Abstract
Background
Pulmonary hypertension (PH) is a lethal syndrome associated with the pathogenic remodeling of the pulmonary vasculature and the emergence of apoptosis-resistant cells. ARC (Apoptosis Repressor with Caspase Recruitment Domain) is an inhibitor of multiple forms of cell death known to be abundantly expressed in striated muscle. We show for the first time that ARC is expressed in arterial smooth muscle cells of the pulmonary vasculature and is markedly up-regulated in several experimental models of PH. In this study, we test the hypothesis that ARC expression is essential for the development of chronic hypoxia-induced PH.
Methods and Results
Experiments in which cells or mice were rendered ARC-deficient revealed that ARC not only protected pulmonary arterial smooth muscle cells from hypoxia-induced death, but also facilitated growth factor-induced proliferation and hypertrophy and hypoxia-induced down-regulation of selective voltage-gated potassium channels, the latter a hallmark of the syndrome in humans. Moreover, ARC-deficient mice exhibited diminished vascular remodeling, increased apoptosis, and decreased proliferation in response to chronic hypoxia, resulting in marked protection from PH in vivo. Patients with PH have significantly increased ARC expression not only in remodeled vessels but also in the lumen-occluding lesions associated with severe disease.
Conclusions
These data show that ARC, previously unlinked to pulmonary hypertension, is a critical determinant of vascular remodeling in this syndrome.
Keywords: Apoptosis, hypertension, pulmonary, muscle, smooth
INTRODUCTION
Pulmonary hypertension (PH), defined clinically as an elevation in the mean pulmonary arterial pressure greater than 25 mmHg, is a life-threatening syndrome involving structural and functional changes in the pulmonary vasculature. These abnormalities result in increased pulmonary vascular resistance (PVR), right ventricular hypertrophy and right heart failure (1–3). Current therapies are costly and have limited efficacy (4,5).
Vascular remodeling in pulmonary hypertension involves the entire vessel and multiple cellular components including smooth muscle cells, endothelial cells, and adventitial fibroblasts. Increased proliferation and hypertrophy in concert with decreased cell death/apoptosis of pulmonary arterial smooth muscle cells (PASMCs) contribute to the medial thickening and neomuscularization of distal arterioles characteristic of all forms of pulmonary hypertension. These changes result in reduction of the inner luminal diameter and/or enhanced vasoconstriction of pulmonary vessels resulting in increased PVR (6,7). PH-associated vascular remodeling can be induced by multiple pathophysiologic stimuli (hypoxemia, anorexigens, aberrant flow) and is influenced by genetics (8).
Although their roles in the pathogenesis of PH are incompletely understood, several molecular changes correlate with its development. Voltage-gated potassium (Kv) channels responsible for maintaining the resting membrane potential of the cell are down-regulated in PH and associated with increased proliferation and decreased apoptosis of PASMCs (9,10). Hypoxia also induces endothelin-1 and 5-hydoxytryptamine (serotonin) release, which respectively increase PASMC proliferation and hypertrophy (11–14). Inhibition of each of these pathways attenuates the development of pulmonary hypertension (15,16).
ARC (Apoptosis Repressor with CARD) is an endogenous inhibitor of cell death that is expressed primarily in cardiac and skeletal myocytes (17,18). It is unusual among death repressors in that it blocks multiple modes of cell death, including that initiated by the extrinsic (death receptor) or intrinsic (mitochondrial/ER) apoptosis pathways, as well as oxidative stress-induced caspase-independent cell death (18–22). Thus, ARC is a tissue-restricted suppressor of multiple cell death pathways.
As the establishment of an apoptosis-resistant state has been implicated in the pathogenesis of PH (23), the purpose of this investigation was to determine whether ARC plays a role in the CH-induced vascular remodeling associated with the development of PH. We show for the first time that ARC is expressed in the lung vasculature and that this expression is increased in the PASMCs of mice and rats exposed to chronic hypoxia or in rats with monocrotaline-induced PH. ARC expression in isolated PASMCs confers resistance to CH-induced death, while promoting proliferative and hypertrophic growth in response to pathophysiologically relevant stimuli. Moreover, mice lacking ARC are resistant to developing CH-induced pulmonary hypertension, exhibiting marked attenuation of pulmonary arterial vascular remodeling associated with increased apoptosis and decreased vascular cell proliferation. The clinical significance of these findings is underscored by increased ARC expression in the pulmonary vasculature and lumen-occluding lesions of patients with PAH. These studies demonstrate that ARC plays an important role in the pathogenesis of CH-induced PH in the mouse and suggest that it may be of similar importance in the development of human PH.
METHODS
Generation of the ARC-deficient (nol3−/−) mouse
A vector containing a neo-TK cassette and three loxP sites flanking mouse genomic nol3 sequences was targeted to the nol3 locus of 129/Sv embryonic stem cells. Chimeric mice were bred to C57Bl/6 mice to achieve heterozygous germline transmission and then to EIIA-Cre mice to create heterozygous nol3 knockout mice. These mice were then bred with C57Bl/6 mice for at least 6 generations. Nol3 heterozygotes were interbred to produce homozygous nol3−/− mice and wild-type controls. Only male mice between 8 and 10 weeks old were used.
Chronic Hypoxia Model
Mice were exposed to 10% O2 in a plexiglass chamber (Biospherix, Inc.) monitored by Pro:Ox oxygen controller (model 350, Reming Bioinstruments, Redfield, NY), as previously described (24).
Hemodynamic Measurements
To obtain in vivo hemodynamic measurements, mice were anesthetized with continuous stream of regulated oxygen containing 2% isoflurane. The mice were then ventilated with continuous isoflurane using a rodent ventilator set to 90 breaths/min with a tidal volume of 6 ml/kg body weight. The chest was opened with a midline incision and then a four-electrode pressure-volume catheter (PVR-1035, IF, 3.5 mm spacing, Millar Instruments, Houston, TX) was placed through the right ventricular apex to record chamber volume by impedance and pressure by micromanometry, as described previously (25,26).
Statistical Analyses
Statistical analysis was performed by two-tailed t-test for pair-wise comparisons or one-way ANOVA for comparison between multiple groups using Bonferroni correction for post-hoc, pair-wise between group comparisons for sample sizes of ≥5 per group. Smaller sample sizes (≤4 per group) were compared using the nonparametric Kruskal-Wallis ANOVA test with Siegel (Bonferroni) correction for post-hoc, pair-wise contrasts. The random effects model ANOVA test was used to statistically evaluate comparisons that involved repeated measures. The minimal level of significance was set at P < 0.05. Statistical analyses were performed using Analyse-It (General, version 1.73) for Microsoft Excel.
Additional details and methods are provided in the Methods Supplement.
RESULTS
ARC is Expressed in the Pulmonary Vasculature and Up-regulated by Chronic Hypoxia (CH)
ARC expression has been shown to be primarily restricted in normal tissues to terminally differentiated cells, such as cardiac and skeletal myocytes. We show here that ARC is also expressed in the large (>200 µm diameter) extraparenchymal pulmonary arteries of rats and that this expression is substantially increased following exposure to chronic hypoxia (CH) (3 weeks, 10% O2) (Figure 1A). Immunofluorescence staining of the intact lung confirmed CH-induced ARC expression in pulmonary arterial vessels, as co-localization of ARC with α-smooth muscle actin (SMA), a molecular marker of PASMCs, was minimal in the large vessels of rats maintained under normoxic conditions (N) (Figure 1B), but markedly elevated in rats exposed to CH (white arrows in Figure 1C). This effect of CH on increasing medial ARC expression was also seen in the extraparenchymal pulmonary arteries (>100 µm) of mice (Supplement Figures 1A, 1B).
