Abstract
Background
Over the past decades, advances in neonatal care have led to substantial increases in survival among preterm infants. With these gains, recent concerns have focused on increases in neurodevelopment morbidity related to the interplay between stressful early life experiences and the immature neuro-immune systems. This interplay between these complex mechanisms is often described as the brain-gut signaling system. The role of the gut microbiome and the brain-gut signaling system have been found to be remarkably related to both short and long term stress and health. Recent evidence supports that microbial species, ligands, and/or products within the developing intestine play a key role in early programming of the central nervous system and regulation of the intestinal innate immunity.
Purpose
The purpose of this state-of-the-science review is to explore the supporting evidence demonstrating the importance of the brain-gut-microbiota axis in regulation of early life experience. We also discuss the role of gut microbiome in modulating stress and pain responses in high-risk infants. A conceptual framework has been developed to illustrate the regulation mechanisms involved in early life experience.
Conclusions
The science in this area is just beginning to be uncovered; having a fundamental understanding of these relationships will be important as new discoveries continue to change our thinking; leading potentially to changes in practice and targeted interventions.
Keywords: early life experience, microbiome, brain-gut-microbiota, neonates
The United States ranks sixth highest in the world for the number of preterm births, with more than 12% of infants born before 37 weeks of gestation, or more than one infant born preterm in nine of all births.1 Over the past decade, advances in neonatal care have contributed to a substantial increase in survival among preterm birth infants.2 Of the 500,000 plus preterm infants who do survive outside the protective intrauterine environment, most are subjected to numerous stressors including repeated painful invasive procedures, infection, daily discomfort care, light, noise, and maternal separation. Neonatal insults yield a high risk of substantial short- and long-term neurological morbidity, e.g., 39.4% of NICU survivors have at least one neurodevelopment deficit.3 Very preterm infants (< 32 weeks gestation) have an especially high risk of early life stress during the highly technical intensive care period which has been linked to serious neurodevelopmental deficits and chronic metabolic conditions, such as diabetes and obesity.4 The result in related costs is at least $26.2 billion per year plus the added burden for families and society.5 Yet, the onset of the altered neuro-immune progress induced by the stressful early life experience is often insidious and the mechanisms remain largely unclear.
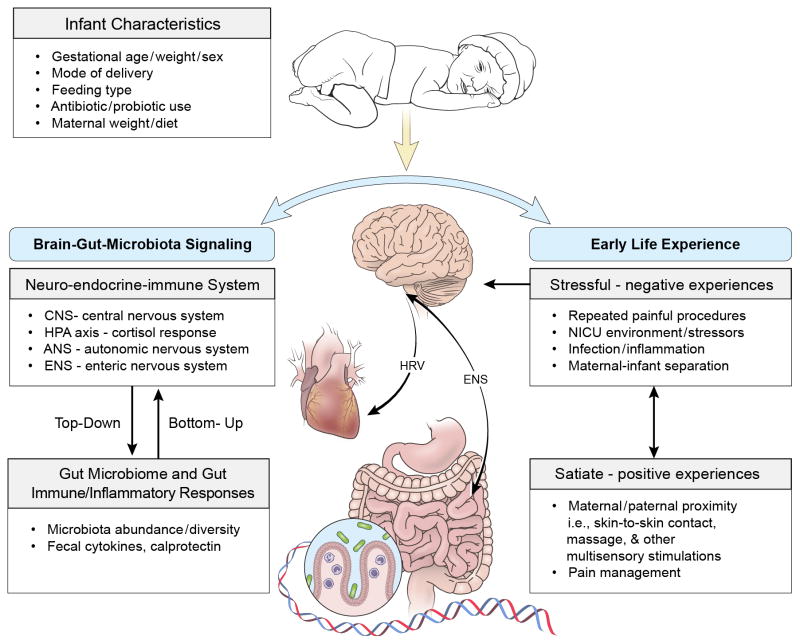
The etiologies of infants’ neurodevelopment and health outcomes are complex and multifactorial.6 Recent research supports a functional communication between the central nervous system (CNS) and gastrointestinal (GI) tract, the brain-gut-microbiota signaling system, in which the intestinal microbiome is proposed to play a key role in regulation of stress and early programming of the neuro-immune system.7 The purpose of this state-of-the-science review is to explore the supporting evidence demonstrating importance of the brain-gut-microbiota axis in the regulation of early life experience and the role of the gut microbiome in modulating stress and pain responses in high-risk infants. A conceptual framework has been developed to illustrate the regulation mechanism involved in early life experience (Figure 1).
Figure 1.
Regulation of early life stress by the brain-gut-microbiota axis.
HRV – heart rate variability system; ENS – enteric nervous system
Gut Microbiome Development in Infancy
The entirety of microorganisms in a particular habitat is identified as the microbiota or microflora, and the natural GI microbiota is predominantly composed of bacteria, as well as nonbacterial organisms, including fungi, viruses, and archaea.8 Microbiome refers to the microbiota and the habitat it colonizes and also refers to the collective genomes of the microbes or the metagenome.8,9 The microbiome and its physiological role have been redefined in recent years through the initiation of the large-scale metagenomic project, Human Microbiome Project (HMP), launched by the National Institute of Health in 2008. The remarkable development of culture-independent molecular techniques has broadened our understanding of the microbiota thereby detecting an expansive microbial world. The GI tract harbors nearly 1013 to 1014 microorganisms, which is more than 10 times the number of cells in the human body and 150 times as many genes as found in the human genome.7 Most interestingly, in addition to skin, oral cavity, airway tract, urogenital tract, and GI tract, microbiota have also been found in the human brain, the immune-privileged site.10 The role of the microbiota in health and disease has been taken to center stage because strong evidence has shown that gut microbiota can greatly influence all aspects of physiology, including brain-gut communication and brain function as well as cognition and behavior.11
For a long time, it has been thought that the human fetal gut is sterile and the colonization begins during and immediately after birth. However, recent reports show interesting results that meconium microbiota are detected in healthy term newborns and suggest that the meconium microbiota have an intrauterine origin and participate in gut colonization.12 The debate about the likelihood of prenatal mother to infant transfer of microbiota has begun from the insights of these recent findings.13 Moreover, other recent findings demonstrate that the placenta harbors a unique microbiome niche, composed of nonpathogenic commensal microbiota. In addition, the placental microbiome is associated with a remote history of antenatal infection and with preterm birth.14 The intrauterine effects on the development of infant gut microbiota are still largely unknown and the hypothesis of microbial prenatal colonization still needs to be rigorously proven.15 This is an area where more research is needed and our understanding is only at the beginning stages.
