Abstract
Epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) have dramatically changed the prognosis of advanced non-small cell lung cancers (NSCLCs) that harbour specific EGFR activating mutations. However, the efficacy of an EGFR-TKI is limited by the onset of acquired resistance, usually within one year, in virtually all treated patients. Moreover, a small percentage of EGFR-mutant NSCLCs do not respond to an EGFR-TKI, thus displaying primary resistance. At the present time, several mechanisms of either primary and acquired resistance have been elucidated, and new drugs are currently under preclinical and clinical development in order to overcome resistance to treatment. Nevertheless, there still remains much to be thoroughly investigated, as so far research has mainly focused on the role of proteincoding genes involved in resistance to EGFR-TKIs. On the other hand, in line with the data underscoring the relevance of non-coding RNAs in the pathogenesis of lung cancer and modulation of response to systemic therapies, microRNAs (miRNAs) have been supposed to play an important role in resistance to EGFR-TKIs. The aim of this review is to briefly summarise the existing relationship between miRNAs and resistance to EGFR-TKIs, and also focusing on the possible clinical applications of miRNAs in reverting and overcoming such resistance.
Keywords: EGFR mutation, EGFR-TKI, NSCLC, miRNAs, resistance
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide, representing 27.2% of all cancer deaths [1]. As many other neoplasms, lung cancer has been recognised as a group of different diseases, each with its own clinical and biological features. On one hand, non-small cell lung cancer (NSCLC), which accounts for approximately 80% of all lung cancers, on the other, small cell lung cancer (SCLC), which accounts for the rest of cases [2]. Even though enormous improvements have been made in the systemic treatment of advanced NSCLC, particularly for the non-squamous subgroup, the prognosis of these patients is still extremely poor, five-year survival being 5% and 1%, respectively [3]. The reason for this poor outcome should be attributed, at least in part, to the fact that platinum-based chemotherapy represents the standard of care for the majority of patients with advanced disease, irrespective of tumour biology, which does not take into account the intra- and inter-individual heterogeneity of each lung cancer case [4]. Nevertheless, recent breakthroughs in the understanding of NSCLC biology have led to the discovery of specific genetic alterations, such as EGFR mutations, anaplastic lymphoma kinase (ALK), and ROS1 gene rearrangements, each of which identifies a distinct disease entity which has been termed ‘oncogene addicted’ in order to reflect its dependence on a single genetic ‘driver’ for proliferation and survival of cancer cells.
With regard to EGFR-mutant NSCLC, the introduction for therapeutic use of EGFR-TKIs, either reversible, first-generation (gefitinib, erlotinib) or irreversible, second-generation (afatinib) agents, has dramatically improved the prognosis of patients whose tumour harbours this specific genetic alteration (Table 1) [5].
Table 1. Phase III studies in which gefitinib, erlotinib, or afatinib were compared with platinum-based chemotherapy as first-line treatment of patients with advanced NSCLC selected according to the presence of EGFR mutation.
| IPASS [6, 7] | First-SIGNAL [8] | NEJ002 [9, 10] | WJTOG3405 [11, 12] | OPTIMAL [13, 14] | EURTAC [15] | LUX-Lung 3 [16, 17] | LUX-Lung 6 [17, 18] | |
|---|---|---|---|---|---|---|---|---|
| EGFR-TKI | Gefitinib versus carboplatin/paclitaxel | Gefitinib versus cisplatin/gemcitabine | Gefitinib versus carboplatin/paclitaxel | Gefitinib versus cisplatin/docetaxel | Erlotinib versus carboplatin/gemcitabine | Erlotinib versus platinum/based chemotherapy | Afatinib versus cisplatin/pemetrexed | Afatinib versus cisplatin/gemcitabin |
| EGFR mutation | All | Common∞ | All≠ | Common∞ | Common∞ | Common∞ | All | All |
| Population | Asiatic | Asiatic | Asiatic | Asiatic | Asiatic | Caucasian | Mixed | Asiatic |
| RR | 71.2% versus 47.3% P = 0.001 | 84.6% versus 37.5% P = 0.002 | 74.7% versus 30.7% P < 0.001 | 62.1% versus 32.2% P < 0.0001 | 83% versus 36% P < 0.0001 | 58% versus 15% P < 0.05 | 56% versus 44% (independent) 69% versus 44% (investigator) P = 0.001 for both | 65.7% versus 23% (independent) 74.4% versus 31.1% (investigator) P < 0.001 for both |
| PFS | 9.5 versus 6.3 mos (HR 0.48) P < 0.001 | 8 versus 6.3 mos (HR 0.54) P = 0.086 | 10.8 versus 5.4 mos (HR 0.30) P < 0.001 | 9.2 versus 6.3 mos (HR 0.48) P < 0.0001 | 13.1 versus 4.6 mos (HR 0.16) P < 00001 | 9.7 versus 5.2 mos (HR 0.37) P < 0.001 | 11.1 vsersus 6.9 mos (HR 0.58) (all mutations, independent) 13.6 versus 6.9 mos (HR 0.47) (del19/Leu858Arg only, independent) P = 0.001 for both | 11 versus 5.6 mos (HR 0.28) (al mutation, independent) 11 versus 5.6 mos (HR 0.25) (del19/Leu858Arg only, independent) P < 0.0001 for both |
| OS | 21.6 versus 21.9 months, (HR 1.00) P = 0.990 | 27.2 versus 25.6 mos (HR 1.04) P = 0.984 | 27.7 versus 26.6 months (HR 0.88) P = 0.48 | 34.8 versus 37.3 months HR 1.25 P = NR | 22.7 versus 28.9 mos (HR 1.04) P = 0.69 | 19.3 versus 19.5 mos (HR 1.04) P = 0.87 | 28.2 versus 28.2 mos (HR 0.88) P = 0.39 | 23.1 versus 23.5 mos (HR 0.93) P = 0.61 |
mos, months – NR, not reported – OS, overall survival – PFS, progression-free survival – RR, response rate
del19 and L858R
T790M excluded
Presented data are based on investigators’ assessment unless otherwise specified
- EGFR-mutation positive subgroup results; median follow-up for PFS 5.6 months [6]; median follow-up for OS 16 months [7]
- EGFR-mutation positive subgroup results; median follow-up for PFS and OS 35 months
- median follow-up for PFS 17.3 months [9]; median follow-up for OS 23.1 months [10]
- median follow-up for PFS 2.3 months [11]; median follow-up for OS 59.1 months [12]
- median follow-up for PFS 15.6 months [13]; median follw-up for OS 32.4 months [14]
- median follow-up not reported for the whole group of study patients
- PFS by investigator assessment for all mutations 11 versus 6.7 months, hazard ratio (HR) 0.49, P < 0.001; PFS by investigator assessment for del19/Leu858Arg only yielded a HR of 0.41, P = 0.001; median follow-up for PFS 16.4 months [16]; median follow-up for OS 41 months [17]
- PFS by investigator assessment 13.7 versus. 5.6 months, HR 0.28, P < 0.0001; median follow-up for PFS 16.6 months [18]; median follow-up for OS 33 months [17]
In this paper, we will briefly summarise the most well known mechanisms of resistance to EGFR-TKIs. Also, our exploration will go beyond traditional mechanisms of resistance, particularly focusing on the ability of microRNAs (miRNAs) to regulate response to EGFR-TKIs. Finally, the possible clinical applications of miRNAs in escaping resistance to EGFR-TKIs will be discussed.
