Abstract
Aims
In about 50–80% of ST-segment elevation myocardial infarction (STEMI) patients there is significant atherosclerotic disease in other coronary arteries in addition to the culprit vessel. There is substantial controversy as to the optimal revascularization approach in these patients. We sought to compare the outcomes of STEMI patients with multi-vessel disease (MVD) treated with culprit-only primary percutaneous coronary intervention (PPCI) without significant ischemia on subsequent non-invasive testing, to those of STEMI patients with single-vessel disease (SVD).
Methods and Results
Between 2001–2010, 1,540 consecutive patients treated with primary PCI for STEMI were prospectively observed and entered into a comprehensive clinical database. The primary end point was a composite of major adverse cardiac events (MACE), consisting of mortality, re-infarction and revascularization within 1 and 3 years following PPCI (excluding events occurring during the first 30 days). Patients with cardiogenic shock were excluded. The study included 720 patients with SVD and 185 patients with MVD who underwent culprit-only PPCI and had no residual ischemia on subsequent non-invasive stress testing. Patients with MVD were older, more likely to have hypertension or previous MI and less likely to be smokers and present with anterior MI than patients with SVD. One and 3-year MACE rates were similar between the groups. On cox proportional-hazards regression MVD without residual ischemia was not independently associated with MACE and its components.
Conclusions
STEMI patients with MVD treated with culprit only-PCI without significant residual ischemia on non-invasive stress testing appear to have similar prognosis to STEMI patients with SVD.
Introduction
The main goal of primary percutaneous coronary intervention (PCI) in the setting of ST elevation myocardial infraction (STEMI) is to re-perfuse the myocardium by opening the culprit (infarct-related) coronary artery. However, in as many as 50–80% of patients with STEMI there is significant atherosclerotic disease in other coronary arteries in addition to the culprit vessel; a state which is associated with adverse outcomes [1–7]. Many studies have examined the value of an early treatment strategy performed upon significant narrowing in the non-culprit vessels during primary PCI [3–14]. Most studies have shown that such intervention does not provide clinical benefit, and at times may even be harmful [3–5,8–14]. By contrast, two recent randomized studies did show benefit for a strategy of non-culprit PCI during the index procedure [14,15]. Despite these two studies, postponing and staging possible treatment of the non-culprit vessels is still the preferable approach by current guidelines on myocardial revascularization and STEMI [16–18]. However, the clinical strategy for staging or postponing PCI in these patients is debatable. Several expert opinions recommend routine elective PCI of significant stenoses in non-culprit vessels several days or weeks after the primary PCI, regardless of clinical features or further studies. Other experts promote performance of non-invasive provocative testing to assess ischemia in the non-culprit territories, such as an exercise nuclear scan or a stress echocardiogram, in order to determine the necessity of performing PCI of the non-culprit vessels [4,13,19]. The latter approach is based on the assumption that during acute MI, due to the release of cytokines and vasoactive agents to the circulation, a temporary vasoconstriction of non-culprit vessels may occur, which may overstress severity and resolve spontaneously after the recovery of the acute phase [5,20].
A central question that may guide decision-making in this controversial issue is what the prognosis is of patients with STEMI with multi-vessel disease undergoing culprit vessel PCI without significant ischemia on subsequent non-invasive testing (and thus they did not undergo further PCI).
We hypothesized that the prognosis of these patients is similar to that of patients with single vessel disease undergoing successful primary PCI. Hence, the aim of this study was to compare the prognosis of these two groups of patients.
Materials and Methods
Between January 2001 and December 2010, 1,540 consecutive patients with STEMI undergoing primary PCI at the Rabin Medical Center, Israel, were prospectively observed and entered into a comprehensive clinical database. Acute STEMI was defined as the presence of typical chest pain and accompanying symptoms for a duration of at least 30 minutes but < 12 hours in the presence of ST-segment elevation ≥1 mm in at least 2 contiguous leads, or new or undetermined duration of left bundle branch block in association with elevated cardiac enzymes (Creatine kinase, Troponin I or T). The registry included demographic, clinical, angiographic, procedural and echocardiographic data. This registry was approved by the ethics committee of the Rabin Medical Centre, in compliance with the Declaration of Helsinki.
Patient records/information was anonymized and de-identified prior to analysis.
