Abstract
Objective
Sex plays an important role in the pathophysiology of cardiovascular diseases. This study aims to investigate how sex impacts on the coronary flow regulation during atherosclerosis.
Methods
ApoE KO mouse fed with western diet were used for atherosclerosis model. Coronary RH and flow response were measured using Langendorff-perfused isolated hearts.
Results
Coronary RH and A23187-induced NO-dependent flow increases were significantly reduced in female (by ~28% and 48%, respectively), but not in male atherosclerotic mice. However, SNP-induced coronary vasodilation remains intact in both sexes of ApoE KO mice. L-NAME (NOS inhibitor) reduced baseline flow and RH to a lesser extent in ApoE KO (by ~19% and 31%) vs. WT (~30% and 59%, respectively), and abolished the sex difference in RH. In contrast, SCH58261 (a selective A2A AR antagonist) reduced the baseline flow and RH to a greater extent in atherosclerotic mice, but did not affect the sex difference. Immunofluorescent staining of coronary arteries showed a similar A2A AR up-regulation in both sexes of ApoE KO mice.
Conclusions
Our results suggest that during atherosclerosis, female mice are more susceptible to NO-dependent endothelial dysfunction and the up-regulation of A2A AR may serve as a compensatory mechanism to counteract the compromised endothelial function.
Keywords: ApoE KO, atherosclerosis, nitric oxide, endothelial dysfunction, A2A adenosine receptor
INTRODUCTION
Evolving experimental and clinical data suggest that sex has an important impact on the pathophysiology of cardiovascular diseases [14, 19, 30]. While young women are believed to be protected against cardiovascular diseases due to the beneficial effect of estrogen, increasing clinical data over the past several decades reveal a higher mortality and cardiovascular event in female vs. male patients with ischemic heart disease [1, 21, 30]. The underlying mechanisms responsible for this observation remains unknown, although aging, variation in risk factors, sex hormones, as well as different pathophysiological changes in coronary vasculature in females vs. males have been proposed [22, 30].
ApoE KO mouse is considered the best available model for human atherosclerosis [15]. This animal model displays increased levels of total plasma cholesterol and extensive lipid deposition in the major arteries, which is accelerated and aggravated by feeding the animals with western diet, suggesting a similarity with human disease [15, 33]. Using this model, while majority of studies have focused on mechanisms responsible for plaque formation in big conductance arteries, less attention has been paid to the impact of hypercholesterolemia/atherosclerosis on coronary vascular function, especially at the level of microvasculature, which is of great physiological significance in cardiac perfusion. Clinical and experimental data showed that cardiac ischemia still occurs despite the absence of atherosclerotic plaque formation [12, 22, 30], suggesting an essential role of alterations in coronary microvasculature in the pathophysiology of cardiac ischemia-associated diseases.
Endothelial dysfunction, characterized by an imbalance between vascular relaxing and contracting factors, pro- and anti-coagulants, and between pro- and anti-inflammatory mediators particularly in the coronary vasculature, is believed to play important roles in the pathophysiology of coronary heart diseases [4, 8, 23]. A decreased NO bioavailability was observed in CAD patients and was correlated with a worse prognosis [4]. Recently, adenosine, one the of the important players in coronary vascular tone regulation [18], have been indicated to be an important compensatory mechanism for cardiac metabolic vasodilation when NO is reduced in human subjects with risk factors of atherosclerosis [17]. In line with the clinical finding, our group has demonstrated an enhanced A2A AR-mediated coronary vasodilation in ApoE-deficient mouse model of atherosclerosis [32], suggesting a potential compensatory mechanism of adenosine in opposing the decreased NO-mediated coronary vasodilation under disease conditions.
Coronary reactive hyperemia (RH) has been suggested to hasten the metabolic and functional recovery of the heart after ischemia, thus preventing ischemia-induced heart injury. Impaired coronary reactive vasodilation has been reported in patients with coronary artery diseases [27, 31] and improvement of coronary RH after angioplasty during acute myocardial infarction has been shown to help the recovery of regional myocardial function [31]. We previously demonstrated that A2A adenosine receptors (AR) and NO play important roles in coronary RH in WT mouse [28, 36]. However, little is known regarding the impact of sex and hypercholesterolemia/atherosclerosis on NO and adenosine-mediated signaling in RH. Therefore, this study aims to explore the impact of sex on hypercholesterolemia/atherosclerosis-associated coronary endothelial dysfunction using isolated Langendorff-perfused mouse hearts. We demonstrated that female ApoE KO mice have a more severely impaired NO-dependent coronary vasodilation compared with males. Additionally, a similar level of A2A AR up-regulation was observed between male and female ApoE KO mice, suggesting a potential compensatory role in regulating coronary vascular tone when NO-dependent mechanism is compromised during atherosclerosis.
MATERIALS AND METHODS
Animals
Institutional Animal Care and Use Committee at West Virginia University School of Medicine approved all experimental protocols. We followed guidelines set forth by the American Physiological Society and National Institutes of Health regarding the care and use of laboratory animals. ApoE KO mice with C57 BL/6 background were purchased from JAX laboratory and were bred in our animal facility. At 8 weeks of age, animals were fed with a high-fat western-type diet (0.2% cholesterol, 21.2% fat, Harlan Teklad, TD88137) for 12 weeks to accelerate atherosclerotic lesion formation. Mice were housed in a 12:12-hr light-dark cycle with free access to standard chow and water.
Langendorff-perfused mouse heart preparation
C57 (WT) and ApoE KO mice with HFD (ApoE-HFD, 20–22 wk) of both sexes were anesthetized with sodium pentobarbital (50 mg/kg of body weight, i.p.) and hearts were excised into heparinized (5 U/ml) ice-cold Krebs-Henseleit buffer containing (mM) 119 NaCl, 11 glucose, 22 NaHCO3, 4.7 KCl, 1.2 KH2PO4, 1.2 MgSO4, 2.5 CaCl2, 2 pyruvate, and 0.5 EDTA. After removal of the lung and surrounding tissue, the aorta was rapidly cannulated with a 20-gauge, blunt-ended needle and continuously perfused with 37°C buffer bubbled with 95% O2 and 5% CO2 at a constant perfusion pressure of 80 mmHg. The left atrium was removed and a fluid-filled balloon made of plastic wrap was inserted into the left ventricle across the mitral valve. The balloon was connected to a pressure transducer for a continuous measurement of left ventricular developed pressure (LVDP). The heart was then immersed in a water-jacketed perfusate bath maintained at 37°C and beat spontaneously. Left ventricular diastolic pressure was adjusted to 2–5 mmHg. Coronary flow was continuously measured with an ultrasonic flow probe (Transonic Systems; Ithaca, NY) placed in the aortic perfusion line. A Power Lab Chart data acquisition system (AD Instruments; Colorado Springs, CO) was used for data acquisition.
