Abstract
Purpose
Interruptions in medical treatment such as dose delays, reductions, or stoppages can lead to suboptimal treatment of cancer. Knowing how and for whom symptom severity and symptom interference with activities of daily living (ADL) are associated with treatment interruptions can guide behavioral interventions for supportive care. The purpose of this analysis is to inform research and clinical practice by bringing attention to specific patient symptoms that may hinder dose completion.
Methods
A secondary analysis of data collected in a randomized clinical trial (RCT) of reflexology for symptom management was performed. The trial enrolled women with advanced breast cancer undergoing treatment (N=385). Outcome data were collected at baseline, weeks 5 and 11 using a valid and reliable measure. Medical records provided data on treatment interruptions and metastasis. The association between alterations in medical treatment during the study period with symptom severity, symptom interference with ADL and metastatic status were tested using generalized estimating equations (GEE) models.
Results
The relationship between dose delays and dose reductions and symptom severity was differential according to metastatic status, with the higher strength of association among women with distant metastasis compared to those with loco-regional disease (p=0.02). The interaction of symptom interference and metastatic status was also significantly related to dose delays and reductions (p=0.04). Severity of pain was a stronger predictor of dose delays or reductions among patients with distant metastasis compared to those with loco-regional disease (p<0.01).
Conclusion
The analysis highlights the importance of understanding symptom outcomes that impact research, practice, and treatment decisions.
Introduction
Symptom burden associated with cancer and its treatment is often the primary reason for treatment interruptions such as dose delays, dose reductions or even stoppage of chemotherapy.1,2 Alterations in medical treatment can lead to suboptimal treatment at the very least,3,4 and life threatening recurrence or shorter overall survival at the extreme.5–7 Yet, few studies have evaluated the association between symptom severity and treatment interruptions.4 Most studies that address treatment interruptions compare specific chemotherapy protocols.4,8 From the patient’s perspective, it is often the severity of symptoms that leads them to request a change in dosage. Patients’ willingness to report their symptoms to oncologists is supported in some literature,9,10 while other papers discuss a lack of concordance between patient reports and provider documentation of patient symptoms.11,12 Patients’ reluctance to report their symptoms to oncologists has also been noted.13,14 When patients either do not report their symptoms or under-report their symptom severity, oncologists may not take symptom information into account when deciding upon dosage. Therefore, the association between the symptom severity experienced by patients and treatment interruptions may or may not exist.
This secondary analysis uses data from a randomized clinical trial (RCT) of a complementary therapy, i.e., reflexology, that was administered to women with advanced breast cancer for symptom management during medical treatment with chemotherapy, hormonal or targeted therapy.15 The purpose of this analysis is to investigate the role that patient-reported symptoms may play in dose delays, dose reductions and stoppages among breast cancer patients. Understanding the factors associated with such changes in treatment is important for improving both the quality of medical treatment and patient symptom management.
Intravenous (IV) chemotherapy involves a dose and duration defined by the patient’s oncologist, and this protocol dose is delivered unless altered by the oncologist or the patient missing scheduled infusions. Predictors of poor patient adherence or under use of treatment are older age, comorbidity, and practical issues such as transportation and distance from the clinic.16–18 Symptoms and toxicities that may contribute to treatment interruptions include nausea, vomiting, neuropathy, myelosuppression, febrile neutropenia, and dermatologic toxicities.1,2,19 Fairclough et al.20 reported that a higher dose intensity of chemotherapy had a negative impact on quality of life, including greater symptom burden. Looking specifically at the population of women with advanced breast cancer, Du et al.21 reported that those with late stage breast cancer had nearly twice as many symptoms as early stage. Thornton et al.22 found no differences in overall quality of life ratings according to the presence or absence of distant metastasis, but significantly lower perceived functional status among metastatic breast cancer patients. Wyatt et al.23 found that breast cancer patients with distant metastasis experience greater pain and poorer physical functioning than those with less advanced disease. Vilhauer24 also reported that women with metastatic breast cancer decreased physical activity due to physical symptoms. Such decreases in physical functioning due to symptom burden among metastatic breast cancer patients may lead to alterations in medical management.
This current investigation of the relationship between symptoms and treatment interruptions may be especially important for women with advanced breast cancer, and has to account for the status of metastasis. In this report, consideration is given to overall symptom burden, as reflected by the symptom severity and interference scores summed across an array of cancer- and treatment-related symptoms. In addition, severities of specific prevalent symptoms in this population (pain, fatigue and dyspnea) are considered in the following research question:
Are dose delays, reductions or stoppages in treatment associated with summed symptom severity, symptom interference with daily activity, and severities of specific prevalent symptoms (pain, fatigue and nausea), and are these associations different according to metastatic status?