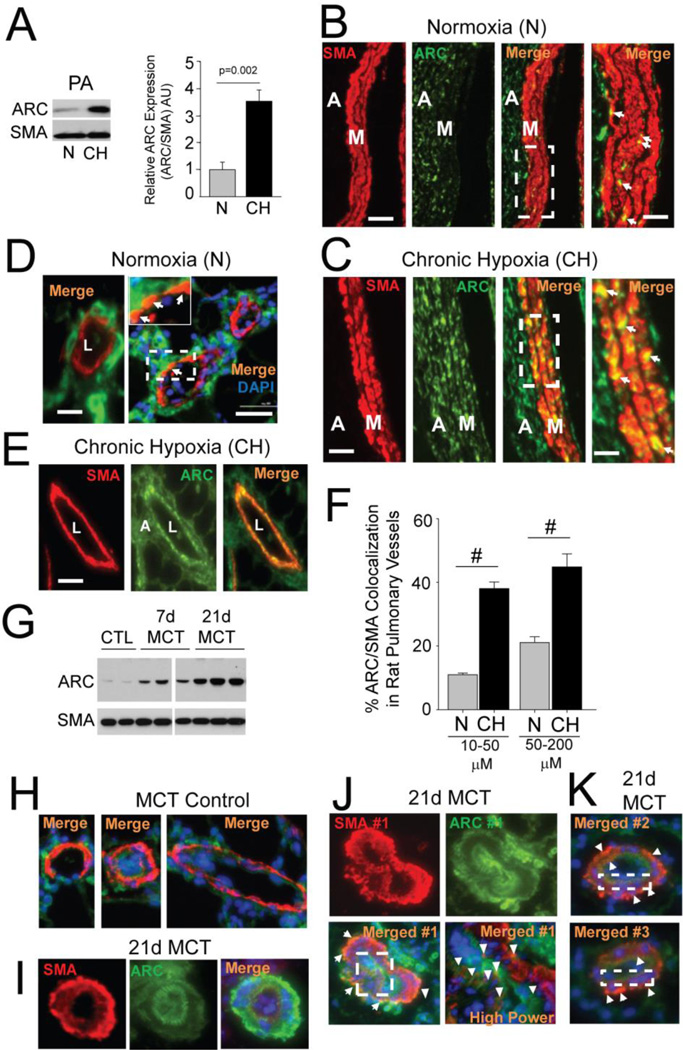
Figure 1. ARC is Expressed in the Rat Pulmonary Vasculature and Increased by Chronic Hypoxia (CH).
A) Western blot of ARC expression in extracts of extraparenchymal pulmonary arteries (PA) from normoxic (N) or chronic hypoxic (CH) rats. Graph displays results from 5 N and 5 CH vessels expressed relative to normoxia and normalized to αSMA (SMA) expression. B,C) Immunostaining of SMA and ARC expression in large pulmonary arteries (>250µm) from normoxic (B) and chronic hypoxic (C) rats. The areas defined by the dashed white boxes are magnified in the Figures to the right. White arrows points to PASMCs co-expressing ARC and SMA. M=medial cell layer; Adv=adventitia. Size bar=100 µm. D) Left panel: Representative image of a small intrapulmonary artery from a normoxic rat showing no detectable expression of ARC in the vessel media. Right panel: Co-localization of ARC and SMA in a distal vessel from a rat exposed to CH as indicated by the arrowheads (yellow/orange pseudocoloring). The area delineated by the white box is magnified in the insert to the figure. Size bars=20 µm (main panel) and 4µm (magnified insert) E) A larger intrapulmonary artery from a CH rat showing expression of ARC throughout the media as revealed in the merged image in the far-right panel. Size bar=50 µm. F) Graph of the percent of ARC-positive cells among SMA-positive vessels, segregated by vessel size (diameter) (#; p< 0.001). G) Western blot of rat pulmonary arteries showing increased ARC expression 7 and 21 days after injection with monocrotaline. H) Merged images of ARC and SMA immunostaining of small intrapulmonary arteries from control rats. I–K) Examples of ARC and SMA staining in muscularized vessels of MCT-treated rats. Size bar=50 µm.
We next assessed ARC expression in small intrapulmonary arteries (diameter <200 µm), the presumed sites of the pathological vascular remodeling in PH. Figure 1D (left panel) shows an example of a distal pulmonary artery (<50 µm) from a normoxic rat in which ARC is clearly expressed in the adventitia (Adv), but only weakly in the medial cell layer (M). When exposed to CH, however, ARC expression is now clearly evident in the media cell layer (Figure 1D, right panel and magnified insert), as well throughout the media of a larger intrapulmonary vessel (>50 µm) (Figure 1E). A systematic analysis of the effect of CH on the expression of ARC revealed that co-localization of ARC and SMA was evident in only 13% (12.6±1.1%) of the small intrapulmonary vessels (20–100 µM) under normoxia, but increased 3-fold (39.2±2.6%; N vs CH, p<0.001) in the vessels of animals exposed to CH (Figure 1F). An approximate 2.5 fold increase in ARC/SMA co-localization in larger intrapulmonary arteries (100–250 µM diameter) was also observed. Similar effects in ARC expression and its co-localization with αSMA were also seen in the small intrapulmonary vessels of the mouse lung (Supplement Figure 1C–1E) and in the vessels from rats with monocrotaline-induced PH (Figures 1G–K). Together, these data show that ARC is expressed in the rat and mouse pulmonary arterial vasculature and that its expression and co-localization with SMA increases in pulmonary resistance vessels in response to CH.
Endogenous Levels of ARC Suppress Hypoxia-induced Cell Death in PASMCs
To assess the functional significance of ARC expression in the pulmonary vasculature, we employed an in vitro hypoxia model using early passage primary rat PASMCs. Hypoxia in vitro (4% O2/5% CO2/91% N2, 24–72 hours) caused a marked increase in ARC mRNA and protein abundance (Figures 2A and 2B, respectively), reaching a maximum within 24 hours of exposure and remaining elevated throughout the period of exposure. In contrast, microvascular endothelial cells expressed much less ARC, which was further reduced by exposure to hypoxia (Supplemental Figure 2A).
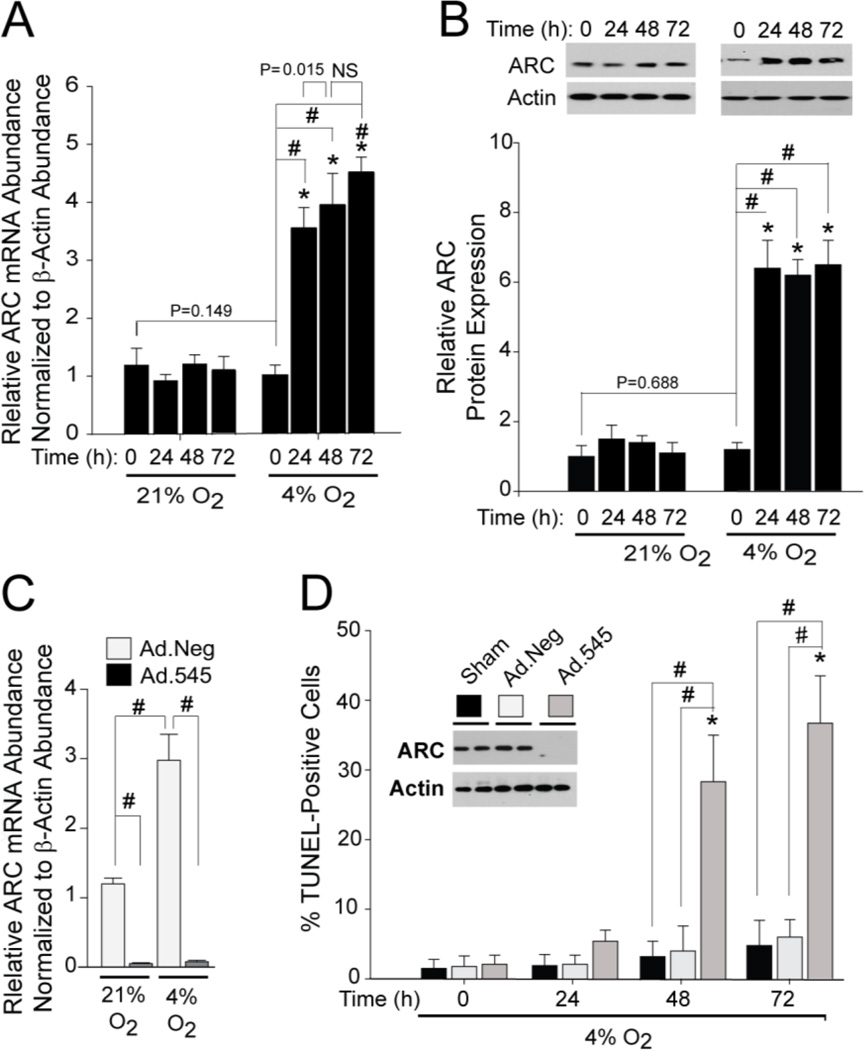
Figure 2. CH Induces ARC Expression in Isolated Pulmonary Arterial Smooth Muscle Cells.
A) qRT-PCR analyses of changes in ARC mRNA abundance in response to CH as a function of oxygen tension (21% O2/5% CO2, balance N2 or 4% O2/5% CO2, balance N2) and time of exposure. Data are expressed relative to ARC mRNA abundance normalized to β-actin mRNA. “#” indicates a significant difference (p<0.05) compared to the zero timepoint, while the asterisk (*) indicates a significant difference relative to same timepoint under normoxia. B). Western blots of ARC protein expression in response to hypoxia and quantitation of the changes in CH-induced ARC protein expression normalized to β-actin expression. (#) and (*) are as indicated above. C) ARC mRNA expression in PASMCs infected with Ad.545 or Ad.NEG and exposed to 4% O2 for 72 hours. E) Apoptosis (TUNEL) in PASMCS during prolonged exposure to 4% O2. Western blots embedded in the Figure show that Ad.545 robustly suppressed ARC protein expression. (#) indicates a significant difference (p>0.05) within treatment groups (sham, Ad.Neg, and Ad.545) at a given timepoint, while (*) indicates a significant difference between treatments during CH and the zero timepoint..
