Abstract
Increasing evidence indicates that cancer stem cells have essential roles in tumor initiation, progression, metastasis and resistance to chemo-radiation. Recent research has pointed out biological importance of microRNAs in cancer stem cell dysregulation. Total number of mature microRNAs in human genome increased to more than 2,500 with the recent up-date of the database. However, currently no information is available regarding microRNA expression profiles of cancer stem cells in head and neck squamous cell carcinoma (HNSCC). Increased ALDH1 activity has been demonstrated as a reliable marker for isolation of cancer stem cells. Therefore, we evaluated the microRNA expression profile of ALDH1-high subpopulations in the HNSCC cell lines UTSCC-9 and UTSCC-90. Initially, we examined cancer stem cell properties of ALDH1-high subpopulations in both cell lines. We analyzed expression of stemness markers, sphere formation capacity and xenograft transplantation into NOD/SCID mice. Our findings validated that ALDH1-high subpopulations showed significantly increased tumor-initiating ability. Furthermore, we investigated the microRNA expression profile of HNSCC stem cells using microRNA array and confirmed the results by quantitative real-time PCR. We found that expressions of miR-424, let-7a, miR-6836, miR-6873 and miR-7152 were downregulated, whereas miR-147b was upregulated with statistical significance in the ALDH1-high subpopulation. In conclusion, we identified a subset of microRNAs that were differentially expressed in ALDH1-high subpopulation, providing new microRNA targets to study dysregulation of HNSCC-initiating cells and develop therapeutic strategies aimed at eradicating the tumorigenic stem cells in HNSCC.
Keywords: head and neck cancer, microRNA, microarray, cancer stem cells, cell line
Introduction
Head and neck squamous cell carcinoma (HNSCC) originates from mucosal epithelial lining of the upper aerodigestive tract. The incidence of the disease has gradually increased over the last three decades, especially associated with HPV infection (1). A typical case presents at an advanced stage as a metastatic cancer, which accounts for the poor mortality rate. Despite the progress in methodology and clinical efficacy of radiotherapy, chemotherapy and surgical approaches up to 50% of patients who die from HNSCC have local or regional recurrence (2). Detailed molecular characterization of HNSCC tumors might help to identify subpopulations for appropriate therapeutic modalities and lead to improvement of the prognosis.
Evidence from xenotransplantation studies revealed that only a small subpopulation in cancer cells has the driving force of cancer growth. Those cells responsible for tumor initiation, carcinogenesis, progression, and metastasis defined to be tumor-initiating cells or cancer stem cells (CSC) (3). In addition, CSCs also possess significant resistance to chemotherapy and radiotherapy (4,5). CSCs have been isolated in several types of epithelial cancers including HNSCC (6). Thus, studies on CSCs may provide clues for improvement of prognosis associated with development of metastases and therapy-resistant local and regional recurrences in HNSCC.
There are a number of methods for isolating and characterizing cancer stem cells, mostly based on fluorescence-activated cell sorting (FACS). Expression of cell surface markers such as CD44, CD133 or drug-detoxifying surface transporter proteins has been used commonly in CSC isolation. However, xenotransplantation study is the gold standard for verification of CSC property, as tumor initiation ability of the isolated cells is evaluated in NOD/SCID mice through serial dilutions (7). Moreover, Aldefluor assay has been developed to isolate CSCs based on aldehyde dehydrogenase 1 (ALDH1) activity (8). Although all these methods were also validated in HNSCC, Aldefluor assay has been found to show higher sensitivity in isolation of CSCs as compared to the expression of cell surface marker, CD44 (9,10).
MicroRNAs (miRNAs) are small non-protein coding RNA molecules, which act to regulate gene expression by post-transcriptional interactions with mRNA. miRNAs are assumed to regulate more than one-third of all genes related with various physiological and pathological processes including carcinogenesis (11). In our previous study, we showed the role of miRNA-200 family in epithelial-mesenchymal-transition, migration and invasion ability of HNSCC (12). Moreover, recent research has indicated the biological importance of miRNAs in CSC dysregulation (13).
In human genome, so far >2,500 mature miRNAs are described according to latest release of miRNA database, miRBase 21 (14). However, currently no data exist concerning miRNA expression profiles of cancer stem cells in HNSCC. Thus, the identification of cancer stem cell-related miRNAs would provide valuable information for a better understanding of cancer stem cell properties and the molecular pathways of oncogenesis.
In the present study, we isolated a subpopulation of HNSCC cells with increased ALDH1 activity and verified their CSC properties in vitro and in vivo conditions. Moreover, we performed microRNA profile analysis to further explore the characteristics and to uncover microRNAs that may serve as novel therapeutic targets in HNSCC.
Materials and methods
Ethics statement
Experimental mice were maintained in accordance with the guidelines, and approval of the Institutional Animal Care and Use Committee of Wakayama Medical University (permit number: 672). Any animal found unhealthy or sick were promptly euthanized.
Cell lines and cultures
UTSCC-9 and UTSCC-90 cell lines (15,16) were kindly provided by Dr R. Grenman (Department of Otolaryngology, Turku University, Finland). UTSCC cells were grown in RPMI-1640 medium supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin and 1 μl/ml amphotericin B (Gibco™, Invitrogen, Japan), and all cell lines were cultured in a humidified incubator with 5% CO2 at 37°C. UTSCC-9 and UTSCC-90 cell lines were established from squamous cell carcinoma of laryngeal carcinoma and tongue carcinoma, respectively.