Methods
Women with advanced breast cancer undergoing chemotherapy, hormonal or targeted therapy (N=385) were enrolled in a RCT of reflexology for symptom management.(N=385). Participants were interviewed at baseline and were randomized to receive 4 weekly sessions of reflexology, lay foot manipulation or standard care. Randomization balanced the groups with respect to recruitment site, levels of pain and fatigue at intake, and goal of therapy (i.e., curative, maintenance, palliative, or uncertain). Data on symptoms were collected via telephone interviews at baseline and study weeks 5 and 11. Medical records were reviewed for data on treatment interruptions during the study. Primary trial results are reported elsewhere.15 Briefly, the reflexology group had a significant improvement in physical function compared to controls, and this improvement was mediated by a reduction in the severity of dyspnea. The present paper leverages the existing large sample data from women with advanced breast cancer for a secondary analysis of treatment interruptions in relation to longitudinal symptom data.
The Sample
Women were eligible to enter the study if they: 1) were diagnosed with advanced breast cancer (stage III or IV); 2) were 21 years of age or older; 3) were able to perform basic activities of daily living (ADLs); 4) were cognitively intact and free of a diagnosis of mental illness on their medical record; 5) were able to speak and understand English; 6) had access to a telephone; 7) were able to hear normal conversation well; and 8) were receiving chemotherapy and/or hormonal therapy at intake into the study. Exclusion criteria were: 1) receiving hospice care at intake; 2) residing in a nursing home or similar care facility; 3) bedridden; 4) regularly using a complementary therapy similar to those used in the study (reflexology or lay foot manipulation); and 5) undergoing bone marrow transplantation.
Data Collection
Participants were recruited at 14 participating oncology clinics in the Midwest. Once recruited, they completed an initial baseline telephone interview. A second interview occurred post-intervention at week 5. At 11 weeks post-baseline, a third telephone interview was completed. Medical records were reviewed following the participant’s completion of the week 11 interview to record data on clinical variables corresponding with the 11 weeks on study.
Measures
Measures of symptoms were obtained using patient self-report during interviews at baseline, weeks 5 and 11. A 25-item cancer symptom inventory created by Given et al.,25 was used. It measures symptom severity and impact on activities of daily living. The inventory included the following symptoms: dyspnea, cough, weakness, numbness or tingling, fever, weight loss (more than 2 pounds a week), muscle wasting, hand or arm swelling, nausea, vomiting, lack of appetite, diarrhea, constipation, dry mouth, changes in taste, mouth sores, bleeding or bruising, sleep disturbance, restlessness, mood changes, difficulty concentrating, dizziness, changes in speech pattern, episodes of confusion, memory problems.25 For each symptom, women rated symptom severity on a 0–3 Likert scale (0=none, 1-mild, 2=moderate, 3=severe), and the interference of the symptom with activities of daily living on a 0–4 Likert scale (0=no extent, 1=small extent, 2=some extent, 3=great extent, and 4=very great extent). The severity scores were summed into a symptom severity index, and the interference with activities of daily living scores were summed into a symptom interference index.26
Measures of pain and fatigue were obtained using the Brief Pain Inventory and Brief Fatigue Inventory.27,28 Items reflecting severity of pain at its worst and severity of fatigue at its worst were rated on a scale from 0=”not present” to 10=”as bad as you can imagine.” These severity ratings were used in the analysis.
A measure of physical function was obtained from the Medical outcomes study short form 36 (MOS SF-36), physical functioning subscale.29 The total scores range from 0 to 100 with higher scores reflecting better functioning. Physical function was considered in the analysis to facilitate the interpretation of the results, since physical functioning is associated with severity of multiple symptoms.29,30
The chart audit covered the same 11 weeks that women were in the study unless they dropped out before week 11, in which case the audit covered the time before attrition. The following data were obtained from charts: comorbidity, cancer recurrence and metastasis status, goal of therapy, agents used, their dates of administration, and dates of dose delays, dose reductions or dose stoppages for each agent, along with reasons. There were a total of 29 different agents used in the sample during the study period, 8 of which were hormonal and 3 were targeted. There were 18 different one-agent protocols, 75 different two-drug combinations, 61 different three drug combinations. Some women received up to 7 different agents given either consecutively or concurrently during the study period. Therefore treatment was summarized in the following categories: hormonal therapy only, singe agent chemotherapy, multiple agent chemotherapy, targeted therapy or targeted therapy and chemotherapy. The latter 5 categories may or may or include additional hormonal therapy.
Treatment interruptions (dose delays, reductions or stoppages) were defined based on their documentation in patients’ medical records. Each of the 14 clinic sites used similar methods for documenting symptoms and changes in medical protocols; however some documentation may have been incomplete.11 Interruptions recorded by the clinicians at each of the study 14 sites were all analyzed. The date of each interruption, i.e., dose delay, dose reduction, or dose stoppage, was used to place it either between the baseline interviews and study week 5, or between study weeks 5 and 11 interviews. This resulted in three binary variables, i.e., dose delay, dose reduction, dose stoppage, with 2 repeated measures for each (yes/no between baseline and week 5, and yes/no between weeks 5 and 11). These repeated measures were investigated in relation to symptoms reported during the interview that preceded each interruption. For women who were on multiple agents, summary variables of treatment interruptions during each time period (baseline to week 5 or week 5 to week 11) were assigned a value “yes” if any of the agents were delayed, reduced in dose, or stopped (respectively) during this time period.
