Abstract
Background and Objectives
There have been few large population-based studies of the association between rheumatoid arthritis (RA) and chronic kidney disease (CKD) and glomerulonephritis. This nationwide cohort study investigated the risks of developing CKD and glomerulonephritis in patients with RA, and the associated risks for cardiovascular complications.
Methods
From the Taiwan National Health Insurance Research Database, we identified a study cohort of 12,579 patients with RA and randomly selected 37,737 subjects without RA as a control cohort. Each subject was individually followed for up for 5 years, and the risk of CKD was analyzed using Cox proportional hazards regression models.
Results
During the follow-up period, after adjusting for traditional cardiovascular risk factors RA was independently associated with a significantly increased risk of CKD (adjusted hazard ratio [aHR] 1.31; 95% confidence interval [CI] 1.23–1.40) and glomerulonephritis (aHR 1.55; 95% CI 1.37–1.76). Increased risk of CKD was also associated with the use of non-steroidal anti-inflammatory drugs, cyclosporine, glucocorticoids, mycophenolate mofetil, and cyclophosphamide. Patients with comorbidities had even greater increased risk of CKD. Moreover, RA patients with concurrent CKD had significantly higher likelihood of developing ischemic heart disease and stroke.
Conclusions
RA patients had higher risk of developing CKD and glomerulonephritis, independent of traditional cardiovascular risk factors. Their increased risk of CKD may be attributed to glomerulonephritis, chronic inflammation, comorbidities, and renal toxicity of antirheumatic drugs. Careful monitoring of renal function in RA patients and tight control of their comorbid diseases and cardiovascular risk factors are warranted.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease that affects many body tissues and leads to joint destruction and other major morbidity and mortality. In particular, previous reports have indicated that patients with RA also have considerable incidence of renal disease. Specifically, there is accruing evidence that a substantial proportion of patients with early RA have proteinuria, hematuria and renal dysfunction [1,2].
Renal disease in RA is clinically important because it not only restricts the management of primary disease, but also increases mortality. In one study, RA patients with renal disease had significantly increased mortality compared to those with normal renal function, with a hazard ratio (HR) of 2.77–4.45 [3]. Other investigators have shown that subjects hospitalized for RA were significantly more likely to die from renal failure than the general population: HR 3.1 (95% confidence interval [CI]: 2.5–3.9) for males, and HR 3.5 (95% CI: 3.0–4.0) for females [2,4]. Autopsy findings in patients with RA have shown that renal failure is a major cause of death in 3–20% of cases [5,6].
However, previous studies evaluated a variety of kidney disorders or used different criteria to define renal abnormality in RA. Further major limitations were small sample sizes, cross-sectional design, sampling frame at consecutive times from a single rheumatology clinic, lack of a comparison group, and short follow-up, all of which make it difficult to determine the true magnitude of risk and potentially limit the generalizability of their results. Few studies have specifically investigated the chronic kidney disease (CKD) in patients with RA and despite evidence that Asians have higher prevalence of CKD than Caucasians, such research focusing on Asian subjects is lacking [7]. Furthermore, as treatment patterns for RA have changed over the years, the true incidence of CKD may be different nowadays and remains unclear. This study was conducted to determine the risk of CKD and glomerulonephritis (GN) and the associated risk for cardiovascular (CV) complications in a nationally-representative cohort of patients with RA from Taiwan.
Materials and Methods
Dataset
This retrospective cohort study used data from the Taiwan Longitudinal Health Insurance Database (LHID) 2005, which is a subset of the National Health Insurance Research Database (NHIRD). NHIRD data are compiled from the Taiwan National Health Insurance (NHI) system, which was launched in 1995 to finance health care for all citizens and provides care for approximately 99% of the Taiwanese population of more than 23 million. In the LHID 2005, approximately 1,000,000 representative individuals were randomly sampled from among all of those in NHIRD that year. The database includes inpatient care, outpatient care, ambulatory care and prescription drugs from 1 January 2000 through 31 December 2010. A multistage stratified systematic sampling design was used and there were no statistically significant differences in gender, age, or average insured payroll-related amount, between the LHID sample and the whole NHIRD population [8]. The disease diagnoses used in our study were coded using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM). The local Investigational Research Bureau approved the study (HCH 103-024-E).
Study Population
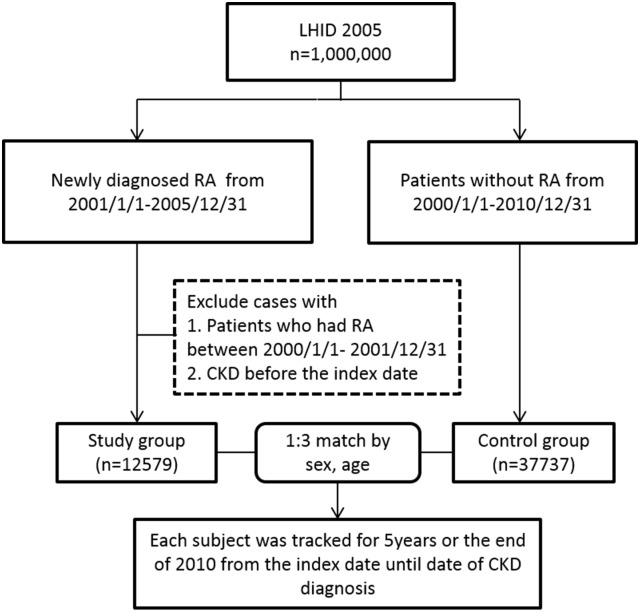
The RA study cohort comprised 12,579 patients newly diagnosed with RA (ICD-9-CM 714.0, 714.2, and 720.0) between 1 January 2001, and 31 December 2005. The initial diagnosis date was defined as the index date of entry into the RA cohort. Patients who were younger than 18 or who had CKD within 1 year before the index date were excluded. A control cohort of 37,737 subjects who had not been diagnosed with RA from year 2000 through 2010, were selected to match each RA patient for gender and age.