Experimental protocols
Coronary flow concentration response curve (CRC) and reactive hyperemia experiments were conducted in Langendorff-perfused hearts. In order to eliminate the effect of heart rate on coronary flow response, each heart was paced at a constant rate (400 beats.min−1) by two Teflon-coated silver wires (AD Instrument, Colorado Spring, CO) connected to the stainless steel cannula and through the apical myocardium using a Grass 48 Stimulator (Grass Medical Instrument, USA). The pulse duration and power was set at 6 millisecond and 4 volts, respectively with a repeated pulse mode. Hearts were allowed to stabilize for 30 min before baseline flow was measured. Hearts with persistent arrhythmias or a LVDP less than 80 mmHg were excluded from the study. The CRC was performed by infusing increasing concentrations of A23187 (Sigma-Aldrich, 10−9–10−5.5 M at a rate of 1% of coronary flow) into the coronary perfusate using a microinjection pump (Harvard Apparatus, Holliston, MA) through an infusion line inserted proximal to the aorta cannula. The working solution of A23187 was freshly prepared by diluting DMSO pre-dissolved stock solution in H2O. The stock solution was used within two days. Each dose was infused for a period that allowed for a maximum response and was followed by a washout step till the flow fell to baseline level before another dosing. The effect of L-NAME (nitric oxide synthase inhibitor, Sigma, 100 μM) on A23187-induced coronary flow response was examined using a concentration that was close to the EC50 (10−6.5 M) of A23187 (Fig. 2A). In these experiments, L-NAME was pre-infused for 15 min and was continuously present during the infusion of A23187.
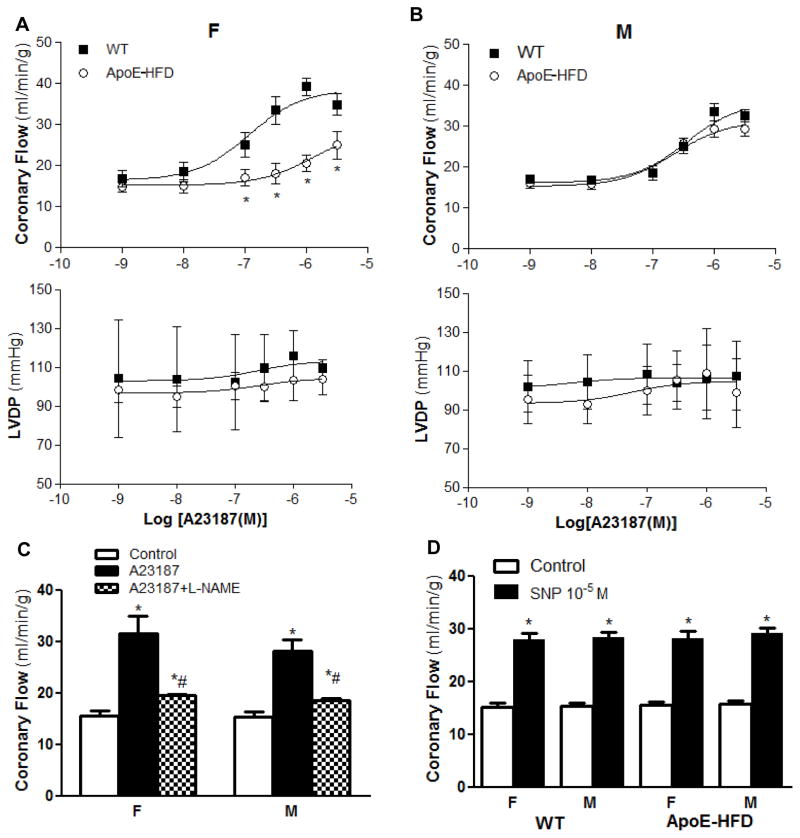
Figure 2.
Sex differences in A23187-induced coronary flow responses in ApoE-HFD mice. Concentration response curve for A23187 (10−9~10−5.5 M)-induced changes in coronary flow (upper panel) and LVDP (middle panel) in female (A) and male (B) WT vs. ApoE-HFD mice (N=5 per group, *p<0.05 vs. WT). C) Summarized effect of L-NAME (100 μM) on A23187 (10−6.5 M)-induced coronary flow response in WT female and male mice (N=3 in each group, *p<0.05 vs. control, #p<0.05 vs. A23187 in each group). D) Summarized coronary flow response after infusion of sodium nitroprusside (SNP, 10−5 M) in female and male WT (F, N=8, M, N=10) vs. ApoE-HFD mice (F, N=7, M, N=8).
Reactive hyperemia response (RH) of isolated hearts was examined in WT, ApoE-HFD mice using the approach described previously [28, 36]. A transient increase in flow was observed after a 15-second total inflow occlusion. Then, L-NAME or SCH58261 (a selective A2A AR antagonist, Tocris Bioscience) was delivered into the aortic perfusion line to achieve a final concentration of 100 and 1 μM, respectively for 15 min, followed by eliciting a second hyperemia response. The response of RH was compared before and after drug treatment. Control experiments were conducted as previously described and showed similar response in the two RH [36].
Fluorescent immunohistochemical staining of A2A AR on isolated mouse coronary arteries
Left and right coronary arteries (main and the first branch, 70–150 μm) of each animal were isolated from mouse hearts of WT and ApoE-HFD and were fixed with 2% ice-cold paraformaldehyde for 30 min, followed by 10 min of permeabilization with 0.1% Triton X-100. The vessels were then blocked with 5% goat serum for 1 hour before overnight incubation at 4 °C with an anti-A2A antibody (1:300, mouse monoclonal, Upstate). After washing with PBS for 1 h, each vessel was incubated with PBS buffer containing Alexa-533 conjugated goat anti-mouse secondary antibodies as well as Draq5 (1 μM, for nuclei staining, Invitrogen,) for 2 h. The vessels were then mounted on slides for imaging after 1 h washing with PBS. Zeiss LSM 510 confocal microscope was used to collect images from two randomly selected segments from the main and first branch. Each stack of images was acquired by optical sectioning at successive X-Y focal planes with a vertical depth of 1 μm using Zeiss objective (EC Plan-Neofluar, 40x/1.3/oil DIC) and 1024×1024 scanning format. The mean FI of each stack of ROIs that cover the area of individual endothelial cells (determined by the shape of nucleus that longitudinally orientated along the vessel axis) and smooth muscle cells (cells with nucleus perpendicularly orientated to the vessel axis, Fig. 4) was quantified using ImageJ [24]. After subtraction of background signal, a mean of the FI averaged from four ROIs of each vessel segment was calculated and was normalized by the mean FI of WT animal in both sexes.
