Microbial colonization research in humans expanded significantly over the last five years with the recognition that the composition and differentiation of the human microbiome is related to the health of individuals.1,2 The human microbiome influences the host immune system and plays a significant role in acquiring as well as preventing disease states.3,4 Humans and microbes form a relationship that starts before birth and evolves throughout the lifespan. High-risk term and preterm infants are some of the most vulnerable to an altered microbiome due to the atypical NICU environment of care following birth.
The human microbiome is defined as the entire collection of naturally occurring bacteria, fungi, and viruses, including their DNA that exists in the human body.5,6 See Table 1 for a list of common terms used in the study of the microbiome. The healthy infant’s microbiome begins prenatally and evolves rapidly after delivery as the infant is exposed to the mother’s microbiota.7,8 The rapid evolution of the infant’s microbiome occurs as microbes are initially introduced from exposure to amniotic fluid, descent through the vaginal canal, intake of human breast milk, and skin-to-skin contact with the mother.7-10 Contact with these initial microbes is beneficial to the health of infants by forming the basis of nutrient utilization, gut barrier function, and immune development.8,10 As the infant matures, the microbiome continues to develop and influence health throughout a lifetime.8,10
Table 1.
Common Terms in the Microbiome Literature
| Keyword | Definition | Examples |
|---|---|---|
| 1. Human Microbiome |
The entire collection of naturally
occurring bacteria, fungi, and viruses, including their DNA, that exists in the human body which are critical for normal development and health. |
Bacteria locations: mouth
(anaerobes); large intestine (aerobes and anaerobes); skin (predominately aerobes) Virus locations: implant into living cells mainly in the respiratory (coxsackie viruses), gastrointestinal (adenovirus), skin- penetrating, and genital routes (human papillomavirus) Fungi locations: superficial including the skin, hair, and nails (candidiasis) |
| 2. Microbiota | Complex communities comprised of many microorganisms including bacteria, fungi, and viruses that colonize various sites in the human body. |
|
| 3. Genera | The usual subdivision of a family or
subfamily in the taxonomic classification of organisms, usually consisting of more than one species. |
Ex: Enterobacter (genus
Enterobacter) is a group of rod-shaped bacteria of the family Enterobacteriaceae, gram-negative facultative anaerobes Ex: Escherichia coli (genus Escherichia) is a gram-negative, facultative anaerobic, rod- shaped bacterium |
| 4. Microbes | Microorganisms such as bacteria,
fungi, and viruses that can perform beneficial and harmful effects. |
|
| 5. Commensal Microbes |
Organisms that produce positive
effects for a host. Indigenous: present on body surfaces covered by epithelial cells and exposed to the external environment. |
Bacteroides, Bifidobacteria,
Enterobacteriaceae, Enterococcaceae Escherichia coli, Lactobacilli Neisseria (all except N. gonorrhea and N. meningitides, Staphlococci spp., Streptococci spp. |
| 6. Pathogenic Microbes |
Organisms that produce negative
effects for a host. Indigenous organisms may induce disease when the host is compromised or with commensal microbe overgrowth. |
Acinectobacter,
Actinobacteria,Citrobacter
Clostridium spp., Firmicutes, Gemella, Geobacillus, Halomonas, Klebsiella spp., Proteobacteria, Pseudomonas aeruginosa, Shewanella , Ureaplasma (commensal in sexually active women) |
Alterations in the formation of the infant microbiome can occur and are associated with adverse gut barrier function and immune response.8,10,11 Infants born via caesarean section have microbial flora similar to environmental microbes, while infants born vaginally have intestinal microbial content similar to the mother’s vaginal and intestinal flora including the commensal bacteria Bacteroides, Bifidobacterium, and Escherichia coli.12,13 This difference in microbial composition is important because commensal bacteria facilitate the development of the infant’s immune system.9 Infants born by caesarean section may take up to six months of age to colonize the commensal bacteria.2 Epidemiological data shows that infants born via caesarean section are also more prone to future childhood disease such as allergic rhinitis, asthma and celiac disease.8 Following delivery infants continue to develop and alter their microbiome based on type of feeding, contact with microbes from the environment, and from economical changes such as improved sanitation, increased antibiotic usage, and immunization prophylaxis.14,15
While the mode of delivery provides the foundation for the infant’s initial microbiota, development of the microbiome continues with ongoing interaction with the mother, family, and home environment. Unfortunately, high-risk infants are separated from their mothers following delivery and for weeks to months are cared for in the complex environment of the neonatal intensive care unit (NICU). Every NICU has its own genera of microbes which can include commensal and pathogenic organisms.16,17 With the NICU genera as the context for microbiome development, the infant’s ongoing interaction with multiple healthcare providers and caregiving processes have the potential to impact microbial colonization. A recent study showed that the risk of acquiring nasal colonization with coagulase-negative Staphylococcus (CoNS) for all neonates admitted in a NICU was 55.9%.18 High-risk term and preterm infants are at an increased risk of infection due to their illness, and in preterm infants due to immature skin and immune systems.19,20 Caregiving equipment not only comes in contact with infants, but items like electrocardiogram (EKG) leads, monitoring probes, adhesive dressings, and chemical burns that can damage the epithelium can also lead to increased risk of microbial invasion.20 Over the past two decades several NICU outbreaks of Pseudomonas aeruginosa and Klebsiella pneumoniae neonatal infections were linked to healthcare providers and caregiving activities,21-24 but they fail to inform us about the development of the infant’s microbiome.
Understanding the colonization patterns of microbes on the skin and in the gastrointestinal tract of infants can help NICU caregivers develop interventions to promote infant health. This systematic review of the literature summarizes what is known about the impact of the NICU environment on microbial colonization of high-risk term and preterm infants and identifies implications for clinical practice and future research.
