Abstract
Recently reporting in Science, Nakagawa et al. describe an unexpected role for Dicer in chromosome fragmentation during apoptosis in C. elegans. They find that cleavage of DCR-1 by the caspase CED-3 redirects its regulatory activity, by destroying its dsRNAse activity while activating an intrinsic DNAse activity.
RNase III enzymes are a widely distributed family of double stranded RNA (dsRNA)-specific ribonucleases. Since the discovery of E. coli RNase III in the 1960s, the functions of this protein family in ribosomal RNA biogenesis and mRNA decay or regulation have been well studied in bacteria and yeast (MacRae et al., 2007). Importance of their homologs in higher eukaryotes was recognized only in the last decade. In particular, Dicer-family RNase III enzymes are central to the biogenesis of Argonaute-associated small regulatory RNAs, including microRNAs (miRNAs) and small interfering RNAs (siRNAs). Because miRNAs play important roles in diverse biological settings, Dicer genes are essential for many aspects of development and physiology. The cell death pathway has critical connections with the miRNA pathway, since many individual miRNAs have pro-apoptotic or anti-apoptotic activities. Deregulation of such miRNAs may contribute to various human cancers (Garzon et al., 2009).
Apoptosis is accompanied by DNA fragmentation, which can be visualized by TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling) staining. In mammals, the endonuclease DFF40 (also known as CAD) initiates DNA fragmentation (Widlak et al., 2005). The DNase activity of DFF40 is normally inhibited by DFF45 (also known as ICAD), but when the cysteine protease caspase-3 is activated, it cleaves DFF45 to release active DFF40. Despite strong conservation of the cell death pathway in C. elegans, including the functional caspase-3 ortholog CED-3 (Miura et al., 1993, Yuan et al., 1993), its genome does not appear to encode homologs of DFF40 and DFF45. Nevertheless, as apoptotic cells in C. elegans exhibit DNA fragmentation (Parrish et al., 2006), some nuclease activity is apparently responsible for initiating this process in nematodes.
The Xue lab aimed to address identify the putative nuclease, and in so doing revealed an unexpected connection between Dicer and DNA degradation during cell death (Nakagawa et al., 2010) (Figure 1). The authors took advantage of sensitized genetic backgrounds that accumulate TUNEL-stained nuclei, such as mutants of the CPS-6 nuclease, and screened for nucleases whose knockdown could reduce TUNEL staining. This approach recovered DCR-1 – the C. elegans Dicer ortholog – as a candidate. This was perhaps surprising as the loss of Dicer in other animal systems often leads to ectopic cell death. They validated that genuine dcr-1 deletion alleles suppressed the accumulation of TUNEL-positive cells in several sensitized backgrounds, and found that dcr-1 mutants exhibited reduced numbers of cell corpses at embryonic stages.
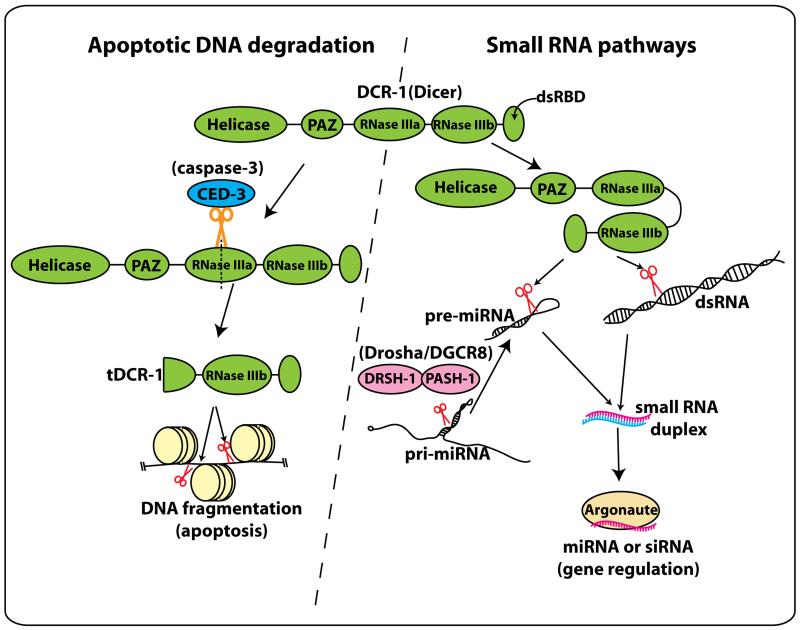
Figure 1.
Roles for C. elegans Dicer in apoptotic DNA degradation and small RNA pathways.
Dicer (DCR-1) is a multidomain protein with helicase, PAZ, RNase III, and dsRNA binding domains. In normal cells, DCR-1 cleaves dsRNAs or pre-miRNA hairpins to produce small regulatory RNAs that guide Argonaute examined proteins during post-transcriptional gene regulation (right). Nakagawa and colleagues showed that in apoptotic cells, activated CED-3 caspase cleaves a specific position of the first RNase III domain of DCR-1 to produce a short isoform named tDCR-1. tDCR-1 can no longer cleave dsRNAs, but instead nicks DNA to initiate chromosomal fragmentation (left).
