Abstract
Background
Household air pollution in low income countries is an important cause of mortality from respiratory infection. We hypothesised that chronic smoke exposure is detrimental to alveolar macrophage function, causing failure of innate immunity. We report the relationship between macrophage function and prior smoke exposure in healthy Malawians.
Methods
Healthy subjects exposed daily to cooking smoke at home volunteered for bronchoalveolar lavage. Alveolar macrophage particulate content was measured as a known correlate of smoke exposure. Phagocytosis and intraphagosomal function (oxidative burst and proteolysis) were measured by a flow cytometric assay. Cytokine responses in macrophages were compared following re-exposure in vitro to wood smoke, before and after glutathione depletion.
Results
Volunteers had a range of alveolar macrophage particulate loading. The macrophage capacity for phagosomal oxidative burst was negatively associated with alveolar macrophage particulate content (n = 29, r2 = 0.16, p = 0.033), but phagocytosis per se and proteolytic function were unaffected. High particulate content was associated with lower baseline CXCL8 release (ratio 0.51, CI 0.29–0.89) and lower final concentrations on re-exposure to smoke in vitro (ratio 0.58, CI 0.34–0.97). Glutathione depletion augmented CXCL8 responses by 1.49x (CI 1.02–2.17) compared with wood smoke alone. This response was specific to smoke as macrophages response to LPS were not modulated by glutathione.
Conclusion
Chronic smoke exposure is associated with reduced human macrophage oxidative burst, and dampened inflammatory cytokine responses. These are critical processes in lung defence against infection and likely to underpin the relationship between air pollution and pneumonia.
Introduction
Environmental smoke exposure is strongly associated with all-cause mortality, even at low concentrations [1]. Household air pollution (HAP) is the greatest source of human particulate exposure, and 3rd most important risk factor for ill-health worldwide [2]. HAP associated respiratory infection results in 900000 excess deaths per year in children under 5 years [3], presumably due to adverse effects on airway immunity. These mechanisms are not understood, but the alveolar macrophage is central to both host defence and particulate handling.
Alveolar macrophages (AM) orchestrate appropriate responses to infective insults. AMs internalise bacteria, such as Streptococcus pneumoniae, activating NADPH oxidase which both kills bacteria and has a role in promoting inflammatory cytokine release [4, 5]. After successful containment, these responses are limited to prevent collateral lung damage [6]. However, inhaled particulate material taken up by AM has the capacity to induce excessive inflammatory signalling [7]. HAP wood smoke particles induce free radicals generation which augment macrophage NF-κB activation [8], reduce intracellular glutathione [9], and potentiate TNFα, IL-6 and CXCL8 release [10]. Adsorbed lipopolysaccharide has an additional pro-inflammatory effect [11].
In animal models, pneumonia mortality is associated with inadequate anti-inflammatory responses and reduced pulmonary macrophage apoptosis [12, 13]. Combined infective and particulate challenges were shown to exaggerate murine pulmonary inflammatory responses, and oxidative stress: phagocytosis of S. pneumoniae was impaired, and survival from pneumococcal pneumonia reduced [14]. In an alternative (high mortality) murine model, particle exposure improved survival, probably due to early neutrophil recruitment [15].
Acute particulate exposures in humans generate pro-inflammatory responses. Firefighters acutely exposed to wood smoke had increased systemic CXCL8 and neutrophilia [16]. Experimental human wood smoke exposure showed increased exhaled nitric oxide and malondialdehyde suggesting pulmonary inflammation and oxidative stress [17].
Chronic exposures appear different. Rats after 70 days of wood smoke exposure have minimal changes in BAL concentrations of cytokines, and lower levels IL-1β than controls [18]. There are few human data on pulmonary responses to chronic ambient particulate exposure, although cigarette smoking causes an hypo-responsive state in the ex vivo human alveolar macrophage, with blunted pro-inflammatory cytokine responses [19].
Previously we have reported preliminary data from a cohort of Malawians, suggesting reduced oxidative burst in alveolar macrophages with high particulate content [20]. We wished to perform a definitive study to extend these findings to other phagosomal functions, and to establish potential cellular explanations. Here, we hypothesise that chronic exposures reduce human macrophage phagosomal capacity to internalise and kill potential pathogens. Our hypothetical mechanism was that chronic exposure to smoke results in antioxidant buffering, altering redox balance in the macrophages, thus disrupting inflammatory responses important for defence against infection. We investigated macrophage function following natural ambient and household air pollution exposure in a biomass fuel burning adult population in Malawi.
Methods
Ethical approval was granted by the Research and Ethics Committees of the College of Medicine, University of Malawi (P.03/10/916) and Liverpool School of Tropical Medicine (09.69). All participants gave informed written consent for their participation. Healthy non-smoking and HIV negative participants were recruited for bronchoscopy in Blantyre, Malawi. Bronchoalveolar lavage (BAL) to obtain alveolar macrophages (AM) was performed as previously described [20]. All assays were done at the site of sample collection (Malawi) excepting cytokine measurement.
Cell culture
BAL was filtered through gauze and centrifuged. The pellet was re-suspended in RPMI1640 with 10% FBS and 2mM L-glutamine (culture medium) with penicillin, streptomycin and neomycin (Sigma-Aldrich, UK). At five hours culture medium was exchanged for that without antibiotics. AM were used on day 1 of culture after 4 hours of incubation at 37°C, 5% CO2.
Peripheral blood mononuclear cells (PBMC) were isolated from heparinised whole blood by Lymphoprep gradient separation (Axis-Shield, UK) and sequentially centrifuged according to manufacturer’s instructions. Cells were seeded at 7.5x105/cm2. Adherent PBMC were incubated at 37°C 5% CO2 in antibiotic-free culture medium for 5 days before use, with fresh medium introduced every 2 days.
Particulate Matter
Dried mopane firewood from Malawi (typically used for cooking) was transported to the UK and burned using a 3-stone open fire method in a contained room. Respirable sized particles (<4micron) were collected by a Higgins-Dewell cyclone at 2.2l/min attached to an Apex pump (Casella CEL, UK), onto polycarbonate isopore filters (Millipore, USA). Particulates were removed from filters by vortexing and sonication in methanol, dried under nitrogen gas and stored at -80°C.