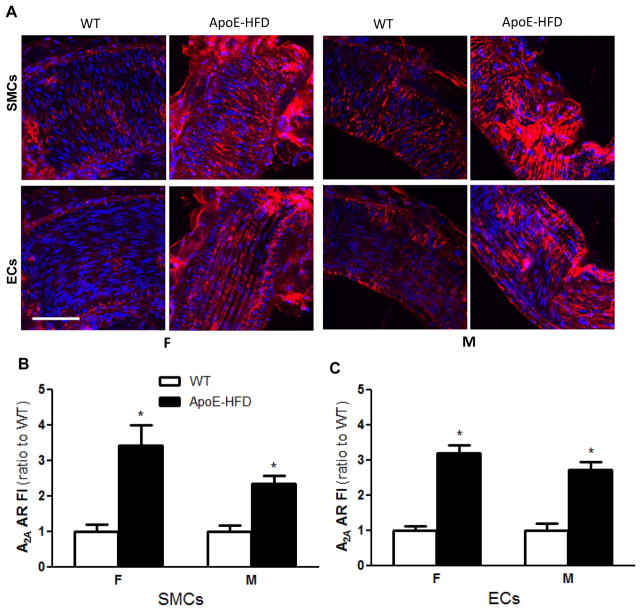
Figure 4.
A2A AR expression in isolated coronary arteries of WT and ApoE-HFD female and male mice. A) Representative confocal immunofluorescence images showing the expression of A2A AR (red) on isolated coronary arteries from WT and ApoE-HFD in female (first two columns) and male (3rd and 4th columns) mice (N=4 animals, 16 vessel segments per group). Confocal images at focal planes with more smooth muscle cells (SMCs, with nuclei orientated perpendicularly to the vessel axis, 1st row) or endothelial cells (ECs, with nuclei orientated longitudinally to the vessel axis, 2nd row) were illustrated at the upper and lower panel, respectively. Summarized quantitative data of A2A AR expression on SMCs (B) and ECs (C) of isolated coronary arteries from female and male WT vs. ApoE-HFD mice, *p<0.05 vs. WT. Scale bar represents 50 μm.
Data analysis and statistics
Flow debt (equal to baseline flow rate × occlusion time) and repayment volume (equal to the integral of hyperemic area above the baseline flow) were calculated using “the integral relative to baseline” function in the data pad of LabChart 7.0 software. Since absolute coronary flow rates change proportionally with heart mass and metabolic rate, the repayment volume and flow debt are represented as ml per gram of wet heart weight (ml/g), and baseline and peak flow rate data are presented as ml per min per gram of wet heart weight (ml/min/g). Values are mean ± SE. Each “N” represents the number of animals unless otherwise indicated. Paired t-test was used for paired data analysis. Two-way ANOVA with Bonferroni post hoc test was used to compare the dose response curve between groups. A probability value of p < 0.05 was considered as statistically significant.
RESULTS
Baseline function of isolated hearts from WT and ApoE KO mice with HFD (ApoE-HFD)
Baseline functional parameters of isolated mouse hearts were recorded in a total of 123 animals (WT, M=34, F=32, ApoE-HFD, M=29, F=28). Body weight, heart weight, and heart/body weight ratio were significantly higher in male vs. female (p<0.05, Table. 1) in both WT and ApoE-HFD animals. However, no significant difference was observed in the baseline coronary flow, LVDP, and dP/dt between sexes. ApoE-HFD mice gained more body and heart weight without alteration of the heart/body weight ratio in both female and male (Table. 1). Cholesterol and triglyceride levels were significantly higher (925 ± 82.2 and 166 ± 27.3 vs. 116 ± 14.0 and 83 ± 34.1 in females, and 1209 ± 309.7 and 174 ± 44.4 vs. 128 ± 10.5 and 69 ± 18.4 mg/dl in males, p<0.05) in ApoE-HFD vs. WT mice without significant difference between sexes (N=8 per group, p>0.05). There were no significant difference in the baseline coronary flow, LVDP, and dP/dt between ApoE-HFD and WT animals (p>0.05, Table. 1).
Table 1.
Baseline functional data of isolated hearts from WT and ApoE KO mice fed with western high fat diet (ApoE-HFD).
| WT
|
ApoE-HFD
|
|||
|---|---|---|---|---|
| M | F | M | F | |
| No. of animals | 34 | 32 | 29 | 28 |
| Age, weeks | 22 ± 0.3 | 22 ± 0.2 | 22 ± 0.4 | 22 ± 0.1 |
| Body weight, g | 27 ± 0.3 | 23 ± 0.3* | 31 ± 0.3* | 26 ± 0.2#& |
| Heart weight, mg | 128 ± 2.3 | 97 ± 2.6* | 150 ± 5.4* | 112 ± 3.4*#& |
| Heart-to-body weight ratio, % | 0.47 ± 0.01 | 0.42 ± 0.01* | 0.48 ± 0.02 | 0.43 ± 0.01*# |
| Coronary flow, ml/min/g | 15.8 ± 0.40 | 15.0 ± 0.52 | 16.0 ± 0.43 | 16.2 ± 0.55 |
| LVDP, mmHg | 106 ± 4.2 | 105 ± 3.0 | 110 ± 4.5 | 101 ± 3.6 |
| Positive dP/dt, mmHg/s | 4875 ± 368 | 4132 ± 446 | 4901 ± 521 | 4151 ± 507 |
| Negative dP/dt, mmHg/s | 4553 ± 342 | 3803 ± 532 | 4066 ± 321 | 3945 ± 226 |
p<0.05 vs. WT male,
p<0.05 vs. ApoE-HFD male,
p<0.05 vs. WT female
Reduced coronary reactive hyperemia in female, but not in male ApoE-HFD mice
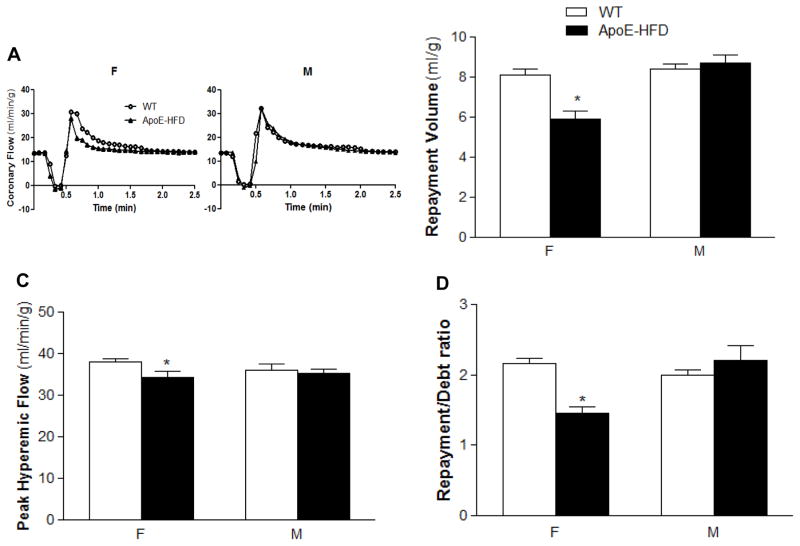
Sex difference in coronary RH was examined in WT and ApoE-HFD mice (N=8 per group). As described above, baseline flow was comparable between WT and ApoE-HFD mice without sex difference. However, repayment volume, peak hyperemic flow rate, as well as repayment/debt ratio were significantly reduced in female from 8.1 ± 0.31, 38.1 ± 0.74, and 2.2 ± 0.08 to 5.9 ± 0.42 ml/g, 34.4 ± 1.49 ml/min/g, and 1.5 ± 0.09, respectively (p<0.05, Fig. 1B,C&D). In contrast, there was no significant reduction in RH in male ApoE-HFD compared with WT mice (Fig. 1).
