Abstract
Hepatocellular carcinoma (HCC) is a significant cause of cancer-related morbidity and mortality worldwide. Despite improvements in local therapies including surgical resection, liver transplantation, and transarterial embolization, the prognosis remains poor for the majority of patients who develop recurrence or present with advanced disease. Systemic therapy with the tyrosine kinase inhibitor sorafenib represents a milestone in advanced HCC, but provides a limited survival benefit. Ongoing efforts to study hepatocarcinogenesis have identified an important role of c-MET signaling in the promotion of tumor growth, angiogenesis, and metastasis. In this review, we summarize the preclinical data from human tissue, cell lines, and animal models that implicate c-MET in the pathogenesis of HCC. We also evaluate potential biomarkers that may estimate prognosis or predict response to c-MET inhibitors for more rational clinical trial design. Finally, we discuss the latest clinical trials of c-MET inhibitors in advanced HCC.
Keywords: hepatocellular carcinoma, c-MET, HGF, receptor tyrosine kinase inhibitors, clinical trials, biomarkers
INTRODUCTION
Liver cancer ranks sixth in incidence and third in mortality among all cancers worldwide (1). The most common primary liver cancer — hepatocellular carcinoma (HCC) — accounts for 70–85% of cases (2). Advanced HCC carries a poor prognosis with a five-year survival of <10% (3). While chemotherapy has shown limited efficacy, two large randomized trials have demonstrated that the small molecule tyrosine kinase inhibitor (TKI) sorafenib (Nexavar®, Bayer and Onyx) improves survival in advanced HCC compared to placebo, but the median overall survival remains less than one year (4–6). As the search for novel and effective treatments continues, the TK receptor c-MET is emerging as a therapeutic target in HCC. In this review, we discuss the role of the c-MET pathway in hepatocarcinogenesis, summarize the preclinical data supporting its potential as a therapeutic target in HCC, and review the prognostic and predictive value of relevant biomarkers. Finally, we will provide an update on c-MET inhibitors currently under investigation in HCC.
THE HGF/c-MET PATHWAY
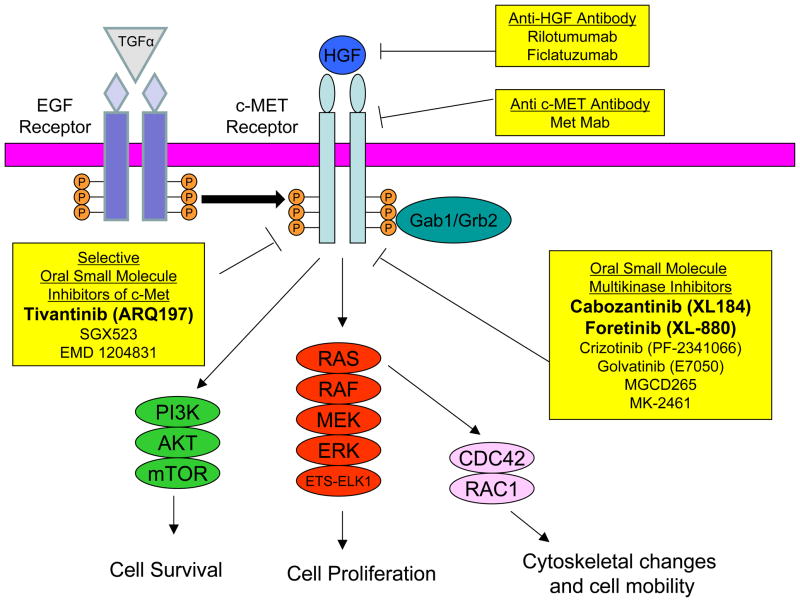
The c-MET proto-oncogene was originally identified as a fusion gene (tpr-met) in a chemically-transformed human osteosarcoma cell line (7). It encodes for the receptor for the ligand hepatocyte growth factor (HGF) (8). In the canonical HGF/c-MET signaling pathway, HGF binding leads to receptor homodimerization, autophosphorylation of tyrosine residues of the carboxy-terminal domain c-MET, and downstream activation of the MAPK, PI3K, and Rac1-Cdc42 pathways (Figure 1, reviewed in (9)). c-MET phosphorylation in the absence of HGF can occur through interactions with the epidermal growth factor receptor (EGFR), cell attachment (10), or binding of the alternate ligand des-gamma-carboxyprothrombin (11). Regardless of the mode of activation, c-MET dimerization, autophosphorylation, and kinase activity appear to be necessary for malignant transformation (12–14).
Figure 1. Therapeutic Inhibitors of the c-MET Signaling Pathway.
Binding of hepatocyte growth factor (HGF) to c-MET, homodimerization, and autophosphorylation (denoted by orange circles containing “P”) elicit downstream signaling mediated by adaptor proteins (Gab1 and Grb2) to induce activation of the phosphotidylinositol-3 kinase (PI3K), Ras/Raf/MEK/ERK, and the Cdc42/Rac1 pathways. HGF-independent signaling can be mediated by phosphorylated epidermal growth factor receptor (EGFR), which can be activated by its ligand transforming growth factor alpha (TGFα). Inhibitors of the HGF/c-MET signaling pathway are shown in yellow boxes with their corresponding targets denoted. Inhibitors in bold have completed phase II trials in advanced HCC.
The HGF/c-MET axis has been shown to exert diverse physiologic effects on cell proliferation, survival, migration, and angiogenesis with important roles in liver development and regeneration. HGF acts as a potent mitogen for primary hepatocytes (15) and promotes cell motility of epithelial cells in vitro (16). c-MET and HGF knockout mice exhibit analogous phenotypes characterized by embryonic lethality due in part to impaired liver formation (17, 18). Finally, liver HGF expression rapidly increases in rodents following partial hepatectomy (19), and mice subject to conditional inactivation of c-MET in mature hepatocytes exhibit deficient liver regeneration (20).