Study Outcomes
The primary study outcomes were new-onset CKD (ICD-9-CM 580, 581, 582, 583, 584, 585, 586, 587, 588, 589, 753, 403, 404, 2504, 2741, 4401, 4421, 4473, 5724, 6421, 6462) and GN (ICD-9-CM codes: 580.0, 580.1, 580.2, 580.3, 580.4, 580.9, 590.81, 582.0, 582.1, 582.4, 582.81, 582.89, 582.9, 583.0, 583.1, 583.2, 583.4, and 583.9); final-stage CKD (end-stage renal disease [ESRD]) (ICD-9-CM 585) was the secondary outcome. Patients were followed-up for 5 years from the index date or until the development of CKD (Fig 1). Comorbidities included diabetes mellitus (ICD-9-CM 250.xx), hypertension (ICD-9-CM 362.11, 401–405, and 437.2), hyperlipidemia (ICD-9-CM 272.x), CV disease (ICD-9-CM 410–429), and obesity (ICD-9-CM 278.0x).
Fig 1. Study cohort.
Subject flow for study cohort.
Statistical Analysis
SPSS software version 19.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. A two-sided p-value <0.05 determined statistical significance. Microsoft SQL Server 2008 software was used for data management and analysis.
Pearson’s chi-square was used to compare the distributions of demographic characteristics between patients with and without RA, and for evaluating differences between categorical variables. Cox proportional hazard regression was performed to estimate the HR and 95% CI of RA associated with CKD. The covariate-adjusted HR was analyzed after adjusting for significant factors (p-value <0.05).
Results
Table 1 shows the baseline distributions of demographic characteristics and clinical features, such as comorbidities and medication use, that occurred throughout the study period among patients with and without RA. There were no significant differences between RA patients and controls in gender or age. However, several comorbidities were significantly more prevalent among RA patients than controls; these included diabetes, hypertension, hyperlipidemia, CV disease, and obesity (all p <0.001). Compared with RA patients, a significantly lower proportion of controls were prescribed glucocorticoids, disease modifying antirheumatic drugs (DMARDs), non-steroidal anti-inflammatory drugs (NSAIDs) and biologics; however, some control patients also received short-term and infrequent glucocorticoids or NSAIDs for common ailments, such as upper respiratory infections, lower back pain and skin allergy (S1 Table).
Table 1. Gender, age, urbanization, geography comorbidity, and medications distributions among individuals with and without rheumatoid arthritis.
| Variable | Numbers (proportions) of individuals | P-value | |
|---|---|---|---|
| Patients with RA(N = 12579) | Patients without RA(N = 37737) | ||
| Sex | 1.00 | ||
| Female | 7580 (60.3%) | 22740 (60.3%) | |
| Male | 4999 (39.7%) | 14997 (39.7%) | |
| Age (years) | 1 | ||
| 18–29 | 1693 (13.5%) | 5079 (13.5%) | |
| 30–39 | 1982 (15.8%) | 5946 (15.8%) | |
| 40–49 | 2889 (23.0%) | 8667 (23.0%) | |
| 50–59 | 2535 (20.2%) | 7605 (20.2%) | |
| 60–69 | 1901 (15.1%) | 5703 (15.1%) | |
| ≥70 | 1579 (12.6%) | 4737 (12.6%) | |
| Income a | <0.001 | ||
| <18,000 | 5269 (41.9%) | 16923 (44.8%) | |
| 18,000–34,000 | 5465 (43.4%) | 15567 (41.3%) | |
| ≥35,000 | 1845 (14.7%) | 5247 (13.9%) | |
| Urbanization | 0.22 | ||
| Provinces | 3232 (25.7%) | 10024 (26.6%) | |
| Counties | 998 (7.9%) | 2975 (7.9%) | |
| Districts | 3255 (25.9%) | 9770 (25.9%) | |
| Urban villages | 5094 (40.5%) | 14968 (39.7%) | |
| Geography | <0.001 | ||
| North | 6510 (51.8%) | 18787 (49.8%) | |
| Central | 2161 (17.2%) | 6686 (17.7%) | |
| South | 3488 (27.7%) | 11357 (30.1%) | |
| East | 420 (3.3%) | 907 (2.4%) | |
| Comorbidity | |||
| Hypertension | 4756 (37.8%) | 12197 (32.3%) | <0.001 |
| Diabetes | 2441 (19.4%) | 5863 (15.5%) | <0.001 |
| Cardiovascular disease | 3999 (31.8%) | 8581 (22.7%) | <0.001 |
| Hyperlipidemia | 3683 (29.3%) | 8530 (22.6%) | <0.001 |
| Obesity | 166 (1.3%) | 296 (0.8%) | <0.001 |
| Drug | |||
| Glucocorticoids | 6055 (48.1%) | 10639 (28.2%) | <0.001 |
| Methotrexate | 615 (4.9%) | 74 (0.2%) | <0.001 |
| Cyclosporine | 99 (0.8%) | 13 (0.0%) | <0.001 |
| Azathioprine | 105 (0.8%) | 29 (0.1%) | <0.001 |
| Mycophenolate Mofetil | 7 (0.1%) | 7 (0.0%) | <0.05 |
| Hydroxyurea | 3 (0.0%) | 15 (0.0%) | 0.41 |
| Cyclophosphamide | 49 (0.4%) | 90 (0.2%) | <0.05 |
| Sulfasalazine | 1453 (11.6%) | 81 (0.2%) | <0.001 |
| Leflunomide | 53 (0.4%) | 4 (0.0%) | <0.001 |
| Penicillamine | 34 (0.3%) | 8 (0.0%) | <0.001 |
| Hydroxychloroquine | 1181 (9.4%) | 160 (0.4%) | <0.001 |
| NSAIDs | 12007 (95.5%) | 28583 (75.7%) | <0.001 |
| Etanercept | 33 (0.3%) | 1 (0.0%) | <0.001 |
| Adalimumab | 12 (0.1%) | 0 (0.0%) | <0.001 |
NSAIDs, non-steroidal anti-inflammatory drugs; RA, rheumatoid arthritis.
