Abstract
Disturbances, both human-induced and natural, may re-shape ecosystems by influencing their composition, structure, and functional processes. Plateau zokor (Eospalax baileyi) is a typical subterranean rodent endemic to Qinghai-Tibetan Plateau (QTP), which are considered ecosystem engineers influencing the alpine ecosystem function. It is also regarded as a pest aggravating the degradation of overgrazed grassland and subject to regular control in QTP since 1950s. Climate change has been predicted in this region but little research exists exploring its impact on such subterranean rodent populations. Using plateau zokor as a model, through maximum entropy niche-based modeling (Maxent) and sustainable habitat models, we investigate zokor habitat dynamics driven by the future climate scenarios. Our models project that zokor suitable habitat will increase by 6.25% in 2050 in QTP. The predication indicated more threats in terms of grassland degradation as zokor suitable habitat will increase in 2050. Distribution of zokors will shift much more in their southern range with lower elevation compare to northern range with higher elevation. The estimated distance of shift ranges from 1 km to 94 km from current distribution. Grassland management should take into account such predictions in order to design mitigation measures to prevent further grassland degradation in QTP under climate change scenarios.
Introduction
Disturbances, both human-induced and natural, may reshape ecosystems by influencing their composition, structure, and functional processes [1, 2, 3]. Climate change, one of the key natural disturbances affecting populations of organisms, has long been considered the greatest challenge to natural and biodiversity conservation [4, 5, 6]. It is widely believed that both historical and contemporary climate change affect populations of organisms. Historical climate change have contributed to the evolutionary process of organisms and even triggered the speciation process [7, 8]. Current and future climate changes are predicted to potentially affect population size, survival and distribution of organisms [4, 9, 10]. Climate has long been considered critical in altering population dynamics through altering the factors such as population growth rate, migration and overwintering [11, 12]. Distributions of mountain biota are predicted to move toward poles or higher altitude as a result of global climate warming [1, 13]. Indeed, many insect species have shifted their ranges to higher latitudes and altitudes during recent climate warming [14].
However, existing studies that investigate the effect of climate change mainly focus on endangered or invasive species [15, 16, 17]. Studies on non-threatened native species are scarce. In areas where the natural ecosystems suffer from degradation due to anthropomorphic disturbances, some native animals are known to become problematic and aggravate the degradation [18]. With the ongoing disturbances from climate changes, many native species potentially become problematic causing further challenges for management of ecosystems and biodiversity [19]. Understanding the effects of climate change on these species is essential for current and future species management and biodiversity conservation under the ongoing climate change scenarios.
Climate change can potentially affect both aboveground and subterranean biota. It is reported that the distribution of subterranean species have been affected by historical climatic factors [20]. But the knowledge on the influence of current and future climate changes on subterranean species is scarce [21].
The Qinghai-Tibetan Plateau (QTP), the largest plateau in the world, occupying 2.5 million km2 with an average altitude of 4000 m above sea level, is a region sensitive to climate changes. QTP is also one of the largest rangeland areas in the world. Like many alpine grassland in world, large areas of rangeland in QTP suffer various degree of grassland degradation [22]. On QTP, large areas are designated as protected conservation area, in which hunting endangered species are prohibited. However, except small proportion of these areas that have been totally protected (core areas), large proportion of these areas are still inhabited by local farming communities where alpine grassland are used for farming (yak, sheep and horse etc) [22, 23]. Rangeland in these areas are degraded and overgrazing is considered the main factor causing grassland degradation, which is aggravated by activities of small mammals such as pika [24] and subterranean rodents such as zokors [23].
Alpine zokors of subfamily Mysopalacinae include two genera, Eospalax and Myospalax. They are typical subterranean rodents that inhabit alpine prairie, meadow and farmland [25, 26, 27]. When present at natural densities, they play important role in the ecosystem function, such as nutrient cycling, soil structure and vegetation composition, and are regarded as ecosystem engineers [28]. However, in some degraded rangeland, they are known to form dense populations, reducing crop production, competing with livestock, causing soil erosion and contributing to further rangeland degradation [23]. Large scale culling of zokors using toxins has been the main method for controlling zokor populations and such control are often conducted in sensitive and fragile ecosystems such as steppe and semi-desert grasslands. Significant population reduction or eradication of these species has had significant negative impacts on biodiversity and potentially on other ecosystem aspects and function such as soil structure, nutrient cycling, tropic interactions and plant community structure [25].
