Abstract
Background
Insulin resistance (IR) is linked with the occurrence of pathological features observed in Alzheimer's disease (AD), including neurofibrillary tangles and amyloid plaques. However, the extent to which IR is associated with AD pathology in the cognitively asymptomatic stages of preclinical AD remains unclear.
Objective
To determine the extent to which IR is linked with amyloid and tau pathology in late-middle-age.
Method
Cerebrospinal fluid (CSF) samples collected from 113 participants enrolled in the Wisconsin Registry for Alzheimer's Prevention study (mean age = 60.6 years), were assayed for AD-related markers of interest: Aβ42, P-Tau181, and T-Tau. IR was determined using the Homeostatic Model Assessment for Insulin Resistance (HOMA-IR). Linear regression was used to test the effect of IR, and APOE ε4, on tau and amyloid pathology. We hypothesized that greater IR would be associated with higher CSF P-Tau181 and T-Tau, and lower CSF Aβ42.
Results
No significant main effects of HOMA-IR on P-Tau181, T-Tau, or Aβ42 were observed; however, significant interactions were observed between HOMA-IR and APOE ε4 on CSF markers related to tau. Among APOE ε4 carriers, higher HOMA-IR was associated with higher P-Tau181 and T-Tau. Among APOE ε4 non-carriers, HOMA-IR was negatively associated with P-Tau181 and T-Tau. We found no effects of IR on Aβ42 levels in CSF.
Conclusion
IR among asymptomatic APOE ε4 carriers was associated with higher P-Tau181 and T-Tau in late-middle age. The results suggest that IR may contribute to tau-related neurodegeneration in preclinical AD. The findings may have implications for developing prevention strategies aimed at modifying IR in mid-life.
Keywords: Insulin Resistance, Cerebrospinal Fluid, Tau protein, Glucose, Insulin, Apolipoprotein ε4, Alzheimer's disease
INTRODUCTION
Type 2 diabetes and insulin resistance (IR) have been linked to cognitive decline[1] and increased risk for developing Alzheimer's disease (AD)[2]. In transgenic mouse models of AD mice, IR promotes the generation of Aβ42 peptides and amyloidosis [3], part of a pathway instigated by cleavage of amyloid precursor protein by β-secretase. IR is also linked with amyloidosis by increasing γ-secretase activity and decreasing insulin degrading enzyme (IDE) activity[3]. IDE regulates Aβ levels in neuronal cells, and IDE knockout mice have hyperinsulinemia, glucose intolerance, and increased cerebral accumulation of Aβ [4]. Diabetic phenotypes in mice are also associated with increased tau phosphorylation [5]. The brain, previously thought to be insulin insensitive, has insulin receptors distributed in several regions including hippocampus, an important site for AD pathology [6]. Furthermore, insulin receptor density decreases in aging, insulin signaling is altered in AD [6, 7] and decreased insulin mRNA in post mortem brain tissue has been observed in AD patients compared to controls [8]. Additionally, prevalence of impaired fasting glucose and type 2 diabetes is increased in AD patients [9]. Some human studies suggest IR and type 2 diabetes are linked with abnormal phosphorylation of tau protein [10] and greater amyloid burden [11], although other studies suggest otherwise [12-14].
The pathological process in AD is now known to begin decades before clinically significant symptoms of cognitive impairment manifest [15]. For many adults, this coincides with midlife. Interestingly, risk factors for AD such as type 2 diabetes and the associated condition of IR at midlife increase the relative risk of developing AD, even when controlling for obesity and other vascular risk factors [16]. Possibilities for little or modest association between type 2 diabetes and postmortem assessment of AD-associated pathology may be due to the use of diabetes medication which may confer neuroprotective effects, development of diabetes in later life which is less associated with dementia risk, or the selective early death of severe diabetic patients attributed to co-morbid conditions such as heart disease and stroke.
In order to examine the contribution of IR to development of AD pathology at the critical juncture of midlife and presumed preclinical phase of AD, this study examined the relationship between IR and cerebrospinal fluid (CSF) markers of AD pathology in asymptomatic late-middle-aged adults with risk factors for AD. Cerebrospinal fluid (CSF) biomarkers show utility for predicting conversion to AD [17] and provide an indirect, but close marker of tissue pathology [18, 19]. Phosphorylated tau (P-tau181) and total tau (T-tau) are elevated in mild cognitive impairment (MCI) and AD while CSF Aβ42 has been shown to be inversely proportional to amyloid in the brain [20] and is decreased in MCI and AD [21]. Participants were from the Wisconsin Registry for Alzheimer's Prevention (WRAP) study, a cohort enriched for AD risk factors, including APOE ε4 genotype (40%) and parental family history of AD (75%). Based on evidence from animal models and human epidemiological studies suggesting IR contributes to AD risk and pathology, we hypothesized that higher IR would be associated with higher P-Tau181 and T-Tau and lower levels of Aβ42 in this preclinical cohort of adults.