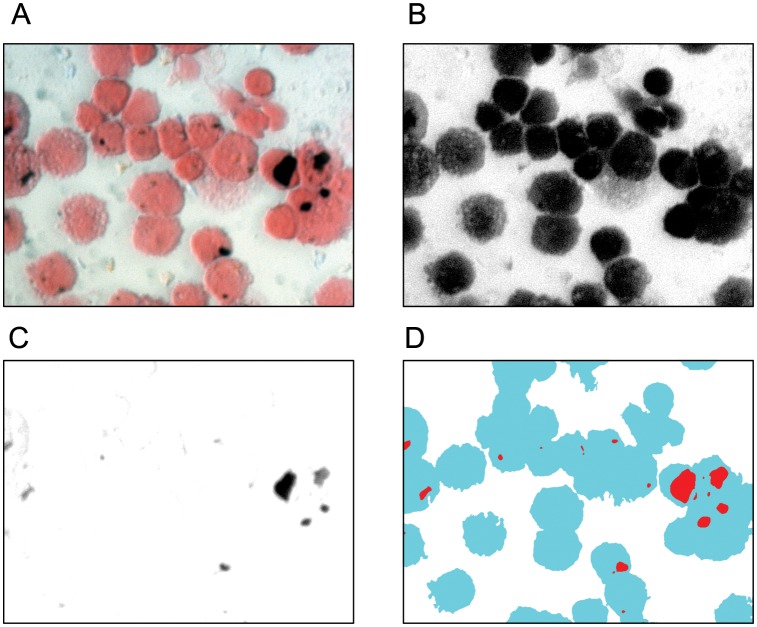
We previously reported that the particulate content of AM correlates with reported exposure to household air pollution [21]. Particulate burden has been used as a biomarker of exposure [22], and was quantified by digital image analysis as previously described [20]. Briefly, cytospin preparations (Thermo Shandon) were imaged at 40x by light microscopy. Fifty fields from each experiment were analysed using freely available software (Image SXM, www.ImageSXM.org.uk)—see Fig 1 for diagrammatic details).
Fig 1. Particulate burden is measured by the percentage of macrophage cytoplasm taken up with particulate matter as calculated by digital image analysis of light microscopy images.
ImageSXM software analyses cytospin images treated with Fields B stain (A), and identifies both cytoplasmic (B) and particulate areas (C). The proportion of particulate to cytoplasm in the output image (D) is used as a measure of recent particulate exposure. Fifty 40x fields are used, and the overall mean particulate burden used as the summary statistic. Panels B and C are generated internally within the software, and are shown here for illustrative purposes only.
Phagosomal assays
We used our previously validated flow cytometric assay to determine intraphagosomal oxidative burst and proteolytic functions [23]: silica beads linked to two fluorogenic probes (a reporter and a calibrator) are coupled to IgG to facilitate Fc receptor mediated uptake. Adherent alveolar macrophages were incubated with beads for 60mins and evaluated by flow cytometry (CyAn ADP, Beckman Coulter). Phagocytosis was defined as the proportion of cells associated with beads by flow cytometry. An example of analysis using FlowJo (Tree Star, Oregon, USA) is shown in supporting information S1 Fig Oxidative burst activity within the phagosome increases the fluorescence of bead-bound “reporter” 2',7'-dichlorodihydrofluoresceindiacetate succinimidyl ester (DCFHDA-SE, Invitrogen, UK). Similarly, proteolytic activity reduces quenching of bead-bound DQ-BSA (Molecular Probes), thereby increasing its fluorescence. For each assay, reporter fluorescence was compared to the stably fluorescent calibrator fluorochrome (Alexa 633, Invitrogen, UK) in order to control for variation in acquisition parameters. Both measures are reported as “activity index”, or the increase in ratio of reporter: calibrator fluorescence at 60 minutes (oxidative burst) and 180 minutes (proteolysis) compared with baseline.
Stimulation experiments
Particulates from wood smoke (WS) were re-suspended in culture medium at 50μg/ml. This dose induces similar particulate burdens in alveolar macrophages to those seen in heavy chronic exposures [20]. Lipopolysaccharide from E. coli 0111:B4 (LPS) was used as a stimulus at a final concentration of 100ng/ml. WS and LPS stimulation of adherent alveolar macrophages was performed after 24 hours of in vitro culture after bronchoscopy, and samples stored at -80°C pending analysis. Cytokines were pre-selected based on their known effects on macrophages, and putative involvement in redox signaling, and assayed from supernatant using the Luminex platform (R&D systems, UK).
To induce oxidative stress in cytokine stimulation experiments, cells were pre-treated for 18hours with 0.2mM buthionine sulfoximine (BSO) causing glutathione depletion. Additional supplementation with 2mM N-acetylcysteine (NAC) provided antioxidant depletion/repletion conditions. BSO related cell toxicity was determined from lactate dehydrogenase concentrations in culture supernatants (Tox7 kit, Sigma).
Redox assessment
Macrophages were lysed in 5% sulfasalicylic acid. Oxidised glutathione (GSSG) and total glutathione were measured in triplicate by a recycling enzymatic assay. Briefly, reduced glutathione is oxidised by 5,5’-dithiobis-2-nitrobenzoic acid (DTNB) to give a yellow product (TNB). Oxidised glutathione is recycled through the assay by NADPH dependent glutathione reductase. Total glutathione is determined from the linear reaction kinetic measured by absorbance at 412nm against known standards. Oxidised glutathione is identically measured, after pre-treatment with 2-vinylpyridine to remove reduced glutathione. Reduced glutathione (GSH) was calculated from [total glutathione = 2GSH + GSSG]. All reagents were obtained from Sigma, UK.
LDH assay for cell toxicity
Toxicity resulting from BSO treatment was measured by lactate dehydrogenase release into culture supernatants using a kit (Tox7, Sigma) according to manufacturer’s instructions. NADH formed in the catalytic reduction of NAD+ reacts with iodonitrotetrazolium producing a blue formazan product which can be quantified by absorption at 492nm. Separately, alveolar macrophages treated with 1% Triton-X for 5 minutes at 21°C were centrifuged at 10000g for 5 minutes, and the supernatant used as a positive control.
Data Analysis
Experiments were performed sequentially due to cell numbers. For each assay, consecutive participants were used to reduce the risk of bias. Due to the ubiquity of particulate exposure in the study population, control groups were not available. Results are therefore reported based of relative exposures within the spectrum of that measured.
Human AM production of cytokines, phagocytosis and phagosomal oxidative function was compared in subjects with variable exposure to household air pollution, measured by AM particulate burden. Cytokine data and particulate densities were normalized by logarithmic transformation, using x’ = log(x+0.05), due to zero values and to enable parametric testing. Groups were compared with paired t-test or ANOVA. Effects on cytokine release were assessed by Sidak’s multiple comparison testing. Analysis used Stata v12 (StatCorp, TX) and GraphPad Prism v6 (GraphPad, CA), with significance at p<0.05 unless stated.