Figure 1.
Sex differences in coronary RH in ApoE KO mice fed with high fat diet (ApoE-HFD). A) Representative tracings of coronary flow changes over time after a 15-sec no flow occlusion in isolated hearts of WT vs. ApoE-HFD mice of both sexes. Summarized sex difference in repayment volume (B), peak hyperemic flow rate (C), and repayment/debt ratio (D) in WT and ApoE-HFD mice (*p<0.05 vs. female WT).
Female instead of male ApoE-HFD mice showed a decreased A23187-induced NO-dependent coronary vasodilation
NO is an important mediator responsible for coronary vasodilation. To determine whether the sex difference in the reduction of coronary vasodilation in ApoE-HFD was associated with NO-dependent mechanism, A23187-induced coronary flow response was compared between male and female in WT vs. ApoE-HFD mice. In WT mice, A23187 induced a similar dose-dependent increase in coronary blood flow between female and male (EC50= 3.0 ±1.2 vs. 2.3 ± 0.8×10−7 M, Emax=33.4 ± 2.2 vs. 39.2 ± 2.2 ml/min/g in male and female, respectively, N=5 per group, p>0.05). However, a right shift of the concentration response curve (CRC) of A23187 from WT was observed in female but not in male ApoE-HFD mice (Fig. 2A&B). Emax significantly decreased from 39.2 ± 2.2 to 25 ± 3.4 ml/min/g, and EC50 increased significantly from 2.3 ± 0.8×10−7 to 1.3 ± 0.5 ×10−6 M (N=5 per group, p<0.05) in female ApoE-HFD mice. A23187 induced a moderate, but not significant increase in LVDP in both WT and ApoE-HFD (p>0.05, Fig. 2A&B) animals. No significant difference in LVDP response was observed between sexes as well as between WT and ApoE-HFD animals (Fig. 2A&B).
NO-dependency of A23187-induced coronary flow response was confirmed in six WT animals (equal sex) using L-NAME (100 μM). L-NAME decreased the baseline flow from 15.6 ± 0.54 to 12.3 ± 0.83 ml/min/g and significantly attenuated the A23187 (10−6.5 M)-induced flow increase to a similar extent in female and male (Emax decreased from 28.2 ± 2.2 to 18.5± 0.43 ml/min/g in male, and from 31.5 ± 3.4 to 19.6 ± 0.24 in female, Fig. 2C).
To exclude the possibility that the sex-associated difference in coronary vasodilation during atherosclerosis is non-endothelial-dependent, nitroprusside (SNP, 10−5 M)-induced coronary flow response was compared between male and female WT (F, N=8, M, N=10) vs. ApoE-HFD (F, N=7, M, N=8) mice. SNP increased the coronary flow to a similar extent in WT male (by 184.5 ± 7.43%) and female (by 184.7 ± 9.87%) vs. ApoE-HFD male (by 185.2 ± 11.8%) and female (by 182.9 ± 12.1%) mice (p>0.05, Fig. 2D).
Less NO-dependent, but more A2A AR-dependent mechanism in coronary flow regulation in ApoE-HFD vs. WT mice
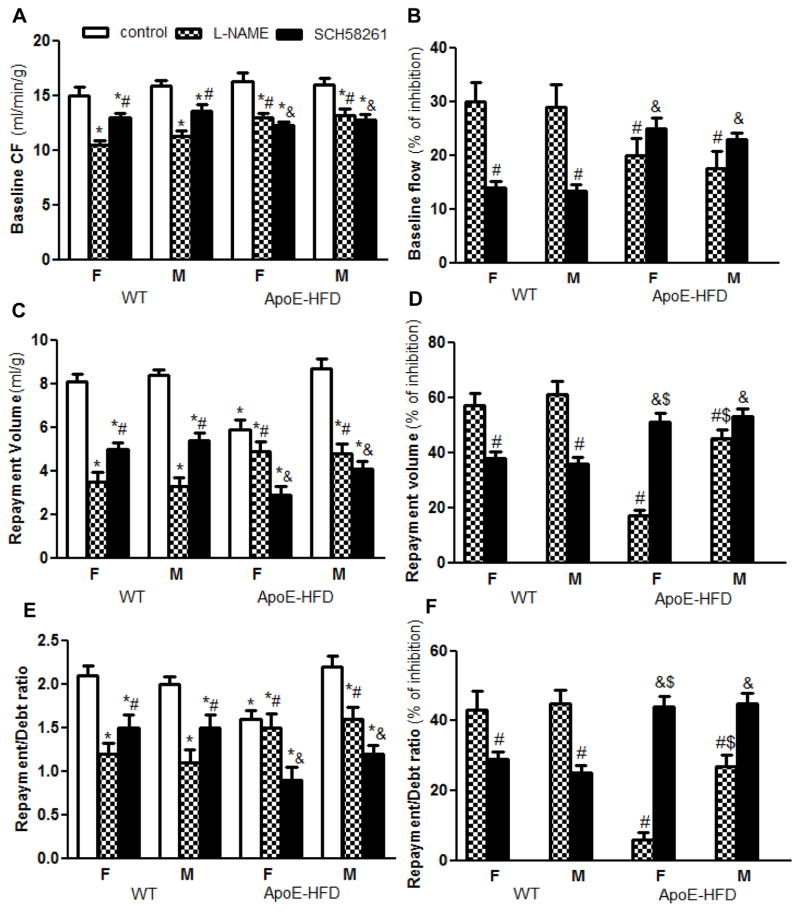
NO and A2A AR play essential roles in coronary RH in isolated mouse hearts [35,36]. To determine whether the sex difference in coronary RH in ApoE-HFD was associated with alterations in NO and/or A2A AR-dependent mechanisms, coronary flow under resting condition and during RH was compared in female and male WT vs. ApoE-HFD mice with or without L-NAME and SCH58261 (a selective A2A AR antagonist). Under resting condition, L-NAME decreased the flow by a similar extent in females vs. males in both WT (baseline flow reduced from 15.9 ± 0.51 to 11.3 ± 0.44 ml/min/g, by ~29% in males, and from 15.0 ± 0.72 to 10.5 ± 0.41 ml/min/g, by ~30% in females) and ApoE-HFD mice (baseline flow reduced from 16.0 ± 0.54 to 13.2 ± 0.52 ml/min/g, by ~18% in males, and from 16.3 ± 0.75 to 13 ± 0.40 ml/min/g, by ~20% in females, N=4 per group, Fig. 3A&B). However, the extent of reduction by L-NAME was significantly less in ApoE-HFD than that in WT mice (by ~20 and 18% vs. ~30 and 29% reduction in females and males, respectively, p<0.05, Fig. 3A&B). In contrast, SCH58261 reduced the baseline flow to a greater extent in ApoE-HFD than in WT mice without sex difference (by~25 and 23% vs. ~14 and 13% in females and males, respectively, N=4 per group, p<0.05, Fig. 3A&B).