METHODS
Participants
This study examined 113 asymptomatic late-middle-aged adults (mean age = 60.6 years, range= 47-75 years, 70% female) from WRAP who had undergone lumbar puncture and fasting blood draw. This continuing study assesses genetic and biological factors postulated to contribute to the development of dementia-related cognitive decline and neural dysfunction. Inclusion criteria for this study entailed no clinical diagnosis of a memory disorder, no current diagnosis of major psychiatric disease or other major medical conditions (e.g., myocardial infarction, or recent history of cancer), and no history of head trauma. Participants were also required to have available fasting insulin and glucose levels, in addition to CSF assays for AD-relevant biomarkers.
Parental family history (FH) of AD classification was based on probable or confirmed AD of one or both parents [22]. A positive family history was defined as having one or both parents with autopsy-confirmed or probable AD as outlined by research criteria [23, 24], reviewed by a multidisciplinary diagnostic consensus panel. Detailed medical history and phone interviews were conducted to confirm absence of parental FH, where absence required that the participant's father survive to at least age 70 and the mother to age 75 without incurring a formal diagnosis of dementia or exhibiting cognitive deterioration. Participants were classified using a binary variable as FH positive or FH negative.
Fasting blood samples were collected following vital sign measurement and processed at the University of Wisconsin Clinical Research Unit and fasting glucose and insulin values were determined at the UW Hospital laboratory. Subjects’ weight and height were measured without shoes. Weight was measured to the nearest tenth of a kilogram using a medical scale balance beam model, Health-O-Meter. Height was measured to the nearest centimeter using a wall-mounted ruler. Body mass index (BMI) was calculated based on height and weight.
Insulin resistance was measured by HOMA-IR (glucose*insulin/405) using fasting blood glucose and insulin from each subject. Currently, cut-off points of HOMA-IR for insulin resistance are not well established. For example, studies in American Indians, American Mexican, Spanish, Japanese, and European populations demonstrate great variability in HOMA-IR cut-offs and cut points have not been determined for a sample comparable to ours [25-28]. Thus, HOMA-IR was analyzed as a continuous variable. Because the raw data were skewed, all analyses used log transformed HOMA-IR. Presence of type 2 diabetes in this sample was assessed by reviewing medication records, self-report, and using American Diabetes Association criteria, where participants with fasting blood glucose over 125 mg/dL were flagged as diabetic. Four participants had previously been diagnosed with type 2 diabetes and were taking Metformin. One participant not diagnosed with diabetes had elevated fasting glucose according to ADA criteria. Medications were also reviewed to identify additional participants with diabetes. In order to test for relationships in the pre-diabetic range, the analyses were repeated excluding participants with diagnosed or possible diabetes. The results did not differ, thus, these 4 participants were retained.
APOE ε4
APOE ε4 extraction and isoform classification have been described previously [29]. Participants were categorized using a binary variable as being non-carriers (zero ε4 alleles) or APOE ε4 carriers (one or two ε4 alleles).
Cerebrospinal Fluid
CSF was collected in the morning after a 12hr fast with a Sprotte 25-or 24-gauge spinal needle at the L3/4 or L4/5 using gentle extraction into propylene syringes. CSF (~22mL) was then combined, gently mixed and centrifuged at 2000g for 10 minutes. Supernatants were frozen in 0.5mL aliquots in polypropylene tubes and stored at -80°C. Samples were analyzed using commercially available enzyme-linked immunosorbent assay (ELISA) methods (INNOTEST assays, Fujiurebio, Ghent Belgium) as described previously in detail [30]. Board-certified laboratory technicians who were blinded to clinical information performed all analyses. All samples were analyzed according to protocols approved by the Swedish Board of Accreditation and Conformity Assessment (SWEDAC) using one batch of reagents (intra-assay coefficients of variation <10%).
Statistics
A multiple regression analysis was conducted using SPSS (IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp) software to examine the effects of HOMA-IR, APOE ε4 genotype and FH status on P-Tau181, T-Tau and Aβ42. Separate analyses were performed for each CSF measure of interest. A secondary analysis also tested the effect of IR on Aβ42/P-Tau181 a ratio that takes into account both facets of pathology with a smaller ratio implying higher AD pathology [31]. Interactions between HOMA-IR and APOE ε4 genotype, and HOMAIR and FH status were also examined. Covariates were age, sex and BMI.
Outliers and collinearity
BMI and IR are positively correlated with one another, which could lead to collinearity artifacts in the regression analyses. We assessed for collinearity effects of BMI and the log of HOMA-IR by calculating the variance inflation factor (1.5), which revealed that the variables were not highly co-linear. Our original N was 115, however two participants were identified as significant outliers (> 3 SD from the mean) on CSF Aβ42 (one participant) and HOMA-IR (one participant), even after log transforming HOMA-IR, and thus were removed from further analysis for a total N of 113.
RESULTS
Demographics
Demographics and clinical characteristics of the participants are shown in Table 1. Mean MMSE across all participants was well within the normal range (29.36 ± .80). Mean HOMA-IR was 2.2 (SD = 1.6), with untransformed values ranging from 0.45-7.65.
Table 1.