Aldefluor assay and fluorescence-activated cell sorting (FACS)
We used an Aldefluor assay kit (Stem Cell Technologies™, Vancouver, Canada) to determine ALDH1 activity of cells according to the manufacturer's protocol. Cells were suspended in Aldefluor assay buffer containing 1 μmol/l per 1×106 cells of the ALDH substrate, boron-dipyrromethene-aminoacetaldehyde (BAAA), and incubated for 45 min at 37°C. Each sample was treated with 45 mmol/l of an ALDH-specific inhibitor, diethylaminobenzaldehyde (DEAB), as a negative control. Stained cells were analyzed by BD FACSAria I™ (BD Biosciences, San Jose, CA, USA). Cells were stained with 1 μg/ml of propidium iodide to evaluate their viability prior to analysis. The brightly fluorescent ALDH1-expressing cells (ALDH1high) were detected in the green fluorescence channel (520–540 nm).
Xenograft transplantation
ALDH1high and ALDH1low cells were isolated by FACS and resuspended at 5.0×103 cells in 100 μl PBS and Matrigel (BD Biosciences) mixture (1:1). Then each mixture was injected subcutaneously into the right/left middle back areas of 6-week-old female non-obese diabetic/severe combined immune-deficiency (NOD/SCID) mice (NOD/ShiJic-scid Jcl, Clea-Japan, Tokyo, Japan) under inhalation anesthesia by isoflurane. Tumor initiation and progression were observed weekly and external tumor volume was calculated as 0.5 × Dmax × (Dmin)2 [mm3] (Dmax:long axis, Dmin:short axis of mass).
Sphere formation assay
ALDH1high and ALDH1low cells were isolated by FACS and then cultured in 6-well ultra-low attachment surface dishes (Corning, Tewksbury, MA, USA) at 5,000 cells per well. The cells were cultured in stem-cell medium, serum-free DMEM/F12 (Life Technologies) supplemented with N-2 supplement (Life Technologies), 10 ng/ml recombinant human epithelial growth factor (R&D Systems, Minneapolis, MN, USA), 10 ng/ml human basic fibroblast growth factor (R&D Systems). Morphological change was observed daily under a light microscope for 28 days. Round cell clusters >100 μm were judged as spheres.
mRNA processing and quantitative real-time PCR
Preparation of cDNA from mRNA was performed directly from cultured cell lysate using the TaqMan® Gene Expression Cells-to-CT™ kit (Ambion, Japan), according to the manufacturer's instructions. Cell lysate were reverse transcribed to cDNA using the Reverse Transcription (RT) Enzyme Mix and appropriate RT buffer (Ambion). Finally the cDNA was amplified by quantitative real-time PCR (qRT-PCR) using the included TaqMan Gene Expression Master Mix and the specific TaqMan primer/probe assay designed for the investigated genes: ALDH1A1 (Hs00946916_m1), NANOG (Hs02387400_g1), OCT4 (Hs03005111_g1), SOX2 (Hs01053049_s1) and GAPDH (Hs99999905_m1), (Applied Biosystems, Tokyo, Japan).
The gene expression levels were normalized to the expression of the housekeeping gene GAPDH and were expressed as fold changes relative to the expression of the untreated cells. The amplification profile was initiated by 10-min incubation at 95°C, followed by two-step amplification of 15 sec at 95°C and 60 sec at 60°C for 40 cycles. All experiments were performed including non-template controls to exclude contamination of the reagents. Quantification was done with the delta/delta Ct calculation method (17). Each sample was analyzed in triplicate on the 7500 Real-Time PCR system in a 96-well plate (Applied Biosystems).
MicroRNA processing and qRT-PCR
Preparation of cDNA from microRNA was also performed directly from cultured cell lysate using the TaqMan® MicroRNA Cells-to-CT™ kits (Ambion) according to the manufacturer's instructions. Cell lysate was reverse transcribed to cDNA using the RT Enzyme Mix, RT buffer and appropriate RT primer (Applied Biosystems). Finally the cDNA of each mature microRNA was amplified separately by qRT-PCR using the TaqMan Universal Master Mix and the specific primer and probe mix included in predesigned TaqMan MicroRNA assays: hsa-miR-424; ID: 000604, hsa-miR-4730; ID: 462061_mat, hsa-miR-6836-5p; ID: 467207_mat, hsa-miR-6873-5p; ID: 467097_mat, hsa-miR-7152-3; ID: 466958_mat, hsa-hsa-let-7a; ID: 000377, hsa-miR-147b; ID: 002262, hsa-miR-3622a-3p; ID: 464955_mat, hsa-miR- miR-1976; ID: 121207_mat (Applied Biosystems). Calculation of microRNA expression was performed with the same method as described above and for normalization RNU6B RNA (RNU6B; ID: 001093, P/N: 4373381, Applied Biosystems) was used as internal control. Each sample was analyzed in triplicate.
Total RNA extraction and microarray profiling of miRNAs
Total RNA was isolated and purified from cell lines using miRNeasy Mini kit (Qiagen, Tokyo, Japan) as recommended by the manufacturer. RNA yield and quality was determined using the Agilent RNA 6000 Nano kit and Agilent 2100 Bioanalyzer (Agilent Technologies, Inc.). For all samples RNA integrity number (RIN) was >7.
Affymetrix GeneChip miRNA 4.0 array were used for miRNA profiling. Total RNA (1 μg per sample) was labeled using the FlashTag™ Biotin HSR RNA Labeling kit (Affymetrix, Inc., USA) as described by the manufacturer. The samples were hybridized on GeneChip miRNA 4.0 arrays (Affymetrix, Inc.) for 18 h at 48°C. Arrays were washed to remove non-specifically bound nucleic acids and stained on Fluidics Station 450 (Affymetrix, Inc.) and then scanned on GeneChip Scanner 3000 7G system (Affymetrix, Inc.). Finally, Microarray Data Analysis Tool version 3.2 (Filgen, Inc., Japan) was used as described in the manufacturer's instructions for all subsequent data analysis.