Despite the control, this species continue to challenge rangeland management due to their roles in grassland ecosystem health. Climate change is known to have affected distribution and life history characteristics of many species, especially species that inhabit alpine regions [29]. The potential effect of climate change on zokor populations has not been examined. Such study is important for effectively planning the future management and preventing further degradation of alpine grassland ecosystem.
The distribution and population density of zokors are limited by elevation, precipitation, vegetation and anthropogenic disturbance. It is generally agreed that climatic changes influence species’ distributions through species-specific physiological thresholds of temperature and precipitation tolerance [4]. With climate change, some habitats that are not currently suitable for zokors may be suitable in the future based on the direction of change in habitat conditions. Global average temperatures have increased by 0.2°C per decade since the 1970s, and global average precipitation increased by 2% in the last 100 years [30]. On the QTP, the climate change is mainly reflected by milder winters [22], which has resulted in phenological changes such as earlier breeding or peak in biomass of plants [4]. Observed impacts of climate change have been reported in relation to grassland ecosystems, its productivity, plant community composition and distribution, and soils [31]. Therefore climate change can potentially alter the temporal and spatial patterns of food availability and soil tunneling condition for zokors and cause the shift in distribution of these species. In this study, we use the plateau zokor (E. baileyi) as the focal species. Through the maximum entropy niche-based (Maxent) modeling and sustainable habitat modeling we analyse the potential effect of predicted climate change on the distributions of alpine subterranean rodents. Our objectives are to: (1) determine the environmental parameters that influence current plateau zokor distribution at QTP; (2) predict future changes in plateau zokor distribution and range shifts and (3) provide baseline information for management of alpine rangeland under climate change scenarios.
Materials and Methods
Study area and presence data collection
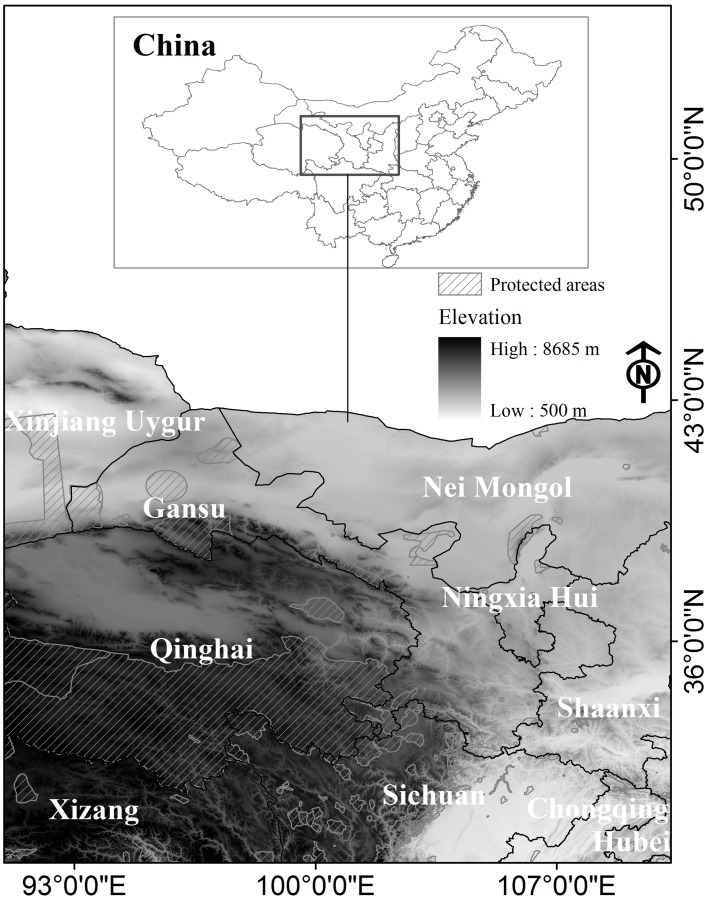
Our study areas covered the Qinghai-Tibetan Plateau (QTP) (Fig 1). We used world protected area network data from IUCN and UNE-WCMC 2015 [32] for this study. We collected the GPS location data of the plateau zokor species from published papers [33, 34], unpublished reports, and field surveys over 5 years from 2008 to 2012 (Fig 1). We identified 194 plateau zokor locations. We used 1-km2 grid cells for the maximum entropy niche-based (Maxent) modeling and sustainable habitat modeling [35]. We use one plateau zokor presence per cell and removed the repeated location data. A final 99 GPS points of plateau zokor presence are included for further analysis.
Fig 1. Study areas with elevation (https://lta.cr.usgs.gov/GMTED2010) and protected areas networks [32].