Healthy Gut Microbiome for Neonates
Although the exact composition of the gut microbiota is still not known, advances in culture-independent DNA-based genomic technologies have recently begun to characterize gut microbial patterns.16 Newborn gut microbial colonization begins with facultative anaerobes, followed by the establishment of anaerobic genera, such as Bifidobacterium, Bacteroides, and Clostridium. Neonatal gut microbiome development is complex and influenced by many factors including mode of delivery, maternal diet and nutrition, infant gestational age, feeding options, environmental factors of care, and use of antibiotics and/or probiotics.13,17,18 Infants born at full-term via vaginal delivery have greater microbial diversity that is often more desirable than pre-term infants born via Caesarian section because they are exposed to normal maternal vaginal, fecal and epithelial flora. Interestingly, a study reported that mothers’ GI microbial community also changes profoundly during pregnancy to support a healthy infant delivery.19 The mother’s intestine is an important source for the vaginally delivered infant’s intestinal microbiota in that the mother’s bifidobacterium strains in the gut can be transmitted and colonize the infant’s intestine shortly after birth.20 Moreover, breastfed infants can gain additional microbiota through milk and contact with the mother’s skin.21 Six Bifidobacterium strains have been isolated from human breast-milk showing phenotypical and genotypical characters of commercial probiotic, which are important specifically for neonates and also potential for use in targeting interventions, such as probiotics for infants.22 The Bifidobacteria are one of the most predominant intestinal microorganisms in full-term breast-fed infants, which is considered the gold standard of a healthy intestinal microbiota for neonates.23 Most recent studies have demonstrated linkages between breastfeeding and early microbial colonization with later growth patterns, and specifically, detection of Bacteroides appears to be associated with protection against obesity.24,25 Derived from these findings, full-term vaginal delivery combined with breast milk feeding is considered ideal for infant gut microbiome development, by which the gut and adaptive immune system tolerate and regulate the microbiota community, and in turn the microbiome shapes gut immune and metabolic function.26
Gut Microbial Characteristics for Preterm Infants
An important concept is that an appropriate balance in the diversity and type of microbes within the microbiota plays a critical role in maintaining the health status of the host. The alteration or imbalance of microbiota, termed dysbiosis or dysbacteriosis, especially in the GI tract has been associated with many acute and chronic health conditions, such as inflammatory bowel disease, obesity, and cardiovascular disease.27,28 The gut microbiome in preterm infants has been found to be different from that in healthy-term infants because of immaturity, antibiotics use and the long stay at the hospital environment instead of a home setting. Preterm infants apparently have a delay in development of commensal bacteria and an increased colonization by potentially pathogenic microorganisms, showing reduced microbiota diversity,29,30 reduced levels of strict anaerobes,31 and a relatively high abundance of proteobacteria.32 Cohort studies exploring the development of the gut microbiota found the abundance of anaerobes, including Bifidobacterium to be low in preterm infants and researchers hypothesize that the delay in anaerobic establishment may detain immune maturation in this population.31,33 Researchers also found that distortions of the gut microbiota were correlated with necrotizing enterocolitis (NEC) and late-onset sepsis (LOS) in preterm infants and showed altered microbiota appeared in NEC and LOS infants several weeks before the diagnosis.34,35 Changing patterns of bacterial dysbiosis, so that Proteobacteria and Firmicutes emerge, are pivotal factors in development of NEC and LOS, two major causes of infant mortality and morbidity.34,35 These findings support the hypothesis that changes in normal microbiota composition, but not an enrichment of potential pathogens are associated with illness in preterm infants; however, the causality has not been proven.34–36 More research about how and when the microbiota changes over time in preterm infants who are healthy and those who develop NEC and LOS is needed.
The Brain-Gut-Microbiota Signaling System
The brain and the gut have been well known to reciprocally affect each other by constant communication. For example, when GI dysfunction occurs it is communicated to the brain and leads to the perception of nausea or pain, and in turn, when stressful events occur the CNS responses lead to altered GI secretions and motility.37 Most recently, the brain-gut-microbiota axis has been recognized as an important mechanism in the regulation of stress response through a bi-directional homeostatic route of communication, and the role of the gut microbiome has been found to be remarkably related to stress and health.27 The components of the brain-gut-microbiota axis includes the CNS, the endocrine-immune system, the hypothalamus-pituitary-adrenal (HPA) axis, the sympathetic-parasympathetic autonomic nervous system (ANS), the enteric nervous system (ENS), and the intestinal microbiota.11,28 This bidirectional communication network enables top-down signaling from the brain to influence the motor, sensory and secretory modalities of the GI tract, and conversely, bottom-up signaling from the gut to affect brain function, most notably the hypothalamus and amygdala that have many functions devoted to stress.
Brain-to-Gut Top-down Signaling
In the past decades, culture-based studies have shown that exposing laboratory animals to novel environments or changes in environmental stimuli can have a significant impact on the stability of the gut microbiota.38 Recent studies further support that the brain-gut signaling system enables the brain to influence GI functions including motility, permeability, secretion and mucin production, as well as modulate immune responses such as with inflammation including cytokine production by cells of the mucosal immune system.38,39 Findings in animals as well as humans indicate that exposure to stressful stimuli results in activation of multiple physiological responses and influences the composition of microbial communities. These changes in microbial communities can have both short and long term effects. For example, stress activates HPA axis and sympathetic system, which leads to increased gut permeability allowing for the crossing of bacteria and bacterial antigens through the epithelial barrier, their presence activates a mucosal immune response; that in turn alters the composition of the microbiome.38,40 Within a mouse model, adult mice with exposure to a social disruption stressor displayed substantial changes to the structure of the gut microbial community immediately after stressor exposure, as well, the stress-induced dysbiosis (meaning alterations in gut microbiota) resulted in a long term increase in circulating inflammatory markers.41 Moreover, bacteria in the genus Lactobacillus, which are considered “healthy” bacteria can be consistently reduced in the gut during stressor exposure, while the relative abundance of different bacterial types are altered during stress.42 Why some bacteria increase and others decrease are areas where more investigation is still needed.