EGFR mutations and EGFR-TKIs in NSCLC
EGFR (ERBB1/HER1) is a member of a transmembrane receptors tyrosine kinase (TK) superfamily, which also includes ERBB2/HER2, ERBB3/HER3, and ERBB4/HER4 [19]. Similarly to other members, EGFR is characterised by an extracellular ligand-binding region, a single transmembrane region, and an intracytoplasmic region with TK activity [20]. When an activating somatic mutation occurs in the EGFR-TK domain, EGFR undergoes ligand-independent homo/hetero-dimerisation with another receptor of the same family. This leads to conformational changes in the three-dimensional structure of EGFR that promote ATP-mediated autophosphorylation of the TK domain, and subsequent receptor activation [21, 22]. As a consequence, multiple intracellular signalling cascades are implemented, such as RAS/RAF/MEK/ERK, JAK/STAT, and PI3K/AKT/mTOR, all of which result into intensification of pro-survival and anti-apoptotic signalling [19, 22].
Interestingly, although EGFR mutations are more commonly associated with specific clinicopathological features such as female gender, Asian ethnicity (where they can be found in up to 30% of advanced NSCLCs as opposed to 15% for the western population), non-smoking history, and adenocarcinoma histology [23], it is not possible to rule out the possibility of an EGFR mutation solely on the basis of the aforementioned characteristics. Also, only activating mutations that affect the TK domain (exons 18→21) are those who predict exquisite sensitivity to treatment with an EGFR-TKI, since EGFR-TKIs and ATP compete for binding to the same pocket on the EGFR-TK domain [24].
EGFR activating mutations include in frame deletions, in frame duplications/insertions, and point mutations [Murray 2008]. Specifically, the most represented and better characterised are the so called classic mutations, which include in frame deletions in exon 19 in correspondence of the LeuArgGluAla sequence (E746-A750), and the exon 21 point mutation Leu858Arg (L858R), together representing 85%–90% of all EGFR mutations in NSCLC [25]. On the other hand, a number of ‘uncommon’ mutations has been reported, such as G719X in exon 18 (G719C, G719S, G719A), L861Q in exon 21, and S768I in exon 20, whose predictive role to treatment with an EGFR-TKI is much less defined. Nevertheless, a growing body of evidence seems to confirm a positive predictive role of these uncommon mutations to treatment with an EGFR-TKI [26–28], although to a less extent than classic mutations [29]. Conversely, exon 20 in frame insertion as well as a de novo T790M point mutation in exon 20 are associated with primary resistance to EGFR-TKIs [26–28].
Recently, six phase III randomised trials comparing either a reversible (gefitinib or erlotinib) or irreversible (afatinib) EGFR-TKI versus platinum-based chemotherapy in patients with untreated EGFR-mutant advanced NSCLC showed superiority for the EGFR-TKI in terms of response rate (RR) and progression free survival (PFS) (Table 1) [6–18], as well as quality of life (QOL) [6, 13, 30]. These results corroborated further those of the earlier IPASS and first-SIGNAL trials, in which gefitinib was compared head-to-head with platinum-based chemotherapy in a population enriched for the presence of an EGFR mutation, such as East Asian and never/light smoker patients (Table 1) [6, 8]. In both studies gefitinib demonstrated a significant increase in RR and PFS only for the EGFR-mutant subgroup, whereas EGFR wild type patients were found to benefit more from standard chemotherapy than gefitinib. Importantly, although first-line afatinib significantly improved OS compared to chemotherapy in patients with EGFR Del19 mutations in two randomised trials, LUX-Lung 3 and LUX-Lung 6, there was no significant difference in OS of patients with EGFR L858R mutation treated with afatinib compared to chemotherapy, both individually and in pooled data [31]. These findings suggest that patients harbouring EGFR Del19 and L858R mutations may represent two separate populations and further studies are required to address this issue.
Unfortunately, despite the impressive activity of EGFR-TKIs in EGFR-mutant NSCLCs, virtually all patients would undergo resistance to treatment, usually within one year of treatment initiation. Against this background, it is of primary importance to understand the molecular mechanisms underlying resistance to EGFR-TKIs in order to develop novel therapeutic strategies aimed at improving further the prognosis of EGFR-mutant NSCLCs.
‘Traditional mechanisms’ of primary and acquired resistance to EGFR-TKIs
Currently, 20–30% of EGFR-mutant NSCLC patients do not undergo tumour shrinkage on treatment with an EGFR-TKI, thus identifying a primary resistant cohort. Furthermore, virtually all patients who have experienced either an objective response or prolonged disease stabilisation (≥ six months) on an EGFR-TKI will eventually develop resistance to the drug, which identifies the acquired resistant cohort [32].
With regard to primary resistance, exon 20 insertions have been invariably associated with lack of response to EGFR-TKIs, as suggested by numerous clinical studies [26–28, 33]. Also, de novo T790M point mutation in exon 20 identifies a group of patients who perform poorly on EGFR-TKIs. Although considered rather uncommon, highly sensitive method based on laser microdissection and peptide nucleic acid (PNA)-clamping polymerase chain reaction (PCR) recently unveiled an unexpected prevalence of de novo T790M substitution in patients with primary resistance to EGFR TKIs (2%–9%) [34–35]. This mutation, which is actually the major determinant of acquired resistance, is worthy of note and will be handled further along. Moreover, various alterations in the EGFR pathway may affect patients with NSCLC, thus conditioning response to EGFR-TKIs. More in detail, loss of phosphatase and tensin homolog PTEN expression, which is an onco-suppressor gene involved in the inhibition of PI3K/AKT/mTOR pathway, as well as PIK3CA activating mutations have both been reported in vitro as inducers of primary resistance to gefitinib and erlotinib through the blockade of EGFR-TKI-induced apoptosis in EGFR-mutant cell lines [34, 39]. In addition, BIM deletion polymorphism has been found to induce resistance to EGFR-TKIs [40]. BIM is a member of the BCL-2 pro-apoptotic proteins family, which is involved in Bax/Bak-mediated cytochrome c release and apoptosis. Of note, the relevance of BIM levels in influencing clinical response to EGFR-TKIs is supported by the results of EURTAC study, which randomised EGFR-mutant patients to erlotinib or platinum-based chemotherapy [15]. Interestingly, the erlotinibtreated patients who had low/intermediate mRNA levels of BIM expression experienced a lower PFS compared with those who had high levels of BIM expression [41]. Conversely, no differences in PFS were observed in the chemotherapy arm according to mRNA levels of BIM.