Data Modeling
We used Maxent model, a widely use tool for Species Distribution Models (SDMs), to predict current and future suitable habitat of zokor [35]. SDMs have been usedto understand different ecological questions relate to habitat preference and suitability, distribution, future prediction, conservation issue and evolutionary study of species [35]. Here, we used Maxent model to understand zokor distribution in different time series. We used current presence points of zokor and extracted nineteen bioclimatic variables of study area from www.worldclim.org [36], land use and land cover [37] (http://www.glcn.org). In ArcGIS, we prepared aspect and slope layers using a digital elevation model (DEM) (https://lta.cr.usgs.gov/GMTED2010) layer and clipped all variables to our study areas. We then extracted the values of each variable corresponding to the occurrence locations of plateau zokor to perform correlation analysis. After removing 11 highly correlated variables (>0.85; S1 Fig), we used the remaining 12 variables for our final analysis.
The MIROC5 (Model for Interdisciplinary Research on Climate) and General Circulation Model (GCM) [38] were used to project the future climate [39, 40]. GCM data were downscaled using the delta method and bias corrected by world clim’s current climate (http://worldclim.org/). We ran the MIROC5 model using the Representative Concentration Pathway 4.5, a “middle-of the-road” GHG (Green House Gas) scenario. We used two time periods for analysis: current and 2050 (average for 2041–2060; http://worldclim.org/cmip5_30s).
Results
Accuracy of species distribution models
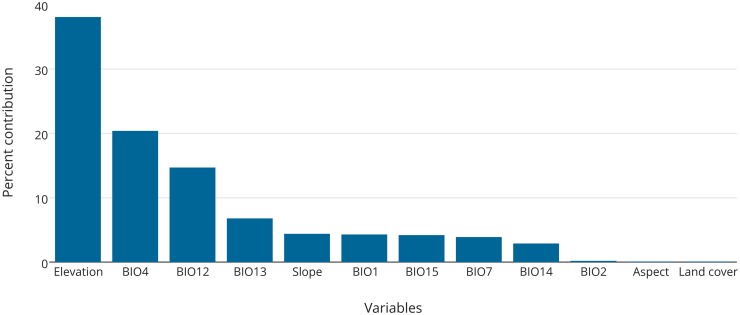
Overall, elevation (38%), precipitation of the driest month (BIO14; 20.4%) and annual precipitation (BIO12; 14.7%) were main contributing factors which contributed 72% for identification of zokor habitats (Fig 2). Habitat suitability of zokor is more related to precipitation related variables with an overall contribution of 50%, followed by temperature (BIO1; 4.3%), slope, aspect, land cover (Fig 2). The jackknife test also showed that elevation and precipitation were the main important variables (S1 Fig). The habitat suitability model was the best model as it has the omission rate very close to the predicted omission (cumulative threshold) (S2 Fig). This model described plateau zokor habitat very well (AUC = 0.99; S3 Fig). Response curves of each environmental variable have affected the distribution and predication of plateau zokor habitat and showed their effect on future prediction and current habitat of suitability of plateau zokor (S4 Fig).
Fig 2. Contribution variables for modeling.
Effect of precipitation, rainfall and other variables on plateau zokor habitat
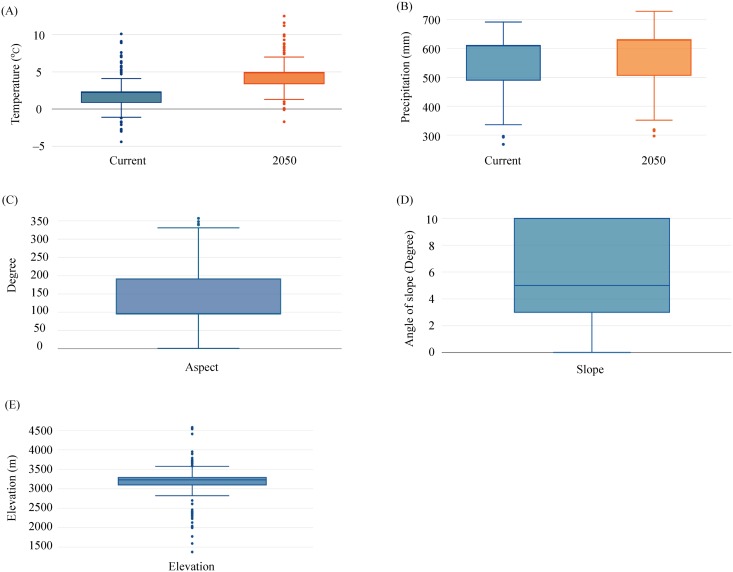
The mean annual temperatures of current suitable habitat of plateau zokor ranges from -4.4°C to 10.1°C (Fig 3A). The mean annual precipitations of current plateau zokor habitat ranges from 268 mm to 692 mm (Fig 3B). Based on the predicted climate change, the mean annual temperature will increase by 2.4°C in 2015 (current: 2.1+2.1°C; 2050: 4.6+2.1°C; means + standard deviation, SD) in plateau zokor habitat (Fig 3A) and mean precipitation will increase by 19 mm (current: 558.63+95.53; 2050: 577.12+98.60; mean+SD) (Fig 3B). The elevation was the main factors for predicting plateau zokor distribution. The mean altitude of areas that suitable as plateau zokor habitat was 3186 m±510; SD, mean aspect 140 (south east) and mean degree of slope 5.8 (Fig 3C and 3E).