Statistical Considerations
The distributions of demographic characteristics, symptom and functioning variables, and clinical variables were summarized for the entire study sample and by metastatic status and compared using chi-square, Fisher’s exact or t-tests. Based on observed counts for dose delays, dose reductions and dose stoppages, dose delays and dose reductions were combined into one category of treatment interruptions, while dose stoppage was kept separate. Reasons for dose delays or reductions and dose stoppages were summarized for each occurrence.
Generalized estimating equations (GEE) modeling were used for the analysis of repeated measures of binary outcome variables. Repeated measures of dose delays and dose reductions (between baseline to week 5, and week 5 to week 11) were entered as an outcome (dependent) variable in the GEE model. Based on the literature review,1,2,16–19 the following explanatory (independent) variables were included: age, number of comorbid conditions, time period, symptom severity, metastatic status, and interaction between symptom severity and metastatic status. Study group was not included in the model to avoid colinearity with the symptom severity variable, since the primary analysis of the trial showed improvements in symptoms according to study group: dyspnea was reduced in the reflexology group, and fatigue was reduced in the lay foot manipulation group.15
First, symptom severity was measured using a summed index from the inventory of multiple symptoms. It was a time-varying covariate so that the severity of symptoms reported at baseline was related to dose delays and reductions that occurred between baseline and week 5, and symptom severity at week 5 was related to dose delays and reductions that occurred between week 5 and week 11. The question about moderating effects of metastatic status on the relationship between symptom severity and treatment interruptions was answered by the test of the significance of the interaction term. When the interaction term was significant, the sample was stratified based on metastatic status, odds ratios (ORs), and the confidence intervals for ORs for the symptom severity index in metastatic and non-metastatic groups were reported. This analysis was then repeated using the symptom interference index instead of the symptom severity index, and using severities of specific prevalent symptoms (pain, fatigue, dyspnea). Dose stoppages were analyzed next following the same approach as described above. To facilitate the interpretation of findings, the physical functioning score was explored as a possible predictor of treatment interruptions, due to the association between symptoms and physical functioning reported in the literature.30–32 All statistical tests were two-sided. SAS 9.4 was used for all analyses.
Results
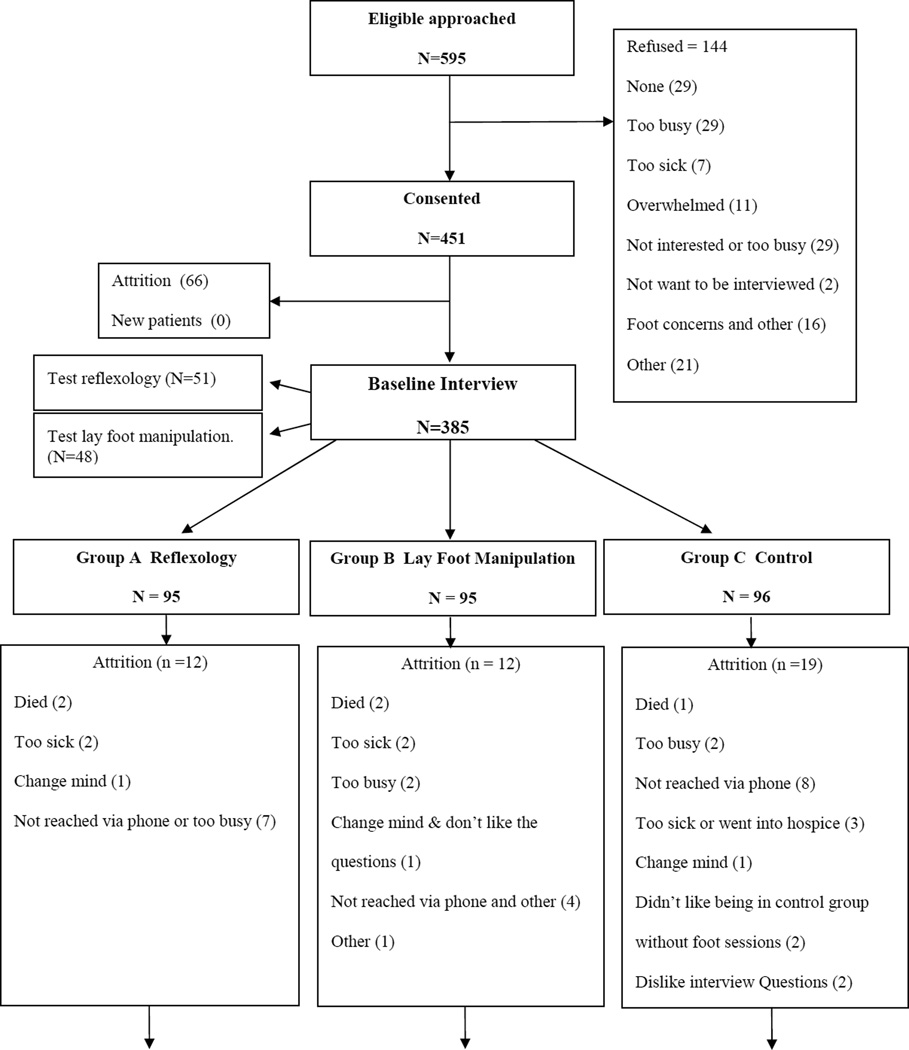
The flow of participants through the study is presented in Figure 1; demographic and clinical characteristics of the study sample are summarized in Table 1. The distributions of outcome variables of dose delays or reductions and dose stoppages are provided according to the timing of these interruptions: between baseline and week 5; and during weeks 5 through 11. As noted in the measures section, summary variables of treatment interruptions were created for women who received multiple agents during each time period, or had multiple interruptions of the same agent. Thus as shown in Table 1, 51 patients had at least one of the drugs reduced or delayed at least once between baseline and week 5, and 21 patients had at least one drug delayed or reduced at least once between weeks 5 and 11. A total of 319 patients (83%) did not have dose delays or reductions in the entire study period. The corresponding numbers for patients with at least one drug stoppage in the respective time periods were 31 and 24 (see Table 1). A total of 332 patients (86%) did not have treatment stoppages. The reasons for interruptions were given for each instance of each interruption of each drug, producing a greater number of interruptions than the count of women who experienced interruptions (see Table 2).