Currently, approximately 60% of cases of acquired resistance appear to be associated with the presence of a secondary missense mutation termed T790M. Such a mutation is characterised by the replacement of a threonine with a methionine at codon 790 of exon 20 of the EGFR gene, in a site that affects the catalytic adenosine 5’ triphosphate (ATP) binding pocket of the EGFR-TK domain [42]. That is because T790M mutation restores the binding affinity between the EGFR-TK domain and ATP, thus identifying what has been called the ‘gatekeeper’ mutation [43]. Of note, Maheswaran et al have shown that in some EGFR-mutation positive NSCLCs who develop T790M-mediated acquired resistance, this mutation could actually be detected at baseline in minor clones with the use of highly sensitive techniques, thus becoming dominant during exposure to EGFR-TKIs as a result of selective pressure induced by treatment with an EGFR-TKI [44]. Nevertheless, a few other EGFR secondary point mutations have been involved in acquired resistance to an EGFR-TKI, namely D716Y (exon 19), L747S (exon 19), and T854A (exon 21) [45–47]. In addition, acquired resistance might be because of the presence of other mutant signalling proteins (e.g. PI3CKA mutation, HER-2 amplification, BRAF mutation) as well as to the activation of EGFR signalling pathways via other aberrant molecules. Among the latter mechanism, the most relevant is certainly MET gene amplification, as it drives roughly 5–10% of cases of acquired resistance to EGFR-TKIs [42]. MET encodes for a transmembrane TK receptor implicated in acquired resistance through the activation of the PI3K/AKT/mTOR pathway via interaction with ERBB3 [48]. Not surprisingly, hepatocyte growth factor (HGF), which is the only known ligand of MET receptor, has also been implicated both in primary and acquired resistance to EGFR-TKIs [49–51]. Finally, in a minority of cases some other additional mechanism may lead to the development of acquired resistance such as phenotypic changes in the tumour. This can be because of SCLC transformation as well as to epithelial-to-mesenchymal transition (EMT) [52–53]. Of note, cells displaying SCLC features still harbour a drug-sensitive EGFR mutation. On the other hand, an increasing body of evidence describes the molecular machinery involved in EMT, which includes a variety of phenotypic and molecular changes (e.g. loss of tight junction and cell adhesion, induction of a mesenchymal phenotype) responsible for the increased aggressiveness and invasiveness of the tumour [54]. Cells with EMT are associated with loss of E-cadherin and β-catenin, though they gain the expression of vimentin and N-cadherin. At a molecular level, NOTCH-1 upregulation, transforming growth factor TGF-β overexpression, activation of MET and AXL pathways have been identified as major determinants of EMT-induced acquired resistance to EGFR-TKIs [55–58]. Even though EGFR mutations are largely homogeneous, mechanisms of resistance are more heterogeneous. Multiple mechanisms of resistance can be detected in a single tumour specimen, and different mechanisms of resistance can emerge from different tumour sites in the same patient, indicating the polyclonal nature of TKIs resistance. For instance, by examining various progressive lesions in an autopsy case from a patient with acquired resistance to EGFR TKI, Balak and colleagues revealed that the seven different sites all harboured the exon 19 deletion, conversely the T790M mutation was found in six out of the seven sites, but was not detected in the brain metastasis, despite the use of highly sensitive procedure [45]. In addition, a recent large series study designed to assess prospectively the frequency of various mechanism of resistance in 155 patients revealed that 98 had a second-site EGFR T790M mutations (63%) and four had small cell transformation, whereas MET and HER-2 amplification were reported in four patients and three patients respectively. Even though acquired mutations in PIK3CA, AKT1, BRAF, ERBB2, KRAS, MEK1, and NRAS were not detected, the coexistence of different mechanisms of acquired resistance was observed in 4% of patients [59].
miRNAs and resistance to EGFR-TKIs
MiRNAs represent a class of 18–25 nucleotides in length, single stranded, endogenous and evolutionarily conserved small non-coding RNAs. They bind protein-coding mRNAs subsequently leading to mRNAs degradation, storage, and translational inhibition [60]. Although miRNAs account for barely 1%–2% of the human genome, they actually regulate and supervise the activity of roughly 50% of all protein-coding genes [61]. Currently, a number of miRNAs has been described which may have a specific role in lung cancer pathogenesis, biological and clinical disease behaviour as well as in modulating response to anticancer treatments, particularly EGFR-TKIs (Table 2) [60–63].
Table 2. Main miRNAs involved in EGFR TKIs resistance.