Psychosocial and physical stressors during the early stages of life, including maternal separation, have been found to be associated with maladaptive stress responses and alterations of the functions of the GI tract which may have a great impact on health in later life.43,44 In animal models, when male rat pups were stressed by separating them from their mothers for 3 hours daily after birth, they were found to have increased plasma corticosterone level, systemic immune responses, visceral sensation and altered fecal microbiota as compared to the control group.45 These findings tell us that early life stress affects the composition of the gut microbiota, strongly suggesting a brain-to-gut regulation of microbiota composition.
In humans, stress or depression can influence the progress of chronic gastrointestinal illnesses such as inflammatory bowel diseases (IBD) and irritable bowel syndrome (IBS) via the brain-gut axis. Physical and emotional factors involving stress have been found to influence the integrity of the intestinal epithelium and alter gut motility, secretions and mucin production, which lead to changing the habitat of resident bacteria and promoting alterations in microbial composition and/or activity. Additionally, release of catecholamines into the gut during stress may influence the microbial community by interfering with interbacterial signaling and with bacterial virulence gene expression.39 The enhanced responsiveness of central stress and emotion circuits through exposure to physical and psychological stressors can occur in IBS patients resulting in manifesting altered modulation of GI function and altered emotional and perceptual responses to visceral events, such as enhanced perception of abdominal discomfort and pain.40,46 The cause of IBS is still not clear, but the current thinking is that the etiology of IBS is multifactorial and may be due to primary changes in the CNS (top-down), or primary alterations in the GI (bottom-up), or by a combination of bidirectional effects.46
Gut-to-Brain Bottom-up Signaling
An exciting concept is emerging that alterations in the composition of the gut microbiota affect a variety of social and emotional behaviors and contribute to brain development and function in rodents 27,39,47 and in humans 48 through the microbiota-gut-brain communication. Complete absence of gut microbiota in germ-free (GF) mice resulted in increased motor activity and altered anxiety-like behavior compared with conventionally reared specific pathogen free (SPF) mice with a normal gut microbiota.49–51 The altered behavior was accompanied by changes in gene expression in brain regions implicated in motor control and anxiety-like behavior, including decreased N-methyl-D-aspartate receptor expression in amygdala, increased brain-derived neurotrophic factor expression, and decreased serotonin receptor expression in the hippocampus.49,51 Interestingly and importantly, the GF mice displayed similar characteristics as SPF mice after exposure to gut microbiota.49 The exaggerated HPA stress response by GF mice was partially corrected by reconstitution with SPF feces containing Bifidobacterium infantis at an early life stage.50 However, the reconstitution exerted at a later stage in life cannot reverse the altered behavior and the anxiolytic behavioral phenotype, which persisted even after the adult GF mice were colonized with SPF feces when the timing wasn’t early enough.49,50 These results therefore indicate that the presence of gut microbiota at an early stage “up regulates” the HPA axis activity and can affect the postnatal development of the stress response in animals. A critical developmental window may exist after which the behavioral phenotype cannot be normalized by the CNS and endocrine-immune systems, even when a conventional gut microbiota are reconstituted at a later life stage.52 Understanding this timing in humans will be important to deciding the timing for targeted interventions to correct or influence the development of the microbiome.
Persons living with neurodevelopmental disorders, such as autism spectrum disorder may display a combination of behavioral and gastrointestinal manifestations. Gut microbiota alterations are hypothesized to be involved in forms of late-onset autism spectrum disorders because of the association of the genus with neurotoxin-mediated tetanus and with prolonged antibiotic usage, which suggests implies a postnatal influence of microbiota on brain development and function.39 Mouse models have shown that compositional and structural alterations of gut microbes result in changes of metabolites in the serum and trigger onset of autism-like behaviors. Moreover, administering a human commensal Bacteroides fragilis, modulated several metabolites as well as corrected GI symptoms, anxiety-like behaviors and sensorimotor functions.47 One human study showed that consumption of fermented milk product with probiotics by healthy women was associated with changes of connectivity and activity in brain regions that control central processing of emotion and sensation, which explained the observed differences in activity and emotions during the task.48 The findings support a bottom-up gut-brain signaling mechanisms that gut microbiota affect on the host metabolome and impact emotional-behavioral symptoms in human neurodevelopment. More research is needed to understand this bottom-up phenomenon and how what we ingest during pregnancy and early in life may have long term effects on neurodevelopment.
The microbiota also has a potential role in immune-mediated CNS diseases, such as multiple sclerosis and Parkinson’s disease, and in neuroinflammation triggered cognitive decline such as dementia.46,53 Research shows that commensal gut flora play key sequential roles in the initiation of a complex spontaneous demyelination in a mouse model of autoimmune disease, which is very similar to early multiple sclerosis in humans.54 Impaired cognitive function in patients with hepatic encephalopathy has been found to be associated with altered bacterial composition, endotoxemia, and increased inflammatory cytokines compared to people with cirrhosis and normal cognitive function.55 These studies provide further evidence that the intestinal microbiota may influence the brain through activation of mucosal immune pathways thereby resulting in significant long term impairment or injury.
In addition to traditional functions of the gut microbiome, such as defending against pathogens, protecting the intestinal epithelial barrier, secretion of IgA, and facilitating nutrient absorption, the recent research has focused on the functions of guiding maturation and functionality of the host immune system in the brain-gut cross talk.9 Strong evidence suggests that the intestinal microbiota is fulfilling key roles in this bidirectional interaction, however, the exact pathways and mediators of this communication need further exploration.