Fig 3. Current and future temperature (a), precipitation (b) and current suitable habitat aspect (c), slope (d) and elevation (e), in plateau zokor habitat.
Predicting current and future habitat for plateau zokor
The total areas of current suitable habitat for plateau zokor species is 270074 km2 in China. Our model showed that currently Qinghai province provides the largest area of suitable habitat of plateau zokor (51.0%; 138223 km2) followed by Gansu province (35.9%; 96996 km2), Sinchuan province (7.6%; 20528 km2) and Xizang province (1.2%; 3428 km2) (Table 1).
Table 1. Current and future suitable zokor habitat in different province of China.
| Province | Current suitable habitat in km2 | Future (2050) suitable habitat in km2 | Change in % | Current suitable habitat in % |
|---|---|---|---|---|
| Gansu | 96996.35 | 101273.31 | +4.22 | 35.91 |
| Ningxia | 9661.61 | 10501.90 | +8.00 | 3.58 |
| Qinghai | 138223.00 | 149243.64 | +7.38 | 51.18 |
| Shaanxi | 1235.43 | 387.41 | -218.89 | 0.46 |
| Sichuan | 20528.76 | 23066.24 | +11.00 | 7.60 |
| Xizang | 3428.19 | 3561.90 | +3.75 | 1.27 |
| Total | 270073.39 | 288034.45 | +6.24% | |
| Suitable habitat within protected area system | 90452.74 | 99116.15 | +8.74% |
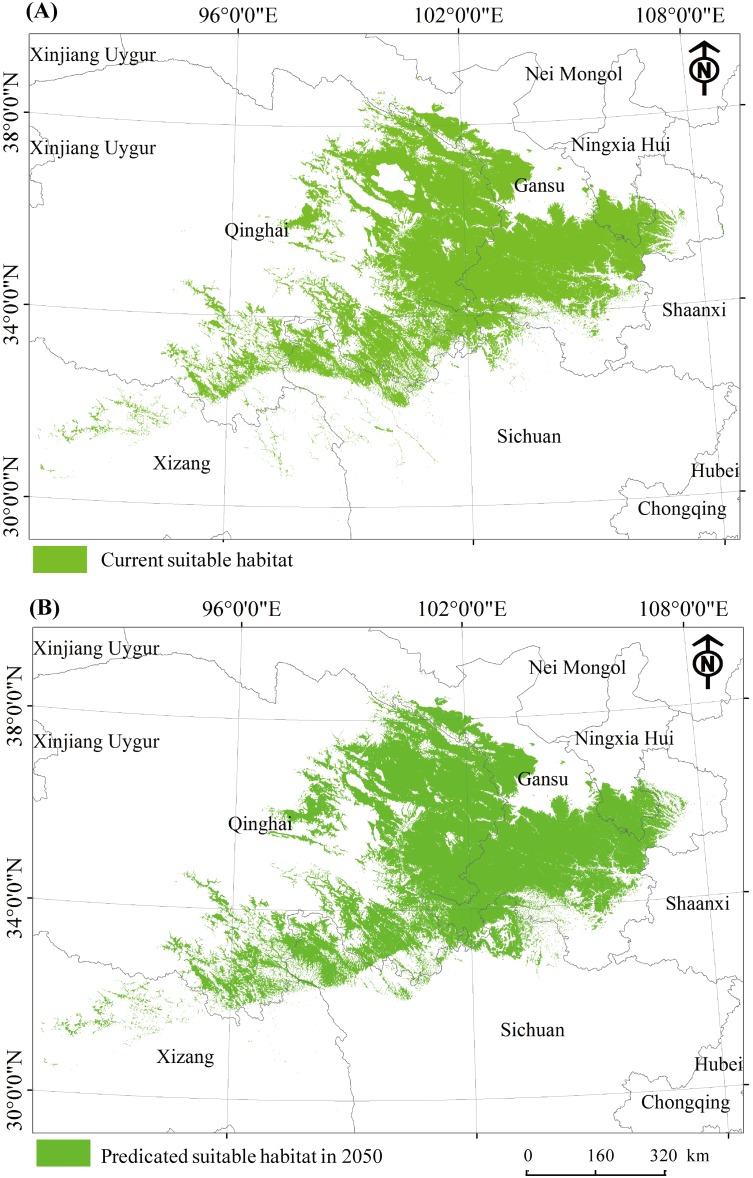
Our model predicated that the plateau zokor suitable habitats will increase by 6.2% (i.e. 288080 km2) according to the scenario of changing in global temperature and precipitation (Fig 4; Table 1). The highest increase in future suitable habitat predicted (7.0%; 138223 km2 to 149243 km2) is in Qinghai province, followed by Gansu province (4.0%; 96996 km2 to 101273 km2) and Sichuan province (11.0%; 20528 km2 to 23066 km2) (Table 1). Currently 33.0% (90452 km2) of total plateau zokor suitable areas lied inside the protected areas. The predicted climate change will results in an 8.7% increase in plateau zokor suitable areas inside the protected areas. The plateau zokor suitable habitat is predicted to reach 99116 km2 inside protected areas by 2050 (Fig 4).
Fig 4. The current (a) and predicated suitable habitat (b) of plateau zokor.
Range shifting
Our result showed that a higher degree of shift of plateau zokor suitable habitat will occur at lower elevation compare to higher elevation (Fig 4). In the southern range of current suitable habitat, plateau zokor is predicted to move further south by 4 km to 70 km distance, however their potential range shift in northern range would be less, ranging from 1 km to 14 km (Fig 4). Most of plateau zokor habitat is predicted to expand and shift towards north-east of current suitable habitat (Fig 4).
Discussion
Factors that influence current zokor distribution
Our best model, the habitat suitability model, was very close to the predicted omission rate, which also described plateau zokor habitat very well. Response curves of each environmental variable have affected the distribution and predication of plateau zokor habitat which showed their effect on the predicted future and current habitat suitability of plateau zokor.
Our SDM model, which incorporates 15 variables, predicted that elevation and precipitation are the most important variables that influence current plateau zokor distribution on QTP. Precipitation related variables, annual precipitation, precipitation of driest month and slope, aspect, land cover make up 50% of overall contributions to habitat suitability.