| miRNA | Genomic location | Target genes relevant for NSCLC | Expression in TKIs resistant NSCLC | Mechanism of TKIs resistance |
|---|---|---|---|---|
| miR-21 | 17q23.2 | PTEN, PDCD4, SPRY/2, APAF1, FASLG, RHOB | Upregulated | PTEN suppression PI3K/Akt pathway stimulation |
| miR-214 | 1q24.3 | PTEN, CADM1 | Upregulated | PTEN suppression CADM1 suppression |
| miR-23a/24/27a | 19p13.12 | CDH1 | Upregulated | TGF-β1-induced EMT Loss of E-cadhein |
| miR-221/222 | Xp11.3 | TRAIL, CDKN1B, PTEN, TIMP3, PUMA, BIM, APAF1 | Upregulated | Escape from TRAIL-induced apoptosis |
| miR-134, miR-487b, miR-655 | 14q32.31 | MAGI2 | Upregulated | TGF-β1-induced EMT Loss of PTEN function |
| miR-200 a/b miR-200c | 1p36.33 12p13.31 | ZEB1/2, FLT1, GATA3, KRAS, MAPK | Downregulated | TGF-β1-induced EMT EGFR-independent Akt activation |
| miR-126, miR-128b | 9q34.3, 2q21.3 | VEGF, CRK, SLC7A5, EGFR | Downregulated | Akt/Erk1/2 activation Angiogenesis |
| miR-103 a/b miR-203 | 5q34/20p13 14q32.33 | SRC, PKC, GSK3β, AKT, ERKs | Downregulated | AKT/ERKs pathway stimulation EMT induction through SRC and PKC-ε upregulation |
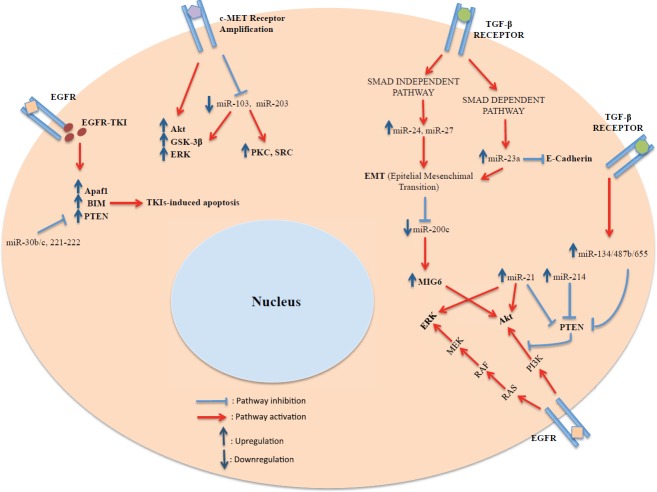
Located on chromosome 17q23.1, miR-21 is one of the most investigated miRNAs. Several studies reported its upregulation in NSCLC, and delineated its involvement in promoting lung cancer cell growth and invasion (Figure 1) [64–66]. Recently, Li et al demonstrated that miR-21 expression levels are higher in the EGFR-mutant (delE746-A750) and EGFR-TKI resistant NSCLC cell line PC9GR, inversely correlating with PTEN and PDCD4 expression [67]. On the other hand, miR-21 was significantly associated with the activation of the PI3K/Akt pathway. Notably, the same investigators found that miR-21 serum levels in EGFR-mutant NSCLC patients treated with an EGFR-TKI were significantly higher at the time of the onset of acquired resistance compared with baseline [67]. Additionally, in human EGFR-mutant NSCLC cell lines, miR-21 has been found to downregulate PTEN expression, a mechanism that could help promote lung carcinogenesis. MiR-21 expression has also been shown to associate with poor response and shorter overall survival (OS) in patients undergoing treatment with an EGFR-TKI [68]. Consistently, miR-21 overexpression decreased gefitinib sensitivity through PTEN downregulation and AKT/ERK pathway activation, whereas miR-21 knockdown restored gefitinib sensitivity through PTEN upregulation and AKT/ERK repression.
Figure 1. Schematic diagram of the relationship existing between miRNAs and resistance to EGFR-TKIs at a cellular level.
Similarly to miR-21, also miR-214 has been involved in acquired resistance to gefitinib through PTEN and PI3K/AKT pathway. More in details, Wang et al obtained gefitinib resistant cell line HCC827/GR through the exposure of normal HCC827 cells (NSCLC cell line with 746E-750A EGFR gene deletion) to increasingly larger concentrations of gefitinib and subsequently found that miR-214 was significantly overexpressed in HCC827/GR, also inversely correlating with the levels of PTEN expression [69]. Furthermore, not only miR-214 knockdown restored PTEN mRNA levels, but also was associated with p-Akt inactivation. Taken together, these data suggest that both miR-21 and miR-214 drive acquired resistance to gefitinib through the downregulation of PTEN expression, and therefore activation of the PI3K/AKT pathway independently of EGFR.
As previously mentioned, EMT represents an important determinant of clinical response to treatment with an EGFR-TKI, which has attracted much of an interest in miRNAs involved in the EMT phenomenon. Among them, miR-23a, miR-24, and miR-27a is a miRNAs cluster located on chromosome 19p13.12 with profound oncogenic properties in several human cancers. Cao et al showed that miR-23a is induced by the TGF-β1/Smad pathway in EGFR-WT A549 lung adenocarcinoma cells with the EMT phenomenon, whereas miR-24 and miR-27a are induced by a Smad-independent mechanism in the same cell lines [70]. These findings suggest that the activation of the TGF-β pathway contributes to EMT also through miR-23a/24/27a upregulation. In support of these data, there is evidence that miR-23a overexpression strengthens EMT and suppresses E-cadherin expression, whereas miR-23a knockdown restores E-cadherin expression [70]. Therefore, since miR-23a overexpression regulates TGF-β1-induced EMT and consequently EMT-related acquired resistance to gefitinib, miR-23a may be a new therapeutic target in both EGFR WT and EGFR-mutant NSCLC patients resistant to EGFR-TKIs.
Still on EMT, the miR-200 family has been found to be downregulated during TGF-β1-induced EMT, leading to mitogen-inducible gene 6 (MIG6) upregulation. MIG6 is a negative regulator of EGFR and its overexpression in TGF-β1-induced EMT changes the molecular profile of NSCLC cells to an AKT-activated/EGFR-independent state. Izumchenko et al showed that expression levels of MIG6 and miR-200 were significantly correlated with EMT, as well as resistance to erlotinib in 25 cancer cell lines originating from different tissues; also the MIG6 mRNA/miR-200 ratio was inversely correlated with response to erlotinib in EGFR wild-type NSCLC patients [71]. In another study, Kitamura et al identified three other miRNAs, namely miR-134, miR-487b, and miR-655, all induced after exposure to TGF-β1 in lung adenocarcinoma cells with EMT [72]. Notably, the upregulation of these mi-RNAs results in loss of PTEN function, as one of the predicted targets of this cluster of miRNAs is the protein MAGI2, which is an essential scaffold protein for the proper functioning of PTEN. Overall, miR-134/487b/655 cluster promotes TGF-β1-induced EMT phenomenon, thus conditioning the development of ETM-driven acquired resistance to EGFR-TKIs [72].
Other authors examined EGFR and MET mediated changes of miRNAs in NSCLC cell lines. More in details, they showed that gefitinib treatment reduces miR-30b/c and miR-221/222 expression levels, consequently leading to the upregulation of APAF and BIM, which are both involved in determining EGFR-TKIs-induced apoptosis in sensitive EGFR-mutant, HCC827, and PC9 NSCLC cell lines [73–74]. Conversely, miR-30b/c and miR-221/222 downregulation was not observed after gefitinib treatment in HCC827/GR and PC9GR resistant clones of gefitinib hypersensitive EGFR exon 19 mutant HCC827 and PC9 NSCLC cell lines [74]. The same authors also showed that MET activation reduces expression levels of miR-103 and miR-203, which normally function as oncosuppressor miRNAs through the inhibition of SRC and PKC activity. Additionally, miR-103 and miR-203 were found to be significantly upregulated in MET knockdown Calu-1 NSCLC cells, whereas their overexpression was strongly related to reduced phosphorylation of Akt, GSK3β, and ERKs. This might suggest that MET amplification and overexpression may drive acquired resistance to EGFR-TKIs by enhancing AKT/ERKs pathway even through miR-103 and miR-203 regulation [74]. Taken together, these studies indicate that miR-30b/c and miR-221/222 upregulation in NSCLC determine resistance to EGFR-TKIs through the repression of APAF-1, BIM, and PTEN, while miR-103 and miR-203 induce apoptosis in EGFR-TKI resistant cells, also promoting mesenchymal to epithelial transition, by downregulating PKC and SRC.