Brain-Gut-Microbiota Signaling System in Regulation of Early Life Stressors
The mechanistic interplays of gut micriobiome development and early life experience are still poorly understood. Dysbiosis in high risk infants is associated with exposure to potentially detrimental factors in the development of the healthy microbiome, including caesarean delivery, use of antibiotics, lack of breast-feeding, and exposure to environmental toxicants.36 Moreover, exposure to early life physiological and psychosocial stressors, such as pain and maternal-infant separation, can lead to oxidative stress within the intestines, which may modulate the process of microbiome establishment in preterm infants.23 Further, absence of normal gut microbiota (such as in an infant born preterm) and its neurochemical profile are less resistant to restoration of a normal gut flora later in life.56 However, the effects of early life stress on modulating the gut microbiome, GI tract function, and neurodevelopment remain largely unexplored. Researchers have uncovered more questions than answers about these relationships and more research is needed to better understand what could be done to prevent disruptions or restore normal gut flora a later time in life.
Accumulated Pain Stressors and Regulation
Neurodevelopmental and health outcomes in vulnerable infants are determined by multifactorial effects and the neonatal intensive care unit (NICU) experience is one of the most crucial factors. Physical and psychosocial early life stress is considered an inherent part of high-tech lifesaving care for high-risk neonates, and these infants usually grow under accumulated stress and in an isolated NICU environment over a prolonged period. Repeated stressful or painful experience is one of the major stressors to which neonates are routinely subjected to in the NICU. Preterm infants may experience an average of 10 – 16 invasive procedures per day, and very preterm infants (gestational age < 32 weeks) may have hundreds of painful procedures during their NICU stay.57 An impressive body of neuroanatomical, neurochemical, and biobehavioral evidence shows that preterm infants possess the ability to detect, perceive and respond to pain and to remember pain experiences.58 Meanwhile, their tactile threshold is lower and descending inhibitory pathways are immature, therefore, they are more sensitive to repeated pain stimulation during this vulnerable period.59 One study demonstrated that a greater numbers of painful procedures directly predicted smaller head circumference and lower brain function in very preterm infants, which indicated that repeated pain stressor during a vulnerable period may activate a downstream cascade of stress signaling that affects later growth and development.60
Limited numbers of human studies exist related to regulation of early life experience by the brain-gut-microbiota signaling system, even though studies using animal models have provided solid support for the relationship between physical and psychological stress and behavior and gut microbiota through the brain-gut signaling system.61,62 The gut microbiota contributes to developmental programming, and there is a “window of vulnerability” within which the microbiota can affect physiological function, with potentially life-long consequences.50 It is now well understood that the development of the human microbiome is greatly influenced during birth and the neonatal period; exactly how it is influenced is complex and further exploration is needed. Since the rapidly developing nervous system of immature preterm neonates differs from that of term infants, preterm infants are particularly vulnerable to the effects of stress and pain experience. Continual adaptation to repeated pain appears to induce functional changes in stress/pain processing systems.63 Furthermore, repeated pain may contribute to long-term changes in generalized stress systems, including altered programming of primary stress hormone (cortisol) levels, long after NICU discharge,64 yet the mechanisms underlying long-term consequences of neonatal noxious insults on pain sensation are still largely unknown. As reviewed above, research shows that the gut microbial content is critical to the development of an appropriate stress response later in life and also that there is a narrow window in early life where colonization must occur to ensure normal development of the HPA axis.7 The early establishment of a healthy microbiota has a profound effect on the future well-being of the individual.65 Microbial colonization patterns in immune naïve premature infants may lay the groundwork for life-long health/disease risk from immune modulation.
Maternal-Infant Separation and Regulation
Another decisive stressor encountered in the NICU is maternal-infant separation. High risk neonates, especially preterm infants, spend much of their early life in the NICU, an experience that deprives them of the benefits of parental proximity and contact. A plethora of animal and human studies show that deprivation of maternal care results in altered neuronal, hormonal, genetic, and bio-behavioral outcomes during the sensitive growth and developmental period.66–68 In mice, maternal separation has been found to trigger the development of severe colitis that was sustained later in life with abnormal colonic epithelial function, elevated proinflammatory cytokines, and enhanced mast cell activation.69 Other animal studies also support a two-hit model, first that long term maternal separation triggered bio-behavioral stress responses including elevated corticosterone levels and altered maze activity in early life, and then later in adulthood, these early maternal separation offspring showed suppressed stress response and higher intestinal permeability when exposed to social stress.44 Therefore, early life events can significantly change animals’ vulnerability in infancy as well as interact with chronic social instability in adult life, though the underlining mechanism of the association of maternal separation experiences with poor neuro-behavioral outcomes is still unclear. To date, studies have shown that exposure to maternal separation stress in newborn animal significantly increases ACTH levels and alters the normal balance of gut microbiota.45,70 Maternal separation caused shifts in neonatal gut microbiota as indicated by fostering an overgrowth of total aerobes and anaerobes, and particularly potential negative bacteria E. coli, enterococci and clostridia. Alterations to neonatal gut microbiota could be explained by the fact that the composition of the postnatal developing microbiota is very susceptible to environmental factors. In addition, during this time the enteric neuro-immune system, i.e., luminal IgA, dynamically interacts with the development of the gut microbiota and then declines at some later time point to allow bacterial colonization.71 More understanding of this change overtime in these relationships is needed. Events such as neonatal stress may potentially affect this process and lead to an imbalance in the microbiota.