The elevation and precipitation are the most important factors affecting soil moisture and plateau zokors’ energetic balance. The plateau zokor burrow systems maintain relatively high moisture content, 79.0–87.3% [41, 42] when aboveground humidity was 31.7–53.3%. The high humidity contributes to economizing water balance by zokors [43]. Zeng et al (1984) found that the water loss of plateau zokor through evaporation, (0.79 mg/g) is lower than that of opening burrow animals such as plateau pika (Ochotona curzoniae) (1.38 mg/g) [41]. In addition, precipitation is also an important factor affecting energetic cost of tunneling by subterranean animals. High precipitation increase soil moisture and decrease soil compaction, which reduce the energetic cost of tunneling, making areas that were unsuitable for tunneling become suitable habitat for subterranean animals. In contrast, for tunneling small mammals such as plateau pikas (O. curzoniae) that are active above ground, temperature, instead of precipitation is the most important factor predicts habitat suitability [44, 45].
Range shifts of plateau zokor under the climate change scenarios
Niche model that correlate known occurrences of species with environmental variables and predict their potential distributions is a good method for assessing the risk of pest establishment [46]. Using this method, we predicted a range expansion of plateau zokor by 1km to 96 km from the current distribution in QTP. Interestingly, we found different patterns of range shift of zokor populations in high altitude and low altitude. In lower range of current suitable habitat, plateau zokor distribution is predicted to shift toward further lower (south) area by 4 km to 70 km. Less degree of range shift is predicted in northern region, by 1km to 14 km towards north (Fig 4). Plateau zokor is the only zokor species inhabit areas above 3000m. Their predicted range shift is likely to realize. At lower altitude, below 3000m, two zokor species, plateau zokor and Gansu zokor coexist. The potential interaction between the two species is likely to happen. Therefore, under climate change scenarios, there are three possible outcomes of their distribution patterns: 1) Increased overlap in distribution between the two species, 2) Gansu zokor would outcompete Plateau zokor resulting in restricting the expansion by plateau zokor at lower altitude or 3) Plateau zokor would out compete Gansu zokor resulting in expansion of plateau zokor in areas of lower altitude and reduction in distribution of Gansu zokor. Range expansion to lower altitude present plateau zokor with a new type of habitat, agricultural land. Abiotic factors, biotic interactions and dispersal factors are three key elements that determine distribution of species and their abundances at different scales [47]. How plateau zokor would respond to this new habitat worth investigation. SDMs are based on a niche conservatism hypothesis and particularly used to project the locations species will occupy following climate change [48, 49]. Niche is evolutionary, but in the evolution of the short history, it’s conservative [46, 50]. Variations in life history and foraging ecology can serve as a basis for grouping species’ responses to climate change [51]. Niche conservatism, adaptation and plasticity play important roles in response to climate change [52]. Current trend of climate change on QTP predicts phenological changes such as earlier thaw of soil and plant germination. This may lead to changes of zokor foraging activities and life history traits such as breeding patterns, and this also worth to investigate.