The Microbiome
Microbiota are complex communities comprised of many microorganisms including bacteria, fungi, and viruses that colonize various sites in the human body.5 In fact, most of the body’s cells are not human, but are microbial in origin with “an estimated microbial/human ratio of 10:1”.2 Of all sites, the human gut houses 10 times as many microorganisms as other areas of the body.9 The skin, bladder, mouth, and vagina are also heavily colonized sites.25
All microbes, which are microorganisms such as bacteria, viruses, and fungi,5 were once thought to be harmful. However, microbes perform a variety of positive functions for the host such as regulating the development of the gut, preventing harmful bacteria from colonizing, and synthesizing vitamins and unused substrates.9 Although bacteria can produce positive effects for a host, a fine line exists between acquiring commensal bacteria and acquiring pathogenic bacteria.25 Pathogenic bacteria can be introduced through a multitude of factors such as altered host immune function, genotype, diet, and the environment.25
The Human Microbiome Project, sponsored by the National Institute of Health, was developed to understand what determines a healthy versus an unhealthy aggregate of genetic material of bacteria, fungi and viruses, and to identify microbial flora that exist in the different areas of the body.10 Current research aims to understand how the microbiome forms and what factors, such as the environment, contribute to commensal and pathogenic microbial colonization. How the microbiome relates to neonatal health is of importance due to high-risk and preterm infants’ increased risk of infection in the environment of the NICU, as well as their risk for altered health and developmental outcomes. The acquisition of the microbiome during infancy can influence immune health over the lifespan.26
This systematic review describes the relationship between factors within the NICU environment and microbial colonization of high-risk infants. For the purpose of this review, environment was defined as the physical environment of care and caregiving practices. The findings will increase healthcare professionals’ awareness of the potential impact of the NICU environment on skin and gut colonization of infants, and identify caregiving interventions to improve infant outcomes.
Literature Search Methods
Data Sources
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRIMSA) standards were used as a framework for this review.27 Electronic databases used to identify relevant articles in English with an available full text or print copy included PubMed BIOSIS Citation Index, Google Scholar, the Cumulative Index for Nursing and Allied Health Literature (CINAHL), and BioMedSearcher. Search terms used to identify research articles relevant to the research question included: microbiome, microbiota, microbe, antibiotic, infant skin care, isolette, delivery mode, environment, infection control, immunity, incubator, necrotizing enterocolitis, infant, premature emollients, nursery, NICU, and gut colonization. No limits were set on research articles regarding the date of publication or country of origin.
Study Selection
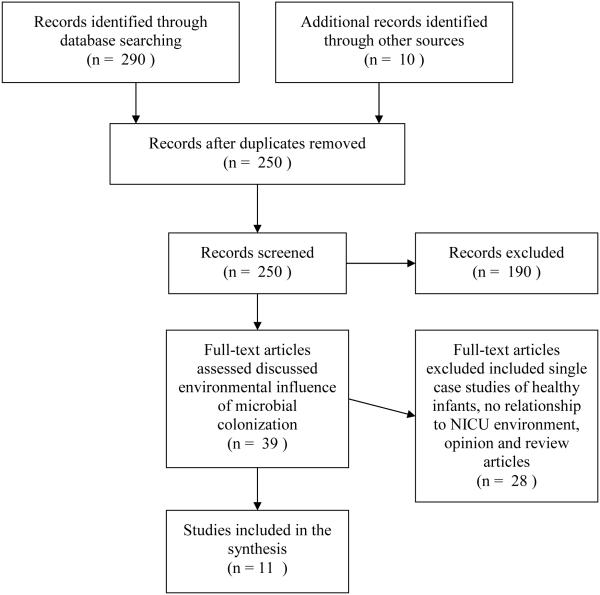
A literature search was conducted in January of 2014, updated in June and November of 2014, and revised in January of 2015. Figure 1 summarizes the study selection. After screening titles and abstracts of 250 articles, 39 articles were chosen to read thoroughly based on discussion of the environmental influence of microbial colonization on infants. Eleven articles were chosen as sources for this review based upon our definition of NICU environment and potential environmental influences on the microbiome of high-risk and preterm infants. These articles included literature about the influence of the mother and mode of delivery on the infant, factors that influence infant gut colonization, and the risks associated with the colonization of the NICU environment.
Figure 1.
Search Strategy and Summary
Data Extraction
Each article was read carefully and categories were identified that had the potential to influence the infant microbiome. The literature analysis presented challenges given the emerging science about the infant microbiome and the identification of few studies specific to the relationship between the physical NICU environment or caregiving practices on the infant microbiome. As evidence of the developing nature of this work, the 11 articles chosen as sources for this review were all published within the last four years (2011-2014). The study designs included six cohort studies, two longitudinal studies, one retrospective descriptive study, one prospective surveillance study, and one descriptive comparative study. No randomized-controlled trials or systematic reviews were found related to the potential environmental influences on the infant microbiome.
Definitions of the microbiome, microbiota, and microbe were used interchangeably in many studies. Consistent use of these terms would make research on this body of knowledge stronger and would decrease variability on this subject.
Data Synthesis
Categories of factors associated with changes in the infant’s microbiome in the 11 articles were parental skin, feeding (human milk vs. formula), environmental surfaces, nursing workspaces, and infant caregiving equipment (e.g. ventilators, incubators and warmers); health care provider skin; and antibiotic use (see Table 2).
Table 2.