ROLE OF c-MET AND HGF IN HCC
HGF/c-MET expression in HCC
The discovery that HGF/c-MET signaling promotes hepatocyte proliferation and regeneration has prompted multiple studies of its role in HCC. Surprisingly, HGF expression is decreased in HCC compared to surrounding tissue (21–25). On the other hand, c-MET transcription is increased in 30–100% of tumors compared to surrounding liver tissue (22, 25–28). Similarly, c-MET is overexpressed at the protein level in 25–100% of HCCs compared to normal liver (26, 28–32), suggesting a potential tumor-promoting role in HCC.
HGF/c-MET manipulation in HCC cell lines
In vitro studies have attempted to establish the effect of HGF/c-MET signaling in HCC cells. Rather than acting as a mitogen, recombinant HGF inhibited growth in most HCC cell lines (33, 34). In contrast, c-MET knockdown by RNA interference decreased cell proliferation, colony formation, and migration in vitro, and suppressed tumor growth in vivo in multiple HCC cell lines (35–37). Similarly, treatment of c-MET-overexpressing HCC cells with the selective c-MET inhibitor PHA665752 resulted in significant growth inhibition in vitro (IC50 = 50–100 nM) and in subcutaneous xenografts in nude mice (38). Treatment was accompanied by inhibition of c-MET phosphorylation and downstream ERK1/2 and Akt activation. PHA665752 did not have significant in vitro or in vivo activity against two low-c-MET-expressing cell lines (38). These data suggest that c-MET may be a promising target in the treatment of HCC and that c-MET overexpression may be a predictive biomarker of response.
HGF/c-MET manipulation in animal models of HCC
Studies in animal models of HCC have been consistent with the in vitro data. Carcinogen-induced rat models to which exogenous HGF is administered (39–41) and transgenic mice in which HGF is endogenously overexpressed in the liver revealed both tumor-promoting and tumor-inhibiting effects of HGF (42–45). On the contrary, transgenic models of c-MET overexpression have consistently induced HCC formation in vivo. Liver-specific overexpression of c-MET in mice led to HCC formation in a ligand-independent fashion, and withdrawal of c-MET overexpression promoted marked tumor regression, suggesting a continued role of c-MET in tumor maintenance in vivo (10). Moreover, overexpression of c-MET cooperated with other oncogenes characteristic of HCC — c-myc or mutant beta-catenin — to generate HCC with shorter latency and survival in mice (46, 47). These data support the role of c-MET in HCC tumor progression and maintenance, providing a rationale for the clinical development of c-MET inhibitors for HCC.
Combined inhibition of HGF/c-MET and VEGF pathways in preclinical models
Several lines of evidence support a significant role of HGF/c-MET in promoting angiogenesis. First, HGF directly promoted the growth of endothelial cells both in vitro and in vivo (48). Second, HGF induced VEGF and suppressed TSP1 (a negative regulator of angiogenesis) expression in cultured breast and leiomyosarcoma cells and in xenografts (49). Third, transgenic mice overexpressing HGF exhibited increased angiogenesis and VEGF transcription in chemically-induced hepatic adenomas and HCC (43). Finally, recent work has revealed significant crosstalk between the HGF/c-MET and VEGF/VEGFR pathways with synergism in enhancing proliferation, cytoskeletal remodeling, and migration in endothelial cells (50). Interestingly, tumor hypoxia, a potential consequence of angiogenesis inhibitors, such as sorafenib, led to increased c-MET expression and potentiated the effect of HGF on c-MET activation, cell migration, and invasiveness (51).
Several in vitro and in vivo studies have validated the utility of combined c-MET and VEGF/VEGFR inhibition in HCC. The addition of the selective c-MET TKI tivantinib (ARQ197, ArQule, Inc.) to sorafenib promoted additive cytotoxicity in HCC cells (52). Moreover, foretinib (GSK1363089, XL880, GlaxoSmithKline), a multi-targeted TKI with activity against c-MET, VEGFR2, RON AXL, KIT, FLT3, PDGFRβ, and Tie2 (53) impaired growth of patient-derived HCC cell lines in vitro and in vivo (54). Finally, cabozantinib (XL184, Exelixis), a TKI with activity against c-MET, VEGFR2, and RET inhibited growth in multiple cancer cell lines including those of the breast, lung, stomach, and prostate with decreased proliferation, metastatic capability, and angiogenesis in xenografts (55). This preclinical evidence supports the clinical application of combined HGF/c-MET and VEGF/VEGFR pathway blockade for HCC.
HGF AND c-MET AS BIOMARKERS IN HCC
Table 1 summarizes our current understanding of HGF and c-MET as biomarkers in HCC. Whereas tissue HGF levels have provided little/no prognostic information, plasma HGF levels were consistently higher in patients with HCC compared to normal subjects (26, 56, 57). Moreover, circulating HGF levels correlated with decreased overall survival in untreated patients (56) and with increased tumor size, grade, recurrence, metastasis, post-operative complications, and worse overall survival after partial hepatectomy (26, 57). A recent biomarker analysis of samples from the pivotal phase III trial of sorafenib showed a trend towards improved survival in patients with lower pre-treatment plasma HGF concentration (58). Elevated circulating HGF levels are observed in many pathologic liver conditions, including hepatitis, and correlate with more severe liver cirrhosis (59). Thus, it remains unclear whether plasma HGF at diagnosis would be predictive of response to anti-HGF/c-MET therapies, and this needs to be prospectively evaluated in clinical trials of c-MET inhibitors.
