Abstract
West Nile virus (WNV) replicates in a wide variety of avian species, which act as amplification hosts. In particular, WNV generates high titers and elicits severe pathology in American crows (AMCRs; Corvus brachyrhynchos), a species that has been used as a sentinel for WNV transmission. Although the specific cellular targets of WNV replication in AMCRs are not well defined, preliminary evidence suggests that leukocytes may be an important target of early replication. Therefore, development of a protocol for ex vivo culture of AMCR leukocytes as a model for assessing differential avian host susceptibility is described herein. WNV growth in these cultures mirrored in vivo viremia profiles. These data indicate that ex vivo leukocyte cultures can be used for preliminary pathological assessment of novel WNV strains and potentially of other flaviviruses that use avian reservoir hosts.
Keywords: West Nile virus, American crow, peripheral blood mononuclear cells, leukocytes, avian
West Nile virus (WNV) is the most common cause of neuroinvasive arboviral disease in the United States, resulting in over 39,000 cases of human illness and over 1500 deaths since its initial identification in New York City in 1999 (Reimann et al., 2008; Lindsey et al., 2013; CDC, 2014). WNV belongs to the genus Flavivirus within the family Flaviviridae. WNV is maintained in an enzootic cycle through transmission between avian reservoir hosts and mosquito vectors (Reisen, 2013). Mammals, particularly humans and horses, are susceptible to WNV infection and disease, but do not develop sufficient viremia to infect mosquitoes and are considered to be dead-end hosts (Komar, 2003; Petersen et al., 2013).
Avian mortality, particularly in corvid species, has been a hallmark of WNV transmission in North America (Bernard et al., 2001; Komar et al., 2003; Nemeth et al., 2007). American crows (AMCRs; Corvus brachyrhynchos) are highly susceptible to WNV, with a high mortality rate, and have been used as a sentinel species (Eidson et al., 2005; Nemeth et al., 2007). A single mutation at amino acid position 249 in the NS3 helicase gene of WNV has been shown to modulate virulence and pathogenicity in AMCRs and other avian species (Brault et al., 2007; Langevin et al., 2014). North American strains of WNV, such as NY99, contain a proline residue at this position and are highly virulent in AMCRs. In contrast, strains such as KN3829, which was isolated in Kenya and contains a threonine residue at NS3-249, elicit limited viremia and mortality in AMCRs (Brault et al., 2004). In the NY99 genetic backbone, an NS3-P249T mutation was found to reduce both mortality rates and magnitude of viremia in AMCRs. Conversely, an NS3-T249P mutation in the KN3829 virus increased viremia and mortality to levels comparable to those of NY99 (Brault et al., 2007).
Both NY99 and KN3829 belong to WNV lineage 1a, in which the ancestral residue at NS3-249 is a threonine. The NS3-249 residue is under positive selective pressure, and has evolved independently to a proline residue multiple times in lineages 1a and 2 (Brault et al., 2007; Papa et al., 2011). Strains from other lineages contain alternative residues at this position, with varying effects on WNV pathogenesis in birds when introduced into the NY99 genetic background (Langevin et al., 2014). This suggests that the presence of a proline residue at this site may be advantageous for the virus, likely due to its effect on replication in birds and subsequent increased infectivity for mosquitoes imbibing high-titered blood meals.
Pathologic assessment of WNV in avian hosts is time-consuming and labor-intensive, requires periodic trapping of wild birds, can result in variable outcomes due to the presence of adventitious infectious agents in outbred avian populations, and is dependent on specialized facilities for housing of birds at biosafety level 3 and approvals for animal use (Pérez-Ramírez et al., 2014). As such, it would be advantageous to have a relatively non-invasive, reproducible model system for assessing the potential avian host competence of novel strains. The replication potential of WNV in avian tissues is not well understood, but WNV replicates in mammalian leukocytes (Garcia-Tapia et al., 2006; Rios et al., 2006; Bai et al., 2010), and preliminary data (not shown) suggested that avian leukocytes may also be targets. Peripheral blood mononuclear cells (PBMCs) can be isolated easily and noninvasively. Therefore, the goal of this study was to determine whether the in vivo phenotypes of NY99, KN3829, and NS3-249 point mutants could be recapitulated in an ex vivo AMCR PBMC culture model.
After hatch-year AMCRs were cannon-netted in 2013 and 2014 under US Fish and Wildlife Services and Colorado Parks and Wildlife permits at two sites in Bellvue, Colorado (40° 38′ 51″, 105° 11′ 15″ W and 40° 37′ 35″ N, 105° 10′ 32″ W). AMCRs were banded, bled to assay for pre-existing neutralizing antibodies against WNV and St. Louis encephalitis virus, and housed at Colorado State University in a large open flight room containing 25-150 crows. Crows were fed dry dog food ad libitum (Brault et al., 2004; Langevin et al., 2014). Animal experiments were approved by the Institutional Animal Care and Use Committee of Colorado State University (Protocol #12-3871A).
Whole blood (0.5 ml) was collected from each AMCR by jugular venipuncture using a 27-gauge syringe and expelled into lithium heparin tubes (BD Vacutainer). Blood from 6 birds was pooled for a total of 3 ml per tube. Pooled blood was diluted with 8 ml of Dulbecco’s phosphate-buffered saline (DPBS) and layered onto 3 ml of Histopaque-1077 (Sigma-Aldrich) in a 15-ml conical tube. This column was centrifuged at 400×g for 30 minutes with no brake. The opaque interface was removed and washed three times in DPBS with centrifugation at 250×g for 10 minutes. The cell pellet was resuspended in growth medium [RPMI containing 10% fetal bovine serum, 100 U/ml penicillin/streptomycin, and 1 μg/ml Fungizone (Life Technologies)], counted with a hemocytometer, and seeded in 12-well plates at a density of 1.5×106 cells/well. The average yield from 3 ml blood was 2.70×107 cells, or approximately 18 wells.
KN3829 virus stocks were passaged once in Vero cells (Kinney et al., 2006). NY99 wild-type and mutant viral stocks were derived from infectious clones as previously described (Kinney et al., 2006). Briefly, the 5′ and 3′ plasmids were digested with NgoMIV, ligated, and linearized with XbaI (New England Biolabs) before in vitro transcription with the Ampliscribe High-Yield T7 Transcription kit (Epicentre Biotechnologies). Viral RNA was transfected into BHK-21 cells by electroporation (Kinney et al., 2006).
PBMCs were incubated with viral stock for 1 hour at 37°C in a total volume of 1.5 ml before being centrifuged at approximately 1500×g and resuspended in fresh growth medium. Supernatant (1.5 ml) was saved as time point 0, and stored at −70°C until titration by plaque assay. One third of the well volume (0.5 ml) was sampled every 24 hours. Cells were centrifuged as above, supernatant was collected, and cells were resuspended in 0.5 ml growth medium and returned to the well.
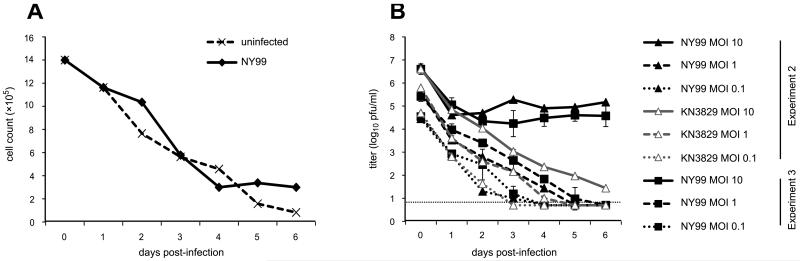
In preliminary experiments, PBMCs were inoculated with wild-type NY99. Cell counts revealed steady mortality of PBMCs over the course of the experiment, regardless of infection (Figure 1A). Viral titers decreased by approximately 2-3 log10 in the first 24 hours post-inoculation, presumably reflecting removal of the initial inoculum and the eclipse phase of viral growth. At subsequent time points, titers of NY99 remained approximately constant when infection was performed at a multiplicity of infection (MOI) of 10, despite daily removal and replacement of growth medium. In contrast, infection with lower MOI resulted in decreases in viral titer, reaching the limit of detection on or before 6 days post-infection (dpi) (Figure 1B). Therefore, subsequent experiments were performed for six days at an MOI of 10.
Figure 1.
(A) Cell death during PBMC culture and infection with WNV NY99 (MOI 0.5; experiment 1). (B) Effect of MOI on viral replication in PBMC culture. Experiment 2 was conducted in single wells, while experiment 3 was conducted in triplicate (error bars depict standard deviation from the mean). The dashed line represents the limit of detection.
Viral growth kinetics were variable between experiments, likely because the source of cells was wild-caught birds of unknown health status. In a number of experiments conducted with NY99, viral titers sometimes plateaued after the initial decline, remaining at approximately 4-5 log10 pfu/ml after 2-4 dpi (e.g. Experiment 7, Figure 2A). Alternatively, titers sometimes increased to 6-7 log10 pfu/ml (e.g. Experiment 6, Figure 2A). In contrast, mean titers of KN3829 and the NY99 NS3-P249T mutant virus, both of which are less pathogenic for AMCRs, either decreased to undetectable levels (Experiment 7), plateaued (Experiment 6; NS3-P249T), or increased slightly late in infection (Experiment 6; KN3829) (Figure 2B). Importantly, although the titers attained in each specific experiment varied, titers of KN3829 and the NS3-P249T mutant were consistently lower than those of NY99 assayed in the same experiment. This effect cannot be attributed to a difference in virion stability, because NY99 and KN3829 decay at equivalent rates in the absence of cells (Kinney et al., 2006).
Figure 2.
Reproducibility of PBMC infection experiments. (A) WNV NY99 and (B) KN3829 and NY99-NS3-P249T were cultured in AMCR PBMCs isolated in multiple independent experiments. Numerical prefixes in the legend represent experiments; experiments with the same number in panels A and B were performed in parallel. The dashed line represents the limit of detection. Experiment 1 was conducted at MOI 0.5; all others were performed at MOI 10. Experiments 1 and 2 were conducted in single wells, while all others were conducted in triplicate (error bars represent standard deviation from the mean). Experiment 8 was conducted in Transwell plates. (C) Individual replicates of KN3829 and NY99 NS3-P249T in experiment 6.
Because wild-type NY99 was included as a positive control in every experiment, the replication potential of each strain in PBMCs could be expressed as a ratio. Specifically, the log-transformed titer attained by KN3829 or NY99-NS3-P249T at 6 dpi was divided by the log-transformed titer attained by NY99 in the same experiment. For samples in which virus was not detected, the titer was assumed conservatively to be the limit of detection (0.7 log10 pfu/ml). The titer ratio was 0.19 and 0.48 in two experiments using NY99-NS3-P249T and ranged from 0.15 to 0.78 in four experiments using KN3829.
The highest value of the ratio for both KN3829 and NY99-NS3-P249T was attained in a single experiment (Experiment 6; Figure 2C), in which the variability among technical replicates was extremely high. Because reversion and compensatory mutations have been identified in in vivo infection experiments with NS3-249 mutants (Langevin et al., 2014), RNA was extracted from the supernatant samples from the last day with detectable virus in this experiment (5 dpi for NY99 NS3-P249T replicate #2; 6 dpi for all others). RT-PCR was performed with primers WNV5032F (GGAACATCAGGCTCACCAATAGTGG) and WNV5497R (CTTTGTGGAAATGTAACCTCTTGCTGC), and the resulting PCR product was sequenced. An NS3-T249A mutation was observed in two of three replicates of KN3829 (replicates #1 and 2, Figure 2C), correlating with increased PBMC replication compared to the third replicate, which retained the wild-type KN3829 sequence. No mutations were observed in the NY99 NS3-P249T samples; however, it is possible that the experimental variability among these samples was caused by mutation(s) in a region that was not sequenced.
In one experiment (Experiment 8; Figure 2), viral growth was assessed in Transwell plates containing a 0.4 μm membrane (Corning). Cells were mixed with virus in the upper well, and media was sampled from the lower well and replaced every 24 hours. This system allows for the removal of virus-containing medium without centrifugation of cells. In this system, titers of NY99 rose early in the experiment, peaking at 2 dpi, while titers of KN3829 began to rise only after 3 dpi. Although the Transwell system was not assessed further in this study, it may provide a convenient alternative to the sampling methods used above, as it reduces the requirement for manipulation and potential for cell loss during centrifugation. However, the day 6 titer ratio described above would not be appropriate in the Transwell system, given that titers of KN3829 had increased to equal those of NY99 by this time point. The difference in titers was greatest at 2 dpi; however, the appropriate time point to use for such a ratio in the Transwell system would likely have to be determined empirically.
To assess the potential of the ex vivo PBMC culture assay to predict avian host competence, AMCR PBMCs were inoculated with a panel of NY99 NS3-249 mutants that have been shown to elicit a variety of viremia magnitudes and levels of mortality in AMCRs. Of these mutants, NS3-P249H and P249D exhibited virulence in AMCRs comparable to the wild-type virus, while P249T elicited limited viremia and low mortality. The P249A and P249N viruses displayed intermediate in vivo viremia and mortality phenotypes (Langevin et al., 2014).
As described above, titers of NS3-P249T in ex vivo PBMC culture decreased to undetectable levels by 5 dpi, while wild-type NY99 titers plateaued at approximately 4 log10 pfu/ml of culture supernatant (Figure 3A). Growth profiles of P249H and P249N mutants were not distinguishable from wild-type at any time point. By 6 dpi, P249A and P249D mutants exhibited titers approximately 1-1.5 log10 pfu/ml supernatant lower than wild type (p < 0.05, Student’s t-test). It is intriguing that P249N and P249D mutants had opposing phenotypes ex vivo compared to their in vivo phenotypes. Viral RNA isolated from PBMC culture supernatants was sequenced as described above to verify the identity of these two strains and to assess the potential for genetic modulation at the NS3-249 loci. All sequencing reactions demonstrated the expected amino acid identity at the NS3-249 loci with no observed genetic mutations.