While hypoxia can be a potent inducer of cell death, our data below show that isolated PASMCs are relatively resistant to cell death caused by hypoxia. To determine if this resistance is due to the expression of ARC, we transduced isolated PASMCs with an adenovirus expressing a shRNA designed to suppress ARC expression (Ad.545). Expression of the shRNA suppressed both the basal and hypoxia-induces in ARC mRNA (Figure 2C) and protein levels (Western blot insert to Figure 2D). This reduction was not seen with sham-transduction (no adenovirus) or transduction with a control shRNA whose target sequence is not present in the human, mouse, or rat genomes (Ad.Neg). While neither sham- nor Ad.Neg-transduced PASMCs showed a significant increase in cell death after 72 h of hypoxia, apoptosis in Ad.545-transduced PASMCs was increased more than 10-fold (Figure 2D). Importantly, Ad.545 transduction in the absence of CH did not cause PASMC apoptosis (Supplement Figure 2B). In addition, nearly identical results were obtained using a second ARC shRNA targeting a different site in the ARC mRNA (Supplement Figures 2C). These data demonstrate that endogenous ARC expression is critical for PASMC resistance to hypoxia-induced death.
ARC Enhances the Proliferative and Hypertrophic Responses of PASMCs and Promotes the Hypoxia-Induced Reduction in Voltage-Gated Potassium Channel mRNA Abundance
While ARC is most closely associated with the suppression of cell death in terminally differentiated cells, recent studies have shown that its abundance is markedly increased in human cancers and may play a role in potentiating growth-promoting pathways (27–30). We, therefore, investigated whether ARC plays a role in the response of PASMCs to endothelin-1 and serotonin (5-HT), growth factors closely linked to pulmonary hypertension (11,12,16). Endothelin-1 elicited robust incorporation of 3H-thymidine (DNA synthesis) in mock- and Ad.Neg-transduced cells (Figure 3A), but only a very modest increase in Ad.545-transduced cells. Likewise, knockdown of ARC by siRNA (Figure 3B) completely prevented the 5-hydroxytryptamine (serotonin)-induced hypertrophy (13–15) of isolated PASMCs (Figure 3C). Thus, in addition to its role in suppressing cell death, ARC is also required for mitogenic and hypertrophic growth of PASMCs.
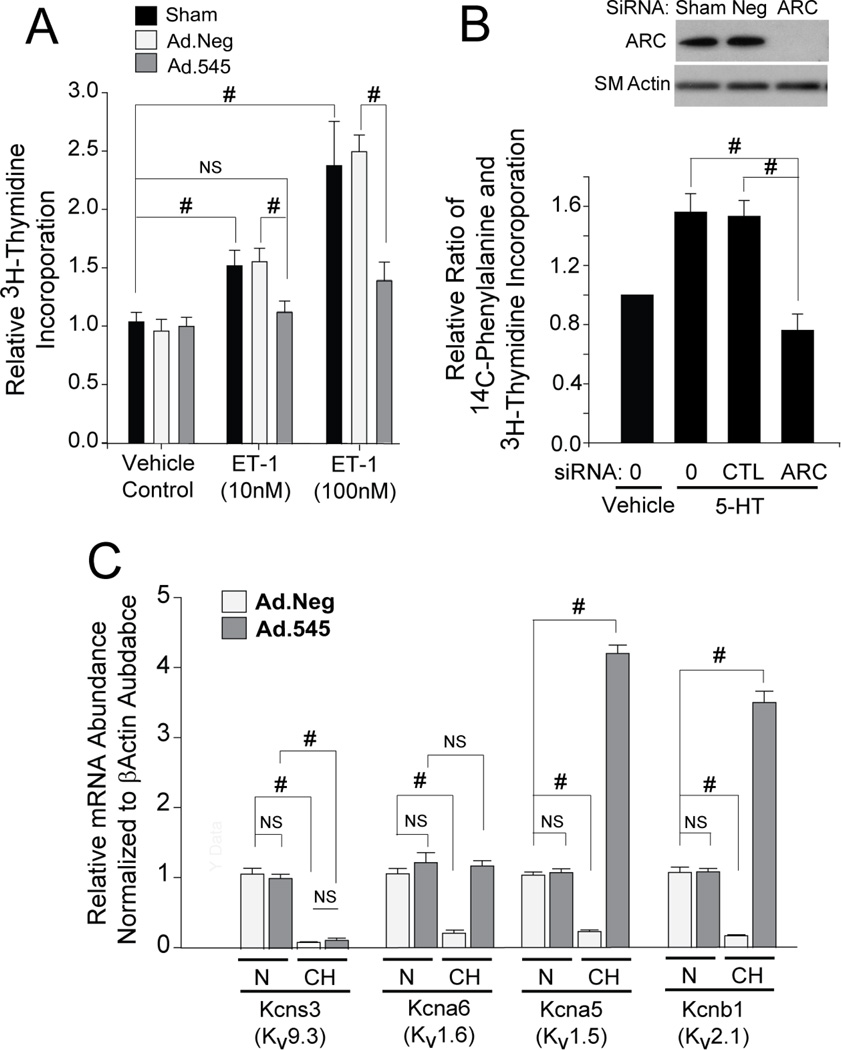
Figure 3. ARC Promotes PASMCs Proliferation and Hypertrophy and is Required for CH-induced Suppression of Select Kv Channels.
A) H3-thymidine incorporation into Sham-, Ad.Neg-, and Ad.545-transduced PASMCs treated with vehicle control, 10 nM ET-1, or 100 nM ET-1. Both Sham and Ad.NEG-transduced cells show a significant and similar uptake in incorporation in response to both ET-1 doses (10 and 100 nM), while Ad.545-transduced cells showed markedly reduced incorporation. B) Western blot showing marked reduction in ARC expression using ARC-specific siRNA but not negative control (CTL) siRNA or sham transfection. C) ARC siRNA resulted in a marked reduction in the ratio of H3-thymidine and C14-phenylalanine incorporation relative to Neg siRNA or sham treatment. D) PASMCs transduced with Ad.Neg or Ad.545 and then exposed to 4% O2 for 72hr were analyzed for expression of Kv1.2, Kv1.5, Kv1.6, and Kv2.1 mRNA expression. The gene symbol for regulatory subunit of each channel is in parenthesis. Data are expressed as the fold change relative to normoxic Ad.Neg-infected PASMCs .(ns, P>0.05; #, P<0.001 for all panels).
Reduction in the expression of the regulatory α-subunits of selected voltage-gated potassium (Kv) channels (e.g, Kv1.5, Kv1.6, Kv2.1 and Kv9.3) is a hallmark of human pulmonary hypertension and multiple animal models of this syndrome (9,10,23,31). While decreases in Kv channel activity caused by acute hypoxia have been linked to vasoconstriction (32), exposure of Ad.Neg-transduced PASMCs to CH (72 hours) caused a significant decrease in the mRNA levels of mRNAs encoding the regulatory α-subunits of Kv9.3 (Kcns3), Kv1.6 (Kcna6), Kv1.5 (Kcna5), and Kv2.1 (Kcnb1) channels (Figure 3D), similar to previous reports (31–34). While knockdown of ARC (Ad.545) had no effect on the hypoxia-induced down-regulation of Kcns3, it ablated the hypoxia-induced decrease in Kcna6 and actually augmented levels of transcripts for Kcna5 and Kcnb1. As the downregulation of Kv channel expression has been linked to increased proliferation and apoptosis-resistance (33), these data along with those in Figures 3A and 3B demonstrate that ARC supports an apoptosis-resistant and growth-promoting environment for PASMCs.
ARC-deficient Mice are Resistant to Chronic Hypoxia-Induced Pulmonary Hypertension
The pro-growth and pro-survival effects of ARC on PASMCs is consistent with a role for ARC in CH-induced pulmonary hypertension in vivo. To test this hypothesis, we created mice with germline deletion of nol3, the gene encoding ARC (Supplement Figure 3). Western blotting of lung extracts and immunostaining of lung tissue sections demonstrate the complete absence of ARC protein expression in null mice (Supplement Figures 3C and D).