Statistical analysis
Data were analyzed with GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA). To evaluate significant differences between two matched pair groups or between two independent groups of samples, paired t-tests and non-paired t-tests were used, respectively. Pearson's correlation coefficient (r) was used to measure correlation, and logarithmic regression was used to calculate the R2 and to create the equation of the slope.
Results
Isolation of ALDH1high cells from HNSCC cell lines
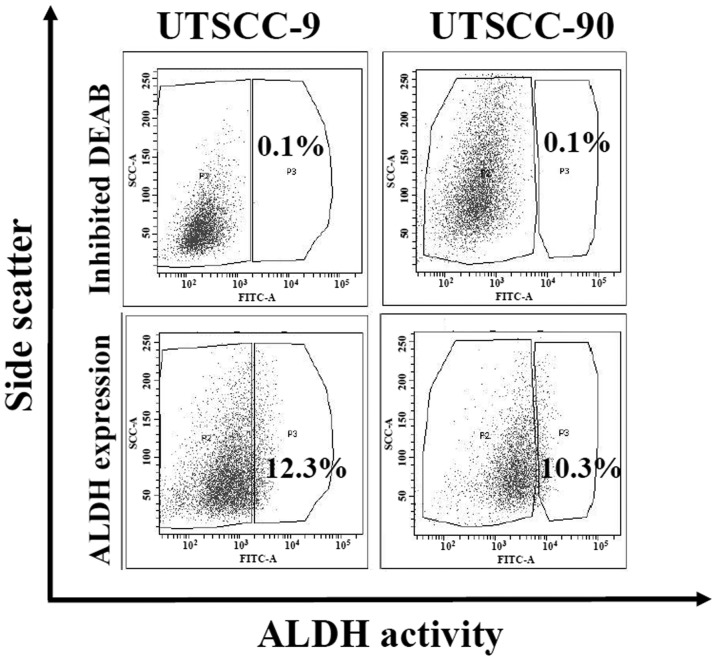
ALDH1 activity is demonstrated to be a reliable marker for the isolation of cancer stem cells in several cancers including HNSCC (10). We analyzed ALDH activity by Aldefluor assay in two human HNSCC cell lines (UTSCC-9 and UTSCC-90). Cancer cells were sorted and isolated into two subpopulations as ALDH1 high (ALDH1high) and low (ALDH1low) by flow cytometry. Proportion of ALDH1high cells was found to be 12.3 and 10.3% for UTSCC-9 and UTSCC-90 cells, respectively (Fig. 1). These results were also confirmed by quantitative real-time PCR (qPCR) analysis. mRNA expression of ALDH1 was significantly higher in ALDH1high cells as compared to ALDH1low in both UTSCC-9 and UTSCC-90 cells (Fig. 2).
Figure 1.
Isolation of ALDH1high cells from HNSCC cell lines. ALDH activity of two human HNSCC cell lines (UTSCC-9 and UTSCC-90) was analyzed by Aldefluor assay. Proportion of ALDH1high cells was isolated using FACS Aria I.
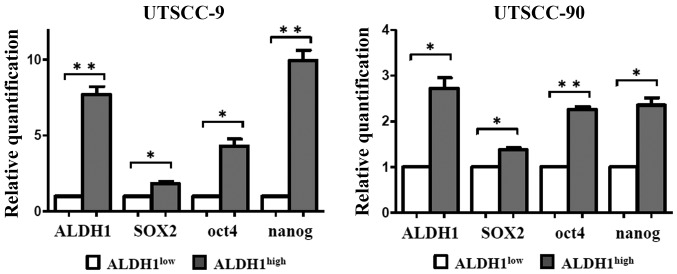
Figure 2.
Expression of stem cell markers in ALDH1high and ALDH1low cells. mRNA purified from UTSCC-9 and UTSCC-90 cells were analyzed by real-time PCR. mRNA expression of stem cell markers including ALDH1, SOX2, Nanog and Oct4 were compared between ALDH1low and ALDH1high cells. The horizontal bars represent the mean expression levels. Error bars represent SD; *P<0.05, **P<0.01, by paired t-test.
Characterization of ALDH1high cells
To analyze whether ALDH1high cells represent the CSC population of HNSCC, several characteristics related to stem cell were evaluated and compared between ALDH1high and ALDH1low portions both for UTSCC-9 and UTSCC-90 cells.
First, the expression levels of stem cell markers including SOX2, Nanog and Oct4 were investigated by qPCR. The ALDH1high population of both HNSCC cell lines demonstrated increased expression levels of SOX2, Nanog and Oct4 as compared to ALDH1low cells with statistical significance (Fig. 2). The results were reproduced in three independent experiments. These results verified that ALDH1high cells derived from each cell line has similar genetic properties with CSCs.