Implications for rangeland management under climate change
Rangeland management should anticipate how disturbance such as climate change may alter the geographic distributions of species that potentially contribute to the rangeland degradation. In areas that new invasion of zokors is predicted, the negative impact of this species on grassland health is also likely. Overgrazing is considered the main factor causing grassland degradation [23, 24], a condition at which the zokors are known to be problematic [53]. Therefore it is important to determine optimal grazing level of alpine grassland so that it can be effectively managed to maintain biodiversity and grassland health, in order to build the resilience against disturbances by such new invaders. Further empirical studies on how zokors and associated native species respond to climate change are important for informing future management and conservation of native alpine ecosystems. Conservation planning should incorporate not only the effects of climate change on the aboveground livings but also on the subterranean biota.
Supporting Information
(TIF)
(TIF)
(TIF)
(TIF)
(XLSX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Funding for this project was provided by the National Natural Science Foundation of China (Grant No. 31460566), National Science Foundation for Post-doctoral Scientists of China (Grant No. 2015M572614) and Agricultural Innovation Research Project of Gansu Province (Grant No. GNCX-2014-27). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421: 37–42. [DOI] [PubMed] [Google Scholar]
- 2. Pearson RG, Dawson TP (2003) Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Global Ecology and Biogeography 12: 361–371. [Google Scholar]
- 3. Thomas CD, Cameron A, Green RE, Bakkenes M, Beaumont LJ, Collingham YC, et al. (2003) Extinction risk from climate change. Nature 427: 145–148. [DOI] [PubMed] [Google Scholar]
- 4. Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, et al. (2002) Ecological responses to recent climate change. Nature 416: 389–395. [DOI] [PubMed] [Google Scholar]
- 5. Heller NE, Zavaleta ES (2009) Biodiversity management in the face of climate change: a review of 22 years of recommendations. Biological Conservation 142: 14–32. [Google Scholar]
- 6. Dawson TP, Jackson ST, House JI, Prentice IC, Mace GM (2011) Beyond predictions: biodiversity conservation in a changing climate. Science 332: 53–58. 10.1126/science.1200303 [DOI] [PubMed] [Google Scholar]
- 7. Davis MB, Shaw RG (2001) Range shifts and adaptive responses to Quaternary climate change. Science 292: 673–679. [DOI] [PubMed] [Google Scholar]
- 8. Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annual Review of Ecology, Evolution, and Systematics 37: 637–669. [Google Scholar]
- 9. Stachowicz JJ, Terwin JR, Whitlatch RB, Osman RW (2002) Linking climate change and biological invasions: ocean warming facilitates nonindigenous species invasions. Proceedings of the National Academy of Sciences 99: 15497–15500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lu XM, Siemann E, He MY, Wei H, Shao X, Ding JQ (2015) Climate warming increases biological control agent impact on a non-target species. Ecology Letters 18: 48–56. 10.1111/ele.12391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cammell ME, Knight JD (1992) Effects of climatic change on the population dynamics of crop pests. Advances in Ecological Research 22: 117–162. [Google Scholar]
- 12. Stenseth NC, Mysterud A, Ottersen G, Hurrell JW, Chan KS, Lima M (2002) Ecological effects of climate fluctuations. Science 297: 1292–1296. [DOI] [PubMed] [Google Scholar]
- 13. Gottfried M, Pauli H, Reiter K, Grabherr G (1999) A fine-scaled predictive model for changes in species distribution patterns of high mountain plants induced by climate warming. Diversity and Distributions 5: 241–251. [Google Scholar]
- 14. Hill JK, Griffiths HM, Thomas CD (2011) Climate change and evolutionary adaptations at species' range margins. Annual Review of Entomology 56: 143–159. 10.1146/annurev-ento-120709-144746 [DOI] [PubMed] [Google Scholar]