a New Taiwan dollars
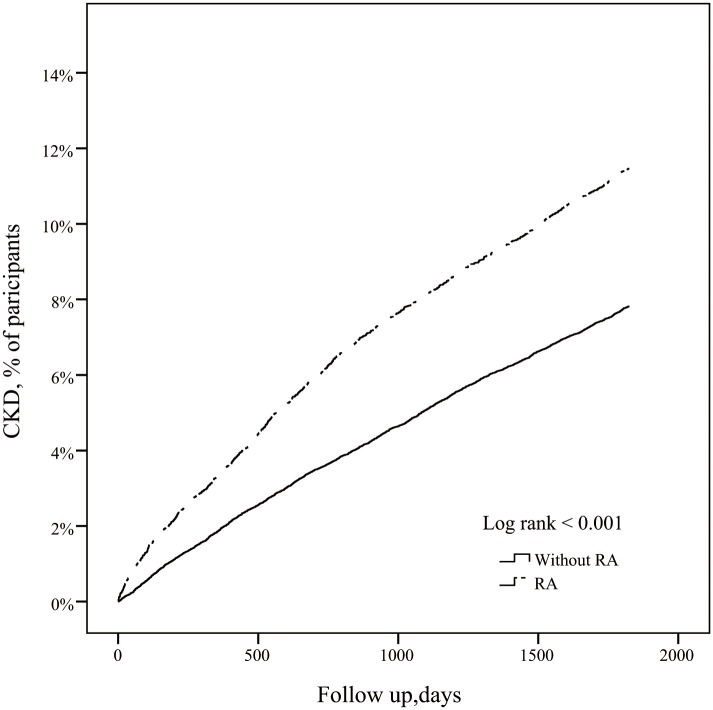
Table 2 shows the incidence and HRs of CKD, GN and ESRD among patients with RA and without. Of 12,759 patients with RA, 1442 (11.5%), 166 (1.3%) and 379 (3.0%) patients respectively, developed CKD, ESRD and GN during the 5 year follow-up period. After adjusting for monthly income, age, gender, urbanization, medication, geography, and comorbidities, the adjusted HR (aHR) among patients with RA versus controls was 1.31 (95% CI 1.23–1.40, p <0.001) for CKD, 1.08 (95% CI 0.90–1.29, p = 0.43) for final- stage CKD (ESRD) and 1.55 (95% CI, 1.37–1.76, p <0.001) for GN. Fig 2 shows Kaplan-Meier survival curves for time to CKD occurrence for RA versus controls. Stratified by age, the aHRs for CKD, GN and ESRD increased with age and were highest in the oldest adults among both RA patients and controls. Moreover, the aHRs for CKD were higher in subjects with comorbidities than those without.
Table 2. Incidence rates and hazard ratios for CKD, ESRD, and GN in patients with rheumatoid arthritis.
| Variable | CKD | ESRD | GN | |||
|---|---|---|---|---|---|---|
| Crude HR (95% CI) | Adjusted HR* (95% CI) | Crude HR (95% CI) | Adjusted HR* (95% CI) | Crude HR (95% CI) | Adjusted HR* (95% CI) | |
| Rheumatoid arthritis | 1.50 (1.41–1.60) ‡ | 1.31 (1.23 to 1.4) ‡ | 1.22 (1.02–1.46) † | 1.08(0.90–1.29) | 1.74 (1.53–1.974) ‡ | 1.55(1.37–1.76) ‡ |
| Incidence rate a | 18.34 | 2.44 | 4.38 | |||
| Gender | ||||||
| Female | 1.00 | ND | 1.00 | 1.00 | 1.00 | 1.00 |
| Male | 1.01 (0.95–1.07) | ND | 1.23 (1.05–1.45) † | 1.47(1.25–1.74) ‡ | 0.87 (0.77–0.99) † | 1.01(0.89–1.15) |
| Age (years) | ||||||
| 18–29 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 30–39 | 1.45 (1.19–1.76) ‡ | 1.27(1.05–1.54) † | 1.85 (0.96–3.58) | 1.75(0.91–3.40) | 1.24 (0.87–1.77) | 1.15(0.80–1.63) |
| 40–49 | 2.44 (2.06–2.89) ‡ | 1.57(1.32–1.87) ‡ | 3.03 (1.67–5.5) ‡ | 2.23(1.22–4.07) ‡ | 2.08 (1.52–2.82) ‡ | 1.55(1.13–2.12) † |
| 50–59 | 4.09 (3.47–4.82) ‡ | 1.83(1.54–2.17) ‡ | 5.82 (3.27–10.34) ‡ | 3.15(1.74–5.69) ‡ | 3.10 (2.30–4.19) ‡ | 1.76(1.29–2.42) ‡ |
| 60–69 | 6.24 (5.3–7.35) ‡ | 2.12(1.78–2.52) ‡ | 11.1 (6.3–19.56) ‡ | 4.49(2.48–8.10) ‡ | 4.45 (3.30–6.00) ‡ | 2.03(1.47–2.80) ‡ |
| ≥70 | 8.8 (7.48–10.36) ‡ | 2.82(2.37–3.37) ‡ | 19.6 (11.2–34.31) ‡ | 7.08(3.93–12.75) ‡ | 5.82 (4.32–7.83) ‡ | 2.53(1.83–3.50) ‡ |
| Comorbidity | ||||||
| Hypertension | 4.37 (4.1–4.66) ‡ | 1.81(1.68–1.96) ‡ | 5.11 (4.28–6.11) ‡ | 1.73(1.40–2.15) ‡ | 3.02 (2.67–3.42) ‡ | 1.34(1.15–1.56) ‡ |
| Diabetes | 4.23 (3.98–4.49) ‡ | 2.04(1.91–2.18) ‡ | 4.57 (3.88–5.38) ‡ | 2.16(1.81–2.59) ‡ | 2.86 (2.52–3.26) ‡ | 1.47(1.28–1.69) ‡ |
| Cardiovascular disease | 3.51 (3.31–3.72) ‡ | 1.60(1.50–1.71) ‡ | 4.1 (3.48–4.83) ‡ | 1.70(1.42–2.05) ‡ | 3.19 (2.83–3.60) ‡ | 1.74(1.52–2.00) ‡ |
| Hyperlipidemia | 3.3 (3.11–3.5) ‡ | 1.57(1.47–1.68) ‡ | 2.82 (2.39–3.31) ‡ | 1.28(1.07–1.53) ‡ | 2.87 (2.54–3.23) ‡ | 1.63(1.42–1.87) ‡ |
| Obesity | 1.81 (1.43–2.3) ‡ | 1.16(0.91–1.47) | 1.76 (0.91–3.39) † | 1.39(0.71–2.69) ‡ | 1.40 (0.81–2.43) | N/A |