Results
Participants
We recruited 128 non-smoking participants with median age 28.4 (IQR 23.6–34.9). 57 (44%) were female. Median bronchoalveolar lavage return was 65% (130ml, IQR 112–140), containing 96.3% macrophages (IQR 93.7–98.1) with 97% viability (IQR 95–99). Tobacco smoking by a household contact was reported in 23 (18%), but smoking in Malawi is outdoors and typically fewer than five cigarettes per day. Baseline details of participants are given in Table 1.
Table 1. Summary details of the participants’ demographics and BAL findings.
| n (%) | |
|---|---|
| Age, mean years (SD) | 30 (7.7) |
| Sex, female (%) | 57 (44%) |
| Body Mass Index, mean kg/m2 (SD) | 22.5 (3.8) |
| African ethnicity, n (%) | 128 (100%) |
| Passive cigarette smoke exposure, n (%) | 23 (18%) |
| Cooking (main source) | |
| Open wood fire, n (%) | 43 (33.6%) |
| Charcoal stove, n (%) | 76 (59.4%) |
| Electricity, n (%) | 9 (7.0%) |
| Cooking location (predominant) | |
| Inside the house, n (%) | 77 (60.6%) |
| Outside, n (%) | 50 (49.4%) |
| Lighting (main source) | |
| Paraffin, n (%) | 45 (35.4%) |
| Candle, n (%) | 47 (37.0%) |
| Electricity, n (%) | 31 (24.4%) |
| Flaming torch, n (%) | 4 (3.2%) |
| Incomplete data | 1 |
| FEV1, mean % predicted (SD) | 94 (13.5) |
| FVC, mean % predicted (SD) | 98 (12.6) |
| FEV1/FVC, % (SD) | 82 (6.7) |
| BAL return*, median ml (IQR) | 130 (112–140) |
| Cell viability, median % (IQR) | 97 (95–99) |
| Percent of BAL macrophages, median (IQR) | 96.3 (93.7–98.1) |
| Macrophage particulate load, median % of cytoplasm (IQR) | 0.4 (0.2–0.9), range 0–8 |
SD standard deviation; IQR interquartile range;
* 200ml instilled.
Particulate loading in macrophages from subjects exposed to HAP
121 (94.5%) participants were routinely exposed to biomass derived household air pollution: 119 (93%) used biomass fuel for cooking of whom 77 (61%) cooked only indoors; 94 (75%) used candle or paraffin as the main source of lighting, the remainder used electricity or battery powered lights. Median macrophage carbon load was 0.4% of total cytoplasmic area (range 0–8.0%, IQR 0.2–0.9%).
Intraphagosomal function
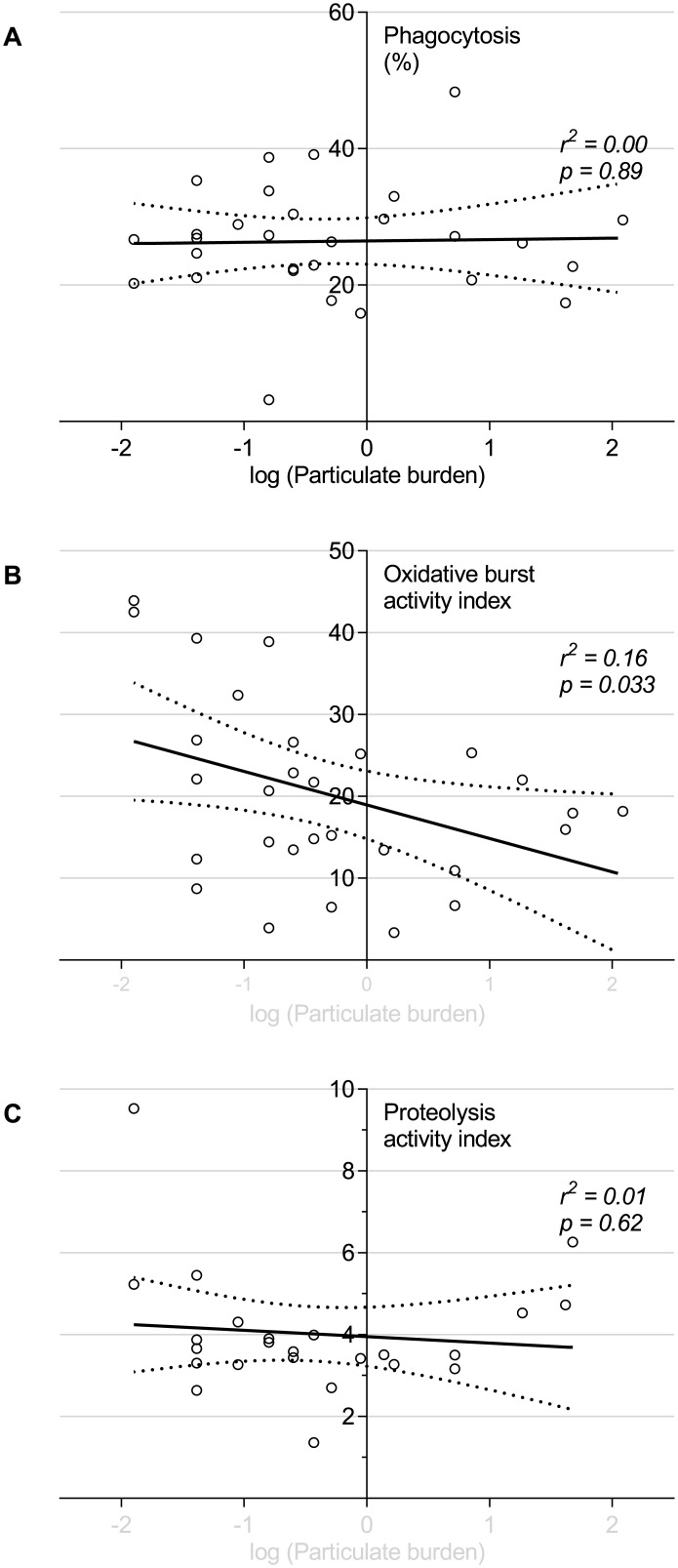
Phagocytosis, proteolysis and oxidative burst were measured in 29 BAL samples. There was a significant negative correlation, between macrophage particulate content and macrophage intraphagosomal oxidative burst (see Fig 2, r2 = 0.16, p = 0.033). There were wide differences between individuals in propensity to generate phagosomal reactive oxygen species (ROS). Neither phagocytosis per se nor proteolytic function were associated with macrophage particulate loading (r2 = 0.00, p = 0.90; r2 = 0.01, p = 0.25 respectively).