Figure 3.
Sex differences in NO and A2A AR-dependent coronary RH in ApoE-HFD mice. A) Summarized effect of L-NAME (NOS inhibitor, 100 μM) and SCH58261 (selective A2A AR antagonist, 1 μM) on baseline coronary flow (A) and % of inhibition (B) of isolated mouse hearts from female and male WT vs. ApoE-HFD mice (N=4 per group). C–F) Summarized effect of L-NAME and SCH58261 on coronary repayment volume (C, the absolute coronary flow, D, % of inhibition), and on repayment/debt ratio (E, the absolute coronary flow; F, % of inhibition) in WT vs. ApoE-HFD mice. *, #, and &p<0.05 vs. control, L-NAME, and SCH58261 treatment in female WT mice, respectively; $p<0.05 vs. L-NAME treatment in female ApoE-HFD mice.
During RH, L-NAME reduced the repayment volume and repayment/debt ratio by a similar extent in female and male WT mice. Repayment volume and repayment/debt ratio was reduced from 8.1 ± 0.31 ml/g and 2.1 ± 0.11, to 3.5 ± 0.42 ml/g (by ~57%) and 1.2 ± 0.12 (by ~43%) in females, and from 8.4 ± 0.25 ml/g and 2.0 ± 0.08, to 3.3 ± 0.36 ml/g (by ~61%) and 1.1 ± 0.15 (by ~45%) in males, respectively (no significant difference in the percentage of inhibition by L-NAME between males and females, p>0.05, Fig. 3C&D). However, in ApoE-HFD mice, L-NAME reduced the RH to a lesser extent in females compared with males and showed less inhibition of RH in both sexes of ApoE-HFD compared with WT animals. L-NAME reduced repayment volume and repayment/debt ratio by ~17 and 6% in female vs. by ~45 and 27% in male ApoE-HFD mice (p<0.05, Fig. 3C, D, E, and F). In contrast, SCH58261 inhibited the RH to a greater extent in ApoE-HFD versus WT mice, but showed no sex difference. Repayment volume was reduced by ~51% vs. ~38% in female, and by ~53% vs. 36% in male, in ApoE-HFD compared with WT animals (p<0.05, Fig. 3C&D). Repayment/debt ratio was decreased by ~44% vs. ~29% in female, and by ~45% vs. 25% in male in ApoE-HFD compared with WT mice (p<0.05, Fig. 2E&F).
Increased A2A AR expression in both male and female ApoE-HFD mice
To examine whether the increased A2AAR-dependency in coronary RH was associated with A2A AR up-regulation, A2A AR expression was compared between WT and ApoE-HFD in both sexes (N=4 animals per group with 2 vessels per animal and 2 segments per vessel). There was no significant difference in A2A AR expression between left and right coronary artery, and between the main (mean diameter=148 ± 10.3 μm) and first branches (92 ± 9.5 μm). A significantly enhanced A2A AR fluorescence staining in both endothelial cells (ECs) and smooth muscle cells (SMCs) of isolated coronary arteries was observed in ApoE-HFD mice (3.2 ± 0.22 and 2.7 ±0.22 fold increase in ECs, and 3.4 ± 0.57 and 2.3 ± 0.22 fold increase in SMCs, in females and males, respectively, Fig. 4, p<0.05). No sex difference in A2A AR up-regulation was observed (p>0.05). The main and first branch of coronary artery showed similar extent of A2A AR up-regulation in ApoE-HFD animals in both sexes (3.1 ± 0.32 vs. 3.1 ± 0.23 fold increase and 2.7 ± 0.6 vs. 3.2 ± 1.13 fold increase in female, and 2.5 ± 0.66 vs. 2.8 ± 0.18 and 2.3 ± 0.11 vs. 2.3 ± 0.34 fold increase in male, in ECs and SMCs of the main compared with the first branch, respectively, p>0.05).
DISCUSSION
In the current study, using Langendorf-perfused hearts, we demonstrated a significant sex difference in atherosclerosis-related endothelial dysfunction in coronary circulation of ApoE KO mice. In contrast to the concept that females tend to be protected against cardiovascular diseases due to the protective role of estrogen [3, 7, 9, 11, 25], our findings indicate that females appear to be more susceptible to NO-dependent coronary endothelial dysfunction, in agreement with the increasingly accumulated clinical evidence showing a higher mortality rate and cardiovascular events in women patients with ischemic heart diseases [1, 21, 30]. Interestingly, a comparable up-regulation of A2A AR in both male and female animals was observed in the coronary vasculature, and pharmacological blockade of A2A AR by SCH58261 unmasked the decreased coronary ischemic vasodilation caused by impairment of NO-dependent mechanism in male and aggravated endothelial dysfunction in female ApoE KO-HFD mice, suggesting a compensatory role of A2A AR-mediated signaling in counteracting the decreased NO-dependent coronary reactive hyperemia during atherosclerosis. Our data suggest that the more impaired NO-mediated signaling, rather than a less compensatory role of A2A AR in coronary vasculature is responsible for the reduced coronary RH in ApoE KO mouse model of atherosclerosis.
