Abstract
Previous research suggests that a history of early life stress (ELS) impacts working memory (WM) in adulthood. Despite the widespread use of WM paradigms, few studies have evaluated whether ELS exposure, in the absence of psychiatric illness, also impacts WM-associated brain activity in ways that might improve sensitivity to these ELS effects or provide insights into the mechanisms of these effects. This study evaluated whether ELS affects WM behavioral performance and task-associated activity by acquiring 3T functional images from 27 medication-free healthy adults (14 with ELS) during an N-back WM task that included 0- and 2-back components. Whole brain voxel-wise analysis was performed to evaluate WM activation, followed by region of interest analyses to evaluate relationships between activation and clinical variables. ELS was associated with poorer accuracy during the 2-back (79 %±19 vs. 92 %±9, p=0.049); accuracy and response time otherwise did not differ between groups. During the 0-back, ELS participants demonstrated increased activation in the superior temporal gyrus/insula, left inferior parietal lobule (IPL) (both corrected p<0.001), and middle temporal and parahippocampal gyrus (MTG/PHG)(corrected p<0.010). During the 2-back, ELS was associated with greater activation in the IPL, MTG/PHG and inferior frontal gyrus (corrected p<0.001), with a trend towards precuneus activation (p=0.080). These findings support previous research showing that ELS is associated with impaired neurobehavioral performance and changes in brain activation, suggesting recruitment of additional cognitive resources during WM in ELS. Based on these findings, ELS screening in future WM imaging studies appears warranted.
Keywords: Life stress, Childhood adversity, Functional magnetic resonance imaging, Working memory, Executive function
Introduction
Trauma during childhood is a common and well-established risk factor for psychiatric illness, but this exposure is not commonly accounted for in neuroimaging studies. Childhood trauma and maltreatment, often called early life stress (ELS), includes a wide range of exposures, including physical, emotional, and sexual abuse, neglect, and parental loss (Teicher and Samson 2013). ELS is commonly associated with impaired development and performance across numerous social, cognitive, and physiological domains that are often a focus of neuroimaging studies (Evans and Schamberg 2009; Lansford et al. 2002; Shonkoff and Garner 2012) is also associated with up to 45 % of childhood-onset and roughly one third of later-onset psychiatric disorders (Green et al. 2010). ELS appears to be a risk factor for some of the most prevalent psychiatric illnesses, such as major depressive disorder and anxiety disorders (Teicher and Samson 2013; Teicher et al. 2006).
A relationship between ELS and cognitive impairment has been well documented outside of the neuroimaging literature. ELS is associated with decreased memory, attention, working memory, and inhibitory control and lower academic performance (for extensive reviews, see (Teicher and Samson 2013; Hart and Rubia 2012; Pechtel and Pizzagalli 2011; Tyrka et al. 2013). Impaired working memory (WM), in particular, has been associated with ELS, and this exposure is related to cognitive impairments that are similar to those observed in many psychiatric illnesses (Majer et al. 2010), such as major depression (Baune et al. 2012; Harvey et al. 2004), bipolar disorder (Bora et al. 2011; Mayer and Park 2012), generalized anxiety disorder, and panic disorder (Butters et al. 2011; Castaneda et al. 2011), obsessive-compulsive disorder (Abramovitch et al. 2013; Roopesh et al. 2013), and schizophrenia (Eich et al. 2013; Silver et al. 2003; Tek et al. 2013). Posttraumatic stress disorder (PTSD) has also been strongly associated with WM impairment (Koso and Hansen 2006; Lagarde et al. 2010; Schuitevoerder et al. 2013; Vasterling et al. 2002). Adults with a history of ELS have shown diminished WM performance on neuropsychological testing, with significant positive correlations between impairment and abuse severity (Majer et al. 2010; Cromheeke et al. 2013). Comparable results, associating ELS with decreased WM functioning, have been found in children (Perna and Kiefner 2012) and young adults with comorbid psychiatric illness (Gould et al. 2012).
Despite the abundance of findings demonstrating WM impairment in ELS, few studies have examined both behavioral and neuroimaging correlates of ELS exposure (McCrory et al. 2011; Raine et al. 2001). In the brain morphometry literature, Hanson et al. (2012) reported smaller volumes of prefrontal cortex and white matter between the anterior cingulate and the frontal poles, which was related to greater cumulative life stress and spatial working memory deficits in adolescents.
Functional magnetic resonance imaging (FMRI) research examining the relationship between ELS and WM also remains limited. FMRI studies of past trauma have predominantly focused on diagnosed PTSD samples. They have observed abnormal activations of frontal brain areas during WM, such as the dorsolateral and ventrolateral prefrontal cortices, and increased recruitment of parietal regions compared to controls (Daniels et al. 2010; Moores et al. 2008; Zhang et al. 2013). However, FMRI research specifically isolating the effects of ELS on WM remains scarce. One neuroimaging study assessed cortical activity in four groups of adult male violent offenders and non-offenders, with and without a history of childhood physical abuse. Men with ELS, regardless of offender status, showed reduced left hemispheric activation during WM. The abused non-offender group demonstrated decreased activation of the left superior temporal gyrus, increased activation of the right superior temporal gyrus, and a strong deficit in behavioral task performance (Raine et al. 2001). However, the study did not report frequency of comorbid psychiatric illness, making it difficult to determine to what extent the results might have reflected possible psychiatric conditions, rather than ELS exposure alone. Other limitations include the use of earlier scanners and inclusion of only men, further complicating interpretation of this data. In another study, youths with a history of interpersonal trauma with post-traumatic symptoms exhibited diminished right hippocampal activity during a verbal declarative memory task, compared to youths without the same exposure (Carrion et al. 2007). Recently, in a preliminary study from our group, adults with a history of childhood maltreatment and abuse demonstrated enhanced deactivations of the default mode network (DMN) (Philip et al. 2013a). Taken together, these findings suggest that ELS exposure is associated with a differential allocation of cognitive resources, as reflected by altered patterns of brain activity during WM tasks.
We hypothesized that ELS-exposed subjects would show inferior performance and require greater cognitive resources – manifested as increased activation in regions associated with executive function and increased default network deactivation – during both 0- and 2 back conditions, as compared to non-exposed participants. This hypothesis reflects our previous work in this population, which found an increased DMN deactivation response to a WM challenge in a smaller ELS sample (Philip et al. 2013a). Our prior study was underpowered to detect whole-brain activation at the voxel level, and we hypothesized that the deactivations observed were, at least in part, compensatory for increased WM-related brain activation. An additional practical rationale for this comparison was to determine whether assessment for ELS should be included in the screening of candidates for participation in WM neuroim-aging studies. More importantly, detection of these shifts in neural response during WM may serve as sensitive markers of risk and yield valuable information on the neural mechanisms that link ELS to altered cognitive function in adulthood.
This study aimed to assess the relationship between ELS and WM-associated brain activity in light of the prevalence of both ELS and reports of impaired WM in ELS, and the relative paucity of current neuroimaging data on ELS-associated WM impairments. To better characterize unique imaging correlates, this study utilized a participant sample without the confounding influence of psychiatric medication or current illness.