miRNAs and new insights in overcoming resistance to EGFR-TKIs
Since miRNAs are involved in resistance to EGFR-TKIs, several studies explored the role of miRNAs in overcoming the mechanisms of resistance, with encouraging preclinical results. Zhou et al analysed miR-130a expression levels in H1975, A549, PC9 gefitinib-sensitive and -resistant NSCLC cell lines treated with different doses of gefitinib [75]. Interestingly, miR-130a levels were significantly lower in the gefitinib-resistant cell lines compared with the gefitinib-sensitive cell lines. Conversely, c-Met mRNA levels were higher in gefitinib-resistant than in gefitinib-sensitive cell lines. Even more importantly, it was demonstrated that transfection of miR-130a mimics could reverse resistance to gefitinib by downregulating c-Met protein levels via direct targeting of the c-Met 3’-UTR, which provides evidence for a new therapeutic strategy in reverting resistance to EGFR-TKIs [75]. Similarly, Acunzo et al highlighted the role of miRNAs in affecting c-Met [76]. More in details, they demonstrated that miR-130a downregulates c-Met, also reducing miR-221 and miR-222 expression. These two miRNAs have already been reported to be upregulated by c-Met activation/amplification, and are involved in cell proliferation, cell growth, and resistance to the TNF-related-apoptosis inducing ligand (TRAIL) protein in NSCLC [76–77]. Therefore, miR-130a overexpression could actually enhance apoptosis in NSCLC cells, and further studies should explore the possibility of a combined therapy with both miR-130a mimics and gefitinib, as both have been shown to revert resistance to TRAIL in human cancers [77].
More recently, Zhou et al also demonstrated that the forced expression of another miRNA, namely miR-34a, can inhibit cell growth and induce apoptosis in HGF-induced gefitinib-resistant HCC827 and PC-9 NSCLC cell lines via targeting c-Met [78]. Interestingly, the same study reported massive tumour regression with miR-34a plus gefitinib in mouse xenograft models of HGF-induced gefitinib-resistant tumours. The development of miR-34 as replacement therapy in EGFR-TKI resistant patients is a topic of growing interest, especially in light of its involvement in targeting c-Met and its oncogenic pathways [79]. Consistently, miR-34 has been found to inversely correlate with c-Met mRNA levels in lung, glioblastoma multiforme, and ovarian cancer [78–79]. Also, miR-34 involvement in the p53 tumour suppression pathway has been well described [80]. The importance of miR-34 in cancer development was further strengthened by Kasinski and Slack who evaluated the feasibility of the delivery of miR-34 lentivirus in order to prevent cancer initiation and progression in a therapeutically resistant mouse model of lung adenocarcinoma harbouring KRAS LSL-G12D/+ and p53 LSL-R172H/+ mutations [81]. Notably, miR-34 restoration induced cell-cycle arrest, apoptosis, and cellular senescence by targeting c-Met, c-myc, and the antiapoptotic protein BCL-2. These findings suggest that miR-34 replacement therapy may restore sensitivity to biological treatments in those patients in which the resistance to EGFR-TKIs is because of the amplification of MET. Moreover, miR-34, which is also able to suppress KRAS-driven cancer progression, could be of great benefit in patients whose acquired resistance depends on KRAS mutation. Finally, Zhao et al showed that miR-34 mimics enhance erlotinib sensitivity in NSCLC cell lines carrying both primary and acquired resistance to EGFR-TKIs [82], and two further studies confirmed that miR-34a might overcome resistance to EGFR-TKIs by targeting MET and AXL, which are both involved in erlotinib resistance [83–84].
More recently, Gao et al reported that miR-138-5 is deeply downregulated in gefitinib-resistant PC9 (harbouring delE746-A750) NSCLC cell lines, while its re-expression sensitise PC9/GR cells and another gefitinib-resistant EGFR-mutated NSCLC cell line, H1975 (harbouring both L858R/T790M mutations), to gefitinib. [85]. This phenomenon, which is mediated by G protein-coupled receptor 124, suggests that miR-138-5 replacement could be a further therapeutic approach in reversing resistance to EGFR-TKIs.
In addition, very attractive results have been obtained by Rai et al who reported that the injection of miR-7–expressing plasmid produces a dramatic growth arrest in either EGFR-mutated TKI-sensitive cell lines (PC9 and H3255) or EGFR TKI-resistant cell lines harbouring T790M mutation (R-PC9 and H1975) [86]. Importantly, no significant aberrant growth suppression was observed into A549 cells carrying wild-type EGFR and K-RAS mutations, thus highlighting that the oncogene addiction of EGFR plays a critical role in miR-7 efficacy.
As previously mentioned, EMT represents an important mechanism of acquired resistance to EGFR TKIs and different studies have shown that this process undergoes a precise regulation by specific miRNAs [54]. As a result, the possibility of reverting this process, leading to mesenchymal-to-epithelial transition through the use of miRNAs, was explored. To this regard, Lee et al found that miR-147 transfection in colon cancer (HCT116, SW480) and lung cancer (A-549) cell lines inhibited cell proliferation, cell migration, and reversed EMT to mesenchymal-to-epithelial transition through CDH1 upregulation and ZEB1 downregulation, thus markedly reestablishing gefitinib sensitivity [87].
Not surprisingly, also members of miRNAs biosynthetic machinery, such as the RNAse III enzyme Dicer, may be involved in TKIs resistance, as was proven by Chen et al who showed that Dicer expression levels are significantly higher in PC9/GR resistant cells than in PC9 cells and that Dicer knockdown restores gefitinib sensitivity in resistant cells through miR-30b/miR-30c and miR-221/miR-222 downregulation [88].
Conclusion and perspectives
Ever since their discovery, EGFR mutations have represented a major step forward in the management of NSCLC patients with this genetic alteration as they were associated with exquisite sensitivity to treatment with an EGFR-TKI. However, no patient was cured, and despite initial response in most of EGFR-mutant patients, resistance to treatment would eventually emerge. The drugs so far developed in order to overcome such resistance, including but not limited to T790M and c-Met inhibitors have been aimed at targeting only protein-coding genes implicated in resistance to treatment. Nevertheless, we also need to explore the universe of non-coding RNAs more thoroughly. In this setting, miRNAs appear to play a crucial role in regulating lung cancer carcinogenesis, sensitivity to chemotherapy, TKIs, as well as radiotherapy [89]. Moreover, their role as diagnostic, prognostic, and predictive biomarkers has been extensively investigated and it is an emerging topic in oncology. Certainly, their clinical use in restoring sensitivity to EGFR-TKIs is corroborated by robust preclinical evidence, which is an issue that needs to be investigated further in clinical trials. In order to achieve this, we must face the challenges regarding the use of miRNAs for NSCLC treatment (e.g. difficulties in identifying the appropriate methods of miRNAs delivery, determining the optimal therapeutic regimen, confirming their safety in humans), with the ultimate goal to benefit our patients.
