucose homeostasis and adrenaline-induced hyperglycaemia. Neuroendocrinology. 2012;96:51–9. doi: 10.1159/000334629. [DOI] [PubMed] [Google Scholar]
- 137.Lerner RL, Porte D., Jr Epinephrine: selective inhibition of the acute insulin response to glucose. J Clin Invest. 1971;50:2453–7. doi: 10.1172/JCI106744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pessina AC, Ciccariello L, Perrone F, et al. Clinical efficacy and tolerability of alpha-blocker doxazosin as add-on therapy in patients with hypertension and impaired glucose metabolism. Nutr Metab Cardiovasc Dis. 2006;16:137–47. doi: 10.1016/j.numecd.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 139.Derosa G, Cicero AF, Gaddi A, Mugellini A, Ciccarelli L, Fogari R. Effects of doxazosin and irbesartan on blood pressure and metabolic control in patients with type 2 diabetes and hypertension. J Cardiovasc Pharmacol. 2005;45:599–604. doi: 10.1097/01.fjc.0000161403.91456.39. [DOI] [PubMed] [Google Scholar]
- 140.Barzilay JI, Davis BR, Bettencourt J, et al. Cardiovascular outcomes using doxazosin vs. chlorthalidone for the treatment of hypertension in older adults with and without glucose disorders: a report from the ALLHAT study. J Clin Hypertens (Greenwich) 2004;6:116–25. doi: 10.1111/j.1524-6175.2004.03216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.DiBona GF. Sympathetic nervous system and hypertension. Hypertension. 2013;61:556–60. doi: 10.1161/HYPERTENSIONAHA.111.00633. [DOI] [PubMed] [Google Scholar]
- 142.Krum H, Schlaich MP, Sobotka PA, et al. Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet. 2014;383:622–9. doi: 10.1016/S0140-6736(13)62192-3. [DOI] [PubMed] [Google Scholar]
- 143.Brotman DJ, Girod JP. The metabolic syndrome: a tug-of-war with no winner. Cleve Clin J Med. 2002;69:990–4. doi: 10.3949/ccjm.69.12.990. [DOI] [PubMed] [Google Scholar]
- 144.Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774–88. doi: 10.1002/hep.23719. [DOI] [PubMed] [Google Scholar]
- 145.Kulick D, Liu DK. Isolation and characterization of polyploid nuclei of R3230AC mammary adenocarcinoma and comparison with normal mammary nuclei. Cancer Res. 1981;41:3907–12. [PubMed] [Google Scholar]
- 146.Unger RH, Clark GO, Scherer PE, Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta. 2010;1801:209–14. doi: 10.1016/j.bbalip.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 147.Ischebeck H, Kuske V. Proceedings: multiple meningiomas. Acta Neurochir (Wien) 1975;31:278. [PubMed] [Google Scholar]
- 148.Eltringham WK, Jenny ME, Morgan AP. Metabolic and tissue effects of prolonged catecholamine infusion. Postgrad Med J. 1969;45:545–9. doi: 10.1136/pgmj.45.526.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kim JK, Kim YJ, Fillmore JJ, et al. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest. 2001;108:437–46. doi: 10.1172/JCI11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kim JK, Fillmore JJ, Sunshine MJ, et al. PKC-theta knockout mice are protected from fat-induced insulin resistance. J Clin Invest. 2004;114:823–7. doi: 10.1172/JCI22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Delarue J, Magnan C. Free fatty acids and insulin resistance. Curr Opin Clin Nutr Metab Care. 2007;10:142–8. doi: 10.1097/MCO.0b013e328042ba90. [DOI] [PubMed] [Google Scholar]
- 152.Sniderman AD, Cianflone K. Substrate delivery as a determinant of hepatic apoB secretion. Arterioscler Thromb. 1993;13:629–36. doi: 10.1161/01.atv.13.5.629. [DOI] [PubMed] [Google Scholar]