All patients were treated with aspirin 300 mg before the PCI and clopidogrel 600 mg. Unfractionated heparin (70 U/Kg loading) was given before PCI and adjusted to achieve an activated clotting time of 200 to 275 seconds during the intervention. Glycoprotein IIb/IIIa receptor inhibition by eptifibatide was used at the discretion of the operator only after crossing the culprit lesion with a guidewire. Coronary angiography was performed through the femoral or radial rout according to the operator’s discretion. All patients had PCI performed only to the culprit vessel according to the institutional policy at the time of data collection (2001–2010). Selection of stent type, pre-dilatation with undersized balloons, and post-dilatation with larger balloons also were left to the operator’s discretion. Procedural success was defined as an angiographic residual stenosis of 20% or less by visual estimation or quantitative coronary angiography with optimized angiographic flow (Thrombolysis In Myocardial Infarction (TIMI) flow 3). All patients were prescribed lifelong aspirin and clopidogrel (75 mg daily) for 12 months. Baseline clinical characteristics, angiographic details, quantitative coronary angiography, TIMI flow and clinical outcomes were collected.
Patients were stratified according to the presence or absence of multi-vessel disease, which was defined as ≥ 70% stenosis of ≥ 2 epicardial coronary arteries or their major branches. Patients with multi-vessel disease were further classified according to whether or not they had undergone staged PCI to the non-culprit vessels based on the results of non-invasive stress testing for residual ischemia performed 4–6 weeks after the index PCI. The test was considered diagnostic when heart rate reached at least 85% of target heart rate.
Exclusion criteria for the current analysis were:
Cardiogenic shock at presentation
Previous coronary artery bypass grafting (CABG)
Death, revascularization or re-infarction within 1 month of the index event
Multivessel disease without non-invasive testing for residual ischemia or with findings of significant ischemia following the index procedure
The study primary endpoint was the 1-year and 3-year cumulative rates of major adverse cardiovascular events (MACE) defined as a composite of all-cause mortality, revascularization (i.e. coronary artery bypass grafting and/or catheter-based target vessel revascularization) and re-infarction (excluding events occurring during the first 30 days after the index PCI). Secondary endpoints included the rates of individual components of MACE at 1 and 3-years.
All events were further adjudicated by a research coordinator and reviewed by an experienced cardiologist from our research team. For each patient, a standardized questionnaire was completed either by telephone or in the outpatient clinic at 1, 6, 12, 24 and 36-month follow-ups. Mortality was confirmed by the records of the Interior Ministry of Israel. Repeat revascularization procedures and episodes of reinfarction were confirmed using the hospital as well as affiliated hospitals databases. These databases were searched for all patients in the study to gather information regarding repeat events. The diagnosis of reinfarction during follow-up was based on recurrent chest pain, suggestive of acute MI, accompanied by re-elevation of the cardiac enzyme with at least one value above the 99th percentile upper reference limit at least 48 hours after PCI and/or new ST elevation, new left bundle branch block or development of pathological Q waves in the electrocardiogram. Target vessel revascularization was defined as any revascularization that involved the target vessel. Stent thrombosis was defined according to the Academic Research Consortium definitions as “definite” in the context of acute coronary syndrome and/or reinfarction in the culprit coronary territory with angiographically proven thrombosis (thrombus or occlusion) of the previously implanted stent.
Statistical Analysis
Data are presented as mean ± standard deviation for normally distributed variables and as median (interquartile range (IQR)) for non-normally distributed variables. Continuous variables were compared using Student’s t testing or Mann Whitney testing, as appropriate. Categorical variables were compared using chi-square statistics or Fischer’s exact testing, as appropriate.
Time-to-event curves using the Kaplan-Meier method were calculated and compared using with the Log-Rank testing. Cox proportional hazards regression model using the enter method to control for confounders that are expected to be related to long-term outcomes (age, sex, glomerular filtration rate, diabetes mellitus, left anterior descending artery disease, pre-TIMI flow grade<2 and left ventricle ejection fraction) was performed.
All tests were two-tailed, and a P-value <0.05 was considered significant. Analyses were performed using SPSS 21.0 statistical software package (IBM SPSS Inc).