Given that DCR-1 is a central player in small RNA pathways (Figure 1), the authors examined mutants of other miRNA/siRNA pathway components. However, none of these mutants exhibited similar defects, suggesting that the involvement of DCR-1 in DNA fragmentation lies outside of its normal requirement for small RNA biogenesis. Since epistasis experiments suggested that DCR-1 functioned downstream of activated CED-3, the authors tested if DCR-1 is a direct target of CED-3 proteolysis. In fact, in vitro tests showed that CED-3 cleaved DCR-1 at a specific position to yield a short isoform termed tDCR-1, which lacks the helicase and PAZ domains, and part of the first RNAse III domain (Figure 1). tDCR-1 was no longer capable of dicing dsRNA, but instead gained the capacity to nick plasmid DNA in vitro. It appears that the catalytic residues in the second RNase III domain of DCR-1 are used for both dsRNA and DNA cleavage, since point mutation of catalytic residues strongly affected both activities.
Endogenous cleaved tDCR-1 was not detected directly, and perhaps the level of such a nuclease might be expected to be kept in limiting amounts. Instead, the authors used genetic rescue assays to assess the importance of different DCR-1 domains in DNA cleavage and small RNA function, using miRNA-directed vulval development as a readout of the latter. As expected, DCR-1 catalytic residues were required to rescue both vulval development and DNA fragmentation phenotypes of dcr-1 mutants. However, these rescues could be separated, since a CED-3-resistant dcr-1 transgene rescued only vulval development. On the other hand, a truncated DCR-1 protein mimicking tDCR-1 rescued the appearance of embryonic cell corpses, but could not support normal development. Finally, overexpression of tDCR-1 induced ectopic TUNEL signals even in ced-3 mutants, suggesting that truncated DCR-1 bypasses CED-3 to nick chromosomal DNA. Still, the authors noted that overexpression of tDCR-1 in the ced-3 background was not sufficient to induce ectopic cell death, perhaps due to the action of other CED-3 substrates during apoptosis induction.
Cleavage of DCR-1 by CED-3 should attenuate small RNA processing in apoptotic cells. Although it is not known if mammalian caspases can cleave Dicer, this process might be conserved if it is beneficial to couple reduced miRNA or siRNA production to the initiation of apoptosis. While this possibility deserves study, it will be important to distinguish any putative alternate Dicer pathway from the myriad characterized roles of animal miRNAs in regulating cell death. It is also worth noting that other functions of RNase III enzyme beyond small RNA production have been reported. It was proposed that the helicase domain of Drosophila Dicer-2 serves as a sensor for RNA viruses, independent of small RNA biogenesis (Deddouche et al., 2008). This notion is supported by the close similarity of the Dicer-2 helicase domain to those found in mammalian virus sensors, the RIG-I-like receptors. The DNase activity of DCR-1 might not be unique since the yeast RNase III enzyme Rnt1 can also cleave the DNA strand of DNA-RNA hybrids in vitro (Lamontagne et al., 2004). Curiously, a bacterial homolog of Argonaute proteins, the effector proteins in small regulatory RNA pathways, can cleave DNA targets as well as RNA targets using guide DNA molecules (Wang et al., 2009). It remains to be seen whether eukaryotic Argonautes can cleave DNA strands guided by small RNA/DNA molecules, or whether other RNases or RNA binding proteins might have additional roles in DNA-mediated biological processes. The work of Nakagawa and colleagues is sure to stimulate searches for non-canonical activities of these and other small RNA factors.
REFERENCES
- MacRae IJ, Doudna JA. Curr Opin Struct Biol. 2007;17:138. doi: 10.1016/j.sbi.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Garzon R, Calin GA, Croce CM. Annu Rev Med. 2009;60:167. doi: 10.1146/annurev.med.59.053006.104707. [DOI] [PubMed] [Google Scholar]
- Widlak P, Garrard WT. J Cell Biochem. 2005;94:1078. doi: 10.1002/jcb.20409. [DOI] [PubMed] [Google Scholar]
- Miura M, Zhu H, Rotello R, Hartwieg EA, Yuan J. Cell. 1993;75:653. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- Yuan J, Shaham S, Ledoux S, Ellis HM, Horvitz HR. Cell. 1993;75:641. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- Parrish JZ, Xue D. Chromosoma. 2006;115:89. doi: 10.1007/s00412-005-0038-0. [DOI] [PubMed] [Google Scholar]
- Nakagawa A, Shi Y, Kage-Nakadai E, Mitani S, Xue D. Science. 2010;328:327. doi: 10.1126/science.1182374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deddouche S, et al. Nat Immunol. 2008;9:1425. doi: 10.1038/ni.1664. [DOI] [PubMed] [Google Scholar]
- Lamontagne B, Hannoush RN, Damha MJ, Abou Elela S. J Mol Biol. 2004;338:401. doi: 10.1016/j.jmb.2004.02.059. [DOI] [PubMed] [Google Scholar]
- Wang Y, et al. Nature. 2009;461:754. doi: 10.1038/nature08434. [DOI] [PMC free article] [PubMed] [Google Scholar]