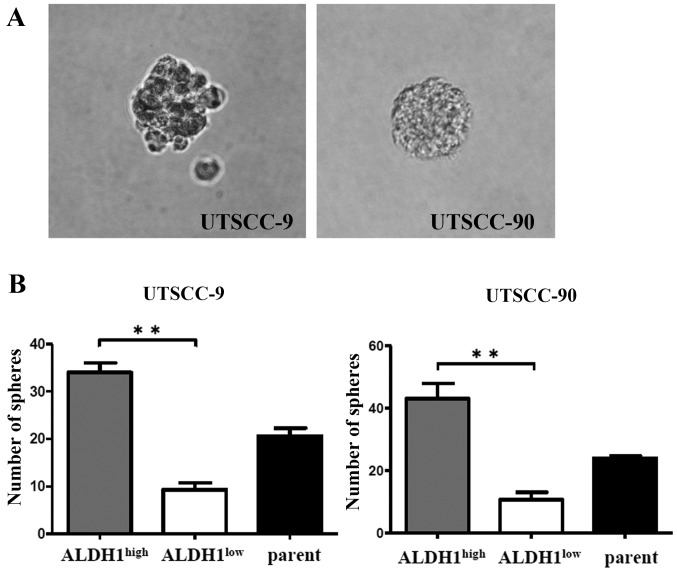
CSCs are known to be able to grow in floating culture conditions as non-adherent, three-dimensional sphere clusters (spheres) (18). Therefore, sphere formation ability of ALDH1high population was compared with ALDH1low and unsorted parent cells of UTSCC-9 and UTSCC-90. Sorted 5×103 cells per well were incubated into a 6-well plate in an anchorage-independent environment. The sphere formation assays were repeated in three independent experiments. Total number of spheres per well were counted after 28 days, and used to calculate the mean number of spheres.
ALDH1high cells derived from UTSCC-9 cells exhibited significantly increased sphere formation efficiency compared to ALDH1low cells (P<0.01). Similar results were obtained also for UTSCC-90 cells (P<0.01) (Fig. 3).
Figure 3.
Sphere formation assay from UTSCC9 and UTSCC90. (A) Sphere formation of ALDH1high cells after cultivation in serum-free cell culture medium. (B) After ALDH1high, ALDH1low and parent 5×103 cells were cultured in 6-well ultra-low attachment dishes, total number of spheres per well was counted. The horizontal bars represent the mean expression levels; *P<0.05, **P<0.01, by unpaired t-test.
Increased tumorigenicity of ALDH1high cells
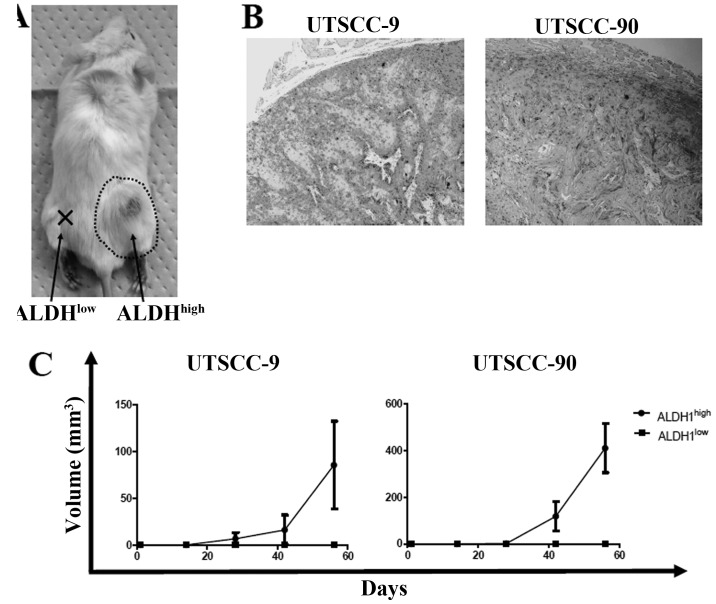
To address the in vivo tumorigenicity of ALDH1high and ALDH1low cells, xenograft transplantation was performed into four NOD/SCID mice for each cell line group (UTSCC-9 and UTSCC-90). Sorted 5×103 cells from ALDH1high and ALDH1low cells were injected simultaneously into the subcutaneous region of right and left back sites for each NOD/SCID mice, respectively. One mouse in UTSCC-9 group died in the third week without any tumor detection. In UTSCC-90 group, ALDH1high cells produced tumors in all four mice, while ALDH1low cells did not produce any tumor. Similarly, tumor formation was observed in 2 of the 3 mice for ALDH1high cells, whereas no tumor occurred in sites injected with ALDH1low cells in UTSCC-9 group (Fig. 4A and C). The histology of tumors formed in NOD/SCID mice was verified to be squamous cell carcinoma (Fig. 4B). These results confirmed higher tumor-initiating ability of ALDH1high cells compared to ALDH1low cells in vivo.
Figure 4.
Tumorigenicity of ALDH1high cells. (A) Representative tumor growth in NOD/SCID mice at the ALDH1high cell injection site (5×103 cells injected). ALDH1high and ALDH1low cells were inoculated subcutaneously into the left and right side of the back, respectively. (B) Histology images of ALDH1high tumors in mice (stained by hematoxylin and eosin). (C) Tumor growth in NOD/SCID mice by ALDH1high and ALDH1low subpopulations of UTSCC-9 and UTSCC-90 cells (5×103 cells injected). Data are reported as means ± SD.