Results
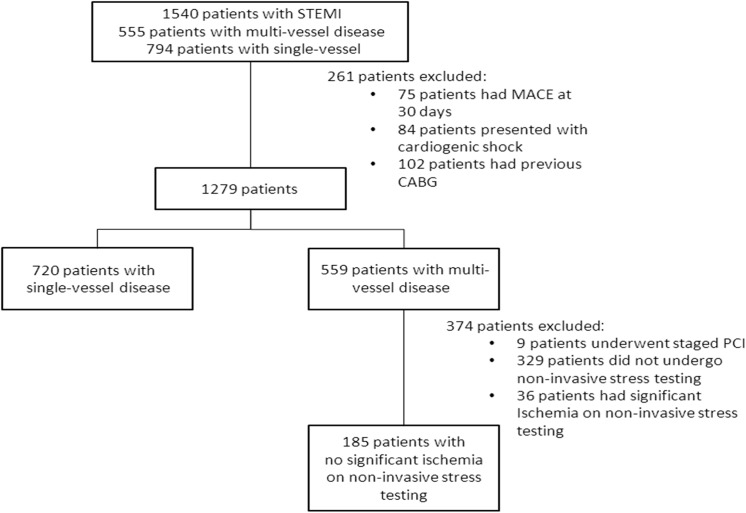
A total of 905 patients were included in the present analysis, of whom 720 had single-vessel disease and 185 had multi-vessel disease (73 with 3-vessel disease) without evidence of significant residual ischemia on subsequent non-invasive stress testing (Fig 1).
Fig 1. Study cohort.
Baseline and angiographic characteristics of the patients with multi-vessel disease vs. patients with single-vessel disease are summarized in Table 1.
Table 1. Baseline and angiographic characteristics of patients with multivessel disease vs. patients with single-vessel disease.
| Single-vessel disease (n = 720) | Multivessel disease (n = 185) | P value | |
|---|---|---|---|
| Mean age (± SD), years | 57 ± 12 | 62 ± 10 | <0.001 |
| Male, % | 80.7 | 85.4 | 0.14 |
| GFR, ml/min | 92±27 | 86±26 | 0.63 |
| GFR <60ml/min, % | 9 | 12.8 | 0.12 |
| Diabetes Mellitus, % | 21.9 | 27.7 | 0.11 |
| Hypertension, % | 40.3 | 53.8 | 0.001 |
| Dyslipidemia, % | 46.5 | 52.7 | 0.13 |
| Smoking, % | 62.4 | 51.1 | 0.007 |
| Current smoker, % | 51.2 | 38.3 | |
| Past smoker, % | 11.2 | 12.8 | |
| History of MI, % | 6.5 | 13.1 | 0.015 |
| History of angioplasty, % | 9.8 | 17.1 | <0.001 |
| Previous CVA, % | 3.2 | 6.1 | 0.06 |
| Peripheral vascular disease, % | 3.5 | 3.4 | 0.94 |
| Killip class≥2, % | 10.3 | 14 | 0.15 |
| Ejection fraction≤40%, % | 39.2 | 38.3 | 0.86 |
| Infarct location, % | 0.005 | ||
| Anterior | 53.3 | 40 | |
| Inferior | 41.7 | 54.1 | |
| Lateral | 5 | 5.9 | |
| LAD culprit | 55.7 | 40 | 0.001 |
| Pre-TIMI Flow 0 | 56.5 | 60 | 0.4 |
| Post-TIMI Flow 3 | 95.6 | 96.2 | 0.66 |
| Thrombus aspiration, % | 17.2 | 14.5 | 0.19 |
| Glycoprotein IIb/IIIa inhibitors, % | 74.8 | 77.3 | 0.31 |
CVA = Cerebrovascular accident, GFR = Glomerular filtration rate; MI = Myocardial infarction, LAD = Left anterior descending artery; TIMI = Thrombolysis In Myocardial Infarction
Patients with multi-vessel disease were older than patients with single vessel disease (mean difference of 4.8 years, p<0.001). They were more likely to have hypertension and less likely to be current or ex-smokers than patients with single-vessel disease. Previous MI was more prevalent amongst patients with multi-vessel disease than in patients with single-vessel disease (13.1% vs. 6.5%, p = 0.015). A greater proportion of patients with single-vessel disease presented with anterior MI as compared with patients with multi-vessel disease (53.3% vs. 40.0%, p = 0.005). Neither the Killip class nor the left ventricular systolic function differed significantly between the 2 groups.