Figure 3.
(A) Growth of WNV NY99 NS3-249 mutants in AMCR PBMCs. NY99 wild-type (NS3-P249) and NS3-P249T mutant data correspond to experiment 7 in Figure 2. The dashed line represents the limit of detection. Error bars represent standard deviation from the mean of three replicates. (B) Scatterplot comparing day 6 PBMC titers to peak in vivo titers in AMCRs. Legend is as in (A), with the addition of KN3829 (black circles) and KN3829-T249P (open diamonds) (Brault et al., 2007; Langevin et al., 2014). R = 0.8726; p = 0.005.
In order to quantify the degree to which the PBMC assay can predict in vivo phenotypes, the titers of the NY99 NS3-249 mutants, as well as the KN3829-T249P mutant, were normalized to the titer of NY99 in the same experiment, as described above. These titers were positively correlated with in vivo peak titers in AMCRs (R = 0.8726; p = 0.005; Figure 3B) (Brault et al., 2007; Langevin et al., 2014).
Overall, these experiments demonstrate that WNV growth in ex vivo culture of AMCR PBMCs is capable of differentiating strains with medium-to-high pathogenicity in AMCRs from those with low pathogenicity. This method will be useful for the initial assessment of the potential avian virulence of novel WNV strains. It can likely be expanded to other avian species and used with related flaviviruses for which birds serve as reservoir hosts, such as St. Louis encephalitis and Japanese encephalitis viruses. Additionally, ex vivo culture of likely target cells will aid in molecular analysis of host factors and pathogen-associated molecular patterns (PAMPs) that could define the competence of reservoir species for WNV and other flaviviruses.
There are some caveats to the potential use of this ex vivo AMCR PBMC culture system for recapitulation of in vivo growth and virulence phenotypes. Inter-experiment variability is quite high (Figure 2), and it will be important to assess novel strains in comparison to control strains with known growth and pathogenicity phenotypes, such as NY99 and KN3829. The reasons for the variability in viral growth are unclear, but they may be related to the genetics and/or infection and immune status of the individual birds sampled in each experiment. It may be possible to reduce variability by limiting the animals bled for each experiment to a set of defined individuals whose PBMCs have been assessed previously for viability and viral replication potential. Alternatively, to reduce inter-experimental variability, attempts are underway to create immortalized cell lines derived from AMCR PBMCs.
High intra-experiment variability was also observed in one experiment. Sequencing revealed a point mutation at the NS3-249 site in KN3829 that correlated with differential replication capacity in this experiment. The possibility of mutation during growth in PBMCs should be taken into account during assessment of novel strains, particularly if high intra-experiment variability is observed.
Intermediate and high pathogenicity strains may not be reliably distinguishable in this system (Figure 3). It is not clear why the NS3-P249N and P249D mutants had different phenotypes in ex vivo leukocyte culture than in vivo. This may be attributable to innate variability in both in vivo and ex vivo systems, or to differential replication of the two mutants in non-leukocyte cell types. It is also possible that there may be subtle differences in the replication of one or more mutants between in vivo and ex vivo leukocytes, depending on media conditions and differentiation status of the cells. If such differences are not uniform between WNV strains and mutants, they could cause the differences observed here. Further work will be necessary to address these questions.
The ex vivo system is clearly not a replacement for in vivo pathological assessment of viral strains. However, in general, it does recapitulate in vivo phenotypes and can be used as a tool to predict in vivo virulence and to help limit the number of avian hosts to be used in more invasive studies.