Conflict of interests
The authors declare no conflict of interests.
Acknowledgments
Supported by the Italian Association for Cancer Research (AIRC).
References
- 1.Siegel R, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD. The 2015 WHO classification of lung tumors. Pathologe. 2014;35(Suppl 2):188. doi: 10.1007/s00292-014-1974-3. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society. Non-small cell lung cancer survival rates by stage. [January 1, 2015]. Available at: http://cancer.org/cancer/lungcancer-non-smallcell/detailedguide/non-small-cell-lung-cancer-survival-rates.
- 4.Reck M, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(suppl 3):iii27–39. doi: 10.1093/annonc/mdu199. [DOI] [PubMed] [Google Scholar]
- 5.Metro G, Crinò L. Advances on EGFR mutation for lung cancer. Transl Lung Cancer Res. 2012;1(1):5–13. doi: 10.3978/j.issn.2218-6751.2011.12.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mok TS, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 7.Fukuoka M, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS) J Clin Oncol. 2011;29(21):2866–74. doi: 10.1200/JCO.2010.33.4235. [DOI] [PubMed] [Google Scholar]
- 8.Han JY, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30(10):1122–8. doi: 10.1200/JCO.2011.36.8456. [DOI] [PubMed] [Google Scholar]
- 9.Maemondo M, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–8. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 10.Inoue A, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naïve non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002) Ann Oncol. 2013;24(1):54–9. doi: 10.1093/annonc/mds214. [DOI] [PubMed] [Google Scholar]
- 11.Mitsudomi T, et al. Gefitinib versus cisplatin plus docetaxel in patient with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 11(2):121–8. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 12.Yoshioka H, et al. Final overall survival results of WJTOG 3405, a randomized phase 3 trial comparing gefitinib (G) with cisplatin plus docetaxel (CD) as the first-line treatment for patients with non-small cell lung cancer (NSCLC) harboring mutations of the epidermal growth factor receptor (EGFR) J Clin Oncol. 2014;32(suppl 5; abstr 8117) [Google Scholar]
- 13.Zhou C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–42. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 14.Zhou C, et al. Overall survival (OS) results from OPTIMAL (CTONG0802), a phase III trial of erlotinib (E) versus carboplatin plus gemcitabine (GC) as first-line treatment for Chinese patients with EGFR mutation-positive advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2012;30(suppl; abstr 7520) [Google Scholar]
- 15.Rosell R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–46. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 16.Sequist LV, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–34. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 17.Yang JC, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16(2):141–51. doi: 10.1016/S1470-2045(14)71173-8. [DOI] [PubMed] [Google Scholar]
- 18.Wu YL, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(2):213–22. doi: 10.1016/S1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]
- 19.Mitsudomi T, Yatabe Y. Epidermal growth factor receptor in relation to tumor development: EGFR gene and cancer. FEBS J. 2010;277(2):301–8. doi: 10.1111/j.1742-4658.2009.07448.x. [DOI] [PubMed] [Google Scholar]
- 20.Sibilia M, et al. The epidermal growth factor receptor: from development to tumorigenesis. Differentiation. 2007;75(9):770–87. doi: 10.1111/j.1432-0436.2007.00238.x. [DOI] [PubMed] [Google Scholar]
- 21.Garrett TP, et al. Crystal structure of a truncated epidermal growth factor receptor extracellular domain bound to transforming growth factor alpha. Cell. 2002;110(6):763–73. doi: 10.1016/S0092-8674(02)00940-6. [DOI] [PubMed] [Google Scholar]
- 22.Burgess AW. EGFR family: structure physiology signalling and therapeutic targets. Growth Factors. 2008;26(5):263–74. doi: 10.1080/08977190802312844. [DOI] [PubMed] [Google Scholar]
- 23.Mitsudomi T, Kosaka T, Yatabe Y. Biological and clinical implications of EGFR mutations in lung cancer. Int J Clin Oncol. 2006;11(3):190–20. doi: 10.1007/s10147-006-0583-4. [DOI] [PubMed] [Google Scholar]
- 24.Sharma SV, et al. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7(3):169–81. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 25.Murray S, et al. Somatic mutations of the tyrosine kinase domain of the epidermal growth factor receptor and tyrosine kinase inhibitor response to TKIs in non-small cell lung cancer: an analytical database. J Thorac Oncol. 2008;3(8):832–9. doi: 10.1097/JTO.0b013e31818071f3. [DOI] [PubMed] [Google Scholar]
- 26.Wu JY, et al. Effectiveness of tyrosine kinase inhibitors on «uncommon» epidermal growth factor receptor mutations of unknown clinical significance in non-small cell lung cancer. Clin Cancer Res. 2011;17(11):3812–21. doi: 10.1158/1078-0432.CCR-10-3408. [DOI] [PubMed] [Google Scholar]
- 27.De Pas T, et al. Activity of epidermal growth factor receptor-tyrosine kinase inhibitors in patients with non-small cell lung cancer harbouring rare epidermal growth factor receptor mutations. J Thorac Oncol. 2011;6(11):1895–901. doi: 10.1097/JTO.0b013e318227e8c6. [DOI] [PubMed] [Google Scholar]
- 28.Yang JC, et al. Activity of afatinib in uncommon epidermal growth factor receptor (EGFR) mutations: findings from three trials of afatinib in EGFR mutation-positive lung cancer. Oral presentation at: World Congress on Lung Cancer. 2013.