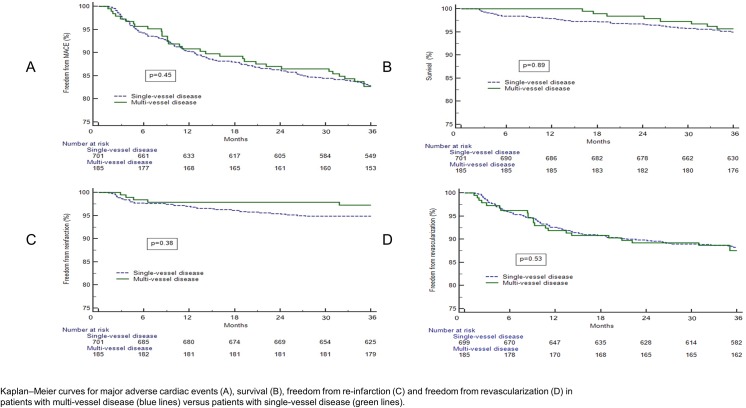
Mean follow up was 83.6 ± 32 months. Clinical outcomes are presented in Table 2, Fig 2.
Table 2. Outcomes of patients with single-vessel disease vs. patients with multi-vessel disease (excluding first 30 days post-PPCI).
| 1-year outcome (%) | Single-vessel disease | Multi-vessel disease | Log-Rank, P value |
| Mortality | 2.5 | 0 | 0.03 |
| Re-infarction | 3.3 | 2.8 | 0.4 |
| Revascularization | 8.1 | 7.6 | 0.83 |
| Major adverse cardiac events | 10.9 | 8.6 | 0.39 |
| 3-year outcome (%) | Single-vessel disease | Multivessel disease | Log-Rank, P value |
| Mortality | 5 | 5.4 | 0.89 |
| Re-infarction | 5.3 | 4.8 | 0.38 |
| Revascularization | 11.7 | 13.5 | 0.53 |
| Major adverse cardiac events | 17.1 | 20 | 0.45 |
Fig 2. Clinical Outcomes of Patients with Multi-Vessel Disease vs. Patients with Single-Vessel Disease.
Kaplan–Meier curves for major adverse cardiac events (A), survival (B), freedom from re-infarction (C) and freedom from revascularization (D) in patients with multi-vessel disease (blue lines) versus patients with single-vessel disease (green lines).
MACE rates calculated by time to first event up to 1 and 3-years were similar between the 2 groups (Table 2, Fig 2A). There were no mortality events in the multi-vessel disease group during the first year after the index procedure (excluding the first 30 days) compared with a 2.5% mortality rate in the single-vessel disease group (p = 0.03). At 3-years after PPCI, mortality rate of patients with multi-vessel disease was similar to that of patients with single-vessel disease. Re-infarction and revascularization rates at 1 and 3-years did not differ between patients with single-vessel disease and those with multi-vessel disease (Fig 2B–2D).
On cox proportional hazards regression model, multi-vessel disease without residual ischemia was not independently associated with MACE and its components at 1 and 3-years, whereas diabetes was independently associated with both MACE at 1 and 3-years and female sex for MACE at 1-year (Tables 3 and 4).
Table 3. Multiple Cox regression models for the association between multi-vessel disease and 1 and 3-year outcomes (excluding first 30 days post-PPCI.
| 1-year Outcome | Unadjusted HR [95%] CI, P value | Adjusted* HR [95%] CI, P value |
| Mortality | N/A** | N/A** |
| Re-infarction | 1.5 [0.52–4.38], P = 0.44 | 1.83 [0.62–5.4], P = 0.27 |
| Revascularization | 0.94 [0.52–1.68], P = 0.83 | 1.15 [0.63–2.1], P = 0.65 |
| MACE | 1.5 [0.88–2.65], P = 0.12 | 1.27 [0.71–2.23], P = 0.42 |
| 3-year Outcome | Unadjusted HR [95%] CI, P value | Adjusted* HR [95%] CI, P value |
| Mortality | 1.03 [0.51–2.08], P = 0.92 | 0.74 [0.33–1.65], P = 0.46 |
| Re-infarction | 0.68 [0.31–1.54], P = 0.36 | 0.80 [0.34–1.86], P = 0.61 |
| Revascularization | 1.15 [0.72–1.76], P = 0.53 | 1.37 [0.86–2.19], P = 0.18 |
| MACE | 1.10 [0.78–1.63], P = 0.51 | 1.10 [0.8–1.75], P = 0.39 |
* Adjusted for age, sex, glomerular filtration rate, diabetes mellitus, left anterior descending artery disease, pre-TIMI flow grade<2 and left ventricle ejection fraction
** No mortality events in the multi-vessel group
CI = Confidence interval; HR = Hazard ratio; MACE = Major adverse cardiac events
Table 4. Multiple Cox regression for MACE at 1 and 3 years.