MicroRNA expression profiles of ALDH1high vs ALDH1low cells
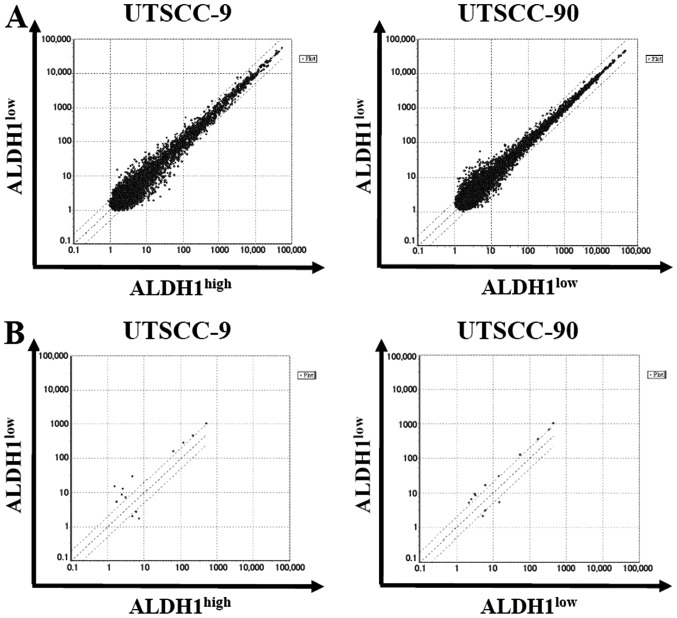
In order to characterize the miRNA expression profile that regulates genes involved in cancer stem cells of HNSCC, we performed a microarray analysis in UTSCC-9 and UTSCC-90 cells. Total RNA samples were prepared from ALDH1high vs ALDH1low subpopulations of each cell type. GeneChip miRNA 4.0 array (Affymetrix, Inc.) was used which contains 2,578 human mature miRNA probe sets annotated in the miRBase 20 database. One hybridization reaction was performed for each cell type.
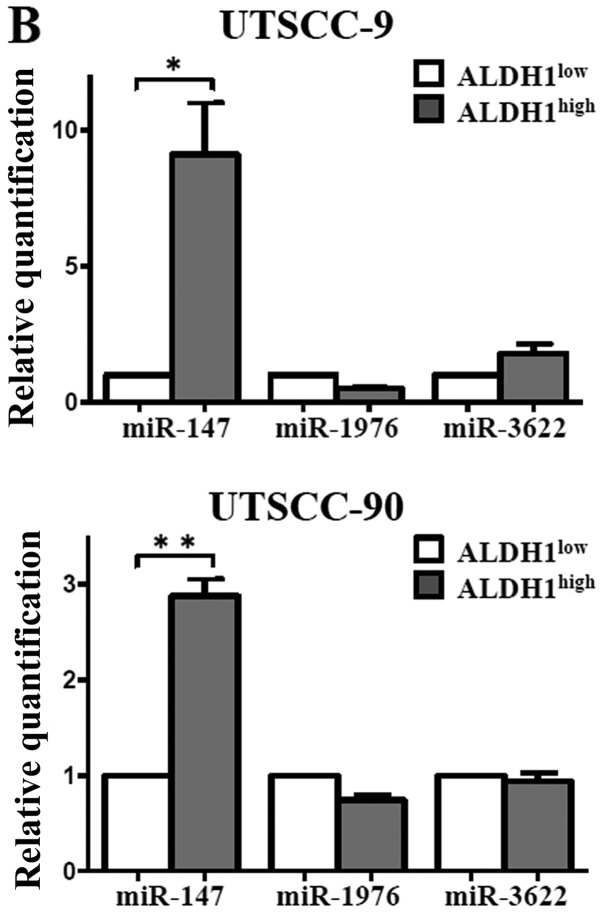
By using stringent significance criteria of a ≥2-fold difference in expression level and P<0.05, we identified that nine miRNAs were differentially expressed between ALDH1high and ALDH1low subpopulations (Fig. 5). In ALDH1high cells, six out of nine miRNAs, including miR-424, let-7a, miR-4730, miR-6836, miR-6873, miR-7152 were significantly downregulated, while three miRNAs including miR-147b, miR-3622a-3p and miR-1976 were significantly upregulated.
Figure 5.
microRNA expression profiles in UTSCC-9 and UTSCC-90. (A) Scatter plot representation of whole mature miRNAs between ALDH1high (x-axis) vs ALDH1low cells (y-axis). (B) Scatter plot representation of differentially expressed miRNAs according to threshold of ≥2-fold difference between ALDH1high (x-axis) vs ALDH1low cells (y-axis).
Validation of microarray results by qPCR
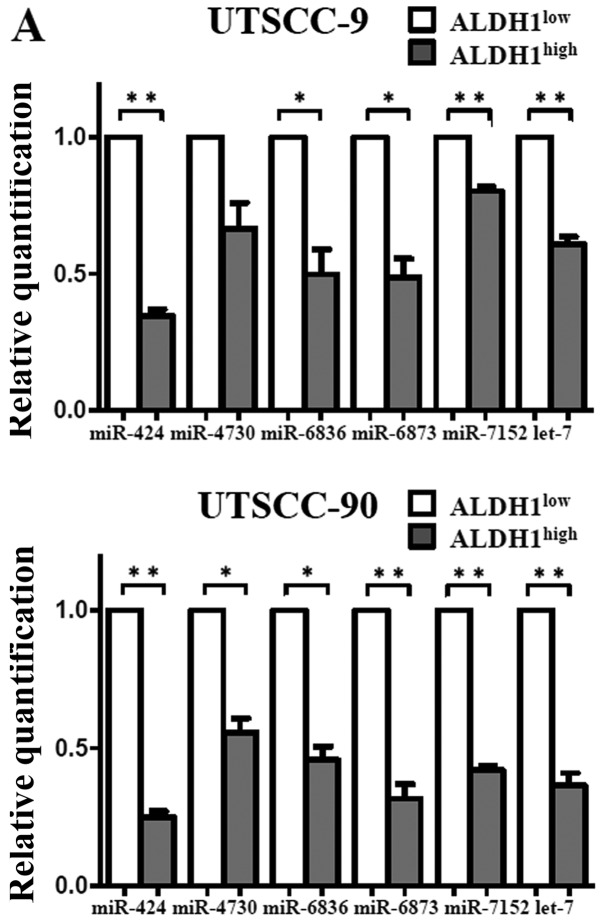
To validate the microarray results, qPCR was performed with nine miRNAs that exhibited >2-fold change in expression. In agreement with the microarray results, expression of miR-424, let-7a, miR-6836, miR-6873 and miR-7152 were downregulated, whereas miR-147b was upregulated with statistical significance in the ALDH1high subpopulation (Fig. 6). Thus, the miRNA expression profiles of the cancer stem cells in HNSCC were confirmed by qPCR.