ApoE KO mouse has been considered the best available model for atherosclerosis due to its similarity in lesion formation to humans.[15] In the current study, ApoE KO mouse fed with western diet for 12 weeks showed a ~9 and 2 folds increase in total cholesterol and triglycerides, respectively, concomitant with accelerated lesion formation compared with WT mice [33]. There was no significant difference in the cholesterol and triglyceride levels between female and male ApoE-HFD mice, suggesting that the sex difference in endothelial dysfunction in ApoE-HFD mice is not a result of the different lipid profile between sexes. However, one previous study reported that while no sex difference in HDL level was observed on C57BL/6 fed with normal chow, female showed a 50% decrease in HDL compared with male when fed with atherogenic diet (1.25% cholesterol, 15% fat, and 0.5% cholic acid) and was correlated with more total lesion area, [20] suggesting that sex may affect the HDL level, thus leading to the sex difference in atherosclerosis. In our previous study, we failed to observe any sex difference in lesion size and surface area in the aortic root, aortic arch, and innominate arteries in ApoE-HFD mice [33], suggesting that the sex difference in coronary endothelial dysfunction observed in the current study appears not to be a result of lipid profile-mediated difference in lesion formation.
Numerous evidences indicate that endothelial dysfunction plays an important role in the pathophysiology of atherosclerosis.[16, 23] While majority of studies regarding the effect of hyperlipidemia/atherosclerosis on vascular reactivity were conducted on conductance vessels, limited information is available in coronary resistance vessels. In the current study, using whole heart preparation to study combined vascular function in both conduit arteries and resistance microvessels, we demonstrated that female ApoE-HFD mice are more prone to NO-dependent endothelial dysfunction compared with male. This is based on the observations that: 1) reduction in coronary RH (Fig. 1) and A23187-induced increase in coronary flow occurred only in female, but not in male ApoE-HFD mice; and 2) SNP (NO donor)-induced endothelial-independent coronary vasodilation was unaffected in ApoE-HFD animals. In line with our results, previous studies on mesenteric and cerebral resistance vessels from ApoE KO mice fed on either normal or western diet reported that while female had an impaired Ach-induced relaxation despite the absence of atherosclerotic lesions, [5, 12] male had a preserved endothelial function. These experimental data support the accumulating clinical evidence that women with lower burden of obstructive coronary artery disease and preserved systolic function, tend to have a greater rate of myocardial ischemia and near-term mortality rate compared with men after adjustment with age and other risk factors [30]. Therefore, it appears that endothelial dysfunction in the coronary microcirculation, rather than in big conductance arteries may play a more critical role in the pathophysiology of cardiac ischemia disease in women.
Although the underlying mechanisms responsible for the sex difference in coronary endothelial dysfunction are not clear, our data suggest that a more severely impaired NO-dependent signaling in females vs. males is involved. This concept is supported by our data that NO-dependent coronary flow increase by A23187 was diminished in female ApoE-HFD mice (Fig. 2A&B) and L-NAME (NOS inhibitor) abolished the sex difference in coronary RH (Fig. 3C, D, E&F). Notably, although L-NAME reduced the RH to a lesser extent in female vs. male, no sex difference in the effect of L-NAME on the baseline coronary flow was observed (baseline flow was reduced by ~18% vs. ~20% between male and female in ApoE-HFD animals, Fig. 3A&B), suggesting that an impaired eNOS signaling during endothelial stimulation rather than a decrease in tonic eNOS activity is responsible for the sex difference in NO-dependent endothelial dysfunction. However, how sex affects the eNOS signaling, e.g., effect of estrogen on eNOS expression, endothelial Ca2+ signaling, eNOS activity, and/or NO bioavailability [6, 16] in coronary vasculature under hypercholesterolemia/atherosclerosis condition, remains to be further explored. A recent study on young women diabetic patients reported that endogenous estrogen contributed to the reduced flow-mediated vasodilation through an increased ROS production in brachial arteries [34], indicating that in contrast to the protective role of estrogen against cardiovascular diseases under physiological conditions, estrogen may exert detrimental effects in the cardiovascular system under disease conditions where a constant state of oxidative stress exists. Therefore, further studies to examine the sex difference in ROS production in coronary vasculature under resting and during RH in ApoE-HFD animals will provide better understanding of the underlying mechanisms.
Adenosine has been demonstrated to play important roles in coronary flow regulation, particularly under ischemic conditions [2, 10, 13, 28]. Clinical study on CAD patients suggested that increased adenosine signaling may be a compensatory mechanism to maintain normal cardiac perfusion when NO-dependent vasodilation is impaired [17]. In support of this concept, the current study showed that atherosclerotic mouse hearts with decreased NO-dependent vasodilation had an up-regulated A2A AR in both endothelium and smooth muscle cells of the coronary vessels (Fig. 4). Functionally, CGS21680 (a selective A2A AR agonist) induced an enhanced coronary flow increase [32], and SCH58261 (a selective A2A AR antagonist) reduced coronary baseline flow and repayment volume to a greater extent in ApoE-HFD vs. WT mouse (Fig. 3). In consistence with these findings, our unpublished in vivo data using pulse-wave Doppler ultrasound study showed an enhanced CGS21680-induced coronary flow increase in ApoE KO (coronary reserve=3.4 ± 0.34, N=8) vs. WT (coronary reserve=2.6 ± 0.31, N=8 with equal number of each sex) mice without sex difference. In addition, blockade of A2A AR unmasked the reduced coronary RH due to the impaired NO-dependent vasodilation in male ApoE-HFD mice and further attenuated the already compromised RH in female ApoE-HFD animals (Fig 3C, D, E, &F). Collectively, our data suggest that the enhanced A2A AR-mediated flow regulation appears to be an important compensatory mechanism to oppose the impaired NO-dependent coronary vasodilation, thus maintaining a normal cardiac perfusion during RH. In regards to the effect of sex on the A2A AR-mediated compensatory role in coronary flow regulation, the comparably up-regulated A2A AR (Fig. 4) and similar extent of reduction in RH by SCH58261 (Fig. 3C, D, E, &F) in females vs. males suggest the absence of sex difference in the functional up-regulation of A2A AR in ApoE-HFD mice. Therefore, the observed sex difference in the coronary endothelial dysfunction in atherosclerotic mice seems to be contributed by a more severely impaired NO-mediated signaling, rather than the difference in the A2A AR-mediated signaling.