Table 1.
Prognostic and Predictive Value of HGF/c-MET Biomarkers in HCC
| Biomarker | Prognostic Value | Predictive Value |
|---|---|---|
| HGF RNA overexpression in tumor tissue | No correlation with tumor size, grade, proliferation, or OS (23). | Insufficient Evidence |
| HGF protein overexpression in tumor tissue | No correlation with tumor size, grade, or intrahepatic metastases, vascular invasion, or OS (30). | Insufficient Evidence |
| Plasma HGF levels | Level at diagnosis inversely correlated with OS in untreated patients (56). High levels post-hepatectomy associated with increase complication rate and worse OS (57). High levels correlate with increased tumor size, higher grade, portal vein thrombosis, and post-operative recurrence (60). | Insufficient Evidence |
| c-MET amplification | Rarely observed (31) with insufficient evidence regarding prognostic value. | Insufficient Evidence |
| c-MET mutations | Observed in some childhood HCC (69) with insufficient evidence regarding prognostic value. | Insufficient Evidence |
| c-MET RNA overexpression in tumor tissue | Frequent overexpression observed (22, 25–28) but insufficient evidence exists regarding prognostic value. | Insufficient Evidence |
| c-MET protein overexpression in tumor tissue | Correlates with higher tumor grade (poorer differentiation) (28, 29), portal vein invasion or thrombosis (26, 31), increased intrahepatic metastases (30, 31), tumor recurrence (26, 31), and worse OS (30). | IHC overexpression correlates with improved OS and TTP in patients treated with Tivantinib (62). |
OS – Overall Survival; TTP – Time-to-Progression.
Tissue c-MET overexpression has shown prognostic promise with direct correlation to tumor grade (28, 29), portal vein invasion or thrombosis (26, 31), intrahepatic metastases (30, 31), tumor recurrence (26, 31), and worse overall survival (30), albeit a limited number of samples were examined in each study. A large retrospective study of 194 patients with HCC <5 cm treated with partial hepatectomy or microwave ablation demonstrated that increased c-MET expression was independently associated with worse survival in multivariate analysis (60). While gene expression profiling of HCC has not consistently shown c-MET overexpression to be an oncogenic driver, a c-MET-positive gene expression signature (identified through comparative microarray analysis of wild-type and c-MET-deficient mouse hepatocytes) has been identified in a subset of human HCCs and was associated with increased vascular invasion and worse outcome (61).
There are two major caveats that limit the prognostic value of c-MET overexpression in HCC. First, the available data are primarily derived from patients with early-stage disease who have undergone partial hepatectomy (28, 31), and the prognostic value of c-MET overexpression has not been definitively evaluated in advanced HCC. Second, the optimal method for evaluating c-MET protein expression remains controversial. One study performed densitometric analysis on western blots of tumor tissue using a median cutoff value to delineate high- versus low-c-MET expressing tumors. Five-year survival was significantly lower in the patients with high compared to low c-MET expression (33.5% and 80.3%, respectively) (30). Another study assessed c-MET expression in tumor tissue by immunohistochemistry (IHC) and showed shortened disease-free survival of patients with c-MET expression versus those without c-MET expression (60). c-MET overexpression assessed by IHC in tumor tissue is the only predictive biomarker that has gained supportive evidence in early-phase trials of HGF/c-MET inhibitors (see below) (62). However, the reproducibility and standardization of these approaches to assess c-MET expression need to be addressed in future studies as has been done previously for HER2 evaluation in breast cancer (63).
Tissue and blood markers are increasingly studied as potential pharmacodynamic biomarkers in early-phase clinical trials. The natural choice for c-MET inhibitors is c-MET phosphorylation in tumor tissue. Evaluation of the activity of signaling molecules such as ERK1/2 and Akt (see Figure 1) is also being pursued, but this analysis may be confounded by the activity of other TKIs, such as sorafenib, that affect similar downstream effectors. Given the role of c-MET in angiogenesis, tissue and serum VEGF and circulating endothelial cells are also being explored as biomarkers of response. Circulating biomarkers are particularly important in HCC patients, as repeat biopsies are challenging due to underlying coagulopathy and thrombocytopenia.
CLINICAL EXPERIENCE
Clinical trials of single agent c-MET inhibitors
In an effort to bring scientific knowledge from the bench to the bedside, several anti-c-MET agents are currently under development and can be broadly categorized into three groups: selective c-MET TKIs, multi-targeted TKIs with activity against c-MET, and monoclonal antibodies against HGF or c-MET (Figure 1). Three oral small molecule c-MET TKIs have demonstrated acceptable toxicity and modest clinical efficacy in Phase II trials in advanced HCC: foretinib, cabozantinib, and tivantinib (Table 2). Below we discuss the unpublished data from conference abstracts that present the clinical experience with these drugs.
Table 2.