Potential Environmental Influences on the Infant Microbiome
| Title/ Authors (Year)/ Country |
Population/ Study Design |
Environmental Factors |
|---|---|---|
|
Effect of skin-to-skin
contact on preterm infant skin barrier function and hospital- acquired infection Ambouelfettoh, Ludington-Hoe, Burant, Visscher (2011)28 USA |
Pretest-test-posttest cohort study N=10 preterm infants with 90 minutes of SSC daily for 5 days Blood cultures results during hospitalization and up to four weeks post-discharge Signs and symptoms of infection after discharge were defined as positive maternal response to one or more of a 3-question questionnaire concerning infection after discharge from the NICU. |
Parental Skin
One infant was blood culture positive seven days after SSC. No infants had signs of infection within 4 weeks of discharge. |
|
Microbes in the neonatal
intensive care unit resemble those found in the gut of premature infants Brooks, Firek, Miller, Sharon, Thomas, Baker, Morowitz, Banfield (2014)29 USA |
Longitudinal descriptive 2 VLBW (<1500 grams) infants cared for in the same area for first month of life Stool samples collected every 3 days; 33 swabs were collected each room surfaces (sink, feeding and intubation tubing, hands of healthcare providers and parents, general surfaces, and nurse station computer keyboard and mouse, and cell phone |
Environmental surfaces,
caregiving
equipment, health care provider skin, parental skin Distinctly different GI tract colonizations in the two infants. Gut organisms were widely distributed throughout the room environment—included Staphylococcus epidermidis, Klebsiella pneumoniae, Bacteroids fragilis, and Escherichia coli. Klebsiella pneumoniae in infant 1 and Finegoldia magna in infant 2 were present in the room prior to detection in the gut. Keyboards, mouse, and telephones had the lowest amount of colonizing organisms detected in the gut Intubation and feeding tubing had the highest amount of colonizing organisms detected in the gut. |
|
Staphylococcus aureus
reservoirs and transmission routes in a Portuguese neonatal intensive care unit: A 30-month surveillance study Conceicao et al. (2012)30 Portugal |
Prospective surveillance 16 clinically symptomatic infants who were Staphylococcus aureus hemo positive; mean gestation age of 31 weeks Swabs from the infants’ parents nares; NICU health care workers nares during the 4 days preceding each infection case; Mothers' nipples in case of breast feeding; NICU environment (cardio-respiratory monitors, incubators, milk pumps, stethoscopes, plastic folder protecting the infants clinical records hanging on the incubators, and telephones) |
Environmental surfaces and
caregiving
equipment, parental skin, health care provider skin Mean time to acquire an S. aureus infection after admission was 15 days (ranging from 2 to 39 days) S. aureus isolates recovered from infant’s hemocultures, catheters, umbilical exudates, wounds, pus of intestinal abscess, pus of skin abscess, and urine. MSSA isolates recovered from plastic folder in the NICU environment Infection cases had the same strain recovered from the infant and other sources such as at least one health care worker, the environment (plastic folder), mother’s nipples. |
|
Bacterial community
structure and functional contributions to emergence of health or necrotizing enterocolitis in preterm infants Claud, Keegan, Brulc, Lu, Bartels, Glass, Chang, Meyer, Antonopoulos (2013)31 USA |
Descriptive cohort study 10 Preterm infants Stool samples of 5 infants with NEC and 5 healthy matched controls |
Feeding type
Control fecal samples had a temporal pattern of microbiota typical of healthy full-term breast-fed infants. Microbiota development in NEC patients diverged from healthy controls beginning three weeks prior to diagnosis. The majority of its genes in the organisms of the NEC patient were associated with carbohydrate metabolism and mapped to members of the family of Enterobacteriaceae. |
|
Intestinal microbial
ecology and environmental factors affecting necrotizing enterocolitis Torrazza, Ukhanova, Wang, Sharma, Hudak, Neu, Mai (2013)32 USA |
Descriptive case controlled cohort N=53 preterm infants ≤ 32 weeks GA; 18 NEC cases; 35 control cases 3 NICUs (1 in Gainesville, FL., 2 in Jacksonville, Fl.) Stool samples at 2, 1, and 0 weeks, prior to the diagnosis of NEC. Samples from matched control infants were chosen during the same week of life at which the samples from the cases were obtained. |
Environmental surfaces, feeding
type,
antibiotic use Microbiota composition differed between the 3 NICUs where antibiotic use also differed. Incidence of NEC was 12.4% for hospital one vs. 6.8% for hospital two and three Control infants received human milk 57.1% of the time while infants with NEC received human milk only 27.8% of the time Abnormal patterns of colonization with predominance of Proteobacteria early in life or later in the days closer to the development of NEC may be associated with a greater risk of developing NEC. |
|
Development of the
preterm gut microbiome in twins at risk of developing necrotising enterocolitis and sepsis Stewart, Marrs, Nelson, Lanyon, Perry, Embelton, Cummins, Berrington (2013)33 Spain |
Longitudinal study 27 infants (12 twin pairs and 1 triplet set) 173 stool samples 18 expressed breast milk samples from 4 mothers |
Feeding type, antibiotics
Staphylococcus, Corynebacterium, and Propionibacterium were found in high abundance in the gut microbiome Gut microbiome was more similar between siblings than unrelated individuals. Reduction in diversity and increasing dominance of Escherichia sp. preceded NEC was not observed in the healthy twin. Antibiotic treatment reduced Escherichia sp. and increased other Enterobacteriaceae in the gut. |
|
Surface microbes in the
neonatal intensive care unit: Changes with routine cleaning and over time. Bokulich, Mills, Underwood (2013)16 USA |
Pre-post cohort study
Environmental cleaning NICU surface samples; N=147 Neonate-associated (e.g. incubator, pacifier, ventilator) Room environment (e.g. computer, space bar, cardiac monitor) |
Environmental surfaces
Fungal populations included Saccharomyces cerevisiae, Cryptococcus albidus, Debaryomyces fabryi, and Candida albicans and were less prominent on neonatal than room surfaces. Cleaning with antibacterial wipes significantly reduced the total microbial load on surfaces and the collection of microbiota shifted to primarily non-pathogenic organisms Levels of common NICU enteric Genera were not altered by cleaning (Enterococcus, Klebsiella, Escherichia, and Pseudomonas) |
|
Cold spots in neonatal
incubators are hot spots for microbial contamination de Goffau, Bergman, de Vries, Meessen, Degener, van Dijl, Harmsen (2011)34 Netherlands |
Prospective Cohort Study 23 Caleo incubators Group 1 N=39 hot; 50 cold spots samples Group 2 N=40 hot; 60 cold spots samples At infant transfer to a clean incubator after 4-7days of use Swab samples from 4 hot and 5 cold spots |
Environmental surfaces and
caregiving
equipment Higher average incubator temperatures (≥34°C) and relative humidity values (≥60%) were associated with higher levels of microbial contamination at cold spots versus hot spots Primary organism found was Staphylococci |
|
Clostridia in premature
neonates’ gut: Incidence, antibiotic susceptibility, and perinatal determinants influencing colonization Ferraris, Butel, Campeotto, Vodovar, Roze, Aires (2012)35 France |
Retrospective descriptive N= 76 preterm neonates; Weekly stool samples |
Environmental surfaces,
antibiotics
79% of infants were colonized by Clostridium sp. colonization Antenatal antibiotic treatment was related to significant decreases in clostridia levels (p<0.01). Neonatal antibiotic therapy was related to significant decreases (p<.01)only when performed during more than 10 days. |
|
Prospective assessment
of the gastroesophageal microbiome in VLBW neonates Milisavljevic, Garg, Vuletic, Miller, Kim, Cunningham, Schroder (2013)36 USA |
Cohort Study 12 Preterm infants Gastroesophageal aspirates were collected during the first 28 days of life to evaluate microbial flora |
Environmental surfaces
Bacteria were detected in 9 of the 12 neonates. By the fourth week, Gram (-) bacteria increased in abundance to account for 50% of the total organisms. Only two distinct species of Staphylococcus epidermis were found, suggesting acquisition from the environment. |
|
Microbiome assembly
across multiple body sites in low-birthweight infants Costello et al. (2013)37 USA |
Descriptive comparative study 6 LBW: Saliva and stool samples, skin swabs, Collected on postnatal days 8, 10, 12, 15, 18, and 21 Healthy NBW: stool Healthy adults: skin |