Positive Early Life Experience and Mechanism
To date there is insufficient evidence for the best methods to effectively treat the negative consequences of prematurity and early persistent maternal separation. Positive or satiate early life experience, such as skin-to-skin contact (SSC) between the mother and her high risk infant as well as other strategies for pain and stress management, has shown beneficial effects on reducing infants’ stressful responses and improving brain maturation and neurodevelopmental outcomes.72,73 The actions of multi-sensory stimulations with parental involvement may activate the neuro-endocrine system and modulate the stress-regulation responses. Maternal infant SSC appears to an influence on autonomic regulation while the infant is undergoing painful procedures and lowers infant serum and salivary cortisol.72,74–76 Although the neurobiological mechanism of beneficial effects from SSC is still unclear, oxytocin release is suggested as one mediator for these effects, with short and long-term consequences on mother-infant interaction, and maternal anxiety and social competence.77,78 Preterm infants would benefit from these strategies directed at favoring the establishment of healthy microbiome regulated by the brain-gut-microbiota axis; and to develop such strategies, knowledge and mechanisms of the microbiota development process in preterm infants as well as how this process differs from the potentially healthy model are needed.31
As reviewed above, early life stressful experiences are significant factors, which influence neuro-bio-behavioral development in infancy as well as a predispose subsequent psycho-social morbidity in adulthood. Early life gut microbiome dysbiosis in high risk infants with negative experiences may represent an accumulated stress response and physiologically traumatic event, which potentially leads specifically to subsequent psychopathology through the brain-gut-microbiota axis.27 Studies from animals and human, directly or indirectly, have shed light on the complex signaling pathways in which the maturing GI microbiome during the critical neurodevelopmental window plays a critical role.79 In order to aide high-risk infants, establishment of a healthful gut microbiota community, early parental contact, such as SSC with stress and pain management can be a most effective bio-behavioral intervention. These strategies not only serve as stress buffers for both infants and parents via activating oxytocin release, but also expose infants to a greater diversity of microbiota when they are held skin-to-skin by their parents. As a counterbalance to the stressful events, satisfying early life experiences provide rich sources to modify and nurture the infant gut microbiome to a normal development pattern at critical neurodevelopmental time points. The healthy gut microbiota in early life has the potential to confer lifelong health benefits because it holds the fundamental pathway of brain-gut communication and modulates the stress response, neuronal growth, plasticity, and neurotransmission in this time frame.79 Conversely, an infant’s abnormal microbial experience during the early unstable phase can pose a risk for future health problems such as asthma, obesity, diabetes, autism, anxiety, depression, and other aspects of pain responses and cognitive function.80 Further studies are needed to more fully explore the complex mechanisms in regulation of early life experience and neuro-bio-behavioral development under the influence of the microbiota at the level of the central nervous system.
Implications and Conclusions
Accumulated infant stress including repeated painful procedures, exposure and development of infection, daily clustered caregiving routines, and maternal separation during critical neurodevelopmental windows are major unsolved problems related to high-tech care. Moreover, the onset of the altered neuro-immune progress induced by the cumulative infant stress/pain is often insidious and the mechanisms remain largely unclear. Along with the significant increase in very preterm infant births, concerns have been raised for the outcomes of stressful early life experience on immature neuro-immune systems and long-term health consequences.2
Until a time when preterm birth becomes preventable, there remain multiple unmet needs to predict and prevent injury to the nervous and other systems while also supporting the infant’s ongoing development. The converging evidence strongly suggests that gut microbiota is playing a role in multiple aspects of human health and disease through the bidirectional brain-gut signaling system, in terms of both top-down and bottom-up effects. However, few human studies have identified clear linkage between these factors and neurodevelopment and/or disease processes. Furthermore, rarely have studies focused on the underlining brain-gut-microbiota mechanism in satiate early life experiences, such as early parent infant contact as a counterbalancing to stressful events and modulating health outcomes in infants. The recent explosion of interest in this area may lead to exponential new findings over the next few years and benefit health from infancy to adulthood by potential providing directions for new diagnostic, preventative and treatment technologies.
BOX 1. Terminology.
Commensals are organisms that benefit from another organism but that have no harm or benefit themselves. Commensal is also frequently used to describe the relationship between humans and their intestinal bacterial community, as many of these bacteria provide benefits to the human host while living in an environment where they proliferate.
Dysbiosis also called dysbacteriosis, is a condition with microbial imbalances on or inside the body. An intestinal dysbiosis refers to quantitative and qualitative microbial alterations in the GI track.
Human genome is the complete set of genetic information for humans (Homo sapiens). The human genome is encoded as DNA sequences within the 23 pairs of chromosomes as well as small DNA molecules in mitochondria.
Human microbiome was originally defined as the collection of the genomes of the microbes that inhabits the human body, on the surface and in deep layers of skin, in the oral cavity, conjunctiva, and GI tracts. Microbiome is now sometimes used to refer to the collective the microbes themselves. A microbiome can be a specific body site, such as the gut microbiome. To clarify the difference between organisms and their genomes the terms, microbiota refers to the microbial organisms and microbiome refers to the catalog of the genes of these microbes.
Metagenomics is the study of a collection of genetic material (genomes) from a mixed community of organisms and usually refers to the study of microbial communities by culture-independent methods.
Pathogenic microbe is the microorganism with the potential to cause infection or disease.
Probiotics are living microorganisms which when administered in adequate amounts confer a health benefit on the host.
Symbiosis is a mutually beneficial relationship involving close physical contact between two organisms in which both organisms benefit. This is one type of relationship seen in the human microbiome.
Summary.
What we know
Gut microbiota is playing a key role in multiple aspects of human health and disease through the bidirectional brain-gut signaling system, in terms of both top-down and bottom-up effects.
Psychosocial and physical stressors during the early stages of life, including accumulated painful procedures and maternal separation may alter the gastrointestinal functions and gut microbiome by the regulation of the brain-gut axis, which may have a great impact on health in in later life.
Intervention strategies, such as early parent-infant contact with stress and pain management can serve as a counterbalance to the stressful events and modify and nurture infant gut microbiome to a normal development pattern at critical neurodevelopmental time points.
What needs to be studied
How changes in degree of stress affect short and long term outcomes.
How decreasing parental separation can regulate the brain-gut axis.
What we can do today
Decrease stress for infants through routine integration of developmental care strategies.
Increase parental participation in caregiving to promote normal developmental patterns for the high-risk infant and their family.
Acknowledgments
Funding Support:
This publication was supported by the National Institute of Nursing Research of the National Institutes of Health under Award Number 1K23NR014674-01.
Footnotes
Conflicts of Interest
There are no declared conflicts of interest for any of this study’s authors.
Contributor Information
Xiaomei Cong, Email: xiaomei.cong@uconn.edu, School of Nursing, University of Connecticut, 231 Glenbrook Road U4026, Storrs, CT 06269, Phone: 860-486-2694.
Wendy A. Henderson, Email: hendersw@mail.nih.gov, Digestive Disorder Unit, Biobehavioral Branch, NINR, NIH.
Joerg Graf, Email: goerg.graf@uconn.edu, Department of Molecular and Cell Biology, University of Connecticut.