Limitations
It should be pointed out that there are some concerns regarding the translational value of our findings due to the limitation of our current experimental model. Firstly, although the buffer-perfused isolated hearts preparation allows us to exclude the effects of neuro-humoral and blood components on coronary flow regulation, it cannot clearly separate the contribution of shear and/or pressure-induced coronary flow changes from the local metabolic effects. In this case, hemodynamic alterations due to coronary vascular phenotypic changes, coronary collateral circulation, and/or stenosis-associated “coronary steal” in atherosclerotic vs. normal mice may complicate the explanation of the current findings. Secondly, continuously oxygenated (95% O2) Krebs buffer used in isolated hearts has much less oxygen carrying capacity than blood, which may not mimic the in vivo situations in humans. However, using the same preparation, we previously demonstrated a correlation between increase in coronary flow and an enhanced myocardial oxygen consumption (MVO2) after the hearts were paced from 400 to 600 bpm [35]. Additionally, coronary flow increased more than two folds over baseline after 15-sec flow occlusion (Fig. 1A) [29, 36]. Therefore, the buffer-perfused isolated hearts preparation only indicates a minimum hypoxia. Nevertheless, due to the relatively lower myoglobin oxygen saturation in buffer-perfused hearts [26], the cardiac interstitial adenosine level might be higher compared with blood-perfused hearts, which may overestimate the role of adenosine in modulating resting coronary blood flow in our model. Additionally, the isolated whole heart preparation does not allow us to dissect out the different contribution of pathological changes in big conduit arteries vs. microvasculature to the sex-associated difference in coronary flow regulation during atherosclerosis. Lastly, due to the relatively low animal numbers (3–10 animals per group) in this study, there is a risk of losing power to detect the true differences between groups, thus complicating the explanations of our findings.
PERSPECTIVES
In summary, using isolated hearts of murine ApoE KO model of atherosclerosis, the current study provided the first line of evidence that female mice is more prone to NO-dependent coronary endothelial dysfunction compared with male mice. In addition, a functional up-regulation of A2A AR-mediated signaling may serve as an important compensatory mechanism to oppose the decreased NO-dependent coronary vasodilation during atherosclerosis. While this compensatory mechanism is sufficient to maintain normal coronary vasodilation during short period of ischemia in males, the same mechanism fails to rescue the more severely impaired NO-dependent coronary vasodilation in females. The more severely impaired coronary vascular dysfunction in females may explain the clinical evidence that female patients with ischemic heart disease tend to have a higher mortality rate and cardiovascular events compared with male patients. Though the underlying cellular and molecular mechanisms involved in the sex difference in coronary vascular dysfunction under atherosclerosis are not fully illustrated in the current study, the findings of this study will shed new light on the sex-based pathophysiology of coronary artery disease in humans.
Acknowledgments
The authors would like to thank Drs. Karen Martin and Amanda Ammer of the Imaging Center for their support. This study was supported by NIH grants HL027339, U54GM104942, and HL09444 to S. Jamal Mustafa.
Abbreviations used
- A2A AR
A2A adenosine receptor
- ApoE KO
Apolipoprotein E-knockout
- CAD
coronary artery disease
- HFD
high fat diet
- LVDP
left ventricular developed pressure
- MVO2
myocardial oxygen consumption
- NO
Nitric oxide
- NOS
nitric oxide synthase
- RH
reactive hyperemia
- ROS
reactive oxygen species
- WT
wild type
- SNP
sodium nitroprusside
References
- 1.Bairey Merz CN, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Pepine CJ, Mankad S, Sharaf BL, Rogers WJ, Pohost GM, Lerman A, Quyyumi AA, Sopko G. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 2006;47:S21–29. doi: 10.1016/j.jacc.2004.12.084. [DOI] [PubMed] [Google Scholar]
- 2.Berwick ZC, Payne GA, Lynch B, Dick GM, Sturek M, Tune JD. Contribution of adenosine A(2A) and A(2B) receptors to ischemic coronary dilation: role of K(V) and K(ATP) channels. Microcirculation. 2010;17:600–607. doi: 10.1111/j.1549-8719.2010.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourassa PA, Milos PM, Gaynor BJ, Breslow JL, Aiello RJ. Estrogen reduces atherosclerotic lesion development in apolipoprotein E-deficient mice. Proc Natl Acad Sci U S A. 1996;93:10022–10027. doi: 10.1073/pnas.93.19.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapidze L, Kapanadze S, Dolidze N, Bakhutashvili Z, Latsabidze N. Endothelial dysfunction in patients with coronary atherosclerosis. Georgian Med News. 2007:20–22. [PubMed]
- 5.Cola MS, Gava AL, Meyrelles SS, Vasquez EC. Endothelial dysfunction of resistance vessels in female apolipoprotein E-deficient mice. Lipids Health Dis. 2010;9:51. doi: 10.1186/1476-511X-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Godecke A, Ziegler M, Ding Z, Schrader J. Endothelial dysfunction of coronary resistance vessels in apoE−/− mice involves NO but not prostacyclin-dependent mechanisms. Cardiovasc Res. 2002;53:253–262. doi: 10.1016/s0008-6363(01)00432-1. [DOI] [PubMed] [Google Scholar]
- 7.Han SH, Bae JH, Holmes DR, Jr, Lennon RJ, Eeckhout E, Barsness GW, Rihal CS, Lerman A. Sex differences in atheroma burden and endothelial function in patients with early coronary atherosclerosis. Eur Heart J. 2008;29:1359–1369. doi: 10.1093/eurheartj/ehn142. [DOI] [PubMed] [Google Scholar]
- 8.Harrison DG. Endothelial dysfunction in atherosclerosis. Basic Res Cardiol. 1994;89 (Suppl 1):87–102. doi: 10.1007/978-3-642-85660-0_8. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi T, Fukuto JM, Ignarro LJ, Chaudhuri G. Gender differences in atherosclerosis: possible role of nitric oxide. J Cardiovasc Pharmacol. 1995;26:792–802. doi: 10.1097/00005344-199511000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Ishibashi Y, Duncker DJ, Zhang J, Bache RJ. ATP-sensitive K+ channels, adenosine, and nitric oxide-mediated mechanisms account for coronary vasodilation during exercise. Circ Res. 1998;82:346–359. doi: 10.1161/01.res.82.3.346. [DOI] [PubMed] [Google Scholar]