* Each variable was adjusted for every other variable listed for which crude HR was significant (p <0.05), and also for income
† p<0.05 for comparison between patients with versus without rheumatoid arthritis.
‡ p<0.001 for comparison between patients with versus without rheumatoid arthritis.
a Incidence rate: per 1000 person-years.
CI, confidence interval; CKD, chronic kidney disease; ESRD, end-stage renal disease; GN, glomerulonephritis; HR, hazard ratio; N/A, not applicable; NSAID, non-steroidal anti-inflammatory drug; OR, odds ratio; ND, not done.
Fig 2. Kaplan-Meier survival curves.
Kaplan-Meier survival curves for time to occurrence of chronic kidney disease among patients with and without rheumatoid arthritis.
Table 3 shows the risks of CKD among RA patients treated with various medications. Adjusted for gender, age, comorbidities, and every other drug listed that showed significant crude HR, patients who received glucocorticoids (p<0.001), mycophenolate mofetil (p<0.05), cyclophosphamide (p<0.05) or NSAIDs (p<0.05) had significantly increased risk of CKD. The overall OR for risk of CKD conferred by DMARDs was 1.22 (p<0.05). Moreover, frequent NSAID users had significantly higher likelihood of developing CKD than infrequent users and non-users.
Table 3. Risks of CKD among rheumatoid arthritis patients treated with medications.
| Medication* | RA with CKD | RA without CKD | Crude OR (95% CI) | Adjusted OR**(95% CI) |
|---|---|---|---|---|
| Glucocorticoids | 888 | 5167 | 1.85 (1.66–2.07) ‡ | 1.49 (1.32–1.68) ‡ |
| Non-users | 554 | 5967 | 1 | 1.00 (ND) |
| Infrequent users a | 650 | 4137 | 1.64 (1.47–1.84) ‡ | 1.40 (1.25–1.57) ‡ |
| Frequent users b | 238 | 1033 | 2.33 (2.01–2.72) ‡ | 1.75 (1.47–2.07) ‡ |
| DMARDs | 264 | 1924 | 1.07 (0.93–1.24) | 1.22 (1.02–1.45) † |
| Methotrexate | 73 | 542 | 1.04 (0.81–1.34) † | 1.07 (0.82–1.41) |
| Azathioprine | 20 | 85 | 2.81 (1.48–5.30) † | 1.74 (0.99–3.05) |
| Mycophenolate mofetil | 4 | 3 | 1.83 (1.12–2.99) † | 10.58 (2.13–52.54) † |
| Hydroxyurea | 2 | 1 | 10.32 (2.31–46.17) † | 13.16 (0.96–180.65) |
| Cyclophosphamide | 13 | 36 | 15.47 (1.40–170.68) | 2.57 (1.27–5.20) † |
| Sulfasalazine | 164 | 1289 | 0.98 (0.83–1.17) | 1.23 (0.99–1.51) |
| Leflunomide | 10 | 43 | 1.80 (0.90–3.59) | 1.87 (0.88–399.00) |
| Penicillamine | 5 | 29 | 1.33 (0.52–3.45) | 0.97 (0.35–2.65) |
| Hydroxychloroquine | 155 | 1026 | 1.19 (0.99–1.42) | 1.15 (0.93–1.43) |
| Gold | 0 | 0 | N/A | N/A |
| Cyclosporine | 17 | 82 | 1.61 (0.95–2.72) | 1.54 (0.84–2.82) |
| Non-users | 1425 | 11055 | 1.00 | 1.00 (ND) |
| Infrequent users a | 7 | 19 | 2.86 (1.20–6.81) † | 3.01(1.18–7.71) † |
| Frequent users b | 10 | 63 | 1.23 (0.63–2.41) | 1.09 (0.51–2.35) |
| NSAIDs | 1418 | 10589 | 3.06 (2.02–4.62) ‡ | 1.68 (1.10–2.56) † |
| Non-users | 24 | 548 | 1.00 | 1.00 (ND) |
| Infrequent users a | 444 | 5678 | 1.79 (1.17–2.72) † | 1.45 (0.95–2.22) |
| Frequent users b | 974 | 4911 | 4.53 (2.99–6.85) ‡ | 2.01 (1.31–3.09) ‡ |
| Biologics | 5 | 40 | 0.97 (0.38–2.45) | 0.86 (0.32–2.34) |
† p <0.05 for comparison between patients with rheumatoid arthritis and without rheumatoid arthritis.
