Abstract
Introduction
Phrenic nerve (PN) stimulation (PNS) frequently limits cardiac resynchronization therapy (CRT). Yet, pacing strategies to minimize PNS have not been systematically compared. We propose to: 1) compare different pacing strategies to minimize PNS in CRT and 2) evaluate differences between PN and left ventricular (LV) capture thresholds among LV pacing configurations.
Methods and Results
PN and LV thresholds were obtained using 6 LV configurations in 28 patients with any PNS during CRT implantation or replacement. Incidence of PNS was compared in all LV configurations by programming pacing output to 1) One Volt above LV threshold, 2) triple pulse width (PW) at LV threshold, and 3) 1.5 times LV threshold for each patient. PN thresholds and PN strength-duration curves were statistically different between configurations (P<0.05). Ring→RVcoil and Ring→Can had the largest difference between PN and LV thresholds. Pacing output programmed to 1.5 times LV threshold, 1 Volt above LV threshold and triple PW at LV threshold had similar probability of PNS between LV configurations. However, 1 volt above LV threshold and triple PW at LV threshold frequently resulted in poor (<30%) LV capture safety margin (14–43% and 53–68%, respectively). Freedom from PNS (programmed output at twice LV threshold) was found in 88%, 84% and 52% with 6, 3, or 2 available LV configurations, respectively.
Conclusion
Multiple LV pacing configurations marginally increase the probability of avoiding PNS by electronic reprogramming. Pacing output programmed to 1.5 times LV threshold is an additional alternative to minimize PNS when electronic reprogramming options are limited.
Keywords: Phrenic nerve stimulation, cardiac resynchronization therapy, pacing thresholds, biventricular pacing, heart failure, implantable cardioverter defibrillator
Introduction
Phrenic nerve stimulation (PNS) occurs in approximately 15% of patients with cardiac resynchronization therapy (CRT) therapy and can limit biventricular pacing1–4. In the presence of a bipolar lead in the coronary sinus, electronic programming of 6 different left ventricular (LV) pacing configurations or vectors is possible. Prior studies comparing PNS in different pacing configurations have only reported voltage thresholds2–5. However, LV thresholds comparing energy or delivered current, are more accurate than voltage for LV pacing due to significant impedance differences between LV pacing configurations6. Since LV threshold may vary significantly with time and clinical circumstances, pacing output is traditionally programmed to twice the LV voltage threshold to allow 100% LV capture safety margin7. However, such programming is not always feasible in the presence of PNS1, 3. Various pacing strategies have been proposed to minimize PNS3, 4, 8. All these pacing strategies use a lower pacing output as a common approach to minimize PNS; however, their effect on LV capture safety margin have not been studied. The main objectives of our study are to: a) compare 1.5 times LV threshold pacing strategy (50% LV capture safety margin) with other proposed strategies (1 Volt above LV threshold and tripling pulse width at LV threshold) to minimize PNS in CRT, b) assess the value of multiple LV configurations in overcoming PNS, and c) evaluate the difference between LV and PN thresholds between LV pacing configurations.
Methods
This prospective study included consecutive patients scheduled to undergo a new LV lead implant or generator replacement of a CRT device at the McGuire Veterans Affairs Medical Center. Patients were only included if PNS was present peri-operatively in at least one of 6 LV pacing configurations at maximum pacing output (10V at 1.5ms). In order to compare all 6 configurations, patients with a unipolar LV lead or surgically-implanted epicardial LV lead were excluded. The LV lead was positioned to target the lateral LV wall and the pacing protocol was performed only at the first LV lead position deemed appropriate based on cardiac venous anatomy in RAO and LAO fluoroscopic views. All patients underwent a PN and LV pacing protocol described below.
We hypothesized that: 1) PN thresholds are significantly different between LV pacing configurations, 2) LV safety margin may be compromised with previously described pacing strategies3, 4, 8 to minimize PNS, and 3) the availability of multiple pacing configurations would help avoid PNS.
Pacing protocol
All patients underwent a pacing protocol intra-operatively using a stimulator (Biotronik Model 3105, GmbH & Co. Berlin, Germany, 5.5μF capacitance) with VVI pacing at a rate of 100 bpm. PN and LV voltage rheobase (pulse width of 1.5ms) and chronaxie (C) were measured in each patient using the following six pacing configurations: 1) tip (−) to ring (+) (Tip→Ring), 2) ring (−) to tip (+) (Ring→Tip), 3) tip (−) to RV coil (+) (Tip→RVcoil), 4) tip (−) to can (+) (Tip→Can), 5) ring (−) to RV coil (+) (Ring→RVcoil) and 6) ring (−) to can (+) (Ring→Can). In addition, voltage LV threshold at 0.4ms was obtained in all configurations. A continuous 12-lead ECG was monitored to assure LV capture and exclude RV cathodal capture during pacing protocol.