Environmental Surfaces
Staphylococcus and Streptococcus, usually found in the saliva and on skin, were abundant at other body sites. Saliva and stool composition diverged over time but were not significantly different until the infants were 15 days old. Escherichia was abundant in NBW infants, which was absent in LBW infants. Despite these differences, over-time LWB infant stool grew more similar to that of 21-day-old NBW stool. Divergence rate was similar to that of age-matched normal birthweight infants. |
Note: GA=gestational age; NICU=neonatal intensive care unit; VLBW: very low birthweight (<1500 grams); LBW: low birthweight (>1500 grams and <2500 grams); NBW: normal birthweight infant (> 2500 grams); NEC=necrotizing enterocolitis; SSC=skin-to-skin care
Parental Skin
Many NICUs encourage skin-to-skin contact, consisting of “chest-to-chest placement of the infant with the mother at an incline of 30-40 degrees” (pg. 37).28 Skin-to-skin care (SSC) allows for positive touch between the infant and mother as well as transfer of microbes. No studies were found that examined the relationship between skin-to-skin contact and the infant microbiome, however one study did examine infection rates after mother skin-to-skin care in preterm infants.28 After 5 consecutive days of skin-to-skin care for 90 minutes, only one infant developed a hospital-acquired infection within 7 days after the skin-to-skin care ended.28 None of the mothers had any signs or symptoms of infection during the first thirty days post discharge.28
Two studies identified microbial transfer from parents to infants cared for in the NICU.29,30 In a NICU with an outbreak of Staphylococcus aureus (S. aureus), S. aureus isolates were identified from two fathers and the nipples of one mother prior to any symptoms of infection in the infant.30 A second study showed that organisms found in the NICU environment were also found on parents hands and in the stools of two infants.29
Feeding Type
The impact of breast milk and formula on the developing microbiome of premature infants has been hypothesized especially in the development of necrotizing enterocolitis (NEC). The relationship between feeding type was addressed in three studies that explored the differences in the gut microbiome of premature infants who developed NEC versus the gut microbiome of healthy control premature infants who did not develop NEC.31-33 In one study, stool samples of NEC cases showed a high proportion of Proteobacteria (61%) at two weeks and Actinobacteria (3%) at one week before diagnosis with NEC, along with lower amounts of Bifidobacteria and Bacteroidetes.32 Stool samples of control infants who did not acquire NEC had less abundance of Proteobacteria (19%) and Actinobacteria (0.4%), and they received human milk 57.1% of the time compared to only 27.8% of the time for infants who developed NEC.32 A second group of infants had stool samples dominated by Proteobacteria with members of the Firmicutes; however, prior to two weeks old, infants who later developed NEC had fewer numbers of Firmicutes compared to infants who did not develop NEC.31 The control infants’ stool samples also had a microbiota similar to healthy full-term, breast-fed infants.31 Lastly, in a related study, 173 stool samples were obtained from 12 sets of twins and one set of triplets, as well as 18 samples of expressed breast milk from 4 mothers.33 Both the expressed breast milk and stool samples shared a common microbiome comprised of Enterobacteriaceae, Enterococcaceae,and Staphylococcaceae, although both had a low abundance of Bifidobacteria and Lactobacilli.33 The expressed breast milk samples had an evolving microbiome throughout lactation as new diversified bacteria continually arose.33
Environmental Surfaces, Nursing Workspaces and Caregiving Equipment
Preterm infants are immunocompromised, making them highly susceptible to hospital-acquired infections. Virtually every object and piece of equipment used in the NICU environment can serve as a reservoir for microbial contamination. Four studies examined several aspects of the NICU environment in an effort to better understand how they might impact the infant microbiome.16,29,30,34 Specific measures utilized within these studies included sampling before and after cleaning with antibacterial wipes,16 cold versus warm spot sampling in incubators,34 predominant NICU organisms living within specific nurseries regardless of routine cleaning and hand washing,29 and lack of hand washing and routine cleaning before and after implementing standard precautions.30 Microbiome samples of environmental surfaces revealed complex and diverse environments and included species associated with nosocomial infections common in the neonatal population.16,29,34 Surfaces that came in direct contact with infants (continuous positive airway pressure machines, stethoscopes, ventilators, incubators, and radiant warmers) were colonized with Streptococcus, Staphylococcus, Neisseria, and Enterobacteriaceae16,30, while room environment surfaces (computer screens, a computer mouse, freezer handles, hand sanitizer bottles, door handles, telephones, heart rate monitor screens, and alarm buttons) were colonized with communities of Geobacillus, Halomonas, Shewanella, Acinetobacter, and Gemella.16 Over time the surfaces with the greatest variation were those exposed to human skin.29
Like other surfaces that come into direct contact with the infant, the microbiome of incubators included Streptococcus16, Staphylococcus16,30, Neisseria, and Enterobacteriaceae.16 The uniformity of heat and humidity was related to the colonization of incubators. In humidified Caleo® [[Dräger, Drägerwerk AG & Co. KGaA, Telford, PA.]] incubators with average temperatures ≥ 34°C and relative humidity levels ≥ 60%, microbial contamination with Staphylococcus was significantly higher at incubator cold spots than at hot spots.34 The cold spots were found at the front, back sides and middle of the incubator where the humidified air did not reach as well. Hot spots were closer to the hot air vents. Across a wide variety of surfaces including incubators, ventilators, monitors and computers, cleaning with disinfectant wipes significantly decreased the bacterial and fungal load on surfaces sampled before and after cleaning.16
Specific infant caregiving supplies like other NICU surfaces had diverse microbiome communities. Pacifiers were colonized with diverse communities of Streptococcus, Staphylococcus, Neisseria, and Enterobacteriaceae16 and stool samples of two infants were similar to gut organisms (Staphylococcus epidermidis, Klebsiella pneumonia, Bacteroids fragilis, and Escherichia coli) prevalent in intubation and feeding tubing.29
The relationship between the general NICU environment and the infant gut microbiome were explored in four studies.32,35-37 Stool colonization patterns in neonates revealed that the diversity of clostridia species increased throughout hospital stay, which included C. perfringens, C. butyricum, C. difficile, and C. paraputrificum.35 Different fecal microbiota composition also existed in neonates housed in different NICUs; one hospital had more colonization with Bacteroidetes and Proteobacteria versus colonization with Firmicutesat another hospital.32 Stool samples of infants who acquired necrotizing enterocolitis (NEC) showed a higher portion of Proteobacteria and Actinobacteria, and lower numbers of Bifidobacteria and Bacteroidetes.32 Gastrointestinal aspirates of very low-birthweight infants revealed that Staphylococci were dominant overall, Ureaplasma were dominant in the first week of life, and by the fourth week of life, gram-negative bacteria increased.36 Firmicutes were present in the majority of the neonates and only two species of Staphylococcus epidermidis were found.36 When stool, skin, and saliva samples were compared among low-birthweight infants, Klebsiella/Enterobacter, Enterococcus, and Citrobacter were present in stool samples; Staphylococcus was present on the skin, and Streptococcus was present in the saliva.37 However, when stool samples of low-birthweight infants were compared to stool samples of normal-birthweight infants, Escherichia was highly present in the stool of normal-birthweight infants, but absent in low-birthweight infants who had an abundance of Enterobacter, Enterobacteriaceae, Enterococcus, and Staphylococcus.37 These findings were related to delay in enteral feedings and increased length of hospital stay in preterm infants.37 Finally when samples were compared to adult microbiota, preterm skin microbiota was most like adult skin microbiota; preterm saliva and stool microbiota were the least like adult microbiota.