Participant Characteristics
| APOE ε4 | ||||
|---|---|---|---|---|
| All | Carrier (38%) | Non-carriers (62%) | p | |
| N | 113 | 43 | 70 | |
| Age | 60.5 ± 5.9 (47-75) | 60.5 ± 6.2 | 60.4 ± 5.8 | .923 |
| Sex (Female) | 69% (78) | 77%(33) | 64% (45) | .164# |
| BMI | 28.5 ± 6.0 (18.9-52.7) | 29.0 ± 5.9 | 28.12 ± 4.8 | .378 |
| Fasting plasma Glucose | 95.3 ± 10.6 (74-132) | 93.98 ± 10.5(74-124) | 95.96 ± 10.2 | .325 |
| Fasting Plasma Insulin | 9.38 ± 7.2 | 10.41 ± 8.9 | 9.0 ± 5.9 | .225 |
| Family History Positive | 77%(89) | 82%(36) | 75%(53) | .312# |
| HOMA-IR | 2.2 ± 1.6 | 2.1 ± 1.4 | 2.2 ± 1.6 | .941 |
| Log of HOMA-IR | .25 ± .28 | .28 ± .30 | .24 ± .27 | .437 |
| Cerebrospinal Fluid (pg/mL) | ||||
| Aβ1-42 | 756.2 ± 222.5 | 703.36 ± 199.5 | 769.0 ± 231.0 | .092 |
| P-Tau181 | 42.9 ± 13.9 | 43.2 ± 14.4 | 42.63 ± 13.6 | .842 |
| T-Tau | 318.6 ± 112.3 | 331.9 ± 104.2 | 310.34 ± 116.9 | .326 |
| Aβ1-42/P-Tau181 | .067 | .059 | .162 | |
Chi-square
Effects of HOMA-IR on P-Tau181, T-Tau and Aβ42
A multiple regression analysis was conducted to test the effect of HOMA-IR on Aβ42, T-Tau, and P-Tau181 including the covariates of sex, age, BMI, FH, APOE ε4 status, HOMA-IR, and the interaction of APOE ε4 status and HOMA-IR. Each CSF measure was tested individually as a dependent variable in a multiple regression analysis. There was no main effect of HOMA-IR on the CSF markers.
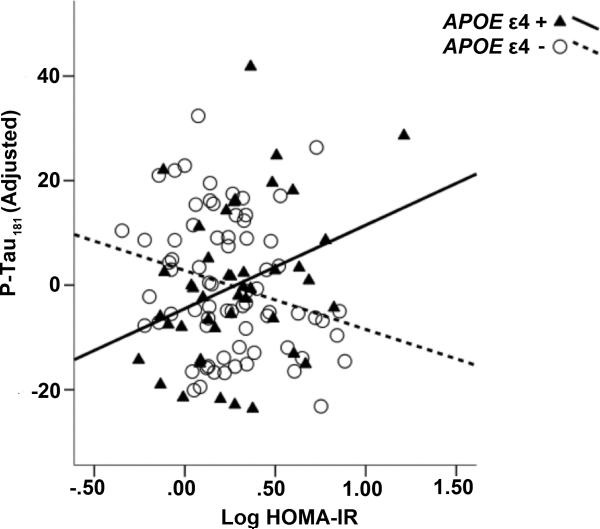
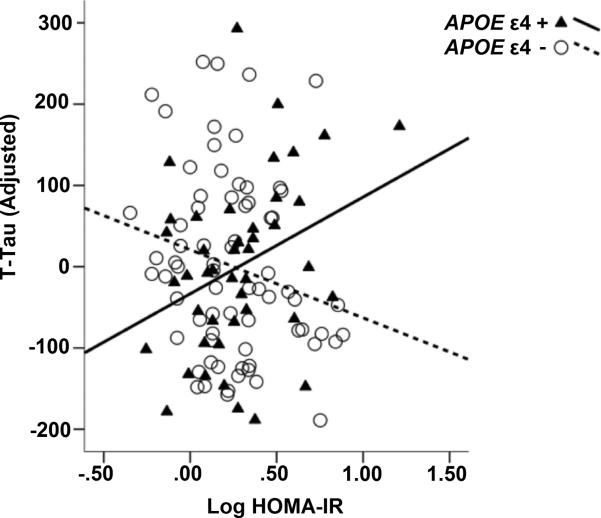
Our regression model indicated a significant interaction between APOE ε4 and HOMA-IR on CSF P-tau181 after adjusting for covariates, (β= 0.369, t(112)= 2.422, p= 0.017) where there was a positive relationship between HOMA-IR and P-Tau181 among APOE ε4 carriers, and a negative relationship between HOMA-IR and P-Tau181 among non-carriers (Figure 1, Table 2). Likewise, there was a significant interaction between HOMA-IR and APOE ε4 status on CSF T-Tau (β= 0.367, t(112)= 2.391, p= 0.002), whereby there was a positive relationship between HOMA-IR and T-Tau among APOE ε4 carriers, but HOMA-IR was negatively associated with T-Tau181 among APOE ε4 non-carriers (Figure 2, Table 3). There was no significant effect of HOMA-IR, APOE ε4, or the interaction term on CSF Aβ42 alone.