Clinical Trials of c-MET inhibitors in HCC
| Drug | Molecular Target (IC-50) | Phase/Arms | Study Population | 1st or 2nd Line | Outcomes/Endpoints | Most Common Adverse Events |
|---|---|---|---|---|---|---|
| Tivantinib (T) (62) | c-Met (0.4 μM) (70) | Phase II T 360 mg PO BID vs T 240 mg PO BID vs Placebo |
n=107 ≤Childs-Pugh A Cirrhosis Geographically unselected |
2nd |
ITT population (placebo vs T):
c-MET high (placebo vs T):
|
|
| Cabozantinib (C) (65) | VEGFR2 (0.04 nM), c-Met (1.3 nM), RET (5.2 nM), KIT (4.6 nM), Flt-4 (6 nM), AXL (7 nM), Flt-3 (11.3 nM), Flt-1 (12 nM), and Tie2 (14.3 nM) (55, 71) | Phase II C 100 mg PO daily |
n=41 ≤Childs-Pugh A Cirrhosis Geographically unselected |
Mixed 1st and 2nd |
|
Most Common Grade 3/4 AEs:
|
| Foretinib (F) (64) | c-Met (0.4 nM), VEGFR2 (0.9 nM), Tie2 (1.1 nM), Flt-4 (2.8 nM), RON (3 nM), Flt-3 (3.6 nM), KIT (6.7 nM), Flt-1 (6.8 nM), PDGFRβ (9.6 nM), and AXL (11 nM) (53, 71) | Phase I/II F 30 mg PO daily |
n=39 ≤Childs-Pugh A Cirrhosis Asian |
1st |
|
Most Common SAEs:
|
ITT – Intention to Treat; TTP – Time to Progression; HR – Hazard Ratio; PFS – Progression Free Survival; OS – Overall Survival; cPR – Confirmed Partial Response; RR – Response Rate; CR – Complete Response; PR – Partial Response; DCR – Disease Control Rate (PR+SD); PPE – Palmar-Plantar Erythrodysesthesia syndrome; ORR – Overall Response Rate (CR+PR); CI – Confidence Interval; SAEs – Serious Adverse Events; SD – Stable Disease
Foretinib
Foretinib was the first c-MET TKI to be evaluated in clinical trials. It has a broad TKI spectrum, which includes c-MET and VEGFR. Foretinib was tested in a Phase I/II trial as first-line therapy for Asian patients with advanced HCC and Child-Pugh A cirrhosis or no cirrhosis. In the 38 of 39 patients treated at the MTD of 30 mg daily and evaluable for efficacy, foretinib exhibited an objective response rate (ORR) of 24%, disease control rate (DCR) of 79%, median time-to-progression (TTP) of 4.2 months, and median overall survival (OS) of 15.7 months. Foretinib had an acceptable toxicity profile, as the most common adverse events (AEs) were hypertension (36%), anorexia (23%), and fever (21%), and the most common serious adverse events (SAEs) were hepatic encephalopathy (10%) and ascites (8%) (64).
Cabozantinib
Cabozantinib is an oral small molecule multi-targeted TKI with activity against c-MET, VEGFR2, and RET. In a Phase II randomized discontinuation trial, 41 patients with advanced HCC and Child-Pugh A cirrhosis received cabozantinib 100 mg daily for 12 weeks during a lead-in phase. Patients who had a partial response (PR) were maintained on open-label cabozantinib, while patients with stable disease were randomized to cabozantinib versus placebo. Patients with progressive disease (PD) discontinued treatment. Two of 36 patients with evaluable disease at 12 weeks had a PR (6%), and a third patient randomized at 12 weeks achieved a PR at 18 weeks. The DCR at week 12 was 68%. A reduction in alpha-fetoprotein (AFP) by greater than 50% from baseline was seen in 26 patients. The median progression-free survival (PFS) was 4.4 months and OS was 15.1 months. Of note, the PFS was similar in patients who were sorafenib naïve (PFS 4.2 months, n=20) and who had prior sorafenib (PFS 5.2 months, n=21). The toxicity profile was acceptable; the most common Grade 3/4 AEs were diarrhea (17%), palmar-plantar erythrodysesthesia (PPE, 15%), and thrombocytopenia (10%) (65).
Tivantinib
Of the oral c-MET TKIs tested in phase II trials of advanced HCC, tivantinib has gained the most experience. Tivantinib is a selective non-ATP competitive inhibitor of c-MET. A phase Ib study evaluating the use of tivantinib in patients with HCC and Child-Pugh A or B cirrhosis demonstrated preliminary evidence of both safety and efficacy (66). The drug then underwent Phase II testing in a randomized, placebo-controlled, multi-national, crossover trial of 107 patients with unresectable HCC who had failed one systemic therapy and had either no cirrhosis or Child-Pugh A cirrhosis (62). Patients were randomized in a 2:1 fashion to tivantinib or placebo. Tivantinib was initially given at 360 mg BID but was changed to 240 mg BID due to a high incidence of neutropenia. The study met its primary endpoint with a modest improvement in TTP in the intent-to-treat population from 1.4 months to 1.6 months (HR=0.64, p=0.04), favoring the tivantinib group. The safety profile was acceptable with the most frequent drug-related AEs in the tivantinib group being neutropenia (25.4%) and anemia (15.5%). Grade 3–4 neutropenia was seen in 21% of patients at the 360 mg PO BID dose and 6% of patients at the 240 mg PO BID dose, so the latter dose was favored.
The most compelling data from this trial came from a subgroup analysis based on tumor c-MET expression. Positive c-MET expression (≥2+ staining intensity in ≥50% of tumor cells by IHC) was associated with improved OS (3.8 vs. 7.2 months, HR=0.38, p=0.01), PFS (1.5 vs. 2.4 months, HR=0.45, p=0.02), and TTP (1.5 vs. 2.9 months, HR=0.43, p=0.03) (62). Despite the early promising results with tivantinib in HCC, it should be cautioned that the sample size was small, the positive signal remained modest, and the data were based on a subgroup analysis (22 patients in the tivantinib arm and 15 patients in placebo arm for the c-MET high group). Currently, a phase III randomized trial comparing tivantinib 240 mg BID to placebo in patients with c-MET-positive advanced HCC in the second line setting is being planned.