- 29.Chiu CH, et al. Epidermal growth factor receptor tyrosine kinase inhibitor treatment response in advanced lung adenocarcinomas with G719X/L861Q/S768I mutations. J Thorac Oncol. 2015;10(5):793–9. doi: 10.1097/JTO.0000000000000504. [DOI] [PubMed] [Google Scholar]
- 30.Yang JC, et al. Symptom control and quality of life in LUX-Lung 3: a phase III study of afatinib or cisplatin/pemetrexed in patients with advanced lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3342–50. doi: 10.1200/JCO.2012.46.1764. [DOI] [PubMed] [Google Scholar]
- 31.Yang JC-H, et al. Overall survival (OS) in patients (pts) with advanced non-small cell lung cancer (NSCLC) harboring common (Del19/L858R) epidermal growth factor receptor mutations (EGFR mut): pooled analysis of two large open-label phase III studies (LUX-Lung 3 [LL3] and LUX-Lung 6 [LL6]) comparing afatinib with chemotherapy (CT) J Clin Oncol. 2014;32(Suppl) abstr 8004. [Google Scholar]
- 32.Jackman D, et al. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol. 2010;28(2):357–60. doi: 10.1200/JCO.2009.24.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu JY, et al. Lung cancer with epidermal growth factor receptor exon 20 mutations is associated with poor gefitinib gefitinib tretment response. Clin Cancer Res. 2008;14(15):4877–82. doi: 10.1158/1078-0432.CCR-07-5123. [DOI] [PubMed] [Google Scholar]
- 34.Su KY, et al. Pretreatment epidermal growth factor receptor (EGFR) T790M mutation predicts shorter EGFR tyrosine kinase inhibitor response duration in patients with non-small-cell lung cancer. J Clin Oncol. 2012;30(4):433–40. doi: 10.1200/JCO.2011.38.3224. [DOI] [PubMed] [Google Scholar]
- 35.Costa C, et al. The impact of EGFR T790M mutations and BIM mRNA expression on outcome in patients with EGFR-mutant NSCLC treated with erlotinib or chemotherapy in the randomized phase III EURTAC trial. Clin Cancer Res. 2014;20(7):2001–10. doi: 10.1158/1078-0432.CCR-13-2233. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto C, et al. Loss of PTEN expression by blocking nuclear translocation of EGR1 in gefitinib-resistant lung cancer cells harboring epidermal growth factor receptor-activating mutations. Cancer Res. 2010;70(21):8715–25. doi: 10.1158/0008-5472.CAN-10-0043. [DOI] [PubMed] [Google Scholar]
- 37.Sos ML, et al. PTEN loss contributes to erlotinib resistance in EGFR-mutant lung cancer by activation of Akt and EGFR. Cancer Res. 2009;69(8):3256–61. doi: 10.1158/0008-5472.CAN-08-4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L, et al. PIK3CA mutations frequently coexist with EGFR/KRAS mutations in non-small cell lung cancer and suggest poor prognosis in EGFR/KRAS wildtype subgroup. PLoS One. 2014;9(2):e88291. doi: 10.1371/journal.pone.0088291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engelman JA, et al. Allelic dilution obscures detection of a biologically significant resistance mutation in EGFR-amplified lung cancer. J Clin Invest. 2006;116(10):2695–706. doi: 10.1172/JCI28656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ng KP, et al. A common BIM deletion polymorphism mediates intrinsic resistance and inferior responses to tyrosine kinase inhibitors in cancer. Nat Med. 2012;18(4):521–8. doi: 10.1038/nm.2713. [DOI] [PubMed] [Google Scholar]
- 41.Costa C, et al. The impact of EGFR T790M mutations and BIM mRNA expression on outcome in patients with EGFR-mutant NSCLC treated with erlotinib or chemotherapy in the randomized phase III EURTAC trial. Clin Cancer Res. 2014;20(7):2001–10. doi: 10.1158/1078-0432.CCR-13-2233. [DOI] [PubMed] [Google Scholar]
- 42.Ohashi K, et al. Epidermal growth factor receptor tyrosine kinase inhibitor-resistant disease. J Clin Oncol. 2013;31(8):1070–80. doi: 10.1200/JCO.2012.43.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yun CH, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci USA. 2008;105(6):2070–5. doi: 10.1073/pnas.0709662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maheswaran S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008;359(4):366–77. doi: 10.1056/NEJMoa0800668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balak MN, et al. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res. 2006;12(21):6494–501. doi: 10.1158/1078-0432.CCR-06-1570. [DOI] [PubMed] [Google Scholar]
- 46.Bean J, et al. ) Acquired resistance to epidermal growth factor receptor kinase inhibitors associated with a novel T854A mutation in a patient with EGFR-mutant lung adenocarcinoma. Clin Cancer Res. 2008;14(22):7519–25. doi: 10.1158/1078-0432.CCR-08-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen KS, Kobayashi S, Costa DB. Acquired Resistance to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Non–Small-Cell Lung Cancers Dependent on the Epidermal Growth Factor Receptor Pathway. Clin Lung Cancer. 2009;10(4):281–9. doi: 10.3816/CLC.2009.n.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Engelman JA, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316(5827):1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 49.Yano S, et al. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res. 2008;68(22):9479–87. doi: 10.1158/0008-5472.CAN-08-1643. [DOI] [PubMed] [Google Scholar]
- 50.Yano S, et al. Hepatocyte growth factor expression in EGFR mutant lung cancer with intrinsic and acquired resistance to tyrosine kinase inhibitors in a Japanese cohort. J Thorac Oncol. 2011;6(12):2011–7. doi: 10.1097/JTO.0b013e31823ab0dd. [DOI] [PubMed] [Google Scholar]
- 51.Turke AB, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 2010;17(1):77–88. doi: 10.1016/j.ccr.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sequist LV, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3(75):75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu HA, et al. Analysis of tumour specimens at the time of acquired resistance to EGFR TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240–7. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7(2):131–42. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 55.Suda K, et al. Epithelial to mesenchymal transition in an epidermal growth factor receptor mutant lung cancer cell line with acquired resistance to erlotinib. J Thorac Oncol. 2011;6(7):1152–61. doi: 10.1097/JTO.0b013e318216ee52. [DOI] [PubMed] [Google Scholar]