Materials and methods
Participants
Participants were recruited for this study from a larger study examining potential endophenotypes for mood/anxiety disorder; this report describes unique data, gathered separately from the parent study. Participants with a history of reported ELS exposure (n=14) and healthy controls (n=13) were recruited from the community. ELS and control participants were matched on age and gender. Study protocols were approved by Institutional Review Boards at Brown University and Butler Hospital, and were performed in accordance with the 1964 Declaration of Helsinki. All participants provided voluntary written informed consent following full explanation of study procedures. Inclusion criteria were report of physical, emotional, or sexual abuse as a child of at least “moderate/severe” intensity using the Childhood Trauma Questionnaire (CTQ) (Bernstein and Fink 1998a, b) for the ELS group, or absence of this history utilizing the same instrument for the control group. The CTQ is a 28-item self-report measure that asks respondents to recollect the frequency of childhood experiences of abuse and neglect using a 5-point Likert-type scale (e.g., “never true,” “rarely true,” “sometimes true,” “often true,” or “very often true”). CTQ items assess different types of maltreatment (emotional, physical, and sexual abuse, and emotional and physical neglect) and the scale generates five subscale scores for each type of ELS. Published cut-off scores are used to determine severity categories (i.e., none to minimal, low to moderate, moderate to severe, and severe to extreme) (Bernstein and Fink 1998a, b). Based on this categorization we calculated a CTQ summary score, for the five subscales, where ELS severity for each subtype was assigned a numerical value: “none/minimal” = 0, “low to moderate” =1, “moderate to severe” = 2, or “severe to extreme” = 3, with total range from 0 to 15.
Other inclusion criteria were right handedness, and absence of a current DSM-IV-TR Axis I or Axis II psychiatric disorder, assessed by the Structured Clinical Interview for DSM-IV-TR (SCID and SCID II) (First et al. 1994); participants were included if they had a prior history (lifetime) of psychiatric illness. Exclusion criteria were contraindications to MRI scanning, current treatment with psychotropic medications, and any evidence of current significant medical or neurological illness (assessed by medical history, physical and neurological examinations, electrocardiogram, and standard blood chemistries), including careful neuroendocrine and cardiac screening as part of the parent study. Study measures included assessment of depressive symptoms using the Inventory of Depressive Symptomatology Self-Report scale (IDSSR) (Rush et al. 2003), and anxiety symptoms using the State-Trait Anxiety Inventory (STAI) (Vythilingam et al. 2002; Spielberg et al. 1983). All rating scale data and diagnostic interviews were administered by trained research staff and results reviewed and confirmed by research psychiatrists. Participants from either group who reported significant life stress (using the Perceived Stress Scale) (Cohen et al. 1983) in the month prior to MRI imaging were also excluded. Since prior data has implicated nicotine use in altered working memory performance (Sweet et al. 2010) and associated neural networks (Sutherland et al. 2011), smoking status was also assessed and no participants were imaged during nicotine withdrawal. Negative toxicology screens were required for study participation, and women of childbearing age were required to have a negative pregnancy test before MRI exposure. Participants were reimbursed $50 for their involvement in the study.
Working memory paradigm: N-back
The N-back task was utilized to evaluate WM. This task is widely used in FMRI research, and provides reliable patterns of brain activation and deactivation (Philip et al. 2013a; Cao et al. 2014; Fernandez-Corcuera et al. 2013; Satterthwaite et al. 2013; Sweet et al. 2008; Sweet et al. 2004). In this study, the N-back included three components: 0-back and 2-back tasks, plus a task-free baseline for comparison (Philip et al. 2014a).
0-back task
During the 0-back, participants responded “yes” when a predetermined target consonant (“H” or “h”) appeared, and “no” when the screen presented other consonants, using the first two fingers of their right hand on a two-button response box. In each block, consonants were presented for 500 ms each, with an interstimulus interval (ISI) of 2500 ms. A total of six 0-back blocks, each containing 9 consonants, were presented.
2-back task
During the 2-back, participants made a “yes” or “no” response, after each consonant presented in the same fashion, to indicate whether the letter on the screen was the same or different from the one presented two previously (e.g., w, N, r, N, R, Q, r, q, N, W etc., with trials warranting a “yes” response in bold). Similar to the 0-back, each consonant was presented for 500 ms each, with an ISI of 2500 ms. A total of six 2-back blocks each containing 15 consonants, were presented.
Overall N-back structure
2-back blocks were shown in alternating order with 0-back blocks. In between each N-back condition, a 30-s task-free baseline condition (i.e., gray fixation cross against a dark background) was presented to allow contrasts between task-associated brain activation against a task-free condition. Two runs of the N-back were presented in counterbalanced order (i.e., 2back, rest, 0back; 0back, rest, 2back). During both 0-and 2-back conditions, the rate of stimulus presentation was identical, with 33 % of targets presented randomly. Capitalization was randomized to limit visual matching.
Task-related accuracy and response time served as metrics of WM behavioral data, and were compared between groups using independent samples t-tests, with statistical significance set at a two-tailed p<0.05. All participants practiced the N-back prior to entering the magnet to avoid confounding influence of learning effects, and data from any in-scanner task performed at less or near chance (i.e., ≤50 % accuracy) was excluded from all subsequent behavioral and imaging analyses.
Image acquisition
All neuroimaging data were acquired at the Brown University MRI Research Facility (mri.brown.edu) using a Siemens TIM TRIO 3T scanner (Siemens, Erlangen, Germany) equipped with a 32-channel head coil. Whole-brain high-resolution (1 mm3) T1 images were acquired for anatomic reference; acquisition parameters were TR=1900, TE=2.98 ms, and FOV 256 mm2. Echoplanar images (EPI) were acquired during two separate 4.8-min epochs. These sequences yielded 116 whole brain volumes for each functional imaging runs. EPI acquisition parameters were TR=2500, TE=28, FOV=192 mm2, and matrix size 642 in 3-mm axial slices.
Image preprocessing
After image acquisition, anatomic data were transformed to standard Talairach stereotaxic space (Talairach and Tournoux 1988). Echoplanar images (EPI) were reconstructed into 3D + time datasets, aligned to anatomic data in original space and then transformed into Talairach space. EPI data were concatenated and registered to the sixth volume of the first series to minimize movement artifact and generate motion correction parameters for use as covariates of no interest in subsequent analyses. Bandpass filtering was performed at 0.009 s < f <0.08 s to reduce the effects of high-frequency noise and low-frequency drift and was applied to each EPI imaging run separately prior to concatenation. Data was smoothed up to a 6-mm full width at half maximum (FWHM) Gaussian distribution. Voxel-based general linear modeling (GLM) was used to quantify task-specific brain activity. Independent variables included in the GLM were the temporal course of the 0- and 2-back tasks (including transitions modeled as a gamma function) and movement parameters, with blood oxygenation level dependent (BOLD) signal over time as the dependent variable. The resultant individual activation maps, which reflected the unique activity of 0- and 2-back conditions compared against a task-free baseline, were registered to high-resolution T1 images at the individual dataset level. Since we previously reported that ELS-exposed individuals showed significant deactivations during the 0-back (Philip et al. 2013a), resultant beta weights from individual datasets of brain response to the 0- and 2-back conditions, were separately used as the metric of brain activity in statistical analyses. Any participants with movement greater than 3 mm were a priori excluded from data analyses; however it was not necessary to exclude participants on this basis. All preprocessing and data analyses utilized Analysis of Functional NeuroImages (AFNI) (Cox 1996), unless otherwise specified.