| Hazard Ratio | 95% Confidence interval | P value | Hazard Ratio | 95% Confidence interval | P value | |
|---|---|---|---|---|---|---|
| Age | 0.99 | 0.97–1.01 | 0.42 | 1.00 | 0.99–1.02 | 0.53 |
| GFR | 0.99 | 0.99–1.000 | 0.49 | 1.01 | 0.99–1.01 | 0.90 |
| MVD | 0.91 | 0.52–1.58 | 0.73 | 1.17 | 0.79–1.73 | 0.43 |
| LAD culprit | 1.07 | 0.52–1.58 | 0.79 | 1.08 | 0.75–1.57 | 0.66 |
| Pre PCI TIMI grade flow<2 | 1.06 | 0.69–1.65 | 0.77 | 1.27 | 0.89–1.79 | 0.18 |
| Diabetes | 1.88 | 1.19–2.94 | 0.006 | 1.79 | 1.27–2.54 | 0.0007 |
| Female sex | 1.69 | 1.02–2.76 | 0.04 | 1.46 | 0.98–2.15 | 0.08 |
| Ejection fraction during index hospitalization | 0.99 | 0.97–1.02 | 0.49 | 0.99 | 0.97–1.01 | 0.46 |
GFR = Glomerular filtration rate; LAD = Left anterior descending artery; MACE = Major adverse cardiac events; MVD = Multi-vessel disease; PCI = Percutaneous coronary intervention; TIMI = Thrombolysis in Myocardial Infarction
Patients with adverse outcomes occurring during the first 30 days following the procedure were not included in the final analysis (as patients underwent non-invasive testing usually within 4 weeks of the index procedure). A sub-analysis of patients who experienced an adverse outcome during the first 30 days after the index event revealed that the MACE rates at 30 days did not differ significantly between patients with single-vessel disease and patients with multi-vessel disease treated with culprit-PCI only (4.6% vs. 6.4%, respectively, Log-Rank p = 0.13). Mortality rates at 30-days were higher in the multi-vessel group, as compared with the single vessel group (3.9% vs. 1.7%,) but after adjustment for confounders multi-vessel disease per se did not remain independently associated with mortality at 30-days.
Discussion
Multi-vessel coronary artery disease is prevalent amongst patients undergoing primary PCI for STEMI [1,2]. While current guidelines recommend that in stable patients primary PCI should be limited to the culprit vessel, there has been an ongoing debate regarding the necessity and timing of revascularization of the non-culprit vessels after the acute event has resolved. Some authors advocate PCI of the non-infarct related vessels with the aim to achieve complete revascularization whenever possible. In contrast, others support ischemia-guided therapy in order to detect functionally significant lesions and to avoid unnecessary interventions. Several meta-analyses have shown that staged-PCI is associated with better outcomes compared with multi-vessel primary PCI and culprit-only PCI [21–23]. However none of these meta-analyses scrutinized the additive value of assessment of ischemia prior to staged-PCI.
We have found that patients with STEMI and multi-vessel coronary disease treated with primary PCI of the culprit vessel-only, who had not demonstrated significant residual ischemia on subsequent non-invasive stress testing, had similar outcomes at 1 and 3-years after the index event to those patients with single vessel disease. Interestingly, 1-year mortality rates were lower in the multi-vessel disease group than in the single-vessel disease group. A possible explanation is the exclusion of patients who experienced an adverse event within the first 30 days following the index event from the analysis.
We have also shown that patients with multi-vessel disease treated with culprit-only PCI had similar 30-day outcomes to patients with single vessel disease. These data may imply that it is safe to postpone stress testing following STEMI until a few weeks have elapsed.
Notably in the vast majority of patients with multi-vessel disease who had undergone non-invasive stress testing in our cohort (83.7%) the non-culprit arteries were not associated with significant residual ischemia on noninvasive stress testing performed 4–6 weeks after the index event. Indeed, Hanratty et al have previously shown that a significant exaggeration of non-culprit stenosis severity occurred at the acute MI angiography in 21% of patients [20]. Moreover, Dambrinkt et al have found that hemodynamic significance of non-culprit lesions, as detected by fractional flow reserve during primary PCI, was overestimated in 40% of the lesions [5]. Several hypotheses on the causes of the exaggeration in non-culprit lesions stenosis have been suggested including increased circulating catecholamine levels, enhanced bioactivity of important coronary vasoconstrictors, such as serotonin, endothelin, angiotensin, and thromboxane and reduced vasodilatory effects of nitric oxide, adenosine and prostacyclin [24–28].