Figure 6.
Real-time PCR analysis of microRNAs differentially expressed in microarray analysis. (A) Upregulated miRNAs in ALDH1high compared to ALDH1low cells: miR-424, let-7a, miR-4730, miR-6836, miR-6873 and miR-7152. (B) Downregulated miRNAs in ALDH1high compared to ALDH1low cells. The horizontal bars represent the mean expression levels; *P<0.05, **P<0.01, by paired t-test.
Discussion
In the present study, we found that the subpopulation of HNSCC cells with increased ALDH1 activity (ALDH1high) represent cancer stem cells of HNSCC. Furthermore, we also revealed that ALDH1high HNSCC cells had a distinct microRNA expression profile as compared to ALDH1low cells. These findings might contribute to understanding miRNA-driven pathways related to the tumor-initiating capability of HNSCC.
Initially, we tested whether ALDH1high subpopulations of two HNSCC cell lines, UTSCC-9 and UTSCC-90, have cancer stemness properties. We isolated ALDH1high subpopulation by FACS and evaluated their stem cell features under in vitro and in vivo conditions. Results of our experiments including expression of stem cell markers (stemness-related genes), sphere formation capacity and xenograft transplantation into NOD/SCID mice supplied evidence that increased ALDH1 activity in HNSCC presents a subpopulation with high tumor-initiating ability.
Several types of approaches have been reported to isolate CSC fraction of cancer cells (19). CD44 is the first and most widely used cell surface biomarker to identify CSC in HNSCC (6). However, validity of CD44 has been questioned recently, since it is abundantly expressed in carcinoma cells and normal epithelium of head and neck region (20,21). Furthermore, Clay et al proposed a new hypothesis on the relation of CD44 and ALDH1 with the cancer stem cell compartment in HNSCC. They suggest that ALDH-high subpopulation of HNSCC cells comprise only a minor fraction among the CD44+ cells, whereas more efficiently represent the CSC subpopulation itself (10). Recently, other reports also demonstrated ALDH1 expression as a reliable method to identify CSC in various epithelial cancers and HNSCC (9,22). Our observations are consistent with previous data on the role of ALDH1 as a promising marker for identification of CSC.
Recently several studies have pointed out the essential role of miRNAs in regulation of CSCs in etiology and progression of various cancers including HNSCC (26–35). Total number of human mature microRNAs has increased remarkably from 1,105 to 2,578 according to the miRBase database in the last 5 years (14). However, only a limited number of studies have been reported on microRNA expression profiles of cancer stem cells in a few types of cancers (23,24). Additionally, almost all of them were designed according to the previous and narrower version of probe entries. Accordingly, any miRNA signature has not been evaluated in HNSCC yet. Only one recent report, evaluated the relation of miRNAs with the radiosensitivity in laryngeal cancer stem cells represented by CD133+ sphere forming subpopulations (25). They studied the alterations of miRNAs before and after radiotherapy exposure in only one cell line, whereas expression profile of miRNAs specific to cancer stem cell itself was not investigated.
In the next step, we evaluated microRNA expression profile of ALDH1high HNSCC cells. The results of microarray analysis in the cell lines of HNSCC showed that nine miRNAs were differentially expressed in ALDH1high HNSCC cells compared to ALDH1low cells. Among them, decreased expression of miR-424, let-7a, miR-4730, miR-6836, miR-6873, miR-7152 and overexpression of miR-147 were confirmed by qPCR assay, confirming the array results. This miRNA expression profile indicates stemness characteristics of HNSCC. Thus, miRNA regulation is intricately related to distinctive features and the biological performance of HNSCC cancer stem cells. By defining miRNAs related to the characteristics of HNSCC stem cells, we will be able to further clarify the regulatory pathway and gain insight into the features of these cancers.
Concerning miRNAs differentially expressed in our ALDH1high cells, there is no data currently in the literature on the function of four miRNAs (miR-4730, miR-6836, miR-6873 and miR-7152). This is due to the fact that these are novel miRNAs revealed by just the recent version of miRBase 20 (14). On the other hand, the role of miR-424, let-7a and miR-147 in carcinogenesis has been described by several studies.
miR-424 has been found to be downregulated in various type of cancers and it is proposed to act as a tumor suppressor in carcinogenesis (26,27). Our data are the first on the expression status of miR-424 in HNSCC, while its downregulation was also observed in squamous cell carcinoma of the cervical region (28). miR-424 is predicted, by TargetScan, to target many mRNAs and recent studies have already validated important targets in cancer cells. miR-424 inhibits Wee1 and Chk1 related with chemo-resistance and radio-resistance in cancer cells (29). It also targets inhibitor of beta-catenin and TCF-4 (ICAT) and leads to inhibition of epithelial-mesenchymal transition (EMT) in hepatocellular carcinoma cells (30). Based on these findings, it is essential to clarify the role of miR-424 in regulation of CSC of HNSCC.