Combination of c-MET inhibition with sorafenib
Based on preclinical data showing the synergy of tivantinib and sorafenib in multiple cell lines (52), a phase I study of 54 patients, including 8 with HCC, was completed and demonstrated preliminary safety and efficacy of the drug combination. An extension cohort of 20 HCC patients with Childs-Pugh A and B cirrhosis, 10 of whom had received ≥ 1 systemic therapy, were treated with tivantinib 240 mg BID and sorafenib 400 mg BID. The combination demonstrated a DCR of 70% with 1 complete response, 1 PR, and 12 patients with stable disease. The toxicities of the combination were manageable with the most common AEs being rash (40%), PPE (35%), fatigue and diarrhea (30% each), and nausea and anorexia (25% each). Neutropenia was reported in two patients (67). These data along with the preliminary phase II results of foretinib and cabozantinib support the further development of combined c-MET and VEGF pathway inhibition in HCC.
FUTURE DEVELOPMENT OF c-MET INHIBITORS IN HCC
The recent failure of several phase III trials with VEGFR TKIs highlights the challenge and need for developing additional targeted agents in HCC. Despite significant preclinical data supporting the role of c-MET as a potential oncogenic driver in HCC, early clinical trials have revealed surprisingly modest benefit of c-MET inhibition. There are several possible explanations. First, HCC is a heterogeneous disease in its predisposing etiologies and tumor microenvironment, which may be insufficiently modeled in cell lines and animal models. Second, there is no clear consensus on how to identify patients whose tumors have high c-MET expression or definitive evidence that this characteristic predicts dependence on c-MET. Third, currently available c-MET TKIs may not be sufficiently selective and potent in vivo. Finally, most patients with advanced HCC have significant underlying cirrhosis, which poses a challenge for the evaluation of targeted therapies. Given the role of the HGF/c-MET pathway in liver regeneration and the fact that most oral c-MET TKIs are metabolized by the liver, the therapeutic window is likely to be small, and the safety profiles of these agents should be carefully examined. The data from the tivantinib trial are interesting but are based on a small sample size with modest improvement in TTP. Target inhibition by tivantinib has not been shown in vivo in HCC tissues.
Thus, future clinical trials of c-MET inhibitors in HCC should seek to determine and standardize the optimal method for delineating c-MET overexpression and validating its role as a predictive biomarker. In addition, more potent and selective c-MET inhibitors should be tested in HCC, and studies that carefully assess phospho-c-MET inhibition as a pharmacodynamic biomarker should be included in the study design. Despite the rationale, interesting preclinical data of combined c-MET and VEGFR inhibition, and preliminary clinical data on foretinib and cabozantinib, the relative contribution of c-MET and VEGFR inhibition remains to be dissected. Finally, the successful development of c-MET inhibitors will rely on carefully designed clinical trials with adequate endpoint selection, correct target population, optimal assessment of clinical efficacy, and robust biomarker discovery as previously recommended (68). Despite these challenges, nearly thirty years after its initial discovery, c-MET is emerging as a biologically rational and druggable target in HCC.
Acknowledgments
We thank Drs. Bruce Chabner and Dan Duda for critical review of the manuscript and helpful suggestions.
Footnotes
Conflict of Interest Statement
AXZ has served consultant/advisory roles for Sanofi-aventis, Bristol-Myers Squibb, Eisai, Daiichi Sankyo, and Exelixis.
Bibliography
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–38. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Altekruse SF, et al. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) National Cancer Institute; Bethesda, MD: Apr, 2012. http://seer.cancer.gov/csr/1975_2009_pops09/, based on November 2011 SEER data submission, posted to the SEER web site. [Google Scholar]
- 4.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 5.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 6.EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–43. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Cooper CS, Park M, Blair DG, Tainsky MA, Huebner K, Croce CM, et al. Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature. 1984;311:29–33. doi: 10.1038/311029a0. [DOI] [PubMed] [Google Scholar]
- 8.Bottaro DP, Rubin JS, Faletto DL, Chan AM, Kmiecik TE, Vande Woude GF, et al. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251:802–4. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- 9.Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat Rev Cancer. 2012;12:89–103. doi: 10.1038/nrc3205. [DOI] [PubMed] [Google Scholar]
- 10.Wang R, Ferrell LD, Faouzi S, Maher JJ, Bishop JM. Activation of the Met receptor by cell attachment induces and sustains hepatocellular carcinomas in transgenic mice. J Cell Biol. 2001;153:1023–34. doi: 10.1083/jcb.153.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki M, Shiraha H, Fujikawa T, Takaoka N, Ueda N, Nakanishi Y, et al. Des-gamma-carboxy prothrombin is a potential autologous growth factor for hepatocellular carcinoma. J Biol Chem. 2005;280:6409–15. doi: 10.1074/jbc.M406714200. [DOI] [PubMed] [Google Scholar]