Health Care Provider Skin
The mechanisms of human-to-human transfer of organisms are well known and are one source of nosocomial infections. Two studies looked at direct transfer of microbes from health care providers’ skin to infants cared for in the NICU.29,30 From 154 surveillance-screening swabs in a NICU with an outbreak of Staphylococcus aureus (S. aureus), 24 S. aureus isolates were identified from 18 healthcare workers.30 This bacteria was identified prior to the symptoms occurring in the infants cared for by the providers with S. aureus isolates.30 With over 50% (n=64) healthcare providers screened, a 28% prevalence of S. aureus colonization was identified.30 In a second study, samples from healthcare providers skin revealed Actinobacteria, Firmicutes, and Proteobacteria.29Caregiver hands were also contributors to the variation of environmental organisms present on environmental surfaces and caregiving supplies. Over time the stools of two infants cared for in the same nursery developed similar microbes.29
Antibiotic Use
Antibiotic use in NICUs remains widely diverse and controversial. Antibiotics are effective in depleting pathogenic bacteria during disease states but simultaneously deplete commensal bacteria. Depleting commensal bacteria negatively alters the infant microbiome and is thought to increase the risk of acquiring NEC.31 The relationship between antibiotic use, gut microbes and NEC was explored in three articles.32,33,35 One study specifically tested for Clostridia strains, which were susceptible to a variety of antibiotics including amoxicillin-clavulanic acid, piperacillin-tazobactam, chloramphenicol, metronidazole, linezolid, and vancomycin.35 Strains were resistant to clindamycin, cefotaxime, tetracycline, and moxifloxacin.35 Antenatal antibiotic treatment was associated with significantly decreased Clostridia levels, while intrapartum antibiotic therapy was unrelated.35 Neonatal antibiotic therapy was also associated with decreased levels of Clostridial colonization when given for more than ten days.35 In a second study, prior to the development of NEC, infants were exposed to different antibiotics and varying durations of treatment at three different NICUs.32 Antibiotic usage was associated with observed differences in the infants’ microbiome. Infants who went on to develop NEC had a higher proportion of Proteobacteria (61%) and Actinobacteria (3%) at two and one weeks prior to the diagnosis, respectively.32 The infants who developed NEC also had lower numbers of Bifidobacteriacounts and Bacteroidetes.32 In the final study, antibiotic therapy was given to a set of twins and showed reduced Escherichia sp. and increased amount of Enterobacteriaceae in stool samples, which reversed in one twin when antibiotics were stopped.33 The other twin developed NEC and received subsequent antibiotic therapy, which altered the bacterial community from the sibling, showing increased levels of Klebsiella sp.and smaller increases in members of the Bifidobacteriaceae family.33
Discussion
The limited studies in this review were not able to answer the complex sequencing questions necessary to determine exactly when and from what the infant acquires its microbiome. However, specific species from the NICU environment are associated with nosocomial infections, and infant complications like NEC reveal patterns of gut microbiota development different from infants who do not develop NEC. Researchers continue to define what constitutes the normal microbiome for healthy term infants, and research techniques in this area are advancing rapidly. What is known about the microbiome in preterm infants remains obscure, and much research is still warranted. The recent literature provides evidence about the diversity of the microbiome in the NICU environment. However, exactly how the microbiome of the NICU environment, caregivers, feeding type, and antibiotics affect the development of the infant’s microbiome over time requires further exploration.
Parental contributions to the healthy infant’s microbiome are typically viewed as positive such as in the practice of skin-to-skin care and breastfeeding. However, the typical parental microbiome may also be altered as they spend significant amounts of time in the NICU environment. Several studies identified bacterial and fungal colonization of NICU surfaces, equipment, skin and caregiving supplies that are associated with nosocomial infections in the neonatal population.16,29,30,34 Intensive care nurseries commonly engage in infection control bundles and routine cleaning of bedside and nursery equipment as a method to prevent nosocomial infections, and at least one study demonstrated a decrease in bacterial communities following routine cleaning practices.16 However, how the routine cleaning might affect colonization of both commensal and pathogenic bacteria in infants is unclear.
Humidified incubators, a mainstay piece of equipment in the treatment of preterm infants in NICUs, are beneficial for skin integrity and temperature regulation, yet the warm moist environment is also a habitat for microbial growth.34 Staphylococci, a well-known infectious agent in preterm infants and gram-negative bacteria, a risk factor for infection related to neonatal death, were found in the cold areas of humidified incubators.34 Yet, the frequency with which incubators should be cleaned to minimize risk of infection while maintaining infant safety is unknown. The transfer of infants into a clean incubator is not without associated risks of dislodgement of catheters and equipment, as well as overstimulation of the infant.