Figure 1.
Flow of the participants throughout the trial
Table 1.
Demographic and clinical characteristics of study participants (N=385)
| Entire sample N=385 |
Non- Metastatic N=87 |
Metastatic N=298 |
P-value | |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Race | ||||
| Caucasian/White | 322 (83%) | 76 (87%) | 246 (83%) | .55 |
| Other | 53 (14%) | 9 (10%) | 44 (15%) | |
| Missing | 10 (3%) | 2 (3%) | 8 (2%) | |
| Employment | ||||
| Employed | 134 (35%) | 40 (46%) | 94 (32%) | .03 |
| Not employed | 249 (65%) | 46 (53%) | 203 (68%) | |
| Missing | 2 (<1%) | 1 (1%) | 1 (<1%) | |
| Education | ||||
| High School or Less | 103 (27%) | 19 (22%) | 84 (28%) | .46 |
| At Least Some College | 279 (72%) | 67 (77%) | 212 (71%) | |
| Missing | 3 (1%) | 1 (1%) | 2 (1%) | |
| Marital Status | ||||
| Married / living with a partner | 246 (64%) | 56 (64%) | 190 (64%) | .40 |
| Not married | 135 (35%) | 29 (33%) | 106 (36%) | |
| Missing | 4 (1%) | 2 (<3%) | 2 (<1%) | |
| Recurrent Disease | ||||
| Yes | 127 (33%) | 22 (25%) | 105 (35%) | .19 |
| No | 247 (64%) | 63 (72%) | 184 (62%) | |
| Missing | 11 (3%) | 2 (<3%) | 9 (3%) | |
| Goal of therapy | ||||
| Uncertain | 37 (10%) | 10 (12%) | 27 (9%) | <.01 |
| Curative | 148 (38%) | 66 (76%) | 82 (28%) | |
| Maintenance | 117 (30%) | 9 (10%) | 108 (36%) | |
| Palliative | 82 (21%) | 2 (2%) | 80 (27%) | |
| Missing | 1 (1%) | 0 (0%) | 1 (<1%) | |
| Type of treatment | .06 | |||
| Hormonal therapy only | 88 (23%) | 15 (17%) | 73 (25%) | |
| Singe agent chemotherapy (with or without hormonal therapy) | 51 (13%) | 9 (10%) | 42 (14%) | |
| Multiple agent chemotherapy (with or without hormonal therapy) | 86 (22%) | 29 (33%) | 57 (19%) | |
| Targeted agent(s) (with or without hormonal therapy) | 35 (9%) | 9 (10%) | 26 (9%) | |
| Targeted agent(s) and chemotherapy agent(s) combinations | 125 (32%) | 25 (29%) | 100 (33%) | |
| Mean (St Dev) Median (Range) | Mean (St Dev) Median (Range) | Mean (St Dev) Median (Range) | ||
| Age | 55.7 (11.05) 54.82 (25–85) |
52 (9.61) 51.5 (33–73) |
56.8 (11.23) 56.1 (25–85) |
<.01 |
| Comorbid conditions | 0.81 (1.07) 0 (0–5) |
1.09 (1.24) 1 (0–4) |
0.73 (1.01) 0 (0–5) |
<.01 |
| Number of symptoms at baseline | 8.66 (4.91) 8 (0–25) |
9.02 (4.52) 9 (0–19) |
8.55 (5.02) 8 (0–25) |
.43 |
| Physical function at baseline | 55.18 (27.36) 55 (0–100) |
60.52 (26.18) 65 (5–100) |
53.62 (27.55) 55 (0–100) |
.04 |
| Patient Count (%) | Patient Count (%) | Patient Count (%) | ||
| Patients with dose delays or reductions | ||||
| Between baseline and week 5 | 51 (13%) | 8 (9%) | 43 (14%) | .21 |
| Between weeks 5 and11 | 21 (5%) | 4 (5%) | 17 (6%) | .69 |
| Patients with dose stoppages | ||||
| Between baseline and week 5 | 31 (8%) | 8 (9%) | 23 (8%) | .66 |
| Between weeks 5 and11 | 24 (6%) | 3 (3%) | 21 (7%) | .22 |
Table 2.