Fig 2. Alveolar macrophages naturally exposed to higher levels of particulate have reduced capacity for intraphagosomal oxidative burst, but an unaffected capacity for phagocytosis and proteolysis.
Unselected participants naturally exposed to inhaled particulates underwent bronchoscopy. The burden particulate within the alveolar macrophage was used as a measure of overall exposure. The capacity of alveolar macrophages to phagocytose, produce oxidative burst and proteolytic responses was tested on the same day by co-incubation with fluorophore labelled silica beads. (A) phagocytosis as indicated by the percentage of macrophages associated with fluorophore labelled beads by flow cytometry. (B) Oxidative burst and (C) Proteolysis were measured using twin labelled beads with calibrator fluor and reporter fluor (DCFH-DA and DQ-bovine serum albumin conjugate respectively), reported as “activity index” (see Methods). Each dot represents an individual (n = 29). Solid and dotted lines show the linear regression model with 95% confidence intervals. The x-axis describes the particulate burden (% of cytoplasm taken up by particulate). This has been log transformed by x’ = log(x+0.05), as described in the methods in order to normalise the data.
Cytokine response and particulate burden
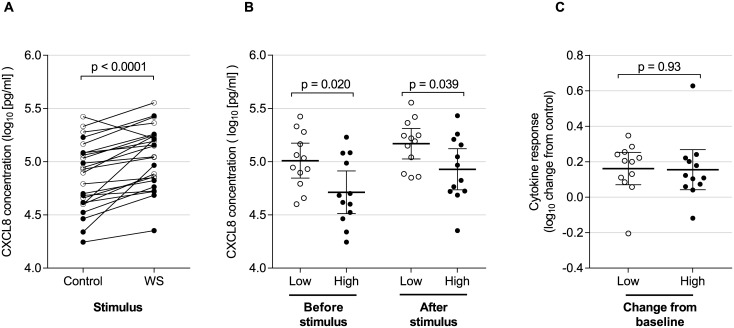
AM from 24 consecutive Malawian participants were stimulated ex vivo with wood smoke extract or media control. Culture supernatant CXCL8 concentrations rose at 6 hours (1.54 fold increase above control [95%CI 1.30–1.82], p<0.0001), see Fig 3A. We compared baseline CXCL8 production between low and high particulate groups as defined by the median macrophage particulate content. AM with high particulate loading had lower CXCL8 release compared with low particulate under unstimulated culture conditions (ratio of means 0.51 (95%CI 0.29–0.89, p = 0.02), see Fig 3B. After stimulation with wood smoke, this difference persisted: the ratio of mean CXCL8 in high compared with low particulate groups was 0.58 (95%CI 0.34–0.97). There was no relationship, however, between high particulate loading and the magnitude of CXCL8 responses (ratio of mean high vs low of 0.99 (95%CI 0.72–1.35, p = 0.93), see Fig 3C.
Fig 3. CXCL8 release is decreased in particulate laden macrophages.
Prior exposure to particulates was measured by HAM particulate burden. Individuals were designated “low” and “high” exposures according to whether their HAM particulate burden was below or above the median value for this group (n = 24). Adherent HAM on day 1 after bronchoscopy were incubated with media only or with additional wood smoke suspension for 6 hours. CXCL8 concentrations in the cell culture supernatant following challenge are shown logarithmically transformed (log10). For all panels, open circles represent individuals with “low” macrophage particulate burden. Filled circles are those with “high” baseline particulate burden. (A) shows concentrations of CXCL8 in media for control and wood smoke stimulated wells for the entire group. There is a significant increase in concentration in stimulated cells (paired t-test, p<0.0001). (B) shows CXCL8 concentrations from untreated (control) and wood smoke treated wells. Here, results are grouped according to baseline particulate burden (low and high as described above). Bars show the mean and 95% confidence intervals. There is a significantly higher CXCL8 concentration from cells with low baseline particulate burden in both control and wood smoke treated wells (two-sided t-test, p = 0.020 and p = 0.039 respectively). (C) represents the increase in CXCL8 (log10 change in wood smoke treated wells compared with control). Bars show the mean and 95% confidence intervals. There is no difference in the magnitude of cytokine response between cells with low and high baseline particulate burden (two sided t-test, p = 0.93).
Cytokine response and redox balance
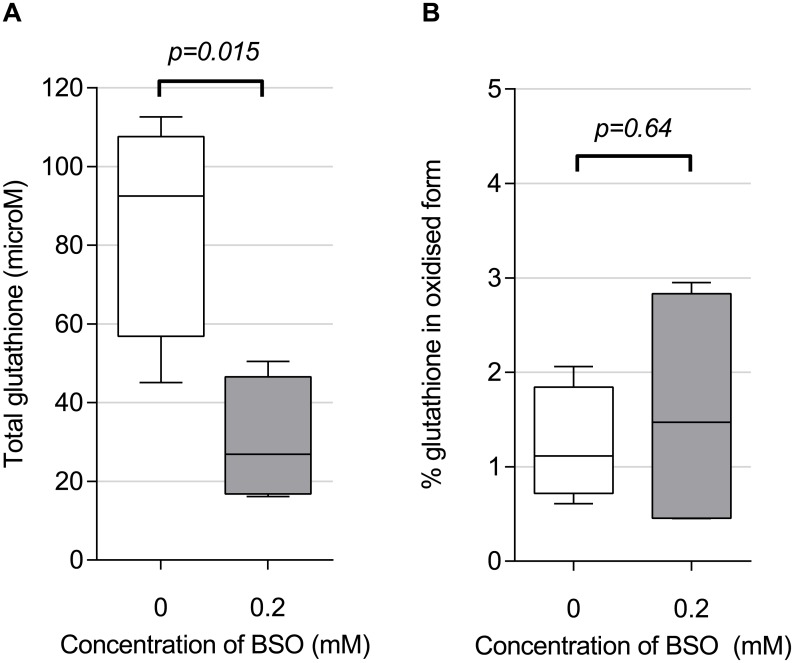
Having described the relation of particulate load to both cytokine production and phagocytic function, we then determined the extent to which AM cytokine responses to particulates (stimulated ex vivo) were dependent on intracellular redox balance. Preliminary experiments (Fig 4A) showed that buthionine sulfoximine (BSO) 0.2mM decreased total glutathione (total glutathione) in HAM to 35% of its baseline (from 85.7μmol [SD 14.3] to 30.1μmol [SD 8.0], p = 0.015). BSO treated AMs therefore had reduced antioxidant buffering capacity (total glutathione). However, the ratio of oxidized to total glutathione (GSSG:total glutathione) expressed as a percentage was unaltered: control and treated mean values were 1.2% (SD 0.30) and 1.6% (SD 0.66) respectively (Fig 4B). This ratio is a frequently used measure of redox signaling [24], and suggests that the macrophage redox potential was not significantly affected, at least under resting culture conditions. There was no evidence for BSO induced toxicity (supporting information S2 Fig).