Data analyses steps included initial t-tests against a hypothetical mean of zero to identify regions of activation or deactivation compared to a task-free baseline (i.e., no difference between active task and baseline). This was followed by group-level contrasts performed using AFNI's 3dttest++ program controlling for differences in behavioral performance (i.e., that statistically differed between groups). Significance threshold for whole-brain contrast was set at two tailed p<0.05, utilizing Family-Wise Error (FWE) correction for multiple comparisons in AFNI's ClusterSim program, which utilizes Monte Carlo simulations to generate the requisite voxel and cluster thresholds for a predetermined alpha level within a specified resolution and matrix size (3dClustSim -dxyz 3 3 3 -nxyz 54 64 50 -fwhmxyz 5.72 5.63 5.62). After performing voxel-wise contrasts, regions that statistically differed between groups on the whole-brain analyses were retained as a mask for subsequent region of interest (ROI) analyses. These explanatory ROI analyses were performed within the ELS group, and used bivariate Pearson r in SPSS (SPSS Statistics 20, IBM Corporation, Armonk NJ) to evaluate correlations between mean beta weights of activity and a) ELS summary score and b) ELS subtype severity score (i.e., physical abuse, sexual abuse, etc.), c) depression and d) anxiety symptoms in order to contextualize the meaning of the observed group differences and to explore any relationships between ELS subtype and imaging findings. Statistical thresholds for these comparisons were set at a two-tailed p<0.05 in SPSS.
Results
Participants and behavioral performance
Demographic information is reported in Table 1; there were no significant group differences in age, gender, education, or race. Five participants in the ELS group had prior history of mood-, anxiety- or depressive disorders-Not Otherwise Specified, and no participants in the control group met criteria for these disorders. Two participants in the ELS group, and one participant in the control group, smoked cigarettes; there were no group differences in number of cigarettes smoked daily (p>0.1). Within the ELS group, two participants met “moderate” severity criteria for only sexual abuse, whereas the rest of the sample met threshold for two or more subtypes of ELS. One participant in the ELS group was excluded due to performance less than chance for both 0- and 2 back tasks (39 and 14 %, respectively); another ELS participant's data was excluded due to performance less than chance on the 2-back (45 %). There were no group differences in 0-back response times or accuracy (both p>0.100). There were no group differences in 2-back response time (p>0.100), but ELS participants' accuracy on the working memory task was significantly lower than the controls' (79 %±19 vs. 92 %±9, p=0.049, for ELS and control groups, respectively). Therefore, accuracy was controlled in the between group voxel-wise contrasts of 2-back related brain activity.
Table 1.
Demographic, clinical and behavioral characteristics
| Characteristic | ELS (n=13)a | Control (n=13) | p |
|---|---|---|---|
| Age (Mean ± SD years) | 38±9 | 30±9 | ns |
| Gender (n, % Female) | 6 (46) | 9 (69) | ns |
| College education (%) | 62 | 46 | ns |
| Race (% Caucasian) | 69 | 84 | ns |
| CTQ | |||
| Category (n, %)a | |||
| Emotional abuse | 2 (15) | – | |
| Physical abuse | 6 (46) | – | |
| Sexual abuse | 8 (62) | – | |
| Emotional neglect | 5 (38) | – | |
| Physical neglect | 5 (38) | – | |
| Summary severity (Mean ± SD)b | 7±4 | ||
| CTQ total score (Mean ± SD) | 59±17 | 31±6 | <0.001 |
| N-back | |||
| Response time (Mean ± SD ms) | |||
| 0-back | 622±112 | 682±158 | ns |
| 2-backc | 929±260 | 806±147 | ns |
| Accuracy (Mean ± SD %) | |||
| 0-back | 95±6 | 96±8 | ns |
| 2-backc | 79±19 | 92±9 | 0.049 |
SD standard deviation, CTQ Childhood Trauma Questionnaire
Participants endorsing at least moderate scores in CTQ categories
Sum of severity scores for the five CTQ categories, where ELS severity is indicated by “none/minimal”=0, “low to moderate”=1, “moderate to severe”=2, or “severe to extreme”=3, with a total range of 0–15
One ELS participant was excluded from the 2-back data analysis
0-back FMRI results
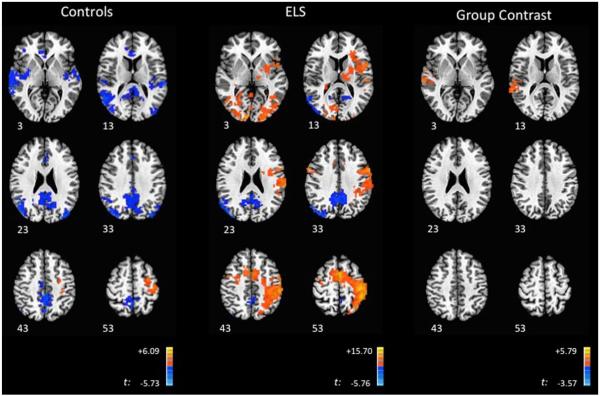
Relative to baseline, during the 0-back, ELS-exposed participants demonstrated statistically significant deactivations in multiple DMN regions, including the PCC and middle temporal gyrus (MTG) extending into the superior occipital gyrus (Fig. 1). Activations peaked in the precentral gyrus, extending into the postcentral gyrus and insula, with an additional peak in the lingual gyrus (Table 2A). Control subjects demonstrated a pattern of statistically significant deactivations predominantly in DMN regions, with activations in the precentral gyrus, medial frontal gyrus and cerebellum (Fig. 1, Table 2B). Group contrasts revealed that ELS participants demonstrated significantly greater activation in three regions: the right superior temporal gyrus (STG), left inferior parietal lobule (IPL), and right MTG (extending into the parahippocampal gyrus (PHG)) (Fig. 1, Table 2C). Examination of the group contrast maps (Fig. 1) suggests that these differences were due to significant activation in the ELS group that was absent in the control group in the STG, IPL, and MTG, and significant deactivation in the control group that was absent from the ELS group in the corresponding regions (Fig. 2).
Fig. 1.
Brain Activity during the 0-back. Axial images of ELS and control participants, with group contrasts during the 0-back. Images are shown using radiologic convention. Z coordinates of each slice are included on the bottom left of the corresponding image
Table 2.