Summary of reasons for dose delays or reductions and dose stoppages.
| Reasons for dose delays or reductions | Total N=155 instances N (%) |
| Symptoms | 51 (33%) |
| Abnormal laboratory values – neutropenia or thrombocytopenia | 32 (22%) |
| Infections | 13 (8%) |
| Patient decision or non-compliance | 11 (7%) |
| Hospitalization | 10 (6%) |
| Disease Progression | 10 (6%) |
| Other | 28 (18%) |
| Reasons for dose stoppages | Total N=106 instances N (%) |
| Disease progression, entering hospice, or death | 62 (58%) |
| Symptoms | 25 (24%) |
| Patient decision to stop treatment | 7 (7%) |
| Hospitalization or surgery | 5 (5%) |
| Other | 7 (6%) |
The GEE models for the outcomes of dose delays or reductions indicated the interaction between the symptom severity index and metastatic status was significant (p=0.01, data for the model with interaction are not shown), and the interaction between the summed symptom interference index and metastatic status was significant as well (p=.04, data for the models with interaction terms are not shown). To clarify the nature of these significant interactions, the sample was stratified on metastatic status, and the ORs for symptom variables in metastatic and non-metastatic groups are summarized in Table 3. The direction of the association between symptom variables and dose delays or reductions was different among women with distant metastasis compared to those with loco-regional disease. For those with no metastasis (first panel of Table 3), there was a negative association between symptom severity and probability of dose delay or reduction reflected by ORs that are less than 1. This association was statistically significant for the summed symptom severity index (OR=0.92, p=.02) and the severity of pain (OR=.60, p<.01). In contrast, among women with metastatic disease (second panel of Table 3), the estimate of the OR was greater than 1 indicating that greater symptom severity was associated with greater probability of dose delays and reductions. This association was also significant for the severity of pain (OR=1.09, p=.02). In the analysis of dose stoppages, the interaction of symptom severity with metastatic status was not statistically significant (data not shown), and in the analysis stratified on metastatic status, the main effect of symptom severity on dose stoppages was also not significant (see Table 4).
Table 3.
Summary of GEE models relating the outcome of dose delays or reductions to symptom severity, symptom interference, severity of pain, severity of fatigue, severity of dyspnea (one at a time, summarized in table rows), adjusted for age and comorbidity
| Outcome: dose delays or reductions | ||||||||
|---|---|---|---|---|---|---|---|---|
| Non-metastatic subgroup | Metastatic subgroup | |||||||
| Predictor | Adjusted OR estimate (SE) |
Confidence interval for OR |
P-value | Adjusted OR estimate (SE) |
Confidence interval for OR |
P-value | ||
| Summed symptom severity index | 0.92 (0.03) | (0.86 | 0.99) | .02 | 1.01 (0.01) | (0.98 | 1.04) | 0.44 |
| Summed symptom interference index | 0.93 (0.03) | (0.87 | 1.00) | .07 | 1.01 (0.01) | (0.99 | 1.04) | 0.26 |
| Severity of pain | 0.60 (0.10) | (0.43 | 0.83) | <.01 | 1.09 (0.04) | (1.01 | 1.17) | 0.02 |
| Severity of fatigue | 0.94 (0.12) | (0.74 | 1.19) | .59 | 1.05 (0.05) | (0.96 | 1.15) | 0.29 |
| Severity of dyspnea | 0.73 (0.21) | (0.41 | 1.28) | .27 | 1.11 (0.13) | (0.88 | 1.42) | 0.38 |
Table 4.
Summary of GEE models relating the outcome of dose stoppages to symptom severity, symptom interference, severity of pain, severity of fatigue, severity of dyspnea (one at a time, summarized in table rows), adjusted for age and comorbidity
| Outcome: dose stoppages | ||||||||
|---|---|---|---|---|---|---|---|---|
| Non-metastatic subgroup | Metastatic subgroup | |||||||
| Predictor | Adjusted OR estimate (SE) |
Confidence interval for OR |
P-value | Adjusted OR estimate (SE) |
Confidence interval for OR |
P-value | ||
| Summed symptom severity index | 1.01 (0.05) | (0.92 | 1.10) | .90 | 1.01 (0.02) | (0.98 | 1.04) | .44 |
| Summed symptom interference index | 1.00 (0.04) | (0.93 | 1.08) | .98 | 1.00 (0.02) | (0.98 | 1.04) | .60 |
| Severity of pain | 1.04 (0.08) | (0.89 | 1.22) | .64 | 1.04 (0.05) | (0.94 | 1.15) | .50 |
| Severity of fatigue | 1.10 (0.12) | (0.90 | 1.36) | .35 | 1.02 (0.06) | (0.91 | 1.14) | .73 |
| Severity of dyspnea | 0.88 (0.21) | (0.56 | 1.40) | .59 | 1.23 (0.16) | (0.95 | 1.58) | .12 |
Finally, because of the association between symptoms and function, findings were assessed to determine if they would hold when symptom severity was replaced with physical function as a predictor of treatment interruptions. The answer was negative, indicating that while many symptoms may affect function, it is symptoms that are associated with dose delays and dose reductions differentially according to metastatic status and not the physical function.