Several studies revealed that the infant gut is colonized differently based upon the composition of the NICU environment.31,32,35-37 Infants appear to acquire organisms in their gut days to weeks after they appear on the surfaces and equipment of the NICU environment.32,35-37 Microbial invasion of the gut from the environment also plays a role in neonates acquiring NEC, although specific pathogens have not been identified.32 However, infants who developed NEC had markedly different microbiota, which could be attributed to the NICU environment, type of feeding, and antibiotic use.32 Along with the microbiome, host physiology, such as how an infant metabolizes milk, may also play a role in the development of NEC.31 Infants who received breast milk had gut microbiota more similar to healthy breast-fed term infants31 revealing that they may be at a lower risk for developing NEC.31,32 Together the findings do not provide definitive evidence to which type of feeding is most beneficial for the development of the gut microbiome; however, given the many other benefits of breast milk it would appear prudent to use breast milk when available. Finally, while the length and criteria for antibiotic therapy remains controversial, antibiotic use appears to be associated with a change in the pattern of the gut microbiome consistent with the development of NEC.32,33,35
The results of this review did not provide information about the development of the infant’s skin microbiome. Although not studied to date, it is known that the skin provides a protective barrier from microbial invasion.38,39 Infants in the NICU are faced with infection risk from EKG leads, monitoring probes, adhesive dressings, and chemical burns that can damage the epithelium, and lead to increased risk of microbial invasion.20 Therefore, skin integrity and knowledge about the skin microbiome in premature and high-risk infants is essential during the first two weeks of life when the epidermis is beginning to mature and neonatal infections are common.19,20,38,39
Implications for Practice
While the findings of this review are unclear about how the NICU environment specifically influences microbial colonization in neonates, the presence of pathogenic bacteria indicates the need for continued diligence in the bundle of practices implemented to reduce nosocomial infections.40,41 Simple techniques such as vigilant hand hygiene, wearing gloves, and practicing sterile technique during invasive procedures can prevent infections especially central-line bloodstream infections40 and should include not only healthcare providers, but also parents.
Routine surface cleaning with disinfectant solutions should be employed to decreased colonization of environmental bacteria on surfaces16 as well as incubators.34 Minimizing condensation on the inside of incubators, wiping the vapor away with a clean cloth, and not leaving the cloth inside the incubator for consecutive use may also prevent excessive colonization.34 Finally, it may be helpful to change incubators at least once a week.34
Evidence-based skin care guidelines should also be implemented to protect the epidermal barrier of all infants, but especially preterm infants.42 Standardization of skin care to facilitate microbiome development is lacking, but in at least one study cleaning the skin of preterm infants between 28 and 36 weeks of age with 0.25% chlorhexidine within three hours of birth reduced axillary skin-colonization at 24 hours.19 While this study did not report any adverse impact on the skin or temperature of the infant, the efficacy of bathing with chlorhexidine is unclear. The same study found that cleaning with saline offered the same reduction in axillary skin colonization.19 The use of topical emollients such as Aquaphor® [[Beiersdorf Inc., CT]] is controversial. Topical emollients have proven to be effective at decreasing transepidermal water loss (TEWL) and maintaining skin integrity in premature infants, but some studies have shown an increased risk of nosocomial infection with emollient use.43 The use of No-Sting® [[3M, MN]] is just as effective as Aquaphor® at decreasing TEWL and maintaining skin integrity, but further studies need to be conducted on its use.44
Labor and delivery nurses as well as NICU nurses should also be strong advocates for new mothers to breast feed or pump breast milk for their infants. The maternal milk microbiome contains beneficial bacteria for infants to help develop a mature immune system and fight infections.45 Formula preparation does try to mimic breast milk properties, although it does not contain the beneficial, natural immunoglobulins, oligosaccharides, and fatty acids contained in maternal breast milk.46,47
Practitioners and providers should be cautious in prescribing antibiotics to neonates if the need for antibiotics is not fully justified. Depending on the institution, many high-risk infants will receive antibiotics on admission immediately after birth. Antibiotics will deplete pathogenic bacteria but also can be detrimental by eliminating all commensal bacteria.48 Antibiotics deplete the diversity of microbes within the microbiome as well as halt the development of commensal bacteria.49 Antibiotic use has also shown to be associated with the establishment of NEC in premature infants.32
Implications for Future Research
The microbiome of neonates is complex and what is considered to be a normal microbiome is not clear. Additional research should attempt to identify normal microbiome development for not only the gut, but also for the skin of high-risk and preterm infants. While a variety of infection control bundles have been put in place, the impact on the microbiome is not known.
Additional research should evaluate how a variety of caregiving activities such as oral suctioning and the feeding tube type and method, may impact the microbiome of the infant’s gut. How long feeding tubes should remain in place and whether in and out placement of feeding tubes promotes or hinders the gut microbiome is unknown. In addition, while breast milk is presumed to be the best for infants, the impact of mother’s own milk versus banked breast milk on the microbiome should be evaluated. Finally, further research is required on the use of probiotics and prebiotics to help form the gut microbiota in infants, as well as determine if they can help prevent infections and the risk of NEC.
Although current research exists focused on neonatal skin care guidelines, more research should focus on evaluating how disinfectants, bathing, wound care products, and skin protection products like No-Sting® and Aquaphor® affect the development of the skin microbiome. Early skin care practices have the potential to not only impact the skin microbiome in the NICU, but may also influence the development of future autoimmune problems such as asthma and atopic dermatitis.
Future studies of the impact of the NICU environment on the developing microbiome should include all infants, not just the infants who develop infections. Larger sample sizes should also be utilized in new studies, as all studies used within this systematic review were of small sample sizes. Microbiome samples from the NICU environment, health care providers, parents and infants collected simultaneously over time would significantly add to our understanding of how the microbiome of the infant cared for in the NICU develops. In addition, researchers should use current genomic DNA standardized procedures rather than cultures to evaluate the impact of the NICU environment on the infant. Finally new research should explore how the infant’s developing microbiome in the NICU impacts the development of the immune system and long-term health outcomes.
Conclusion
The microbiome of high-risk and preterm neonates may be altered by the environment in a variety of ways beginning with the mode of delivery. Potential influencing factors from the NICU environment include the genera of the NICU itself and essential caregiving contact between the infant and the parents, and health care providers. Not all of these environmental influences are harmful, yet some were related to an increased risk of obtaining pathogenic microbes. How these factors influence long term similarities or differences in the microbiome of the infant hospitalized in the NICU compared to infants who are discharged home with their family following delivery remains unknown.
What we know:
The environmental surfaces in the NICU, human milk and caregiver skin influences the microbiome of neonates.