- 11.Kauser K, Rubanyi GM. Gender difference in endothelial dysfunction in the aorta of spontaneously hypertensive rats. Hypertension. 1995;25:517–523. doi: 10.1161/01.hyp.25.4.517. [DOI] [PubMed] [Google Scholar]
- 12.Kitayama J, Faraci FM, Lentz SR, Heistad DD. Cerebral vascular dysfunction during hypercholesterolemia. Stroke. 2007;38:2136–2141. doi: 10.1161/STROKEAHA.107.481879. [DOI] [PubMed] [Google Scholar]
- 13.Kleppisch T, Nelson MT. Adenosine activates ATP-sensitive potassium channels in arterial myocytes via A2 receptors and cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1995;92:12441–12445. doi: 10.1073/pnas.92.26.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maas AH, Appelman YE. Gender differences in coronary heart disease. Neth Heart J. 2011;18:598–603. doi: 10.1007/s12471-010-0841-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meir KS, Leitersdorf E. Atherosclerosis in the apolipoprotein-E-deficient mouse: a decade of progress. Arterioscler Thromb Vasc Biol. 2004;24:1006–1014. doi: 10.1161/01.ATV.0000128849.12617.f4. [DOI] [PubMed] [Google Scholar]
- 16.Meyrelles SS, Peotta VA, Pereira TM, Vasquez EC. Endothelial dysfunction in the apolipoprotein E-deficient mouse: insights into the influence of diet, gender and aging. Lipids Health Dis. 2011;10:211. doi: 10.1186/1476-511X-10-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minamino T, Kitakaze M, Matsumura Y, Nishida K, Kato Y, Hashimura K, Matsu-Ura Y, Funaya H, Sato H, Kuzuya T, Hori M. Impact of coronary risk factors on contribution of nitric oxide and adenosine to metabolic coronary vasodilation in humans. J Am Coll Cardiol. 1998;31:1274–1279. doi: 10.1016/s0735-1097(98)00095-3. [DOI] [PubMed] [Google Scholar]
- 18.Mustafa SJ, Morrison RR, Teng B, Pelleg A. Adenosine receptors and the heart: role in regulation of coronary blood flow and cardiac electrophysiology. Handb Exp Pharmacol. 2009:161–188. doi: 10.1007/978-3-540-89615-9_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;286:R233–249. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- 20.Paigen B, Holmes PA, Mitchell D, Albee D. Comparison of atherosclerotic lesions and HDL-lipid levels in male, female, and testosterone-treated female mice from strains C57BL/6, BALB/c, and C3H. Atherosclerosis. 1987;64:215–221. doi: 10.1016/0021-9150(87)90249-8. [DOI] [PubMed] [Google Scholar]
- 21.Pepine CJ. Ischemic heart disease in women. J Am Coll Cardiol. 2006;47:S1–3. doi: 10.1016/j.jacc.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 22.Pepine CJ, Kerensky RA, Lambert CR, Smith KM, von Mering GO, Sopko G, Bairey Merz CN. Some thoughts on the vasculopathy of women with ischemic heart disease. J Am Coll Cardiol. 2006;47:S30–35. doi: 10.1016/j.jacc.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 23.Poredos P. Endothelial dysfunction in the pathogenesis of atherosclerosis. Int Angiol. 2002;21:109–116. [PubMed] [Google Scholar]
- 24.Rasband WS. ImageJ. U. S. National Institutes of Health; Bethesda, Maryland, USA: 1997–2012. http://rsbinfonihgov/ij/ [Google Scholar]
- 25.Rubanyi GM, Johns A, Kauser K. Effect of estrogen on endothelial function and angiogenesis. Vascul Pharmacol. 2002;38:89–98. doi: 10.1016/s0306-3623(02)00131-3. [DOI] [PubMed] [Google Scholar]
- 26.Schenkman KA. Cardiac performance as a function of intracellular oxygen tension in buffer-perfused hearts. Am J Physiol Heart Circ Physiol. 2001;281:H2463–2472. doi: 10.1152/ajpheart.2001.281.6.H2463. [DOI] [PubMed] [Google Scholar]
- 27.Serruys PW, Juilliere Y, Zijlstra F, Beatt KJ, De Feyter PJ, Suryapranata H, Van Den Brand M, Roelandt J. Coronary blood flow velocity during percutaneous transluminal coronary angioplasty as a guide for assessment of the functional result. Am J Cardiol. 1988;61:253–259. doi: 10.1016/0002-9149(88)90926-5. [DOI] [PubMed] [Google Scholar]
- 28.Sharifi-Sanjani M, Zhou X, Asano S, Tilley S, Ledent C, Teng B, Dick GM, Mustafa SJ. Interactions between A(2A) adenosine receptors, hydrogen peroxide, and KATP channels in coronary reactive hyperemia. Am J Physiol Heart Circ Physiol. 2013;304:H1294–1301. doi: 10.1152/ajpheart.00637.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharifi Sanjani M, Zhou X, Asano S, Tilley SL, Ledent C, Teng B, Dick GM, Mustafa SJ. Interactions between A2A adenosine receptors, hydrogen peroxide, and KATP channels in coronary reactive hyperemia. Am J Physiol Heart Circ Physiol. 2013 doi: 10.1152/ajpheart.00637.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaw LJ, Bugiardini R, Merz CN. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol. 2009;54:1561–1575. doi: 10.1016/j.jacc.2009.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suryapranata H, Zijlstra F, MacLeod DC, van den Brand M, de Feyter PJ, Serruys PW. Predictive value of reactive hyperemic response on reperfusion on recovery of regional myocardial function after coronary angioplasty in acute myocardial infarction. Circulation. 1994;89:1109–1117. doi: 10.1161/01.cir.89.3.1109. [DOI] [PubMed] [Google Scholar]
- 32.Teng B, Mustafa SJ. A(2A) adenosine receptor-mediated increase in coronary flow in hyperlipidemic APOE-knockout mice. J Exp Pharmacol. 2011;2011:59–68. doi: 10.2147/JEP.S18945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teng B, Smith JD, Rosenfeld ME, Robinet P, Davis ME, Morrison RR, Mustafa SJ. A(1) adenosine receptor deficiency or inhibition reduces atherosclerotic lesions in apolipoprotein E deficient mice. Cardiovasc Res. 2014;102:157–165. doi: 10.1093/cvr/cvu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diabetes and Estrogen: The Role of Oxidative Stress on Endothelial Function in Women. The FASEB Journal. 2015:820.822. [Google Scholar]
- 35.Zhou X, Teng B, Tilley S, Ledent C, Mustafa SJ. Metabolic hyperemia requires ATP-sensitive K+ channels and H2O2 but not adenosine in isolated mouse hearts. Am J Physiol Heart Circ Physiol. 2014;307:H1046–1055. doi: 10.1152/ajpheart.00421.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou X, Teng B, Tilley S, Mustafa SJ. A1 adenosine receptor negatively modulates coronary reactive hyperemia via counteracting A2A-mediated H2O2 production and KATP opening in isolated mouse hearts. Am J Physiol Heart Circ Physiol. 2013;305:H1668–1679. doi: 10.1152/ajpheart.00495.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]