Fig 4. BSO treatment of HAM reduced total intracellular glutathione concentrations but did not alter overall proportion of glutathione in the oxidised state.
Ex vivo adherent macrophages (3x106 per well) were cultured for 18 hours in medium only (control), or with addition of 0.2mM BSO. Concentrations of total and oxidised glutathione were measured using an enzymatic recycling assay (see Methods). (A) Total glutathione (a measure of buffering capacity against oxidative stress) is reduced by BSO treatment compared with control. (B) Oxidised glutathione, as conventionally expressed as a percentage of total (a measure of oxidative stress), was not significantly altered by BSO treatment. n = 4 in each group. Boxes indicate 25th to 75th centile with median, and whiskers at 95% CI. Two sided t-test used for comparison.
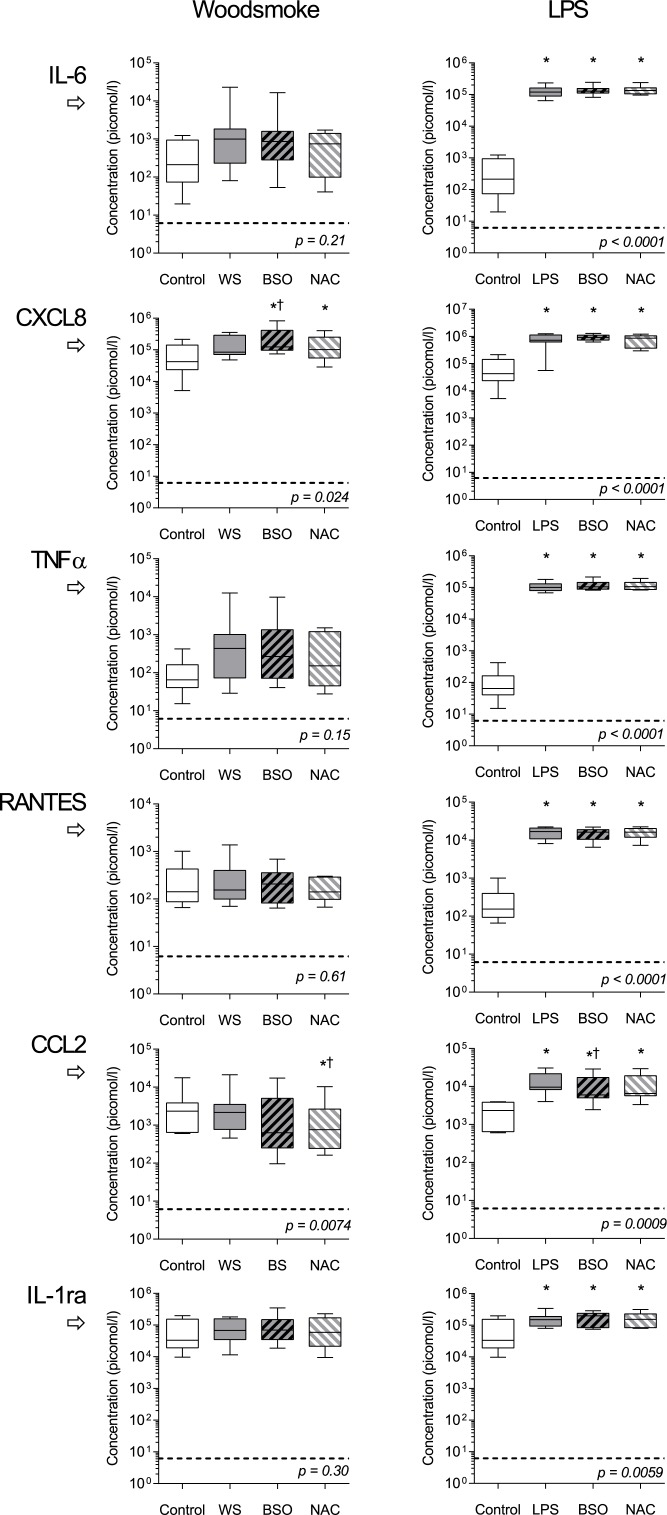
Cytokine release from BSO treated AM was measured following ex vivo incubation with WS particles and LPS (Fig 5). CXCL8 production was augmented by BSO pre-treatment (mean fold increase of 1.49 [95%CI 1.02–2.17]). NAC offset this increase. CCL2 release was also altered by redox balance (p = 0.0074 by ANOVA), and was significantly reduced in response to wood smoke in cells pretreated with BSO/NAC (mean fold difference 0.40 [CI 0.24–0.66]. Other inflammatory mediators demonstrated no statistical difference after redox manipulation. All measured cytokines were increased following lipopolysaccharide treatment (p<0.05 by repeated measures).
Fig 5. Cytokine responses to wood smoke particulates and LPS: the effect of intracellular redox balance.
Ex-vivo adherent alveolar macrophages were pre-incubated with media alone (control), 0.2mM BSO (glutathione depletion), or BSO plus 2mM NAC (antioxidant depletion-repletion) for 18 hours, followed by stimulation with either media alone (control), wood smoke (left panels, WS) or lipopolysaccharide (right panels, LPS). After WS stimulus, low level changes were seen in CXCL8 and CCL2 release, while other cytokines were no significantly different. Maximal responses were seen with LPS, with no consistent effect of redox manipulation. Boxes show mean cytokine concentration in cell culture supernatants with 25th to 75th centile, and whiskers at 95% CI. n = 8 for each group. p values represent repeated measures ANOVA on log-transformed data. * denotes difference from control. † denotes difference from stimulated condition without pre-treatment. Significance testing by Sidak’s multiple comparisons test (p<0.05 significance level). Horizontal broken line represents limit of detection for each cytokine.