The let-7 family has been widely studied to have an essential function in stem cell differentiation and tumor-suppressive activity. Recently, let-7a was shown to negatively modulate the expression of stemness genes, and inhibit chemo-resistance and tumor initiating ability of ALDH1 (+) cell population in HNSCC (31). Our findings supported the tumor suppressive role of let-7a through cancer stem cells in HNSCC.
Profiling studies demonstrated that miR-147 was significantly overexpressed in gastric cancers and tongue cancers, consistent with our findings (32,33), contrarily it was shown to be underexpressed in colon carcinomas (34). Interestingly, miR-147 was also found significantly associated with chemo-resistance in small cell lung cancer (35).
The limitation of this study is the lack of functional studies to verify the potential role of those miRNAs differentially expressed in ALDH1high HNSCC cells. As a further step, designs of loss-of-function and gain-of-function experiments are required for each miRNA to evaluate their effect on cancer stem-like phenotypes such as drug resistance and cell invasion.
In conclusion, we validated cancer stem cell properties of ALDH1high subpopulation in HNSCC through in vivo and in vitro experiments. Furthermore, we identified a subset of miRNAs that were differentially expressed in ALDH1high subpopulation of HNSCC. These findings may provide new microRNA targets to study dysregulation of HNSCC-initiating cells and develop therapeutic strategies aimed at eradicating the tumorigenic subpopulation of cells in HNSCC. Thus, further investigation is required to validate the role of these microRNA candidates in HNSCC.
Acknowledgements
This study was supported in part by Doctorate Program Research Grant of Wakayama Medical University.
References
- 1.Dufour X, Beby-Defaux A, Agius G, Lacau St Guily J. HPV and head and neck cancer. Eur Ann Otorhinolaryngol Head Neck Dis. 2012;129:26–31. doi: 10.1016/j.anorl.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Marur S, Forastiere AA. Head and neck cancer: Changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83:489–501. doi: 10.4065/83.4.489. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: An evolving concept. Nat Rev Cancer. 2012;12:133–143. doi: 10.1038/nrc3184. [DOI] [PubMed] [Google Scholar]
- 4.Bertolini G, Roz L, Perego P, Tortoreto M, Fontanella E, Gatti L, Pratesi G, Fabbri A, Andriani F, Tinelli S, et al. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci USA. 2009;106:16281–16286. doi: 10.1073/pnas.0905653106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumann M, Krause M, Hill R. Exploring the role of cancer stem cells in radioresistance. Nat Rev Cancer. 2008;8:545–554. doi: 10.1038/nrc2419. [DOI] [PubMed] [Google Scholar]
- 6.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Brien CA, Kreso A, Jamieson CH. Cancer stem cells and self-renewal. Clin Cancer Res. 2010;16:3113–3120. doi: 10.1158/1078-0432.CCR-09-2824. [DOI] [PubMed] [Google Scholar]
- 8.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen YC, Chen YW, Hsu HS, Tseng LM, Huang PI, Lu KH, Chen DT, Tai LK, Yung MC, Chang SC, et al. Aldehyde dehydrogenase 1 is a putative marker for cancer stem cells in head and neck squamous cancer. Biochem Biophys Res Commun. 2009;385:307–313. doi: 10.1016/j.bbrc.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 10.Clay MR, Tabor M, Owen JH, Carey TE, Bradford CR, Wolf GT, Wicha MS, Prince ME. Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck. 2010;32:1195–1201. doi: 10.1002/hed.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shcherbata HR, Hatfield S, Ward EJ, Reynolds S, Fischer KA, Ruohola-Baker H. The MicroRNA pathway plays a regulatory role in stem cell division. Cell Cycle. 2006;5:172–175. doi: 10.4161/cc.5.2.2343. [DOI] [PubMed] [Google Scholar]
- 12.Tamagawa S, Beder LB, Hotomi M, Gunduz M, Yata K, Grenman R, Yamanaka N. Role of miR-200c/miR-141 in the regulation of epithelial-mesenchymal transition and migration in head and neck squamous cell carcinoma. Int J Mol Med. 2014;33:879–886. doi: 10.3892/ijmm.2014.1625. [DOI] [PubMed] [Google Scholar]
- 13.DeSano JT, Xu L. MicroRNA regulation of cancer stem cells and therapeutic implications. AAPS J. 2009;11:682–692. doi: 10.1208/s12248-009-9147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozomara A, Griffiths-Jones S. miRBase: Annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014;42(D1):D68–D73. doi: 10.1093/nar/gkt1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minn H, Clavo AC, Grénman R, Wahl RL. In vitro comparison of cell proliferation kinetics and uptake of tritiated fluorode-oxyglucose and L-methionine in squamous-cell carcinoma of the head and neck. J Nucl Med. 1995;36:252–258. [PubMed] [Google Scholar]