- 15. Carpenter KE, Abrar M, Aeby G, Aronson RB, Banks S, Bruckner A, et al. (2008) One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science 321: 560–563. 10.1126/science.1159196 [DOI] [PubMed] [Google Scholar]
- 16. Hellmann JJ, Byers JE, Bierwagen BG, Dukes JS (2008) Five potential consequences of climate change for invasive species. Conservation Biology 22: 534–543. 10.1111/j.1523-1739.2008.00951.x [DOI] [PubMed] [Google Scholar]
- 17. Davis MA, Douglas MR, Webb CT, Collyer ML, Holycross AT, Painter CW, et al. (2015) Nowhere to go but up: Impacts of climate change on demographics of a short-range endemic (Crotalus willardi obscurus) in the sky-islands of Southwestern North America. PLoS ONE 10: e0131067 10.1371/journal.pone.0131067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arthur AD, Pech RP, Jiebu, Zhang YM, Lin H (2007) Grassland degradation on the Tibetan Plateau: The role of small mammals and methods of control ACIAR Technical Reports No. 67, ACIAR, Canberra, pp 35. [Google Scholar]
- 19. Ficetola GF, Thuiller W, Miaud C (2007) Prediction and validation of the potential global distribution of a problematic alien invasive species-the American bullfrog. Diversity and Distributions 3: 476–485. [Google Scholar]
- 20. Tang LZ, Yu L, Lu WD, Wang JJ, Ma M, Shi XD, et al. (2013) Comparative analyses of past population dynamics between two subterranean zokor species and the response to climate changes. Turkish Journal of Zoology 37: 143–148. [Google Scholar]
- 21. Cannon RJ (1998) The implications of predicted climate change for insect pests in the UK, with emphasis on non-indigenous species. Global Change Biology 4: 785–796. [Google Scholar]
- 22. Harris RB (2010) Rangeland degradation on the Qinghai-Tibetan plateau: a review of the evidence of its magnitude and causes. Journal of Arid Environments 74: 1–12. [Google Scholar]
- 23. Zhou HK, Zhao XQ, Tang YH, Gu S, Zhou L (2005) Alpine grassland degradation and its control in the source region of the Yangtze and Yellow Rivers, China. Grassland Science 51: 191–203. [Google Scholar]
- 24. Wang QY, Bian JH, Shi YZ (1993) Influence of plateau zokors on the vegetation and soil nutrients in alpine meadow. Acta Theriologica Sinica 13: 31–37. [Google Scholar]
- 25. Fan NC. Rodent pest management in the Qinghai-Tibet alpine meadow ecosystem In: Singleton GR, Hind LA, Leirs H, Zhang ZB editors. Ecologically-based rodent management. Canberra: Australian Centre for International Agricultural Research; 1999. pp. 285–309. [Google Scholar]
- 26. Smith AT, Xie Y, Hoffmann RS, Lunde D, MacKinnon J, Wilson DE, et al. A guide to the mammals of China. New Jersey: Princeton University Press; 2010. [Google Scholar]
- 27. Su JH, Ji WH, Howitt R, Hua LM, Gleeson D, Duan HL (2014) Novel microsatellite markers obtained from Gansu zokor (Eospalax cansus) and cross-species amplification in Plateau zokor (Eospalax baileyi). Biochemical Systematics and Ecology 57: 128–132. [Google Scholar]
- 28. Zhang YM, Liu JK (2003) Effects of plateau zokors (Myospalax fontanierii) on plant community and soil in an alpine meadow. Journal of Mammalogy 84: 644–651. [Google Scholar]
- 29. Giorgi F, Lionello P (2008) Climate change projections for the Mediterranean region. Global and Planetary Change 63: 90–104. [Google Scholar]
- 30. IPCC. Climate Change 2007-The Physical Science Basis. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- 31. Wilkes A (2008) Towards mainstreaming climate change in grassland management policies and practices on the Tibetan Plateau WP number 67. Beijing, China, World Agroforestry Centre-ICRAF China, 43 [Google Scholar]
- 32. IUCN, UNEP-WCMC. The World Database on Protected Areas (WDPA) [On-line]. Cambridge, UK: UNEPWCMC; 2014. Available: www.protectedplanet.net Accessed 2014 November 21. [Google Scholar]
- 33. Tang LZ, Wang LY, Cai ZY, Zhang TZ, Ci HX, Lin GH, et al. (2010) Allopatric divergence and phylogeographic structure of the plateau zokor (Eospalax baileyi), a fossorial rodent endemic to the Qinghai-Tibetan Plateau. Journal of Biogeography 37: 657–668. [Google Scholar]