- 12.Rodrigues GA, Park M. Dimerization mediated through a leucine zipper activates the oncogenic potential of the met receptor tyrosine kinase. Mol Cell Biol. 1993;13:6711–22. doi: 10.1128/mcb.13.11.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodrigues GA, Park M. Autophosphorylation modulates the kinase activity and oncogenic potential of the Met receptor tyrosine kinase. Oncogene. 1994;9:2019–27. [PubMed] [Google Scholar]
- 14.Jeffers M, Fiscella M, Webb CP, Anver M, Koochekpour S, Vande Woude GF. The mutationally activated Met receptor mediates motility and metastasis. Proc Natl Acad Sci U S A. 1998;95:14417–22. doi: 10.1073/pnas.95.24.14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura T, Nishizawa T, Hagiya M, Seki T, Shimonishi M, Sugimura A, et al. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989;342:440–3. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- 16.Stoker M, Gherardi E, Perryman M, Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature. 1987;327:239–42. doi: 10.1038/327239a0. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, et al. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- 18.Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–71. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura T, Nawa K, Ichihara A. Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun. 1984;122:1450–9. doi: 10.1016/0006-291x(84)91253-1. [DOI] [PubMed] [Google Scholar]
- 20.Borowiak M, Garratt AN, Wustefeld T, Strehle M, Trautwein C, Birchmeier C. Met provides essential signals for liver regeneration. Proc Natl Acad Sci U S A. 2004;101:10608–13. doi: 10.1073/pnas.0403412101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiss A, Wang NJ, Xie JP, Thorgeirsson SS. Analysis of transforming growth factor (TGF)-alpha/epidermal growth factor receptor, hepatocyte growth Factor/c-met, TGF-beta receptor type II, and p53 expression in human hepatocellular carcinomas. Clin Cancer Res. 1997;3:1059–66. [PubMed] [Google Scholar]
- 22.Daveau M, Scotte M, Francois A, Coulouarn C, Ros G, Tallet Y, et al. Hepatocyte growth factor, transforming growth factor alpha, and their receptors as combined markers of prognosis in hepatocellular carcinoma. Mol Carcinog. 2003;36:130–41. doi: 10.1002/mc.10103. [DOI] [PubMed] [Google Scholar]
- 23.Tavian D, De Petro G, Benetti A, Portolani N, Giulini SM, Barlati S. u-PA and c-MET mRNA expression is co-ordinately enhanced while hepatocyte growth factor mRNA is down-regulated in human hepatocellular carcinoma. Int J Cancer. 2000;87:644–9. [PubMed] [Google Scholar]
- 24.Selden C, Farnaud S, Ding SF, Habib N, Foster C, Hodgson HJ. Expression of hepatocyte growth factor mRNA, and c-met mRNA (hepatocyte growth factor receptor) in human liver tumours. J Hepatol. 1994;21:227–34. doi: 10.1016/s0168-8278(05)80400-3. [DOI] [PubMed] [Google Scholar]
- 25.Noguchi O, Enomoto N, Ikeda T, Kobayashi F, Marumo F, Sato C. Gene expressions of c-met and hepatocyte growth factor in chronic liver disease and hepatocellular carcinoma. J Hepatol. 1996;24:286–92. doi: 10.1016/s0168-8278(96)80006-7. [DOI] [PubMed] [Google Scholar]
- 26.Wu F, Wu L, Zheng S, Ding W, Teng L, Wang Z, et al. The clinical value of hepatocyte growth factor and its receptor--c-met for liver cancer patients with hepatectomy. Dig Liver Dis. 2006;38:490–7. doi: 10.1016/j.dld.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Boix L, Rosa JL, Ventura F, Castells A, Bruix J, Rodes J, et al. c-met mRNA overexpression in human hepatocellular carcinoma. Hepatology. 1994;19:88–91. [PubMed] [Google Scholar]
- 28.Suzuki K, Hayashi N, Yamada Y, Yoshihara H, Miyamoto Y, Ito Y, et al. Expression of the c-met protooncogene in human hepatocellular carcinoma. Hepatology. 1994;20:1231–6. [PubMed] [Google Scholar]
- 29.D’Errico A, Fiorentino M, Ponzetto A, Daikuhara Y, Tsubouchi H, Brechot C, et al. Liver hepatocyte growth factor does not always correlate with hepatocellular proliferation in human liver lesions: its specific receptor c-met does. Hepatology. 1996;24:60–4. doi: 10.1002/hep.510240112. [DOI] [PubMed] [Google Scholar]
- 30.Ueki T, Fujimoto J, Suzuki T, Yamamoto H, Okamoto E. Expression of hepatocyte growth factor and its receptor, the c-met proto-oncogene, in hepatocellular carcinoma. Hepatology. 1997;25:619–23. doi: 10.1002/hep.510250321. [DOI] [PubMed] [Google Scholar]
- 31.Kondo S, Ojima H, Tsuda H, Hashimoto J, Morizane C, Ikeda M, et al. Clinical impact of c-Met expression and its gene amplification in hepatocellular carcinoma. Int J Clin Oncol. 2012 doi: 10.1007/s10147-011-0361-9. [DOI] [PubMed] [Google Scholar]
- 32.Chen Q, Seol DW, Carr B, Zarnegar R. Co-expression and regulation of Met and Ron proto-oncogenes in human hepatocellular carcinoma tissues and cell lines. Hepatology. 1997;26:59–66. doi: 10.1002/hep.510260108. [DOI] [PubMed] [Google Scholar]
- 33.Tajima H, Matsumoto K, Nakamura T. Hepatocyte growth factor has potent anti-proliferative activity in various tumor cell lines. FEBS Lett. 1991;291:229–32. doi: 10.1016/0014-5793(91)81291-f. [DOI] [PubMed] [Google Scholar]