‡ p <0.001 for comparison between patients with rheumatoid arthritis and without rheumatoid arthritis.
* Rheumatoid arthritis patients with one or more drug prescription during 5-year follow-up
** Adjusted for gender, age group, comorbidities and every other listed drug for which crude hazard ratio was significant (p <0.05).
CI, confidence interval; CKD, chronic kidney disease; N/A, not applicable; NSAID, non-steroidal anti-inflammatory drug; OR, odds ratio; RA, rheumatoid arthritis; ND, not done.
a Prescribed <90 days.
b Prescribed ≥90 days.
Table 4 shows the comparative risks of CV complications in RA patients with and without CKD. Adjusted for gender, age, diabetes, hypertension, and hyperlipidemia, RA patients with versus without CKD had significantly increased risks of ischemic heart disease (p<0.001) and stroke (p<0.05).
Table 4. The risk of cardiovascular complications in rheumatoid arthritis patients with versus without CKD.
| Variable | RA with CKD | RA without CKD | Crude HR | Adjusted HR* |
|---|---|---|---|---|
| Ischemic heart disease | 590 | 2119 | 2.95 (2.63–3.31) ‡ | 1.57 (1.38–1.79) ‡ |
| Stroke | 351 | 1274 | 2.49 (2.18–2.85) ‡ | 1.24 (1.06–1.43) † |
† p <0.05 for comparison between rheumatoid arthritis patients with versus without.
‡ p <0.001 for comparison between rheumatoid arthritis patients with versus without.
*Adjusted for gender, age group, diabetes, hypertension, and hyperlipidemia.
CI, confidence interval; CKD, chronic kidney disease; HR, hazard ratio; RA, Rheumatoid arthritis.
Discussion
This national population-based cohort study of more than 12, 000 RA patients who were followed for up to 5 years, not only affords generalizability in comparing the incidence of CKD in patients with versus without RA, but also makes it possible to assess the temporal relationship between RA and CKD.
The reported prevalence of kidney disease in patients with RA ranges from 5–50%, reflecting wide variations in the diagnostic criteria and definitions of renal disease, and different study designs [1,5,9,10]. In a cross-sectional population-based cohort study of 604 Finnish patients with RA, 17% had evidence of nephropathy (defined as hematuria, proteinuria, or kidney failure) [11]. The prevalence of kidney disease among 129 consecutive RA patients in a more recent study was 46.3% as measured by the Modification of Diet in Renal Disease formula, and 57% according to Cockcroft-Gault formula [2]. Another recent study of 350 consecutive RA patients in England found that 53% had mild renal impairment, with glomerular filtration rate (GFR) of 60–90 mL/min/1.73 m2, and 13% had moderate renal impairment, with GFR below 60 mL/min/1.73m2 [12]. Among another 400 RA patients, 11.5% had GFR below 60, and only four had severe renal impairment (GFR <30) [13]. However, specific data on the incidence of CKD in Asian patients with RA are lacking.
The cause of renal disease in RA patients is contentious, and may be attributable to nephrotoxic pharmacotherapies, secondary renal diseases induced by amyloidosis and/or GN, and associated comorbidities [5,13]. The etiologic role of chronic inflammation has also been highlighted [14,15,16,17]. Accumulating data reveal that CKD is more prevalent in patients with chronic inflammatory disorders, such as psoriasis [17,18] and ankylosing spondylitis [19], compared to the general population. Systemic inflammation may contribute to progressive loss of kidney function and anti-inflammatory drugs, such as TNF-α antagonists, have therapeutic potential in preventing CKD progression in RA [20,21,22]. Concerning comorbidities in CKD, Daoussis and coworkers showed renal dysfunction to be strongly associated with classic CV risk factors [13]. Likewise, our analysis found that diabetes, hypertension, hyperlipidemia, and CV disease were associated with the development of CKD in RA. Moreover, our results demonstrate that RA is associated with increased risk of CKD independently of traditional CV risk factors. In a recent study, excess weight rather than hypertension and diabetes appeared to be more strongly associated with CKD in RA [9]; however, we did not find a significant association between obesity and CKD after adjustment, probably due to ethnic differences or relatively lower prevalence of obesity in Asian populations.
Previous reports suggested that RA may be complicated by renal disease secondary to GN. Renal biopsies in 158 Japanese and 110 Finnish RA patients with clinical renal diseases, suggested that mesangial GN is the most frequent type of GN (34–36%), followed by membranous GN [23,24]. Other types of GN, including rapidly progressive GN, minimal change glomerulopathy, and immunoglobulin A nephropathy have also been reported in RA [1,2,23,24]. Since GN may progress to CKD and CKD is diagnosed based on abnormal urinalysis and GFR results, irrespective of the specific cause, we further investigated the relationship among GN, ESRD and RA. Our patients with RA were at higher risk of both GN and ESRD than controls. The increased risk of CKD may be partly attributed to higher incidence of GN in patients with RA.