Rheobase voltage for PN and LV was determined in each configuration at fixed PW of 1.5ms by increasing stimulus voltage from 0.5V in increments of 0.1–0.5V. Rheobase voltage for PN threshold and LV threshold were defined as the lowest voltage that resulted in any PNS (regardless of severity and duration) and 100% LV capture throughout at least 3 normal respiratory cycles (inspiration and expiration), respectively. PN and LV chronaxie for each pacing configuration was obtained at twice PN or LV rheobase voltage (fixed voltage), respectively, increasing PW in 0.1ms increments until capture. PN and LV chronaxie was defined as the shortest pulse duration that resulted in any PNS or 100% LV capture, respectively.
The following parameters were calculated for all 6 LV pacing configuration in each patient: 1) pacing energy (mJ) at PN and LV thresholds at 0.4ms; 2) voltage and energy strength-duration curves for PN and LV capture; and 3) difference between PN and LV thresholds (PN threshold - LV threshold difference). Delivered energy (E) was calculated by the formula: E= V2/R x PW, where V is voltage, R is impedance and PW is pulse width of pacing impulse7. PN and LV voltage strength-duration curve for all 6 pacing configurations were constructed using Lapicque’s formula7: V(t) = Vr (1 + C/t), where V(t) is the threshold stimulus voltage at pulse duration (t), Vr is the voltage rheobase, and C is the chronaxie7.
The prevalence of PNS was assessed in each LV pacing configuration at maximum pacing output (10V at 1.5ms) regardless of LV threshold. The probability of PNS (defined as LV threshold/PN threshold ≥ 1) was calculated based on a programmed LV pacing output at twice LV threshold at 0.4ms (LV threshold × 2) that provides 100% LV capture safety margin. Similarly, the probability of PNS and LV capture safety margin were estimated according to two previously published pacing strategies to minimize PNS: 1) 1V above LV threshold (LV threshold+1V) and 2) tripling pulse width (PW) at LV threshold (0.4ms) 3, 4, 8, 9 (Figure 1). LV capture safety margin for a specific programmed LV pacing output was calculated by formula, LV capture safety margin (%) = (LV pacing output – LV threshold)/LV threshold. In addition, we estimated the probability of PNS with pacing output programmed to: 1) 1.5 times LV threshold at 0.4ms (LV threshold × 1.5) and 2) LV threshold at 0.4ms (pacing output equal to LV threshold) (Figure 1). Finally, we estimated the probability of overcoming PNS with the availability of 2 (Tip→RVcoil and Tip→Ring), 3 (Tip→RVcoil, Tip→Ring and Ring→RVcoil) or 6 LV pacing configurations based on the different pacing strategies described above.
Figure 1.
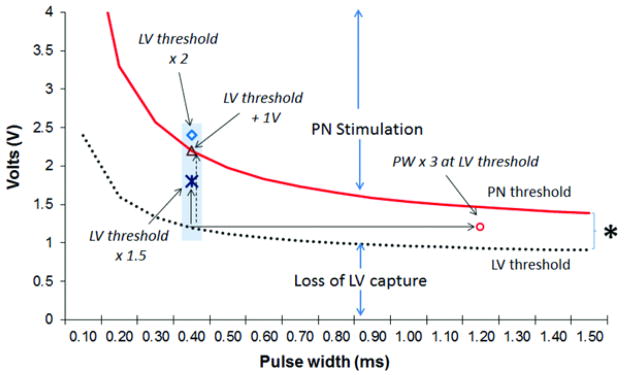
Pacing output strategies compared to minimize phrenic nerve stimulation (PNS). Pacing output is programmed to LV threshold at 0.4ms: (a) plus 1 Volt (LV threshold +1V), (b) multiplied by 2 (LV threshold × 2), (c) multiplied by 1.5 (LV threshold × 1.5), or (d) LV output is left at LV threshold but pulse width (PW) is tripled (PW × 3 at LV threshold), PNS will be present above phrenic nerve (PN, solid) strength duration curve, whereas LV capture will be absent below LV (dashed) strength duration curve.
Statistical analysis
Continuous variables were expressed as mean ± SEM and group means were compared using a two-tailed unpaired Student’s t tests. Categorical variables were compared using either a Chi-square test or, where appropriate, Fisher’s Exact test. Ordinal variables were compared using a non-parametric Wilcoxon Rank-sum (Mann-Whitney U) test. One-way and two-way repeated measures ANOVA compared the differences in pacing impedances, minimal threshold energy, voltage, energy, current drain and density at phrenic nerve and LV threshold at 1.5ms and 0.4ms between LV pacing configurations. If the repeated measures ANOVA indicated a significant difference (P<0.05) between LV pacing configurations, a Bonferroni multiple comparison corrections was performed to identify individual differences between LV configurations. A p value <0.05 was considered significant. Statistical analysis was performed using SAS/STAT® Software (SAS Institute, Inc. Cary, NC).