- 153.Pervanidou P, Chrousos GP. Metabolic consequences of stress during childhood and adolescence. Metabolism. 2012;61:611–19. doi: 10.1016/j.metabol.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 154.Golden SH. A review of the evidence for a neuroendocrine link between stress, depression and diabetes mellitus. Curr Diabetes Rev. 2007;3:252–9. doi: 10.2174/157339907782330021. [DOI] [PubMed] [Google Scholar]
- 155.Abboud F, Kumar R. Obstructive sleep apnea and insight into mechanisms of sympathetic overactivity. J Clin Invest. 2014;124:1454–7. doi: 10.1172/JCI70420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Barcelo A, Pierola J, de la Pena M, et al. Free fatty acids and the metabolic syndrome in patients with obstructive sleep apnoea. Eur Respir J. 2011;37:1418–23. doi: 10.1183/09031936.00050410. [DOI] [PubMed] [Google Scholar]
- 157.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 158.Henrion J, Colin L, Schapira M, Heller FR. Hypoxic hepatitis caused by severe hypoxemia from obstructive sleep apnea. J Clin Gastroenterol. 1997;24:245–9. doi: 10.1097/00004836-199706000-00013. [DOI] [PubMed] [Google Scholar]
- 159.Trakada G, Gogos C, Tsiamita M, Siagris D, Goumas P, Spiropoulos K. A case of ischemic hepatitis. Sleep Breath. 2004;8:155–9. doi: 10.1007/s11325-004-0155-7. [DOI] [PubMed] [Google Scholar]
- 160.Lentini S, Manka R, Scholtyssek S, Stoffel-Wagner B, Luderitz B, Tasci S. Creatine phosphokinase elevation in obstructive sleep apnea syndrome: an unknown association? Chest. 2006;129:88–94. doi: 10.1378/chest.129.1.88. [DOI] [PubMed] [Google Scholar]
- 161.Ucar ZZ, Taymaz Z, Erbaycu AE, Kirakli C, Tuksavul F, Guclu SZ. Nocturnal hypoxia and arterial lactate levels in sleep-related breathing disorders. South Med J. 2009;102:693–700. doi: 10.1097/SMJ.0b013e3181a93897. [DOI] [PubMed] [Google Scholar]
- 162.Hira HS, Shukla A, Kaur A, Kapoor S. Serum uric acid and lactate levels among patients with obstructive sleep apnea syndrome: which is a better marker of hypoxemia? Ann Saudi Med. 2012;32:37–42. doi: 10.5144/0256-4947.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Vanuxem D, Badier M, Guillot C, Delpierre S, Jahjah F, Vanuxem P. Impairment of muscle energy metabolism in patients with sleep apnoea syndrome. Respir Med. 1997;91:551–7. doi: 10.1016/s0954-6111(97)90089-5. [DOI] [PubMed] [Google Scholar]
- 164.Choi JB, Loredo JS, Norman D, et al. Does obstructive sleep apnea increase hematocrit? Sleep Breath. 2006;10:155–60. doi: 10.1007/s11325-006-0064-z. [DOI] [PubMed] [Google Scholar]
- 165.King AJ, Eyre T, Littlewood T. Obstructive sleep apnoea does not lead to clinically significant erythrocytosis. BMJ. 2013;347:f7340. doi: 10.1136/bmj.f7340. [DOI] [PubMed] [Google Scholar]
- 166.Solmaz S, Duksal F, Ganidagli S. Is obstructive sleep apnoea syndrome really one of the causes of secondary polycythaemia? Hematology. 2014 doi: 10.1179/1607845414Y.0000000170. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 167.Reinke C, Bevans-Fonti S, Drager LF, Shin MK, Polotsky VY. Effects of different acute hypoxic regimens on tissue oxygen profiles and metabolic outcomes. J Appl Physiol. 2011;111:881–90. doi: 10.1152/japplphysiol.00492.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Almendros I, Farre R, Planas AM, et al. Tissue oxygenation in brain, muscle, and fat in a rat model of sleep apnea: differential effect of obstructive apneas and intermittent hypoxia. Sleep. 2011;34:1127–33. doi: 10.5665/SLEEP.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Semenza GL. Regulation of physiological responses to continuous and intermittent hypoxia by hypoxia-inducible factor 1. Exp Physiol. 2006;91:803–6. doi: 10.1113/expphysiol.2006.033498. [DOI] [PubMed] [Google Scholar]