Anti-inflammatory drugs have also been implicated in causing renal disease in RA patients [1,9,25,26]. Likewise, we found that use of NSAIDs was associated with the development of CKD in patients with RA in a dose-dependent manner. Also consistent with earlier studies, cyclosporine [26,27] and cyclophosphamide [28,29] were associated with nephrotoxicity that would lead to CKD in patients with RA. However, our data showed a higher likelihood of developing CKD in infrequent versus frequent cyclosporine users. Further analysis showed that infrequent users are older than frequent users, and the probable explanation for our result is that physicians may avoid prescribing frequent cyclosporine in elderly patients with RA who tend to be more likely to progress to CKD (S2 Table). Our observation that glucocorticoids, mycophenolate mofetil and cyclophosphamide were associated with increased risk of CKD might be because prescription of these drugs indicates higher disease activity or presence of comorbidity, resulting in rapidly declining renal function. Similarly, Hickson and coworkers found corticosteroids to be associated with increased risk of estimated GFR (eGFR) <45 mL/min/1.73 m2 in RA [9]. Nevertheless, the small sample size in the mycophenolate mofetil and cyclophosphamide subgroups may be confounding.
Patients with RA have higher CV mortality than non-RA controls, independent of traditional CV risk factors, has been underestimated [30]. Moreover, hematuria, proteinuria, or CKD are associated with three- to four-fold higher mortality in patients with RA [3]. A recent study showed that in RA, renal dysfunction is associated with a higher risk of CV disease, independently of traditional CV risk factors [31]. However, the small number of CV events, confounding variables, and the lack of healthy control subjects limited the generalizability of these results [32].
Study Strengths and Limitations
NHIRD data are generally accurate and reliable because the Taiwan NHI Bureau performs regular cross-checks and imposes heavy fines for false claims, overcharging, or malpractice. Earlier reports have affirmed the reliability of performing epidemiological research using the NHIRD LHID [33,34,35], and also that of the specific ICD-9-CM codes used to assess CKD outcomes [36,37,38].
By using a large national cohort with a longitudinal design and adjusting for several confounders, we have been able to affirm that CKD in RA patients is significantly associated with increased risk of ischemic heart disease and stroke, independent of traditional CV risk factors. Our findings concur with others which showed that CKD was independently associated with atherosclerosis and endothelial activation in RA, suggesting that endothelial dysfunction is central to the pathogenesis of CV complications in RA patients with CKD [39].
Our study had limitations. First, diagnoses of CKD, GN and ESRD were based entirely on secondary claim data, rather than eGFR. Thus, we were unable to stratify the risk of CKD in RA by CKD stage. However, others have documented the similarity of CKD diagnosis based on large administrative data sets and on eGFR [18,36]. Second, we have no renal biopsy pathology data. Third, because NHIRD lacks a reliable severity index for RA, we did not correlate disease activity with the prevalence of CKD. Although we were unable to adjust for potential unmeasured confounders, previous research suggests that such factors contribute far less to CKD than traditional risk factors, namely aging, diabetes, and hypertension [40], which were included in our analysis.
Conclusions
This national cohort study demonstrates that the risks of CKD, GN and ESRD are significantly higher in patients with RA than the general population. The development of CKD in patients with RA is multifactorial and may result from several ongoing processes, including primary or secondary renal involvement associated with RA (eg, GN), chronic inflammation, comorbidities, and nephrotoxic antirheumatic drugs. Moreover, concurrent CKD disease predicts CV complications in RA patients, independent of other risk factors. Physicians should monitor the renal function of patients with RA regularly and intervene to tightly control CV risk factors and the progression of CKD, particularly in patients who are older, NSAID users, or have comorbidities.
Supporting Information
(DOCX)
(DOCX)
Acknowledgments
This study is based in part on data from the Taiwan National Health Insurance Research Database, which is provided by the National Health Insurance Administration, Ministry of Health and Welfare, and managed by National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of the National Health Insurance Administration, Ministry of Health and Welfare, or National Health Research Institutes. This work was funded by Cathay General Hospital (Gant number:MR-A10324), National Science Council of Taiwan under the contract number MOST-103-2221-E-009-117-, and "Center for Bioinformatics Research of Aiming for the Top University Program" of the National Chiao Tung University and Ministry of Education, Taiwan, R.O.C. for the project 104W962. This work was also supported in part by UST-UCSD International Center of Excellence in Advanced Bioengineering sponsored by the Ministry of Science and Technology with I-RiCE Program under Grant Number: MOST 103-2911-I-009-101.