Figure 1. Interaction between insulin resistance and APOEε4 predicts P-Tau181.
We found a significant interaction between HOMA-IR and APOE ε4 on P-Tau181, adjusting for age, sex, family history, and BMI. Carriers are shown with triangles and solid black line. Plotted P-Tau values are adjusted for covariates.
Table 2.
Summary of Multiple Regression Analysis for CSF P-Tau181 (N = 113)
| Dependent Variable | Predictor Variable | B | SE(B) | β | t | Sig.(p) |
|---|---|---|---|---|---|---|
| P-Tau181 | Age | 4.021 | 1.892 | .211 | 2.126 | .050 |
| Gender | −17.040 | 23.526 | −.070 | −.724 | .628 | |
| Family History | −3.763 | 25.727 | −.014 | −.146 | .272 | |
| BMI | −1.617 | 2.543 | −.076 | −.636 | .520 | |
| APOE ε4 | −31.076 | 29.296 | −.135 | −1.061 | .117 | |
| HOMA-IR | −111.865 | 55.039 | −.281 | −2.032 | .005* | |
| (APOE ε4 )X(HOMA-IR) | 218.175 | 77.190 | .447 | 2.826 | .017* |
Regression Model Statistics
R2 = .120 F(7,105) = 1.888 p=.056
Figure 2. Interaction between insulin resistance and APOEε4 predicts T-Tau.
We found a significant interaction between HOMA-IR and APOE ε4 on T-Tau, adjusting for age, sex, family history, and BMI. Carriers are shown with triangles and solid black line. Plotted T-Tau values are adjusted for covariates.
Table 3.
Summary of Multiple Regression Analysis for CSF Total-tau (N = 113)
| Dependent Variable | Predictor Variable | B | SE(B) | β | t | Sig.(p) |
|---|---|---|---|---|---|---|
| Total-tau | Age | .441 | .232 | .187 | 1.304 | .032 |
| Gender | −1.093 | 2.884 | −.036 | −.379 | .429 | |
| Family History | −3.073 | 3.154 | −.092 | −.975 | .842 | |
| BMI | .109 | .312 | .041 | .351 | .645 | |
| APOE ε4 | −6.722 | 3.591 | −.236 | −1.872 | .365 | |
| HOMA-IR | −17.899 | 6.747 | −.364 | −2.653 | .030* | |
| (APOE ε4 )X(HOMA-IR) | 29.434 | 9.462 | .488 | 3.111 | .019* |
Regression model Statistics
R2 = .105 F(7,105) = 1.768 p=.101
Our secondary analysis of Aβ42/P-Tau181 showed a significant interaction between APOE ε4 and HOMA-IR (β= -0.410, t(112)= -2.80 p= 0.006) on the Aβ42/P-Tau181 ratio. Among APOE ε4 carriers only, there was a negative relationship between HOMA-IR and Aβ42 /P-Tau181 as HOMA-IR increased, the ratio decreased.
We found no significant effects of family history on CSF biomarkers, nor were any significant interactions with family history observed.
DISCUSSION
Higher IR in this sample of asymptomatic late-middle-aged adults was associated with increased T-Tau and P-Tau181, and decreased Aβ142/P-Tau181 in APOE ε4 carriers. These results suggest a possible preclinical mechanism that may contribute to increased risk for AD related neurodegeneration among late middle-aged adults with glucoregulatory impairment.
Insulin resistance potentially promotes abnormal phosphorylation of tau in cognitively healthy middle-aged APOE ε4 carriers. Phosphorylation of tau decreases binding to microtubules and hyperphosphorylation eventually leads to formation of paired helical filaments aggregating to form neurofibrillary tangles. Impaired insulin signaling has been shown to enhance hyperphosphorylation of tau, which mediates dissociation of the protein from microtubules in the axons and association into tangles [32, 33]. IR has been implicated in tau hyperphosphorylation through upregulation of kinases, particularly downstream kinases that are known to phosphorylate tau protein, including glycogen synthase kinase 3β (GSK3β) [34, 35].
The relationship between type 2 diabetes, IR, and brain pathology in humans has been mixed, but may in part be explained by APOE ε4. In a postmortem study conducted by Malek-Ahmadi et al [36], AD patients who were diagnosed with type 2 diabetes and who were APOE ε4 carriers, showed higher plaque and tangle burden compared to non-carriers, suggesting that type 2 diabetes may exacerbate AD neuropathology in the presence of APOE ε4. This is consistent with the current findings, where APOE ε4 carriers showed greater CSF tau levels. Conversely, the general prevalence of type 2 diabetes among AD patients is actually higher in APOE ε4 non-carriers compared to APOE ε4 carriers [37].