Discussion
This study has shown that human AM obtained from participants most exposed to HAP have altered macrophage function: macrophage cytokine responses to wood smoke particles are reduced, and phagosomal oxidative burst responses are diminished. Further, we have shown that experimental alteration of human AM redox balance augments the prominent cytokine response to wood smoke (CXCL8). We did not show any alteration in macrophage phagocytosis associated with chronic particulate exposure, which is different from reports of acute exposure but consistent with the clinical differences between acute and chronic smoke exposure in human subjects.
A large number of participants underwent bronchoscopy, allowing confidence in the findings. We carefully characterised alveolar macrophage particulate burden as a measure of exposure, and determined intraphagosomal function. While in vitro challenge models are by their nature different from in vivo conditions, and relevant concentrations at cellular interface are debated, our model is consistent with in vivo findings: we used respirable fraction particles at concentrations which mimicked cellular appearances of macrophages exposed in vivo. Smoke particles were produced from the same firewood sources used by the exposed population, thus providing a relevant exposure model.
Phagosomal function
We identified a specific defect in phagosomal function associated with natural particulate loading of alveolar macrophages. Despite known crosstalk between oxidative burst and proteolysis pathways, particulate burden was independently associated with reduced oxidative burst, but proteolytic and phagocytic functions were preserved.
These results differ from models of acute exposure which have generally demonstrated decreased phagocytosis [25]. Cellular reactive oxygen species increase following acute exposure, but many methods measure ROS release throughout the cell, including potentially damaging extra-phagosomal production. Compared with our intraphagosomal assay, such methods are more likely to measure cellular oxidative stress than phagocytic killing. In mice, chronic PM2.5 exposure causes increased NADPH oxidase activity, by infiltration of bone marrow derived monocytes [26]. In our study, chronic exposures in humans do not have this effect. Particulate toxicity could explain a reduction in oxidative burst, but this does not explain the differential effect on phagocytosis and proteolysis. Other studies report no difference on macrophage viability at the same particulate dose and time interval [27].
Our data provide novel evidence that innate pulmonary immunity is specifically impaired by in vivo particulate exposure. Reduced oxidative burst could result in less robust bacterial handling, which is key to innate defence during early pulmonary infection[4]. NADPH oxidase is important in both bacterial killing, and in limiting inflammatory responses by limiting NF-κB activity and the effect of LPS on pro-inflammatory cascades (reduction of NADPH oxidase activity, dampens the pro-inflammatory effect of particulates [28]). These mechanisms are likely to be important in the major observed associations between pulmonary infection and smoke exposure [2].
HAP particulates from biomass fuel combustion contain endotoxin concentrations one hundred times higher than those which cause childhood respiratory disease [11]. In view of the lower oxidative burst in particulate laden macrophages, we hypothesize that chronic inhaled endotoxin down-regulates M1-“activation” phenotype, reducing ROS production analogous to the endotoxin tolerance seen in cigarette smokers [19].
Release of inflammatory mediators
Wood smoke particles induced AM to produce CXCL8 in vitro, which is the prominent systemic cytokine response in wood smoke exposed firefighters [16]. We noted non-significant rises in IL-6 and TNFα which might be explained by our use of lower particulate concentrations than previous studies [10]. Particulate type also alters the effect: WS generates lower level inflammatory responses compared with other particulates in animals [29]. However, we have for the first time used alveolar macrophages obtained from chronically exposed adults who have down-regulated inflammatory AM responses when compared with monocytic cells used in many in vitro models [30].
Dampening of LPS cytokine responses has been demonstrated in COPD patients’ mucosa (CXCL8) [31], and alveolar macrophages (TNFα but not CXCL8) [32]. In our study, LPS induced rises in IL-6, CXCL8 and TNFα within bronchoalveolar lavage fluid were reduced by the antioxidant, N-acetylcysteine [33]. We tested the effect of oxidative stress, and showed that CXCL8 was increased in BSO pre-treated cells. The differential in effect (the absence of a measurable effect on IL-6 and TNFα) might be due to relatively larger absolute changes in CXCL8, or the involvement of separate (non-NFΚB) induction pathways. Recent study has shown that redox changes induce CXCL8 in other ways, including Lyn (an Src family kinase), AP-1 binding and histone 3 modifications in the promotor region, [34]
In contrast to CXCL8, CCL2 responses to LPS stimulation were reduced by glutathione depletion. CCL2 is released following acute inflammatory lung injury, reducing AM oxidant production [35]. Under conditions of oxidative stress, reducing CCL2 may therefore prevent excessive inflammation.
Gosset et al noted that macrophage glutathione depletion by 90% augments LPS stimulated release of CXCL8 and TNFα [36]. Our BSO treatment effect was less profound (65% reduction), which might explain our observed lack of redox effect. However, Gosset et al also demonstrate CXCL8 changes with glutathione reduction of 45%. CXCL8 production is known to be influenced by oxygen metabolites. We are investigating the possibility that our participants’ macrophages had already up-regulated antioxidant defences due to chronic particulate-induced oxidative stress.
Summary
AM obtained from subjects with prior exposure to high concentrations of respirable particulates show reduced cytokine production at baseline and after further stimulation with particulate generated from wood combustion. Reducing intracellular glutathione increased CXCL8 responses and decreased CCL2 responses in human AM in a similar pattern to that observed in chronically particulate exposed subjects. With LPS stimulation, we did not see an increase in cytokine in BSO treated AM, perhaps due to chronic oxidative stress caused by HAP exposure in this population [36].
In conclusion, particulate exposure in vivo is directly associated with impaired macrophage function. It is unlikely that these cellular changes can be specifically altered by antioxidant therapy. These data should, however, provide biological plausibility for public health interventions to reduce HAP with consequent reductions in the burden of pulmonary infection among adults and children living in homes using biomass fuel.
Supporting Information
(EPS)
Culture medium content of lactate dehydrogenase (LDH) following human alveolar macrophage culture for 18 hours, in the presence BSO 0.2mM or media alone (control). LDH given as proportion of lysed HAM positive control (see Methods). n = 6 in each group.
(EPS)
Acknowledgments
The authors would like to thank the participants at Queen Elizabeth Central Hospital, Blantyre and staff at MLW especially Rose Malamba and Mr A. Kankwatira for their help with recruitment and bronchoscopy. We would also like to thank Dr Brian Faragher for statistical advice and support.