Results
Fifty-two consecutive patients participated in the study. Three patients did not complete the LV pacing protocol due to hemodynamic instability or recurrent VT during the pacing protocols. Twenty-one patients lacked PNS in any pacing configuration at maximum pacing output (10V at 1.5ms) and were excluded from analysis. The remaining 28 patients (57%) manifested PNS in at least one pacing configuration and were included for analysis. Patient demographics and LV lead characteristics are shown in Supplemental table 1.
Voltage and delivered energy at phrenic nerve thresholds
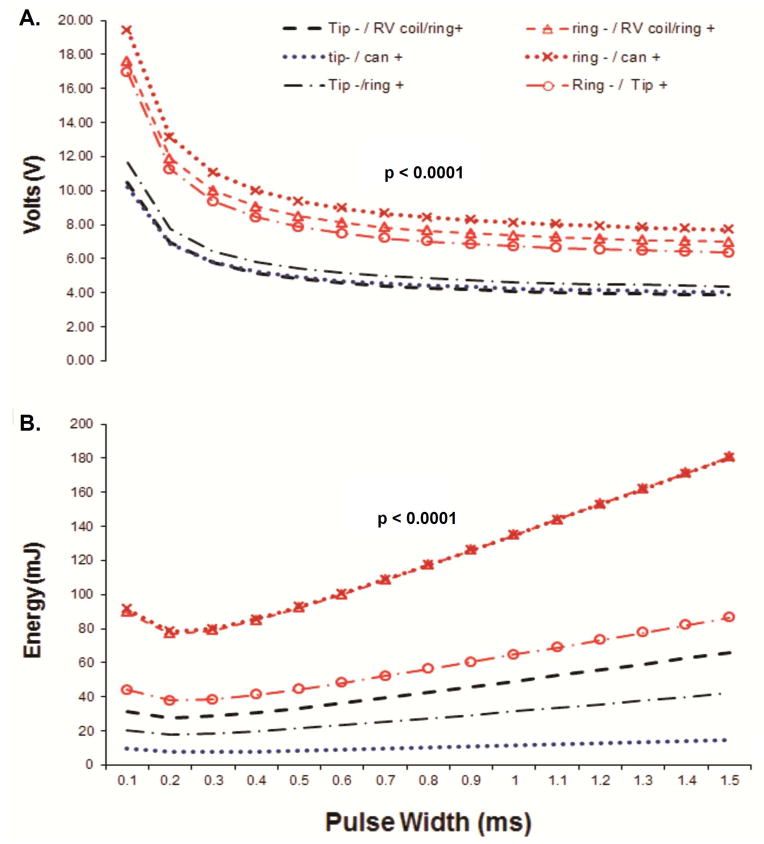
PN threshold (0.4ms) and strength-duration curves (conveyed in voltage and delivered energy) were significantly different between LV pacing vectors (Table 1, Figure 2). Overall, voltage and delivered energy at PN threshold were lower in the configurations using the tip electrode as cathode (Tip→RVcoil Tip→Can and Tip→Ring configurations, Table 1 and Figure 2). Ring→Can configuration had significantly higher delivered energy at PN threshold (0.4ms) when compared to Tip→Ring configuration (Table 1).
Table 1.
PN threshold and PN threshold – LV threshold difference at 0.4ms between LV pacing configurations in 28 patients conveyed in voltage and delivered energy. Data expressed in mean ± SEM.
| Tip→RV | Tip→Can | Tip→Ring | Ring→RV | Ring→Can | Ring→Tip | P value | |
|---|---|---|---|---|---|---|---|
| Voltage | |||||||
| PN (Volts) | 5.2 ± 0.8 | 5.3 ± 0.8 | 5.8 ± 0.7 | 9.1 ± 1.1 | 10 ± 1.1 | 8.4 ± 0.9 | 0.033 |
| PN–LV difference | 3 ± 1.2 | 2.8 ± 1.3 | 2.6 ± 1.2| | 7.2 ± 1.3 | 7.9 ± 1.2‡ | 5.2 ± 1.2 | 0.019 |
| Delivered energy | |||||||
| PN (mJ) | 30.9 ± 9 | 29.4 ± 9.1 | 19.9 ± 5.2| | 84.6 ± 19 | 85.6 ± 17‡ | 41.1 ± 9.4 | 0.001 |
| PN–LV difference | 26.4 ± 11 | 23.5 ± 12 | 14.2 ± 7§,| | 80.8 ± 20‡ | 80.3 ± 18‡ | 35.4 ± 11 | 0.0003 |
Note: P value refers to an overall test (repeated measures ANOVA) between all configurations. Repeated measures ANOVA showed that there was a significant configuration effect. Statistical difference between individual configurations is depicted as follows:
Tip→Ring;
Ring→RVcoil;
Ring→Can. Ring→Can vs. Tip→Ring configurations were statistically different in PN threshold-LV threshold difference (voltage and delivered energy), whereas Ring→RVcoil vs. Tip→Ring were statistically different in energy but not in voltage PN threshold-LV threshold difference. Bonferroni correction failed to identify statistical difference in voltage at PN threshold between individual configurations. Voltage and energy of PN thresholds at 0.4ms were derived using Lapique’s formula. mA, miliamperes; mJ, miliJoules.
Figure 2.
PN voltage (A) and Energy (B) strength-duration curves in all 6 LV pacing configurations. Details of the analysis in Supplemental Material.
Difference between PN and LV thresholds
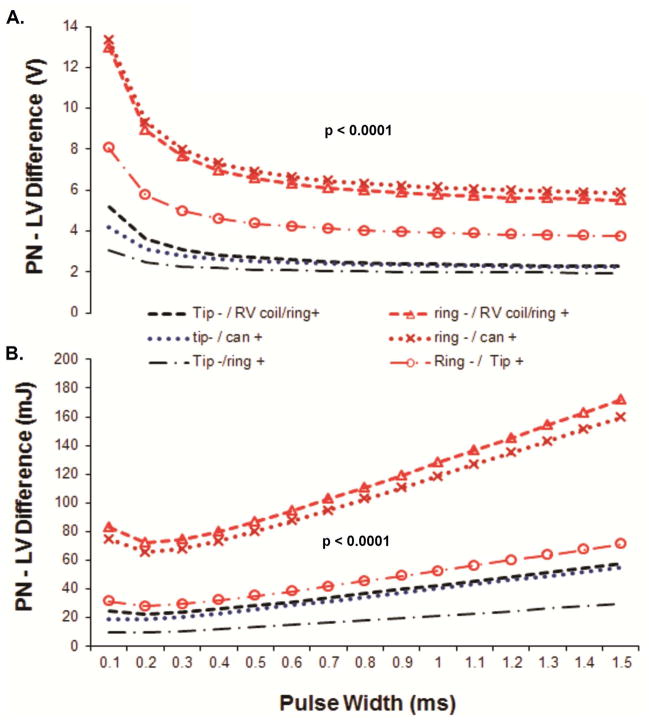
The PN threshold-LV threshold difference at 0.4ms and strength-duration curve differences between PN and LV thresholds (conveyed in voltage and energy; Table 1 and Figure 3, respectively) were significantly different between pacing configurations. Overall, voltage and energy PN threshold-LV threshold difference were higher in Ring→RVcoil and Ring→Can configurations (Table 1, Figure 3).
Figure 3.
Voltage (A) and energy (B) strength-duration curves differences between PN & LV thresholds (PN threshold-LV threshold difference) in all 6 LV pacing configurations. Details of the analysis in Supplemental Material.
Prevalence of phrenic nerve stimulation
The prevalence of PNS at maximum output (10 volts at 1.5ms) or at LV threshold was not statistically different between pacing configurations (Supplemental Table 2).
Probability of PNS between different pacing strategies
A trend towards lower probability of PNS was noted if pacing output was programmed at 1.5 times LV threshold, LV threshold+1V and triple PW of LV threshold when compared to the traditional twice LV threshold (100% LV capture safety margin) (Supplemental Table 2). Regardless of the programmed pacing strategy to minimize PNS, the probability of PNS remained lower in Ring→Can and Ring→RVcoil configurations. The LV threshold+1V strategy had a wide range of LV capture safety margin (10 – 350%). In contrast to the 1.5 times LV threshold pacing strategy (providing a 50% LV capture safety margin), a programmed pacing output strategy of LV threshold+1V and triple PW at LV threshold frequently demonstrated poor (<30%) LV capture safety margin (range within various configurations of 14–42% and 53–68%, respectively; Table 2).
Table 2.
Proportion of patients with poor (<30%) LV capture safety margin (LVSM) with different pacing strategies (LV threshold +1V and triple PW at LV threshold, 0.4ms). PW, pulse width.
| LV Pacing Configurations | Pacing output at LV threshold + 1 Volt % patients LVSM < 30% |
Pacing output at triple PW (1.2ms) at LV threshold % patients LVSM < 30% |
|---|---|---|
|
| ||
| Tip→RVcoil | 21% | 67% |
| Tip→Can | 25% | 53% |
| Tip→Ring | 42% | 61% |
| Ring→RVcoil | 14% | 68% |
| Ring→Can | 14% | 64% |
| Ring→Tip | 42% | 68% |
Note: LV capture safety margin refers to the relationship between LV pacing output and LV threshold (see methods for details calculating LVSM).
Avoiding phrenic nerve stimulation based on availability of 2, 3 or 6 LV pacing configurations
The probability of avoiding PNS (lack of PNS in at least one pacing configuration) at maximum output (10V at 1.5ms) and twice LV threshold at 0.4ms (100% LV capture safety margin) were 21% and 56%, 50% and 84%, and 57% and 88% in 2 (Tip→RVcoil and Tip-Ring), 3 (Tip→RVcoil, Tip-Ring and Ring→RVcoil) or 6 available LV pacing configurations, respectively (Table 3). The availability of 6 versus 3 pacing configurations avoided PNS in one patient only, based solely on the lack of PN capture on the Ring→Can configuration with a pacing output programmed at twice LV threshold at 0.4ms (LV threshold × 2).
Table 3.
Lack of phrenic nerve stimulation (no PNS in at least one configuration) at different programmed LV pacing outputs based on the number of configurations available.
| Phrenic Nerve stimulation | Tip→RVcoil & Tip→Ring | Tip→RVcoil, Ring→RVcoil & Tip→Ring | All 6 vectors |
|---|---|---|---|
| Max output (10V, 1.5ms) | 21 % | 50 % | 57 % |
| 100% LVSM (LVt×2, 0.4ms) | 56 % | 84 % | 88 % |
| 50% LVSM (LVt ×1.5, 0.4ms) | 68 % | 96 % | 96 % |
| LVt + 1V (0.4ms) | 64 % | 96 % | 100 % |
| Triple PW of LVt (1.2ms) | 68 % | 96 % | 100 % |
| No LVSM (0.4ms) | 80 % | 96 % | 100 % |
LVt, LV threshold; LVSM, LV capture safety margin.
Discussion
PNS is frequently reported (33–37%) and clinically relevant in 3–26% of patients undergoing CRT implantation1–4, 10–12. It is estimated that up to 5% of all patients with CRT devices undergo repeat surgery for PNS, and at least 1–2% have their LV lead deactivated due to PNS1, 3, 9. Electronic reprogramming has been an important tool to minimize PNS without compromising the efficacy of CRT1–3, 5. Ideally, a given LV pacing configuration should have a low LV threshold without PNS and/or large PN threshold-LV threshold difference in order to allow program pacing output at twice LV threshold (ideal 100% LV capture safety margin). However, pacing output programmed to twice LV threshold is achieved in only a small percentage of patients with PNS1, 3. If pacing output at twice LV threshold is not feasible due to PNS, alternative strategies1, 8, 9 have been proposed, such as reprogramming LV pacing output to: a) 1 Volt above LV threshold (LV threshold+1V), or b) tripling the PW at the LV threshold obtained at 0.4ms. Until now, the effects these pacing strategies on LV capture safety margin has not been studied. Additionally, we investigated an alternative strategy to minimize PNS by programming pacing output to 1.5 times LV threshold (LV threshold x 1.5 at 0.4ms,), while maintaining a constant 50% LV capture safety margin.
Our main findings include: a) voltage and energy PN thresholds and strength-duration curves demonstrate statistical difference between LV pacing configurations; b) Ring→Can and Ring→RVcoil configurations are least likely to have PNS since they have the highest PN threshold and largest PN threshold-LV threshold difference; c) LV output programmed at 1.5 times LV threshold and 1 V above LV threshold are appropriate alternatives to eliminate PNS when twice LV threshold (providing 100% LV capture safety margin) is not possible; however, a pacing output at 1V above LV threshold has frequently a borderline and unacceptably wide range of LV capture safety margin depending on LV threshold; d) LV output programmed at triple PW at LV threshold can similarly avoid PNS, at the expense of an unacceptably low (<30%) LV capture safety margin despite higher energy delivered; and e) multiple LV pacing configurations marginally increase the probability of avoiding PNS by electronic reprogramming.
Phrenic nerve thresholds and difference with LV thresholds between LV configurations
Few studies have compared PN voltage thresholds in different LV pacing configurations4. We have previously shown that voltage thresholds cannot be equally compared between LV pacing configurations due to significant impedance differences between lead configurations6, 7. Our study expands on this principle, as we compared voltage and energy of PN threshold in all 6 configurations in the same patient, which corroborates that Tip→Ring has a low energy PN threshold despite a high voltage PN threshold, as previously reported4.