Acknowledgments
We thank the Colorado Department of Fish and Game and private landowners in Bellvue, Colorado for allowing AMCR trapping on their respective grounds. Angela Bosco-Lauth, Nisha Duggal, and Hannah Romo assisted with bird trapping and care. Todd Felix (USDA) assisted with trapping. Funding for this study was provided by the U.S. Centers for Disease Control and Prevention. E.A.D. was supported by an American Society for Microbiology/CDC postdoctoral fellowship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- AMCR
American crow (Corvus brachyrhynchos)
References
- Bai F, Kong K-F, Dai J, Qian F, Zhang L, Brown CR, Fikrig E, Montgomery RR. A paradoxical role for neutrophils in the pathogenesis of West Nile virus. J. Infect. Dis. 2010;202:1804–1812. doi: 10.1086/657416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard KA, Maffei JG, Jones SA, Kauffman EB, Ebel GD, Dupuis AP, Ngo KA, Nicholas DC, Young DM, Shi P-Y, et al. West Nile virus infection in birds and mosquitoes, New York State, 2000. Emerg. Infect. Dis. 2001;7:679–685. doi: 10.3201/eid0704.010415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault AC, Langevin SA, Bowen RA, Panella NA, Biggerstaff BJ, Miller BR, Komar N. Differential virulence of West Nile strains for American crows. Emerg. Infect. Dis. 2004;10:2161–2168. doi: 10.3201/eid1012.040486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault AC, Huang CY-H, Langevin SA, Kinney RM, Bowen RA, Ramey WN, Panella NA, Holmes EC, Powers AM, Miller BR. A single positively selected West Nile viral mutation confers increased virogenesis in American crows. Nat. Genet. 2007;39:1162–1166. doi: 10.1038/ng2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . West Nile virus disease cases and deaths reported to CDC by year and clinical presentation, 1999-2013. 2014. [Google Scholar]
- Eidson M, Schmit K, Hagiwara Y, Anand M, Backenson PB, Gotham I, Kramer L. Dead crow density and West Nile virus monitoring, New York. Emerg. Infect. Dis. 2005;11:1370–1375. doi: 10.3201/eid1109.040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Tapia D, Loiacono CM, Kleiboeker SB. Replication of West Nile virus in equine peripheral blood mononuclear cells. Vet. Immunol. Immunopathol. 2006;110:229–244. doi: 10.1016/j.vetimm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Kinney RM, Huang CY-H, Whiteman MC, Bowen RA, Langevin SA, Miller BR, Brault AC. Avian virulence and thermostable replication of the North American strain of West Nile virus. J. Gen. Virol. 2006;87:3611–3622. doi: 10.1099/vir.0.82299-0. [DOI] [PubMed] [Google Scholar]
- Komar N. West Nile virus: epidemiology and ecology in North America. Adv. Virus Res. 2003;61:185–234. doi: 10.1016/s0065-3527(03)61005-5. [DOI] [PubMed] [Google Scholar]
- Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen RA, Bunning M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg. Infect. Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin SA, Bowen RA, Reisen WK, Andrade CC, Ramey WN, Maharaj PD, Anishchenko M, Kenney JL, Duggal NK, Romo H, et al. Host competence and helicase activity differences exhibited by West Nile viral variants expressing NS3-249 amino acid polymorphisms. PLoS One. 2014;9:e100802. doi: 10.1371/journal.pone.0100802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey NP, Lehman JA, Staples JE, Fischer M. West nile virus and other arboviral diseases - United States, 2012. MMWR. Morb. Mortal. Wkly. Rep. 2013;62:513–517. [PMC free article] [PubMed] [Google Scholar]
- Nemeth NM, Beckett S, Edwards E, Klenk K, Komar N. Avian mortality surveillance for West Nile virus in Colorado. Am. J. Trop. Med. Hyg. 2007;76:431–437. [PubMed] [Google Scholar]
- Papa A, Bakonyi T, Xanthopoulou K, Vázquez A, Tenorio A, Nowotny N. Genetic characterization of West Nile virus lineage 2, Greece, 2010. Emerg. Infect. Dis. 2011;17:920–922. doi: 10.3201/eid1705.101759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Ramírez E, Llorente F, Jiménez-Clavero MÁ. Experimental infections of wild birds with West Nile virus. Viruses. 2014;6:752–781. doi: 10.3390/v6020752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen LR, Brault AC, Nasci RS. West Nile virus: review of the literature. JAMA. 2013;310:308–315. doi: 10.1001/jama.2013.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann CA, Hayes EB, DiGuiseppi C, Hoffman R, Lehman JA, Lindsey NP, Campbell GL, Fischer M. Epidemiology of neuroinvasive arboviral disease in the United States, 1999-2007. Am. J. Trop. Med. Hyg. 2008;79:974–979. [PubMed] [Google Scholar]
- Reisen WK. Ecology of West Nile virus in North America. Viruses. 2013;5:2079–2105. doi: 10.3390/v5092079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios M, Zhang MJ, Grinev A, Srinivasan K, Daniel S, Wood O, Hewlett IK, Dayton AI. Monocytes-macrophages are a potential target in human infection with West Nile virus through blood transfusion. Transfusion. 2006;46:659–667. doi: 10.1111/j.1537-2995.2006.00769.x. [DOI] [PubMed] [Google Scholar]