It is noteworthy, that in contrast to the aforementioned studies, the recent PRAMI study, has found that preventive PCI at time of the index procedure (in culprit and non-culprit coronary arteries with significant stenosis) was superior to PCI limited to the culprit artery in reduction of MACE [14]. However, the study did not address the question of immediate preventive PCI during the index procedure versus staged PCI within days-weeks of the MI. Additionally, its relatively small sample size (n = 465) and premature cessation might cause the end points to appear more significant than they might be with a longer follow-up. Likewise, the CvLPRIT trial, has found that complete revascularization at the time of the PPCI or during the index hospitalization yielded better outcomes at 12 months than culprit-only PCI [15]. However, similar to the PRAMI trial this was a relatively small sized study (n = 278) that did not differentiate between an early staged vs. multivessel PCI strategies during the course of STEMI. Two meta-analyses of randomized controlled trials, conducted following the PRAMI and CvLPRIT trials by Elgendy et al and Dahal et al, suggested that multi-vessel PCI at the time of PPCI for STEMI resulted in better outcomes than culprit-vessel only PCI [29,30]. However, both included limited number of studies (i.e. 4 and 6) with a relatively small number of patients in each study except for the PARMI trial, which, thus, had the greatest weight of the sample size. Additionally, the design and follow-up time in the studies were variable. Furthermore, the meta-analysis by Elgendy et al. was underpowered for hard outcomes such as mortality and non-fatal MI [29]. Notably, although Dahal et al. found reduced MACE with multi-vessel PCI compared with culprit-only PCI, there was no significant difference in MACE between staged-PCI and multi-vessel PCI during the index procedure [30].
Limitations
The current study has several limitations. First, it was a retrospective and nonrandomized single center study that was subjected to our local mode of practice. Second, only 40% of patients with multi-vessel disease underwent non-invasive stress testing. Third, we excluded patients who had an adverse event during the first 30 days following the index procedure, as patients underwent non-invasive testing usually within 4 weeks following the index procedure. Hence, the outcome analysis did not include events occurring up to 30 days after the index procedure. Information about the cause of death was not available; therefore we could only assess the rate of all cause mortality. Pervious studies have shown that approximately 30% of patients have residual stress defects following PCI and are known to be at higher risk of adverse events. However, since performing non-invasive stress testing following PPCI in patients with SVD is not common at our medical center we cannot rule out residual ischemia in patients with SVD.
Conclusions
We have shown that patients with STEMI and multi-vessel disease treated with culprit-only PCI without significant residual myocardial ischemia on non-invasive stress testing have similar short- and long-term prognosis to STEMI patients with single vessel disease. These findings support a strategy of employing non-invasive stress testing for the assessment of ischemia in patients with STEMI and multi-vessel disease following the culprit artery PCI. Such strategy can guide the management and prevent unnecessary interventions in this STEMI patient population.