Two studies reported that Ring→RVcoil and Tip→Ring had a significantly higher PN voltage threshold compared to Tip→RVcoil 4, 5. These studies included devices with only 3 available pacing configurations. Our study expands current literature with a) threshold energy data and b) analysis of 6 LV pacing configurations. We found that Ring→RVcoil and Ring→Can had the highest voltage and energy at PN threshold and the largest PN threshold − LV threshold difference, whereas Tip→Can and Tip→Ring configurations had the lowest voltage and energy at PN threshold and the lowest PN threshold − LV threshold difference (Table 1, Figure 2 and 3). These findings suggest that Tip→Can and Tip→Ring configurations are more likely to have PNS, whereas Ring→Can and Ring→RVcoil are less likely to have PNS. The most probable explanation for this finding is the more apical course of the PN and the closer physical location to the tip electrode position13.
Our study was performed peri-operatively in the supine position only. PN threshold and PN threshold – LV threshold difference can vary with position4. Thus, these findings may not apply to other postures. Unfortunately, limited programmability of LV pacing configurations, as well as a limited amplitude and time (pulse width) programmability from different device manufacturers obligated us to perform this study intra-operatively.
Avoiding phrenic nerve stimulation
Consistent with our findings (Supplemental Table 2), Champagne et al2 failed to demonstrate a statistically significant difference in the prevalence of PNS among all 6 LV pacing configurations. Our study is unique in that we had a priori knowledge of existence of PNS in all our patients. Despite such knowledge, our results demonstrate minimal incremental benefit with the availability of 6 versus 3 LV pacing configurations (Table 3). However, one could posit a situation where the availability of a particular configuration is necessary to achieve a satisfactory clinical result from CRT in the presence of PNS. Based on our data, these situations would be predicted to be very rare.
Based on our results, we propose a strategy to minimize PNS by programming a pacing output to 1.5 times LV threshold (LV threshold × 1.5; provides a 50% LV capture safety margin) as the best compromise between eliminating PNS and maintaining an acceptable LV capture safety margin when PNS is present at ideal twice LV threshold (100% LV capture safety margin). We propose this pacing strategy because delivered energy (E) doubles when pacing output is programmed at 1.5 times LV threshold, since E is a factor of a squared voltage (E=V2/R x PW, where V is voltage, R is impedance, PW is pulse width of pacing impulse). Our findings support that a pacing output programmed at triple PW at LV threshold may be an alternative to minimize PNS; however, this strategy provides only minimal LV capture safety above LV threshold at markedly higher energy cost (higher E compared to pacing output of 1.5 times LV threshold near chronaxie). Similarly, a pacing output programmed to 1 Volt above LV threshold is not an ideal strategy to eliminate PNS due to a frequently variable and marginal LV capture safety depending on baseline LV threshold (Table 2). Supplemental Figure 1 illustrates an example of the marginal and variable LV capture safety margin by these pacing strategies.
Limitations
The pacing stimulator had a maximum output of 10V. We cannot exclude some pacing configurations could have PNS at higher pacing outputs.
Our study included 5 different LV lead types; therefore, our conclusions may not apply to all LV leads. There are differences in the surface area of the LV lead tip and right ventricular coil depending on the manufacturer.
Pacing protocol was performed primarily in fluoroscopically “ideal” lead positions, but not necessarily the final LV lead position.
Conclusions
Voltage and energy PN thresholds and PN threshold strength-duration curves demonstrate statistical difference between LV pacing configurations. Ring→Can and Ring→RVcoil are the preferred configurations to avoid PNS during LV pacing since they have the highest PN threshold and the largest PN-LV threshold difference. If an ideal pacing output programmed to twice LV threshold (providing 100% LV capture safety margin) is not feasible due to PNS, a pacing output strategy of 1.5 times LV threshold (providing a 50% LV capture safety margin) is an additional alternative to LV threshold + 1V or tripling pulse width at LV threshold. However, LV threshold + 1V may have substantial variability in LV capture safety margin (depending on baseline LV threshold), whereas tripling pulse width of LV threshold frequently compromises LV capture safety margin (< 30%) despite a substantial increase in delivered energy. Electronic reprogramming with multiple LV pacing configurations marginally increases the probability of avoiding PNS.
Supplementary Material
Figure 1. Phrenic nerve and LV strength-duration curves on 2 different pacing configurations in the same patient. This illustrates the different LV capture safety margin (LVSM) that can result from different pacing strategies to minimize phrenic nerve stimulation.