Discussion
In response to the research question posed for these data, three key findings were obtained on the associations between symptom severity, dosage alterations, interference with daily activity and metastatic status. First, summed symptom severity and pain severity were significantly associated with dose delays or dose reductions, and the direction of these associations was dependent on women having metastatic disease. The dependence on metastatic status may reflect clinician’s reluctance to modify the dose in patients treated with curative intent, as opposed to patients treated for palliation. While this is speculation on such clinical decisions, it is known that dose reductions and delays, if leading to a substantial reduction in relative dose intensity, have been found to potentially decrease overall survival.33
Second, the fact that summed symptom severity was not significantly related to treatment stoppages in this sample of patients with advanced disease reflects the finding that 58% of all drug stoppages were due to disease progression, admittance to hospice or death. In other words, clinical judgments determined these women too ill to continue medical therapy; even though symptom-related reasons accounted for only 24% of stoppages.
Finally, none of the three dose indicators, i.e., delays, reductions or stoppages, were significantly related to physical functioning. While other investigators have reported a link between symptoms and physical functioning and activities of daily living,34 physical function was not a predictor of alterations in medical management based on these data.
In summary, this secondary analysis bridges the symptom experience of women with advanced stage breast cancer to treatment outcomes important to clinical practice. Clinicians may make dosage decisions based on metastatic status as well as patient-reported symptoms, especially pain.
Limitations
Limitations of this report include a primarily Caucasian sample of women and a fairly large number of medications. Due to this large number, specific symptoms were not assessed as to whether they were associated with delays or reductions in the dose of each specific agent Second, data on supportive medical care such as transfusions and symptom medications were not uniformly available across all clinical sites.11 For example, patients with stage III disease might have received Neulasta, which could cause considerable pain, but despite this pain, the dose intensity was maintained. By contrast, in the situation of metastatic disease, Neulasta is typically not given, and pain can be a reflection of disease progression rather than treatment toxicity. This could explain why more severe pain was associated with lower probability of dose delays and reductions among non-metastatic subgroup, and with higher probability in the metastatic subgroup. Possibilities such as this underscore the importance of collecting detailed chart data to complement patient-reported outcomes in symptom management trials.
Conclusions
The finding of no significant relationship between symptom severity and dose delays/reductions among non-metastatic breast cancer patients by no means diminishes the importance of supportive symptom management interventions. Symptom management interventions improve quality of life during cancer treatment, but may not go as far as reducing treatment interruptions among patients with loco-regional disease. In this reflexology trial, the symptom of dyspnea was improved in the reflexology arm, and fatigue was improved in the lay foot manipulation arm,15 but neither dyspnea nor fatigue severity were found to be related to treatment interruptions in the present analysis.15 In limited evidence from past trials that included both the assessments of patient-reported symptoms and treatment interruptions based on medical charts, dose delays and reductions moderated the effect of a cognitive-behavioral intervention on symptom severity, i.e., the intervention was more successful in the absence of treatment interruptions.35 Neutropenia had similar moderating effects among patients with solid tumors undergoing chemotherapy.36 In future trials of symptom management interventions that target pain or other symptoms, assessing treatment interruptions may be a valuable addition to patient-reported outcomes. As types of treatment evolve and the resulting patient symptom experience changes, the analyses of treatment interruptions data from symptom management trials may provide further evidence on the links between symptom severity and treatment interruptions, and ways to maintain both medical protocols and quality of life during cancer treatment.
Acknowledgement
National Cancer Institute
Grant1R01CA104883-01A1
Footnotes
Conflict of Interest
The research being reported in this publication was supported by the National Cancer Institute. No conflicts of interest exist. Authors have control of the primary data and agree to allow the journal to review their data if requested.
Contributor Information
Gwen Wyatt, College of Nursing, Michigan State University, 1355 Bogue Street; Room C345, East Lansing, MI 48824, gwyatt@msu.edu, p: (517) 353-6672, f: (517) 353-4587.
Alla Sikorskii, Department of Statistics and Probability, Michigan State University, 619 Red Cedar Road, Room C423, East Lansing, MI 48824.
Irena Tesnjak, Department of Statistics and Probability, Michigan State University, 619 Red Cedar Road, Room C423, East Lansing, MI 48824.
David Victorson, Department of Medical Social Sciences, Feinberg School of Medicine, Northwestern University, 2205 Tech Drive, Hogan Building (Suite 2-120), Evanston, IL 60208.
Gordan Srkalovic, Director, Clinical Trials, Sparrow Health System, 1215 E. Michigan Ave, Lansing, MI 48912.