Underscoring the importance of APOE ε4 status specifically in relation to insulin, AD and MCI patients have shown differences in memory performance in response to intranasal insulin therapy based on APOE ε4 carriage. In one study, intranasal insulin therapy led to improved memory performance among APOE ε4 carriers [38], while in another study, facilitated memory function in APOE ε4 non-carriers [39]. Like intranasal insulin, intravenous insulin infusion shows differential effects among APOE ε4 carriers and non-carriers. Specifically, APOE ε4 non-carriers show memory facilitation in hyperinsulinemic conditions, an effect not observed among APOE ε4 carriers [40]. Basal levels of plasma and CSF insulin in AD patients also differ by genotype, with APOE ε4 homozygotes showing normal basal levels of insulin compared to heterozygotes and non-carriers [41], suggesting an importance of APOE ε4 load. Animal work suggests that insulin signaling may differ depending on APOE ε4 status. A study of APOE ε4 knock-in mice revealed altered insulin signaling when compared to APOE ε3 mice. Effector molecules including IRS-1, PI-3K and Akt phosphorylation were all decreased in the liver of APOE ε4 mice suggesting an APOE ε4 mediated alteration in insulin signaling [42]. More research is needed to determine whether the product of APOE, apolipoprotein E, is involved in molecular processes modulating the effect of insulin resistance on pathological markers of AD.
Interesting interactions have also been reported between insulin levels and cognitive status. Among non-demented older adults, higher serum insulin levels are associated with a deleterious effect on cognitive function in addition to greater global and hippocampal atrophy [43]. In contrast, among patients diagnosed with AD, higher insulin levels are associated with better cross-sectional and longitudinal outcomes, including less decline in cognitive function, and lower global and hippocampal atrophy [43, 44]. Taken together, these studies suggest the importance of considering both the non-linear relationships between IR and neural outcome across the spectrum of cognitive function, and genotype, in treatments that target insulin signaling.
With the exception of an interaction between IR and APOE ε4 on Aβ42/P-Tau181 ratio, we did not find a relationship between IR and CSF Aβ42. Our group has previously found higher amyloid burden among normoglycemic WRAP participants with higher HOMA-IR, using Pittsburg compound B position emission tomography (PiB-PET) scanning [11]; although at least one prior study has found a negative relationship between IR and amyloid levels measured with PiB-PET [11]. Further work will be needed to determine the extent to which CSF levels of Aβ42 correlate with regional amyloid deposition as shown with amyloid-PET in this preclinical population.
This study examined participants who were cognitively asymptomatic, and largely normal on fasting glucose and fasting insulin levels. Cut-offs for insulin resistance vary by study and the population under consideration, however, for comparison purposes, 85% of our sample fell below the cut-point of 3.8 for IR, while 75% of the sample was below the cut-point of 2.6 for IR [45-47]. Three participants were taking metformin for diabetes and one participant was identified who had elevated fasting glucose; however, removing these participants from the analyses did not change the relationship between IR and CSF markers of tau among APOE ε4 carriers, suggesting these relationships can be observed within the relatively normal variation of IR. While we have framed IR as key player in the generation of AD pathology we must note that the observed variation in IR itself could be the result of preclinical AD having an effect on metabolic regulation, for example, due to AD pathology in brain regions which regulate metabolic pathways [48]. Alternatively, AD pathology and peripheral insulin resistance could be co-occurring manifestations of either brain, or systemic metabolic dysfunction. This notion is supported by studies showing commonalities among diabetes, insulin resistance, and AD, including mitochondrial dysfunction [49-51]. The fact that these relationships can be detected in preclinical stages of glucoregulatory impairment however, does suggest the potential importance of preclinical detection and prevention strategies for mitigating age and disease associated brain changes in later life.
It is important to note that while CSF tau levels are known to associate with postmortem tangle burden [52], T-Tau is a non-specific marker and we cannot conclude that an AD-specific process is at play. The vascular effects of insulin resistance also cannot be discounted. Cerebrovascular pathology could lead to neural injury that in turn could lead to elevated levels of tau protein in CSF, particularly T-Tau. Combined CSF and blood flow brain imaging studies are expected to shed further light on vascular contributions to AD pathology, as well as provide a method for localizing pathology. Furthermore, although P-Tau is more specific to AD, P-Tau levels were only assessed for tau phosphorylated at site 181. There are over 80 sites for phosphorylation of tau and some of these sites may be more closely linked with insulin signaling disruption [35].
Other potential limitations of this study include the small sample size and the cohort's demographics, particularly in this sub-sample that underwent lumbar puncture. Participants were largely Caucasian, female, and had high levels of educational attainment, and thus are not representative of the general population. Finally, it is important to note that longitudinal investigation will be necessary to assess the long-term impacts of insulin resistance on disease progression.
Conclusion
This study demonstrates for the first time that pre-diabetic levels of IR are associated with elevated levels of CSF tau protein in an asymptomatic population with genetic risk for AD. This study suggests that insulin resistance may enhance tau pathology, especially in APOE ε4 carriers. Understanding modifiable factors during the preclinical stage is expected to contribute to treatment and prevention strategies in this devastating disease.