Ring→RVcoil configuration demonstrated a pacing impedance of 600 Ohms, LV threshold of 1.2V at 0.4ms and 0.93V at 1.2ms, and PN threshold of 2.2V at 0.4ms (PN threshold – LV threshold difference 1 V), whereas Tip→Ring configuration demonstrated a pacing impedance of 1200 Ohms, LV threshold of 3V at 0.4ms and 2.2V at 1.2ms and PN threshold of 5V at 0.4ms (PN threshold – LV threshold difference 2V), which would argue in favor of using Tip→Ring configuration with programmed pacing output of 1 volt above LV threshold (4V) to avoid PNS. However, this would provide a marginal 33% LV capture safety margin (LV capture safety margin = (4–3)/3). Similarly, tripling PW at LV threshold obtained at 0.4ms (Ring→RVcoil 1.2V at 1.2ms; Tip→Ring 3V at 1.2ms) would provide a marginal LV capture safety margin (Ring→RVcoil ,LV capture safety margin = [1.2–0.93]/0.93 = 29%; Tip→Ring, LV capture safety margin = [3−2.2]/2.2 = 36%) despite increase in delivered energy (Ring→RVcoil 2.9mJ; Tip→Ring 9mJ). In this case, we could use either Ring→RVcoil or Tip→Ring programmed at 1.5 times LV threshold (Ring→RVcoil LV threshold × 1.5 = 1.2V×1.5= 1.8V at 0.4ms; Tip→Ring LV threshold × 1.5 = 3×1.5= 4.5V at 0.4ms) to avoid PNS without compromising (50%) LV capture safety margin.
Table 1. Patient demographics and LV lead characteristics. All data presented in mean ± SEM.
Table 2. Probability of phrenic nerve stimulation (PNS) at maximum pacing output (10V, 1.5ms), at LV threshold and different pacing strategies to minimize PNS between LV pacing configurations. LVSM, LV capture safety margin; LVt, LV threshold; PW, pulse width
Acknowledgments
Funding source: NIH Grant UL1TR00058 to VCU Center for Advancing Translational Sciences (LRT).
JFH reports grant support from St. Jude Medical and Boston Scientific. KK is an investigator for Boston Scientific, St. Jude Medical & Sorin. JNK received honoraria from Medtronic. KAE received grant support & honoraria and is a consultant for Boston Scientific, Medtronic, St. Jude Medical & Biotronik.
This manuscript is dedicated to our late friend and colleague, Dr. Mark A. Wood, a superb clinician, teacher and researcher who assisted in the study design and analysis. He is greatly missed. We are also grateful to the Electrophysiology Laboratory staff at McGuire VA Medical Center, especially Donna Sargent, R.N.
Non-standard abbreviations
- PN
Phrenic nerve
- PNS
Phrenic nerve stimulation
- CRT
Cardiac resynchronization therapy
- LV
left ventricle
- RV
Right ventricle
- PW
pulse width (ms)
- V
volts
- R
Impedance (Ohms)
- E
Energy delivered (mJ)
References
- 1.Biffi M, Moschini C, Bertini M, Saporito D, Ziacchi M, Diemberger I, Valzania C, Domenichini G, Cervi E, Martignani C, Sangiorgi D, Branzi A, Boriani G. Phrenic stimulation: A challenge for cardiac resynchronization therapy. Circ Arrhythm Electrophysiol. 2009;2:402–410. doi: 10.1161/CIRCEP.108.836254. [DOI] [PubMed] [Google Scholar]
- 2.Champagne J, Healey JS, Krahn AD, Philippon F, Gurevitz O, Swearingen A, Glikson M. The effect of electronic repositioning on left ventricular pacing and phrenic nerve stimulation. Europace. 2011;13:409–415. doi: 10.1093/europace/euq499. [DOI] [PubMed] [Google Scholar]
- 3.Gurevitz O, Nof E, Carasso S, Luria D, Bar-Lev D, Tanami N, Eldar M, Glikson M. Programmable multiple pacing configurations help to overcome high left ventricular pacing thresholds and avoid phrenic nerve stimulation. Pacing Clin Electrophysiol. 2005;28:1255–1259. doi: 10.1111/j.1540-8159.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 4.Seifert M, Schau T, Moeller V, Neuss M, Meyhoefer J, Butter C. Influence of pacing configurations, body mass index, and position of coronary sinus lead on frequency of phrenic nerve stimulation and pacing thresholds under cardiac resynchronization therapy. Europace. 2010;12:961–967. doi: 10.1093/europace/euq119. [DOI] [PubMed] [Google Scholar]
- 5.Klein N, Klein M, Weglage H, Przibille O, Fischer S, Trappe HJ, Birkenhauer F, Pfeiffer D. Clinical efficacy of left ventricular pacing vector programmability in cardiac resynchronization therapy defibrillator patients for management of phrenic nerve stimulation and/or elevated left ventricular pacing thresholds: Insights from the efface phrenic stim study. Europace. 2012 doi: 10.1093/europace/eur412. [DOI] [PubMed] [Google Scholar]