- 34.Shiota G, Rhoads DB, Wang TC, Nakamura T, Schmidt EV. Hepatocyte growth factor inhibits growth of hepatocellular carcinoma cells. Proc Natl Acad Sci U S A. 1992;89:373–7. doi: 10.1073/pnas.89.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang SZ, Pan FY, Xu JF, Yuan J, Guo SY, Dai G, et al. Knockdown of c-Met by adenovirus-delivered small interfering RNA inhibits hepatocellular carcinoma growth in vitro and in vivo. Mol Cancer Ther. 2005;4:1577–84. doi: 10.1158/1535-7163.MCT-05-0106. [DOI] [PubMed] [Google Scholar]
- 36.Salvi A, Arici B, Portolani N, Giulini SM, De Petro G, Barlati S. In vitro c-met inhibition by antisense RNA and plasmid-based RNAi down-modulates migration and invasion of hepatocellular carcinoma cells. Int J Oncol. 2007;31:451–60. [PubMed] [Google Scholar]
- 37.Xie B, Xing R, Chen P, Gou Y, Li S, Xiao J, et al. Down-regulation of c-Met expression inhibits human HCC cells growth and invasion by RNA interference. J Surg Res. 2010;162:231–8. doi: 10.1016/j.jss.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 38.You H, Ding W, Dang H, Jiang Y, Rountree CB. c-Met represents a potential therapeutic target for personalized treatment in hepatocellular carcinoma. Hepatology. 2011;54:879–89. doi: 10.1002/hep.24450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu ML, Mars WM, Michalopoulos GK. Hepatocyte growth factor inhibits cell proliferation in vivo of rat hepatocellular carcinomas induced by diethylnitrosamine. Carcinogenesis. 1995;16:841–3. doi: 10.1093/carcin/16.4.841. [DOI] [PubMed] [Google Scholar]
- 40.Yaono M, Hasegawa R, Mizoguchi Y, Futakuchi M, Nakamura T, Ito N, et al. Hepatocyte growth factor enhancement of preneoplastic hepatic foci development in rats treated with diethylnitrosamine and N-ethyl-N-hydroxyethylnitrosamine. Jpn J Cancer Res. 1995;86:718–23. doi: 10.1111/j.1349-7006.1995.tb02459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogasawara H, Hiramoto J, Takahashi M, Shirahama K, Furusaka A, Hiyane S, et al. Hepatocyte growth factor stimulates DNA synthesis in rat preneoplastic hepatocytes but not in liver carcinoma cells. Gastroenterology. 1998;114:775–81. doi: 10.1016/s0016-5085(98)70591-8. [DOI] [PubMed] [Google Scholar]
- 42.Sakata H, Takayama H, Sharp R, Rubin JS, Merlino G, LaRochelle WJ. Hepatocyte growth factor/scatter factor overexpression induces growth, abnormal development, and tumor formation in transgenic mouse livers. Cell Growth Differ. 1996;7:1513–23. [PubMed] [Google Scholar]
- 43.Horiguchi N, Takayama H, Toyoda M, Otsuka T, Fukusato T, Merlino G, et al. Hepatocyte growth factor promotes hepatocarcinogenesis through c-Met autocrine activation and enhanced angiogenesis in transgenic mice treated with diethylnitrosamine. Oncogene. 2002;21:1791–9. doi: 10.1038/sj.onc.1205248. [DOI] [PubMed] [Google Scholar]
- 44.Shiota G, Wang TC, Nakamura T, Schmidt EV. Hepatocyte growth factor in transgenic mice: effects on hepatocyte growth, liver regeneration and gene expression. Hepatology. 1994;19:962–72. [PubMed] [Google Scholar]
- 45.Santoni-Rugiu E, Preisegger KH, Kiss A, Audolfsson T, Shiota G, Schmidt EV, et al. Inhibition of neoplastic development in the liver by hepatocyte growth factor in a transgenic mouse model. Proc Natl Acad Sci U S A. 1996;93:9577–82. doi: 10.1073/pnas.93.18.9577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tward AD, Jones KD, Yant S, Cheung ST, Fan ST, Chen X, et al. Distinct pathways of genomic progression to benign and malignant tumors of the liver. Proc Natl Acad Sci U S A. 2007;104:14771–6. doi: 10.1073/pnas.0706578104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amicone L, Terradillos O, Calvo L, Costabile B, Cicchini C, Della Rocca C, et al. Synergy between truncated c-Met (cyto-Met) and c-Myc in liver oncogenesis: importance of TGF-beta signalling in the control of liver homeostasis and transformation. Oncogene. 2002;21:1335–45. doi: 10.1038/sj.onc.1205199. [DOI] [PubMed] [Google Scholar]
- 48.Grant DS, Kleinman HK, Goldberg ID, Bhargava MM, Nickoloff BJ, Kinsella JL, et al. Scatter factor induces blood vessel formation in vivo. Proc Natl Acad Sci U S A. 1993;90:1937–41. doi: 10.1073/pnas.90.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang YW, Su Y, Volpert OV, Vande Woude GF. Hepatocyte growth factor/scatter factor mediates angiogenesis through positive VEGF and negative thrombospondin 1 regulation. Proc Natl Acad Sci U S A. 2003;100:12718–23. doi: 10.1073/pnas.2135113100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sulpice E, Ding S, Muscatelli-Groux B, Berge M, Han ZC, Plouet J, et al. Cross-talk between the VEGF-A and HGF signalling pathways in endothelial cells. Biol Cell. 2009;101:525–39. doi: 10.1042/BC20080221. [DOI] [PubMed] [Google Scholar]
- 51.Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S, Comoglio PM. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3:347–61. doi: 10.1016/s1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 52.Chen C-R, Szwaya J, Rojnuckarin A, Uppalapati U, Huang L, Enkeleda N, et al. Abstract #820: Combination studies of tyrosine kinase inhibitors (TKIs): Assessment of potential cytotoxic synergy of ARQ197 with sorafenib and sunitinib. Proc Am Assoc Cancer Res. 2009 [Google Scholar]