-The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank Dr. David Neil (PhD), of Content Ed Net Taiwan Ltd., for professional medical editing services.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was funded by the Cathay General Hospital (Grant number: MR-A10324; http://www.cgh.org.tw/index.html), the National Science Council of Taiwan under contract number MOST-103-2221-E-009-117-, and the Center forBioinformatics Research of Aiming for the Top University Program of the National Chiao Tung University and Ministry of Education, Taiwan, R.O.C. for the project 104W962. This work was also supported in part by UST-UCSD International Center of Excellence in Advanced Bioengineering, sponsored by the Ministry of Science and Technology with I-RiCE Program under Grant Number: MOST 103-2911-I-009-101. 2. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Koseki Y, Terai C, Moriguchi M, Uesato M, Kamatani N. (2001) A prospective study of renal disease in patients with early rheumatoid arthritis. Ann Rheum Dis 60: 327–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Karie S, Gandjbakhch F, Janus N, Launay-Vacher V, Rozenberg S, Mai Ba CU, et al. (2008) Kidney disease in RA patients: prevalence and implication on RA-related drugs management: the MATRIX study. Rheumatology (Oxford) 47: 350–354. [DOI] [PubMed] [Google Scholar]
- 3. Sihvonen S, Korpela M, Mustonen J, Laippala P, Pasternack A. (2004) Renal disease as a predictor of increased mortality among patients with rheumatoid arthritis. Nephron Clin Pract 96: c107–114. [DOI] [PubMed] [Google Scholar]
- 4. Thomas E, Symmons DP, Brewster DH, Black RJ, Macfarlane GJ. (2003) National study of cause-specific mortality in rheumatoid arthritis, juvenile chronic arthritis, and other rheumatic conditions: a 20 year followup study. J Rheumatol 30: 958–965. [PubMed] [Google Scholar]
- 5. Haroon M, Adeeb F, Devlin J, D OG, Walker F. (2011) A comparative study of renal dysfunction in patients with inflammatory arthropathies: strong association with cardiovascular diseases and not with anti-rheumatic therapies, inflammatory markers or duration of arthritis. Int J Rheum Dis 14: 255–260. 10.1111/j.1756-185X.2011.01594.x [DOI] [PubMed] [Google Scholar]
- 6. Boers M, Croonen AM, Dijkmans BA, Breedveld FC, Eulderink F, Cats A, et al. (1987) Renal findings in rheumatoid arthritis: clinical aspects of 132 necropsies. Ann Rheum Dis 46: 658–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang QL, Rothenbacher D. (2008) Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health 8: 117 10.1186/1471-2458-8-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Health Insurance Database. LHID 2005. 2014. Available: http://nhird.nhri.org.tw/en/Data_Subsets.html.
- 9. Hickson LJ, Crowson CS, Gabriel SE, McCarthy JT, Matteson EL. (2014) Development of reduced kidney function in rheumatoid arthritis. Am J Kidney Dis 63: 206–213. 10.1053/j.ajkd.2013.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boers M, Dijkmans BA, Breedveld FC, Camps JA, Chang PC, van Brummelen P, et al. (1990) Subclinical renal dysfunction in rheumatoid arthritis. Arthritis Rheum 33: 95–101. [DOI] [PubMed] [Google Scholar]
- 11. Karstila K, Korpela M, Sihvonen S, Mustonen J. (2007) Prognosis of clinical renal disease and incidence of new renal findings in patients with rheumatoid arthritis: follow-up of a population-based study. Clin Rheumatol 26: 2089–2095. [DOI] [PubMed] [Google Scholar]
- 12. Daoussis D, Panoulas V, Toms T, John H, Antonopoulos I, Nightingale P, et al. (2009) Uric acid is a strong independent predictor of renal dysfunction in patients with rheumatoid arthritis. Arthritis Res Ther 11: R116 10.1186/ar2775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Daoussis D, Panoulas VF, Antonopoulos I, John H, Toms TE, Wong P, et al. (2010) Cardiovascular risk factors and not disease activity, severity or therapy associate with renal dysfunction in patients with rheumatoid arthritis. Ann Rheum Dis 69: 517–521. 10.1136/ard.2008.105049 [DOI] [PubMed] [Google Scholar]
- 14. Fried L, Solomon C, Shlipak M, Seliger S, Stehman-Breen C, Bleyer AJ, et al. (2004) Inflammatory and prothrombotic markers and the progression of renal disease in elderly individuals. J Am Soc Nephrol 15: 3184–3191. [DOI] [PubMed] [Google Scholar]
- 15. Tonelli M, Sacks F, Pfeffer M, Jhangri GS, Curhan G. (2005) Biomarkers of inflammation and progression of chronic kidney disease. Kidney Int 68: 237–245. [DOI] [PubMed] [Google Scholar]
- 16. Stuveling EM, Hillege HL, Bakker SJ, Gans RO, De Jong PE, De Zeeuw D. (2003) C-reactive protein is associated with renal function abnormalities in a non-diabetic population. Kidney Int 63: 654–661. [DOI] [PubMed] [Google Scholar]
- 17. Chiu H-Y, Cheng Y-P, Tsai T-F. (2012) T helper type 17 in psoriasis: From basic immunology to clinical practice. Dermatologica Sinica 30: 136–141. [Google Scholar]
- 18. Chiu HY, Huang HL, Li CH, Yin YJ, Chen HA, Hsu ST, et al. (2015) Increased risk of glomerulonephritis and chronic kidney disease in relation to the severity of psoriasis, concomitant medication, and comorbidity: A nationwide population-based cohort study. Br J Dermatol. 173:146–54. 10.1111/bjd.13599 [DOI] [PubMed] [Google Scholar]