Acknowledgements
This project was supported by the National Institute on Aging (R01 AG037639 , R01 AG027161, R01 AG021155, ADRC P50 AG033514), the National Institute of General Medical Sciences (R25 GM083252), the Waisman Center Core grant P30 HD003352-45 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the University of Wisconsin Institute for Clinical and Translational Research, funded through a National Center for Research Resources/National Institutes of Health Clinical and Translational Science Award, 1UL1RR025011, a program of the National Center for Research Resources, United States National Institutes of Health. The authors gratefully acknowledge Amy Hawley, Sandra Harding, Caitlin Cleary, Jay Fruehling, Paul Cary, Jennifer Oh, and Chuck Illingworth. Above all, we wish to thank our dedicated volunteers for their participation in this research.
REFERENCES
- 1.Rawlings AM, Sharrett AR, Schneider AL, Coresh J, Albert M, Couper D, Griswold M, Gottesman RF, Wagenknecht LE, Windham BG, Selvin E. Diabetes in midlife and cognitive change over 20 years: a cohort study. Ann Intern Med. 2014;161:785–793. doi: 10.7326/M14-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 3.Ho L, Qin W, Pompl PN, Xiang Z, Wang J, Zhao Z, Peng Y, Cambareri G, Rocher A, Mobbs CV, Hof PR, Pasinetti GM. Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer's disease. FASEB J. 2004;18:902–904. doi: 10.1096/fj.03-0978fje. [DOI] [PubMed] [Google Scholar]
- 4.Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ, Guenette S. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci U S A. 2003;100:4162–4167. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim B, Backus C, Oh S, Hayes JM, Feldman EL. Increased tau phosphorylation and cleavage in mouse models of type 1 and type 2 diabetes. Endocrinology. 2009;150:5294–5301. doi: 10.1210/en.2009-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frolich L, Blum-Degen D, Bernstein HG, Engelsberger S, Humrich J, Laufer S, Muschner D, Thalheimer A, Turk A, Hoyer S, Zochling R, Boissl KW, Jellinger K, Riederer P. Brain insulin and insulin receptors in aging and sporadic Alzheimer's disease. J Neural Transm. 1998;105:423–438. doi: 10.1007/s007020050068. [DOI] [PubMed] [Google Scholar]
- 7.Frolich L, Blum-Degen D, Riederer P, Hoyer S. A disturbance in the neuronal insulin receptor signal transduction in sporadic Alzheimer's disease. Ann N Y Acad Sci. 1999;893:290–293. doi: 10.1111/j.1749-6632.1999.tb07839.x. [DOI] [PubMed] [Google Scholar]
- 8.Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease--is this type 3 diabetes? J Alzheimers Dis. 2005;7:63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- 9.Janson J, Laedtke T, Parisi JE, O'Brien P, Petersen RC, Butler PC. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 2004;53:474–481. doi: 10.2337/diabetes.53.2.474. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Brain glucose transporters, O-GlcNAcylation and phosphorylation of tau in diabetes and Alzheimer's disease. J Neurochem. 2009;111:242–249. doi: 10.1111/j.1471-4159.2009.06320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willette AA, Johnson SC, Birdsill AC, Sager MA, Christian B, Baker LD, Craft S, Oh J, Statz E, Hermann BP, Jonaitis EM, Koscik RL, La Rue A, Asthana S, Bendlin BB. Insulin resistance predicts brain amyloid deposition in late middle-aged adults. Alzheimers Dement. 2014 doi: 10.1016/j.jalz.2014.03.011. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thambisetty M, Jeffrey Metter E, Yang A, Dolan H, Marano C, Zonderman AB, Troncoso JC, Zhou Y, Wong DF, Ferrucci L, Egan J, Resnick SM, O'Brien RJ. Glucose intolerance, insulin resistance, and pathological features of Alzheimer disease in the Baltimore Longitudinal Study of Aging. JAMA Neurol. 2013;70:1167–1172. doi: 10.1001/jamaneurol.2013.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alafuzoff I, Aho L, Helisalmi S, Mannermaa A, Soininen H. Beta-amyloid deposition in brains of subjects with diabetes. Neuropathol Appl Neurobiol. 2009;35:60–68. doi: 10.1111/j.1365-2990.2008.00948.x. [DOI] [PubMed] [Google Scholar]
- 14.Heitner J, Dickson D. Diabetics do not have increased Alzheimer-type pathology compared with age-matched control subjects. A retrospective postmortem immunocytochemical and histofluorescent study. Neurology. 1997;49:1306–1311. doi: 10.1212/wnl.49.5.1306. [DOI] [PubMed] [Google Scholar]