- 170.Jaakkola P, Mole DR, Tian YM, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–72. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 171.Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR. Induction of HIF-1alpha expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol. 2008;217:674–85. doi: 10.1002/jcp.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol. 2001;13:167–71. doi: 10.1016/s0955-0674(00)00194-0. [DOI] [PubMed] [Google Scholar]
- 173.Kaczmarek E, Bakker JP, Clarke DN, et al. Molecular biomarkers of vascular dysfunction in obstructive sleep apnea. PLoS One. 2013;8:e70559. doi: 10.1371/journal.pone.0070559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–7. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 175.da Rosa DP, Forgiarini LF, Baronio D, Feijo CA, Martinez D, Marroni NP. Simulating sleep apnea by exposure to intermittent hypoxia induces inflammation in the lung and liver. Mediators Inflamm. 2012;2012:879419. doi: 10.1155/2012/879419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yuan G, Nanduri J, Bhasker CR, Semenza GL, Prabhakar NR. Ca2+/calmodulin kinase-dependent activation of hypoxia inducible factor 1 transcriptional activity in cells subjected to intermittent hypoxia. J Biol Chem. 2005;280:4321–8. doi: 10.1074/jbc.M407706200. [DOI] [PubMed] [Google Scholar]
- 177.Young PM, Rose MS, Sutton JR, Green HJ, Cymerman A, Houston CS. Operation Everest II: plasma lipid and hormonal responses during a simulated ascent of Mt. Everest. J Appl Physiol. 1989;66:1430–5. doi: 10.1152/jappl.1989.66.3.1430. [DOI] [PubMed] [Google Scholar]
- 178.Gautier H. Interactions among metabolic rate, hypoxia, and control of breathing. J Appl Physiol. 1996;81:521–7. doi: 10.1152/jappl.1996.81.2.521. [DOI] [PubMed] [Google Scholar]
- 179.Jun JC, Shin MK, Yao Q, et al. Acute hypoxia induces hypertriglyceridemia by decreasing plasma triglyceride clearance in mice. Am J Physiol Endocrinol Metab. 2012;303:E377–88. doi: 10.1152/ajpendo.00641.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jun JC, Shin MK, Yao Q, Devera R, Fonti-Bevans S, Polotsky VY. Thermoneutrality modifies the impact of hypoxia on lipid metabolism. Am J Physiol Endocrinol Metab. 2013;304:E424–35. doi: 10.1152/ajpendo.00515.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Pasarica M, Rood J, Ravussin E, Schwarz JM, Smith SR, Redman LM. Reduced oxygenation in human obese adipose tissue is associated with impaired insulin suppression of lipolysis. J Clin Endocrinol Metab. 2010;95:4052–5. doi: 10.1210/jc.2009-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pasarica M, Sereda OR, Redman LM, et al. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58:718–25. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hosogai N, Fukuhara A, Oshima K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–11. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 184.He Q, Gao Z, Yin J, Zhang J, Yun Z, Ye J. Regulation of HIF-1{alpha} activity in adipose tissue by obesity-associated factors: adipogenesis, insulin, and hypoxia. Am J Physiol Endocrinol Metab. 2011;300:E877–85. doi: 10.1152/ajpendo.00626.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Halberg N, Khan T, Trujillo ME, et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29:4467–83. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jiang C, Qu A, Matsubara T, et al. Disruption of hypoxia-inducible factor 1 in adipocytes improves insulin sensitivity and decreases adiposity in high-fat diet-fed mice. Diabetes. 2011;60:2484–95. doi: 10.2337/db11-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Magalang UJ, Cruff JP, Rajappan R, et al. Intermittent hypoxia suppresses adiponectin secretion by adipocytes. Exp Clin Endocrinol Diabetes. 2009;117:129–34. doi: 10.1055/s-2008-1078738. [DOI] [PubMed] [Google Scholar]
- 188.Wang B, Wood IS, Trayhurn P. Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch. 2007;455:479–92. doi: 10.1007/s00424-007-0301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang B, Wood IS, Trayhurn P. Hypoxia induces leptin gene expression and secretion in human preadipocytes: differential effects of hypoxia on adipokine expression by preadipocytes. J Endocrinol. 2008;198:127–34. doi: 10.1677/JOE-08-0156. [DOI] [PubMed] [Google Scholar]
- 190.Boppidi H, Daram SR. Nonalcoholic fatty liver disease: hepatic manifestation of obesity and the metabolic syndrome. Postgrad Med. 2008;120:E01–7. doi: 10.3810/pgm.2008.07.1800. [DOI] [PubMed] [Google Scholar]
- 191.Baze MM, Schlauch K, Hayes JP. Gene expression of the liver in response to chronic hypoxia. Physiol Genomics. 2010;41:275–88. doi: 10.1152/physiolgenomics.00075.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Piguet AC, Stroka D, Zimmermann A, Dufour JF. Hypoxia aggravates non-alcoholic steatohepatitis in mice lacking hepatocellular PTEN. Clin Sci (Lond) 2010;118:401–10. doi: 10.1042/CS20090313. [DOI] [PubMed] [Google Scholar]
- 193.Moon JO, Welch TP, Gonzalez FJ, Copple BL. Reduced liver fibrosis in hypoxia-inducible factor-1alpha-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2009;296 (3):G582–92. doi: 10.1152/ajpgi.90368.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Copple BL, Bai S, Moon JO. Hypoxia-inducible factor-dependent production of profibrotic mediators by hypoxic Kupffer cells. Hepatol Res. 2010;40:530–9. doi: 10.1111/j.1872-034X.2010.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Copple BL, Bai S, Burgoon LD, Moon JO. Hypoxia-inducible factor-1 alpha regulates the expression of genes in hypoxic hepatic stellate cells important for collagen deposition and angiogenesis. Liver Int. 2011;31:230–44. doi: 10.1111/j.1478-3231.2010.02347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Okamoto R, Hatani M, Tsukitani M, et al. The effect of oxygen on the development of atherosclerosis in WHHL rabbits. Atherosclerosis. 1983;47:47–53. doi: 10.1016/0021-9150(83)90070-9. [DOI] [PubMed] [Google Scholar]
- 197.Martin JF, Booth RF, Moncada S. Arterial wall hypoxia following thrombosis of the vasa vasorum is an initial lesion in atherosclerosis. Eur J Clin Invest. 1991;21:355–9. doi: 10.1111/j.1365-2362.1991.tb01382.x. [DOI] [PubMed] [Google Scholar]
- 198.Drager LF, Bortolotto LA, Lorenzi MC, Figueiredo AC, Krieger EM, Lorenzi-Filho G. Early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:613–18. doi: 10.1164/rccm.200503-340OC. [DOI] [PubMed] [Google Scholar]