Brain activity during 0-back task
| Peak region | BA | Coordinates (x,y,z) | Cluster size | t-scove | p-value |
|---|---|---|---|---|---|
| A. ELS group | |||||
| L Precentral gyrus | 4 | 38, 26, 63 | 2944 | 3.98 | <0.001 |
| R Lingual gyrus | 17 | −8, 92, 9 | 2454 | 4.28 | <0.001 |
| L/R Precuneus/PCC | 31 | 2, 47, 39 | 665 | −2.71 | <0.001 |
| R Middle temporal gyrus | 19 | −44, 77, 27 | 276 | −4.01 | <0.001 |
| B. Control group | |||||
| R Precuenus | 19 | −26, 79, 38 | 1089 | −2.20 | <0.001 |
| L PCC/Precuneus | 7 | 2,53,51 | 1001 | −2.30 | <0.001 |
| L Superior temporal gyrus | 21 | 62, 20, −1 | 255 | −2.93 | <0.001 |
| R Declive (Cerebellum) | −44, 65, −22 | 170 | 2.50 | 0.040 | |
| L Sup. occ. gyrus/Precuneus | 19 | 35, 80, 33 | 168 | −2.81 | 0.045 |
| L Precentral gyrus | 6 | 35, 17, 63 | 168 | 3.10 | 0.045 |
| R Medial frontal gyrus | 32 | −2, −44, 9 | 155 | −2.38 | 0.075 |
| C. Group comparisons | |||||
| R Superior temporal gyrus | 42 | −62, 8, 9 | 238 | 2.62 | <0.001 |
| L Inferior parietal lobule | 40 | 53, 35, 48 | 232 | 2.31 | <0.001 |
| R middle temporal gyrus | 37 | −53, 59, 3 | 201 | 2.29 | 0.010 |
Abbreviations: BA Brodmann area, p-value FWE-corrected p-value, L left, R right, PCC posterior cingulate cortex, Sup superior, Occ occipital. Peak voxel coordinates based in the atlas of Talairach and Tournoux
Fig. 2.
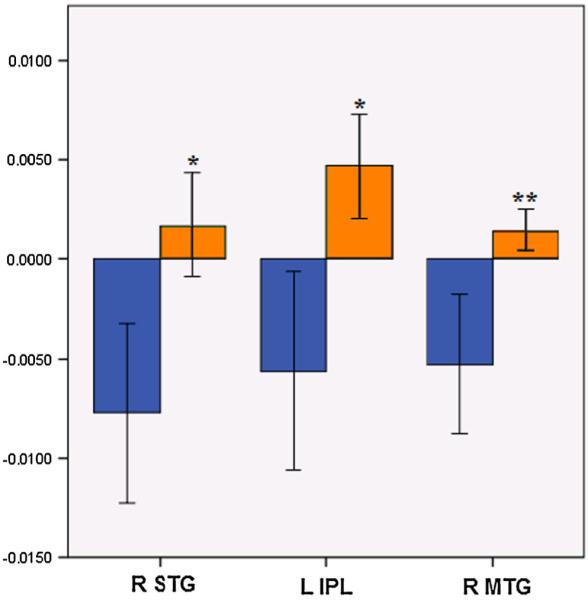
Effect of ELS on 0-back associated activity. Mean effect of ELS on 0-back associated activity in regions that exhibited significant group differences, derived from voxelwise contrasts. Blue bars indicate the control group and orange bars indicate the ELS group. Y-axis indicates beta weight, as an estimation of percent signal change. Error bars indicate standard error of the mean. X-axis indicates regions that significantly differed between groups during whole-brain voxel-wise group contrasts. * p<0.001, ** p<0.01, corrected for multiple comparisons; ELS early life stress, STG superior temporal gyrus, IPL inferior parietal lobule, MTG middle temporal region
2-back FMRI results
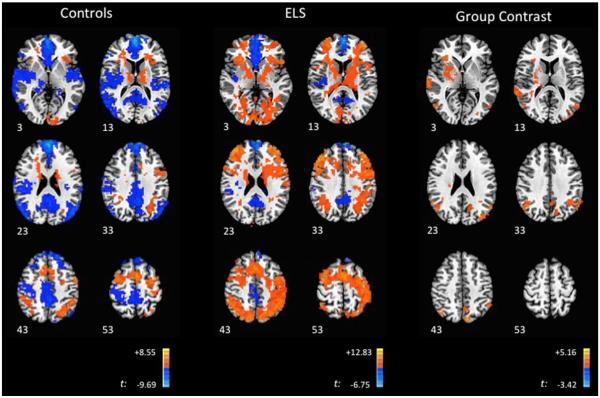
During the 2-back, ELS participants demonstrated significant deactivation in regions associated with the DMN, including the medial prefrontal cortex, PCC and medial temporal regions, alongside deactivation within the inferior frontal gyrus. Widespread activations were observed, which peaked in the superior parietal lobule and dorsolateral frontal and medial frontal cortices (Fig. 3; Table 3A). This was in contrast with findings in the control group, which showed widespread DMN deactivations, alongside activations in the lateral and medial frontal cortices, cerebellum, inferior frontal gyrus, and insula (Fig. 3, Table 3B). In group contrasts, the ELS group demonstrated significantly greater activation peaking within four regions, the right inferior frontal gyrus (IFG) (extending into the thalamus), right IPL, left IPL (with activation extending into the STG), left PCC/precuneus, IPL (Fig. 3; Table 3C). Examination of the group activation maps suggests that these differences were due to significant activation in the ELS group that was absent in controls in these four regions, with deactivations observed in the controls that were absent in the ELS group (Fig. 4).
Fig. 3.
Brain Activity during the 2-back. Axial images of ELS and control participants, with group contrasts during the 2-back. Images are shown using radiologic convention. Z coordinates of each slice are included on the bottom left of the corresponding image
Table 3.
Brain activity during 2-back task
| Peak region | BA | Coordinates (x,y,z) | Cluster size | t-scove | p-value |
|---|---|---|---|---|---|
| A. ELS group | |||||
| R Medial frontal gyrus | 8 | −41, −29, 39 | 10433 | 2.68 | <0.001 |
| L Medial prefrontal cortex | 10 | 2, −56, 12 | 961 | −3.57 | <0.001 |
| R Superior parietal lobule | 7 | −35, 58, 50 | 671 | 4.74 | <0.001 |
| R Inferior parietal lobule | 13 | −47, 32, 21 | 606 | −2.61 | <0.001 |
| LPCC | 23 | 2, 47, 24 | 535 | −4.11 | <0.001 |
| L Inferior frontal gyrus | 47 | 47, −32, −4 | 520 | −4.40 | <0.001 |
| B. Control group | |||||
| R Medial frontal gyrus | 10 | −2, −56, 21 | 8542 | −4.95 | <0.001 |
| R Declive (Cerebellum) | −2, 38, −40 | 925 | 3.18 | <0.001 | |
| L Superior frontal gyrus | 6 | 2, −5, 51 | 498 | 5.89 | <0.001 |
| L Middle temporal gyrus | 39 | 47, 71, 24 | 424 | −2.98 | <0.001 |
| L Superior parietal lobule | 7 | 32, 65, 51 | 350 | 2.80 | <0.001 |
| R Insula/Inf. frontal gyrus | 13 | −32, −20, 9 | 325 | 3.74 | <0.001 |
| L Middle frontal gyrus | 6 | 32, 8, 60 | 323 | 3.41 | <0.001 |
| R Superior parietal lobule | 7 | −35, 62, 48 | 194 | 2.25 | 0.015 |
| L Culmen (Cerebellum) | 35, 56, −19 | 188 | 4.06 | 0.020 | |
| L Insula | 13 | 29, −20, 12 | 159 | 4.39 | 0.065 |
| L Thalamus | 8, 8, 18 | 153 | 2.98 | 0.085 | |
| C. Group comparison | |||||
| R Inferior frontal gyrus | 47 | −44, −23, −4 | 387 | 2.54 | <0.001 |
| R Inferior parietal lobule | 39 | −44, 65, 42 | 313 | 2.91 | <0.001 |
| L Superior temporal gyrus/IPL | 39 | 53, 62, 27 | 284 | 2.71 | <0.001 |
| L PCC/precuneus | 7 | 11, 77, 45 | 153 | 2.86 | 0.080 |
Abbreviations: BA Brodmann area, p-value FWE-corrected p-value, L left, R right, IPL inferior parietal lobule, PCC posterior cingulate cortex. Peak voxel coordinates based in the atlas of Talairach and Tournoux
Fig. 4.