References
- 1.Barbour SY. Caring for the treatment-experienced breast cancer patient: the pharmacist's role. Am J Health Syst Pharm. 2008 May 15;65(10) Suppl 3:S16–S22. doi: 10.2146/ajhp080090. [DOI] [PubMed] [Google Scholar]
- 2.Leonard K. A European survey relating to cancer therapy and neutropenic infections: nurse and patient viewpoints. Eur J Oncol Nurs. 2012 Sep;16(4):380–386. doi: 10.1016/j.ejon.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Chirivella I, Bermejo B, Insa A, et al. Optimal delivery of anthracycline-based chemotherapy in the adjuvant setting improves outcome of breast cancer patients. Breast Cancer Res Treat. 2009 Apr;114(3):479–484. doi: 10.1007/s10549-008-0018-1. [DOI] [PubMed] [Google Scholar]
- 4.Kubicek GJ, Kimler BF, Wang F, Reddy EK, Girod DA, Williamson SK. Chemotherapy in head and neck cancer: clinical predictors of tolerance and outcomes. Am J Clin Oncol. 2011 Aug;34(4):380–384. doi: 10.1097/COC.0b013e3181e9c0a2. [DOI] [PubMed] [Google Scholar]
- 5.Yood MU, Owusu C, Buist DS, et al. Mortality impact of less-than-standard therapy in older breast cancer patients. J Am Coll Surg. 2008 Jan;206(1):66–75. doi: 10.1016/j.jamcollsurg.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 6.Kim J, Kim Y, Lee K, et al. The early discontinuation of palliative chemotherapy in older patients with cancer. Supportive Care in Cancer. 2014;22(3):773–781. doi: 10.1007/s00520-013-2033-y. [DOI] [PubMed] [Google Scholar]
- 7.Lyman GH. Impact of chemotherapy dose intensity on cancer patient outcomes. Journal of the National Comprehensive Cancer Network. 2009;7(1):99–108. doi: 10.6004/jnccn.2009.0009. [DOI] [PubMed] [Google Scholar]
- 8.McLellan B, Kerr H. Cutaneous toxicities of the multikinase inhibitors sorafenib and sunitinib. Dermatol Ther. 2011 Jul-Aug;24(4):396–400. doi: 10.1111/j.1529-8019.2011.01435.x. [DOI] [PubMed] [Google Scholar]
- 9.Trotti A, Colevas A, Setser A, Basch E. Patient-reported outcomes and the evolution of adverse event reporting in oncology. J Clin Oncol. 2007;25(32):5121–5127. doi: 10.1200/JCO.2007.12.4784. [DOI] [PubMed] [Google Scholar]
- 10.Basch E, Artz D, Dulko D, Scher K, Sabbatini P. Patient Online Self-Reporting of Toxicity Symptoms During Chemotherapy. J Clin Oncol. 2005;23(15):3552–3561. doi: 10.1200/JCO.2005.04.275. [DOI] [PubMed] [Google Scholar]
- 11.Sikorskii A, Wyatt G, Tamkus D, Victorson D, Rahbar M, Ahn S. Concordance between patient reports of cancer-related symptoms and medical records documentation. Journal of Pain and Symptom Management. 2012;44(3):362–372. doi: 10.1016/j.jpainsymman.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stromgren AS, Groenvold M, Pedersen L, Olsen AK, Spile M, Sjogren P. Does the medical record cover the symptoms experienced by cancer patients receiving palliative care? A comparison of the record and patient self-rating. Journal of Pain & Symptom Management. 2001;21(3):189–196. doi: 10.1016/s0885-3924(01)00264-0. [DOI] [PubMed] [Google Scholar]
- 13.Volgezan NJ, Breitbart W, Cella DF, et al. Patient, caregiver, and oncologist perception of cancer-related fatigue: Resuslts of a tri-part assessment survey. Seminars in Hematology. 1997;34:4–12. [PubMed] [Google Scholar]
- 14.Mazzotti E, Cappellini GCA, Buconovo S, et al. Treatment-related side effects and quality of life in cancer patients. Supportive Care in Cancer. 2012;20(10):2553–2557. doi: 10.1007/s00520-011-1354-y. [DOI] [PubMed] [Google Scholar]
- 15.Wyatt G, Sikorskii A, Rahbar M, Victorson D, You M. Health-related quality of life outcomes: A reflexology trial with patients with advanced-stage breast cancer. Oncology Nursing Forum. 2012 Nov;39(6):568–577. doi: 10.1188/12.ONF.568-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Margalit DN, Mamon HJ, Ancukiewicz M, et al. Tolerability of combined modality therapy for rectal cancer in elderly patients aged 75 years and older. Int J Radiat Oncol Biol Phys. 2011 Dec 1;81(5):e735–e741. doi: 10.1016/j.ijrobp.2010.12.056. [DOI] [PubMed] [Google Scholar]
- 17.MA AMT, Barone J, Wallis AE, et al. Noncompliance with adjuvant radiation, chemotherapy, or hormonal therapy in breast cancer patients. The American Journal of Surgery. 2008;196(4):500–504. doi: 10.1016/j.amjsurg.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 18.Salloum RG, Smith TJ, Jensen GA, Lafata JE. Factors associated with adherence to chemotherapy guidelines in patients with non-small cell lung cancer. Lung Cancer. 2012;75:255–260. doi: 10.1016/j.lungcan.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gandhi M, Oishi K, Zubal B, Lacouture M. Unanticipated toxicities from anicancer therapies: survivors' perspectives. Supportive Care in Cancer. 2010;18(11):1461–1468. doi: 10.1007/s00520-009-0769-1. [DOI] [PubMed] [Google Scholar]