- 15.Blennow K, de Leon MJ, Zetterberg H. Alzheimer's disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 16.Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimer's disease risk with obesity, diabetes, and related disorders. Biol Psychiatry. 2010;67:505–512. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Vos S, van Rossum I, Burns L, Knol D, Scheltens P, Soininen H, Wahlund LO, Hampel H, Tsolaki M, Minthon L, Handels R, L'Italien G, van der Flier W, Aalten P, Teunissen C, Barkhof F, Blennow K, Wolz R, Rueckert D, Verhey F, Visser PJ. Test sequence of CSF and MRI biomarkers for prediction of AD in subjects with MCI. Neurobiol Aging. 2012;33:2272–2281. doi: 10.1016/j.neurobiolaging.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 18.Tapiola T, Alafuzoff I, Herukka SK, Parkkinen L, Hartikainen P, Soininen H, Pirttila T. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol. 2009;66:382–389. doi: 10.1001/archneurol.2008.596. [DOI] [PubMed] [Google Scholar]
- 19.Buerger K, Ewers M, Pirttila T, Zinkowski R, Alafuzoff I, Teipel SJ, DeBernardis J, Kerkman D, McCulloch C, Soininen H, Hampel H. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer's disease. Brain. 2006;129:3035–3041. doi: 10.1093/brain/awl269. [DOI] [PubMed] [Google Scholar]
- 20.Bibl M, Gallus M, Welge V, Esselmann H, Wolf S, Ruther E, Wiltfang J. Cerebrospinal fluid amyloid-beta 2-42 is decreased in Alzheimer's, but not in frontotemporal dementia. J Neural Transm. 2012;119:805–813. doi: 10.1007/s00702-012-0801-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5:228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- 22.Sager MA, Hermann B, La Rue A. Middle-aged children of persons with Alzheimer's disease: APOE genotypes and cognitive function in the Wisconsin Registry for Alzheimer's Prevention. J Geriatr Psychiatry Neurol. 2005;18:245–249. doi: 10.1177/0891988705281882. [DOI] [PubMed] [Google Scholar]
- 23.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr., Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 25.Aradillas-Garcia C, Rodriguez-Moran M, Garay-Sevilla ME, Malacara JM, Rascon-Pacheco RA, Guerrero-Romero F. Distribution of the homeostasis model assessment of insulin resistance in Mexican children and adolescents. Eur J Endocrinol. 2012;166:301–306. doi: 10.1530/EJE-11-0844. [DOI] [PubMed] [Google Scholar]
- 26.Singh Y, Garg MK, Tandon N, Marwaha RK. A study of insulin resistance by HOMA-IR and its cut-off value to identify metabolic syndrome in urban Indian adolescents. J Clin Res Pediatr Endocrinol. 2013;5:245–251. doi: 10.4274/Jcrpe.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamada C, Mitsuhashi T, Hiratsuka N, Inabe F, Araida N, Takahashi E. Optimal reference interval for homeostasis model assessment of insulin resistance in a Japanese population. J Diabetes Investig. 2011;2:373–376. doi: 10.1111/j.2040-1124.2011.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gayoso-Diz P, Otero-Gonzalez A, Rodriguez-Alvarez MX, Gude F, Garcia F, De Francisco A, Quintela AG. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: effect of gender and age: EPIRCE cross-sectional study. BMC endocrine disorders. 2013;13:47. doi: 10.1186/1472-6823-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson SC, La Rue A, Hermann BP, Xu G, Koscik RL, Jonaitis EM, Bendlin BB, Hogan KJ, Roses AD, Saunders AM, Lutz MW, Asthana S, Green RC, Sager MA. The effect of TOMM40 poly-T length on gray matter volume and cognition in middle-aged persons with APOE epsilon3/epsilon3 genotype. Alzheimers Dement. 2011;7:456–465. doi: 10.1016/j.jalz.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmqvist S, Zetterberg H, Blennow K, Vestberg S, Andreasson U, Brooks DJ, Owenius R, Hagerstrom D, Wollmer P, Minthon L, Hansson O. Accuracy of brain amyloid detection in clinical practice using cerebrospinal fluid beta-amyloid 42: a cross-validation study against amyloid positron emission tomography. JAMA Neurol. 2014;71:1282–1289. doi: 10.1001/jamaneurol.2014.1358. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Li TQ, Andreasen N, Wiberg MK, Westman E, Wahlund LO. Ratio of Abeta42/P- tau181p in CSF is associated with aberrant default mode network in AD. Sci Rep. 2013;3:1339. doi: 10.1038/srep01339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schubert M, Gautam D, Surjo D, Ueki K, Baudler S, Schubert D, Kondo T, Alber J, Galldiks N, Kustermann E, Arndt S, Jacobs AH, Krone W, Kahn CR, Bruning JC. Role for neuronal insulin resistance in neurodegenerative diseases. Proc Natl Acad Sci U S A. 2004;101:3100–3105. doi: 10.1073/pnas.0308724101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Planel E, Tatebayashi Y, Miyasaka T, Liu L, Wang L, Herman M, Yu WH, Luchsinger JA, Wadzinski B, Duff KE, Takashima A. Insulin dysfunction induces in vivo tau hyperphosphorylation through distinct mechanisms. J Neurosci. 2007;27:13635–13648. doi: 10.1523/JNEUROSCI.3949-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de la Monte SM, Tong M. Brain metabolic dysfunction at the core of Alzheimer's disease. Biochem Pharmacol. 2014;88:548–559. doi: 10.1016/j.bcp.