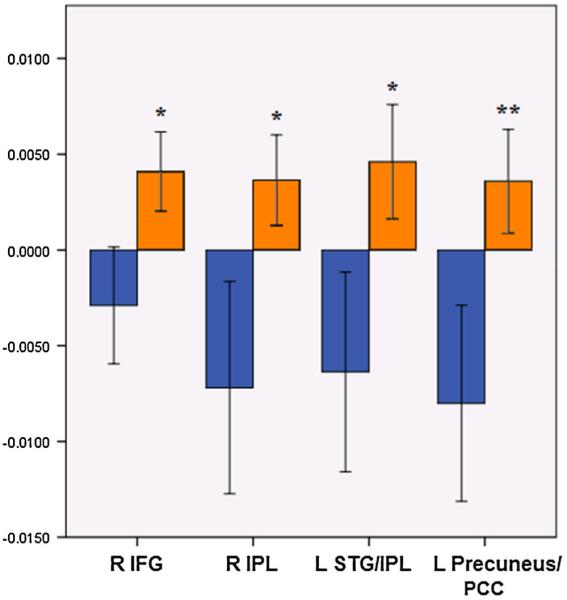
Effect of ELS on 2-back associated activity. Mean effect of ELS on 2-back associated activity in regions that exhibited significant group differences, derived from voxelwise contrasts. Blue bars indicate the control group and orange bars indicate the ELS group. Y-axis indicates beta weight, as an estimation of percent signal change. Error bars indicate standard error of the mean. X-axis indicates regions that significantly differed between groups during whole-brain voxel-wise group contrasts. * p<0.001, ** p<0.08, corrected for multiple comparisons; ELS early life stress, IFG inferior frontal gyrus, IPL inferior parietal lobule, STG superior temporal gyrus, PCC posterior cingulate cortex
Relationship between clinical rating scales and ELS effects observed on FMRI
ELS participants had higher mean scores on the IDSSR (10±6 vs. 3±2, ELS vs. non-ELS, respectively, p<0.01). There was higher trait anxiety in the ELS group (32±9 vs. 26±5, ELS vs. non-ELS, respectively, p=0.04), but no group differences in state anxiety (p>0.1). Mean IDSSR and STAI scores in each group were below cutoffs indicative of clinically significant symptoms (Rush et al. 2003; Spielberg et al. 1983).
There was a significant correlation between ELS severity and right MTG/PHG activity during the 0-back (r=0.418, p= 0.034), and a trend was noted for correlations between severity and activity in the right STG/insula (r=0.333, p=0.096). During the 2-back there were significant correlations between severity and activity in the right IFG (r=0.452, p=0.023) and left MTG/IPL (r=0.414, p=0.04). Trends were also observed for correlations between severity and activity in the right MTG/IPL (r=0.377, p=0.063) and left precuneus (r= 0.371, p=0.068). There were no statistically significant correlations between severity of ELS subtype (i.e., emotional abuse, physical abuse, sexual abuse, etc.) and 0- or 2-back associated activations in these regions.
When evaluating the relationship between reported depressive or anxiety symptoms, during the 0-back there were significant correlations between Trait Anxiety and activity in the right STG (r=0.475, p=0.014), left IPL (r=0.392, p= 0.048), and right MTG (r=0.416, p=0.035). On the 2-back, trend-level correlations were observed between IDSSR scores and activity in the left precuneus (r=0.353, p= 0.084) and right inferior frontal region (r=0.329, p= 0.108). Trend-level associations were also found between left precuneus activity and Trait Anxiety scores during the 2-back (r=0.376, p=0.064).
Discussion
To our knowledge, this is the first study to evaluate WM-associated performance and FMRI brain activity patterns in a sample of ELS-exposed participants without psychiatric illness or medication use, compared to controls. We found decreased WM accuracy in ELS-exposed participants, relative to controls, and these cognitive deficits were seen alongside increased activation in temporal-parietal DMN nodes and frontal inhibitory regions; these patterns of activation were also modestly correlated with anxiety and depressive symptoms. These findings indicate that FMRI may complement behavioral assessments and yield potentially more sensitive markers of ELS.
0-back: ELS is associated with altered Insula and DMN activity
The neuroimaging findings during the 0-back task in the control group are consistent with previous findings during this task, which include DMN deactivations (Philip et al. 2013a) and premotor and motor activation (Sweet et al. 2004; Sweet et al. 2006; Owen et al. 2005). These results stand in contrast to findings in the ELS group, which showed qualitatively reduced extent of significant DMN deactivation with a larger extent of significant activation including the insula, premotor and motor regions.
Group differences during the 0-back revealed a significant absence of deactivation in the right STG and insula in the ELS group. A similar pattern was observed in the left hemisphere, where there was significant deactivation in the control group and significant activation of the in the ELS group. However, these differences did not reach statistical significance in the direct group contrast. Insula involvement has been previously reported during WM in healthy participants (Hanson et al. 2012) and patients with bipolar depression (Fernandez-Corcuera et al. 2013), and the increased involvement in the ELS group is consistent with the hypothesis that these participants required greater salience-related resources to perform the 0-back.
ELS-associated activation of the MTG/PHG region, which is associated with the DMN, was an unexpected finding during 0-back. The PHG would be expected to exhibit deactivation during external cognitive demands (Andrews-Hanna et al. 2010), as was observed in the control group. This result suggests that ELS-participants are recruiting more cortical areas to perform the 0-back, further indicating that they required greater cognitive resources to perform even this relatively simple task. While the difficulty level might not have been great enough to elicit differences in behavioral measures, enhanced recruitment may be apparent before changes in behavioral measures are observed (Sweet et al. 2006; Charlet et al. 2013).
0-back group contrasts additionally demonstrated significantly greater IPL activation in the ELS group. This region is involved in spatial memory and visual-spatial processing (Pardo et al. 1991; Petersen et al. 1988; Posner et al. 1988), and is traditionally recruited during the 2-back. Prior studies have highlighted how the IPL may serve to integrate information processing in otherwise separated canonical brain networks (Braga et al. 2013), and the IPL developmentally matures later than other brain regions (Barber et al. 2013). This may result in a longer period of vulnerability to neurotoxic effects of prolonged glucocorticoid secretion associated with trauma exposure (Conrad et al. 2007; Patel and Finch 2002). To our knowledge, this is the first study to demonstrate IPL over-activation during the 0-back in an ELS-exposed sample compared to a control group, although related studies have implicated IPL dysfunction in stress-related conditions. Our previous research has found diminished resting state connectivity (Philip et al. 2013b) associated with this area, suggesting a convergence of results using multiple analytic methods. Interestingly, associations between anxiety and structural integrity have also recently been reported in this region. Nardo et al. (2013) found increased grey matter volume in the IPL in a sample with PTSD and dissociative symptoms, which suggests that the relationship between stress, structure and function within the IPL may be an important area for further research to characterize the neuroimaging correlates of stress exposure.
2back: ELS is associated with altered activity in the DMN and frontal inhibitory regions
Images from the control group during the 2-back demonstrated DMN deactivation during this task, alongside executive network activation. This is consistent with the previous DMN (Philip et al. 2013a; Philip et al. 2013b) and WM (Owen et al. 2005; Fernandez-Corcuera et al. 2013; Satterthwaite et al. 2013) literature, and supports the validity of the activity that was associated with the 2-back task in this study. Similar to the 0-back results, during the 2-back the ELS group demonstrated qualitatively different patterns of activation and deactivation compared to controls.
Group contrasts demonstrated a statistically significant pattern of bilateral activation in MTG/IPL regions associated with ELS that overlap with the right-sided findings during the 0-back, albeit with a greater spatial extent into adjacent areas. This likely reflects increased cognitive effort, an interpretation supported by the decreased 2-back accuracy in the ELS group.
Furthermore what is notable is a differential pattern of activation observed between ELS and control participants. The significant regional brain activity patterns observed in ELS groups were due to activations, compared to deactivation in the control groups, during both the 0- and 2-back tasks. This indicates that ELS participants demonstrated brain activation patterns that were fundamentally different than the non-exposed controls. This suggests that the N-back may be a sensitive measure of ELS exposure.
An alternative interpretation of these findings is that IPL activation, in a region known to be an intermediate node between executive and default networks (Braga et al. 2013), is reflective of inadequate communication between these networks, such that larger areas of local cortex need to be recruited in order to shift internal resources from internal (i.e., DMNrelated) to external (i.e., executive) functions during WM. This interpretation would be consistent with our previous findings of reduced prefrontal to IPL connectivity in ELS (Philip et al. 2014b). Future studies, perhaps evaluating task-associated connectivity during WM, might address this hypothesis.
Findings from this study have relevance for interpretation of the current literature characterizing WM in psychiatric disorders. Our principal finding is that ELS exposure, even in the absence of medications or psychiatric illness, is associated with significantly inferior WM, in terms of both neuropsychological performance during the N-back and task-associated changes in brain activity. This work, using a prospective design, larger sample size, and more detailed ELS assessment, expands upon our prior study that showed greater DMN deactivation associated with ELS, and highlights that activation and deactivation patterns may be substantially different in ELS samples compared to non-exposed controls. In this larger sample we also found differences in ELS-related WM performance that was previously not observed. This work indicates the need to formally control healthy, ELS-exposed individuals with both non-exposed controls and those with ELS-associated psychiatric disorders. As reviewed above, impaired WM is common across samples with psychiatric illnesses. Since ELS is also strongly associated with the development of these illnesses, results from this study suggest that at least a component of the previous findings in the WM literature may be due to the common denominator of ELS exposure. This theory is supported by results from this study, which found WM-related activations in the ELS group overlapping with some regions reported in previous WM imaging studies of various psychiatric disorders (e.g., the IPL, found in Moores et al. 2008 and Daniels et al. 2010), but not overlapping in lateral and medial prefrontal regions found in these studies. Based on these findings, future WM neuroimaging studies should include ELS screening during participant recruitment, and should endeavor to either control for childhood maltreatment severity or recruit only those without an ELS history, in order to ensure valid comparisons. Maternal stress exposure should also be measured, as recent data indicates stress during pregnancy can affect WM (Plamondon et al. 2015), as well as evaluating for potential interactions between WM performance and stress-related polymorphisms (Fuge et al. 2014).
This study had several important limitations. A retrospective assessment of ELS was employed, which is a standard method but nonetheless vulnerable to recall bias. This primary limitation is shared among other studies utilizing retrospective childhood assessments. Secondly, this study provided a cross-sectional assessment of participants, and as such it is difficult to draw conclusions regarding longitudinal stability of neuro-imaging findings. Other limitations, by design, were that patients with active psychiatric illness were not included, and we not exclude based on prior psychiatric history. Direct comparisons between these findings and, for example, findings generated in samples with PTSD or depression patients, are not possible. The sample included in this study was also majority Caucasian, reflecting the geographic location of the study, but limiting its generalizability. The mild ELS exposure reported in the control group might have served to attenuate group differences, although these reports validate the representativeness of our sample. Major strengths of this study include the well-characterized nature of current symptoms in a healthy population, and no apparent confounding influences of medications or psychiatric and medical illnesses.
In summary, we found that previous ELS exposure is associated with altered patterns of task-associated activation during both 0- and 2-back tasks, and that the observed differences from the control group occurred largely in important hub regions shared by the default mode and executive networks. These results also confirm results from previous studies, using different imaging modalities, of ELS-associated IPL dysfunction, suggesting this region may have a unique role in ELS-associated psychopathology. Results from this study indicate that trauma exposure – even in the absence of diagnosed psychiatric illness – can have a significant impact on WM imaging. This raises the question of how this has impacted prior WM imaging studies, and indicates that evaluation of ELS should be included in future neuropsychological and imaging studies.
Acknowledgments and conflicts of interest
This study was supported in part by NIH grant 5R01MH068767 (LLC), Veterans Administration grant 1IK2CX000724 (NSP) and grants from the Brown MRI Research Facility (NSP) and Rhode Island Foundation (NSP). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the NIH or Department of Veterans Affairs. Noah S. Philip, Lawrence H. Sweet, Audrey R. Tyrka, S. Louisa Carpenter, Sarah E. Albright, Lawrence H. Price, and Linda L. Carpenter have no relevant conflicts of interest to disclose. We thank all of the participants.
References
- Abramovitch A, Abramowitz JS, Mittelman A. The neuropsychology of adult obsessive-compulsive disorder: a meta-analysis. Clinical Psychology Review. 2013;33:1163–71. doi: 10.1016/j.cpr.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65:550–62. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AD, Caffo BS, Pekar J, Mostofsky SH. Developmental changes in within- and between-network connectivity between late childhood and adulthood. Neuropsychologia. 2013;51:156–167. doi: 10.1016/j.neuropsychologia.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baune BT, Czira ME, Smith AL, Mitchell D, Sinnamon G. Neuropsychological performance in a sample of 13–25 year olds with a history of non-psychotic major depressive disorder. Journal of Affective Disorders. 2012;14:441–8. doi: 10.1016/j.jad.2012.02.041. [DOI] [PubMed] [Google Scholar]
- Bernstein D, Fink L. Childhood trauma questionnaire: A retrospective self-report. Pearson Education, Inc; San Antonio: 1998a. [Google Scholar]
- Bernstein DP, Fink LA. Childhood trauma questionnaire: A retrospective self-report manual. The Psychological Corporation; San Antonio: 1998b. [Google Scholar]
- Bora E, Yucel M, Pantelis C, Berk M. Meta-analytic review of neurocognition in bipolar II disorder. Acta Psychiatrica Scandinavica. 2011;123:165–74. doi: 10.1111/j.1600-0447.2010.01638.x. [DOI] [PubMed] [Google Scholar]
- Braga RM, Sharp DJ, Leeson C, Wise RJ, Leech R. Echoes of the brain within default mode, association, and heteromodal cortices. Journal of Neuroscience. 2013;33:14031–9. doi: 10.1523/JNEUROSCI.0570-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butters MA, Bhalla RK, Andreescu C, et al. Changes in neuropsychological functioning following treatment for late-life generalised anxiety disorder. British Journal of Psychiatry. 2011;199:211–8. doi: 10.1192/bjp.bp.110.090217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Plichta MM, Schafer A, et al. Test-retest reliability of fMRI-based graph theoretical properties during working memory, emotion processing, and resting state. NeuroImage. 2014;84:888–900. doi: 10.1016/j.neuroimage.2013.09.013. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Haas BW, Garrett A, Song S, Reiss AL. Reduced hippocampal activity in youth with posttraumatic stress symptoms: an FMRI study. Journal of Pediatric Psychology. 2007;35:559–69. doi: 10.1093/jpepsy/jsp112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda AE, Suvisaari J, Marttunen M, et al. Cognitive functioning in a population-based sample of young adults with anxiety disorders. European Psychiatry. 2011;26:346–53. doi: 10.1016/j.eurpsy.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Charlet K, Beck A, Jorde A, et al. Increased neural activity during high working memory load predicts low relapse risk in alcohol dependence. Addiction Biology. 2013;19:402–14. doi: 10.1111/adb.12103. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–96. [PubMed] [Google Scholar]
- Conrad CD, McLaughlin KJ, Harman JS, et al. Chronic glucocorticoids increase hippocampal vulnerability to neurotoxicity under conditions that produce CA3 dendritic retraction but fail to impair spatial recognition memory. Journal of Neuroscience. 2007;27:8278–85. doi: 10.1523/JNEUROSCI.2121-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cromheeke S, Herpoel LA, Mueller SC. Childhood abuse is related to working memory impairment for positive emotion in female university students. Child Maltreatment. 2013;19:38–48. doi: 10.1177/1077559513511522. [DOI] [PubMed] [Google Scholar]
- Daniels JK, McFarlane AC, Bluhm RL, et al. Switching between executive and default mode networks in posttraumatic stress disorder: alterations in functional connectivity. Journal of Psychiatry and Neuroscience. 2010;35:258–66. doi: 10.1503/jpn.090175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eich TS, Nee DE, Insel C, Malapani C, Smith EE. Neural correlates of impaired cognitive control over working memory in schizophrenia. Biological Psychiatry. 2013;76:146–53. doi: 10.1016/j.biopsych.2013.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, Schamberg MA. Childhood poverty, chronic stress, and adult working memory. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:6545–9. doi: 10.1073/pnas.0811910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Corcuera P, Salvador R, Monte GC, et al. Bipolar depressed patients show both failure to activate and failure to deactivate during performance of a working memory task. Journal of Affective Disorders. 2013;148:170–8. doi: 10.1016/j.jad.2012.04.009. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structural clinical interview for Axis I DSM-IV disorders: Biometrics research department. New York State Psychiatric Institute; New York: 1994. [Google Scholar]
- Fuge P, Aust S, Fan Y, Weigand A, Gärtner M, Feeser M, Bajbouj M, Grimm S. Interaction of early life stress and corticotropin-releasing hormone receptor gene: effects on working memory. Biological Psychiatry. 2014;76(11):888–94. doi: 10.1016/j.biopsych.2014.04.016. doi:10.1016/j. biopsych.2014.04.016. Epub 2014 May 14. [DOI] [PubMed] [Google Scholar]
- Gould F, Clarke J, Heim C, Harvey PD, Majer M, Nemeroff CB. The effects of child abuse and neglect on cognitive functioning in adulthood. Journal of Psychiatric Research. 2012;46:500–6. doi: 10.1016/j.jpsychires.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, et al. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Archives of General Psychiatry. 2010;67:113–23. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Chung MK, Avants BB, et al. Structural variations in prefrontal cortex mediate the relationship between early childhood stress and spatial working memory. Journal of Neuroscience. 2012;32:7917–25. doi: 10.1523/JNEUROSCI.0307-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H, Rubia K. Neuroimaging of child abuse: a critical review. Frontiers in Human Neuroscience. 2012;6:52. doi: 10.3389/fnhum.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PO, Le Bastard G, Pochon JB, et al. Executive functions and updating of the contents of working memory in uni-polar depression. Journal of Psychiatric Research. 2004;38:567–76. doi: 10.1016/j.jpsychires.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Koso M, Hansen S. Executive function and memory in post-traumatic stress disorder: a study of Bosnian war veterans. European Psychiatry. 2006;21:167–73. doi: 10.1016/j.eurpsy.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Lagarde G, Doyon J, Brunet A. Memory and executive dysfunctions associated with acute posttraumatic stress disorder. Psychiatry Research. 2010;177:144–9. doi: 10.1016/j.psychres.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Lansford JE, Dodge KA, Pettit GS, Bates JE, Crozier J, Kaplow J. A 12-year prospective study of the long-term effects of early child physical maltreatment on psychological, behavioral, and academic problems in adolescence. Archives of Pediatrics and Adolescent Medicine. 2002;156:824–30. doi: 10.1001/archpedi.156.8.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majer M, Nater UM, Lin JM, Capuron L, Reeves WC. Association of childhood trauma with cognitive function in healthy adults: a pilot study. BMC Neurology. 2010;10:61. doi: 10.1186/1471-2377-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer JS, Park S. Working memory encoding and false memory in schizophrenia and bipolar disorder in a spatial delayed response task. Journal of Abnormal Psychology. 2012;121:784–94. doi: 10.1037/a0028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory E, De Brito SA, Viding E. The impact of childhood maltreatment: a review of neurobiological and genetic factors. Frontiers Psychiatry. 2011;2:48. doi: 10.3389/fpsyt.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moores KA, Clark CR, McFarlane AC, Brown GC, Puce A, Taylor DJ. Abnormal recruitment of working memory updating networks during maintenance of trauma-neutral information in post-traumatic stress disorder. Psychiatry Research. 2008;163:156–70. doi: 10.1016/j.pscychresns.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Nardo D, Hogberg G, Lanius RA, et al. Gray matter volume alterations related to trait dissociation in PTSD and traumatized controls. Acta Psychiatrica Scandinavica. 2013;128:222–33. doi: 10.1111/acps.12026. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Human Brain Mapping. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo JV, Fox PT, Raichle ME. Localization of a human system for sustained attention by positron emission tomography. Nature. 1991;349:61–4. doi: 10.1038/349061a0. [DOI] [PubMed] [Google Scholar]
- Patel NV, Finch CE. The glucocorticoid paradox of caloric restriction in slowing brain aging. Neurobiology of Aging. 2002;23:707–17. doi: 10.1016/s0197-4580(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology. 2011;214:55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perna RB, Kiefner M. Long-term cognitive sequelae: abused children without PTSD. Applied Neuropsychology Child. 2012;2:1–5. doi: 10.1080/09084282.2011.595460. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;331:585–9. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Philip NS, Sweet LH, Tyrka AR, et al. Early life stress is associated with greater default network deactivation during working memory in healthy controls: a preliminary report. Brain Imaging and Behavior. 2013a;7:204–12. doi: 10.1007/s11682-012-9216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip NS, Sweet LH, Tyrka AR, Price LH, Bloom RF, Carpenter LL. Decreased default network connectivity is associated with early life stress in medication-free healthy adults. European Neuropsychopharmacology. 2013b;23:24–32. doi: 10.1016/j.euroneuro.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip NS, Carpenter SL, Sweet LH. Developing neuroimaging phenotypes of the default mode network in PTSD: integrating the resting state, working memory, and structural connectivity. Journal of Visualized Experiments. 2014a;89:51651. doi: 10.3791/51651. doi:10.3791/51651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip NS, Valentine TR, Sweet LH, Tyrka AR, Price LH, Carpenter LL. Early life stress impacts dorsolateral prefrontal cortex functional connectivity in healthy adults: Informing future studies of antidepressant treatments. Journal of Psychiatric Research. 2014b;52:63–9. doi: 10.1016/j.jpsychires.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plamondon A, Akbari E, Atkinson L, Steiner M, Meaney MJ, Fleming AS. MAVAN research team. Spatial working memory and attention skills are predicted by maternal stress during pregnancy. Early Human Development. 2015;91(1):23–9. doi: 10.1016/j.earlhumdev.2014.11.004. doi:10.1016/j.earlhumdev.2014.11.004. Epub 2014 Nov 26. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE, Fox PT, Raichle ME. Localization of cognitive operations in the human brain. Science. 1988;240:1627–31. doi: 10.1126/science.3289116. [DOI] [PubMed] [Google Scholar]
- Raine A, Park S, Lencz T, et al. Reduced right hemisphere activation in severely abused violent offenders during a working memory task: an fMRI study. Aggressive Behavior. 2001;27:111–29. [Google Scholar]
- Roopesh BN, Janardhan Reddy YC, Mukundan CR. Neuropsychological deficits in drug naive, non-depressed obsessive-compulsive disorder patients. Asian Journal Psychiatry. 2013;6:162–70. doi: 10.1016/j.ajp.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Rush AJ, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biological Psychiatry. 2003;54:573–83. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Erus G, et al. Functional maturation of the executive system during adolescence. Journal of Neuroscience. 2013;33:16249–61. doi: 10.1523/JNEUROSCI.2345-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuitevoerder S, Rosen JW, Twamley EW, et al. A meta-analysis of cognitive functioning in older adults with PTSD. Journal of Anxiety Disorders. 2013;27:550–8. doi: 10.1016/j.janxdis.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Garner AS. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129:e232–46. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- Silver H, Feldman P, Bilker W, Gur RC. Working memory deficit as a core neuropsychological dysfunction in schizophrenia. The American Journal of Psychiatry. 2003;160:1809–16. doi: 10.1176/appi.ajp.160.10.1809. [DOI] [PubMed] [Google Scholar]
- Spielberg CD, Gorsuch RL, Lushene RE. Manual for the state-trait anxiety inventory consulting. Consulting Psychologists Press; Palo Alto: 1983. [Google Scholar]
- Sutherland MT, Ross TJ, Shakleya DM, Huestis MA, Stein EA. Chronic smoking, but not acute nicotine administration, modulates neural correlates of working memory. Psychopharmacology (Berl) 2011;213(1):29–42. doi: 10.1007/s00213-010-2013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet LH, Rao SM, Primeau M, Mayer AR, Cohen RA. Functional magnetic resonance imaging of working memory among multiple sclerosis patients. Journal of Neuroimaging. 2004;14:150–7. [PubMed] [Google Scholar]
- Sweet LH, Rao SM, Primeau M, Durgerian S, Cohen RA. Functional magnetic resonance imaging response to increased verbal working memory demands among patients with multiple sclerosis. Human Brain Mapping. 2006;27:28–36. doi: 10.1002/hbm.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet LH, Paskavitz JF, Haley AP, et al. Imaging phonological similarity effects on verbal working memory. Neuropsychologia. 2008;46:1114–23. doi: 10.1016/j.neuropsychologia.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Sweet LH, Mulligan RC, Finnerty CE, Jerskey BA, David SP, Cohen RA, Niaura RS. Effects of nicotine withdrawal on verbal working memory and associated brain response. Psychiatry Research. 2010;183:69–74. doi: 10.1016/j.pscychresns.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: An approach to cerebral imaging. Thieme Medical Publishers, Inc; Stuggart: 1988. [Google Scholar]
- Teicher MH, Samson JA. Childhood maltreatment and psychopathology: a case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. The American Journal of Psychiatry. 2013;170:1114–33. doi: 10.1176/appi.ajp.2013.12070957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Tomoda A, Andersen SL. Neurobiological consequences of early stress and childhood maltreatment: are results from human and animal studies comparable? Annals of the New York Academy of Sciences. 2006;1071:313–23. doi: 10.1196/annals.1364.024. [DOI] [PubMed] [Google Scholar]
- Tek C, Gold J, Blaxton T, Wilk C, McMahon RP, Buchanan RW. Visual perceptual and working memory impairments in schizophrenia. Archives of General Psychiatry. 2013;59:146–53. doi: 10.1001/archpsyc.59.2.146. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Burgers DE, Philip NS, Price LH, Carpenter LL. The neurobiological correlates of childhood adversity and implications for treatment. Acta Psychiatrica Scandinavica. 2013;128:434–47. doi: 10.1111/acps.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasterling JJ, Duke LM, Brailey K, Constans JI, Allain AN, Jr., Sutker PB. Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology. 2002;16:5–14. doi: 10.1037//0894-4105.16.1.5. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Heim C, Newport J, et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. The American Journal of Psychiatry. 2002;159:2072–80. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JN, Xiong KL, Qiu MG, et al. Negative emotional distraction on neural circuits for working memory in patients with posttraumatic stress disorder. Brain Research. 2013;1531:94–101. doi: 10.1016/j.brainres.2013.07.042. [DOI] [PubMed] [Google Scholar]