- 6.Huizar JF, Kaszala K, Koneru J, Kowalski M, Thacker LR, Wood MA, Ellenbogen KA. Disparity in left ventricular stimulation among different pacing configurations in cardiac resynchronization therapy. Circ Arrhythm Electrophysiol. 2011 doi: 10.1161/CIRCEP.111.965475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellenbogen KA. Clinical cardiac pacing, defibrillation, and resynchronization therapy. Philadelphia, PA: Elsevier/Saunders; 2011. [Google Scholar]
- 8.Oh S, Kim WY, Kim HC, Oh IY, Choi EK. Left ventricular pacing with long pulse duration can avoid phrenic nerve stimulation. Heart Rhythm. 2011;8:1637–1640. doi: 10.1016/j.hrthm.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Jastrzebski M, Bacior B, Wojciechowska W, Czarnecka D. Left ventricular lead implantation at a phrenic stimulation site is safe and effective. Europace. 2011;13:520–525. doi: 10.1093/europace/euq505. [DOI] [PubMed] [Google Scholar]
- 10.Alonso C, Leclercq C, d’Allonnes FR, Pavin D, Victor F, Mabo P, Daubert JC. Six year experience of transvenous left ventricular lead implantation for permanent biventricular pacing in patients with advanced heart failure: Technical aspects. Heart. 2001;86:405–410. doi: 10.1136/heart.86.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biffi M, Bertini M, Ziacchi M, Gardini B, Mazzotti A, Massaro G, Diemberger I, Martignani C, Valzania C, Boriani G. Management of phrenic stimulation in crt patients over the long term: Still an unmet need ? Pacing Clin Electrophysiol. 2011;34:1201–1208. doi: 10.1111/j.1540-8159.2011.03147.x. [DOI] [PubMed] [Google Scholar]
- 12.Knight BP, Desai A, Coman J, Faddis M, Yong P. Long-term retention of cardiac resynchronization therapy. J Am Coll Cardiol. 2004;44:72–77. doi: 10.1016/j.jacc.2004.03.054. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez-Quintana D, Cabrera JA, Climent V, Farre J, Weiglein A, Ho SY. How close are the phrenic nerves to cardiac structures? Implications for cardiac interventionalists. J Cardiovasc Electrophysiol. 2005;16:309–313. doi: 10.1046/j.1540-8167.2005.40759.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1. Phrenic nerve and LV strength-duration curves on 2 different pacing configurations in the same patient. This illustrates the different LV capture safety margin (LVSM) that can result from different pacing strategies to minimize phrenic nerve stimulation.
Ring→RVcoil configuration demonstrated a pacing impedance of 600 Ohms, LV threshold of 1.2V at 0.4ms and 0.93V at 1.2ms, and PN threshold of 2.2V at 0.4ms (PN threshold – LV threshold difference 1 V), whereas Tip→Ring configuration demonstrated a pacing impedance of 1200 Ohms, LV threshold of 3V at 0.4ms and 2.2V at 1.2ms and PN threshold of 5V at 0.4ms (PN threshold – LV threshold difference 2V), which would argue in favor of using Tip→Ring configuration with programmed pacing output of 1 volt above LV threshold (4V) to avoid PNS. However, this would provide a marginal 33% LV capture safety margin (LV capture safety margin = (4–3)/3). Similarly, tripling PW at LV threshold obtained at 0.4ms (Ring→RVcoil 1.2V at 1.2ms; Tip→Ring 3V at 1.2ms) would provide a marginal LV capture safety margin (Ring→RVcoil ,LV capture safety margin = [1.2–0.93]/0.93 = 29%; Tip→Ring, LV capture safety margin = [3−2.2]/2.2 = 36%) despite increase in delivered energy (Ring→RVcoil 2.9mJ; Tip→Ring 9mJ). In this case, we could use either Ring→RVcoil or Tip→Ring programmed at 1.5 times LV threshold (Ring→RVcoil LV threshold × 1.5 = 1.2V×1.5= 1.8V at 0.4ms; Tip→Ring LV threshold × 1.5 = 3×1.5= 4.5V at 0.4ms) to avoid PNS without compromising (50%) LV capture safety margin.
Table 1. Patient demographics and LV lead characteristics. All data presented in mean ± SEM.
Table 2. Probability of phrenic nerve stimulation (PNS) at maximum pacing output (10V, 1.5ms), at LV threshold and different pacing strategies to minimize PNS between LV pacing configurations. LVSM, LV capture safety margin; LVt, LV threshold; PW, pulse width