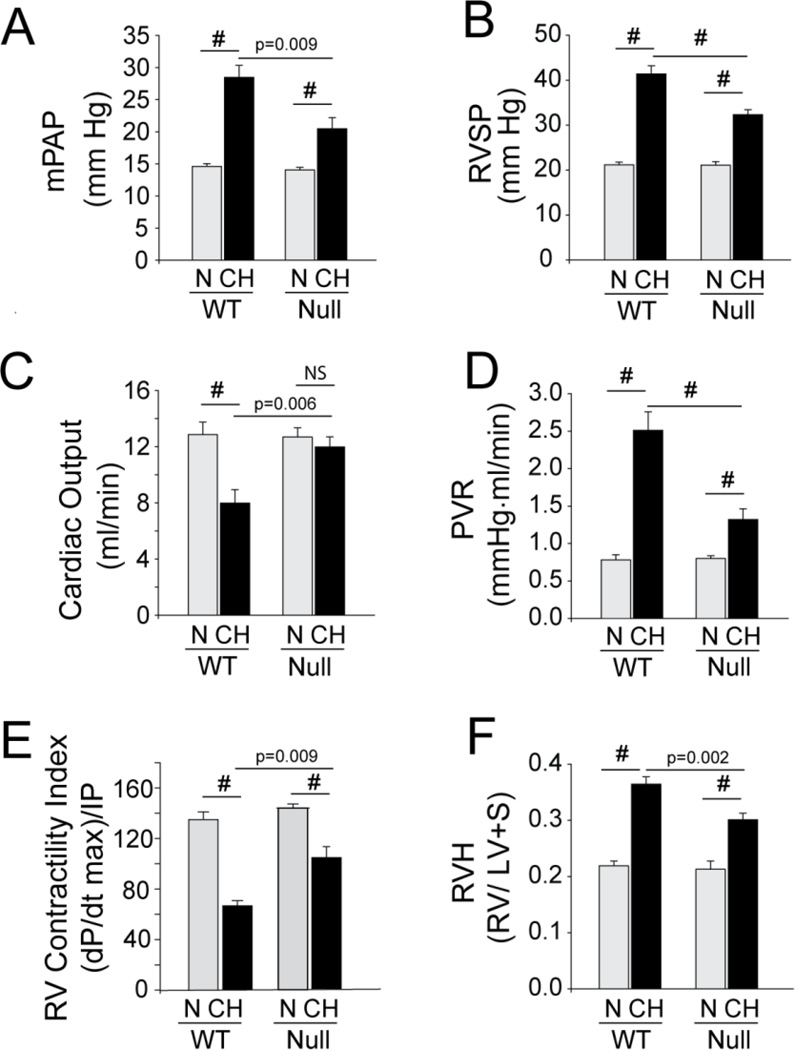
Under normoxic (N) conditions, nol3+/+ (Wild type, WT) and nol 3−/− (Knockout, KO) mice had similar baseline hemodynamic characteristics (Supplement Table 2) and exhibited similar changes in hematocrit in response to CH (Supplement Figure 3E). However, while CH induced a significant elevation in mean pulmonary arterial and RV systolic pressures in WT mice, only modest increases were observed in KO mice (Figures 4A and B and Supplement Table 2). In a similar manner, the right ventricles of CH-WT mice exhibited hypertrophy that was more prominent than that seen in CH-KO mice (Figure 4F). CH-WT mice also exhibited a substantial decrease in cardiac output (CO) and the various indices dependent on it (contractility index and pulmonary vascular resistance) (Figures 4D and E), while CO in CH-KO mice was not statistically different from N-KO controls (Figure 4C). Overall, these data show that the hemodynamic response to CH-induced pulmonary hypertension was markedly attenuated in the ARC KO mouse.
Figure 4. ARC-Deficient/Null Mice Exposed to Chronic Hypoxia Have Reduced Hemodynamic Responses and Right Ventricular Hypertrophy.
Bar graphs comparing changes in mean pulmonary artery pressure (mPAP) (A), right ventricular end systolic pressure (RVSP) (B), cardiac output (C), pulmonary vascular resistance (PVR) (D), contractility index (E), and right ventricular hypertrophy (RVH) (F) in ARC WT and null mice under normoxic (N) and chronic hypoxic (CH) conditions. (#:p<0.001; ns: p>0.05; others as indicated; n=8 animals/experimental arm).
Absence of ARC Attenuates Hypoxia-Induced Remodeling of the Pulmonary Vasculature
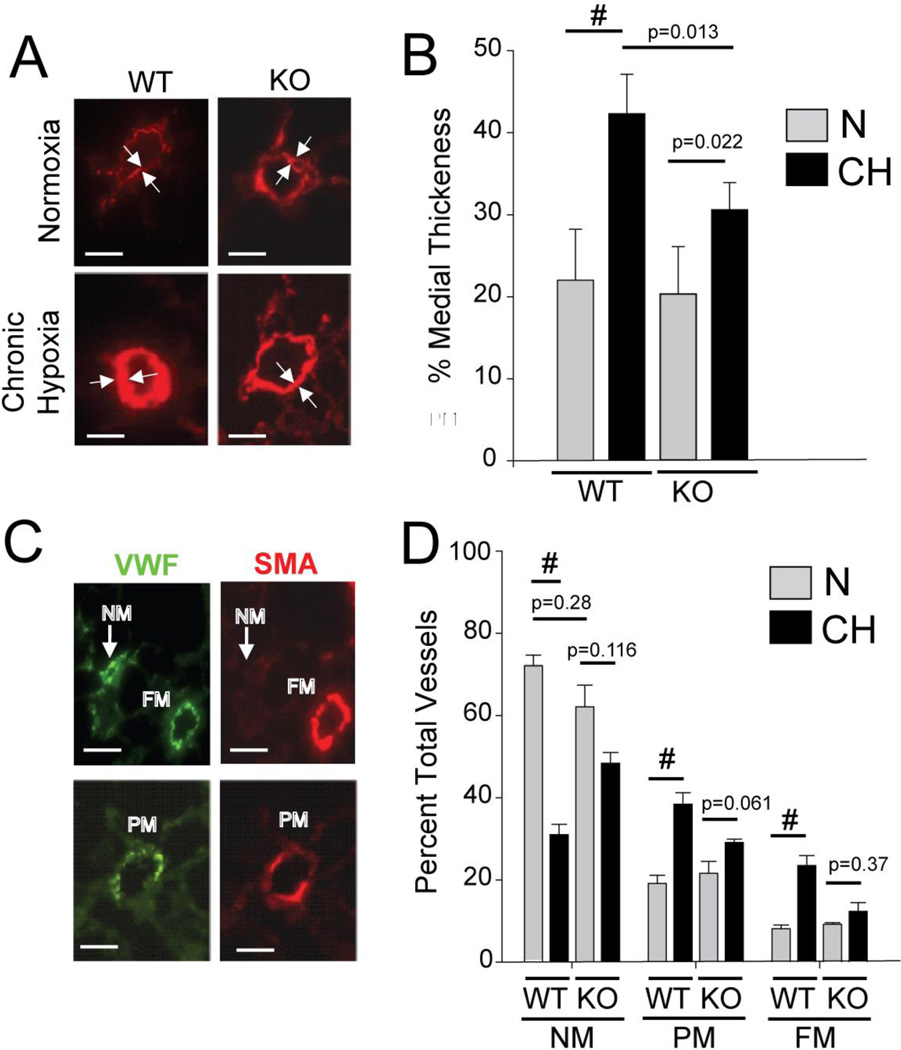
The marked reduction in mPAP, RVSP, and RV hypertrophy in CH-KO mice is reduced CH-induced vascular remodeling in the KO compared to WT mice. We, therefore, compared CH-induced vascular remodeling in WT and KO mice. Pulmonary vascular changes in chronic hypoxic mice include moderate thickening (25–100%) of the medial cell layer of resistance pulmonary arteries and neo-muscularization of more distal vessels. Figure 5A shows representative images of medial thickening in small vessels (<50 µM) of CH-WT and CH-KO mice, as delineated by the space between opposing arrows. While under normoxic conditions, no significant differences in medial thickness were observed between WT and KO mice, CH induced a near doubling in medial thickness in WT mice while eliciting only a ~30% increase in KO mice (Figure 5B).
Figure 5. Nol3−/− (ARC-Deficient) Mice have Markedly Attenuated CH-Induced Vascular Remodeling.
A) Representative images of αSMA-stained distal intrapulmonary arteries from wild type (WT) and ARC-deficient (KO) mice exposed to normoxia (N) or chronic hypoxia (CH) showing a marked increase in medial thickening in WT vessels exposed to CH (lower left panel) that is significantly reduced in CH-KO mice (lower right panel). Size markers = 25µm. B) Percent medial thickening of small arterial blood vessels from normoxic (N) and chronic hypoxic (CH) WT and ARC-deficient (KO) mice. (# = p<0.001; others as indicated; ns, p>0.05; 30–50 vessels analyzed per animal, n=8 animals per study arm). C and D) Representative images of distal intrapulmonary vessels stained for VWF and SMA, showing fully muscularized (FM) and non-muscularized (NM) (C) vessels in the upper panels and partially muscularized vessels in the lower panels. Size markers = 25µm. D) Quantification of the percentage of NM, PM, and FM vessels in lungs from WT and ARC-deficient (KO) mice. (# = p<0.001; others as indicated, ns, p>0.05; 150–200 vWF-positive vessels examined per mouse, n = 8 mice per study arm).