- 34. Zhao F, Deng XG, Zhang TZ, Su JP, Lin GH (2015) Molecular authentication of Sailonggu and its resource distribution in Qinghai-Tibet Plateau. China Journal of Chinese Materia Medica 40: 399–403. [PubMed] [Google Scholar]
- 35. Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of species geographic distributions. Ecological Modelling 190: 231–259. [Google Scholar]
- 36. Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25: 1965–1978. [Google Scholar]
- 37. Latham J, Cumani R, Rosati I, Bloise M (2014) Global Land Cover SHARE (GLC-SHARE) database Beta-Release Version 1.0–2014. FAO, Land and Water Division. [Google Scholar]
- 38. Watanabe M, Suzuki T, O'ishi R, Komuro Y, Watanabe S, Emori S, et al. (2010) Improved climate simulation by MIROC5: mean states, variability, and climate sensitivity. Journal of Climate 23: 6312–6335. [Google Scholar]
- 39. Sperber KR, Annamalai H, Kang IS, Kitoh A, Moise A, Turner A, et al. (2013) The Asian summer monsoon: an intercomparison of CMIP5 vs. CMIP3 simulations of the late 20th century. Climate Dynamics 41: 2711–2744. [Google Scholar]
- 40. Sharmila S, Joseph S, Sahai AK, Abhilash S, Chattopadhyay R (2015) Future projection of Indian summer monsoon variability under climate change scenario: An assessment from CMIP5 climate models. Global and Planetary Change 124: 62–78. [Google Scholar]
- 41. Zeng JX, Wang ZW, Shi ZX (1984) Metabolic characteristics and some physiological parameters of the mole rat (Myospalax baileyi) in an alpine area. Acta Biol. Plat. Sin. 3: 163–171. [Google Scholar]
- 42. Zhang YM. The biology and ecology of plateau zokors (Eospalax fontanierii) In: Begall S, Burda H, Schleich CE, editors. Subterranean rodents: news from underground. New York: Springer Berlin Heidelberg; 2007. pp. 237–249. [Google Scholar]
- 43. Frank C (1988) The effects of moldiness level on seed selection by Dipodomys spectabilis . Journal of Mammalogy 69:358–362. [Google Scholar]
- 44. Calkins MT, Beever EA, Boykin KG, Frey JK, Andersen MC (2012) Not-so-splendid isolation: modelling climate-mediated range collapse of a montane mammal Ochotona princeps across numerous ecoregions. Ecography 35: 780–791. [Google Scholar]
- 45. Phillips SJ, Dudík M (2008) Modelling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography 31: 161–175. [Google Scholar]
- 46. Peterson AT (2011) Ecological niche conservatism: A time-structured review of evidence. Journal of Biogeography 38: 817–827. [Google Scholar]
- 47. Kneitel JM, Chase JM (2004) Trade-offs in community ecology: linking spatial scale & species coexistence. Ecology Letter 7, 69–80. [Google Scholar]
- 48. Araújo MB, Alagador D, Cabeza M, Nogués-Bravo D, Thuiller W (2011) Climate change threatens European conservation areas. Ecology Letters 14: 484–492. 10.1111/j.1461-0248.2011.01610.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Elith J, Leathwick JR (2009) Species distribution models: ecological explanation and prediction across space and time. Annual Review of Ecology, Evolution, and Systematics 40: 677. [Google Scholar]
- 50. Wiens JJ, Ackerly DD, Allen AP, Anacker BL, Buckley LB, Cornell HV, et al. (2010) Niche conservatism as an emerging principle in ecology and conservation biology. Ecology Letters 3: 1310–1324. [DOI] [PubMed] [Google Scholar]
- 51. Winkler DW, Dunn PO, McCulloch CE (2002) Predicting the effects of climate change on avian life-history traits. Proceedings of the National Academy of Sciences 99: 13595–13599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ackerly DD (2003) Community assembly, niche conservatism, and adaptive evolution in changing environments. International Journal of Plant Sciences 164: S165–S184. [Google Scholar]
- 53. Zhang YM, Zhang ZB, Liu JK (2003) Burrowing rodents as ecosystem engineers: the ecology and management of plateau zokors Myospalax fontanierii in alpine meadow ecosystems on the Tibetan Plateau. Mammal Review 33: 284–294. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(TIF)
(TIF)
(XLSX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.