Supporting Information
(XLS)
(DOCX)
Abbreviations
- MACE
Major adverse cardiac events
- STEMI
ST-segment elevation myocardial infarction
- PCI
Percutaneous coronary angioplasty
- TIMI
Thrombolysis In Myocardial Infarction
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Sorajja P, Gersh BJ, Cox DA, McLaughlin MG, Zimetbaum P, Costantini C, et al. Impact of multivessel disease on reperfusion success and clinical outcomes in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Eur Heart J 2007; 28: 1709–16. [DOI] [PubMed] [Google Scholar]
- 2. Webb JG, Lowe AM, Sanborn TA, White HD, Sleeper LA, Carere RG, et al. Percutaneous coronary intervention for cardiogenic shock in the SHOCK trial. J Am Coll Cardiol 2003; 42: 1380–6. [DOI] [PubMed] [Google Scholar]
- 3. Di Mario C, Sansa M, Airoldi F, Sheiban I, Manari A, Petronio A, et al. Single vs multivessel treatment during primary angioplasty: results of the multicentre randomized HEpacoat for cuLPrit or multivessel stenting for Acute Myocardial Infarction (HELP AMI) Study. Int J Cardiovasc Intervent 2004; 6:128–133. [DOI] [PubMed] [Google Scholar]
- 4. Corpus RA, House JA, Marso SP, Grantham JA, Huber KC, Laster SB, et al. Multivessel percutaneous coronary intervention in patients with multivessel disease and acute myocardial infarction. Am Heart J 2004; 148:493–500. [DOI] [PubMed] [Google Scholar]
- 5. Dambrink JH, Debrauwere JP, van 't Hof AW, Ottervanger JP, Gosselink AT, Hoorntje JC, et al. Non-culprit lesions detected during primary PCI: treat invasively or follow the guidelines? EuroIntervention. 2010;5(8):968–75. [PubMed] [Google Scholar]
- 6. Varani E, Balducelli M, Aquilina M, Vecchi G, Hussien MN, Frassineti V, et al. Single or multivessel percutaneous coronary intervention in ST elevation myocardial infarction patients. Cathet Cardiovasc Interv 2008;72:927–33 [DOI] [PubMed] [Google Scholar]
- 7. Qarawani D, Nahir M, Abboud M, Hazanov Y, Hasin Y. Culprit only versus complete coronary revascularization during primary PCI. Int J Cardiol. 2008. January 24;123(3):288–92. [DOI] [PubMed] [Google Scholar]
- 8. Kornowski R, Mehran R, Dangas G, Nikolsky E, Assali A, Claessen BE, et al. Prognostic impact of staged versus “One-Time” Multivessel Percutaneous Intervention in acute myocardial infarction- Analysis from the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) Trial. J Am Coll Cardiol: 58, 7. [DOI] [PubMed] [Google Scholar]
- 9. Toma M, Buller CE, Westerhout CM, Fu Y, O’Neill WW, Holmes DR, et al. Non-culprit coronary artery percutaneous coronary intervention during acute ST-segment elevation myocardial infarction: insights from the APEX-AMI trial. European Heart Journal 2010; 31:1701–1707 10.1093/eurheartj/ehq129 [DOI] [PubMed] [Google Scholar]
- 10. Cavender MA, Milford-Beland S, Roe MT, Peterson ED, Weintraub WS, Rao SV. Prevalence, predictors, and in-hospital outcomes of non-infarct artery intervention during primary percutaneous coronary intervention for ST- segment elevation myocardial infarction (from the National CardiovascularData Registry). Am J Cardiol 2009;104:507–13. 10.1016/j.amjcard.2009.04.016 [DOI] [PubMed] [Google Scholar]
- 11. Hannan EL, Samadashvili Z, Walford G, Holmes DR Jr, Jacobs AK, Stamato NJ, et al. Culprit Vessel Percutaneous Coronary Intervention Versus Multivessel and Staged Percutaneous Coronary Intervention for ST-Segment Elevation Myocardial Infarction Patients With Multivessel Disease. J Am Coll Cardiol Intv 2010;3:22–31 [DOI] [PubMed] [Google Scholar]
- 12. Ijsselmuiden AJ, Ezechiels J, Westendorp IC, Tijssen JG, Kiemeneij F, Slagboom T, et al. Complete versus culprit vessel percutaneous coronary intervention in multivessel disease: A randomized comparison. Am Heart J 2004;148:467–74 [DOI] [PubMed] [Google Scholar]
- 13. Vlaar PJ, Mahmoud KD, Holmes DR Jr, van Valkenhoef G, Hillege HL, van der Horst IC, et al. Culprit vessel only versus multivessel and staged percutaneous coronary intervention for multivessel disease in patients presenting with ST-segment elevation myocardial infarction: a pairwise and network meta-analysis. J Am Coll Cardiol. 2011. August 9;58(7):692–703 10.1016/j.jacc.2011.03.046 [DOI] [PubMed] [Google Scholar]