- 53.Qian F, Engst S, Yamaguchi K, Yu P, Won KA, Mock L, et al. Inhibition of tumor cell growth, invasion, and metastasis by EXEL-2880 (XL880, GSK1363089), a novel inhibitor of HGF and VEGF receptor tyrosine kinases. Cancer Res. 2009;69:8009–16. doi: 10.1158/0008-5472.CAN-08-4889. [DOI] [PubMed] [Google Scholar]
- 54.Huynh H, Ong R, Soo KC. Foretinib demonstrates anti-tumor activity and improves overall survival in preclinical models of hepatocellular carcinoma. Angiogenesis. 2012;15:59–70. doi: 10.1007/s10456-011-9243-z. [DOI] [PubMed] [Google Scholar]
- 55.Yakes FM, Chen J, Tan J, Yamaguchi K, Shi Y, Yu P, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10:2298–308. doi: 10.1158/1535-7163.MCT-11-0264. [DOI] [PubMed] [Google Scholar]
- 56.Vejchapipat P, Tangkijvanich P, Theamboonlers A, Chongsrisawat V, Chittmittrapap S, Poovorawan Y. Association between serum hepatocyte growth factor and survival in untreated hepatocellular carcinoma. J Gastroenterol. 2004;39:1182–8. doi: 10.1007/s00535-004-1469-8. [DOI] [PubMed] [Google Scholar]
- 57.Mizuguchi T, Nagayama M, Meguro M, Shibata T, Kaji S, Nobuoka T, et al. Prognostic impact of surgical complications and preoperative serum hepatocyte growth factor in hepatocellular carcinoma patients after initial hepatectomy. J Gastrointest Surg. 2009;13:325–33. doi: 10.1007/s11605-008-0711-8. [DOI] [PubMed] [Google Scholar]
- 58.Llovet JM, Pena CE, Lathia CD, Shan M, Meinhardt G, Bruix J. Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2012;18:2290–300. doi: 10.1158/1078-0432.CCR-11-2175. [DOI] [PubMed] [Google Scholar]
- 59.Shiota G, Okano J, Kawasaki H, Kawamoto T, Nakamura T. Serum hepatocyte growth factor levels in liver diseases: clinical implications. Hepatology. 1995;21:106–12. [PubMed] [Google Scholar]
- 60.Wang ZL, Liang P, Dong BW, Yu XL, Yu de J. Prognostic factors and recurrence of small hepatocellular carcinoma after hepatic resection or microwave ablation: a retrospective study. J Gastrointest Surg. 2008;12:327–37. doi: 10.1007/s11605-007-0310-0. [DOI] [PubMed] [Google Scholar]
- 61.Kaposi-Novak P, Lee JS, Gomez-Quiroz L, Coulouarn C, Factor VM, Thorgeirsson SS. Met-regulated expression signature defines a subset of human hepatocellular carcinomas with poor prognosis and aggressive phenotype. J Clin Invest. 2006;116:1582–95. doi: 10.1172/JCI27236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rimassa L, Porta C, Borbath I, Daniele B, Salvagni S, Van Laethem JL, et al. Tivantinib (ARQ 197) versus placebo in patients with hepatocellular carcinoma who failed one systemic therapy: Results of a randomized controlled phase II trial [abstract] J Clin Oncol. 2012;30(suppl):abstr 4006. [Google Scholar]
- 63.Turashvili G, Leung S, Turbin D, Montgomery K, Gilks B, West R, et al. Inter-observer reproducibility of HER2 immunohistochemical assessment and concordance with fluorescent in situ hybridization (FISH): pathologist assessment compared to quantitative image analysis. BMC Cancer. 2009;9:165. doi: 10.1186/1471-2407-9-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yau T, Sukeepaisarnjaroen W, Chao Y, Yen C, Lausoontornsiri W, Chen P, et al. A phase I/II study of foretinib, an oral multikinase inhibitor targeting MET, RON, AXL, TIE-2, and VEGFR in advanced hepatocellular carcinoma (HCC) [abstract] J Clin Oncol. 2012;30(suppl):abstr 4108. [Google Scholar]
- 65.Verslype C, Cohn AL, Kelley RK, Yang T, Su W, Ramies DA, et al. Activity of cabozantinib (XL184) in hepatocellular carcinoma: Results from a phase II randomized discontinuation trial (RDT) [abstract] J Clin Oncol. 2012;30(suppl):abstr 4007. [Google Scholar]
- 66.Zucali P, Santoro A, Rodriguez-Lope C, Simonelli M, Camacho LH, Senzer NN, et al. Final results from ARQ 197–114: A phase Ib safety trial evaluating ARQ 197 in cirrhotic patients (pts) with hepatocellular carcinoma (HCC) [abstract] J Clin Oncol. 2010;28(suppl):abstr 4137. [Google Scholar]
- 67.Martell RE, Puzanov I, Ma WW, Santoro A, Dy GK, Goff LW, et al. Safety and efficacy of MET inhibitor tivantinib (ARQ 197) combined with sorafenib in patients (pts) with hepatocellular carcinoma (HCC) from a phase I study [abstract] J Clin Oncol. 2012;30(suppl):abstr 4117. [Google Scholar]
- 68.Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 69.Park WS, Dong SM, Kim SY, Na EY, Shin MS, Pi JH, et al. Somatic mutations in the kinase domain of the Met/hepatocyte growth factor receptor gene in childhood hepatocellular carcinomas. Cancer Res. 1999;59:307–10. [PubMed] [Google Scholar]
- 70.Munshi N, Jeay S, Li Y, Chen CR, France DS, Ashwell MA, et al. ARQ 197, a novel and selective inhibitor of the human c-Met receptor tyrosine kinase with antitumor activity. Mol Cancer Ther. 2010;9:1544–53. doi: 10.1158/1535-7163.MCT-09-1173. [DOI] [PubMed] [Google Scholar]
- 71.You WK, Sennino B, Williamson CW, Falcon B, Hashizume H, Yao LC, et al. VEGF and c-Met blockade amplify angiogenesis inhibition in pancreatic islet cancer. Cancer Res. 2011;71:4758–68. doi: 10.1158/0008-5472.CAN-10-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]