Data Availability
All data are available from http://dx.doi.org/10.5061/dryad.89nj3.
Funding Statement
This work was funded and supported by a Wellcome Trust grant 086756/B/08/Z (JR); a Wellcome Trust Project Grant 083606/A/07/Z (RSH); and Malawi-Liverpool-Wellcome Trust Clinical Research Programme 084679/Z/08/Z (RSH). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Schwartz J, Zanobetti A. Using meta-smoothing to estimate dose-response trends across multiple studies, with application to air pollution and daily death. Epidemiology. 2000;11(6):666–72. Epub 2000/10/31. . [DOI] [PubMed] [Google Scholar]
- 2. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–60. Epub 2012/12/19. 10.1016/S0140-6736(12)61766-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dherani M, Pope D, Mascarenhas M, Smith KR, Weber M, Bruce N. Indoor air pollution from unprocessed solid fuel use and pneumonia risk in children aged under five years: a systematic review and meta-analysis. Bull World Health Organ. 2008;86(5):390–8C. Epub 2008/06/12. S0042-96862008000500017 [pii]. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gordon SB, Irving GR, Lawson RA, Lee ME, Read RC. Intracellular trafficking and killing of Streptococcus pneumoniae by human alveolar macrophages are influenced by opsonins. Infect Immun. 2000;68(4):2286–93. Epub 2000/03/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu F, Droemann D, Rupp J, Shen H, Wu X, Goldmann T, et al. Modulation of the inflammatory response to Streptococcus pneumoniae in a model of acute lung tissue infection. Am J Respir Cell Mol Biol. 2008;39(5):522–9. Epub 2008/05/17. 10.1165/rcmb.2007-0328OC . [DOI] [PubMed] [Google Scholar]
- 6. Dockrell DH, Marriott HM, Prince LR, Ridger VC, Ince PG, Hellewell PG, et al. Alveolar macrophage apoptosis contributes to pneumococcal clearance in a resolving model of pulmonary infection. J Immunol. 2003;171(10):5380–8. Epub 2003/11/11. . [DOI] [PubMed] [Google Scholar]
- 7. Jimenez LA, Drost EM, Gilmour PS, Rahman I, Antonicelli F, Ritchie H, et al. PM(10)-exposed macrophages stimulate a proinflammatory response in lung epithelial cells via TNF-alpha. Am J Physiol Lung Cell Mol Physiol. 2002;282(2):L237–48. Epub 2002/01/17. 10.1152/ajplung.00024.2001 . [DOI] [PubMed] [Google Scholar]
- 8. Leonard SS, Wang S, Shi X, Jordan BS, Castranova V, Dubick MA. Wood smoke particles generate free radicals and cause lipid peroxidation, DNA damage, NFkappaB activation and TNF-alpha release in macrophages. Toxicology. 2000;150(1–3):147–57. Epub 2000/09/21. S0300483X00002560 [pii]. . [DOI] [PubMed] [Google Scholar]
- 9. Kubatova A, Dronen LC, Picklo MJ Sr., Hawthorne SB. Midpolarity and nonpolar wood smoke particulate matter fractions deplete glutathione in RAW 264.7 macrophages. Chem Res Toxicol. 2006;19(2):255–61. Epub 2006/02/21. 10.1021/tx050172f . [DOI] [PubMed] [Google Scholar]
- 10. Kocbach A, Namork E, Schwarze PE. Pro-inflammatory potential of wood smoke and traffic-derived particles in a monocytic cell line. Toxicology. 2008;247(2–3):123–32. Epub 2008/04/15. S0300-483X(08)00085-1 [pii] 10.1016/j.tox.2008.02.014 . [DOI] [PubMed] [Google Scholar]
- 11. Semple S, Devakumar D, Fullerton DG, Thorne PS, Metwali N, Costello A, et al. Airborne Endotoxin Concentrations in Homes Burning Biomass Fuel. Environ Health Perspect. 2010. Epub 2010/03/24. 10.1289/ehp.0901605 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Knapp S, Leemans JC, Florquin S, Branger J, Maris NA, Pater J, et al. Alveolar macrophages have a protective antiinflammatory role during murine pneumococcal pneumonia. Am J Respir Crit Care Med. 2003;167(2):171–9. Epub 2002/10/31. 10.1164/rccm.200207-698OC 200207-698OC [pii]. . [DOI] [PubMed] [Google Scholar]
- 13. Marriott HM, Hellewell PG, Cross SS, Ince PG, Whyte MK, Dockrell DH. Decreased alveolar macrophage apoptosis is associated with increased pulmonary inflammation in a murine model of pneumococcal pneumonia. J Immunol. 2006;177(9):6480–8. Epub 2006/10/24. 177/9/6480 [pii]. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sigaud S, Goldsmith CA, Zhou H, Yang Z, Fedulov A, Imrich A, et al. Air pollution particles diminish bacterial clearance in the primed lungs of mice. Toxicol Appl Pharmacol. 2007;223(1):1–9. Epub 2007/06/15. S0041-008X(07)00209-8 [pii] 10.1016/j.taap.2007.04.014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tellabati A, Fernandes VE, Teichert F, Singh R, Rylance J, Gordon S, et al. Acute exposure of mice to high-dose ultrafine carbon black decreases susceptibility to pneumococcal pneumonia. Part Fibre Toxicol. 2010;7:30 Epub 2010/10/21. 10.1186/1743-8977-7-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Swiston JR, Davidson W, Attridge S, Li GT, Brauer M, van Eeden SF. Wood smoke exposure induces a pulmonary and systemic inflammatory response in firefighters. Eur Respir J. 2008;32(1):129–38. Epub 2008/02/08. 09031936.00097707 [pii] 10.1183/09031936.00097707 . [DOI] [PubMed] [Google Scholar]
- 17. Barregard L, Sallsten G, Andersson L, Almstrand AC, Gustafson P, Andersson M, et al. Experimental exposure to wood smoke: effects on airway inflammation and oxidative stress. Occup Environ Med. 2008;65(5):319–24. Epub 2007/08/21. oem.2006.032458 [pii] 10.1136/oem.2006.032458 . [DOI] [PubMed] [Google Scholar]
- 18. Tesfaigzi Y, McDonald JD, Reed MD, Singh SP, De Sanctis GT, Eynott PR, et al. Low-level subchronic exposure to wood smoke exacerbates inflammatory responses in allergic rats. Toxicol Sci. 2005;88(2):505–13. Epub 2005/09/16. kfi317 [pii] 10.1093/toxsci/kfi317 . [DOI] [PubMed] [Google Scholar]