- 14. Wald DS, Morris JK, Wald NJ, Chase AJ, Edwards RJ, Hughes LO, et al. Randomized Trial of Preventive Angioplasty in Myocardial Infarction. N Engl J Med 2013; 369:1115–1123 10.1056/NEJMoa1305520 [DOI] [PubMed] [Google Scholar]
- 15. Gershlick AH, Khan JN, Kelly DJ, Greenwood JP, Sasikaran T, Curzen N, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015. March 17;65(10):963–72. 10.1016/j.jacc.2014.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction. J Am Coll Cardiol. 2013;61(4):e78–e140. 10.1016/j.jacc.2012.11.019 [DOI] [PubMed] [Google Scholar]
- 17.Authors/Task Force m, Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35(37):2541–619. 10.1093/eurheartj/ehu278 [DOI] [PubMed] [Google Scholar]
- 18. The Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J (2012) 33, 2569–2619 10.1093/eurheartj/ehs215 [DOI] [PubMed] [Google Scholar]
- 19. Biondi-Zoccai G, Lotrionte M, Sheiban I. Management of multivessel coronary disease after ST-elevation myocardial infarction treated by primary coronary angioplasty. Am Heart J. 2010. December;160(6 Suppl):S28–35 10.1016/j.ahj.2010.10.013 [DOI] [PubMed] [Google Scholar]
- 20. Hanratty CG, Koyama Y, Rasmussen HH, Nelson GIC, Hansen PS, Ward MR. Exaggeration of nonculprit stenosis during acute myocardial infarction: implication for immediate multivessel revascularization. J Am Coll Cardiol 2002; 40:911–6. [DOI] [PubMed] [Google Scholar]
- 21. Vlaar PJ, Mahmoud KD, Holmes DR Jr, van Valkenhoef G, Hillege HL, van der Horst IC, et al. Culprit vessel only versus multivessel and staged percutaneous coronary intervention for multivessel disease in patients presenting with ST-segment elevation myocardial infarction: a pairwise and network meta-analysis. J Am Coll Cardiol. 2011. August 9;58(7):692–703 10.1016/j.jacc.2011.03.046 [DOI] [PubMed] [Google Scholar]
- 22. Bainey KR, Mehta SR, Lai T, Welsh RC. Complete vs culprit-only revascularization for patients with multivessel disease undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: a systematic review and meta-analysis. Am Heart J. 2014. January;167(1):1–14.e2. 10.1016/j.ahj.2013.09.018 [DOI] [PubMed] [Google Scholar]
- 23. Bagai A, Thavendiranathan P, Sharieff W, Al Lawati HA, Cheema AN. Non- infarct-related artery revascularization during primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: a systematic review and meta-analysis. Am Heart J. 2013. October;166(4):684–693.e1 10.1016/j.ahj.2013.07.027 [DOI] [PubMed] [Google Scholar]
- 24. Gibson CM, Ryan KA, Murphy SA, Mesley R, Marble SJ, Giugliano RP, et al. Impaired coronary blood flow in nonculprit arteries in the setting of acute myocardial infarction The TIMI Study Group. Thrombolysis In Myocardial Infarction. J Am Coll Cardiol 1999;34:974–82 [DOI] [PubMed] [Google Scholar]
- 25. Fuster V, Stein B, Ambrose JA, Badimon L, Badimon JJ, Chesebro JH. Atherosclerotic plaque rupture and thrombosis. Evolving concepts. Circulation 1990;82:47–59. [PubMed] [Google Scholar]
- 26. Stewart DJ, Kubac G, Costello KB, Cernacek P. Increased plasma endothelin-1 in the early hours of acute myocardial infarction. J Am Coll Cardiol 1991;18:38–43. [DOI] [PubMed] [Google Scholar]
- 27. Reilly MP, Delanty N, Roy L, Rokach J, Callaghan PO, Crean P, et al. Increased formation of the isoprostanes IPF2alpha-I and 8- epi-prostaglandin F2alpha in acute coronary angioplasty: evidence for oxidant stress during coronary reperfusion in humans. Circulation 1997;96:3314–20. [DOI] [PubMed] [Google Scholar]
- 28. Hempel SL, Wessels DA, Spector AA. Effect of glutathione of endothelial prostacyclin synthesis after anoxia. Am J Physiol 1993;264:1448–57. [DOI] [PubMed] [Google Scholar]
- 29. Elgendy IY, Huo T, Mahmoud A, Bavry A. Complete versus culprit-only revascularization in patients with multi-vessel disease undergoing primary percutaneous coronary intervention: A meta-analysis of randomized trials. Int J Cardiol. 2015. May 1;186:98–103 10.1016/j.ijcard.2015.03.163 [DOI] [PubMed] [Google Scholar]
- 30. Dahal K, Rijal J, Panta R, Lee J, Azrin M, Lootens R. Multi-vessel versus culprit-vessel and staged percutaneous coronary intervention in STEMI patients with multivessel disease: a meta-analysis of randomized controlled trials. Cardiovasc Revasc Med. 2014. Nov-Dec;15(8):408–13. 10.1016/j.carrev.2014.10.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.