Neomuscularization of distal arteries in response to CH was assessed by scoring von Willebrand Factor (vWF)-positive vessels as either fully (FM), partially (PM), or not (NM) muscularized. Only arterioles adjacent to alveoli were selected for analysis because these are, in general, less than 50 µm in diameter. The images in Figure 5C show examples of each category, while the combined data from the analysis of the vessels show that CH dramatically increased the proportion of PM and FM vessels in WT mice at the expense of NM vessels, indicative of increased neomuscularization (Figure 5D). In contrast, these changes were significantly blunted in CH-KO mice and not statistically different from N-KO mice. These data show that vascular remodeling of resistance vessels in CH-KO mice is significantly reduced from that seen in CH-WT mice, demonstrating that endogenous levels of ARC are critical for CH-induced remodeling of pulmonary vessels.
Absence of ARC Interferes with Cellular Processes that Mediate Pulmonary Vascular Remodeling in vivo
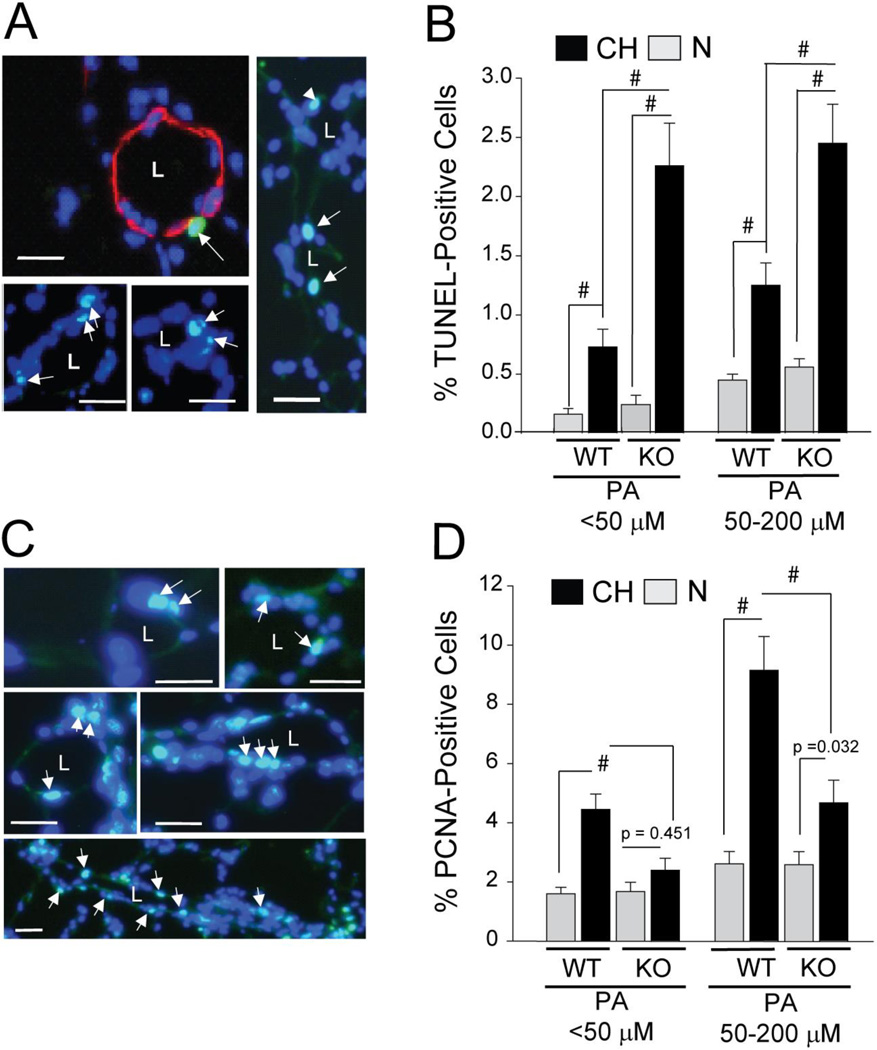
To identify cellular mechanisms through which ARC promotes pulmonary vascular remodeling in vivo, we assessed the effects of its absence on apoptosis and proliferation in small intrapulmonary vessels of CH mice. While baseline apoptosis was not significantly affected by loss of ARC (Figure 6E), CH increased apoptosis in both small (<50µm) and larger (50–200µm) intrapulmonary vessels was markedly elevated in KO vs WT mice (Figures 6A–E). Similarly, the absence of ARC severely curtailed hypoxia-induced increases in the percentage of PCNA-positive cells in both small and larger intrapulmonary vessels PASMCs in vivo, without affecting baseline levels (Figures 6F–K). Taken together, these data show that ARC suppresses cell death and stimulates proliferation in the distal vasculature to promote vascular remodeling.
Figure 6. Increased Apoptosis and Decreased Proliferation in the Pulmonary Vasculature of ARC-KO Mice.
A) Apoptosis-positive cells as detected by TUNEL in the lungs from animals exposed to normoxia (N) or chronic hypoxia (CH) for four days. Distal intrapulmonary arteries stained for nuclear DNA/DAPI (blue) and TUNEL (green). Co-expression produces a light blue pseudo-colored image (arrows). The vessel in Figure 6A was also stained for SMA (red) Size markers = 10mm. B) Quantitation of the percentage of TUNEL-positive nuclei in large (50–200 µm) and small (<50mm) pulmonary vessels (# = P<0.001; 1000–3000 nuclei examined per mouse; n = 9–12 mice per study arm). C) Nuclear PCNA staining in different intrapulmonary arteries. D) Quantitation of the percentage of PCNA-positive nuclei (# = P<0.001, other p values as indicated. NS;. P>0.05; 1000–3000 nuclei examined per mouse; n = 9–12 mice per study arm). Size markers: 10µm.
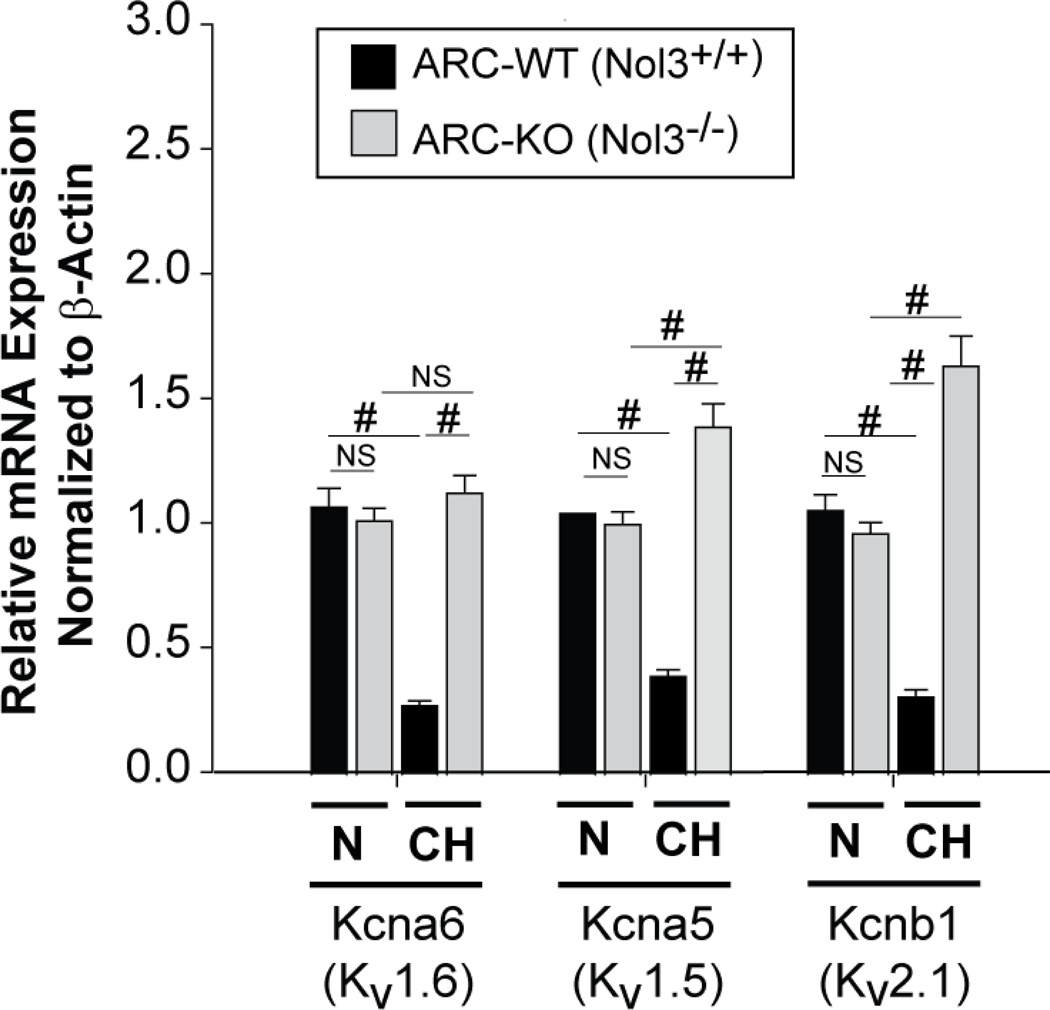
In Figure 3D, we showed that ARC plays an essential role in the CH-induced down-regulation of the mRNAs encoding select hypoxia-sensitive Kv channels in isolated PASMCs. We show in Figure 7 that absence of ARC expression in vivo also prevented the CH-induced decreases in the expression of Kv1.6, Kv1.5, and Kv2.1 regulatory subunits in intact distal vessels without influencing baseline levels (Figure 7). As in the cell culture experiments (Figure 3D), lack of ARC did not prevent the CH-induced downregulation of Kv9.3 transcripts. These data show that endogenous levels of ARC are critical for the chronic hypoxia-induced down-regulation of mRNAs encoding select Kv channels in response to hypoxia in vivo.