Jacqueline M. McGrath, Email: jacqueline.mcgrath@uconn.edu, School of Nursing, University of Connecticut, Connecticut Children’s Medical Center.
References
- 1.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012 Jun 9;379(9832):2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 2.Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010 Sep;126(3):443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mwaniki MK, Atieno M, Lawn JE, Newton CR. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet. 2012 Feb 4;379(9814):445–452. doi: 10.1016/S0140-6736(11)61577-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith GC, Gutovich J, Smyser C, et al. Neonatal intensive care unit stress is associated with brain development in preterm infants. Annals of neurology. 2011 Oct;70(4):541–549. doi: 10.1002/ana.22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morken NH. Preterm birth: new data on a global health priority. Lancet. 2012 Jun 9;379(9832):2128–2130. doi: 10.1016/S0140-6736(12)60857-5. [DOI] [PubMed] [Google Scholar]
- 6.Behrman RE, Butler AS, editors. Committee on Understanding Premature Birth and Assuring Healthy Outcomes IoMotNA. Preterm Birth: Causes, Consequences, and Prevention. Washington, D.C: The National Academies Press; 2007. [PubMed] [Google Scholar]
- 7.Dinan TG, Cryan JF. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology. 2012 Sep;37(9):1369–1378. doi: 10.1016/j.psyneuen.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Weinstock GM. Genomic approaches to studying the human microbiota. Nature. 2012 Sep 13;489(7415):250–256. doi: 10.1038/nature11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Kasper LH. The role of microbiome in central nervous system disorders. Brain Behav Immun. 2014 May;38:1–12. doi: 10.1016/j.bbi.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Branton WG, Ellestad KK, Maingat F, et al. Brain microbial populations in HIV/AIDS: alpha-proteobacteria predominate independent of host immune status. PloS one. 2013;8(1):e54673. doi: 10.1371/journal.pone.0054673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins SM, Bercik P. Gut microbiota: Intestinal bacteria influence brain activity in healthy humans. Nature reviews Gastroenterology & hepatology. 2013 Jun;10(6):326–327. doi: 10.1038/nrgastro.2013.76. [DOI] [PubMed] [Google Scholar]
- 12.Gosalbes MJ, Llop S, Valles Y, Moya A, Ballester F, Francino MP. Meconium microbiota types dominated by lactic acid or enteric bacteria are differentially associated with maternal eczema and respiratory problems in infants. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2013 Feb;43(2):198–211. doi: 10.1111/cea.12063. [DOI] [PubMed] [Google Scholar]
- 13.Valles Y, Gosalbes MJ, de Vries LE, Abellan JJ, Francino MP. Metagenomics and development of the gut microbiota in infants. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2012 Jul;18 (Suppl 4):21–26. doi: 10.1111/j.1469-0691.2012.03876.x. [DOI] [PubMed] [Google Scholar]
- 14.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Science translational medicine. 2014 May 21;6(237):237ra265. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerr CA, Grice DM, Tran CD, et al. Early life events influence whole-of-life metabolic health via gut microflora and gut permeability. Critical reviews in microbiology. 2014 Mar 19; doi: 10.3109/1040841X.2013.837863. [DOI] [PubMed] [Google Scholar]
- 16.Turroni F, Peano C, Pass DA, et al. Diversity of bifidobacteria within the infant gut microbiota. PloS one. 2012;7(5):e36957. doi: 10.1371/journal.pone.0036957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reid G, Younes JA, Van der Mei HC, Gloor GB, Knight R, Busscher HJ. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nature reviews Microbiology. 2011 Jan;9(1):27–38. doi: 10.1038/nrmicro2473. [DOI] [PubMed] [Google Scholar]
- 18.Jakobsson HE, Abrahamsson TR, Jenmalm MC, et al. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut. 2014 Apr;63(4):559–566. doi: 10.1136/gutjnl-2012-303249. [DOI] [PubMed] [Google Scholar]
- 19.Koren O, Goodrich JK, Cullender TC, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012 Aug 3;150(3):470–480. doi: 10.1016/j.cell.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makino H, Kushiro A, Ishikawa E, et al. Mother-to-infant transmission of intestinal bifidobacterial strains has an impact on the early development of vaginally delivered infant’s microbiota. PloS one. 2013;8(11):e78331. doi: 10.1371/journal.pone.0078331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Latuga MS, Stuebe A, Seed PC. A review of the source and function of microbiota in breast milk. Seminars in reproductive medicine. 2014 Jan;32(1):68–73. doi: 10.1055/s-0033-1361824. [DOI] [PubMed] [Google Scholar]
- 22.Arboleya S, Ruas-Madiedo P, Margolles A, et al. Characterization and in vitro properties of potentially probiotic Bifidobacterium strains isolated from breast-milk. International journal of food microbiology. 2011 Sep 1;149(1):28–36. doi: 10.1016/j.ijfoodmicro.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 23.Arboleya S, Salazar N, Solis G, et al. Assessment of intestinal microbiota modulation ability of Bifidobacterium strains in in vitro fecal batch cultures from preterm neonates. Anaerobe. 2012 Nov 12; doi: 10.1016/j.anaerobe.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 24.White RA, Bjornholt JV, Baird DD, et al. Novel developmental analyses identify longitudinal patterns of early gut microbiota that affect infant growth. PLoS computational biology. 2013;9(5):e1003042. doi: 10.1371/journal.pcbi.1003042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergstrom A, Skov TH, Bahl MI, et al. Establishment of intestinal microbiota during early life: a longitudinal, explorative study of a large cohort of Danish infants. Applied and environmental microbiology. 2014 May;80(9):2889–2900. doi: 10.1128/AEM.00342-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Echarri PP, Gracia CM, Berruezo GR, et al. Assessment of intestinal microbiota of full-term breast-fed infants from two different geographical locations. Early Hum Dev. 2011 Jul;87(7):511–513. doi: 10.1016/j.earlhumdev.