- 16.Lansford CD, Grenman R, Bier H, Somers KD, Kim S-Y, Whiteside TL, Clayman GL, Welkoborsky H-J, Carey TE. Head and neck cancers. In: Masters JR, Palsson B, editors. Human Cell Culture, Vol. 2, Cancer Cell Lines Part 2. Norwell: Kluwer Academic Publishers; 1999. pp. 185–255. [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (−Delta Delta C (T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Pastrana E, Silva-Vargas V, Doetsch F. Eyes wide open: A critical review of sphere-formation as an assay for stem cells. Cell Stem Cell. 2011;8:486–498. doi: 10.1016/j.stem.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Major AG, Pitty LP, Farah CS. Cancer stem cell markers in head and neck squamous cell carcinoma. Stem Cells Int. 2013;2013:319489. doi: 10.1155/2013/319489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen C, Wei Y, Hummel M, Hoffmann TK, Gross M, Kaufmann AM, Albers AE. Evidence for epithelial-mesenchymal transition in cancer stem cells of head and neck squamous cell carcinoma. PLoS One. 2011;6:e16466. doi: 10.1371/journal.pone.0016466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mack B, Gires O. CD44s and CD44v6 expression in head and neck epithelia. PLoS One. 2008;3:e3360. doi: 10.1371/journal.pone.0003360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng S, Yang X, Lassus H, Liang S, Kaur S, Ye Q, Li C, Wang LP, Roby KF, Orsulic S, et al. Distinct expression levels and patterns of stem cell marker, aldehyde dehydrogenase isoform 1 (ALDH1), in human epithelial cancers. PLoS One. 2010;5:e10277. doi: 10.1371/journal.pone.0010277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nam EJ, Lee M, Yim GW, Kim JH, Kim S, Kim SW, Kim YT. MicroRNA profiling of a CD133 (+) spheroid-forming subpopulation of the OVCAR3 human ovarian cancer cell line. BMC Med Genomics. 2012;5:18. doi: 10.1186/1755-8794-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun JG, Liao RX, Qiu J, Jin JY, Wang XX, Duan YZ, Chen FL, Hao P, Xie QC, Wang ZX, et al. Microarray-based analysis of microRNA expression in breast cancer stem cells. J Exp Clin Cancer Res. 2010;29:174. doi: 10.1186/1756-9966-29-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang CX, Zhu Y, Duan GL, Yao JF, Li ZY, Li D, Wang QQ. Screening for MiRNAs related to laryngeal squamous carcinoma stem cell radiation. Asian Pac J Cancer Prev. 2013;14:4533–4537. doi: 10.7314/APJCP.2013.14.8.4533. [DOI] [PubMed] [Google Scholar]
- 26.Oneyama C, Kito Y, Asai R, Ikeda J, Yoshida T, Okuzaki D, Kokuda R, Kakumoto K, Takayama K, Inoue S, et al. MiR-424/503-mediated Rictor upregulation promotes tumor progression. PLoS One. 2013;8:e80300. doi: 10.1371/journal.pone.0080300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu J, Li Y, Wang F, Wang X, Cheng B, Ye F, Xie X, Zhou C, Lu W. Suppressed miR-424 expression via upregulation of target gene Chk1 contributes to the progression of cervical cancer. Oncogene. 2013;32:976–987. doi: 10.1038/onc.2012.121. [DOI] [PubMed] [Google Scholar]
- 28.Cheung TH, Man KN, Yu MY, Yim SF, Siu NS, Lo KW, Doran G, Wong RR, Wang VW, Smith DI, et al. Dysregulated microRNAs in the pathogenesis and progression of cervical neoplasm. Cell Cycle. 2012;11:2876–2884. doi: 10.4161/cc.21278. [DOI] [PubMed] [Google Scholar]
- 29.Pouliot LM, Chen YC, Bai J, Guha R, Martin SE, Gottesman MM, Hall MD. Cisplatin sensitivity mediated by WEE1 and CHK1 is mediated by miR-155 and the miR-15 family. Cancer Res. 2012;72:5945–5955. doi: 10.1158/0008-5472.CAN-12-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Li T, Guo P, Kang J, Wei Q, Jia X, Zhao W, Huai W, Qiu Y, Sun L, et al. MiR-424-5p reversed epithelial-mesenchymal transition of anchorage-independent HCC cells by directly targeting ICAT and suppressed HCC progression. Sci Rep. 2014;4:6248. doi: 10.1038/srep06248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu CC, Chen YW, Chiou GY, Tsai LL, Huang PI, Chang CY, Tseng LM, Chiou SH, Yen SH, Chou MY, et al. MicroRNA let-7a represses chemoresistance and tumourigenicity in head and neck cancer via stem-like properties ablation. Oral Oncol. 2011;47:202–210. doi: 10.1016/j.oraloncology.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Yao Y, Suo AL, Li ZF, Liu LY, Tian T, Ni L, Zhang WG, Nan KJ, Song TS, Huang C. MicroRNA profiling of human gastric cancer. Mol Med Rep. 2009;2:963–970. doi: 10.3892/mmr_00000199. [DOI] [PubMed] [Google Scholar]
- 33.Wong TS, Liu XB, Wong BY, Ng RW, Yuen AP, Wei WI. Mature miR-184 as potential oncogenic microRNA of squamous cell carcinoma of tongue. Clin Cancer Res. 2008;14:2588–2592. doi: 10.1158/1078-0432.CCR-07-0666. [DOI] [PubMed] [Google Scholar]
- 34.Gaedcke J, Grade M, Camps J, Søkilde R, Kaczkowski B, Schetter AJ, Difilippantonio MJ, Harris CC, Ghadimi BM, Møller S, et al. The rectal cancer microRNAome - microRNA expression in rectal cancer and matched normal mucosa. Clin Cancer Res. 2012;18:4919–4930. doi: 10.1158/1078-0432.CCR-12-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ranade AR, Cherba D, Sridhar S, Richardson P, Webb C, Paripati A, Bowles B, Weiss GJ. MicroRNA 92a-2*: A biomarker predictive for chemoresistance and prognostic for survival in patients with small cell lung cancer. J Thorac Oncol. 2010;5:1273–1278. doi: 10.1097/JTO.0b013e3181dea6be. [DOI] [PubMed] [Google Scholar]