- 20.Fairclough DL, Fetting JH, Cella D, Wonson W, Moinpour CM. Quality of life and quality adjusted survival for breast cancer patients receiving adjuvant therapy. Quality of Life Research. 1999;8(8):723–731. doi: 10.1023/a:1008806828316. [DOI] [PubMed] [Google Scholar]
- 21.Du XL, Osborne C, Goodwin JS. Population-based assessment of hospitalizations for toxicity from chemotherapy in older women with breast cancer. J Clin Oncol. 2002 Dec 15;20(24):4636–4642. doi: 10.1200/JCO.2002.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thornton LM, Madlensky L, Flatt SW, Kaplan RM, Pierce JP. The impact of a second breast cancer diagnosis on health related quality of life. Breast Cancer Research and Treatment. 2005;92(1):25–33. doi: 10.1007/s10549-005-1411-7. [DOI] [PubMed] [Google Scholar]
- 23.Wyatt G, Sikorskii A, Tamkus D, You M. Quality of life among advanced breast cancer patients with and without distant metastasis. European Journal of Cancer Care. 2013;22:272–280. doi: 10.1111/ecc.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vilhauer RP. A qualitative study of the experiences of women with metastatic breast cancer. Palliative and Supportive Care. 2008;6:249–258. doi: 10.1017/S1478951508000382. [DOI] [PubMed] [Google Scholar]
- 25.Given CW, Stommel M, Given B, Osuch J, Kurtz ME, Kurtz JC. The influence of cancer patients' symptoms and functional status on patients' depression and family caregivers' reaction and depression. Health Psychol. 1993;12(4):277–285. doi: 10.1037//0278-6133.12.4.277. [DOI] [PubMed] [Google Scholar]
- 26.Given C, Given B, Rahbar M, et al. Effect of a cognitive behavioral intervention on reducing symptom severity during chemotherapy. Journal of Clinical Oncology. 2004;22(3):507–516. doi: 10.1200/JCO.2004.01.241. [DOI] [PubMed] [Google Scholar]
- 27.Cleeland CS. Assessment of pain in cancer: Measurement issues. Advances in Pain Research and Therapy. 1990;16:47–55. [Google Scholar]
- 28.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999 Mar 1;85(5):1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 29.Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. Boston: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 30.Kurtz M, Kurtz J, Stommel M, Given C, Given B. Symptomatology and loss of physical functioning among geriatric patients with lung cancer. Journal of Pain and Symptom Management. 2000;19(4):249–256. doi: 10.1016/s0885-3924(00)00120-2. [DOI] [PubMed] [Google Scholar]
- 31.Van Onselen C, Cooper BA, Lee K, et al. Identification of distinct subgroups of breast cancer patients based on self-reported changes in sleep disturbance. Supportive Care Cancer. 2012;20(10):2611–2619. doi: 10.1007/s00520-012-1381-3. [DOI] [PubMed] [Google Scholar]
- 32.Gerber L, Stout N, McGarvey C, et al. Factors predicting clinically significant fatigue in women following treatment for primary breast cancer. Supportive Care in Cancer. 2011;19(10):1581–1591. doi: 10.1007/s00520-010-0986-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vavra KL, Saadeh CE, Rosen AL, Uptigrove CE, Srkalovic G. Improving the relative dose intensity of systemic chemotherapy in a community-based outpatient cancer center. Journal of Oncology Practice. 2013 doi: 10.1200/JOP.2012.000810. Published ahead of print. [DOI] [PubMed] [Google Scholar]
- 34.Bertheussen G, Kaasa S, Hokstad A, et al. Deasibility and canges in skymptoms and functioning following inpatient cancer rehabilitation. Acta Oncologica. 2012;51(8):1070–1080. doi: 10.3109/0284186X.2012.699684. [DOI] [PubMed] [Google Scholar]
- 35.Sikorskii A, Given C, Given B, Jeon S, McCorkle R. Testing the effects of treatment complications on a cognitive behavioral intervention for reducing symptom severity. Journal of Pain and Symptom Management. 2006;32(2):129–139. doi: 10.1016/j.jpainsymman.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Given BA, Given CW, Jeon S, Sikorskii A. The effect of neutropenia on the impact of a cognitive-behavioral intervention for symptom management. Cancer. 2005;104(3):869–878. doi: 10.1002/cncr.21240. [DOI] [PubMed] [Google Scholar]