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nature reviews. 2012 Oct;13(10):701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 28.De Palma G, Collins SM, Bercik P, Verdu EF. The Microbiota-Gut-Brain axis in gastrointestinal disorders: Stressed bugs, stressed brain or both? J Physiol. 2014 Apr 22; doi: 10.1113/jphysiol.2014.273995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacquot A, Neveu D, Aujoulat F, et al. Dynamics and clinical evolution of bacterial gut microflora in extremely premature patients. J Pediatr. 2011 Mar;158(3):390–396. doi: 10.1016/j.jpeds.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Rouge C, Goldenberg O, Ferraris L, et al. Investigation of the intestinal microbiota in preterm infants using different methods. Anaerobe. 2010 Aug;16(4):362–370. doi: 10.1016/j.anaerobe.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Arboleya S, Binetti A, Salazar N, et al. Establishment and development of intestinal microbiota in preterm neonates. FEMS microbiology ecology. 2012 Mar;79(3):763–772. doi: 10.1111/j.1574-6941.2011.01261.x. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Hoenig JD, Malin KJ, et al. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. The ISME journal. 2009 Aug;3(8):944–954. doi: 10.1038/ismej.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stewart CJ, Marrs EC, Magorrian S, et al. The preterm gut microbiota: changes associated with necrotizing enterocolitis and infection. Acta Paediatr. 2012 Nov;101(11):1121–1127. doi: 10.1111/j.1651-2227.2012.02801.x. [DOI] [PubMed] [Google Scholar]
- 34.Claud EC, Keegan KP, Brulc JM, et al. Bacterial community structure and functional contributions to emergence of health or necrotizing enterocolitis in preterm infants. Microbiome. 2013;1(1):20. doi: 10.1186/2049-2618-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mai V, Torrazza RM, Ukhanova M, et al. Distortions in development of intestinal microbiota associated with late onset sepsis in preterm infants. PloS one. 2013;8(1):e52876. doi: 10.1371/journal.pone.0052876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berrington JE, Stewart CJ, Cummings SP, Embleton ND. The neonatal bowel microbiome in health and infection. Current opinion in infectious diseases. 2014 Jun;27(3):236–243. doi: 10.1097/QCO.0000000000000061. [DOI] [PubMed] [Google Scholar]
- 37.Drossman DA. Presidential address: Gastrointestinal illness and the biopsychosocial model. Psychosom Med. 1998 May-Jun;60(3):258–267. doi: 10.1097/00006842-199805000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Bonaz BL, Bernstein CN. Brain-gut interactions in inflammatory bowel disease. Gastroenterology. 2013 Jan;144(1):36–49. doi: 10.1053/j.gastro.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nature reviews Microbiology. 2012 Nov;10(11):735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- 40.Bailey MT. The contributing role of the intestinal microbiota in stressor-induced increases in susceptibility to enteric infection and systemic immunomodulation. Hormones and behavior. 2012 Aug;62(3):286–294. doi: 10.1016/j.yhbeh.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 41.Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun. 2011 Mar;25(3):397–407. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galley JD, Bailey MT. Impact of stressor exposure on the interplay between commensal microbiota and host inflammation. Gut microbes. 2014 Apr 1;5(3) doi: 10.4161/gmic.28683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Mahony SM, Hyland NP, Dinan TG, Cryan JF. Maternal separation as a model of brain-gut axis dysfunction. Psychopharmacology (Berl) 2011 Mar;214(1):71–88. doi: 10.1007/s00213-010-2010-9. [DOI] [PubMed] [Google Scholar]
- 44.Oines E, Murison R, Mrdalj J, Gronli J, Milde AM. Neonatal maternal separation in male rats increases intestinal permeability and affects behavior after chronic social stress. Physiol Behav. 2012 Feb 28;105(4):1058–1066. doi: 10.1016/j.physbeh.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 45.O’Mahony SM, Marchesi JR, Scully P, et al. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009 Feb 1;65(3):263–267. doi: 10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 46.Daulatzai MA. Chronic functional bowel syndrome enhances gut-brain axis dysfunction, neuroinflammation, cognitive impairment, and vulnerability to dementia. Neurochemical research. 2014 Apr;39(4):624–644. doi: 10.1007/s11064-014-1266-6. [DOI] [PubMed] [Google Scholar]
- 47.Hsiao EY, McBride SW, Hsien S, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013 Dec 19;155(7):1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tillisch K, Labus J, Kilpatrick L, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013 Jun;144(7):1394–1401. 1401 e1391–1394. doi: 10.1053/j.gastro.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heijtz RD, Wang S, Anuar F, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011 Feb 15;108(7):3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sudo N, Chida Y, Aiba Y, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004 Jul 1;558(Pt 1):263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2011 Mar;23(3):255–264. e119. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 52.Neufeld KA, Kang N, Bienenstock J, Foster JA. Effects of intestinal microbiota on anxiety-like behavior. Communicative & integrative biology. 2011 Jul;4(4):492–494. doi: 10.4161/cib.4.4.15702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caracciolo B, Xu W, Collins S, Fratiglioni L. Cognitive decline, dietary factors and gut-brain interactions. Mechanisms of ageing and development. 2014 Mar-Apr;136–137:59–69. doi: 10.1016/j.mad.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 54.Berer K, Mues M, Koutrolos M, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011 Nov 24;479(7374):538–541. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- 55.Bajaj JS, Ridlon JM, Hylemon PB, et al. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol. 2012 Jan 1;302(1):G168–175. doi: 10.1152/ajpgi.00190.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clarke G, Grenham S, Scully P, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Molecular psychiatry. 2012 Jun 12; doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 57.Carbajal R, Rousset A, Danan C, et al. Epidemiology and treatment of painful procedures in neonates in intensive care units. Jama. 2008 Jul 2;300(1):60–70. doi: 10.1001/jama.300.1.60. [DOI] [PubMed] [Google Scholar]