- 19. Levy AR, Szabo SM, Rao SR, Cifaldi M, Maksymowych WP. (2014) Estimating the occurrence of renal complications among persons with ankylosing spondylitis. Arthritis Care Res (Hoboken) 66: 440–445. [DOI] [PubMed] [Google Scholar]
- 20. Kim HW, Lee CK, Cha HS, Choe JY, Park EJ, Kim J. (2015) Effect of anti-tumor necrosis factor alpha treatment of rheumatoid arthritis and chronic kidney disease. Rheumatol Int 35:727–34. 10.1007/s00296-014-3146-4 [DOI] [PubMed] [Google Scholar]
- 21. Goicoechea M, Garcia de Vinuesa S, Quiroga B, Verdalles U, Barraca D, Yuste C, et al. (2012) Effects of pentoxifylline on inflammatory parameters in chronic kidney disease patients: a randomized trial. J Nephrol 25: 969–975. 10.5301/jn.5000077 [DOI] [PubMed] [Google Scholar]
- 22. Vielhauer V, Mayadas TN. (2007) Functions of TNF and its receptors in renal disease: distinct roles in inflammatory tissue injury and immune regulation. Semin Nephrol 27: 286–308. [DOI] [PubMed] [Google Scholar]
- 23. Nakano M, Ueno M, Nishi S, Shimada H, Hasegawa H, Watanabe T, et al. (1998) Analysis of renal pathology and drug history in 158 Japanese patients with rheumatoid arthritis. Clin Nephrol 50: 154–160. [PubMed] [Google Scholar]
- 24. Helin HJ, Korpela MM, Mustonen JT, Pasternack AI. (1995) Renal biopsy findings and clinicopathologic correlations in rheumatoid arthritis. Arthritis Rheum 38: 242–247. [DOI] [PubMed] [Google Scholar]
- 25. Moller B, Pruijm M, Adler S, Scherer A, Villiger PM, Finckh A. (2013) Chronic NSAID use and long-term decline of renal function in a prospective rheumatoid arthritis cohort study. Ann Rheum Dis 74:718–23. 10.1136/annrheumdis-2013-204078 [DOI] [PubMed] [Google Scholar]
- 26. Dijkmans BA, van Rijthoven AW, Goei The HS, Boers M, Cats A. (1992) Cyclosporine in rheumatoid arthritis. Semin Arthritis Rheum 22: 30–36. [DOI] [PubMed] [Google Scholar]
- 27. Yocum DE, Klippel JH, Wilder RL, Gerber NL, Austin HA 3rd, Wahl SM, et al. (1988) Cyclosporin A in severe, treatment-refractory rheumatoid arthritis. A randomized study. Ann Intern Med 109: 863–869. [DOI] [PubMed] [Google Scholar]
- 28. Abraham P, Rabi S. (2011) Aminoguanidine, a selective nitric oxide synthase inhibitor, attenuates cyclophosphamide-induced renal damage by inhibiting protein nitration and poly(ADP-Ribose) polymerase activation. Chemotherapy 57: 327–334. 10.1159/000330463 [DOI] [PubMed] [Google Scholar]
- 29. Sugumar E, Kanakasabapathy I, Abraham P. (2007) Normal plasma creatinine level despite histological evidence of damage and increased oxidative stress in the kidneys of cyclophosphamide treated rats. Clin Chim Acta 376: 244–245. [DOI] [PubMed] [Google Scholar]
- 30. del Rincon ID, Williams K, Stern MP, Freeman GL, Escalante A. (2001) High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum 44: 2737–2745. [DOI] [PubMed] [Google Scholar]
- 31. van Sijl AM, van den Oever IA, Peters MJ, Boers M, Dijkmans BA, van Halm VP, et al. (2012) Subclinical renal dysfunction is independently associated with cardiovascular events in rheumatoid arthritis: the CARRE Study. Ann Rheum Dis 71: 341–344. 10.1136/annrheumdis-2011-200051 [DOI] [PubMed] [Google Scholar]
- 32. Kawada T. (2012) Reply to: subclinical renal dysfunction is independently associated with cardiovascular events in rheumatoid arthritis: the CARRE Study. Ann Rheum Dis 71: e3 10.1136/annrheumdis-2012-202595 [DOI] [PubMed] [Google Scholar]
- 33. Cheng CL, Lee CH, Chen PS, Li YH, Lin SJ, Yang YH. (2014) Validation of acute myocardial infarction cases in the national health insurance research database in taiwan. J Epidemiol 24: 500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hsieh CY, Chen CH, Li CY, Lai ML. (2015) Validating the diagnosis of acute ischemic stroke in a National Health Insurance claims database. J Formos Med Assoc 114: 254–259. 10.1016/j.jfma.2013.09.009 [DOI] [PubMed] [Google Scholar]
- 35. Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML. (2011) Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf 20: 236–242. 10.1002/pds.2087 [DOI] [PubMed] [Google Scholar]
- 36. Kuo HW, Tsai SS, Tiao MM, Yang CY. (2007) Epidemiological features of CKD in Taiwan. Am J Kidney Dis 49: 46–55. [DOI] [PubMed] [Google Scholar]
- 37. Chen YC, Lin HY, Li CY, Lee MS, Su YC. (2014) A nationwide cohort study suggests that hepatitis C virus infection is associated with increased risk of chronic kidney disease. Kidney Int 85: 1200–1207. 10.1038/ki.2013.455 [DOI] [PubMed] [Google Scholar]
- 38. Huang HL, Ho SY, Li CH, Chu FY, Ciou LP, Lee HC, et al. (2014) Bronchial asthma is associated with increased risk of chronic kidney disease. BMC Pulm Med 14: 80 10.1186/1471-2466-14-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dessein PH, Hsu HC, Tsang L, Millen AM, Woodiwiss AJ, Norton GR, et al. (2015) Kidney function, endothelial activation and atherosclerosis in black and white Africans with rheumatoid arthritis. PLoS One 10: e0121693 10.1371/journal.pone.0121693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lai MN, Lai JN, Chen PC, Hsieh SC, Hu FC, Wang JD. (2010) Risks of kidney failure associated with consumption of herbal products containing Mu Tong or Fangchi: a population-based case-control study. Am J Kidney Dis 55: 507–518. 10.1053/j.ajkd.2009.10.055 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper.