SUMMARY
Although chronic sleep restriction frequently produces long-lasting behavioural and physiological impairments in humans, the underlying neural mechanisms are unknown. Here we used a rat model of chronic sleep restriction to investigate the role of brain adenosine and noradrenaline systems, known to regulate sleep and wakefulness, respectively. The density of adenosine A1 and A2a receptors and β-adrenergic receptors before, during and following 5 days of sleep restriction was assessed with autoradiography. Rats (n = 48) were sleep-deprived for 18 h day–1 for 5 consecutive days (SR1–SR5), followed by 3 unrestricted recovery sleep days (R1–R3). Brains were collected at the beginning of the light period, which was immediately after the end of sleep deprivation on sleep restriction days. Chronic sleep restriction increased adenosine A1 receptor density significantly in nine of the 13 brain areas analysed with elevations also observed on R3 (+18 to +32%). In contrast, chronic sleep restriction reduced adenosine A2a receptor density significantly in one of the three brain areas analysed (olfactory tubercle which declined 26–31% from SR1 to R1). A decrease in b-adrenergic receptors density was seen in substantia innominata and ventral pallidum which remained reduced on R3, but no changes were found in the anterior cingulate cortex. These data suggest that chronic sleep restriction can induce long-term changes in the brain adenosine and noradrenaline receptors, which may underlie the long-lasting neurocognitive impairments observed in chronic sleep restriction.
Keywords: [3H]-ZM 241385, [3H]-DPCPX, basal forebrain, [3H]-DHA, receptor autoradiography
INTRODUCTION
A poorly understood but important problem in our 24/7 society is the increasing prevalence of chronic sleep restriction (CSR), the daily insufficient sleep over a long period. CSR leads to elevated daytime sleepiness and deteriorated cognitive performance as well as impaired health, including hypertension, obesity, neuronal diseases and even increased mortality (Banks and Dinges, 2007). Although many of these detrimental effects of CSR have been documented in humans, there has been surprisingly little work on the brain mechanisms that underlie the long-lasting behavioural and physiological impairments during and following CSR. Here we used a rat model of CSR to investigate the role of brain adenosine and noradrenaline systems, known to regulate sleep and wakefulness, respectively.
The inhibitory neuromodulator adenosine has been proposed as an endogenous sleep factor that mediates the sleepiness associated with prolonged wakefulness. Extracellular adenosine levels increase during spontaneous wakefulness as well as sleep deprivation (SD), especially in the basal forebrain (BF), leading to inhibition of wake-active BF neurones, which have widespread projections to the cerebral cortex and other brain areas (Basheer et al., 2004; Brown et al., 2012).
The locus coeruleus noradrenaline neurones are known to mediate wakefulness. The firing rate of these neurones is high during waking (especially during high vigilance), slow and irregular during non-rapid eye movement (NREM) sleep and almost absent during REM sleep (Jones, 2005). These neurones project diffusely to the forebrain, brain stem and spinal cord, and are thought to initiate and maintain waking state (Berridge, 2008; Jones, 2005). A recent optogenetic study also confirmed that locus coeruleus noradrenaline neurones play a key role in modulating sleep/wake time and electroencephalography (EEG) power of different arousal states (Carter et al., 2010).
With the novel rat model of CSR that we developed (Kim et al., 2007), we have investigated changes in receptor mRNA of these two neurochemical systems (Kim et al., 2012, 2013). Throughout five sleep restriction (SR) days and on the first day of recovery sleep we found that adenosine A1 receptor (A1R) mRNA levels were elevated in the BF region, whereas A2a receptor (A2aR) mRNA levels were reduced in the frontal cortex (Kim et al., 2012). In contrast, β-adrenergic receptor (β-AR) mRNA levels were reduced only on the first SR day and returned to the baseline level from the third SR day in the anterior cingulate cortex (Kim et al., 2013).
The present study used autoradiography and this rat CSR model to measure membrane-bound A1R in 13 brain regions. A more limited analysis of A2aR and β-AR was conducted in three brain regions. The extension of our previous mRNA findings is essential, because mRNA levels do not always predict neuronal receptor densities accurately (Rizzo et al., 2014). Receptor density changes are also more physiologically relevant than mRNA. Indeed, the findings reveal brain region- and receptor-specific differences in receptor protein density on the third day of recovery sleep that were not seen in previous mRNA studies.
METHODS
Animals
Three-month-old male Sprague–Dawley rats were housed individually and maintained on a 12 : 12-h light–dark cycle (lights on at 10:00 hours) with free access to food and water. All animal procedures were approved by the Institutional Animal Care and Use Committee at the VA Boston Health-care System.
Experimental design
The experimental design followed our previous studies (Kim et al., 2012, 2013). Rats (n = 48) were moved to the experimental room at least 4 days before the experiments began and were habituated to the SD wheels for 2 h day–1 for 2 consecutive days (without rotation on the first day and with rotation on the second day). Rats were assigned to one of the six conditions (baseline = BL, SR1, SR3, SR5, R1, R3) and each condition had eight rats that were run in two cohorts. The protocol was designed with daily 6-h blocks of sleep opportunity (SO) starting at light onset, i.e. zeitgeber time 0 (ZT0 = 10:00 hours), the beginning of the rats’ rest period in order to model typical human CSR. For up to 5 consecutive days (SR1–SR5), animals were sleep-deprived for 18 h (ZT6–24) per day, followed by a daily 6-h SO (ZT0– 6). Following the last day of SR, animals in the R1 and R3 conditions were allowed unrestricted recovery sleep for up to 3 days. As shown in Fig. 1, brains were collected at light onset (i.e. immediately following 18-h SD on SR days) on BL, SR1, SR3, SR5, R1 and R3 days.
Figure 1.
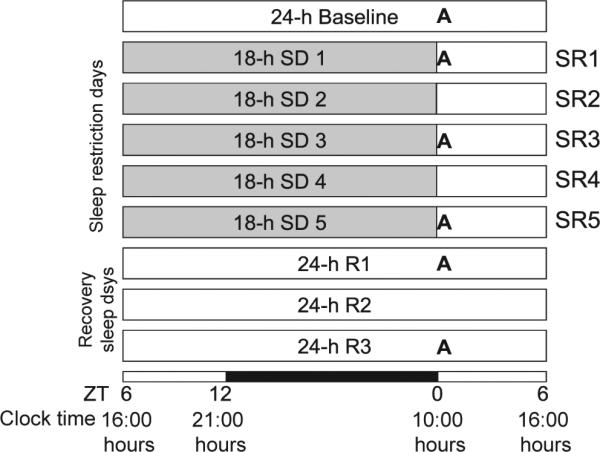
Schematic diagram of experimental design. For five sleep restriction (SR) days, 18-h sleep deprivation (SD) was started 6 h after light onset [zeitgeber time 6 (ZT6) = 16:00 hours] followed by a 6-h sleep opportunity (ZT0-6). Thereafter, animals were given a 3-day full recovery sleep period (R1–R3). The 12 : 12-h light–dark cycle is indicated at the bottom (open bar = light phase; black bar = dark phase). ‘A’ represents brain tissue collection time-points (ZT0) for receptor autoradiography.
Sleep deprivation
Sleep deprivation methods have been reported previously in detail (Kim et al., 2012). Briefly, animals were sleep-deprived by placing each animal in a periodically rotating wheel (product no. 80860; Lafayette Instrument Co., Lafayette, IN, USA) programmed on a repeated cycle of 4 s on (3 m min–1) and 12 s off during the daily 18-h periods of SD (Animal Wheel Monitor software; Lafayette Instrument Co.). Animals had free access to food and water throughout the SD and SO periods. After the daily 18-h SD period, animals were returned quickly to their home cage for the 6-h SO. This CSR protocol has been shown previously to reduce the amount of daily sleep to about half the normal sleep time for 5 consecutive days followed by 3 days of unrestricted recovery sleep (Kim et al., 2012).
Autoradiography
Rats (n = 48 in total) were anaesthetized briefly with isoflurane and then decapitated, and their brains removed rapidly, frozen in 2-methylbutane (–40 °C) and stored at –80 °C. The temperature of the brains (–80 °C) was equilibrated to the temperature at which they were to be sliced (–20 °C) prior to slicing. Brain sections (20 μm thick) were thaw-mounted onto silane-coated slides (Starfrost adhesive; Knittel GmbH, Braunschweig, Germany). The sections were then dried on a warming plate at 35 °C before they were sorted into slide cassettes, vacuum-packed and stored at –80 °C. To obtain an anatomical overview of the brain sections, aiding the evaluation of the autoradiograms, cresyl fast violet staining was performed in adjacent sections. Autoradiographic analysis for the A1R started after a preincubation step in Tris-HCl buffer (170 mm; pH 7.4) for 15 min at 4 °C. Three sections per coronal level per rat were incubated for 2 h at room temperature (RT) in buffer containing 1 nm of [3H]-DPCPX (8-cyclopentyl-1,3-dipropylxanthine) and 2 units mL–1 adenosine deaminase and 100 μm Gpp(NH)p. One batch of incubation solution was prepared and distributed in four vials that were used for the incubation of 60 slides. For the assessment of non-specific binding, three sections per coronal level per animal were incubated with R-phenyl-iso-propyl-adenosine (100 μm) in the medium. Binding steps were followed by two washes in preincubation buffer (5 min, 4 °C) and a rapid rinse in ice-cold water (15 s). Then the sections were dried with a stream of air.
For the A2aR analysis, preincubation was performed with Tris-HCl buffer (170 mm; pH 7.4 and ethylediamine tetraacetic acid (1 mm) for 30 min at 37 °C. Thereafter a first wash with buffer and MgCl2 (10 mm) was performed twice for 10 min at RT followed by incubation for the total binding with buffer and adenosine deaminase (2 units mL–) and the ligand [3H]-ZM 241385 (0.42 nM) for 120 min at RT. For nonspecific binding, 2-chloro-adenosine (20 μm) was added to the above-mentioned solution. A second washing step was performed with buffer twice for 5 min at 4 °C, followed by a rapid rinse with distilled water at 4 °C before drying as described above.
The β-AR distributions were determined by preincubation with a Tris-HCl buffer (50 mm, pH 7.4, 25 mm MgCl2) at 22 °C for 10 min. Then, incubation for the total binding was performed with the buffer and the ligand [3H]-DHA (dihydroalprenolol) (1 nm) for 60 min at 22 °C to determine the fraction of β1 and 2 receptors. To measure only β1 receptors, the same setup was used but the β2 selective antagonist ICI 118551 (0.5 μm; Tocris Bioscience, Bristol, UK) was added. For the non-specific binding, propranolol-hydrochloride (50 μm; Sigma Aldrich, Taufkirchen, Germany) was used. Afterwards, the slides were washed once with the buffer for 1 min at RT and twice for 5 min at 4 °C, followed by a rapid rinse with distilled water at 4 °C before drying.
Glass slides were cut closely around the brain sections to reduce the size and taped to paper sheets. Sections were organized to have two to three sets of all conditions (BL-R3) per sheet. Each phosphor-imaging plate (BAS2025; Fuji, Tokyo, Japan; AIDA 2.31; Raytest-Fuji, Straubenhardt, Germany) was exposed to approximately 100 labelled slides together with industrial tritium activity standards (Amersham Biosciences, Piscataway, NJ, USA). After 72 h of exposure, the stored information was retrieved with an image plate reader (spatial resolution of 50 νm; BAS 5000; Fuji).
The digital autoradiograms were processed further with respective image analysis software (Image Gauge 4.0; Fuji). Regions of interest (ROI) were defined based on the cresyl fast violet-stained brain sections and according to a standard rat brain atlas (Paxinos and Watson, 2007). To determine the distribution of cholinergic neurones in caudate-putamen striasomes, acetylcholinesterase staining was performed on sections used previously in autoradiographic experiments. Two sections containing the caudate-putamen per animal were analysed. Specific binding was calculated as the difference of total and non-specific binding. Using the known values of the radioactive standard, a calibration straight line (R2 > 0.99) was created, allowing the assignment of a quantified value to each original image value. These ROI values were used to calculate receptor density values (pmol per mg protein) using the following equation: Bmax = B × (KD + L)/L × 18.4, where B = specific ligand binding (total binding – non-specific binding) (pmol mg– wet weight) and the KD values were: [3H]-DPCPX = 1 nm; [3H]-DHA = 2.3 nm; [3H]-ZM 241385 = 0.42 nm (as determined previously for rats) and L = ligand concentrations as listed above; the factor 18.4 is an experimentally determined conversion factor for wet weight to protein dry per weight. The mean receptor density value of each brain region was averaged from values of that brain region (both hemispheres) from all eight animals within a condition group (except n = 7 for the olfactory bulb A2aR BL and SR5 groups).
Statistics
To test for the significance of the treatments, a one-way analysis of variance (anova) was performed on the ligand binding data followed by a pairwise least significant difference test and a linear contrast test for post-hoc analysis (SPSS version 22; IBM, Rochester, MN, USA). For all tests, P < 0.05 was considered statistically significant. All reported values are mean ± standard error of the mean.
RESULTS
Fig. 2 shows representative baseline examples of autoradio-grams of the adenosine and noradrenaline receptor distributions and the outline of ROI. The effects of the CSR protocol on the receptor densities in all brain areas analysed are shown in Table 1.
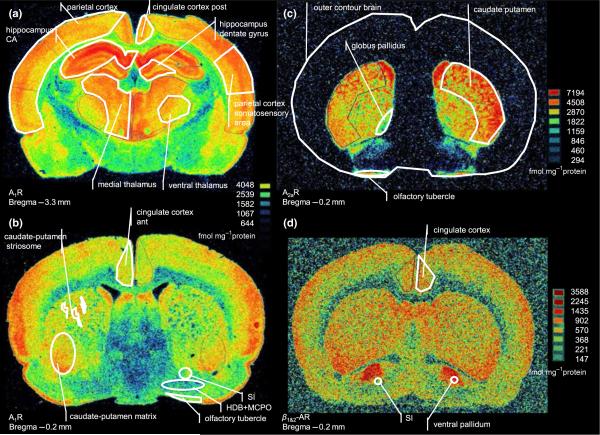
Figure 2.
Representative autoradiograms showing the receptor distribution in the baseline condition and how the regions of interest were defined. (a,b) [3H]-DPCPX (8-cyclopentyl-1,3-dipropylxanthine) binding to adenosine A1 receptors at two coronal sections. (c) [3H]-ZM 241 385 binding to adenosine A2a receptors. (d) [3H]-DHA (dihydroalprenolol) binding to adrenergic β1- and β2-adrenergic receptors. The shape and size of some brain regions varied markedly between the three individual sections analysed at each anatomical level. For example, the globus pallidus often appeared much bigger than it does in the –0.2 AP level shown in (c). CA, cornu amonis; SI, substantia innominata; HDB, horizontal limb of diagonal band; MCPO, magnocellular preoptic nuclei.
Table 1.
Receptor density (pmol mg–1 protein) of adenosine A1 (A1R) and A2a receptors (A2aR), and β-adrenergic receptors (β-AR; types 1 and 2) during baseline (BL), sleep restriction (SR1, SR3 and SR5) and recovery sleep days (R1 and R3)
| BL | SR1 | SR3 | SR5 | R1 | R3 | ANOVA P-value | Linear contrast | |
|---|---|---|---|---|---|---|---|---|
| A1R | ||||||||
| Basal forebrain | ||||||||
| HDB + MCPO | 2.70 ± 0.14 | 2.81 ± 0.17 | 2.66 ± 0.15 | 3.04 ± 0.20 | 3.04 ± 0.25 | 3.03 ± 0.13 | 0.420 | 0.069 |
| SI | 3.74 ± 0.24 | 3.86 ± 0.25 | 3.48 ± 0.25 | 4.42 ± 0.27 | 4.33 ± 0.39 | 4.62 ± 0.16 | 0.029 | 0.004 |
| Cortex | ||||||||
| Ant. cingulate | 5.85 ± 0.36 | 5.76 ± 0.45 | 4.92 ± 0.41 | 6.27 ± 0.48 | 6.93 ± 0.80 | 7.56 ± 0.49 | 0.015 | 0.003 |
| Post-cingulate | 7.24 ± 0.42 | 7.49 ± 0.34 | 6.08 ± 0.28 | 7.25 ± 0.46 | 7.65 ± 0.84 | 8.31 ± 0.48 | 0.083 | 0.110 |
| Parietal | 7.85 ± 1.14 | 7.97 ± 0.97 | 6.21 ± 1.07 | 8.00 ± 1.53 | 8.68 ± 1.60 | 9.25 ± 1.65 | 0.002 | 0.009 |
| Somatosensory | 8.53 ± 1.20 | 8.51 ± 1.06 | 6.64 ± 1.09 | 8.62 ± 1.70 | 9.13 ± 1.96 | 9.85 ± 1.75 | 0.005 | 0.023 |
| Hippocampus | ||||||||
| CA | 12.39 ± 0.56 | 12.21 ± 0.53 | 9.91 ± 0.66 | 12.74 ± 0.79 | 13.38 ± 1.11 | 14.14 ± 0.70 | 0.008 | 0.021 |
| Dentate gyrus | 9.74 ± 0.58 | 9.34 ± 0.52 | 7.35 ± 0.46 | 10.08 ± 0.61 | 10.77 ± 0.71 | 10.72 ± 0.46 | 0.001 | 0.015 |
| Thalamus | ||||||||
| Medial | 9.28 ± 0.43 | 9.50 ± 0.43 | 8.04 ± 0.48 | 9.84 ± 0.58 | 10.54 ± 0.64 | 11.10 ± 0.65 | 0.006 | 0.004 |
| Ventral | 6.76 ± 0.36 | 6.78 ± 0.33 | 5.99 ± 0.44 | 6.99 ± 0.50 | 7.38 ± 0.43 | 7.30 ± 0.48 | 0.253 | 0.135 |
| Caudate-putamen | ||||||||
| Matrix | 5.47 ± 0.33 | 5.77 ± 0.38 | 4.69 ± 0.36 | 5.88 ± 0.42 | 6.21 ± 0.66 | 7.19 ± 0.27 | 0.006 | 0.003 |
| Striasomes | 6.38 ± 0.34 | 6.44 ± 0.43 | 5.60 ± 0.31 | 6.45 ± 0.39 | 7.19 ± 0.54 | 7.79 ± 0.35 | 0.009 | 0.004 |
| Olfactory tubercle | 4.79 ± 0.31 | 5.02 ± 0.42 | 4.47 ± 0.26 | 5.29 ± 0.28 | 5.61 ± 0.40 | 5.72 ± 0.30 | 0.086 | 0.014 |
| A2aR | ||||||||
| Olfactory tubercle | 4.11 ± 0.32 | 3.03 ± 0.31 | 2.90 ± 0.27 | 2.72 ± 0.35 | 2.81 ± 0.24 | 3.45 ± 0.34 | 0.031 | 0.162 |
| Globus pallidus | 3.33 ± 0.16 | 3.04 ± 0.19 | 3.14 ± 0.06 | 3.05 ± 0.10 | 3.29 ± 0.15 | 3.10 ± 0.18 | 0.606 | 0.699 |
| Caudate-putamen | 7.26 ± 0.14 | 7.14 ± 0.09 | 6.65 ± 0.37 | 6.45 ± 0.13 | 6.47 ± 0.42 | 6.33 ± 0.49 | 0.208 | 0.012 |
| β1-AR | ||||||||
| Ant. cingulate cortex | 0.57 ± 0.03 | 0.57 ± 0.02 | 0.57 ± 0.02 | 0.53 ± 0.05 | 0.58 ± 0.02 | 0.62 ± 0.01 | 0.427 | 0.308 |
| SI | 0.87 ± 0.04 | 0.83 ± 0.05 | 0.83 ± 0.02 | 0.83 ± 0.05 | 0.77 ± 0.06 | 0.71 ± 0.06 | <0.001 | <0.001 |
| Ventral pallidum | 1.74 ± 0.15 | 1.72 ± 0.17 | 1.61 ± 0.14 | 1.57 ± 0.16 | 1.69 ± 0.15 | 1.47 ± 0.11 | 0.007 | 0.002 |
| β1& β2-AR | ||||||||
| Ant. cingulate cortex | 1.08 ± 0.05 | 1.14 ± 0.04 | 1.08 ± 0.05 | 1.02 ± 0.06 | 1.08 ± 0.04 | 1.05 ± 0.05 | 0.621 | 0.252 |
| SI | 0.99 ± 0.06 | 1.04 ± 0.06 | 0.96 ± 0.04 | 1.03 ± 0.15 | 0.90 ± 0.04 | 0.88 ± 0.05 | <0.001 | <0.001 |
| Ventral pallidum | 2.20 ± 0.33 | 2.30 ± 0.22 | 2.11 ± 0.24 | 1.98 ± 0.42 | 1.96 ± 0.30 | 2.01 ± 0.37 | 0.239 | 0.030 |
Numbers in bold type represent values statistically different from BL. HDB, horizontal limb of diagonal band; MCPO, magnocellular preoptic nuclei; CA, cornu amonis; SI, substantia innominata; ANOVA, analysis of variance.
A1R density
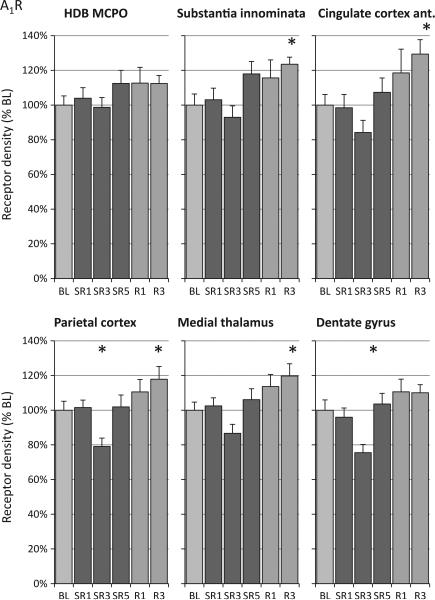
Among the brain areas analysed, A1R density was highest at the baseline condition in the hippocampal formation (CA region) and lowest in the BF region (Table 1). The CSR protocol produced a similar pattern of A1R density changes in all brain areas analysed characterized by a gradual elevation of A1R, which appeared highest on SR5, R1 and R3 at the end of the CSR protocol, and a decrease in A1R on SR3 (Fig. 3 and Table 1). Analysis of the average A1R density of all ROI analysed (weighted for the area of each ROI) revealed a significant effect of CSR (F(5,42) = 4.67, P = 0.002), with post-hoc tests indicating that only SR3 and R3 were significantly different from baseline (P = 0.022 and P = 0.047, respectively). Analysis of the individual brain regions revealed that CSR-induced changes in A1R density were significant in nine of the 13 regions evaluated (Table 1, anova column). Testing for a linear relationship of the time– course of the A1R density revealed a significant linear increase over time in all regions where the significant time effect was detected by the anova (Table 1, linear contrast column). The pattern of elevated A1R density was most noticeable on SR5 and the recovery days, with post-hoc tests revealing that R3 was significantly higher than baseline in six brain regions (Table 1, R3 column). The decline in A1R density on SR3 was seen in all brain regions and was significant with post-hoc tests compared to baseline in the following four regions: two hippocampal regions, somatosensory cortex and parietal cortex (Table 1, SR3 column). The density of A1R in caudate-putamen subregions (striosomes and matrix) showed a comparable time–course, with post-hoc tests showing that R3 was significantly different from baseline.
Figure 3.
Relative changes of adenosine A1 receptor (A1R) density during sleep restriction (SR1, SR3 and SR5) and recovery sleep days (R1 and R3) compared to baseline (BL). Chronic sleep restriction produced a pattern in all brains of increased A1R receptor density throughout SR and recovery days, except on SR3. A1R density was elevated significantly on R3 in six of the 13 brain regions examined. The six brain regions shown are representative of all A1R temporal patterns observed. HDB, horizontal limb of diagonal band; MCPO, magnocellular preoptic nuclei. See Table 1 for analysis of variance (anova) and linear contrast statistics; the asterisks (*) indicate statistical significance of post-hoc comparisons to BL (P < 0.05, n = 8).
A2aR density
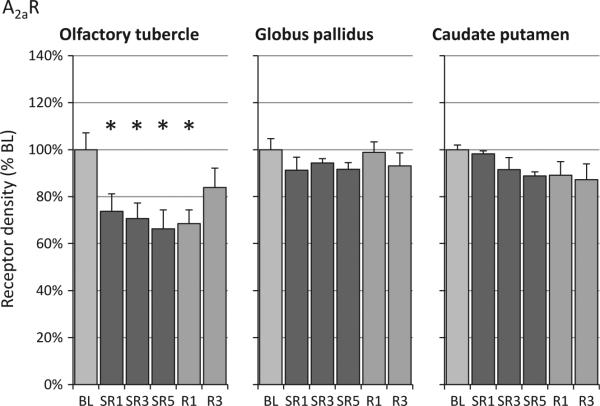
As shown in the literature (Demet and Chicz-Demet, 2002) and as shown in Fig. 2, the A2aR binding is so low in many brain regions that it cannot be measured reliably with autoradiography. Hence, only three brain regions with high A2aR levels were analysed (olfactory tubercle, globus pallidus and caudate-putamen). The A2aR density in the olfactory tubercle decreased significantly on all SR days (–25 to –34%) and remained reduced on the first recovery day (Fig. 4). A2aR levels in the olfactory tubercle on R3 were not significantly different from baseline or from any of the other experimental days. In addition, no significant linear trend was observed in olfactory tubercle. Although the anova result was not significant in caudate putamen (P = 0.2), a significant linear contrast (Table 1) was found, which was supported additionally by a significant linear regression (P = 0.009).
Figure 4.
Relative changes of adenosine A2a receptor (A2aR) density during sleep restriction (SR1, SR3 and SR5) and recovery sleep days (R1 and R3) compared to baseline (BL). A2aR receptor density in the olfactory tubercle decreased on SR days as well as on R1. The decline in A2aR density in the caudate-putamen was significant with the linear contrast analysis, but not with analysis of variance (anova) (see Table 1 for anova and linear contrast statistics). The asterisks (*) indicate statistical significance of post-hoc comparisons to BL (P < 0.05, n = 8 except for olfactory tubercle for BL and SR5 (n = 7).
β-AR density
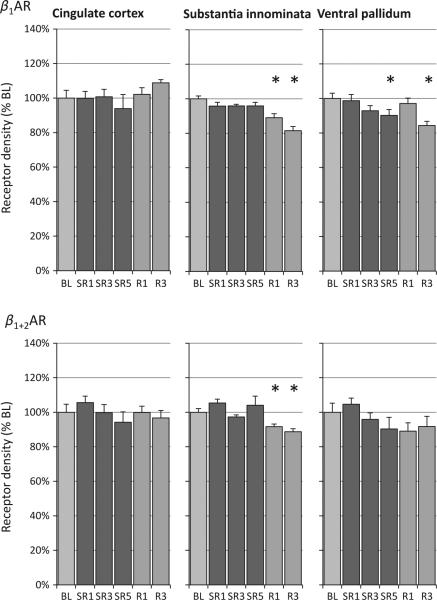
The ventral pallidum exhibited the highest β-AR density of the three regions analysed (Table 1). A significant change in β1-AR was detected over experimental days in the substantia innominata and ventral pallidum, but not in the anterior cingulate cortex (Table 1, anova column and Fig. 5, top panel). In the substantia innominata region, β-AR densities were reduced significantly on both recovery days (for β1, –11% on R1 and –18% on R3; for β1 and β2, –9% on R1 and –11% on R3). For the ventral pallidum, β1-AR density declined gradually over SR days, reaching statistical significance on SR5 (–10%), and remained at a lower level on R3 (–16%). In addition, significant linear decreases in β1-AR density were detected in the substantia innominata and ventral pallidum regions (linear contrasts, Table 1).
Figure 5.
Relative changes of β-adrenergic receptor (β-AR) density during sleep restriction (SR1, SR3 and SR5) and recovery sleep days (R1 and R3) compared to baseline (BL). Chronic sleep restriction decreased β1-AR density (top panel) in the substantia innominata and ventral pallidum; this reduction remained at R3. No changes were found in the anterior cingulate cortex. A similar pattern was seen with a ligand that binds to both β1-AR and β2-AR (bottom panel; see text for details). See Table 1 for analysis of variance (anova) and linear contrast statistics; the asterisks (*) indicate statistical significance of post-hoc comparisons to BL (P < 0.05, n = 8).
DISCUSSION
The CSR protocol used in this study produced the following changes in brain adenosine and noradrenaline receptors: (i) A1R density significantly increased in a linear fashion in 10 of the 13 brain areas analysed and remained significantly elevated on the third day of recovery sleep (in six regions). (ii) In contrast, A2aR density was reduced from SR1 to R1 in the olfactory tubercle, declined steadily in the caudate-putamen (significant with linear contrast but not anova), and not changed in the globus pallidus. (iii) A decrease in β-AR density was observed in substantia innominata and ventral pallidum which remained reduced on R3, but no changes were found in the anterior cingulate cortex. The most important finding is that CSR induced changes in the brain adenosine and noradrenaline receptors that often persisted through the 3-day period of recovery sleep.
The direction of CSR-induced changes in receptor density may depend upon the functional characteristics of the receptor
The net effect of physiological adenosine concentrations is considered inhibitory, but which action is dominant depends upon receptor distribution and the affinity of adenosine to different receptor subtypes (Dunwiddie and Masino, 2001). In the brain, adenosine is typically inhibitory at the ubiquitous A1R (Boison, 2012) and excitatory at the more regionally localized A2aR (Rebola et al., 2008; Van Dort et al., 2009). Noradrenaline acting at the β-AR increases the firing rate of cortical pyramidal neurones during wakefulness (Haas and Konnerth, 1983). In the present study, the most obvious pattern of changes we observed is that CSR induced increases in the density of inhibitory receptors (e.g. A1R) and decreases in excitatory receptors (e.g. A2aR and β-AR).
Although this is the first study to examine the effect of CSR on A1R density, several studies have looked at the effects of acute SD on A1R density. Specifically, in previous A1R autoradiography experiments no changes were detected in A1R density in most cortical, striatal and BF regions after 3, 6 and 12 h of acute SD (Basheer et al., 2001, 2007; Elmenhorst et al., 2009a). However, after 24 h of SD significant increases in the range of 7–15% were observed in these three regions (Basheer et al., 2007; Elmenhorst et al., 2009a). In the present study, the SR1 condition is essentially 18 h of acute SD. The A1R density changes in the SR1 condition were non-signifi-cant, and thus resemble the previous findings that used 12 h or less of acute SD.
The CSR A1R density findings herein also can be compared directly to our CSR study that measured A1R mRNA (Kim et al., 2012). The main difference observed is the sustained elevation of A1R density seen on the third day of recovery sleep, which was not seen in any brain areas in the CSR A1R mRNA study (Kim et al., 2012). A1R mRNA elevations are likely to temporally precede changes in receptor protein and also may return to baseline sooner. For example, BF A1R mRNA levels are increased consistently by 3, 6 or 12 h of acute SD, whereas 24 h of SD is needed to produce elevations of A1R density. In addition, mRNA changes do not always predict accurately changes in protein expression (reviewed in Rizzo et al., 2014).
Overall, the direction and magnitude of the receptor density changes described herein is largely consistent with the results of our previous studies of receptor mRNA and density using short-term SD (Basheer et al., 2001, 2007; Elmenhorst et al., 2007, 2009a) and with our recent CSR studies of receptor mRNA changes (Kim et al., 2012, 2013).
An exception to the pattern of CSR-induced elevation of A1R elevation was the decrease in A1R seen on the third day of SR, which was observed to some extent in all brain areas. The decline in A1R density on SR3 was not seen for A2aR or β-AR (Figs 4 and 5), or in our previous A1R mRNA studies (Kim et al., 2012, 2013). It is extremely unlikely that the dip in A1 receptors on SR3 is due to environmental factors, because the experimental design of the sample collection included multiple cohorts for each experimental condition and autoradiography was performed on all experimental conditions in a single run to prevent any systematic error. The neurobiological mechanisms of this transient down-regulation are not clear. Hence, assessing A1R density on SR days 2 and 4 might be an important first step to determine if a time lag between the events that determine receptor function (e.g. receptor production, affinity, internalization, etc.) plays a role, as A1R have a much slower half-life than do A2aR (Klaasse et al., 2008).
The changes of receptor densities found after CSR were in the range of 20–30%, which is comparable to the receptor changes induced by pharmacological blockade of receptors resulting in physiological consequences. For example, chronic treatment of rats with a high dose of the nonspecific adenosine receptor antagonist, caffeine (corresponding to seven and 50 cups of coffee in humans) led to a 17 and 28% increase in A1R density (Hawkins et al., 1988; Johansson et al., 1993). Similar magnitudes of change (40% reduction) were found in autoradiographic investigations of β-AR after chronic exposure to high doses (~10 times of human dosage) of a noradrenaline reuptake inhibitor (Goodnough and Baker, 1994).
More specifically, in the present study we observed a maximal receptor concentration increase of approximately 30% for the A1R. The physiological relevance of this increase in A1R can be compared to studies of receptor occupancy after caffeine consumption. To provoke an acute occupancy of 30% of the A1R in humans the consumption of two to three cups of caffeine is necessary (Elmenhorst et al., 2013). This amount of caffeine is consumed commonly as a countermeasure against the effects of SD. Thus, a change of receptor density in the range of 30% is likely to have significant functional consequences.
CSR-induced cognitive performance impairment and receptor density changes persist into the period of recovery sleep
In humans, CSR has been shown to produce cumulative and long-lasting increases in objective sleepiness and sustained impairment of cognitive performance (Belenky et al., 2003; Carskadon and Dement, 1981; Elmenhorst et al., 2009b; Kim et al., 2012; Van Dongen et al., 2003). The study by Belenky et al. (2003) found that psychomotor vigilance performance did not return to normal during a 3-day period of recovery sleep. Specifically, human subjects experienced 7 days of SR with 3, 5 or 7 h time in bed per night followed by 3 days of 8-h SO per day (Belenky et al., 2003). The subjects’ sustained attention performance in the psychomotor vigilance task did not return to the pre-CSR levels during the 3 days of recovery sleep period in the 3- and 5-h time-in-bed conditions. It is not known how many days of recovery sleep are needed for performance to return to baseline in man and, to date, animal studies have not replicated the persistence of cognitive impairments into the period of recovery sleep.
Recent human CSR studies using forced desynchrony protocols (Cohen et al., 2010; Zhou et al., 2011) also reported cumulative deterioration in cognitive performance, which is evident especially when performance is measured during periods of forced wakefulness occurring lateduring the circadian ‘night’(Cohen et al.,2010).Cohen et al.(2010)andourprevious report (Kim et al., 2012) hypothesized that homeostatic regulation of alertness and cognitive performance is composed of at least two distinct biological processes: one reacts to short-term SD and the other reacts to chronic sleep loss. The authors postulated further that acute SD induces an accumulation in extracellular adenosine in the BF and other brain areas, while repeated SD/restriction over multiple days changes adenosine receptor density, further increasing sleep drive. The present study supports the above hypothesis by showing that in some brain regions A1R density remains elevated and β-AR are still reduced on day 3 of recovery sleep. The findings herein are correlative and experiments demonstrating direct causal evidence that receptor changes cause the long-term cognitive impairments are needed. It would be most interesting to see if current mathematical models on CSR-induced changes in cognitive performance and EEG (McCauley et al., 2009; Rajdev et al., 2013) integrate these neuroreceptor data.
A recent mouse CSR study (Clasadonte et al., 2014) reported that CSR produced long-lasting effects on sleep homeostasis and adenosine tone. They found that 3 days of SR reduced SD-induced compensatory increases in NREM sleep time and slow wave activity that were still present after 2 weeks of recovery. Adenosine tone, assessed indirectly in an electrophysiological hippocampal slice preparation, was increased by the first 4 h of SD but was reduced thereafter, and remained reduced at the 2-week recovery time-point. A modest but non-significant increase in A1R sensitivity was also seen. Hence, the authors concluded that the reduction in adenosine tone was mediated by decreased extracellular adenosine levels rather than changes in receptor sensitivity/density. However, in our present investigation, the increase in A1R sensitivity observed is consistent with the modest and non-significant elevation of hippocampal A1R density observed on recovery days. Here we also report significant elevations of A1R in other brain regions on recovery day 3. The combined findings suggest that CSR may both decrease adenosine tone and increase A1R density and/or sensitivity. Additional studies are needed to determine the physiological importance of these CSR-induced elevations of A1R density.
Chronic sleep restriction-induced changes in A1R and β-AR receptors are slow to develop, being greatest in the last days of SR and the period of recovery sleep. Additional studies are needed to determine how many days of recovery sleep are needed for these neuroreceptors to return to baseline levels. It is also unknown how many days of recovery sleep are needed to normalize CSR-induced cognitive performance deficits (Belenky et al., 2003). A study combining CSR-induced cognitive performance impairments with in-vivo positron emission tomography imaging of receptor density would address these issues.
Additional work is needed to characterize fully the effects of CSR on these receptors and other receptors. This study analysed receptors in only a limited number of regions that were present in two anterior–posterior levels of the forebrain. Brain stem and other regions are of interest. For example, the significant decrease in A2aR in the olfactory tubercle observed suggests that additional A2aR brain regions should be analysed. Adenosine A2a and dopamine receptors influence each other by forming dimers (Fuxe et al., 2005), and their role in the regulation of sleep has been reviewed recently (Lazarus et al., 2013). It has been shown in humans that dopamine D2/D3 receptors are down-regulated in the ventral striatum (including nucleus accumbens) after acute SD (Volkow et al., 2012). Thus, it would be of interest to investigate changes in receptor density of both adenosine and dopamine systems in the nucleus accumbens.
Finally, given the pattern of receptor changes observed, it is unlikely that the non-specific effects of SD procedure such as stress or locomotor behaviour are responsible for the effects. For example, the greatest receptor changes are seen in the recovery sleep days for A1R and β-AR and not on the first couple of days of SR. In addition, we previously reported non-specific pattern of changes in AR mRNA levels in the exercise control group (Kim et al., 2013), which was not correlated with the total amount of locomotor activity.
In conclusion, the present findings reveal brain region-and receptor-specific differences in receptor protein density on the third day of recovery sleep that were not seen in our previous mRNA studies. Specifically, in response to CSR, the density of inhibitory receptors increases (e.g. A1R) and that of excitatory receptors decreases (e.g. A2aR and β-AR), which continued even during 3 recovery sleep days for the A1R and β-AR. The net effect of these CSR-induced receptor changes may reduce neuronal excitability as well as mediate the long-lasting neurocognitive impairments produced by CSR.
ACKNOWLEDGEMENTS
We thank Maxine Dibue, Sabine Wilms, Yunren Bolortuya and Patrick Schelenz for technical assistance and Dr Radhika Basheer for helpful discussions. This research was supported by the Department of Veterans Affairs Medical Research Service Award (to RES), HL060292 (to RES and RWM), MH039683 and HL095491 (to RWM), NHLBI–T32 HL07901 (to YK).
Footnotes
AUTHOR CONTRIBUTIONS
YK, DE and RES designed the study, analysed the data and wrote the manuscript. AB designed the study. YK, AW, FW and TK performed the experiment. RWM wrote the manuscript.
CONFLICT OF INTEREST
No conflicts of interest declared.
REFERENCES
- Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J. Clin. Sleep Med. 2007;3:519–528. [PMC free article] [PubMed] [Google Scholar]
- Basheer R, Halldner L, Alanko L, McCarley RW, Fredholm BB, Porkka-Heiskanen T. Opposite changes in adenosine A1 and A2A receptor mRNA in the rat following sleep deprivation. NeuroReport. 2001;12:1577–1580. doi: 10.1097/00001756-200106130-00013. [DOI] [PubMed] [Google Scholar]
- Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep–wake regulation. Prog. Neurobiol. 2004;73:379–396. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Basheer R, Bauer A, Elmenhorst D, Ramesh V, McCarley RW. Sleep deprivation upregulates A1 adenosine receptors in the rat basal forebrain. NeuroReport. 2007;18:1895–1899. doi: 10.1097/WNR.0b013e3282f262f6. [DOI] [PubMed] [Google Scholar]
- Belenky G, Wesensten NJ, Thorne DR, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose–response study. J. Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- Berridge CW. Noradrenergic modulation of arousal. Brain Res. Rev. 2008;58:1–17. doi: 10.1016/j.brainresrev.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D. Adenosine dysfunction in epilepsy. Glia. 2012;60:1234–1243. doi: 10.1002/glia.22285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol. Rev. 2012;92:1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981;18:107–113. doi: 10.1111/j.1469-8986.1981.tb02921.x. [DOI] [PubMed] [Google Scholar]
- Carter ME, Yizhar O, Chikahisa S, et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat. Neurosci. 2010;13:1526–1533. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clasadonte J, McIver SR, Schmitt LI, Halassa MM, Haydon PG. Chronic sleep restriction disrupts sleep homeostasis and behavioral sensitivity to alcohol by reducing the extracellular accumulation of adenosine. J. Neurosci. 2014;34:1879–1891. doi: 10.1523/JNEUROSCI.2870-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DA, Wang W, Wyatt JK, et al. Uncovering residual effects of chronic sleep loss on human performance. Sci. Transl. Med. 2010;2:14ra3. doi: 10.1126/scitranslmed.3000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demet EM, Chicz-Demet A. Localization of adenosine A2A-receptors in rat brain with [3H]ZM-241385. Naunyn Schmiedebergs Arch. Pharmacol. 2002;366:478–481. doi: 10.1007/s00210-002-0613-3. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu. Rev. Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Elmenhorst D, Meyer PT, Winz OH, et al. Sleep deprivation increases A1 adenosine receptor binding in the human brain: a positron emission tomography study. J. Neurosci. 2007;27:2410–2415. doi: 10.1523/JNEUROSCI.5066-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmenhorst D, Basheer R, McCarley RW, Bauer A. Sleep deprivation increases A(1) adenosine receptor density in the rat brain. Brain Res. 2009a;1258:53–58. doi: 10.1016/j.brainres.2008.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmenhorst D, Elmenhorst EM, Luks N, et al. Performance impairment during four days partial sleep deprivation compared with the acute effects of alcohol and hypoxia. Sleep Med. 2009b;10:189–197. doi: 10.1016/j.sleep.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Elmenhorst D, Meyer PT, Matusch A, Winz OH, Bauer A. Caffeine occupancy of human cerebral A1 adenosine receptors: in vivo quantification with 18F-CPFPX and PET. J. Nucl. Med. 2013;53:1723–1729. doi: 10.2967/jnumed.112.105114. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Ferre S, Canals M, et al. Adenosine A2A and dopamine D2 heteromeric receptor complexes and their function. J. Mol. Neurosci. 2005;26:209–220. doi: 10.1385/JMN:26:2-3:209. [DOI] [PubMed] [Google Scholar]
- Goodnough DB, Baker GB. 5-Hydroxytryptamine2 and beta-adrenergic receptor regulation in rat brain following chronic treatment with desipramine and fluoxetine alone and in combination. J. Neurochem. 1994;62:2262–2268. doi: 10.1046/j.1471-4159.1994.62062262.x. [DOI] [PubMed] [Google Scholar]
- Haas HL, Konnerth A. Histamine and noradrenaline decrease calcium-activated potassium conductance in hippocampal pyramidal cells. Nature. 1983;302:432–434. doi: 10.1038/302432a0. [DOI] [PubMed] [Google Scholar]
- Hawkins M, Dugich MM, Porter NM, Urbancic M, Radulovacki M. Effects of chronic administration of caffeine on adenosine A1 and A2 receptors in rat brain. Brain Res. Bull. 1988;21:479–482. doi: 10.1016/0361-9230(88)90162-1. [DOI] [PubMed] [Google Scholar]
- Johansson B, Ahlberg S, Van Der Ploeg I, et al. Effect of long term caffeine treatment on A1 and A2 adenosine receptor binding and on mRNA levels in rat brain. Naunyn Schmiedebergs Arch. Pharmacol. 1993;347:407–414. doi: 10.1007/BF00165391. [DOI] [PubMed] [Google Scholar]
- Jones BE. From waking to sleeping: neuronal and chemical substrates. Trends Pharmacol. Sci. 2005;26:578–586. doi: 10.1016/j.tips.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kim Y, Laposky AD, Bergmann BM, Turek FW. Repeated sleep restriction in rats leads to homeostatic and allostatic responses during recovery sleep. Proc. Natl Acad. Sci. USA. 2007;104:10697–10702. doi: 10.1073/pnas.0610351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Bolortuya Y, Chen L, Basheer R, McCarley RW, Strecker RE. Decoupling of sleepiness from sleep time and intensity during chronic sleep restriction: evidence for a role of the adenosine system. Sleep. 2012;35:861–869. doi: 10.5665/sleep.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Chen L, McCarley RW, Strecker RE. Sleep allostasis in chronic sleep restriction: the role of the norepinephrine system. Brain Res. 2013;1531:9–16. doi: 10.1016/j.brainres.2013.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaasse EC, Ijzerman AP, De Grip WJ, Beukers MW. Internalization and desensitization of adenosine receptors. Purinergic Signal. 2008;4:21–37. doi: 10.1007/s11302-007-9086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus M, Chen JF, Urade Y, Huang ZL. Role of the basal ganglia in the control of sleep and wakefulness. Curr. Opin. Neurobiol. 2013;23:780–785. doi: 10.1016/j.conb.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley P, Kalachev LV, Smith AD, Belenky G, Dinges DF, Van Dongen HP. A new mathematical model for the homeostatic effects of sleep loss on neurobehavioral performance. J. Theor. Biol. 2009;256:227–239. doi: 10.1016/j.jtbi.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th edn. Academic Press; San Diego, CA: 2007. [Google Scholar]
- Rajdev P, Thorsley D, Rajaraman S, et al. A unified mathematical model to quantify performance impairment for both chronic sleep restriction and total sleep deprivation. J. Theor. Biol. 2013;331:66–77. doi: 10.1016/j.jtbi.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Rebola N, Lujan R, Cunha RA, Mulle C. Adenosine A2A receptors are essential for long-term potentiation of NMDAEPSCs at hippocampal mossy fiber synapses. Neuron. 2008;57:121–134. doi: 10.1016/j.neuron.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Rizzo G, Veronese M, Heckemann RA, et al. The predictive power of brain mRNA mappings for in vivo protein density: a positron emission tomography correlation study. J. Cereb. Blood Flow Metab. 2014;34:827–835. doi: 10.1038/jcbfm.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose–response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- Van Dort CJ, Baghdoyan HA, Lydic R. Adenosine A(1) and A(2A) receptors in mouse prefrontal cortex modulate acetylcholine release and behavioral arousal. J. Neurosci. 2009;29:871–881. doi: 10.1523/JNEUROSCI.4111-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Tomasi D, Wang GJ, et al. Evidence that sleep deprivation downregulates dopamine D2R in ventral striatum in the human brain. J. Neurosci. 2012;32:6711–6717. doi: 10.1523/JNEUROSCI.0045-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Ferguson SA, Matthews RW, et al. Sleep, wake and phase dependent changes in neurobehavioral function under forced desynchrony. Sleep. 2011;34:931–941. doi: 10.5665/SLEEP.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]