Figure 7. ARC Regulates Kv Channel Expression in vivo.
Expression of Kv9.3, Kv1.6, Kv1.5, and Kv2.1 mRNA in intrapulmonary arteries of WT and KO mice after 3 weeks CH. Results are presented for each relative to the average PCR cycle number of the normoxic WT samples. (NS, p>0.05; # p<0.001; 8 arteries per experimental group).
ARC is a Component of Human Pulmonary Hypertension
The in vitro and in vivo loss of function studies demonstrate that absence of ARC disrupts CH-induced remodeling of the pulmonary arterial vasculature in the mouse. To explore a possible role for ARC in human PH, immunohistochemical analyses were performed on lung tissue obtained from patients undergoing surgical biopsy to evaluate a lung nodule (controls; N1–N4) and explants from patients with WHO group I pulmonary arterial hypertension undergoing lung transplantation (PH1–PH5). The official pathologic assessment confirmed normal lung architecture in the controls and changes consistent with PH in the explanted lungs. In controls, there was little to no staining for ARC in the vessel wall or lumen (Figure 8A). In contrast, 4 of the 5 PH samples showed robust staining in both muscularized vessels (Figure 8B, black arrows) and lumen-occluding lesions (Figure 8C, white arrows) associated with severe PAH in humans (Figure 8B). Thus, ARC abundance is markedly increased in the remodeled vessels of patients with PH. The high level of ARC expression in these lesions is not unexpected ARC is highly expressed in many different cancers (27–30) and others have characterized the lumen-occluding lesions in severe PH as having multiple cancer-like properties (35).
Figure 8. ARC Expression is Elevated in Vascular Lesions of Patients with Pulmonary Hypertension.
Detection of ARC in human lung tissue performed on archival paraffin-embedded section of lungs from patients with pulmonary hypertension undergoing lung transplantation or control patients undergoing surgical biopsy to evaluate a lung nodule. A) Lung sections from control non-PAH patients show weak immunoreactivity (arrows mark brown DAB precipitate) in small (<100 mm) pulmonary arteries. B and C) Lung sections from 5 PAH patients showing intense immunoreactivity for ARC in the medial wall (B) and lumen-occluding lesions (C) in patients PH1–PH5. PH5 samples shows only moderate immunoreactivity in the vascular wall and the occluded lumen.
DISCUSSION
Suppression of cellular apoptosis and increased proliferation has been proposed as essential mechanisms in the remodeling of the pulmonary vasculature associated with PH (23,33,36,37). We show here for the first time that ARC, a suppressor of multiple forms of cell death, is expressed in the medial smooth muscle cells of the pulmonary vasculature of both mice and rats. In addition, we found that ARC’s expression was markedly increased in the pulmonary arteries and resistance arterioles in different experimental models of PAH and in the pathologic vascular lesions observed in patients with PAH (Figures 1–4 and 8; Supplement Figure 1). The absence of ARC in isolated PASMCs 1) sensitized them to hypoxia-induced cell death (Figure 2D), 2) reduced their responsiveness to the growth-promoting effects of ET-1 and 5-HT (Figures 3A and B), and 3) reversed the CH-induced suppression of select hypoxia-sensitive Kv channel subunits (Figure 3C). All of these attributes are consistent with a role for ARC in promoting pathologic vascular remodeling in PH. This role is further supported by the demonstration that ARC-deficient (nol3−/−) animals have a markedly attenuated hemodynamic response to CH that is associated with reduced vascular remodeling, increased vascular cell apoptosis, and decreased vascular cell proliferation. Collectively, this work provides strong evidence in support of a critical role for ARC as a previously unrecognized determinant of the pathological vascular remodeling in CH-induced PH.
The mechanism(s) through which ARC promotes vascular remodeling in PH may be through its ability to induce a state of marked apoptosis resistance in cells (18–22). When expressed in PASMCs, ARC upsets the balance between vascular cell growth and death in favor of increased muscularization of the vasculature. ARC, however, is not unique in this regard, as other anti-apoptosis/pro-survival molecules, such as survivin (Birc5) (23), Bcl2 (36), and Bclxl (37) have also been implicated in PH. While the upregulation of multiple death repressors in PH is strong testament to the importance of suppressing cell death in the progression of this disease, it is not clear if these repressors have redundant, interdependent, or unique roles.
Nonetheless, ARC is unique among this cadre of death repressors in that it is also a critical regulator of PASMC growth (Figure 3A and B). The mechanism(s) underlying the ARC’s pro-growth effects have not been established, although recent studies have revealed that ARC functionally inactivates p53 in several cancer cell lines, thereby indirectly promoting cell growth (38). As p53 can also suppress ARC expression (39), a dynamic antagonism may exist between the two molecules to strictly control and fine-tune PASMC growth and death. This antagonism is apparent in vivo in the opposite responses to CH in mice deficient for ARC (reduced CH-PH) or p53 (exaggerated CH-PH)(40).
We also show that ARC is necessary for the hypoxia-induced down-regulation of mRNAs encoding the regulatory subunits of select Kv channels (Figure 3C and 7), placing ARC upstream of changes in Kv channel expression. A connection between ARC and Kv channel activity was suggested by Ekheterae et al (41) who observed chronic suppression of Kv channel activity in ARC-overexpressing cells, although the molecular basis of this effect was not elucidated. Our data clearly show that ARC can suppress the accumulation of mRNAs encoding select Kv channels (Figures 3C and 7). One mechanism through which ARC might affect Kv channel mRNA abundance is through its regulation of endothelin-1 signaling (Figure 3A), as this pathway has been shown to link CH to Kv channel expression (42).
Other anti-apoptosis/pro-survival proteins implicated in PH (survivin and Bcl-2) also promote decreased Kv channel activity and/or expression (23,43). Although these molecules and ARC are structurally unrelated, a common denominator among them is their ability to prevent depolarization of the mitochondrial membrane potential (Δψm) (22,44,45). This suggests that changes in select Kv channel activity and/or expression may be closely linked to changes in Δψm. If so, ARC on its own or in concert with survivin and/or Bcl2 would promote a more hyperpolarized Δψm, resulting in reduced expression/activity of select Kv channels, perhaps through changes in mitochondrial reactive oxygen species production, as proposed by others (46,47).
Our study has some limitations. CH in the mouse produces relatively mild PH with few of the histological landmarks (e.g., lumen-occluding lesions) characteristic of human PH. Our studies on ARC, however, provide an important connection to the pathobiology of human PH as we demonstrate increased ARC expression within the apoptosis-resistant lumen-occluding lesions in the pulmonary arteries of PH patients (Figure 8). Also, while ARC is expressed in the heart, the separate contributions of ARC deficiency on vasculature remodeling and right heart function cannot be ascertained from our current genetic model, a concern because others have shown that deficiency of ARC in the left ventricle leads to reduced contractile performance and decreased cardiac output in response to sustained pressure-overload (48). This does not, however, exclude direct effects of ARC deficiency on RV function, the presence of which would be best addressed by examining the effects of fixed pulmonary arterial stenosis in WT and KO mice.
In summary, we have shown that ARC is a novel upstream regulator of several critical factors implicated in the development of PH, and that suppression of ARC in vivo protects mice from CH-induced PH. These effects of ARC are also likely to be important to human PAH as patient data show that ARC is expressed in the lumen-occluding lesions of severe human PH. Additional studies are needed to determine the role of ARC in other animal models of PH and how signaling through ARC integrates with other molecular signaling pathways contributing to PH.
Supplementary Material
Clinical Perspective.