- 56.Zavadil J, Bottinger EP. TGF-β and epithelial-to-mesenchymal transitions. Oncogene. 2005;24(37):5764–74. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 57.Xie M, et al. Activation of Notch-1 enhances epithelial-mesenchymal transition in gefitinib acquired resistant lung cancer cells. J Cell Biochem. 2012;113(5):1501–13. doi: 10.1002/jcb.24019. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Z, et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet. 2012;44(8):852–60. doi: 10.1038/ng.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu HA, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240–7. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin PY, Yu SL, Yang PC. MicroRNA in lung cancer. Br J Cancer. 2010;103(8):1144–8. doi: 10.1038/sj.bjc.6605901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11(9):597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 62.Qi J, Mu D. MicroRNAs and lung cancers: from pathogenesis to clinical implications. Front Med. 2012;6(2):134–55. doi: 10.1007/s11684-012-0188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grignani F, et al. Correlation of circulating miRNA levels with progression-free survival (PFS) and overall survival (OS) in early-stage lung adenocarcinoma. J Clin Oncol. 2014;32(suppl 5; abstr 11099) [Google Scholar]
- 64.Pan X, Wang ZX, Wang R. MicroRNA-21 A novel therapeutic target in human cancer. Cancer Biol Ther. 2010;10(12):1224–32. doi: 10.4161/cbt.10.12.14252. [DOI] [PubMed] [Google Scholar]
- 65.Frezzetti D, et al. Upregulationof miR-21 by Ras in vivo and its role in tumor growth. Oncogene. 2011;30(3):275–86. doi: 10.1038/onc.2010.416. [DOI] [PubMed] [Google Scholar]
- 66.Seike M, et al. MiR-21 is an EGFR-regulated antiapoptotic factor in lung cancer in never-smokers. Proc Natl Acad Sci U S A. 2009;106(29):12085–90. doi: 10.1073/pnas.0905234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li B, et al. MiR-21 overexpression is associated with acquired resistance of EGFR-TKI in non-small cell lung cancer. Lung Cancer. 2014;83(2):146–53. doi: 10.1016/j.lungcan.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 68.Shen H, et al. Alteration in Mir-21/PTEN Expression Modulates Gefitinib Resistance in Non-Small Cell Lung Cancer. PLoS One. 2014;9(7):e103305. doi: 10.1371/journal.pone.0103305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang YS, et al. MicroRNA-214 regulates the acquired resistance to gefitinib via the PTEN/AKT pathway in EGFR-mutant cell lines. Asian Pac J Cancer Prev. 2012;13(1):255–60. doi: 10.7314/APJCP.2012.13.1.255. [DOI] [PubMed] [Google Scholar]
- 70.Cao M, et al. MiR-23a regulates TGF-beta-induced epithelial-mesenchymal transition by targeting E-cadherin in lung cancer cells. Int J Oncol. 2012;41(3):869–75. doi: 10.3892/ijo.2012.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Izumchenko E, et al. The TGFβ-miR200-MIG6 pathway orchestrates the EMT-associated kinase switch that induces resistance to EGFR inhibitors. Cancer Res. 2014;74(14):3995–4005. doi: 10.1158/0008-5472.CAN-14-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kitamura K, et al. MiR-134/487b/655 cluster regulates TGF-β-induced epithelial-mesenchymal transition and drug resistance to gefitinib by targeting MAGI2 in lung adenocarcinoma cells. Mol Cancer Ther. 2014;13(2):444–53. doi: 10.1158/1535-7163.MCT-13-0448. [DOI] [PubMed] [Google Scholar]
- 73.Cragg MS, et al. Gefitinib-induced killing of NSCLC cell lines expressing mutant EGFR requires BIM and can be enhanced by BH3 mimetics. PLoS Med. 2007;4(10):1681–9. doi: 10.1371/journal.pmed.0040316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garofalo M, et al. EGFR and MET receptor tyrosine kinase-altered microRNA expression induces tumorigenesis and gefitinib resistance in lung cancers. Nat Med. 2011;18(1):74–82. doi: 10.1038/nm.2577. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 75.Zhou YM, Liu J, Sun W. MiR-130a Overcomes Gefitinib Resistance by Targeting Met in Non-Small Cell Lung Cancer Cell Lines. Asian Pac J Cancer Prev. 2014;15(3):1391–6. doi: 10.7314/APJCP.2014.15.3.1391. [DOI] [PubMed] [Google Scholar]
- 76.Acunzo M, et al. miR-130a targets MET and induces TRAIL-sensitivity in NSCLC by downregulating miR-221 and 222. Oncogene. 2012;31(5):634–42. doi: 10.1038/onc.2011.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garofalo M, et al. miR-221&222 regulate TRAIL resistance and enhance tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell. 2009;16(6):498–509. doi: 10.1016/j.ccr.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 78.Zhou JY, et al. MicroRNA-34a overcomes HGF-mediated gefitinib resistance in EGFR mutant lung cancer cells partly by targeting MET. Cancer Lett. 2014;351(2):265–71. doi: 10.1016/j.canlet.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 79.Gomez GG, Wykosky J, Zanca C. Therapeutic resistance in cancer: microRNA regulation of EGFR signaling networks. Cancer Biol Med. 2013;10(4):192–205. doi: 10.7497/j.issn.2095-3941.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Raver-Shapira N, et al. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol Cell. 2007;26(5):731–43. doi: 10.1016/j.molcel.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 81.Kasinski AL, Slack FJ. miRNA-34 prevents cancer initiation and progression in a therapeutically resistant K-ras and p53- induced mouse model of lung adenocarcinoma. Cancer Res. 2012;72(21):5576–87. doi: 10.1158/0008-5472.CAN-12-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao J, Kelnar K, Bader AG. In-depth analysis shows synergy between erlotinib and miR-34a. PLoS One. 2014;9(2):e89105. doi: 10.1371/journal.pone.0089105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mudduluru G, et al. Regulation of Axl receptor tyrosine kinase expression by miR-34a and miR-199a/b in solid cancer. Oncogene. 2011;30(25):2888–99. doi: 10.1038/onc.2011.13. [DOI] [PubMed] [Google Scholar]
- 84.Kaller M, et al. Genome-wide characterization of miR-34a induced changes in protein and mRNA expression by a combined pulsed SILAC and microarray analysis. Mol Cell Proteomics. 2011;10(8) doi: 10.1074/mcp.M111.010462. M111.010462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gao Y, et al. miR-138-5p reverses gefitinib resistance in non-small cell lung cancer cells via negatively regulating G protein-coupled receptor 124. Biochem Biophys Res Commun. 2014;446(1):179–86. doi: 10.1016/j.bbrc.2014.02.073. [DOI] [PubMed] [Google Scholar]
- 86.Rai K, et al. Liposomal delivery of MicroRNA-7-expressing plasmid overcomes epidermal growth factor receptor tyrosine kinase inhibitor-resistance in lung cancer cells. Mol Cancer Ther. 2011;10(9):1720–7. doi: 10.1158/1535-7163.MCT-11-0220. [DOI] [PubMed] [Google Scholar]
- 87.Lee CG, et al. MicroRNA-147 induces a mesenchymal-to-epithelial transition (MET) and reverses EGFR inhibitor resistance. PLoS One. 2014;9(1):e84597. doi: 10.1371/journal.pone.0084597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen JC, et al. Suppression of dicer increases sensitivity to gefitinib in human lung cancer cells. Ann Surg Oncol. 2014;21(Suppl 4):555–63. doi: 10.1245/s10434-014-3673-y. [DOI] [PubMed] [Google Scholar]
- 89.Ricciuti B, et al. Non-coding RNAs in lung cancer. Oncoscience. 2014;1(11):674–705. doi: 10.18632/oncoscience.98. [DOI] [PMC free article] [PubMed] [Google Scholar]