- 58.Fitzgerald M, Beggs S. The neurobiology of pain: developmental aspects. Neuroscientist. 2001 Jun;7(3):246–257. doi: 10.1177/107385840100700309. [DOI] [PubMed] [Google Scholar]
- 59.Slater R, Fabrizi L, Worley A, Meek J, Boyd S, Fitzgerald M. Premature infants display increased noxious-evoked neuronal activity in the brain compared to healthy age-matched term-born infants. NeuroImage. 2010 Aug 15;52(2):583–589. doi: 10.1016/j.neuroimage.2010.04.253. [DOI] [PubMed] [Google Scholar]
- 60.Vinall J, Miller SP, Chau V, Brummelte S, Synnes AR, Grunau RE. Neonatal pain in relation to postnatal growth in infants born very preterm. Pain. 2012 Jul;153(7):1374–1381. doi: 10.1016/j.pain.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 61.Caso JR, Leza JC, Menchen L. The effects of physical and psychological stress on the gastro-intestinal tract: lessons from animal models. Current molecular medicine. 2008 Jun;8(4):299–312. doi: 10.2174/156652408784533751. [DOI] [PubMed] [Google Scholar]
- 62.Bonaz BL, Bernstein CN. Brain-Gut Interactions in Inflammatory Bowel Diseases. Gastroenterology. 2012 Oct 11; doi: 10.1053/j.gastro.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 63.Slater R, Worley A, Fabrizi L, et al. Evoked potentials generated by noxious stimulation in the human infant brain. European journal of pain (London, England) 2010 Mar;14(3):321–326. doi: 10.1016/j.ejpain.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 64.Grunau RE, Haley DW, Whitfield MF, Weinberg J, Yu W, Thiessen P. Altered basal cortisol levels at 3, 6, 8 and 18 months in infants born at extremely low gestational age. J Pediatr. 2007 Feb;150(2):151–156. doi: 10.1016/j.jpeds.2006.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Conroy ME, Shi HN, Walker WA. The long-term health effects of neonatal microbial flora. Current opinion in allergy and clinical immunology. 2009 Jun;9(3):197–201. doi: 10.1097/ACI.0b013e32832b3f1d. [DOI] [PubMed] [Google Scholar]
- 66.Anand KJ, Scalzo FM. Can adverse neonatal experiences alter brain development and subsequent behavior? Biol Neonate. 2000 Feb;77(2):69–82. doi: 10.1159/000014197. [DOI] [PubMed] [Google Scholar]
- 67.Higham JP, Barr CS, Hoffman CL, Mandalaywala TM, Parker KJ, Maestripieri D. Mu-opioid receptor (OPRM1) variation, oxytocin levels and maternal attachment in free-ranging rhesus macaques Macaca mulatta. Behav Neurosci. 2011 Apr;125(2):131–136. doi: 10.1037/a0022695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weller A, Feldman R. Emotion regulation and touch in infants: the role of cholecystokinin and opioids. Peptides. 2003 May;24(5):779–788. doi: 10.1016/s0196-9781(03)00118-9. [DOI] [PubMed] [Google Scholar]
- 69.Lennon EM, Maharshak N, Elloumi H, Borst L, Plevy SE, Moeser AJ. Early life stress triggers persistent colonic barrier dysfunction and exacerbates colitis in adult IL-10−/− mice. Inflammatory bowel diseases. 2013 Mar-Apr;19(4):712–719. doi: 10.1097/MIB.0b013e3182802a4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barouei J, Moussavi M, Hodgson DM. Effect of maternal probiotic intervention on HPA axis, immunity and gut microbiota in a rat model of irritable bowel syndrome. PloS one. 2012;7(10):e46051. doi: 10.1371/journal.pone.0046051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Inoue R, Ushida K. Development of the intestinal microbiota in rats and its possible interactions with the evolution of the luminal IgA in the intestine. FEMS microbiology ecology. 2003 Jul 1;45(2):147–153. doi: 10.1016/S0168-6496(03)00134-X. [DOI] [PubMed] [Google Scholar]
- 72.Cong X, Ludington-Hoe SM, Walsh S. Randomized crossover trial of kangaroo care to reduce biobehavioral pain responses in preterm infants: a pilot study. Biol Res Nurs. 2011 Apr;13(2):204–216. doi: 10.1177/1099800410385839. [DOI] [PubMed] [Google Scholar]
- 73.Scher MS, Ludington-Hoe S, Kaffashi F, Johnson MW, Holditch-Davis D, Loparo KA. Neurophysiologic assessment of brain maturation after an 8-week trial of skin-to-skin contact on preterm infants. Clin Neurophysiol. 2009 Oct;120(10):1812–1818. doi: 10.1016/j.clinph.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cong X, Ludington-Hoe SM, McCain G, Fu P. Kangaroo Care modifies preterm infant heart rate variability in response to heel stick pain: pilot study. Early Hum Dev. 2009 Sep;85(9):561–567. doi: 10.1016/j.earlhumdev.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cong X, Cuss on RM, Walsh S, Hussein N, Ludington-Hoe SM, Zhang D. Effects of skin-to-skin contact on autonomic pain responses in preterm infants. J Pain. 2012 Jul;13(7):636–645. doi: 10.1016/j.jpain.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 76.New M, Laudenslager ML, Robinson J. Coregulation in salivary cortisol during maternal holding of premature infants. Biol Res Nurs. 2009 Jan;10(3):226–240. doi: 10.1177/1099800408327789. [DOI] [PubMed] [Google Scholar]
- 77.Uvnas-Moberg K, Arn I, Magnusson D. The psychobiology of emotion: the role of the oxytocinergic system. Int J Behav Med. 2005;12(2):59–65. doi: 10.1207/s15327558ijbm1202_3. [DOI] [PubMed] [Google Scholar]
- 78.Cong X, Cuss on RM, Walsh W, Vazquez V. Oxytocin Mechanism in Modulating Parental Stress and Anxiety during Skin-to-Skin Contact with Preterm Infants. Advances in Neonatal Care. 2014;14(3):214. [Google Scholar]
- 79.Clarke G, O’Mahony S, Dinan T, Cryan J. Priming for health: gut microbiota acquired in early life regulates physiology, brain and behaviour. Acta Paediatr. 2014 May 6; doi: 10.1111/apa.12674. [DOI] [PubMed] [Google Scholar]
- 80.Douglas-Escobar M, Elliott E, New J. Effect of intestinal microbial ecology on the developing brain. JAMA pediatrics. 2013 Apr;167(4):374–379. doi: 10.1001/jamapediatrics.2013.497. [DOI] [PubMed] [Google Scholar]