Pulmonary arterial hypertension (PAH) remains a devastating disease with an unacceptably high mortality. While initial therapies focused on the regulators of pulmonary vascular tone, few patients have a clinical response to acute pulmonary vasodilators and chronic therapy rarely modifies pulmonary vascular resistance due to the structural alterations in the pulmonary vasculature. Pulmonary vascular remodeling results from dysregulated endothelial and smooth muscle cell function and phenotype. Altered rates of proliferation and apoptosis result in increased PASMC proliferation/hypertrophy and contribute to remodeling in animal models and clinical samples. Parallels between these phenotypic alterations and the changes characteristic of a neoplastic process have been increasingly recognized. Identification of novel molecular determinants of pathologic remodeling are critical for the development of new pharmacologic targets. Here, we provide the first demonstration that the apoptosis repressor with CARD domain (ARC), an intracellular inhibitor of apoptosis, is selectively upregulated in the pulmonary arterial circulation in both rodent models of PAH and in patients with idiopathic PAH. Loss of function studies both in vivo and in isolated cells demonstrate that ARC is a critical determinant of pathologic vascular remodeling contributing to altered smooth muscle cell proliferation and apoptosis during disease development. Further, we provide evidence that ARC is obligatory for hypoxia-induced alterations of select potassium channels critical for alterations of pulmonary vascular tone and smooth muscle cell phenotypic changes. Thus, ARC represents a novel determinant of pathologic vascular remodeling obligatory for multiple molecular and cell biological derangements critical for pathologic remodeling in PAH.
ACKNOWLEDGEMENTS
The authors acknowledge the excellent technical assistance of Mr. James Watkins and Mr. Andre Robinson of the Hopkins Cardiopulmonary Histology Core.
FUNDING SOURCES
NIH grants P50 HL084946 (PH), R01 HL060665 (RNK), R01 HL073935 (MTC), and AHA Grant-in-Aid (MTC). RNK acknowledges the generous support of the Wilf Family Cardiovascular Research Institute and the Dr. Gerald and Myra Dorros Chair in Cardiovascular Disease of the Albert Einstein School of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES. None
REFERENCES
- 1.Gaine SP, Rubin LJ. Primary pulmonary hypertension. Lancet. 1998;352:719–725. doi: 10.1016/S0140-6736(98)02111-4. [DOI] [PubMed] [Google Scholar]
- 2.Badesch DB, Champion HC, Sanchez MA, Hoeper MM, Loyd JE, Manes A, McGoon M, Naeije R, Olshewski H, Oudiz RJ, Torbicki A. Diagnosis and assessment of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S55–S66. doi: 10.1016/j.jacc.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 3.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT, Levy PS, Pietra GS, Reid LM, Reeves JT, Stuart R, Vreim CE, Williams GW, Wu M. Survival in patients with primary pulmonary hypertension: Results from a national prospective registry. Ann Intern Med. 1991;115:343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin VV, Shillington A, Rich S. Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation. 2002;106:1477–1482. doi: 10.1161/01.cir.0000029100.82385.58. [DOI] [PubMed] [Google Scholar]
- 5.McLaughlin VV. Survival in patients with pulmonary arterial hypertension treated with first-line bosentan. Eur J Clin Invest. 2006;36(Suppl 3):10–15. doi: 10.1111/j.1365-2362.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- 6.Mandegar M, Fung YC, Huang W, Remillard CV, Rubin LJ, Yuan JX. Cellular and molecular mechanisms of pulmonary vascular remodeling: role in the development of pulmonary hypertension. Microvasc Res. 2004;68:75–103. doi: 10.1016/j.mvr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing ZC, Krowka MJ, Langleben D, Nakahashi N, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–S54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Pak O, Aldashev A, Welsh D, Peacock A. The effects of hypoxia on the cells of the pulmonary vasculature. Eur Respir J. 2007;30:364–372. doi: 10.1183/09031936.00128706. [DOI] [PubMed] [Google Scholar]
- 9.Yuan JX, Aldinger AM, Juhaszova M, Wang J, Conte JV, Jr, Gaine SP, Orens JB, Rubin LJ. Dysfunctional voltage-gated K+ channels in pulmonary artery smooth muscle cells of patients with primary pulmonary hypertension. Circulation. 1998;98:1400–1406. doi: 10.1161/01.cir.98.14.1400. [DOI] [PubMed] [Google Scholar]
- 10.Bonnet S, Archer SL. Potassium channel diversity in the pulmonary arteries and pulmonary veins: implications for regulation of the pulmonary vasculature in health and during pulmonary hypertension. Pharmacol Ther. 2007;115:56–69. doi: 10.1016/j.pharmthera.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Chen SJ, Chen YF, Meng QC, Durand J, Oparil S, Elton TS. Enhanced endothelin-1 and endothelin receptor gene expression in chronic hypoxia. J Appl Physiol. 1994;77:1451–1459. doi: 10.1152/jappl.1994.77.3.1451. [DOI] [PubMed] [Google Scholar]
- 12.Chen YF, Oparil S. Endothelin and pulmonary hypertension. J Cardiovasc Pharmacol. 2000;35:S49–S53. doi: 10.1097/00005344-200000002-00012. [DOI] [PubMed] [Google Scholar]
- 13.Eddahibi S, Humbert M, Fadel E, Raffestin B, Darmon M, Capron F, Simonneau G, Dartevelle P, Hamon M, Adnot S. Hyperplasia of pulmonary artery smooth muscle cells is causally related to overexpression of the serotonin transporter in primary pulmonary hypertension. Chest. 2002;121:97S–98S. [PubMed] [Google Scholar]
- 14.Esteve JM, Launay JM, Kellermann O, Maroteaux L. Functions of serotonin in hypoxic pulmonary vascular remodeling. Cell Biochem Biophys. 2007;47:33–44. doi: 10.1385/cbb:47:1:33. [DOI] [PubMed] [Google Scholar]
- 15.Eddahibi S, Hanoun N, Lanfumey L, Lesch KP, Raffestin B, Hamon M, Adnot S. Attenuated hypoxic pulmonary hypertension in mice lacking the 5-hydroxytryptamine transporter gene. J Clin Invest. 2000;105:1555–1562. doi: 10.1172/JCI8678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan SH, Dai DZ, Guan L, Dai Y, Ji M. CPU0507, an endothelin receptor antagonist, improves rat hypoxic pulmonary artery hypertension and constriction in vivo and in vitro. Clin Exp Pharmacol Physiol. 2006;33:1066–1072. doi: 10.1111/j.1440-1681.2006.04488.x. [DOI] [PubMed] [Google Scholar]
- 17.Geertman R, McMahon A, Sabban EL. Cloning and characterization of cDNAs for novel proteins with glutamic acid-proline dipeptide tandem repeats. Biochim Biophys Acta. 1996;1306:147–152. doi: 10.1016/0167-4781(96)00036-x. [DOI] [PubMed] [Google Scholar]
- 18.Koseki T, Inohara N, Chen S, Nunez G. ARC, an inhibitor of apoptosis expressed in skeletal muscle and heart that interacts selectively with caspases. Proc Natl Acad Sci USA. 1998;95:5156–5160. doi: 10.1073/pnas.95.9.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nam YJ, Mani K, Ashton AW, Peng CF, Krishnamurthy B, Hayakawa Y, Lee P, Korsmeyer SJ, Kitsis RN. Inhibition of both the extrinsic and intrinsic death pathways through nonhomotypic death-fold interactions. Mol Cell. 2004;15:901–912. doi: 10.1016/j.molcel.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 20.Gustafsson AB, Tsai JG, Logue SE, Crow MT, Gottlieb RA. Apoptosis repressor with caspase recruitment domain protects against cell death by interfering with Bax activation. J Biol Chem. 2004;279:21233–21238. doi: 10.1074/jbc.M400695200. [DOI] [PubMed] [Google Scholar]
- 21.Ekhterae D, Lin Z, Lundberg MS, Crow MT, Brosius FC, III, Nunez G. ARC inhibits cytochrome c release from mitochondria and protects against hypoxia-induced apoptosis in heart-derived H9c2 cells. Circ Res. 1999;85:e70–e77. doi: 10.1161/01.res.85.12.e70. [DOI] [PubMed] [Google Scholar]
- 22.Neuss M, Monticone R, Lundberg MS, Chesley AT, Fleck E, Crow MT. The apoptotic regulatory protein ARC (apoptosis repressor with caspase recruitment domain) prevents oxidant stress-mediated cell death by preserving mitochondrial function. J Biol Chem. 2001;276:33915–33922. doi: 10.1074/jbc.M104080200. [DOI] [PubMed] [Google Scholar]