Microbes found within the NICU environment resembles microbes identified within the gut microbiome of neonates and may be associated with NEC.
Infection control cleaning practices may decrease infant exposure to disease-causing pathogens.
What needs to be studied:
-
Relationship between the development of the infant microbiome in high-risk and preterm infants and NICU
○ Infection control measures,
○ Antibiotic prescribing practices,
○ Prebiotic and probiotic practices,
○ And caregiving practices such as oral suctioning and the feeding tube type, dwell time and method.
What we can do today:
Follow evidence-based infection control and skin care practices in the NICU to minimize pathogen entry in high-risk preterm infant hosts.
REFERENCES
- 1.Azad MB, Konya T, Maughan H, et al. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ. 2013 Mar 19;185(5):385–394. doi: 10.1503/cmaj.121189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faa G, Gerosa C, Fanni D, Nemolato S, van Eyken P, Fanos V. Factors influencing the development of a personal tailored microbiota in the neonate, with particular emphasis on antibiotic therapy. J. Matern. Fetal Neonatal Med. 2013 Oct;26(Suppl 2):35–43. doi: 10.3109/14767058.2013.829700. [DOI] [PubMed] [Google Scholar]
- 3.Young VB. The intestinal microbiota in health and disease. Current opinion in gastroenterology. 2012 Jan;28(1):63–69. doi: 10.1097/MOG.0b013e32834d61e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012 Sep 13;489(7415):231–241. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ursell LK, Metcalf JL, Parfrey LW, Knight R. Defining the human microbiome. Nutr. Rev. 2012 Aug;70(Suppl 1):S38–44. doi: 10.1111/j.1753-4887.2012.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shafquat A, Joice R, Simmons SL, Huttenhower C. Functional and phylogenetic assembly of microbial communities in the human microbiome. Trends Microbiol. 2014 May;22(5):261–266. doi: 10.1016/j.tim.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saavedra JM, Dattilo AM. Early development of intestinal microbiota: implications for future health. Gastroenterol. Clin. North Am. 2012 Dec;41(4):717–731. doi: 10.1016/j.gtc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Collado MC, Cernada M, Bauerl C, Vento M, Perez-Martinez G. Microbial ecology and host-microbiota interactions during early life stages. Gut Microbes. 2012 Jul-Aug;3(4):352–365. doi: 10.4161/gmic.21215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neu J, Rushing J. Cesarean versus vaginal delivery: long-term infant outcomes and the hygiene hypothesis. Clin. Perinatol. 2011 Jun;38(2):321–331. doi: 10.1016/j.clp.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prince AL, Antony KM, Ma J, Aagaard KM. The Microbiome and Development: A Mother’s Perspective. Semin. Reprod. Med. 2014 Jan;32(1):14–22. doi: 10.1055/s-0033-1361818. [DOI] [PubMed] [Google Scholar]
- 11.Rigon G, Vallone C, Lucantoni V, Signore F. Maternal factors pre- and during delivery contribute to gut microbiota shaping in newborns. Front Cell Infect Microbiol. 2012;2:93. doi: 10.3389/fcimb.2012.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biasucci G, Rubini M, Riboni S, Morelli L, Bessi E, Retetangos C. Mode of delivery affects the bacterial community in the newborn gut. Early Hum. Dev. 2010 Jul;86(Suppl 1):13–15. doi: 10.1016/j.earlhumdev.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Marques TM, Wall R, Ross RP, Fitzgerald GF, Ryan CA, Stanton C. Programming infant gut microbiota: influence of dietary and environmental factors. Curr. Opin. Biotechnol. 2010 Apr;21(2):149–156. doi: 10.1016/j.copbio.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Arrieta MC, Stiemsma LT, Amenyogbe N, Brown EM, Finlay B. The intestinal microbiome in early life: health and disease. Front. Immunol. 2014;5:427. doi: 10.3389/fimmu.2014.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koenig JE, Spor A, Scalfone N, et al. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. U. S. A. 2011 Mar 15;108(Suppl 1):4578–4585. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bokulich NA, Mills DA, Underwood MA. Surface microbes in the neonatal intensive care unit: changes with routine cleaning and over time. J. Clin. Microbiol. 2013 Aug;51(8):2617–2624. doi: 10.1128/JCM.00898-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hewitt KM, Mannino FL, Gonzalez A, et al. Bacterial diversity in two Neonatal Intensive Care Units (NICUs) PLoS One. 2013;8(1):e54703. doi: 10.1371/journal.pone.0054703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ternes YM, Lamaro-Cardoso J, Andre MC, et al. Molecular epidemiology of coagulase-negative Staphylococcus carriage in neonates admitted to an intensive care unit in Brazil. BMC Infect. Dis. 2013;13:572. doi: 10.1186/1471-2334-13-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sankar MJ, Paul VK, Kapil A, et al. Does skin cleansing with chlorhexidine affect skin condition, temperature and colonization in hospitalized preterm low birth weight infants?: a randomized clinical trial. J. Perinatol. 2009 Dec;29(12):795–801. doi: 10.1038/jp.2009.110. [DOI] [PubMed] [Google Scholar]
- 20.Ness MJ, Davis DM, Carey WA. Neonatal skin care: a concise review. Int. J. Dermatol. 2013 Jan;52(1):14–22. doi: 10.1111/j.1365-4632.2012.05687.x. [DOI] [PubMed] [Google Scholar]
- 21.Foca M, Jakob K, Whittier S, et al. Endemic Pseudomonas aeruginosa infection in a neonatal intensive care unit. N. Engl. J. Med. 2000 Sep 7;343(10):695–700. doi: 10.1056/NEJM200009073431004. [DOI] [PubMed] [Google Scholar]
- 22.Gupta A, Della-Latta P, Todd B, et al. Outbreak of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae in a neonatal intensive care unit linked to artificial nails. Infect. Control Hosp. Epidemiol. 2004 Mar;25(3):210–215. doi: 10.1086/502380. [DOI] [PubMed] [Google Scholar]