- 199.Drager LF, Bortolotto LA, Figueiredo AC, Krieger EM, Lorenzi GF. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2007;176:706–12. doi: 10.1164/rccm.200703-500OC. [DOI] [PubMed] [Google Scholar]
- 200.Savransky V, Nanayakkara A, Li J, et al. Chronic intermittent hypoxia induces atherosclerosis. Am J Respir Crit Care Med. 2007;175:1290–7. doi: 10.1164/rccm.200612-1771OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jun J, Reinke C, Bedja D, et al. Effect of intermittent hypoxia on atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 2010;209:381–6. doi: 10.1016/j.atherosclerosis.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Drager LF, Yao Q, Hernandez KL, et al. Chronic intermittent hypoxia induces atherosclerosis via activation of adipose angiopoietin-like 4. Am J Respir Crit Care Med. 2013;188:240–8. doi: 10.1164/rccm.201209-1688OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kayser B, Verges S. Hypoxia, energy balance and obesity: from pathophysiological mechanisms to new treatment strategies. Obes Rev. 2013;14:579–92. doi: 10.1111/obr.12034. [DOI] [PubMed] [Google Scholar]
- 204.Mackenzie R, Maxwell N, Castle P, Elliott B, Brickley G, Watt P. Intermittent exercise with and without hypoxia improves insulin sensitivity in individuals with type 2 diabetes. J Clin Endocrinol Metab. 2012;97:E546–55. doi: 10.1210/jc.2011-2829. [DOI] [PubMed] [Google Scholar]
- 205.Mackenzie R, Maxwell N, Castle P, Brickley G, Watt P. Acute hypoxia and exercise improve insulin sensitivity (S(I) (2*)) in individuals with type 2 diabetes. Diabetes Metab Res Rev. 2011;27:94–101. doi: 10.1002/dmrr.1156. [DOI] [PubMed] [Google Scholar]
- 206.Lee EJ, Alonso LC, Stefanovski D, et al. Time-dependent changes in glucose and insulin regulation during intermittent hypoxia and continuous hypoxia. Eur J Appl Physiol. 2013;113:467–78. doi: 10.1007/s00421-012-2452-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160:521–30. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 208.Mehta V, Vasu TS, Phillips B, Chung F. Obstructive sleep apnea and oxygen therapy: a systematic review of the literature and meta-analysis. J Clin Sleep Med. 2013;9:271–9. doi: 10.5664/jcsm.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Pokorski M, Jernajczyk U. Nocturnal oxygen enrichment in sleep apnoea. J Int Med Res. 2000;28:1–8. doi: 10.1177/147323000002800101. [DOI] [PubMed] [Google Scholar]
- 210.Norman D, Loredo JS, Nelesen RA, et al. Effects of continuous positive airway pressure versus supplemental oxygen on 24-hour ambulatory blood pressure. Hypertension. 2006;47:840–5. doi: 10.1161/01.HYP.0000217128.41284.78. [DOI] [PubMed] [Google Scholar]
- 211.Mills PJ, Kennedy BP, Loredo JS, Dimsdale JE, Ziegler MG. Effects of nasal continuous positive airway pressure and oxygen supplementation on norepinephrine kinetics and cardiovascular responses in obstructive sleep apnea. J Appl Physiol. 2006;100:343–8. doi: 10.1152/japplphysiol.00494.2005. [DOI] [PubMed] [Google Scholar]
- 212.Fava C, Dorigoni S, Dalle Vedove F, et al. Effect of CPAP on blood pressure in patients with OSA/hypopnea a systematic review and meta-analysis. Chest. 2014;145:762–71. doi: 10.1378/chest.13-1115. [DOI] [PubMed] [Google Scholar]
- 213.Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010;137:95–101. doi: 10.1378/chest.09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Tasali E, Ip MS. Obstructive sleep apnea and metabolic syndrome: alterations in glucose metabolism and inflammation. Proc Am Thorac Soc. 2008;5:207–17. doi: 10.1513/pats.200708-139MG. [DOI] [PubMed] [Google Scholar]