- 19. Chen H, Cowan MJ, Hasday JD, Vogel SN, Medvedev AE. Tobacco smoking inhibits expression of proinflammatory cytokines and activation of IL-1R-associated kinase, p38, and NF-kappaB in alveolar macrophages stimulated with TLR2 and TLR4 agonists. J Immunol. 2007;179(9):6097–106. Epub 2007/10/20. . [DOI] [PubMed] [Google Scholar]
- 20. Rylance J, Fullerton DG, Scriven J, Aljurayyan AN, Mzinza D, Barrett S, et al. Household Air Pollution Causes Dose-dependent Inflammation and Altered Phagocytosis in Human Macrophages. Am J Respir Cell Mol Biol. 2014. Epub 2014/09/26. 10.1165/rcmb.2014-0188OC . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fullerton DG, Jere K, Jambo K, Kulkarni NS, Zijlstra EE, Grigg J, et al. Domestic smoke exposure is associated with alveolar macrophage particulate load. Trop Med Int Health. 2009;14(3):349–54. Epub 2009/03/13. 10.1111/j.1365-3156.2009.02230.x . [DOI] [PubMed] [Google Scholar]
- 22. Kulkarni N, Pierse N, Rushton L, Grigg J. Carbon in airway macrophages and lung function in children. N Engl J Med. 2006;355(1):21–30. Epub 2006/07/11. 355/1/21 [pii] 10.1056/NEJMoa052972 . [DOI] [PubMed] [Google Scholar]
- 23. Russell DG, Vanderven BC, Glennie S, Mwandumba H, Heyderman RS. The macrophage marches on its phagosome: dynamic assays of phagosome function. Nat Rev Immunol. 2009;9(8):594–600. Epub 2009/07/11. 10.1038/nri2591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30(11):1191–212. Epub 2001/05/23. S0891584901004804 [pii]. . [DOI] [PubMed] [Google Scholar]
- 25. Barlow PG, Brown DM, Donaldson K, MacCallum J, Stone V. Reduced alveolar macrophage migration induced by acute ambient particle (PM10) exposure. Cell Biol Toxicol. 2008;24(3):243–52. Epub 2007/09/12. 10.1007/s10565-007-9033-y . [DOI] [PubMed] [Google Scholar]
- 26. Kampfrath T, Maiseyeu A, Ying Z, Shah Z, Deiuliis JA, Xu X, et al. Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways. Circ Res. 2011;108(6):716–26. Epub 2011/01/29. 10.1161/CIRCRESAHA.110.237560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stone WL, Qui M, Smith M. Lipopolysaccharide enhances the cytotoxicity of 2-chloroethyl ethyl sulfide. BMC cell biology. 2003;4:1 Epub 2003/01/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Becher R, Bucht A, Ovrevik J, Hongslo JK, Dahlman HJ, Samuelsen JT, et al. Involvement of NADPH oxidase and iNOS in rodent pulmonary cytokine responses to urban air and mineral particles. Inhal Toxicol. 2007;19(8):645–55. Epub 2007/05/19. 10.1080/08958370701353528 . [DOI] [PubMed] [Google Scholar]
- 29. Seagrave J, McDonald JD, Reed MD, Seilkop SK, Mauderly JL. Responses to subchronic inhalation of low concentrations of diesel exhaust and hardwood smoke measured in rat bronchoalveolar lavage fluid. Inhal Toxicol. 2005;17(12):657–70. Epub 2005/08/10. HUV3427G2508135N [pii] 10.1080/08958370500189529 . [DOI] [PubMed] [Google Scholar]
- 30. Aberdein JD, Cole J, Bewley MA, Marriott HM, Dockrell DH. Alveolar macrophages in pulmonary host defence the unrecognized role of apoptosis as a mechanism of intracellular bacterial killing. Clin Exp Immunol. 2013;174(2):193–202. Epub 2013/07/12. 10.1111/cei.12170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nadigel J, Audusseau S, Baglole CJ, Eidelman DH, Hamid Q. IL-8 production in response to cigarette smoke is decreased in epithelial cells from COPD patients. Pulmonary pharmacology & therapeutics. 2013;26(5):596–602. Epub 2013/03/19. 10.1016/j.pupt.2013.03.002 . [DOI] [PubMed] [Google Scholar]
- 32. Metcalfe HJ, Lea S, Hughes D, Khalaf R, Abbott-Banner K, Singh D. Effects of cigarette smoke on Toll-like receptor (TLR) activation of chronic obstructive pulmonary disease (COPD) macrophages. Clin Exp Immunol. 2014;176(3):461–72. Epub 2014/02/18. 10.1111/cei.12289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Antonicelli F, Brown D, Parmentier M, Drost EM, Hirani N, Rahman I, et al. Regulation of LPS-mediated inflammation in vivo and in vitro by the thiol antioxidant Nacystelyn. Am J Physiol Lung Cell Mol Physiol. 2004;286(6):L1319–27. Epub 2004/05/12. 10.1152/ajplung.00329.2003 286/6/L1319 [pii]. . [DOI] [PubMed] [Google Scholar]
- 34. de Oliveira S, Boudinot P, Calado A, Mulero V. Duox1-derived H2O2 modulates Cxcl8 expression and neutrophil recruitment via JNK/c-JUN/AP-1 signaling and chromatin modifications. J Immunol. 2015;194(4):1523–33. 10.4049/jimmunol.1402386 . [DOI] [PubMed] [Google Scholar]
- 35. Okuma T, Terasaki Y, Sakashita N, Kaikita K, Kobayashi H, Hayasaki T, et al. MCP-1/CCR2 signalling pathway regulates hyperoxia-induced acute lung injury via nitric oxide production. International journal of experimental pathology. 2006;87(6):475–83. Epub 2007/01/16. 10.1111/j.1365-2613.2006.00502.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gosset P, Wallaert B, Tonnel AB, Fourneau C. Thiol regulation of the production of TNF-alpha, IL-6 and IL-8 by human alveolar macrophages. Eur Respir J. 1999;14(1):98–105. Epub 1999/09/18. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(EPS)
Culture medium content of lactate dehydrogenase (LDH) following human alveolar macrophage culture for 18 hours, in the presence BSO 0.2mM or media alone (control). LDH given as proportion of lysed HAM positive control (see Methods). n = 6 in each group.
(EPS)
Data Availability Statement
All data are available from http://dx.doi.org/10.5061/dryad.89nj3.