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stoothoff WH, Johnson GV. Tau phosphorylation: physiological and pathological consequences. Biochim Biophys Acta. 2005;1739:280–297. doi: 10.1016/j.bbadis.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 36.Malek-Ahmadi M, Beach T, Obradov A, Sue L, Belden C, Davis K, Walker DG, Lue L, Adem A, Sabbagh MN. Increased Alzheimer's disease neuropathology is associated with type 2 diabetes and ApoE epsilon.4 carrier status. Curr Alzheimer Res. 2013;10:654–659. doi: 10.2174/15672050113109990006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Profenno LA, Faraone SV. Diabetes and overweight associate with non-APOE4 genotype in an Alzheimer's disease population. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:822–829. doi: 10.1002/ajmg.b.30694. [DOI] [PubMed] [Google Scholar]
- 38.Claxton A, Baker LD, Hanson A, Trittschuh EH, Cholerton B, Morgan A, Callaghan M, Arbuckle M, Behl C, Craft S. Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer's disease dementia. J Alzheimers Dis. 2015;44:897–906. doi: 10.3233/JAD-141791. [DOI] [PubMed] [Google Scholar]
- 39.Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B, Fishel MA, Plymate SR, Breitner JC, DeGroodt W, Mehta P, Craft S. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008;70:440–448. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- 40.Craft S, Asthana S, Schellenberg G, Baker L, Cherrier M, Boyt AA, Martins RN, Raskind M, Peskind E, Plymate S. Insulin effects on glucose metabolism, memory, and plasma amyloid precursor protein in Alzheimer's disease differ according to apolipoprotein-E genotype. Ann N Y Acad Sci. 2000;903:222–228. doi: 10.1111/j.1749-6632.2000.tb06371.x. [DOI] [PubMed] [Google Scholar]
- 41.Craft S, Peskind E, Schwartz MW, Schellenberg GD, Raskind M, Porte D., Jr. Cerebrospinal fluid and plasma insulin levels in Alzheimer's disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology. 1998;50:164–168. doi: 10.1212/wnl.50.1.164. [DOI] [PubMed] [Google Scholar]
- 42.Ong QR, Chan ES, Lim ML, Wong BS. Expression of human apolipoprotein E4 reduces insulin-receptor substrate 1 expression and Akt phosphorylation in the ageing liver. FEBS open bio. 2014;4:260–265. doi: 10.1016/j.fob.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burns JM, Honea RA, Vidoni ED, Hutfles LJ, Brooks WM, Swerdlow RH. Insulin is differentially related to cognitive decline and atrophy in Alzheimer's disease and aging. Biochim Biophys Acta. 2012;1822:333–339. doi: 10.1016/j.bbadis.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burns JM, Donnelly JE, Anderson HS, Mayo MS, Spencer-Gardner L, Thomas G, Cronk BB, Haddad Z, Klima D, Hansen D, Brooks WM. Peripheral insulin and brain structure in early Alzheimer disease. Neurology. 2007;69:1094–1104. doi: 10.1212/01.wnl.0000276952.91704.af. [DOI] [PubMed] [Google Scholar]
- 45.Marques-Vidal P, Mazoyer E, Bongard V, Gourdy P, Ruidavets JB, Drouet L, Ferrieres J. Prevalence of insulin resistance syndrome in southwestern France and its relationship with inflammatory and hemostatic markers. Diabetes Care. 2002;25:1371–1377. doi: 10.2337/diacare.25.8.1371. [DOI] [PubMed] [Google Scholar]
- 46.Qu HQ, Li Q, Rentfro AR, Fisher-Hoch SP, McCormick JB. The definition of insulin resistance using HOMA-IR for Americans of Mexican descent using machine learning. PloS one. 2011;6:e21041. doi: 10.1371/journal.pone.0021041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ascaso JF, Pardo S, Real JT, Lorente RI, Priego A, Carmena R. Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care. 2003;26:3320–3325. doi: 10.2337/diacare.26.12.3320. [DOI] [PubMed] [Google Scholar]
- 48.Standaert DG, Lee VM, Greenberg BD, Lowery DE, Trojanowski JQ. Molecular features of hypothalamic plaques in Alzheimer's disease. Am J Pathol. 1991;139:681–691. [PMC free article] [PubMed] [Google Scholar]
- 49.Moreira PI, Carvalho C, Zhu X, Smith MA, Perry G. Mitochondrial dysfunction is a trigger of Alzheimer's disease pathophysiology. Biochim Biophys Acta. 2010;1802:2–10. doi: 10.1016/j.bbadis.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 50.Swerdlow RH, Khan SM. A “mitochondrial cascade hypothesis” for sporadic Alzheimer's disease. Med Hypotheses. 2004;63:8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 51.Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008;102:401–414. doi: 10.1161/CIRCRESAHA.107.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kandimalla RJ, Prabhakar S, Wani WY, Kaushal A, Gupta N, Sharma DR, Grover VK, Bhardwaj N, Jain K, Gill KD. CSF p-Tau levels in the prediction of Alzheimer's disease. Biology Open. 2013;2:1119–1124. doi: 10.1242/bio.20135447. [DOI] [PMC free article] [PubMed] [Google Scholar]