- 23.Moolenaar RL, Crutcher JM, San Joaquin VH, et al. A prolonged outbreak of Pseudomonas aeruginosa in a neonatal intensive care unit: did staff fingernails play a role in disease transmission? Infect. Control Hosp. Epidemiol. 2000 Feb;21(2):80–85. doi: 10.1086/501739. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez-Carrillo C, Padilla B, Marin M, et al. Contaminated feeding bottles: the source of an outbreak of Pseudomonas aeruginosa infections in a neonatal intensive care unit. Am. J. Infect. Control. 2009 Mar;37(2):150–154. doi: 10.1016/j.ajic.2008.04.259. [DOI] [PubMed] [Google Scholar]
- 25.Gregory KE. Microbiome aspects of perinatal and neonatal health. J. Perinat. Neonatal Nurs. 2011 Apr-Jun;Quiz 163-154;25(2):158–162. doi: 10.1097/JPN.0b013e3182169346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mueller NT, Bakacs E, Combellick J, Grigoryan Z, Dominguez-Bello MG. The infant microbiome development: mom matters. Trends Mol. Med. 2015 Feb;21(2):109–117. doi: 10.1016/j.molmed.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Abouelfettoh A, Ludington-Hoe SM, Burant CJ, Visscher MO. Effect of skin-to-skin contact on preterm infant skin barrier function and hospital-acquired infection. J. Clin. Med. Res. 2011 Feb 12;3(1):36–46. doi: 10.4021/jocmr479w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brooks B, Firek BA, Miller CS, et al. Microbes in the neonatal intensive care unit resemble those found in the gut of premature infants. Microbiome. 2014;2(1):1. doi: 10.1186/2049-2618-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conceicao T, Aires de Sousa M, Miragaia M, et al. Staphylococcus aureus reservoirs and transmission routes in a Portuguese Neonatal Intensive Care Unit: a 30-month surveillance study. Microb Drug Resist. 2012 Apr;18(2):116–124. doi: 10.1089/mdr.2011.0182. [DOI] [PubMed] [Google Scholar]
- 31.Claud EC, Keegan KP, Brulc JM, et al. Bacterial community structure and functional contributions to emergence of health or necrotizing enterocolitis in preterm infants. Microbiome. 2013;1(1):20. doi: 10.1186/2049-2618-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torrazza RM, Ukhanova M, Wang X, et al. Intestinal microbial ecology and environmental factors affecting necrotizing enterocolitis. PLoS One. 2013;8(12):e83304. doi: 10.1371/journal.pone.0083304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stewart CJ, Marrs EC, Nelson A, et al. Development of the preterm gut microbiome in twins at risk of necrotising enterocolitis and sepsis. PLoS One. 2013;8(8):e73465. doi: 10.1371/journal.pone.0073465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Goffau MC, Bergman KA, de Vries HJ, et al. Cold spots in neonatal incubators are hot spots for microbial contamination. Appl. Environ. Microbiol. 2011 Dec;77(24):8568–8572. doi: 10.1128/AEM.06015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferraris L, Butel MJ, Campeotto F, Vodovar M, Roze JC, Aires J. Clostridia in premature neonates’ gut: incidence, antibiotic susceptibility, and perinatal determinants influencing colonization. PLoS One. 2012;7(1):e30594. doi: 10.1371/journal.pone.0030594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milisavljevic V, Garg M, Vuletic I, et al. Prospective assessment of the gastroesophageal microbiome in VLBW neonates. BMC Pediatr. 2013;13:49. doi: 10.1186/1471-2431-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costello EK, Carlisle EM, Bik EM, Morowitz MJ, Relman DA. Microbiome assembly across multiple body sites in low-birthweight infants. MBio. 2013;4(6):e00782–00713. doi: 10.1128/mBio.00782-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fluhr JW, Darlenski R, Taieb A, et al. Functional skin adaptation in infancy - almost complete but not fully competent. Exp. Dermatol. 2010 Jun;19(6):483–492. doi: 10.1111/j.1600-0625.2009.01023.x. [DOI] [PubMed] [Google Scholar]
- 39.Stamatas GN, Nikolovski J, Mack MC, Kollias N. Infant skin physiology and development during the first years of life: a review of recent findings based on in vivo studies. Int J Cosmet Sci. 2011 Feb;33(1):17–24. doi: 10.1111/j.1468-2494.2010.00611.x. [DOI] [PubMed] [Google Scholar]
- 40.Polin RA, Denson S, Brady MT. Strategies for prevention of health care-associated infections in the NICU. Pediatrics. 2012 Apr;129(4):e1085–1093. doi: 10.1542/peds.2012-0145. [DOI] [PubMed] [Google Scholar]
- 41.Mahmood A, Fazal ur R, Chughtai F. A survey of infection control practices in the delivery room and nursery to investigate and control the high rate of neonatal sepsis: an experience at a secondary care hospital. J. Pak. Med. Assoc. 2008 May;58(5):237–240. [PubMed] [Google Scholar]
- 42.New neonatal skin care evidence-based practice guideline. Nurs. Womens Health. 2013 Dec;17(6):545–546. [Google Scholar]
- 43.Afsar FS. Skin care for preterm and term neonates. Clin. Exp. Dermatol. 2009 Dec;34(8):855–858. doi: 10.1111/j.1365-2230.2009.03424.x. [DOI] [PubMed] [Google Scholar]
- 44.Brandon DH, Coe K, Hudson-Barr D, Oliver T, Landerman LR. Effectiveness of No-Sting skin protectant and Aquaphor on water loss and skin integrity in premature infants. J. Perinatol. 2010 Jun;30(6):414–419. doi: 10.1038/jp.2009.174. [DOI] [PubMed] [Google Scholar]
- 45.Arboleya S, Ang L, Margolles A, et al. Deep 16S rRNA metagenomics and quantitative PCR analyses of the premature infant fecal microbiota. Anaerobe. 2012 Jun;18(3):378–380. doi: 10.1016/j.anaerobe.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 46.Guaraldi F, Salvatori G. Effect of breast and formula feeding on gut microbiota shaping in newborns. Front Cell Infect Microbiol. 2012;2:94. doi: 10.3389/fcimb.2012.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernandez L, Langa S, Martin V, et al. The human milk microbiota: origin and potential roles in health and disease. Pharmacol. Res. 2013 Mar;69(1):1–10. doi: 10.1016/j.phrs.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 48.Fouhy F, Ross RP, Fitzgerald GF, Stanton C, Cotter PD. Composition of the early intestinal microbiota: knowledge, knowledge gaps and the use of high-throughput sequencing to address these gaps. Gut Microbes. 2012 May-Jun;3(3):203–220. doi: 10.4161/gmic.20169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torrazza RM, Neu J. The altered gut microbiome and necrotizing enterocolitis. Clin. Perinatol. 2013 Mar;40(1):93–108. doi: 10.1016/j.clp.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]