- 215.Carrington MJ, Trinder J. Blood pressure and heart rate during continuous experimental sleep fragmentation in healthy adults. Sleep. 2008;31:1701–12. doi: 10.1093/sleep/31.12.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Silvani A, Dampney RA. Central control of cardiovascular function during sleep. Am J Physiol Heart Circ Physiol. 2013;305:H1683–92. doi: 10.1152/ajpheart.00554.2013. [DOI] [PubMed] [Google Scholar]
- 217.van den Berg JF, Knvistingh Neven A, Tulen JH, et al. Actigraphic sleep duration and fragmentation are related to obesity in the elderly: the Rotterdam Study. Int J Obes (Lond) 2008;32:1083–90. doi: 10.1038/ijo.2008.57. [DOI] [PubMed] [Google Scholar]
- 218.Lofaso F, Coste A, Gilain L, Harf A, Guilleminault C, Goldenberg F. Sleep fragmentation as a risk factor for hypertension in middle-aged nonapneic snorers. Chest. 1996;109:896–900. doi: 10.1378/chest.109.4.896. [DOI] [PubMed] [Google Scholar]
- 219.Grahnstedt S, Ursin R. Platform sleep deprivation affects deep slow wave sleep in addition to REM sleep. Behav Brain Res. 1985;18:233–9. doi: 10.1016/0166-4328(85)90031-2. [DOI] [PubMed] [Google Scholar]
- 220.Hilakivi I, Peder M, Elomaa E, Johansson G. Validation of the cuff pedestal technique for rapid eye movement sleep (REMs) deprivation by electrophysiological recordings. Physiol Behav. 1984;32:945–7. doi: 10.1016/0031-9384(84)90283-x. [DOI] [PubMed] [Google Scholar]
- 221.Mendelson WB, Guthrie RD, Frederick G, Wyatt RJ. The flower pot technique of rapid eye movement (REM) sleep deprivation. Pharmacol Biochem Behav. 1974;2:553–6. doi: 10.1016/0091-3057(74)90018-5. [DOI] [PubMed] [Google Scholar]
- 222.Brooks D, Horner RL, Kimoff RJ, Kozar LF, Render-Teixeira CL, Phillipson EA. Effect of obstructive sleep apnea versus sleep fragmentation on responses to airway occlusion. Am J Respir Crit Care Med. 1997;155:1609–17. doi: 10.1164/ajrccm.155.5.9154865. [DOI] [PubMed] [Google Scholar]
- 223.Brooks D, Horner RL, Kozar LF, Render-Teixeira CL, Phillipson EA. Obstructive sleep apnea as a cause of systemic hypertension. Evidence from a canine model. J Clin Invest. 1997;99:106–9. doi: 10.1172/JCI119120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Polotsky VY, Rubin AE, Balbir A, et al. Intermittent hypoxia causes REM sleep deficits and decreases EEG delta power in NREM sleep in the C57BL/6J mouse. Sleep Med. 2006;7:7–16. doi: 10.1016/j.sleep.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 225.Barf RP, Meerlo P, Scheurink AJ. Chronic sleep disturbance impairs glucose homeostasis in rats. Int J Endocrinol. 2010;2010:819414. doi: 10.1155/2010/819414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zhang SX, Khalyfa A, Wang Y, et al. Sleep fragmentation promotes NADPH oxidase 2-mediated adipose tissue inflammation leading to insulin resistance in mice. Int J Obes (Lond) 2014;38:619–24. doi: 10.1038/ijo.2013.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Baud MO, Magistretti PJ, Petit JM. Sustained sleep fragmentation affects brain temperature, food intake and glucose tolerance in mice. J Sleep Res. 2013;22:3–12. doi: 10.1111/j.1365-2869.2012.01029.x. [DOI] [PubMed] [Google Scholar]
- 228.Wang Y, Carreras A, Lee S, et al. Chronic sleep fragmentation promotes obesity in young adult mice. Obesity. 2014;22:758–62. doi: 10.1002/oby.20616. [DOI] [PMC free article] [PubMed] [Google Scholar]