- 23.McMurtry MS, Archer SL, Altieri DC, Bonnet S, Haromy A, Harry G, Bonnet S, Puttagunta L, Michelakis ED. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J Clin Invest. 2005;115:1479–1491. doi: 10.1172/JCI23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Girgis RE, Li X, Zhan X, Garcia JG, Tuder RM, Hassoun PM, Johns RA. Attenuation of chronic hypoxic pulmonary hypertension by simvastatin. Amer J Respir Cell Mol Biol. 2003;285(3):H938–H945. doi: 10.1152/ajpheart.01097.2002. [DOI] [PubMed] [Google Scholar]
- 25.Hemnes AR, Zaiman A, Champion HC. PDE5A inhibition attenuates bleomycin-induced pulmonary fibrosis and pulmonary hypertension through inhibition of ROS generation and RhoA/Rho kinase activation. Am J Physiol Lung Cell Mol Physiol. 2008;294:L24–L33. doi: 10.1152/ajplung.00245.2007. [DOI] [PubMed] [Google Scholar]
- 26.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y, Kass DA. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11:214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 27.Mercier I, Vuolo M, Madan R, Xue X, Levalley AJ, Ashton AW, Jasmin JF, Czaja MT, Lin EY, Armstrong RC, Pollard KW, Kitsis RN. ARC, an apoptosis suppressor limited to terminally differentiated cells, is induced in human breast cancer and confers chemo- and radiation-resistance. Cell Death Differ. 2005;12:682–686. doi: 10.1038/sj.cdd.4401631. [DOI] [PubMed] [Google Scholar]
- 28.Wu L, Nam YJ, Kung G, Crow MT, Kitsis RN. Induction of the apoptosis inhibitor ARC by Ras in human cancers. J Biol Chem. 2010;285:19235–19245. doi: 10.1074/jbc.M110.114892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mercier I, Vuolo M, Jasmin JF, Medina CM, Williams M, Mariadason JM, Qian H, Xue X, Pestell RG, Lisanti MP, Kitsis RN. ARC (apoptosis repressor with caspase recruitment domain) is a novel marker of human colon cancer. Cell Cycle. 2008;7:1640–1647. doi: 10.4161/cc.7.11.5979. [DOI] [PubMed] [Google Scholar]
- 30.Carter BZ, Qui YH, Zhang N, Coombes KR, Mak DH, Thomas DA, Ravandi F, Kantarjian HM, Andreeff M, Kornblau SM. 2011 Expression of ARC (apoptosis repressor with caspase recruitment domain), an antiapoptotic protein, is strongly prognostic in AML. Blood. 2011;117:780–787. doi: 10.1182/blood-2010-04-280503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J, Weigand L, Wang W, Sylvester JT, Shimoda LA. Chronic hypoxia inhibits Kv channel gene expression in rat distal pulmonary artery. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1049–L1058. doi: 10.1152/ajplung.00379.2004. [DOI] [PubMed] [Google Scholar]
- 32.Archer SL, Souil E, Nh-Xuan AT, Schremmer B, Mercier JC, El YA, Nguyen-Huu L, Reeve HL, Hampl V. Molecular identification of the role of voltage-gated K+ channels, Kv1.5 and Kv2.1, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes. J Clin Invest. 1998;101:2319–2330. doi: 10.1172/JCI333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burg ED, Remillard CV, Yuan JX. Potassium channels in the regulation of pulmonary artery smooth muscle cell proliferation and apoptosis: pharmacotherapeutic implications. Br J Pharmacol. 2008;153(Suppl 1):S99–S111. doi: 10.1038/sj.bjp.0707635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Platoshyn O, Remillard CV, Fantozzi I, Mandegar M, Sison TT, Zhang S, Burg E, Yuan JX. Diversity of voltage-dependent K+ channels in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L226–L238. doi: 10.1152/ajplung.00438.2003. [DOI] [PubMed] [Google Scholar]
- 35.Rai PR, Cool CD, King JA, Stevens T, Burns N, Winn RA, Kasper M, Voelkel NF. The cancer paradigm of severe pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;178:558–564. doi: 10.1164/rccm.200709-1369PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonnet S, Rochefort G, Sutendra G, Archer SL, Harmony A, Webster L, Hashimoto K, Bonnet SN, Mickelakis ED. The nuclear factor of activated T cells in pulmonary arterial hypertension can be therapeutically targeted. Proc Natl Acad Sci USA. 2007;104:11418–11423. doi: 10.1073/pnas.0610467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki YJ, Nagase H, Wong CM, Kumar SV, Jain V, Park AM, Day RM. Regulation of Bcl-xL expression in lung vascular smooth muscle. Am J Respir Cell Mol Biol. 2007;36:678–687. doi: 10.1165/rcmb.2006-0359OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foo RS, Nam YJ, Ostreicher MJ, Metzl MD, Whelan RS, Peng CF, Ashton AW, Fu W, Mani K, Chin SF, Provenzano E, Ellis I, Figg N, Pinder S, Bennett MR, Caldas C, Kitsis RN. Regulation of p53 tetramerization and nuclear export by ARC. Proc Natl Acad Sci USA. 2007;104:20826–20831. doi: 10.1073/pnas.0710017104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li YZ, Lu DY, Tan WQ, Wang JX, Li PF. p53 initiates apoptosis by transcriptionally targeting the antiapoptotic protein ARC. Mol Cell Biol. 2008;28:564–574. doi: 10.1128/MCB.00738-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mizuno S, Bogaard HJ, Kraskauskas D, Alhussaini A, Gomez-Arroyo J, Voelkel NF, Ishizaki T. p53 gene deficiency promotes hypoxia-induced pulmonary hypertension and vascular remodeling in mice. Am J Physiol Lung Cell Mol Physiol. 2011;300:L753–L761. doi: 10.1152/ajplung.00286.2010. [DOI] [PubMed] [Google Scholar]
- 41.Ekhterae D, Platoshyn O, Zhang S, Remillard CV, Yuan JX. Apoptosis repressor with caspase domain inhibits cardiomyocyte apoptosis by reducing K+ currents. Am J Physiol Cell Physiol. 2003;284:C1405–C1410. doi: 10.1152/ajpcell.00279.2002. [DOI] [PubMed] [Google Scholar]
- 42.Whitman EM, Pisarcik S, Luke T, Fallon M, Wang J, Sylvester JT, Semenza GL, Shimoda LA. Endothelin-1 mediates hypoxia-induced inhibition of voltage-gated K+ channel expression in pulmonary artery myocytes. Am J Physiol Lung Cell Mol Physiol. 2008;294:L309–L318. doi: 10.1152/ajplung.00091.2007. [DOI] [PubMed] [Google Scholar]
- 43.Ekhterae D, Platoshyn O, Krick S, Ying Y, Sharon SS, Yuan J-X. Bcl-2 decreases voltage-gated K+ channel activity and enhances survival in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2001;281:C157–C165. doi: 10.1152/ajpcell.2001.281.1.C157. [DOI] [PubMed] [Google Scholar]
- 44.Blanc-Brude OP, Mesri M, Wall NR, Plescia J, Dohi T, Altieri DC. Therapeutic targeting of the survivin pathway in cancer: initiation of mitochondrial apoptosis and suppression of tumor-associated angiogenesis. Clin Cancer Res. 2003;7(9):2683–2692. [PubMed] [Google Scholar]
- 45.Shimuzu S, Eguchi Y, Kamiiki W, Funahashi Y, Mignon M, Lacronique V, Matsuda H, Tsujimoto Y. Bcl-2 prevents apoptotic mitochondrial dysfunction by regulating proton flux. Proc Nat Acad Sci USA. 1998;95:1455–1459. doi: 10.1073/pnas.95.4.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Michelakis ED, McMurtry MS, Wu XC, Dyck JR, Moudgil R, Hopkins TA, Lopaschuk GD, Puttagunta L, Waite R, Archer SL. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: role of increased expression and activity of voltage-gated potassium channels. Circulation. 2002;105(2):244–250. doi: 10.1161/hc0202.101974. [DOI] [PubMed] [Google Scholar]
- 47.Pozeg ZI, Michelakis ED, McMurtry MS, Thébaud B, Wu XC, Dyck JR, Hashimoto K, Wang S, Moudgil R, Harry G, Sultanian R, Koshal A, Archer SL. In vivo gene transfer of the O2-sensitive potassium channel Kv1.5 reduces pulmonary hypertension and restores hypoxic pulmonary vasoconstriction in chronically hypoxic rats. Circulation. 2003;107(15):2037–2044. doi: 10.1161/01.CIR.0000062688.76508.B3. [DOI] [PubMed] [Google Scholar]
- 48.Donath S, Li P, Willenbockel C, Al-Saadi N, Gross V, Willnow T, Bader M, Martin U, Bauersach J, Wollert KC the German Heart Network. Apoptosis repressor with caspase recruitment domain is required for cardioprotection in response to biomechanical and ischemic stress. Circulation. 2006;113:1203–1212. doi: 10.1161/CIRCULATIONAHA.105.576785. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.